Article Summary
HMG-CoA reductase inhibitors (statins) have been proposed to both positively and negatively alter the cognitive trajectory of older adults. To further investigate the disparate findings (provided by the FDA black box warning, adverse event reports, case-control studies, observational studies, and randomized controlled trials) that have led to such proposals, this study explored the association between statin use and longitudinal cognitive change, as well as time to diagnostic conversion, using prospective longitudinal (i.e., 24 months follow-up) cohort data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI). No associations between statin use and cognitive change were found for participants with cognitively normal status, late mild cognitive impairment, or dementia due to Alzheimer’s disease. However, there was an association between statin use and slower decline on a memory composite in participants with early mild cognitive impairment. Lastly, results did not support an association between statin use and conversion to a more severe diagnostic category for any diagnostic group. Thus, these findings do not provide support for the FDA black box warning that claims statin use causes cognitive deficits, but rather, suggest that additional randomized trials investigating statin use in participants in the prodromal early mild cognitive impairment stage of Alzheimer’s disease may be warranted.
Objective:
To investigate associations between statin use and cognitive change, as well as diagnostic conversion, in individuals with cognitively normal (CN) status, mild cognitive impairment (MCI), and dementia due to Alzheimer’s disease (AD-dementia).
Methods:
A multi-center cohort study with 1629 adults 48 to 91 years-old with CN status, early MCI (EMCI), late MCI (LMCI), or AD-dementia at baseline followed prospectively for 24 months. Statin use was assessed at baseline, and cognition was measured over time with a composite memory score, a composite executive function score, and a global cognition score (Alzheimer’s Disease Assessment Scale). Conversion to a more impaired diagnostic category was determined by clinician assessment. Repeated measures linear mixed effects models were used to evaluate associations between statin use and change in cognition over time. Cox proportional hazards models were used to evaluate associations between statin use and time to diagnostic conversion. All models were stratified by baseline diagnostic group.
Results:
Statin use was not associated with change in cognitive measures for CN, LMCI, or AD-dementia participants. Among EMCI participants, statin use was associated with a significantly slower rate of decline on the memory composite, but no other cognitive measure. Statin use was not associated with time to conversion for any diagnostic group.
Conclusions:
This study did not support an association between statin use and diagnostic conversion but suggested a possible association between statin use and cognitive change in EMCI. Additional randomized clinical trials of statins may be warranted in the prodromal EMCI stage of AD.
Keywords: ADNI, Alzheimer’s Disease, statin, memory, executive function
I. Objective
The 2013 American College of Cardiology and American Heart Association practice guidelines recommend physicians prescribe HMG-CoA reductase inhibitors (statins) to patients with high cholesterol and to patients with or at high risk for atherosclerotic cardiovascular disease, regardless of cholesterol levels [1]. According to the National Center for Health Statistics (NCHS), from 2011 to 2012, 1 in 4 adults ages 40 years and older were taking prescription cholesterol lowering medications, 93% of which were statins [2]. The NCHS recently reported that cholesterol-lowering drugs continue to be one of the most commonly used prescriptions in adults ages 40–79 during the year 2019 [3].
Statins have been proposed to alter the cognitive trajectory of older adults in several ways. Adverse event reports and two early randomized controlled trials suggested an association between short term statin use and cognitive deficits [4, 5]. These findings led to the black box FDA warning that prescribing statins could lead to cognitive changes, including memory loss and confusion, despite a preponderance of evidence showing no effect of statins on cognition [6].
Alternatively, long-term statin use has been theorized to lower the risk of dementia, including dementia due to Alzheimer’s disease (AD-dementia), and to slow the rate of clinical deterioration. That is, since cardiovascular disease is associated with an increased risk of dementia, treatment with statins has been proposed to reduce dementia risk by reducing cardiovascular burden [7]. Furthermore, certain statins may cross the blood-brain barrier to cause an anti-inflammatory effect within the central nervous system, purportedly reducing the risk of dementia [8]. Finally, substantial preclinical research [9] and some small clinical biomarker trials [10, 11] suggest that lowering cholesterol may modify AD pathogenesis thereby slowing clinical decline.
Several [12, 13, 14], but not all [15, 16], cohort and case-control studies have suggested a protective effect of statins against incident AD. However, randomized controlled trials in participants with CN status [17, 18] or AD-dementia [19, 20] have failed to confirm a beneficial effect of statins. To investigate further the association between statin use and cognitive change at various stages of AD, we utilized data collected in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) study. ADNI is a multi-center observational cohort study that enrolls individuals as having cognitively normal (CN) status, early MCI (EMCI), late MCI (LMCI), or AD-dementia. We tested associations between statin use and both change in cognitive performance over time and conversion to a more impaired diagnostic category.
II. Methods
Data used in the preparation of this article were obtained from the ADNI database (adni.loni.usc.edu) on September 28, 2014. ADNI was launched in 2003 as a public-private partnership led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD-dementia. A committee on human research at each participating institution approved the study protocol, and all participants gave their informed consent. At the time of download, the dataset contained 1629 adults (48 to 91 years-old) with the following baseline diagnostic classification: 418 CN, 308 EMCI, 561 LMCI, and 342 AD-dementia. The present study analyzed data up to 24 months follow-up, with follow-up timepoints at 6 months, 12 months, and 24 months. In addition, data regarding diagnostic conversion was collected for LMCI participants at 18 months. All 1629 participants were included in statistical analyses as they had data from at least one follow-up visit over the course of 24-months follow-up. CN participants had no memory complaints, normal memory performance, and an absence of impairment in cognition or function. EMCI participants had a subjective memory concern, mildly abnormal memory performance, and preserved functional performance such that a diagnosis of AD-dementia could not be made. LMCI participants had a subjective memory concern, memory performance that was abnormal and below that of EMCI participants, and preserved functional performance such that a diagnosis of AD-dementia could not be made [21]. AD-dementia participants had a subjective memory concern, abnormal memory performance, and functional impairment meeting NINCDS/ADRDA criteria for probable AD-dementia [22]. Detailed information describing diagnostic criteria can be found at www.adniinfo.org.
Outcomes
Statin use was assessed at baseline by reviewing participant medication lists for current use of any HMG-CoA reductase inhibitor (e.g., simvastatin, atorvastatin, rosuvastatin, etc.) at any dose. As in our previous report [23], primary outcomes used in this analysis included a composite z-score of memory performance (ADNI-Mem), a composite z-score of executive function (ADNI-EF), and a global cognition score measured with the Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-11) [24, 25, 26]. To test the additional primary outcome of time to diagnostic conversion, visit diagnosis was used to determine if a participant converted from CN to MCI (early or late) or MCI to AD-dementia. Apolipoprotein E (APOE) ε4 allele number, Clinical Dementia Rating Scale sum of boxes (CDRsb), ADAS-11, Mini-Mental State Exam (MMSE), and cardiovascular comorbidities listed in Table 1 were utilized to characterize the study cohort.
Table 1A:
Baseline Participant Characteristics
| CN | EMCI | |||||||
|---|---|---|---|---|---|---|---|---|
| No Statin | Statin | F or χ2 (df) | p-value | No Statin | Statin | F or χ2 (df) | p-value | |
| N (%) | 221 (53%) | 197 | - | - | 148 (48%) | 160 | - | - |
| Age (years) | 74.4 (5.9) | 75.0 (5.6) | 1.16 (416) | 0.688 | 70.8 (7.7) | 71.6 (7.4) | 0.62 (306) | 0.431 |
| Sex (% male) | 42.1 | 58.9 | 11.76 (1) | 0.001* | 50.0 | 60.6 | 3.52 (1) | 0.061 |
| Years of Education | 16.1 (2.9) | 16.5 (2.6) | 0.82 (416) | 0.366 | 16.1 (2.6) | 15.8 (2.7) | 0.13 (306) | 0.717 |
| APOE ε4 Alleles (%) | ||||||||
| 0 ε4 | 71.4 | 73.8 | 2.07 (2) | 0.356 | 61.4 | 52.5 | 2.62 (2) | 0.269 |
| 1 ε4 | 26.8 | 22.6 | - | - | 33.1 | 39.2 | - | - |
| 2 ε4 | 1.8 | 3.6 | - | - | 5.5 | 8.2 | - | - |
| CDRsb | 0.04 (0.13) | 0.03 (0.13) | 1.44 (416) | 0.231 | 1.15 (0.65) | 1.43 (0.83) | 4.95 (306) | 0.027* |
| ADAS-11 | 5.8 (2.8) | 6.2 (3.2) | 0.45 (416) | 0.505 | 7.5 (3.7) | 8.2 (3.4) | 0.14 (306) | 0.709 |
| MMSE | 29.1 (1.0) | 29.0 (1.2) | 0.71 (416) | 0.402 | 28.3 (1.6) | 28.3 (1.5) | 1.29 (306) | 0.257 |
| Comorbid Diagnoses (%) | ||||||||
| HTN | 38.9 | 52.8 | 8.09 (1) | 0.004* | 39.2 | 56.9 | 9.63 (1) | 0.002* |
| DM | 4.5 | 10.7 | 5.71 (1) | 0.017* | 4.1 | 16.9 | 13.21 (1) | <0.001* |
| HCL | 14.0 | 76.6 | 166.16 (1) | <0.001* | 16.2 | 78.8 | 120.34 (1) | <0.001* |
| CAD | 1.8 | 4.6 | 2.63 (1) | 0.105 | 0.7 | 5.6 | 6.00 (1) | 0.014* |
| Means (SD) are reported for continuous variables stratified by statin use for each group, F-value from ANOVA, χ2 from chi-square test, *p < 0.05. SD = standard deviation, CN = cognitively normal, EMCI = early Mild Cognitive Impairment, df = degrees of freedom, CDRsb = Clinical Dementia Rating Scale-Sum of Boxes Score, ADAS-11 = Alzheimer’s Disease Assessment Scale-Cognitive Subscale, MMSE = Mini-Mental State Exam, HTN = Hypertension, DM = Diabetes Mellitus, HCL = Hypercholesterolemia, CAD = Coronary Artery Disease | ||||||||
| Table 1B: Baseline Participant Characteristics | ||||||||
|---|---|---|---|---|---|---|---|---|
| LMCI | AD-dementia | |||||||
| No Statin | Statin | F or χ2 (df) | p-value | No Statin | Statin | F or χ2 (df) | p-value | |
| N (%) | 271 (48%) | 290 | - | - | 169 (49%) | 173 | - | - |
| Age (years) | 74.0 (8.0) | 73.9 (7.2) | 2.08 (559) | 0.150 | 75.0 (8.2) | 75.0 (7.4) | 1.42 (340) | 0.969 |
| Sex (% male) | 60.5 | 61.7 | 0.09 (1) | 0.769 | 50.3 | 60.1 | 3.33 (1) | 0.082 |
| Years of Education | 15.8 (3.1) | 16.0 (2.8) | 3.26 (559) | 0.071 | 15.2 (3.2) | 15.2 (2.8) | 6.30 (340) | 0.939 |
| APOE ε4 Alleles (%) | ||||||||
| 0 ε4 | 52.6 | 39.1 | 14.56 (2) | 0.001* | 35.1 | 32.4 | 0.78 (2) | 0.110 |
| 1 ε4 | 38.9 | 43.6 | - | - | 44.6 | 49.4 | - | - |
| 2 ε4 | 8.5 | 17.3 | - | - | 20.2 | 18.2 | - | - |
| CDRsb | 1.64 (0.87) | 1.65 (0.97) | 2.69 (559) | 0.101 | 4.48 (1.77) | 4.31 (1.55) | 1.58 (340) | 0.329 |
| ADAS-11 | 11.4 (4.7) | 11.6 (4.4) | 2.82 (559) | 0.094 | 19.4 (7.0) | 19.4 (6.7) | 0.00 (340) | 0.996 |
| MMSE | 27.2 (1.8) | 27.1 (1.8) | 0.09 (559) | 0.765 | 23.2 (2.1) | 23.3 (2.0) | 0.50 (340) | 0.854 |
| Comorbid Diagnoses (%) | ||||||||
| HTN | 37.6 | 59.0 | 25.51 (1) | <0.001* | 41.4 | 59.0 | 10.52 (1) | 0.007* |
| DM | 3.7 | 12.4 | 14.16 (1) | <0.001* | 6.5 | 12.7 | 3.78 (1) | 0.056 |
| HCL | 12.2 | 77.9 | 243.71 (1) | <0.001* | 21.3 | 82.7 | 129.02 (1) | <0.001* |
| CAD | 2.6 | 5.2 | 2.49 (1) | 0.114 | 0.6 | 4.6 | 5.43 (1) | 0.306 |
| Means (SD) are reported for continuous variables stratified by statin use for each group, F-value from ANOVA, χ2 from chi-square test, *p < 0.05. SD = standard deviation, LMCI = late Mild Cognitive Impairment, AD-dementia = dementia due to Alzheimer’s disease, df = degrees of freedom, CDRsb = Clinical Dementia Rating Scale - Sum of Boxes Score, ADAS-11 = Alzheimer’s Disease Assessment Scale-Cognitive Subscale, MMSE = Mini-Mental State Exam, HTN = Hypertension, DM = Diabetes Mellitus, HCL = Hypercholesterolemia, CAD = Coronary Artery Disease | ||||||||
Statistical Analyses
Independent analyses were performed for each of the 4 baseline diagnostic groups: CN, EMCI, LMCI, or AD-dementia. Baseline characteristics were compared between statin users and nonusers with analysis of variance (ANOVA) for continuous variables and chi-square (χ2) for categorical variables. To assess for attrition bias, a comparison of baseline characteristics, including statin use, was performed between participants with 24 months of follow-up data and those without 24 months of follow-up (Supplementary Tale 1).
Three repeated measures linear mixed effects models with random subject-specific intercepts were used to test for associations between statin use and longitudinal cognitive change, with ADNI-Mem, ADNI-EF, or ADAS-11 as the outcome variable and the interaction of statin use and time (measured continuously in months) as the main explanatory variable. Covariates in the models included age, sex, APOE ε4 allele number, years of education, and presence of hypertension (HTN), diabetes mellitus (DM), hypercholesterolemia (HCL), and coronary artery disease (CAD), as well as all interactions with time. Primary analyses were uncorrected for multiplicity, although false discovery rate (FDR) corrections were also applied to control for type I error in the case of multiple comparisons [27]. FDR corrections were applied separately for each of the baseline diagnostic groups for a total of three hypothesis tests per group. Parameter estimates are reported for each model and its respective cognitive outcome and can be interpreted as rate of change over time in either ADAS-score, memory score, or executive function score.
Survival curves of conversion from CN to MCI and MCI to AD-dementia were calculated using the Kaplan-Meier method and used to assess the proportional hazards assumption. Cox proportional hazards models were used to assess the time to diagnostic conversion for statin users versus nonusers. The models were adjusted for the same covariates as in the linear mixed effects models, with the addition of baseline ADAS-11 scores to account for baseline disease stage. The confidence level for statistical inference was 95% (p < 0.05), applied either before or after FDR correction. Statistical analyses were performed using SPSS Statistics Version 24.0.
Power Analyses
Power analyses were performed to investigate the effect sizes corresponding to various levels of type II error. For repeated measures linear mixed effects models, power analyses were performed using simulation with MATLAB R2015a and the Statistics Toolbox (see Supplementary Methods for more details). Study data parameters were used for baseline mean cognitive scores (ADNI-Mem, ADNI-EF, ADAS-11), standard deviation, and standard error of the parameter estimate for change in cognitive score over time. Statin use was the main explanatory variable and covariates were not included. Power to detect group differences was estimated by simulation over a range of rates of decline.
III. Results
CN Participants.
Of the 418 CN participants who had a baseline visit, 197 (47%) were taking a statin. Statin users included a larger percentage of males (Table 1). As might be expected, statin users also had a higher incidence of comorbid diagnoses of HTN, DM, and HCL at baseline than nonusers (Table 1). There were no significant differences between statin users and nonusers in age, education, APOE ε4 allele number, CDRsb, ADAS-11, or comorbid diagnosis of CAD at baseline (Table 1). There was no significant difference (t(416) = 1.27, p = 0.205) in the average follow-up time between statin users (22.05 months) and nonusers (21.26 months).
EMCI Participants.
Of the 308 EMCI participants who had a baseline visit, 160 (52%) were taking a statin. Statin users had higher CDRsb scores at baseline, compared to nonusers (Table 1). Statin users had a higher incidence of HTN, DM, HCL, and CAD at baseline (Table 1). There were no significant differences between statin users and nonusers in age, sex, education, APOE ε4 allele number, or ADAS-11 at baseline (Table 1). There was no significant difference (t(306) = 0.79, p = 0.430) in the average follow-up time between statin users (20.70 months) and nonusers (20.03 months).
LMCI Participants.
Of the 561 LMCI participants who had a baseline visit, 290 (52%) were taking a statin. Statin users were more likely to be a carrier of one or two APOE ε4 alleles than nonusers (Table 1). In addition, statin users had a higher incidence of HTN, DM, and HCL at baseline (Table 1). There were no significant differences between statin users and nonusers in age, sex, education, CDRsb, ADAS-11, or comorbid diagnosis of CAD at baseline (Table 1). There was no significant difference (t(559) = 1.51, p = 0.131) in the average follow-up time between statin users (20.79 months) and nonusers (19.90 months).
AD-dementia Participants.
Of the 342 AD-dementia participants who had a baseline visit, 173 (51%) were taking a statin. Statin users had a higher incidence of HTN and HCL diagnoses at baseline (Table 1). There were no significant differences between statin users and nonusers in age, sex, education, ADAS-11, APOE ε4 allele number, CDRsb, or comorbid diagnosis of DM or CAD at baseline (Table 1). There was no significant difference (t(339) = 0.10, p = 0.921) in the average follow-up time between statin users (16.23 months) and nonusers (16.32 months). Supplementary Table 1 contains a detailed summary of participant characteristics relative to attrition and stratified by diagnostic group.
Association Between Statin Use and Longitudinal Cognitive Performance: Repeated Measures Linear Mixed Effects Models
The association between statin use and change in memory, executive function, or global cognitive performance over time was investigated using a linear repeated measures mixed effects model with the interaction of statin use (users vs. nonusers) and time (in months) as the main explanatory variable and either ADNI-Mem score, ADNI-EF score, or ADAS-11 score over time as the outcome variable (Table 2). All covariates, and their interactions with time, that contributed significantly to the model variance are listed in Supplementary Table 2.
Table 2:
Association between Statin Use and Change in Cognition over Time
| CN | EMCI | |||||
|---|---|---|---|---|---|---|
| Parameter Estimate (SE) | F (dfN, dfD) | p-value | Parameter Estimate (SE) | F (dfN, dfD) | p-value | |
| Memory | 0.000 (0.002) | 0.001 (1, 1155) | 0.980 | 0.006 (0.003) | 5.55 (1, 799) | 0.019* |
| EF | −0.002 (0.003) | 0.67 (1, 1150) | 0.415 | −0.004 (0.004) | 1.44 (1, 795) | 0.231 |
| ADAS-11 | 0.004 (0.017) | 0.05 (1, 1161) | 0.818 | −0.033 (0.027) | 1.51 (1, 791) | 0.220 |
| LMCI | AD-dementia | |||||
| Parameter Estimate (SE) | F (dfN, dfD) | p-value | Parameter Estimate (SE) | F (dfN, dfD) | p-value | |
| Memory | −0.002 (0.002) | 1.11 (1, 1482) | 0.293 | −0.004 (0.003) | 2.11 (1, 765) | 0.147 |
| EF | 0.002 (0.002) | 0.40 (1, 1467) | 0.526 | −0.003 (0.003) | 0.99 (1, 742) | 0.321 |
| ADAS-11 | 0.035 (0.025) | 1.97 (1, 1489) | 0.160 | 0.078 (0.057) | 1.86 (1, 778) | 0.173 |
Parameter Estimates, F-values, and p-values are for separate repeated measures linear mixed effects models in which the interactions of statin use and time are the main explanatory variables. Parameter estimates can be interpreted as rate of change over time in either ADAS-score, memory score, or executive function score. Separate models were fit for each diagnostic group with each cognitive outcome (12 models total). The models were adjusted for age, sex, education, APOE ε4 allele number, and comorbid cardiovascular diagnoses, including HTN, DM, HCL, and CAD, and all interactions with time,
p < 0.05 prior to (false discovery rate [FDR]) correction for multiple comparisons.
CN = cognitively normal, EMCI = early Mild Cognitive Impairment, LMCI = late Mild Cognitive Impairment, AD-dementia = dementia due to Alzheimer’s disease, EF = Executive Function, ADAS-11 = Alzheimer’s Disease Assessment Scale-Cognitive Subscale, SE = standard error, dfN = numerator degrees of freedom, dfD = denominator degrees of freedom
CN Participants.
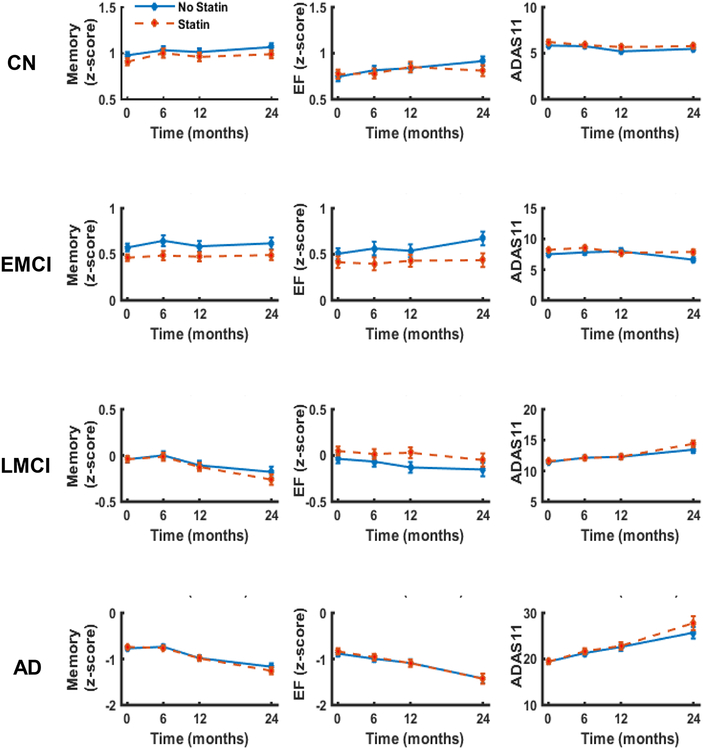
Statin users and nonusers did not differ in any measure of longitudinal cognitive performance (Table 2), including ADNI-Mem score (Figure 1A), ADNI-EF score (Figure 1B), or ADAS-11 (Figure 1C).
Figure 1: Change in cognition over time for statin users and nonusers.
Memory (ADNI-Mem), executive function (ADNI-EF), and global cognition (ADAS-11) over time for statin users and nonusers stratified by baseline diagnosis of cognitively normal (CN), early MCI (EMCI), late MCI (LMCI), or dementia due to AD (AD-dementia). Repeated measures linear mixed effects models were used to test associations between statin use and change in cognitive outcome over time. Plotted values are unadjusted means with error bars for standard error of the mean.
EMCI Participants.
Statin use was associated with a slower rate of ADNI-Mem decrease (i.e., worsening; Figure 1D, Table 2). However, this association was not significant after FDR correction for multiple comparisons. Statin users and nonusers did not differ in longitudinal cognitive performance based on ADNI-EF score (Figure 1E) or ADAS-11 (Figure 1F).
LMCI Participants.
Statin users and nonusers did not differ in any measure of longitudinal cognitive performance (Table 2), including ADNI-Mem score (Figure 1G), ADNI-EF score (Figure 1H), or ADAS-11 (Figure 1I).
AD-dementia Participants.
Statin users and nonusers did not differ in any measure of longitudinal cognitive performance (Table 2), including ADNI-Mem score (Figure 1J), ADNI-EF score (Figure 1K), or ADAS-11 (Figure 1L).
A sensitivity analysis was performed as above with the exception that the sample was not stratified by baseline diagnoses. Statin users and nonusers did not differ in longitudinal cognitive performance (Supplementary Table 3A).
Association between Statin Use and Diagnostic Conversion: Cox Proportional Hazards Models
The association between statin use and conversion to a more impaired diagnostic category was investigated using Cox regression with statin use as the main explanatory variable. Age, sex, education, APOE ε4 allele number, the same comorbid cardiovascular diagnoses, and baseline ADAS-11 score were included as covariates. Those covariates that contributed significantly to the model variance are listed in Supplementary Table 4.
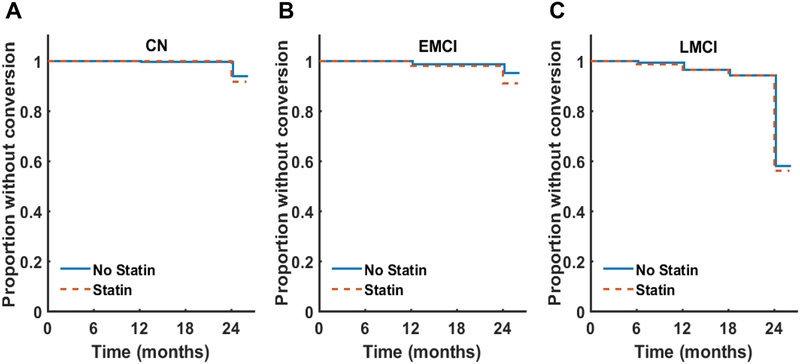
Twenty-six out of 393 participants (6.6%) with a baseline diagnosis of CN converted to a diagnosis of MCI (EMCI or LMCI) over the 24-month follow-up period (367 participants were censored). Eighteen out of 281 (6.4%) participants with a baseline diagnosis of EMCI converted to a diagnosis of AD-dementia (263 participants were censored). One hundred and ninety-nine out of 540 participants (35.5%) with a baseline diagnosis of LMCI converted to AD-dementia (391 were censored).
In the CN participants, statin use was not associated with conversion to MCI (HR [hazard ratio] = 0.98, χ2(1) = 0.002, p = 0.967, 95% CI for HR = 0.35 – 2.74; Figure 2A). Statin use was not associated with conversion to AD-dementia in the EMCI participants (HR = 1.67, χ2(1) = 0.63, p = 0.427, 95% CI for HR = 0.47 – 5.87; Figure 2B). Finally, in the LMCI participants, statin use was not associated with conversion to AD-dementia (HR = 1.12, χ2(1) = 0.39, p = 0.532, 95% CI for HR = 0.78–1.61; Figure 2C).
Figure 2: Conversion to a more impaired diagnostic category.
Survival curves were constructed using the Kaplan-Meier method.
An additional analysis was performed as above with the exception that CN, EMCI, and LMCI were combined as a non-dementia cohort. Statin use was not associated with conversion to either MCI or AD-dementia (Supplementary Table 3B).
Power Analyses
Power analyses were performed to aid in the interpretation of our results. Depending on baseline diagnosis, the study had 80% power to detect group differences of 0.004 – 0.007 ADNI-Mem per year, 0.006 – 0.010 ADNI-EF per year, and 0.05 – 0.40 ADAS-11 per year using repeated measures linear mixed effects models. Power to detect group differences was estimated by simulation over a range of rates of decline (Supplementary Table 5; Supplementary Figure 1).
IV. Conclusions
We investigated the relationship between statin use and cognitive change over time or conversion to a more impaired diagnostic category in participants with CN status, MCI (EMCI or LMCI), or AD-dementia enrolled in the ADNI study. In general, statin use was not associated with the rate of change in measures of memory, executive function, or global cognition. Notably, our analyses did show an association between statin use and slower decline in a composite memory score for the EMCI group. However, if FDR correction was applied, this association did not remain significant. Further, statin use was not associated with conversion to a more impaired diagnostic category in any diagnostic group. Since the majority of our analyses did not yield statistical significance, we performed power analyses to investigate the likelihood of type II error. These analyses indicated that our use of continuous cognitive outcome measures with linear mixed models may be relatively sensitive for detecting differences in cognitive change between groups.
One previous study utilized the ADNI cohort to examine the association between statin use and cognitive decline. Smith and colleagues [28] analyzed ADNI MCI participants (combining EMCI and LMCI groups) with a similar sample size to ours (N = 768) stratified by APOE genotype and statin use. They assessed rates of cognitive decline on the ADAS-11, among other cognitive measures, over 12-months follow-up and observed non-significant trends for less cognitive decline in statin users than nonusers (by t-tests) and suggested that statin use may have a potential subtle treatment benefit on cognitive functioning in individuals with MCI. The larger effect size in our analysis of EMCI participants may be attributable to the separation of EMCI and LMCI subgroups, use of more sensitive outcome measures (i.e., memory and executive function-specific composite z-scores in addition to global cognition scores) and statistical models (i.e., repeated measures linear mixed effects models), and longer follow-up (i.e., 24 months).
Apart from the ADNI study, a number of prospective cohort and case-control studies have investigated the possibility that statins could reduce the risk of developing dementia or AD. A meta-analysis of such studies by Zhou and colleagues [16] did not show a beneficial overall effect of statins on the risk of dementia or AD. Subsequent to this meta-analysis, however, larger and longer cohort studies have suggested that statins may have a protective effect against incidence of dementia, AD, or MCI [12, 13, 14], although one study did not find such an association [15].
Several previous randomized controlled trials have evaluated the effects of statins on cognition in participants with CN status or AD dementia. Short-term (6 month) trials of statin treatment in CN participants with HCL have suggested small performance decrements on some neuropsychological measures of attention and psychomotor speed relative to placebo, the clinical significance of which is unclear [4, 5]. Conversely, one short-term trial reported that participants treated with simvastatin showed greater improvement on tests of working memory and attention, but not cerebrospinal fluid (CSF) biomarkers, over the course of three months [10]. Two studies that resulted from a more definitive trial (the PROSPER study) with a longer follow-up period (over 36 months) in older adults at risk for vascular disease (N > 5,000) have found no effect of statin use on global and domain-specific cognitive change [17, 18]. At the other end of the diagnostic spectrum in AD-dementia, an early randomized trial of simvastatin primarily assessed CSF biomarkers but also reported a modest slowing in the rate of decline in MMSE scores over 6.5 months [10]. However, more conclusive multi-center trials in participants with AD-dementia have shown no effect of atorvastatin [19] or simvastatin [20] on cognitive, functional, or global outcomes in participants with AD-dementia over 18 months.
Thus, several cohort and case-control studies have suggested a protective effect of statins against incident AD, but randomized controlled trials in participants with CN status or AD-dementia have failed to confirm a beneficial effect of statins. In conjunction with these prior observational studies and clinical trials, our results suggest that the intermediate stage of prodromal EMCI may be worthy of further study in large-scale trials. Our results and those of Smith et al. [28] with the same ADNI cohort suggest a potential neuroprotective or symptomatic cognitive benefit of statin use at this stage. The previous trial in CN older adults at high cardiovascular risk (PROSPER) [17, 18] may have failed because few of the participants were at near-term risk for AD-related symptoms and may have shown insufficient decline during the follow-up period. Conversely, previous multi-center trials of statins in AD-dementia [14, 15] may have failed for the reasons cited for other failed AD therapies [29]—that is, they come too late in AD pathogenesis when neurodegeneration is irrevocably triggered. Our results suggest that a trial in early AD at a prodromal, or even a preclinical stage, when AD biomarkers are present would be worthwhile.
Our study design has several strengths, including the combined use of multiple cognitive outcomes that are sensitive to cognitive change [24, 25, 26], as well as time to diagnostic conversion. Moreover, our use of separate analyses by disease stage may provide greater sensitivity for varying degrees of diagnostic certainty and rates of diagnostic conversion at different stages. Finally, our use of data from the multi-center ADNI study may enhance external validity, and the low attrition rate in ADNI over a 24-month follow up-period minimizes the risk of attrition bias (Supplementary Table 1).
An important limitation of the present study is the somewhat restricted follow-up period (up to 24 months), which, however, exceeds the average period for non-epidemiologic studies [28]. Additionally, the ADNI cohort, like many samples recruited for observational studies of AD, is self-selected and may contain participants at disproportionate risk of cognitive decline. Another important limitation is the potential for survival bias due to the observed rates of attrition in each baseline diagnostic group over the 24-month follow-up [30]. However, few participant demographics or characteristics differed significantly between groups with and without 24-month follow-up, and only the comorbid diagnosis of DM differed between follow-up groups within the AD-dementia group (Supplementary Table 1). Finally, our study defined statin use according to treatment status at baseline (without regard to subsequent discontinuation) with any statin drug at any dose. Stratifying by statin type, dose, or duration may be more informative, but such an analysis would be underpowered using the ADNI cohort.
In summary, these results bolster the existing observational and clinical trial findings that conclude statin use is not associated with cognitive impairment, as cautioned by the FDA black box warning. In general, statin use was not associated with the rate of change in measures of memory, executive function, or global cognition but did suggest a possible slowing of decline in a composite memory score for the EMCI group. With this, additional randomized trials may be warranted in the prodromal EMCI stage of AD.
Supplementary Material
Highlights.
What is the primary question addressed by this study?
The primary purpose of this study was to investigate associations between statin use and cognitive change, as well as diagnostic conversion, in individuals with cognitively normal (CN) status, mild cognitive impairment (MCI), and dementia due to Alzheimer’s disease (AD-dementia).
What is the main finding of this study?
Statin use was not associated with longitudinal cognitive change in asymptomatic (cognitively normal [CN]) or symptomatic (late mild cognitive impairment [LMCI] or dementia due to Alzheimer’s disease [AD-dementia]) participants, however, there was an association between statin use and slower decline on a composite memory score in prodromal (early mild cognitive impairment [EMCI]) participants. Statin use was not associated with time to conversion to a more impaired diagnostic category for any diagnostic group (i.e., conversion to EMCI, LMCI, or AD-dementia).
What is the meaning of the finding?
In summary, these results support extant observational and randomized clinical trial findings that conclude statin use is not a risk factor for cognitive worsening (as indicated by the FDA black box warning) but that additional randomized trials in the prodromal EMCI stage may be warranted.
Acknowledgements
APM was supported in part by the NIH/NIA K23-AG057794. This work was also supported by the National Institute on Aging (P50-AG047270). MCM was supported in part by an award from Health Resources and Services Administration Geriatric Workforce Enhancement Program to Yale University School of Medicine and the Veterans Affairs Patient Safety Center for Medication Safety in Aging and Dementia.
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI; National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research &Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Conflicts of Interest and Source of Funding: The authors declare no conflicts of interest. APM was supported in part by the NIH/NIA K23-AG057794. This work was also supported by the National Institute on Aging (P50-AG047270). MCM was supported in part by an award from Health Resources and Services Administration Geriatric Workforce Enhancement Program to Yale University School of Medicine and the Veterans Affairs Patient Safety Center for Medication Safety in Aging and Dementia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None of the authors have actual or potential conflicts of interest to disclose that are related to this research.
References
- 1.Stone NJ, et al. , 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation, 2014. 129(25 Suppl 2): p. S1–45. [DOI] [PubMed] [Google Scholar]
- 2.Gu Q, et al. , Prescription cholesterol-lowering medication use in adults aged 40 and over: United States, 2003–2012. NCHS data brief, 2014. 177: p. 1–8. [PubMed] [Google Scholar]
- 3.Hales Craig M., M.D., M.P.H, Servais Jennifer, B.Sc., Martin Crescent B., M.P.H., M.A., and and Dafna Kohen PD, M.Sc., Prescription Drug Use Among Adults Aged 40–79 in the United States and Canada, in NCHS Data Brief. 2019. [PubMed] [Google Scholar]
- 4.Muldoon MF, et al. , Effects of lovastatin on cognitive function and psychological well-being. Am J Med, 2000. 108(7): p. 538–46. [DOI] [PubMed] [Google Scholar]
- 5.Muldoon MF, et al. , Randomized trial of the effects of simvastatin on cognitive functioning in hypercholesterolemic adults. Am J Med, 2004. 117(11): p. 823–9. [DOI] [PubMed] [Google Scholar]
- 6.FDA Drug Safety Communication: Important safety label changes to cholesterol-lowering statin drugs. 2016. 1/19/2016; Available from: https://www.fda.gov/Drugs/DrugSafety/ucm293101.htm.
- 7.Power MC, et al. , Statins, cognition, and dementia—systematic review and methodological commentary. Nature Reviews Neurology, 2015. 11(4): p. 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zamrini E, McGwin G, and Roseman JM, Association between statin use and Alzheimer’s disease. Neuroepidemiology, 2004. 23(1–2): p. 94–8. [DOI] [PubMed] [Google Scholar]
- 9.Hartmann T, Kuchenbecker J, and Grimm MO, Alzheimer’s disease: the lipid connection. J Neurochem, 2007. 103 Suppl 1: p. 159–70. [DOI] [PubMed] [Google Scholar]
- 10.Carlsson CM, et al. , Effects of simvastatin on cerebrospinal fluid biomarkers and cognition in middle-aged adults at risk for Alzheimer’s disease. J Alzheimers Dis, 2008. 13(2): p. 187–97. [DOI] [PubMed] [Google Scholar]
- 11.Simons M, et al. , Treatment with simvastatin in normocholesterolemic patients with Alzheimer’s disease: A 26-week randomized, placebo-controlled, double-blind trial. Ann Neurol, 2002. 52(3): p. 346–50. [DOI] [PubMed] [Google Scholar]
- 12.Beydoun MA, et al. , Statins and serum cholesterol’s associations with incident dementia and mild cognitive impairment. J Epidemiol Community Health, 2011. 65(11): p. 949–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cramer C, Haan MN, Galea S, Langa KM, & Kalbfleisch JD, Use of statins and incidence of dementia and cognitive impairment without dementia in a cohort study. Neurology, 2008. 71(5): p. 344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haag MD, et al. , Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity. The Rotterdam Study. J Neurol Neurosurg Psychiatry, 2009. 80(1): p. 13–7. [DOI] [PubMed] [Google Scholar]
- 15.Arvanitakis Z, et al. , Statins, incident Alzheimer disease, change in cognitive function, and neuropathology. Neurology, 2008. 70(19 Pt 2): p. 1795–802. [DOI] [PubMed] [Google Scholar]
- 16.Zhou B, Teramukai S, and Fukushima M, Prevention and treatment of dementia or Alzheimer’s disease by statins: a meta-analysis. Dement Geriatr Cogn Disord, 2007. 23(3): p. 194–201. [DOI] [PubMed] [Google Scholar]
- 17.Shepherd J, et al. , Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet, 2002. 360(9346): p. 1623–30. [DOI] [PubMed] [Google Scholar]
- 18.Trompet S, et al. , Pravastatin and cognitive function in the elderly. Results of the PROSPER study. J Neurol, 2010. 257(1): p. 85–90. [DOI] [PubMed] [Google Scholar]
- 19.Feldman HH, et al. , Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology, 2010. 74(12): p. 956–64. [DOI] [PubMed] [Google Scholar]
- 20.Sano M, et al. , A randomized, double-blind, placebo-controlled trial of simvastatin to treat Alzheimer disease. Neurology, 2011. 77(6): p. 556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aisen PS, et al. , Clinical Core of the Alzheimer’s Disease Neuroimaging Initiative: progress and plans. Alzheimers Dement, 2010. 6(3): p. 239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKhann G, et al. , Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology, 1984. 34(7): p. 939–44. [DOI] [PubMed] [Google Scholar]
- 23.Mecca AP, et al. , Sleep Disturbance and the Risk of Cognitive Decline or Clinical Conversion in the ADNI Cohort. Dement Geriatr Cogn Disord, 2018. 45(3–4): p. 232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crane PK, et al. , Development and assessment of a composite score for memory in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Brain Imaging Behav, 2012. 6(4): p. 502–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibbons LE, et al. , A composite score for executive functioning, validated in Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging Behav, 2012. 6(4): p. 517–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosen WG, Mohs RC, and Davis KL, A new rating scale for Alzheimer’s disease. Am J Psychiatry, 1984. 141(11): p. 1356–64. [DOI] [PubMed] [Google Scholar]
- 27.Benjamini Y and Hochberg Y, Controlling the False Discovery Rate: a Practical and Powerful Approach to Multiple Testing. Journal of the Royal statistical society: series B (Methodological), 1995. 57(1): p. 289–300. [Google Scholar]
- 28.Smith KB, et al. , The Effect of Statins on Rate of Cognitive Decline in Mild Cognitive Impairment. Alzheimers Dement (N Y), 2017. 3(2): p. 149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The Lancet N, Solanezumab: too late in mild Alzheimer’s disease? Lancet Neurol, 2017. 16(2): p. 97. [DOI] [PubMed] [Google Scholar]
- 30.Delgado-Rodriguez M and Llorca J, Bias. J Epidemiol Community Health, 2004. 58(8): p. 635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.