Abstract
Introduction:
We evaluated the heterogeneity of outcomes among heart failure patients with ventricular recovery.
Methods:
The BEST trial studied patients with left ventricular ejection fraction (LVEF) ≤35%. Serial LVEF assessment were performed at baseline, 3 months, and 12 months. Heart failure with better ejection fraction (HFbEF) was defined as an LVEF > 40% at any point.
Results:
Of the patients who survived to one year, 399 (21.3%) had HFbEF. Among subjects with HFbEF, 173 (43.4%) had “Extended”-recovery, 161 (40.4%) had “Late”-recovery and 65 (16.3%) patients had “Transient”-recovery. Subjects with HFbEF had an improved event-free survival from death or first HF-hospitalization compared to subjects without recovery (HR 0.50, 95%CI, 0.39–0.64, p<0.001). Compared to “Transient”-recovery, “Late”-and “Extended”-recovery were associated with an improved event-free survival from all-cause death and HF-hospitalization (HR 0.55, 95%CI, 0.34 – 0.90, p=0.016).
Discussion:
Our study shows patients with HFbEF to be a heterogeneous population with differing prognoses.
Keywords: Heart failure with reduced ejection fraction, heart failure with better ejection fraction, recovery
Introduction
More than 6.5 million Americans are afflicted with heart failure and the prevalence is expected to increase by more that 46% over the next 20 years (1). With therapeutic advances over the past two decades, the median survival after a diagnosis of heart failure with reduced ejection fraction (HFrEF) has increased from 1.5 years to 1.9 years (2). However, the absolute mortality among patients with HFrEF remains high and is comparable to other virulent diseases such as cancer (3). In contrast to the majority of patients with HFrEF, a subset of patients recover ventricular function. These patients with heart failure with better ejection fraction (HFbEF) have better survival compared to those patients with HFrEF who do not recover ventricular function (4–7). Thus, recovery of ventricular function should be considered a major goal in the contemporary management of patients with HFrEF.
Recovery of ventricular function is not uncommon, reported to occur in 10–70% of patients with HFrEF (5,6,8). Prior work has consistently identified patients who recover ventricular function to have a shorter duration of HF, more likely to be hypertensive, and more likely to have a non-ischemic cardiomyopathy. However, less is known about which patients have durable functional recovery and how the stability of recovery influences outcomes. Of critical importance is whether patients with HFbEF are truly recovered from their HF or simply have HF in remission with a risk for subsequent relapse (9). Results of the recent TRED-HF study suggest that patients with HFbEF who discontinue HF treatment are at increased risk for relapse to HFrEF (10). These findings have led to the recommendation that HF therapies be continued indefinitely until patients at risk for relapse can be differentiated from those with recovery (10). Here, using a large well-characterized cohort of patients with HFrEF, we used stability of ventricular function to identify prognostically distinct phenotypes of HFbEF.
Methods
Study population
We obtained the dataset for the Beta-blocker in Evaluation Survival Trial (BEST) from the NHLBI’s BioLINCC clinical trial repository (11). The design and primary results of the trial have been previously published (12,13). Briefly, BEST randomized 2708 patients with left-ventricular ejection fraction (LVEF) ≤35% and New York Heart Association (NYHA) class III/IV symptoms to bucindolol or placebo. The BEST study was carried out at 90 clinical sites across the United States and Canada from 1995–1998 and co-sponsored by the NHLBI and Department of Veterans Affairs. Randomized subjects were >18 years of age and had a mean follow-up of 49 months. The majority of subjects received angiotensin converting enzyme inhibitors (ACE-I) or angiotensin receptor blockers (ARB) (90%) and diuretics (90%) during the study. Use of optimal medical therapy was required for at least 1 month prior to randomization. At the time of the BEST trial, mineralocorticoid receptor antagonists, implanted cardiac defibrillators (ICDs), or cardiac resynchronization therapy were not widely used for HFrEF. Patients were not eligible for the study if they were awaiting heart transplantation or found to be in acute decompensated HF. The BEST trial was stopped early due to non-significant changes in mortality between the study arms.
Assessment of Left Ventricular Ejection Fraction and other Functional Parameters
Serial assessments of LVEF, were performed using gated-equilibrium radionuclide ventriculography (MUGA) at baseline, at 3 months, and then again at 12 months after study enrollment. For this study, we only included patients with MUGA data for all three time points, meaning that all subjects included in our analysis were alive for at least one-year after study enrollment. As a quality control measure during the study, the first two LVEF examinations at each site were also evaluated by a central core laboratory. Subsequently, 5% of all the LVEF studies were randomly reevaluated by the core laboratory.
In addition to LVEF, MUGA was used to measure right ventricular function and peak filling rate. Peak filling rate measures the end-diastolic volume per second and is a marker of diastolic function. Both were measured serially as described for LVEF but were not evaluated by the core laboratory.
Study Definitions and Outcomes
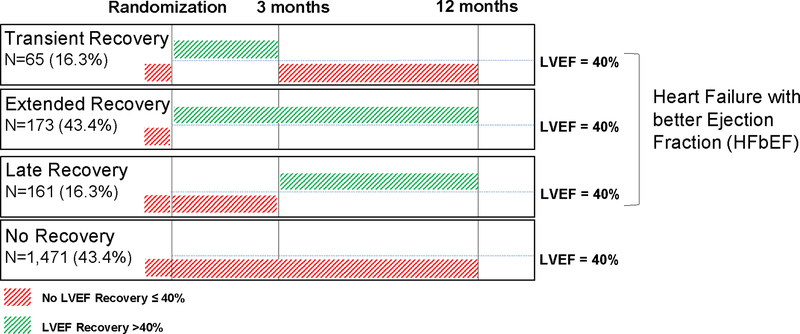
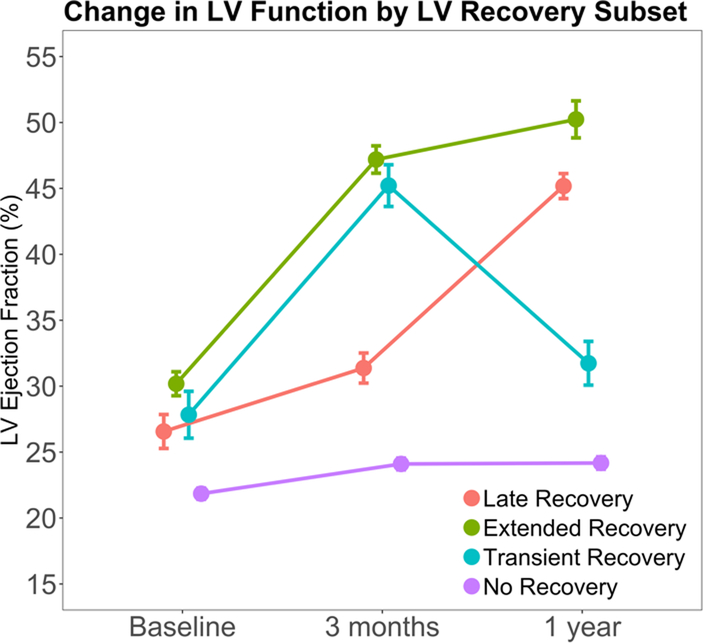
We divided the study cohort into groups based on LVEF dynamics over time (Figure 1). Patients with HFbEF were defined to have an LVEF > 40% at any point during the study. We chose 40% as a threshold, because these patients would no longer meet criteria for a primary prevention implantable cardiac defibrillator, potentially linking the HFbEF phenotype to a clinical decision node (14). By contrast, patients with LVEF < 40% through the first 12 months of the study were classified as having “No recovery.” We further defined patients with HFbEF to have 1) “Transient” recovery if LVEF was > 40% at 3 months, but ≤ 35% at 12 months; 2) “Extended” recovery if patients had an EF > 40% at 3 months and at 12 months and 3) “Late” recovery if patients had an LVEF of ≤ 35% at 3 months, but > 40% at 12 months (Figure 1). Actual change in LVEF for each individual group is presented in Figure 2.
Figure 1.
Longitudinal trend of left ventricular ejection fraction in patients enrolled to the BEST trial.
Figure 2.
Change in LVEF by recovery subset. Abbreviations: LV = left ventricular
In the present study, the primary outcome was a composite of all-cause mortality (the BEST primary outcome) and time to first HF hospitalization (a BEST secondary outcome). Our secondary outcomes were cardiovascular (CV) mortality, time to first HF hospitalization, and the composite of CV mortality and time to first HF hospitalization. Outcomes were adjudicated by an independent committee as a part of the BEST trial.
Statistical analysis
Baseline continuous variables are displayed using the median with 25th and 75th percentiles, while categorical variables are summarized using counts with percentages for non-missing variables. Comparisons were done using either the Kruskal-Wallis test or the chi-square test for continuous and categorical variables, respectively, to identify differences in baseline characteristics between recovery versus no recovery groups (Table 1) and across recovery type groups (Table 2).
Table 1.
Baseline characteristics between patients with and without left ventricular ejection fraction recovery using both matched and unmatched groups.
| No Recovery† (N=1471) | No Recovery‡ (N=399, Matched) | Recovery (N=399) | P-value (std.)† | P-value (std.)‡ | |
|---|---|---|---|---|---|
| Patient age, years | 62 (53, 70) | 62 (52, 68) | 60 (50, 68) | 0.028 (0.13) | 0.886 (0.02) |
| Male sex | 1177 (80.0%) | 289 (72.4%) | 289 (72.4%) | 0.001 (0.18) | 0.750 (0.02) |
| Race | 0.751 (0.08) | 0.948 (<0.01) | |||
| White | 1059 (72.0%) | 283 (70.9%) | 284 (71.2%) | ||
| Black, not Hispanic | 319 (21.7%) | 89 (22.3%) | 88 (22.1%) | ||
| Hispanic | 71 (4.8%) | 20 (5.0%) | 18 (4.5%) | ||
| Other | 22 (1.5%) | 7 (1.8%) | 9 (2.3%) | ||
| Baseline EF, percentage | 22 (17, 27) | 28 (24, 32) | 30 (25, 33) | <0.001 (1.00) | <0.001 (0.23) |
| HF Duration, months | 37 (15, 72) | 27 (10, 60) | 17 (4, 50) | <0.001 (0.34) | <0.001 (0.18) |
| Atrial fibrillation or flutter | 344 (23.4%) | 91 (22.8%) | 88 (22.1%) | 0.576 (0.03) | 0.799 (0.02) |
| History of Diabetes | 533 (36.2%) | 150 (37.6%) | 142 (35.6%) | 0.812 (0.01) | 0.557 (0.04) |
| History of Hypertension | 822 (55.9%) | 223 (55.9%) | 251 (62.9%) | 0.012 (0.14) | 0.044 (0.14) |
| CAD etiology | 907 (61.7%) | 187 (46.9%) | 157 (39.3%) | <0.001 (0.46) | 0.032 (0.15) |
| **Other Etiologies | n=575 | n=214 | n=246 | ||
| Drug induced etiology | 13 (2.3%) | 4 (1.9%) | 7 (2.8%) | 0.619 (0.04) | 0.494 (0.06) |
| Alcoholic cardiomyopathy | 80 (13.9%) | 21 (9.8%) | 36 (14.6%) | 0.786 (0.02) | 0.118 (0.15) |
| Hypertension induced | 127 (22.1%) | 53 (24.8%) | 68 (27.6%) | 0.087 (0.13) | 0.485 (0.07) |
| Viral etiology | 39 (6.8%) | 12 (5.6%) | 18 (7.3%) | 0.783 (0.02) | 0.459 (0.07) |
| Idiopathic etiology | 386 (67.1%) | 149 (69.6%) | 153 (62.2%) | 0.173 (0.10) | 0.094 (0.16) |
| Familial etiology | 19 (3.3%) | 6 (2.8%) | 13 (5.3%) | 0.179 (0.10) | 0.182 (0.13) |
| BUN, mg/dL | 20 (15, 27) | 18 (14, 25) | 18 (14, 25) | 0.003 (0.19) | 0.921 (0.05) |
| Creatinine, mg/dL | 1.1 (1.0, 1.4) | 1.1 (0.9, 1.4) | 1.1 (0.9, 1.3) | 0.004 (0.15) | 0.515 (0.06) |
| Hemoglobin, g/dL | 14.2 (13.0, 15.2) | 14.2 (13.0, 15.3) | 14.1 (13.0, 15.1) | 0.391 (0.03) | 0.515 (0.02) |
| Potassium, mEq/L | 4.3 (4.0, 4.6) | 4.3 (4.0, 4.6) | 4.2 (4.0, 4.6) | 0.349 (0.03) | 0.712 (<0.01) |
| Sodium, mEq/L | 139 (137, 141) | 139 (137, 141) | 139 (137, 141) | 0.178 (0.08) | 0.443 (0.05) |
| **PNE level | n=1160 | n=311 | n=310 | 0.022 (0.16) | 0.827 (0.02) |
| 410 (293, 598) | 387 (270, 536) | 398 (270, 548) | |||
| BMI, kg/m2 | 27.2 (24.0, 31.2) | 27.6 (24.4, 31.9) | 28.5 (24.2, 33.2) | 0.004 (0.16) | 0.313 (0.05) |
| Systolic BP, mmHg | 115 (104, 130) | 120 (110, 140) | 126 (112, 140) | <0.001 (0.53) | 0.037 (0.15) |
| Diastolic BP, mmHg | 70 (64, 80) | 73 (68, 80) | 76 (68, 84) | <0.001 (0.36) | 0.138 (0.11) |
| JVD at 30 degrees | 671 (45.7%) | 168 (42.1%) | 159 (40.1%) | 0.045 (0.11) | 0.571 (0.05) |
| Conduction LBBB | 398 (27.1%) | 82 (20.6%) | 72 (18.1%) | <0.001 (0.22) | 0.370 (0.06) |
| Baseline QRS, msec | 120 (100, 160) | 110 (100, 140) | 100 (90, 120) | <0.001 (0.54) | 0.021 (0.14) |
| Treatment = Bucindolol | 693 (47.1%) | 221 (55.4%) | 252 (63.2%) | <0.001 (0.33) | 0.026 (0.16) |
Note: Data reported as median (25th, 75th percentiles) or count (percentage). EF = ejection fraction; HF = heart failure; CAD = coronary artery disease; BUN = blood urea nitrogen; PNE = plasma norepinephrine; BMI = body mass index; BP =blood pressure; JVD = jugular venous distention; LBBB = left bundle branch block.
Variables not used for propensity matching due to missing values.
P-value and standardized difference (std.) comparing the recovery and unmatched no recovery groups (all patients).
P-value and standardized difference (std.) comparing the recovery and propensity matched no recovery groups.
Table 2.
Baseline characteristics by recovery type.
| Total (N=1870) | No Recovery (N=1471) | Extended Recovery (N=173) | Late Recovery (N=161) | Transient Recovery (N=65) | P-value | |
|---|---|---|---|---|---|---|
| Patient age, years | 61 (52, 69) | 62 (53, 70.0) | 59 (50, 67) | 60 (49, 67) | 64 (55, 72) | 0.004 |
| Male sex | 1466 (78.4%) | 1177 (80.0%) | 120 (69.4%) | 126 (78.3%) | 43 (66.2%) | 0.001 |
| Race | 0.163 | |||||
| White | 1343 (71.8%) | 1059 (72.0%) | 132 (76.3%) | 109 (67.7%) | 43 (66.2%) | |
| Black, not Hispanic | 407 (21.8%) | 319 (21.7%) | 34 (19.7%) | 36 (22.4%) | 18 (27.7%) | |
| Hispanic | 89 (4.8%) | 71 (4.8%) | 6 (3.5%) | 11 (6.8%) | 1 (1.5%) | |
| Other | 31 (1.7%) | 22 (1.5%) | 1 (0.6%) | 5 (3.1%) | 3 (4.6%) | |
| Baseline EF, percentage | 24 (18, 29) | 22 (17, 27) | 32 (28, 34) | 28 (21, 32) | 30 (27, 33) | <0.001 |
| Duration of HF, months | 36 (12, 67) | 37 (15, 72) | 14 (4, 42) | 16 (5, 54) | 36 (9, 60) | <0.001 |
| Atrial fibrillation or flutter | 432 (23.1%) | 344 (23.4%) | 33 (19.1%) | 42 (26.1%) | 13 (20.0%) | 0.423 |
| History of Diabetes | 675 (36.1%) | 533 (36.2%) | 68 (39.3%) | 45 (28.0%) | 29 (44.6%) | 0.059 |
| History of Hypertension | 1073 (57.4%) | 822 (55.9%) | 113 (65.3%) | 95 (59.0%) | 43 (66.2%) | 0.045 |
| CAD etiology | 1064 (56.9%) | 907 (61.7%) | 61 (35.3%) | 65 (40.4%) | 31 (47.7%) | <0.001 |
| **Other Etiologies | n=821 | n=575 | n=115 | n=96 | n=35 | |
| Drug induced etiology | 20 (2.4%) | 13 (2.3%) | 5 (4.3%) | 2 (2.1%) | 0 (0.0%) | 0.429 |
| Alcoholic cardiomyopathy | 116 (14.1%) | 80 (13.9%) | 16 (13.9%) | 15 (15.6%) | 5 (14.3%) | 0.977 |
| Hypertension induced | 195 (23.8%) | 127 (22.1%) | 37 (32.2%) | 18 (18.8%) | 13 (37.1%) | 0.017 |
| Viral etiology | 57 (6.9%) | 39 (6.8%) | 8 (7.0%) | 9 (9.4%) | 1 (2.9%) | 0.614 |
| Idiopathic etiology | 539 (65.7%) | 386 (67.1%) | 68 (59.1%) | 64 (66.7%) | 21 (60.0%) | 0.352 |
| Familial etiology | 32 (3.9%) | 19 (3.3%) | 5 (4.3%) | 6 (6.3%) | 2 (5.7%) | 0.507 |
| BUN, mg/dL | 19 (15, 27) | 20 (15, 27) | 19 (15, 25) | 18 (14, 25) | 19 (13, 27) | 0.022 |
| Creatinine, mg/dL | 1.1 (0.9, 1.4) | 1.1 (1.0, 1.4) | 1.1 (0.9, 1.3) | 1.1 (0.9, 1.3) | 1.1 (0.9, 1.3) | 0.031 |
| Hemoglobin, g/dL | 14.2 (13.0, 15.2) | 14.2 (13.0, 15.2) | 13.9 (12.7, 15.1) | 14.2 (13.3, 15.2) | 14.2 (13.1, 14.8) | 0.321 |
| Potassium, mEq/L | 4.3 (4.0, 4.6) | 4.3 (4.0, 4.6) | 4.2 (4.0, 4.6) | 4.2 (4.0, 4.6) | 4.3 (3.9, 4.6) | 0.766 |
| Sodium, mEq/L | 139 (137, 141) | 139 (137, 141) | 139 (137, 141) | 139 (137, 141) | 141 (138, 142) | 0.021 |
| PNE level | n=1470 | n=1160 | n=138 | n=122 | n=50 | 0.145 |
| 407 (286, 583) | 410 (293, 598) | 391 (278, 517) | 397 (281, 548) | 410 (225, 575) | ||
| Body mass index, kg/m2 | 27.4 (24.0, 31.5) | 27.2 (24.0, 31.2) | 29.6 (24.7, 34.0) | 26.9 (24.1, 30.6) | 28.6 (25.1, 33.0) | 0.001 |
| Systolic BP, mmHg | 118 (106, 130) | 115 (104, 130) | 130 (112, 142) | 122 (110, 140) | 126 (112, 140) | <0.001 |
| Diastolic BP, mm Hg | 70 (64, 80) | 70 (64, 80) | 76 (67, 84) | 76 (68, 84) | 72 (66, 80) | <0.001 |
| JVD at 30 degrees | 830 (44.5%) | 671 (45.7%) | 72 (42.1%) | 65 (40.4%) | 22 (33.8%) | 0.150 |
| Conduction LBBB | 470 (25.2%) | 398 (27.1%) | 25 (14.5%) | 36 (22.5%) | 11 (16.9%) | 0.001 |
| Baseline QRS (ms) | 120 (100, 150) | 120 (100, 160) | 100 (90, 120) | 110 (90, 140) | 100 (90, 120) | <0.001 |
| Treatment = Bucindolol | 945 (50.5%) | 693 (47.1%) | 129 (74.6%) | 86 (53.4%) | 37 (56.9%) | <0.001 |
Note: Data presented as median (25th, 75th percentiles) or as count (percentage). EF = ejection fraction; HF = heart failure; CAD = coronary artery disease; BUN = blood urea nitrogen; PNE = plasma norepinephrine; BMI = body mass index; BP = blood pressure; JVD = jugular venous distention; LBBB = left bundle branch block.
Kaplan-Meier methods, including the log-rank test, were used to compare HFbEF groups (Figures 3, 4). Cox proportional hazard models were utilized to determine the prognostic importance of recovery (HFbEF vs no recovery), as well across HFbEF categories and the primary and secondary clinical outcomes, including CV death, all-cause death, HF hospitalization, as well as combined outcomes. The Kolmogorov–type supremum test and graphical evaluation of the hazard function over time were used to evaluate the proportional hazard and linearity assumptions of the Cox models. Fine and Gray’s method for competing risks were used to estimate the cumulative incidence function for models evaluating HF hospitalization or CV death to adjust for all-cause deaths that prevent the event of interest from occurring. Results are presented using the hazard ratio (HR) with 95% CI (Table 3).
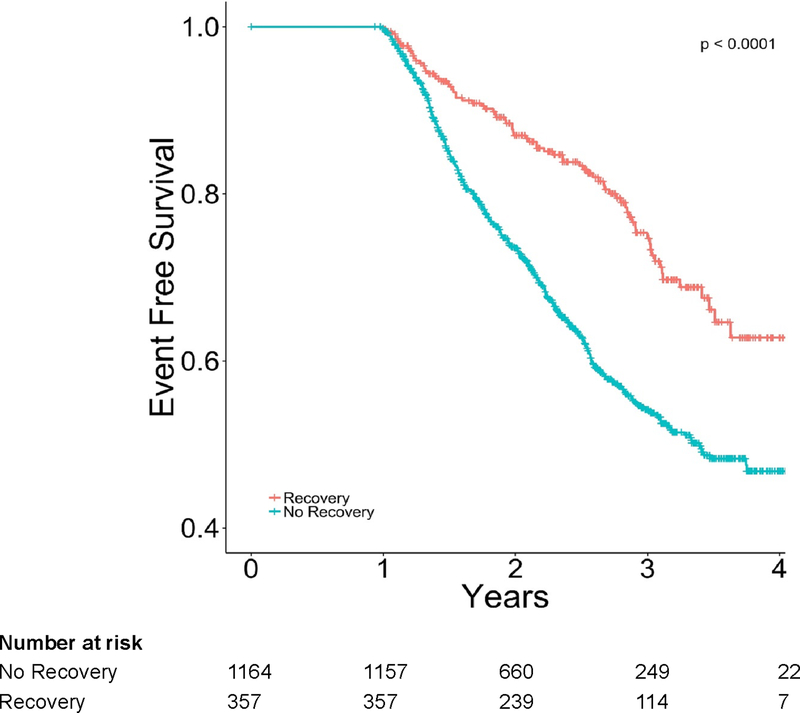
Figure 3.
Kaplan-Meier Plot for freedom from death or first heart failure hospitalization by recovery status.
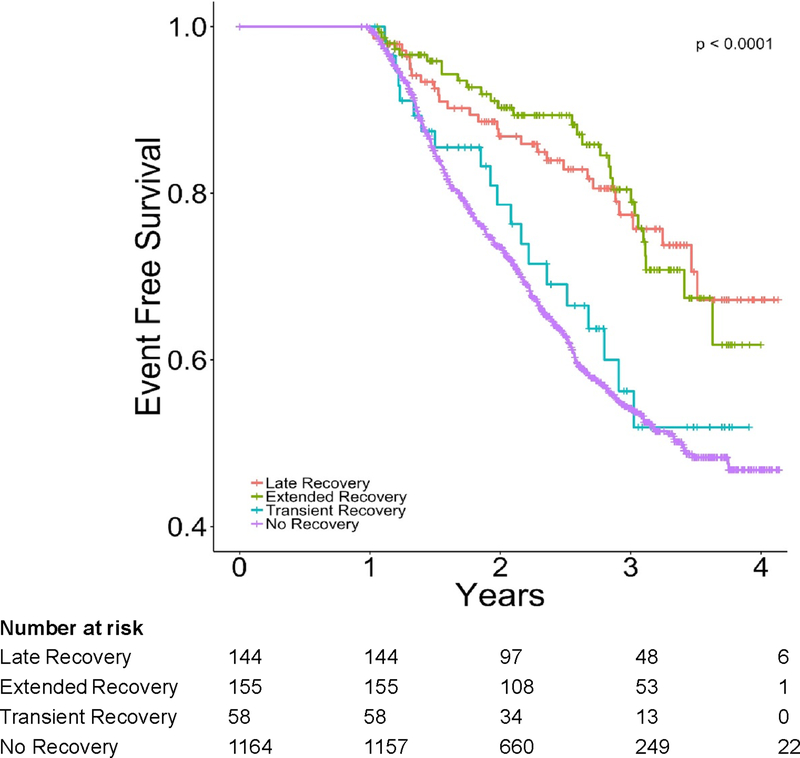
Figure 4.
Kaplan-Meier plot for freedom from death or first heart failure hospitalization by recovery subtype.
Table 3:
Cox proportional hazards (PH) models for all-cause death, cardiovascular death, heart failure re-hospitalization, and combined events by individual recovery groups and no recovery versus any recovery.
| Endpoint | Individual Recovery Groups | No Recovery vs Any Recovery | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | **Adjusted | |||||||
| Group | HR (95% CI) | P-value | Group | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Combined (All-Cause Death or HF Re-hosp.) | No Recovery | 1.0 | No Recovery | 1.0 | 1.0 | |||
| Extended | 0.44 (0.30–0.64) | <0.001 | 0.50 (0.39–0.64) | <0.001 | 0.56 (0.41–0.76) | <0.001 | ||
| Late | 0.44 (0.30–0.64) | <0.001 | Recovery | |||||
| Transient | 0.86 (0.55–1.35) | 0.511 | ||||||
| Combined (CV Death or HF Re-hosp.) | No Recovery | 1.0 | No Recovery | 1.0 | 1.0 | |||
| Extended | 0.34 (0.23–0.52) | <0.001 | 0.46 (0.36–0.60) | <0.001 | 0.59 (0.44–0.79) | <0.001 | ||
| Late | 0.46 (0.32–0.68) | <0.001 | Recovery | |||||
| Transient | 0.81 (0.51–1.30) | 0.384 | ||||||
| All-Cause Death | No Recovery | 1.0 | No Recovery | 1.0 | 1.0 | |||
| Extended | 0.46 (0.30–0.71) | <0.001 | 0.44 (0.33–0.60) | <0.001 | 0.60 (0.42–0.85) | 0.004 | ||
| Late | 0.36 (0.22–0.58) | <0.001 | Recovery | |||||
| Transient | 0.63 (0.35–1.15) | 0.133 | ||||||
| CV Death | No Recovery | 1.0 | No Recovery | 1.0 | 1.0 | |||
| Extended | 0.32 (0.19–0.56) | <0.001 | 0.36 (0.25–0.51) | <0.001 | 0.55 (0.36–0.84) | 0.005 | ||
| Late | 0.33 (0.19–0.57) | <0.001 | Recovery | |||||
| Transient | 0.54 (0.27–1.08) | 0.081 | ||||||
| HF Re-hosp. | No Recovery | 1.0 | No Recovery | 1.0 | <0.001 | 1.0 | 0.004 | |
| Extended | 0.38 (0.23–0.62) | <0.001 | 0.52 (0.38–0.69) | 0.58 (0.40–0.84) | ||||
| Late | 0.54 (0.35–0.84) | 0.006 | Recovery | |||||
| Transient | 0.83 (0.48–1.42) | 0.495 | ||||||
Note: HF = heart failure; CV = cardiovascular; HR = hazard ratio; CI = confidence interval.
Propensity matched analysis using N=399 matched pairs as described in Table 1 and further adjusted by variables with standardized differences >0.10 after matching.
Propensity score matching (1:1) of recovery and no recovery groups was then conducted using a logistic regression model consisting of baseline characteristics as detailed in Table 1. The maximum allowable absolute caliper difference between propensity scores was 0.20. The relationship between recovery group and each clinical outcome was then evaluated by a multivariable Cox model with a robust sandwich covariance to account for intracluster dependence further adjusted for any baseline variables with standardized differences >0.10 after matching.
Multivariable logistic regression models were used to identify independent predictors of any HFbEF versus no recovery after adjusting for confounders found significantly different at baseline within Table 1, as well as amongst HFbEF recovery subgroups. A univariable p < 0.10 was required to enter the model and p < 0.05 to stay in the backward regression models. Regression splines were used for non-linear predictors (e.g. HF duration) and results presented as odds ratio (OR) with 95% confidence interval (CI) (Table 4, Supplemental Table 1).
Table 4.
Independent predictors of HFbEF.
| Variable | OR (95% CI) | P-value |
|---|---|---|
| Ischemic cardiomyopathy | 0.354 (0.272–0.462) | < 0.001 |
| Treatment with bucindolol | 2.194 (1.680–2.865) | < 0.001 |
| Baseline LVEF | 1.157 (1.131–1.184) | < 0.001 |
| Systolic blood pressure | 1.017 (1.010–1.024) | < 0.001 |
| Heart failure duration (linear spline) | ||
| < 30 months | 0.956 (0.943–0.969) | < 0.001 |
| ≥ 30 months | 1.002 (0.998–1.006) | 0.435 |
| QRS Duration | 0.986 (0.982–0.991) | < 0.001 |
Note: OR = odds ratio; CI = confidence interval; LVEF = left ventricular ejection fraction.
All statistical tests were two-sided with a p < 0.05 considered statistically significant. Data analyses were performed by the Duke Department of Biostatistics and Bioinformatics (Durham, NC) using SAS version 9.4 (SAS, Institute, Inc., Cary, NC).
Results
Baseline Characteristics and Heart Failure Classification of the Study Population
Among the patients enrolled in the BEST trial with HF and baseline EF ≤ 35% at the index visit, 1870 survived to one year and had longitudinal imaging data (69% of the original trial cohort). “No recovery” of LVEF was seen in 1471 (78.7%) of the patients. The remaining 399 patients (21.3%) were classified as having HFbEF. In comparison to patients with “No recovery,” patients with HFbEF were younger (median age, 60 years [25th, 75th %iles: 50–68] vs 62 [25th, 75th %iles: 53–70], p=0.028), were less likely to be male (72.4% vs 80%, p=0.001), had a shorter duration of HF [median = 17 months (25th, 75th %iles: 4–50) vs 37 months (25th, 75th %iles: 15–72), p<0.001], had a higher LVEF at randomization [median = 30% (25th, 75th %iles: 25–33) vs 22% (25th, 75th %iles: 17–27), p<0.001], were less likely to have an ischemic cardiomyopathy (39% vs 62%, p<0.001), and were more likely to be randomized to bucindolol (63% vs 47%, p<0.001) (Table 1). Duration of heart failure, baseline LVEF, absence of an ischemic cardiomyopathy, and treatment with bucindolol were also significant predictors of HFbEF in a separate propensity-matched analysis (Table 1). Among patients with HFbEF, 173 (43.4%) had “Extended” recovery, 161 (40.4%) had “Late” recovery and 65 (16.3%) patients had “Transient” recovery. Compared to patients who experienced “Late” or “Transient” recovery, patients with “Extended” recovery had a shorter duration of HF, were less likely to have an ischemic cardiomyopathy, had a higher LVEF at baseline, had a higher blood pressure, and were more likely to have been randomized to treatment with bucindolol (Table 2).
Additional parameters captured on MUGA such as right ventricular function and peak filling rate (PFR) (end diastolic volume/sec) followed changes to left ventricular function (Supplemental Figure 1).
HFbEF Phenotypes and Outcomes
Subjects with HFbEF lived longer and had fewer HF hospitalizations compared to subjects with “No recovery,” (Figure 3). The hazard ratio for event-free survival from death or first HF hospitalization of the HFbEF group compared to the group with “No recovery” was 0.50 (95% CI, 0.39–0.64, p<0.001). The favorable outcomes of patients with HFbEF compared to “No recovery” extended across all other combined or individual clinical endpoints such as all-cause death (HR 0.44, [95% CI 0.33–0.60], p<0.001), CV death (HR 0.36, [95% CI 0.25–0.51], p<0.001), and time to first HF hospitalization (HR 0.52, [95% CI 0.38–0.69], p<0.001) (Table 3). In a separate propensity-matched analysis, patients with HFbEF had persistently better outcomes (Table 3). When HFbEF was analyzed by subtype, the composite endpoints of all-cause death and time to first HF hospitalization were very similar between the “No recovery” and “Transient” recovery groups (HR 0.86, [95% CI 0.55–1.35], p=0.511) (Table 3). By contrast, outcomes were more favorable in the “Extended” and “Late” recovery subgroups, which appeared to be comparable to each other. The risk for event-free survival from all-cause death and time to first HF hospitalization with either “Late” or “Extended” recovery compared to the “Transient” recovery group was HR 0.55 (95% CI, 0.34 – 0.90, p=0.016). The findings were comparable across all composite and individual clinical endpoints (Table 3).
Independent Predictors of Heart Failure Recovery Phenotypes
We next evaluated independent predictors of HFbEF through the first year of study follow-up. Multivariable analyses revealed that patients with HFbEF were more likely to have a non-ischemic cardiomyopathy, shorter duration of HF, higher baseline LVEF, and to be treated with bucindolol compared to those who did not recover their LVEF (Table 4). Factors predictive of “Transient” recovery versus either “Extended” or “Late” recovery were older age (HR 1.028 [95% CI 1.003–1.053], p=0.029), shorter duration of HF (HR 1.024 [95% CI 1.010–1.038], p<0.001), and higher baseline serum sodium (≥ 137 mEq/L versus < 137 mEq/L) (HR 1.612 [95% CI 1.144–2.272], p=0.006) (Supplemental Table 1).
Discussion
Recovery of LVEF among patients with HFrEF has been widely associated with a favorable prognosis (5,6,8). Indeed, a previous analysis of the BEST study demonstrated that as little as a ≥5% change in LVEF over the 12 months is associated with reduced heart failure hospitalizations and improved survival (7). Yet, although HFbEF has been widely described, the time course of recovery and relapse is not well defined. Key elements for devising care strategies for patients with HFbEF include a better understanding of the long-term outcomes and the determinants of relapse to HFrEF (10). Our secondary analysis of the BEST study extends prior work by linking the dynamics of functional recovery and relapse to clinical outcomes. We found that HFbEF comprises a heterogeneous group of patients, not only with variation in the timing of left ventricular recovery but also the persistence of ventricular recovery. Notably, these findings also extend to right ventricular systolic and left ventricular diastolic function suggesting that LVEF improvement is a surrogate for global myocardial recovery. Despite better outcomes in patients with HFbEF as a whole (50% reduction in all-cause mortality and HF hospitalization), our study demonstrates specific phenotypes amongst patients with HFbEF. Our most striking finding is that “Transient” LVEF recovery was not associated with improved clinical outcomes, a unique result of our study, not previously demonstrated in a large cohort. Indeed, we found relapse of ventricular function to be associated with outcomes comparable to patients without recovery. Because interruption of medical therapy is linked to a recurrent drop in LVEF, our findings support the notion that patients with HFbEF should be continued on guideline directed medical therapy and periodically monitored for functional relapse (10,15).
In our study population, HFbEF patients were more likely to have a non-ischemic cardiomyopathy, HF of shorter duration, higher baseline LVEF, and to be treated with bucindolol compared to those who did not recover their LVEF. These findings are highly consistent with the results of other work (5,16,17). Such a strong signature of recovery potential suggests an innate biology that underlies functional recovery. Unique to our analysis, we found that shorter duration of HF was also associated with “Extended” recovery compared to “Transient” recovery. Thus, we postulate that duration of heart failure serves as a key threshold for predicting the potential for relapse. How and why duration of heart failure modifies recovery potential is unclear, but may be related to scar burden and the cumulative level of myocyte injury. This premise is consistent with prior work suggesting that baseline scar burden provides important prognostic information on subsequent left ventricular remodeling and adverse events (18,19). Somewhat unexpectedly, we found treatment with bucindolol to be strongly associated with recovery of ventricular function, although bucindolol failed to reduce mortality in the original analysis of the BEST trial and is not considered to be an evidence-based therapy for HFrEF (13). Subsequent work has suggested that bucindolol has different levels of efficacy in patients with HFrEF based on polymorphisms of the ADRB1 gene (20). Whether our association of bucindolol treatment with HFbEF signals a primary effect of bucindolol or is an indicator for genetic variants that promote HFbEF is uncertain, but likely highlights a role for innate myocyte biology in modulating reverse remodeling. Additional translational studies will be needed to further elucidate mechanisms and predictors of functional recovery.
Our study drew its strength from a large sample size and a prolonged follow-up with repeated high fidelity LVEF measurement. Further, clinical events were adjudicated by an independent committee. However, our analysis has some limitations that need to be acknowledged. Background therapy in the BEST trial was limited to ACE inhibitors and ARBs. MRAs and cardiac resynchronization therapy were not widely utilized at the time of the study. Since these therapies are well-known to affect reverse remodeling and recovery of LVEF, our findings might underestimate recovery potential in current practice. Of note, similar analyses in a more contemporary trial and real-world cohort appear to observe a similar if not higher proportion of LVEF improvement despite similar predictors of LVEF recovery (6,17,21). A second potential issue is the definition of recovery. We chose to define HFbEF as an increase in LVEF from ≤ 35% at baseline to > 40% at 3 or 12 months based on LVEF thresholds used to guide the implantation of primary prevention ICDs. While this in congruent with prior studies, other cut-offs used to define improved LVEF include >45% (22) and >50% (23). Standardization of HFbEF definitions is likely needed for prospective studies focused on patients with HFbEF.
Conclusions
Patients who recover their LVEF are a heterogenous cohort with approximately 20% likely to relapse to HFrEF. Understanding what factors differentiate patients with “Transient” recovery from more durable recovery may lead to better approaches for promoting true recovery from HFrEF compared to remission. Additionally, because patients with “Transient” recovery of their LVEF go on to have similar outcomes to patients without recovery, patients with HFbEF should undergo ongoing treatment and surveillance to prevent relapse of ventricular dysfunction.
Supplementary Material
Footnotes
Conflict of Interest: MF is supported by AHA Grant 17MCPRP33460225 and the NHLBI T32 postdoctoral training grant 5T32HL007101-42. Consulting for Coridea, AxonTherapies and Galvani. RK is supported by an NIH Mentored Clinical Scientist Award (K08-HL116485), a Duke University School of Medicine Strong Start Award, the Walker P. Inman Endowment, and the Edna and Fred L. Mandel, Jr. Foundation.
No human or animal studies were carried out by the authors for this article
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vasan RS, Xanthakis V, Lyass A et al. Epidemiology of Left Ventricular Systolic Dysfunction and Heart Failure in the Framingham Study: An Echocardiographic Study Over 3 Decades. JACC Cardiovascular imaging 2018;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Askoxylakis V, Thieke C, Pleger ST et al. Long-term survival of cancer patients compared to heart failure and stroke: a systematic review. BMC Cancer 2010;10:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basuray A, French B, Ky B et al. Heart failure with recovered ejection fraction: clinical description, biomarkers, and outcomes. Circulation 2014;129:2380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Florea VG, Rector TS, Anand IS, Cohn JN. Heart Failure With Improved Ejection Fraction: Clinical Characteristics, Correlates of Recovery, and Survival: Results From the Valsartan Heart Failure Trial. Circulation Heart failure 2016;9. [DOI] [PubMed] [Google Scholar]
- 6.Kalogeropoulos AP, Fonarow GC, Georgiopoulou V et al. Characteristics and Outcomes of Adult Outpatients With Heart Failure and Improved or Recovered Ejection Fraction. JAMA Cardiol 2016;1:510–8. [DOI] [PubMed] [Google Scholar]
- 7.Breathett K, Allen LA, Udelson J, Davis G, Bristow M. Changes in Left Ventricular Ejection Fraction Predict Survival and Hospitalization in Heart Failure With Reduced Ejection Fraction. Circ Heart Fail 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Punnoose LR, Givertz MM, Lewis EF, Pratibhu P, Stevenson LW, Desai AS. Heart failure with recovered ejection fraction: a distinct clinical entity. Journal of cardiac failure 2011;17:527–32. [DOI] [PubMed] [Google Scholar]
- 9.Mann DL, Barger PM, Burkhoff D. Myocardial recovery and the failing heart: myth, magic, or molecular target? J Am Coll Cardiol 2012;60:2465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halliday BP, Wassall R, Lota AS et al. Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED-HF): an open-label, pilot, randomised trial. Lancet 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. https://biolincc.nhlbi.nih.gov/studies/best/?q=BEST.
- 12.Design of the Beta-Blocker Evaluation Survival Trial (BEST). The BEST Steering Committee. The American journal of cardiology 1995;75:1220–3. [DOI] [PubMed] [Google Scholar]
- 13.Beta-Blocker Evaluation of Survival Trial I, Eichhorn EJ, Domanski MJ, Krause Steinrauf H, Bristow MR, Lavori PW. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med 2001;344:1659–67. [DOI] [PubMed] [Google Scholar]
- 14.Al-Khatib SM, Stevenson WG, Ackerman MJ et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2017. [DOI] [PubMed] [Google Scholar]
- 15.Moon J, Ko YG, Chung N et al. Recovery and recurrence of left ventricular systolic dysfunction in patients with idiopathic dilated cardiomyopathy. The Canadian journal of cardiology 2009;25:e147–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilcox JE, Fonarow GC, Yancy CW et al. Factors associated with improvement in ejection fraction in clinical practice among patients with heart failure: findings from IMPROVE HF. American heart journal 2012;163:49–56 e2. [DOI] [PubMed] [Google Scholar]
- 17.Chang KW, Beri N, Nguyen NH et al. Heart Failure With Recovered Ejection Fraction in African Americans: Results From the African-American Heart Failure Trial. Journal of cardiac failure 2018;24:303–309. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Guallar E, Weiss RG et al. Associations between scar characteristics by cardiac magnetic resonance and changes in left ventricular ejection fraction in primary prevention defibrillator recipients. Heart Rhythm 2016;13:1661–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon DH, Halley CM, Carrigan TP et al. Extent of left ventricular scar predicts outcomes in ischemic cardiomyopathy patients with significantly reduced systolic function: a delayed hyperenhancement cardiac magnetic resonance study. JACC Cardiovascular imaging 2009;2:34–44. [DOI] [PubMed] [Google Scholar]
- 20.Liggett SB, Mialet-Perez J, Thaneemit-Chen S et al. A polymorphism within a conserved beta(1)-adrenergic receptor motif alters cardiac function and beta-blocker response in human heart failure. Proc Natl Acad Sci U S A 2006;103:11288–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lupon J, Diez-Lopez C, de Antonio M et al. Recovered heart failure with reduced ejection fraction and outcomes: a prospective study. European journal of heart failure 2017;19:1615–1623. [DOI] [PubMed] [Google Scholar]
- 22.de Groote P, Fertin M, Duva Pentiah A, Goeminne C, Lamblin N, Bauters C. Long-term functional and clinical follow-up of patients with heart failure with recovered left ventricular ejection fraction after beta-blocker therapy. Circulation Heart failure 2014;7:434–9. [DOI] [PubMed] [Google Scholar]
- 23.Joyce E, Chung C, Badloe S et al. Variable Contribution of Heart Failure to Quality of Life in Ambulatory Heart Failure With Reduced, Better, or Preserved Ejection Fraction. JACC Heart Fail 2016;4:184–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.