Abstract
Topical instillation of eye drops remains the most common and for most the easiest route of ocular drug administration, representing the treatment of choice for many ocular diseases. Nevertheless, low ocular bioavailability of topically applied drug molecules can considerably limit their efficacy. Over the last several decades, numerous drug delivery systems (DDS) have been developed in order to improve drug bioavailability on the ocular surface. This review systematically covers the most recent advances of DDS applicable by topical instillation, that have shown better performance on in vivo models compared to standard eye drop formulations. These delivery systems are based on in situ forming gels, nanoparticles and combinations of both. Most of the DDS have been developed using natural or synthetic polymers. Polymers offer many advantageous properties for designing advanced DDS including biocompatibility, gelation properties and/or mucoadhesiveness. However, despite the high number of studies published over the last decade, there are several limitations for clinical translation of DDS. The potential challenges for commercialization of new DDS are also presented in this review.
Keywords: Ocular surface, ocular drug delivery, eye drop, gel, nanoparticle, microparticle
1. INTRODUCTION
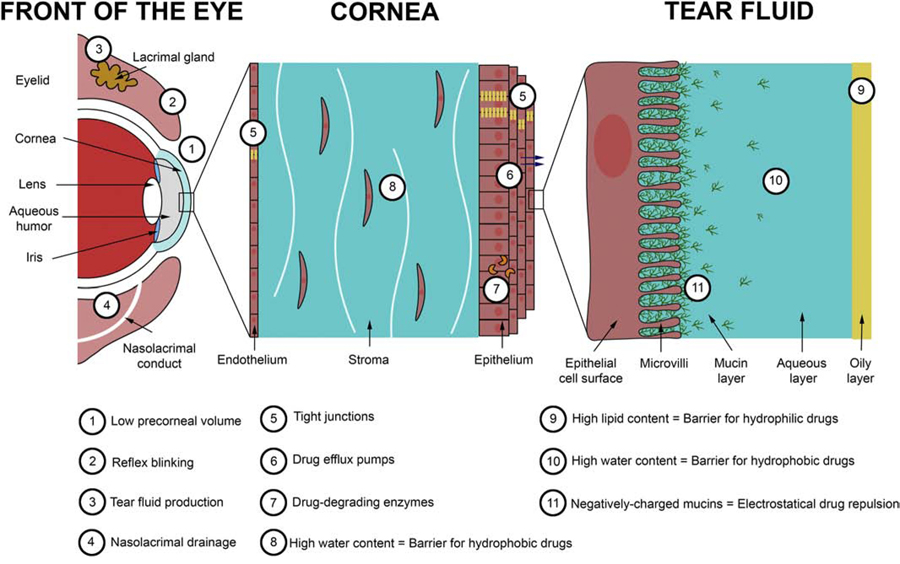
Topical administration represents the easiest and least invasive route to deliver drugs to the anterior segment of the eye. Therefore, eye drops are the treatment of choice for many ocular diseases such as infection, inflammation, glaucoma, dry eye and allergy, representing 90% of the commercialized products in the global ophthalmic drug market [1]. However, the major limitation of topical administration remains their relatively low efficacy. Drug delivery through the anterior segment is limited due to the unique physiology and anatomy of the eye, providing low bioavailability [2] (Fig. 1).
Fig. 1.
Main static and dynamic barriers for ocular drug delivery.
The first barrier of drug delivery is the limited volume (~30 µL) of the eye drop that can be applied onto the ocular surface, due to the limited precorneal surface area. Moreover, most of the volume applied is eliminated during the first reflex blinking, triggered by the abrupt increase of tear volume [3]. The remaining volume of drug left on the eye then mixes with the tear film produced by the lacrimal and Meibomian glands. The tear film is a thin transparent fluid layer composed of three phases including an outer oily phase, an intermediate aqueous phase, and an inner mucin layer (Fig. 1). The oily phase and the aqueous phase represent another barrier for hydrophilic and hydrophobic drugs, respectively. Moreover, the aqueous phase is composed of proteins and enzymes that can fix and degrade drugs. The inner layer of the tear fluid is composed of mucins that are high-molecular weight and highly glycosylated proteins secreted by the epithelial cells of the cornea. Their primary function is to protect the ocular surface against external noxious stimuli and invading pathogens. Mucins are negatively-charged macromolecules that can attract or repulse drugs via electrostatic interactions depending on the charge of the drug molecule or carrier system [4]. An additional factor that limits drug bioavailability is the tear film turnover (between 0.5 and 2.2 µL/min under normal conditions in human) increases after topical instillation, causing a rapid clearance (within 1–2 min) of the drug molecules via the nasolacrimal drainage [5]. Two minutes after eye drop installation, it is estimated that 60% of the active ingredient is eliminated via all these mechanisms. After 8 min, the active ingredient is diluted at 1/1000 and after 15–25 min, all the active ingredient is eliminated on the corneal surface [6].
For some conditions such as glaucoma and uveitis, drugs need to diffuse through the anterior ocular tissues (cornea, sclera) to achieve adequate intraocular levels in order to induce their therapeutic effect. It is, however, estimated that less than approximately 5% of drugs applied by this route are can efficiently be delivered to the anterior chamber [3,7]. Corneal and scleral/conjunctival tissues also represent a major barrier of drug delivery into the anterior chamber. The cornea is a transparent lens-shaped tissue responsible for two thirds of the refractive power of the eye. It is composed of three layers: the outer epithelium, the intermediate stroma and the inner endothelium (Fig 1). The corneal epithelium is a hydrophobic layer, composed of a stratified squamous cell layer. The high expression of tight junctions between epithelial cells forms a strong permeation barrier for hydrophilic drugs [8,9]. Also, the presence of drug efflux pumps and cytochrome P450 (drug-degrading enzyme) in the epithelium represents another cause of low drug bioavailability [10–12]. The stroma represents 90% of the corneal volume. In contrast to the epithelium, the stroma is highly hydrophilic, due to its high water content (80%), which limits the penetration of hydrophobic drugs. Finally, the endothelium is also considered as a hydrophobic barrier due to the presence of tight junctions; however, because of its lower cell thickness, the endothelium represents a weaker permeation barrier compared to the epithelium. The conjunctiva and sclera are tissues surrounding the cornea; they also consist of low-permeable barriers that limit drug permeation into the anterior chamber. Conjunctiva and sclera are less drug resistant compared to the cornea tissue. However, the presence of blood vessels promotes drug elimination via the systemic route [13].
To improve the efficacy of drug delivery via the topical route, high drug concentrations and repeated instillations are often required in order to reach the desired therapeutic effects, which can result in side effects and poor patient compliance. [14]. Two main strategies have been followed in order to improve ocular bioavailability upon topical administration: a) increasing precorneal retention time, and b) enhancing corneal, scleral and/or conjunctival drug permeability.
A variety of drug delivery systems (DDS) have been investigated and marketed during the past decades including prodrugs, permeation enhancers, gels, ointments and liposomes nanocarriers [15]. More recent advances in nanotechnology and biomaterial sciences led to the development of new DDS such as in situ gelling systems, polymeric nanoparticles, polymeric/lipidic nanoparticles or a combination of these strategies. Most of these recent DDS have been developed using natural and/or synthetic polymers, which are macromolecules composed of many repeated subunits [16]. The physicochemical properties of polymers such as molecular mass, charge, hydrophobicity and type of functional groups, make them suitable material for a broad range of applications. In this review, we will give an overview of the recent development of DDS applicable by topical instillation, which showed successful results on in vivo models. This review will also highlight current challenges towards the commercial development of new DDS formulated in eye drops.
2. In situ gelling systems
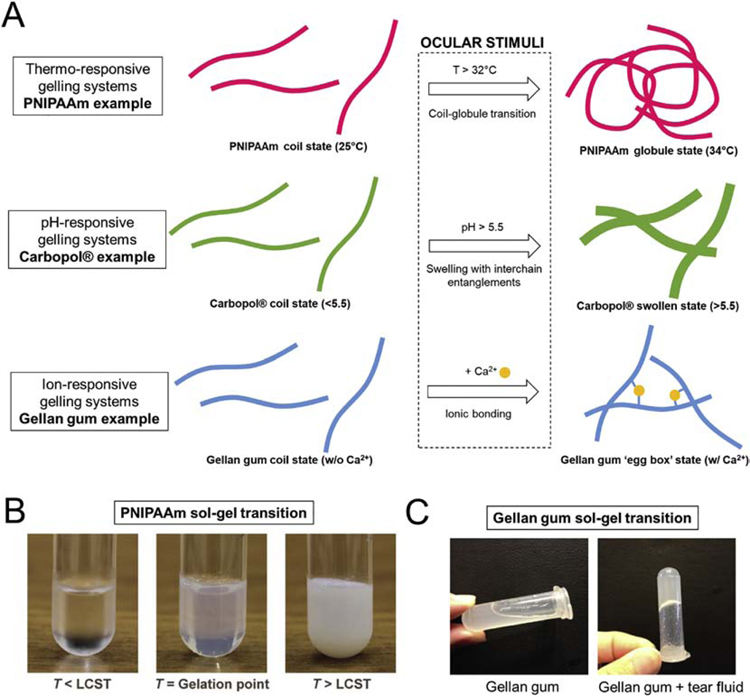
The use of viscous formulations, such as gels and ointments, have been widely used to increase the retention time of drugs on the ocular surface by limiting the drug elimination via the nasolacrimal drainage. However, gels and ointments are less accurate and less reproducible to apply, and can induce blurred vision, eyelids crusting, and lacrimation [15]. More recently, stimuli-responsive materials have been used to develop in situ gelling systems as an alternative to standard liquid and viscous formulations. In situ gels are administrated as a liquid and form a gel upon contact of the eye. This solution-gelation (sol-gel) transition is triggered by the environmental stimuli of the ocular surface, including the temperature, pH and the presence of ions in the tear fluid (Fig. 2).
Fig. 2. Principle of ‘sol-gel transition’ of different types of in situ gel used for ocular drug delivery.
(A) Schematic principle of sol-gel transition of different types of stimuli-responsive materials. Images of sol-gel transition of thermo-responsive PNIPAAm (from [17]) (B) and ion-responsive gellan gum (C) (from [18]).
Thermo-responsive, pH-responsive and ion-responsive materials are the three main types of stimuli-responsive materials that are most widely used for the development of gelling systems for ocular drug delivery (Table 1).
Table 1.
In situ gelling systems used for ophthalmic drug delivery.
| Material | Drug model | Animal model | In vivo studies | In vivo results | Ref |
|---|---|---|---|---|---|
|
Thermo-responsive gelling systems | |||||
| Poloxamer® | Loteprednol | Rabbit | Determination of drug concentration of aqueous humor by HPLC | AUC(0–10h) and Cmax values was found 2.55-fold and 4.34-fold higher, respectively, for in situ gel compared with marketed formulation. | [19] |
| MethazolamideRabbit | Rabbit | Determination of drug concentration of aqueous humor by HPLC. Measurement of intraocular pressure by indentation tonometer. | AUC(0–12h) was found 1.58-fold higher for in situ gel compared with Azopt®. No significant difference in the IOP lowering effect was found between in situ gel and Azopt®. | [20] | |
| Timolol | Rabbit | Biocompatibility study (slit lamp test and histopathology study). Determination of drug concentration of aqueous humor by HPLC. Measurement of intraocular pressure by indentation tonometer. | Good biocompatibility and no sign of irritation. AUC(0–240min) and Cmax was found 1.1-fold higher and 1.33-fold lower, respectively, for in situ gel compared with standard eye drop. No significant difference in the IOP lowering effect was found between in situ gel and standard eye drop. | [21] | |
| Poloxamer®-HPMC or Poloxamer®-HEC | Ciprofloxacin HCl | Rabbit | Assessment of antimicrobial efficacy by scoring system | Significant improvement of scoring for Poloxamer-HPMC and poloxamer-HEC in situ gels compared with Ciprofloxacin®. | [22] |
| PNIPAAm | Epinephrine | Rabbit | Measurement of intraocular pressure by ophthalmic tonometer. | In situ gel decreased IOP for 24h with a minimum of 8.9 mmHg at 4h. Standard eye drop decreased IOP for 6–7h with a minimum of 7.2 mmHg at 2h. | [23] |
| PNIPAAm-HA | Ketoconazole | Rabbit | Eye irritation test (Draize test). Assessment of antimicrobial efficacy by scoring system. | No sign of ocular irritation. 91.7% and 66.7% of eyes were cured with in situ gel and commercial eye drops, respectively. | [24] |
| Cyclosporine A | Rabbit | Eye irritation test (Draize test). Determination of drug concentration in ocular tissues by HPLC. | No sign of ocular irritation. Significant increase of drug concentration levels in corneas (1455.8 ng/g of tissue) compared with castor oil formulation and commercial eye drops. | [25] | |
| Xyloglucan | Pilocarpine | Rabbit | Assessment of pupil diameter. | AUC(0–270min) values were found higher for in situ gel compared with standard solution. | [26] |
| Glycerol 2-phosphatechitosan-gelatin | Levocetirizine | Rabbit and guinea pig | Eye irritation test. Precorneal drainage assessment by slit lamps and blue light. Assessment of antiallergic conjunctivitis efficacy by Evans Blue(EB) extravastion quantification. | No sign of ocular irritation. Residence time was found 2.94-fold higher for in situ gel compared with aqueous solution. Extravasted amounts of EB in ocular tissues were found 1.75-fold and 2.56-fold lower for aqueous solution and in situ gel, compared with physiological saline. | [27] |
| Timolol | Rabbit | Eye irritation test. Precorneal retention time by fluorescein staining. Measurement of intraocular pressure by tonometer. | No sign of ocular irritation. Precorneal retention was around 10 min for standard eye drops and at least 60 min for in situ gel. The maximum IOP lowering effect was observed at 0.5h and 1h for standard eye drops and in situ gel, respectively. The IOP lowering effect lasted 12h and 24h for standard eye drops and in situ gel, respectively. | [28] | |
| Latanoprost | Rabbit | Measurement of intraocular pressure by tonometer. | Weekly administration of in situ gel showed similar IOP lowering effect pattern compared with daily administration of Xalatan®. | [29] | |
|
pH-responsive gelling systems | |||||
| Carbopol®-HPMC | Puerarin | Rabbit | Determination of drug concentration of aqueous humor by HPLC. | AUC(0–24h) and Cmax values were found 2.17-fold and 1.29-fold higher, respectively, for in situ gel compared with aqueous solution. | [30] |
| Baicalin | Rabbit | Eye irritation test (Draize test). Determination of drug concentration in ocular tissues by HPLC. | AUC and Cmax values were found 6.1-fold and 3.6-fold higher, respectively, for in situ gel compared with aqueous solution. | [31] | |
| Pefloxacin | Rabbit | Determination of drug concentration in ocular tissues by HPLC. | Drug concentration was found above MIC (minimum inhibitory concentration, 2 ng/mL) for 24h for in situ gel and for 12h for marketed eye drops. | [32] | |
| Timolol and brimonidine | Rabbit | Eye irritation test. Measurement of intraocular pressure by Schiotz tonometer. | No sign of ocular irritation. Maximum ΔIOP (IOP treated eye – IOP untreated eye) achieved 17.75±0.050 mmHg at 12h and 13.12±0.034 mmHg at 4h for in situ gel and COMBIGEN®, respectively. 14h after instillation, ΔIOP was found 2.51-fold higher for in situ gel compared with COMBIGEN®. | [33] | |
| Carbopol®-Chitosan | Timolol | Rabbit | Measurement of intraocular pressure by Schiotz tonometer. | AUC(0–9h) values were found 1.71-fold and 2.48-fold higher for Carbomer® in situ gel and Carbomer®-Chitosan in situ gel, respectively, compared with GLUCOMOL®. | [34] |
|
Ion-responsive gelling systems | |||||
| Gellan gum | Moxifloxacin | Rabbit | Determination of drug concentration in ocular tissues by HPLC. Bacterial infection study. | AUC(0–∞) and Cmax values were found 6-fold higher for in situ gel compared with Vigamox®. In situ gel cured corneal infection after 4 days compared to 7 days of photodynamic therapy. | [35] |
| Brinzolamide | Rabbit | Eye irritation test (Draize test). Measurement of intraocular pressure by tonometer. | 1h after instillation, IOP was found 18.2% and 27% lower for in situ gel and standard solution, respectively. After 6h, IOP was found lower for in situ gel (18.6 mmHg) compared to standard solution (21.2 mmHg). | [18] | |
| Gellan gum-NaCMC | Gatifloxacin | Rabbit | Eye irritation test (Draize test). Assessment of antimicrobial efficacy on a S. aureus infection model by clinical symptoms scoring. | No sign of ocular irritation. Significant improvement in the observed symptoms for in situ gel compared with marketed solution. | [36] |
| Gellan gum-Kcarrageenan | Econazole | Rat | Eye irritation test (HET-CAM). Biopermanence PET study | No sign of ocular irritation. AUC(0–∞) was found 2.33-fold higher for in situ gel compared to standard solution. | [37] |
| Alginate-HPMC | Gatofloxacin | Rabbit | Eye irritation test (Draize test). Precorneal drainage assessment by gamma scintigraphy. | No sign of ocular irritation. AUC(0–10h) values were found 3.58-fold higher for in situ gel compared with standard eye drop. | [38] |
| Alginate-NaCMC | Gatifloxain | Rabbit | Eye irritation test (Draize test). Assessment of antimicrobial efficacy on a S. aureus infection model by clinical symptoms scoring. | No sign of ocular irritation. Significant improvement in the observed symptoms for in situ gel compared with marketed solution. | [36] |
| Alginate-Gellan gum | Matrine | Rabbit | Eye irritation test (Draize test). Determination of drug concentration in tear fluid by HPLC. | AUC(0–30) values were found 4.65-fold, 3.44-fold and 2.83-fold higher for alginate-gellan gum, alginate and gellan gum in situ gels respectively, compared to standard drug solution. | [39] |
|
Multi-stimuli responsive gelling systems | |||||
| Alginate-Poloxamer® | Pilocarpine | Rabbit | Assessment of pupil diameter. | AUC(0–360h) values were found 4.38-fold, 2.85-fold and 1.36-fold higher for alginate-poloxamer, poloxamer and alginate in situ gels, respectively, compared with standard solution. | [40] |
| Carbomer®-xanthan gum | Ofloxacin | Rabbit | Eye irritation test (Draize test). Determination of drug concentration in tear fluid by HPLC. | No sign of ocular irritation. In situ gel and Oflox® showed significant difference in residence time at all point intervals. | [41] |
| Alginate-chitosan | Levofloxacin | Rabbit | Eye inflammation with infra-red camera. Precorneal drainage assessment by gamma scintigraphy. | Standard eye drop cleared more rapidly from the corneal region and reached systemic circulation via nasolacrimal drainage, compared with in situ gel. | [42] |
| Chitosan-PNIPAAm | Timolol | Rabbit | Measurement of intraocular pressure by Schiotz tonometer. | At all time points, in situ gels exhibited stronger IOP lowering effect compared with standard solution. Maximum IOP decrease was found higher for in situ gel (3.375 kPa) compared with standard solution (2.395 kPa). | [43] |
Abbreviations: AUC = Area under the curve; EB = Evans blue; HA = Hyaluronic acid; HCl = Hydrochloric acid; HEC = Hydroxyethyl cellulose; HET-CAM = Hen’s egg-chorioallantoic membrane test; HPLC = High performance liquid chromatography; HPMC = Hydroxypropyl methyl cellulose; IOP = Intraocular pressure; NaCMC = Sodium carboxymethycellulose; PET = Positron emission tomography; pNIPAAm = Poly(N-isopropylacrylamide); ΔIOP = Intraocular pressure variation
2.1. Thermo-responsive gelling systems.
Thermo-responsive materials have been the first type of stimuli-responsive materials used for the development of in situ gels. They have been investigated for many biomedical applications [44]. These materials undergo a sol-gel transition above a certain temperature called the lower critical solution temperature (LCST). The gelation is usually due to an increase in hydrophobicity by the formation of intermolecular hydrogen bonding, hydrophobic interactions and physical entanglements of polymer chains [45,46]. The temperature of the human ocular surface is around 33.7°C in normal subjects [47,48]. Some thermo-responsive materials exhibit a LCST around the eye temperature, making them suitable for the development of in situ gels for ocular drug delivery. Among them, Poloxamers®, poly(N-isopropylacrylamide) (PNIPAAm), xyloglucan and glycerol-2-phosphate showed promising results in vivo.
2.1.1. Poloxamer®-based gelling systems
Poloxamers®, also known by the trade name Pluronic®, are synthetic, nonionic and amphiphilic polymers composed of a hydrophobic block of poly(propylene oxide) (PPO) flanked by two hydrophilic blocks of poly(ethylene oxide) (PEO), poly(ethylene oxide)- poly(propylene oxide)-poly(ethylene oxide) (PEO-PPO-PEO). Poloxamers® 188 and 407 are both approved by the Food and Drug Administration (FDA) utilized in various cosmetic, industrial and pharmaceutical applications. Since 1970’s, these polymers have been used as inactive ingredients of numerous marketed eye drops due to their excellent biocompatibility, non-toxicity, biodegradability and surfactant properties. When dispersed in aqueous solution at low concentration, Poloxamers® form colloidal formulations that reduce surface tension and thus increase drug permeation [49]. At concentrations above 15% (w/w), poloxamers® are liquid at cold (~4°C) or room (~20C) temperature and form a colorless and transparent gel at the temperature of the eye (~32°C) [50]. In an in vivo rabbit model, poloxamer-based in situ gels showed a significantly higher absorption to the aqueous humor of loteprednol [19], methazolamide [20] and timolol [21], compared to standard formulations. However, it has been shown that the delivery of methazolamide and timolol, both anti-glaucoma drugs, did not significantly reduce the intraocular pressure (IOP) compared with standard formulations [20,21]. Besides its excellent biocompatibility, poloxamers® exhibited low mechanical strength and rapid erosion. This intrinsic instability is due to the weak hydrophobic interactions between the PPO blocks [51].
Precorneal retention time increases with poloxamer® concentration but the high concentration necessary to formulate in situ gels can cause ocular irritation [49]. Thereby, viscosifiers have been added to poloxamer® in order to reduce its concentration without modifying the gelling properties. Cellulose is a natural polysaccharide and represents the most abundant polymer on earth. Many of its derivatives, such as ethylcellulose (EC), methylcellulose (MC), hypromellose (INN, also called hydroxypropyl methyl cellulose (HPMC)) or hydroxyethyl cellulose (HEC), are currently used as viscosifiers in numerous commercialized eye drops to increase their viscosity. It has been shown that the addition of HPMC or HEC to poloxamer-based in situ gels significantly increased its mucoadhesive properties and the release of ciprofloxacin, allowing an enhanced antimicrobial effect compared to commercial formulations [22].
2.1.2. PNIPAAm-based gelling systems
Poly(N-isopropylacrylamide) (PNIPAAm) is a synthetic polymer that can undergo a reversible thermo-sensitive coil-globule transition in aqueous solutions at approximatively 33°C [52] (Fig. 2). Below this temperature, PNIPAAm is water-soluble and hydrophilic, and above this temperature, it is able to form inter- and intrachain associations resulting in an insoluble and hydrophobic aggregate [53]. Compared to the other polymers used for in situ gels, PNIPAAm is not FDA-approved. A PNIPAAm-based in situ gel has been tested to deliver epinephrine in an in vivo rabbit model. Results have demonstrated that the IOP-lowering effect was 4-fold longer compared to standard eye drops [23]. However, PNIPAAm is not biodegradable, limiting its use for eye drop formulations [54]. Thereby, PNIPAAm has been grafted to natural polymers in order to obtain a safe and biodegradable in situ gels.
Hyaluronic acid (HA), also named hyaluronan, is a glycosaminoglycan composed of repeating disaccharide units of D-glucuronic acid and N-acetyl glucosamine which are negatively charged at physiologic conditions [55]. It represents another natural polymer particularly used in the field of ophthalmology due to its excellent biocompatibility and biodegradability. Naturally biocompatible and biodegradable, HA is a component of vitreous and aqueous humor and can be degraded by hyaluronases present in ocular tissues. In some studies, PNIPAAm has been grafted to HA to develop in situ forming gels for the delivery of ketaconazole [24] and cyclosporin A [25] to the anterior segment of ocular tissue. Results showed that the use of PNIPAAm-HA gel improved precorneal retention time and thus increased drug levels on the cornea, compared with marketed eye drops [25]. Moreover, a significantly higher cure rate of Candida albicans infections was observed after delivery of ketaconazole loaded in PNIPAAm-HA in situ gel, compared with marketed solution [24]. Also, studies showed no sign of ocular irritation after instillation [24,25]. Compared to other thermo-responsive polymers, PNIPAAm has a highly efficient sol-gel transition independently of the concentration and the molecular weight used. However, PNIPAAm is not transparent after sol-gel transition, and therefore, can alter vision of the patient.
2.1.3. Xyloglucan-based gelling systems
Xyloglucan is a highly soluble natural polysaccharide derived from tamarind seeds. When partially degraded by β-galactosidase, this polymer exhibits thermo-responsive properties [56]. The temperature of the sol-gel transition and the degradation rate can be modulated by varying the polymer concentration [57].
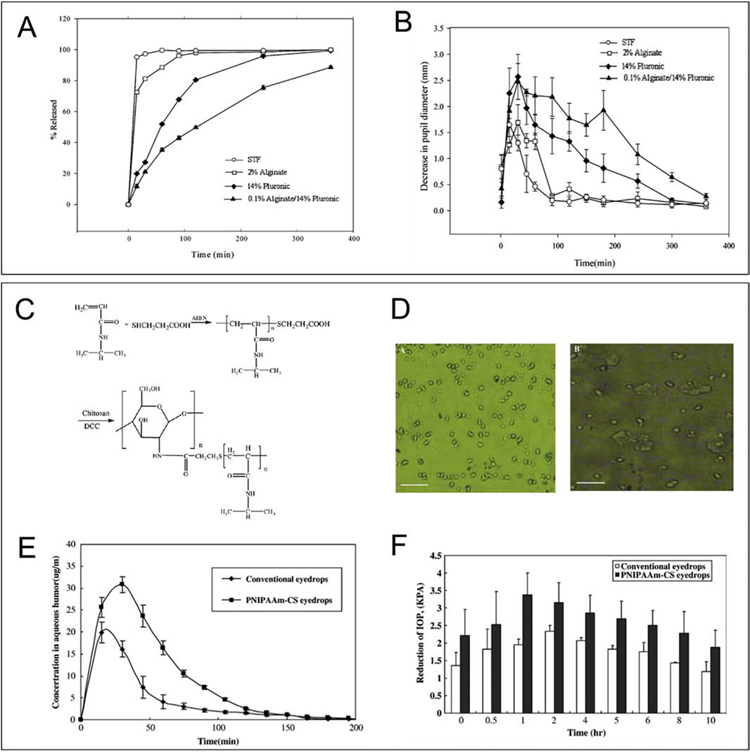
Xyloglucan-based in situ gels were assessed for ocular delivery of pilocarpine, a drug used to enlarge the pupil (miotic response). Results showed that the in situ gel had a greater effect on the miotic response compared to standard pilocarpine formulations. Moreover, similar effects on the miotic response have been observed between xyloglucan-based in situ gel at a concentration of 1.5 wt% and a Poloxamer®-based gel at a concentration of 25 wt% [26] (Fig. 3).
Fig. 3. Sustained drug release and improved therapeutic effect by using in situ gels compared with conventional eye drops.
(A) Cumulative amount of pilocarpine released as a function of time from various pilocarpine-containing solutions. All measurements were performed in triplicate, and the standard deviations were all within 3% (From [40]). (B) Decrease in pupil diameter vs time profiles for various pilocarpine-containing solutions. All the measurements were performed in triplicate (From [40]). (C) Thermosensitive PNIPAAm–CS synthesis outline. (D) Morphology change of the PNIPAAm–CS gel forming solution below and upon LCST by using an optical microscope. Scale bar, 20 μm. (E) Timolol maleate concentration in aqueous humor after instillation of 0.5% timolol maleate conventional and thermosensitive PNIPAAm–CS gel forming solution (n=5) (From [43]). (F) The IOP-lowering effect of timolol maleate in thermosensitive PNIPAAm–CS and conventional eye drop (n=4) (From [43]).
2.1.4. Glycerol phosphate-based gelling systems
Recently, glycerol phosphate has been used to modify the thermo-responsive properties of natural polymers, including chitosan and gelatin. For this process, glycerol phosphate interacts with protonated amines of polymers, inducing higher solubility at low temperatures [58].
Several researchers have successfully developed chitosan-gelatin-glycerol 2-phosphate in situ gels, with a sol-gel transition at body temperature. These formulations were assessed for the delivery of levocetirizine [27], timolol [28] and latanoprost [29]. Precorneal retention of levocetirizine and antiallergic conjunctivitis efficacy were found higher for in situ gels compared with aqueous solutions [27]. For the delivery of timolol, precorneal retention has been shown to be around 10 min for standard eye drops and at least 60 min for the in situ gel. Moreover, IOP lowering effect lasted 12h and 24h for standard eye drops and the in situ gel, respectively [28]. Finally, it has been found that the delivery of latanoprost was more efficient for the in situ gel compared with Xalatan®, a marketed formulation. Interestingly, IOP measurement showed that a weekly administration of the in situ gel showed a similar IOP lowering effect pattern when compared with daily administration of Xalatan® [29] (Fig. 3). This result confirmed that chitosan-gelatin-glycerol 2-phosphate in situ gels represent a promising DDS by remarkably reducing repetitive instillations and thus increasing the patient compliance for glaucoma treatment.
2.2. pH-responsive gelling systems
The pH of the ocular surface is neutral. Some pH-responsive materials have the property to be liquid at an acidic pH and undergo the sol-gel transition when the pH increases. Carbopol® and chitosan are both pH-responsive materials that have been extensively used for the development of in situ gels for ocular drug delivery.
2.2.1. Carbopol®-based gelling systems
Carbopol®, also known by the generic name Carbomer®, is a synthetic polymer derived from cross-linking of poly(acrylic acid). A high purity grade version of this polymer, Carbopol® 934P, was designed for the pharmaceutical industry in the 1960’s by Lubrizol (Wickliffe, OH) and have been used in many commercial ophthalmic gels and ointments. Carbopol® is a pH-sensitive polymer that is in a liquid form at a pH lower than 5.5, and is able to form a semi-solid gel above this pH. The sol-gel transition occurs with the formation of a three dimensional (3D) network swollen in aqueous solution due to electrostatic repulsion and osmotic forces within the polymer backbone [59] (Fig. 2). Due to its synthetic nature, physical and chemical properties of Carbopol® can be fine-tuned to make it suitable for various biomedical applications. However, high concentration of Carbopol® is required to formulate in situ gels and its acidic nature can be toxic for the eye. To reduce Carbopol® concentration without compromising the gelation efficiency, cellulose derivatives such as HPMC have been added into the formulation [30]. Carbopol®-HPMC in situ gels have been developed to deliver puerarin [30], baicalin [31], pefloxacin [32] and timolol and brimonidine simultaneously [33]. Compared to standard solutions, higher drug concentrations were delivered in ocular tissues by these in situ gels, showing their ability to increase precorneal retention time [30,31]. It has also been shown that after instillation, the pefloxacin concentration was found above the minimum inhibitory concentration for 24h for in situ gels, whereas it was only 12h for commercial eye drops [32]. Moreover, the simultaneous delivery of two anti-glaucoma drugs, timolol and brimonidine, by the Carbopol®-HPMC in situ gel allowed a sustained and higher IOP-lowering effect compared to COMBIGEN® [33]. Finally, Carbopol®-HPMC in situ gels showed no sign of ocular irritation after instillation [33].
2.2.2. Chitosan-based gelling systems
Chitosan is a linear amino polysaccharide derived from chitin, the main component of shells of crustaceans, insects and microorganisms, representing the second most abundant natural polymer on earth after cellulose. Chitosan can be solubilized in aqueous solutions only in acidic environments. When the pH exceeds 6.2, chitosan is neutralized and forms a gel. This property allows chitosan to form a gel at immediate contact with the cornea, where the pH is neutral. The combination of Carbopol® and chitosan have been used to form an in situ gel for the delivery of timolol. Results demonstrated an increased and more sustained IOP-lowering effect for the formulated in situ gel compared with GLUCOMOL® [34]. However, the low purity and batch-to-batch reproducibility of chitosan considerably limits its application into market compared to other synthetic polymers such as Carbopol®. Moreover, the use of pH-responsive materials for ocular application requires the instillation of acidic formulations on the ocular surface which can induce discomfort and lacrimation for the patient [60].
2.3. Ion-responsive gelling systems
The human tear fluid is composed of different mono or divalent cations, particularly Na+, Mg+ and Ca2+. The sol-gel transition of ion-responsive materials occurs in the presence of cations that generate ionic bonds within the polymer backbone, creating an ‘egg box’ structure [61]. Among these ionic materials, gellan, xanthan gum and alginate have been widely used to develop in situ gels for ocular drug delivery.
2.3.1. Gellan and xanthan gum-based gelling systems
Gellan gum (also known by the trade name Gelrite®) and xanthan gum are both naturally derived anionic polymers produced by the bacterium Sphingomonas elodea and Xanthomonas campestris, respectively. Gellan gum is an anionic linear polysaccharide composed of repeating units of tetrasacharide composed of two units D-glucose, one of D-glucuronic acid and L-rhamnose, while xanthan gum is composed of pentasaccharide repeating units of mannose, glucose and glucuronic acid. These polymers can be stored in a liquid state and form a gel upon contact of the eye due to the presence of cations in the tear film. The sol-gel transition occurs by the formation of ionic bonds of the polymer backbone. Gellan and xanthan gum are already used in clinic as in situ gels, such as TIMOLOL MALEATE EX® (Timolol 0.25%, Sandoz Inc., Switzerland), TIMOPTIC-XE® (Timolol 0.25%, Valeant Pharms LLC, USA), TIMOLOL L.P.® (Timolol 0.25% and 0.5%, Santen Oy, Japan) and MOXEZA® (Moxifloxacin 0.5%, Novartis Pharms Corp, Switzerland). On an in vivo rabbit model, gellan gum was used to deliver moxifloxacin [35] and brinzolamide [18]. It was shown that the gellan gum-based in situ gel could deliver a 6-fold higher concentration of moxifloxacin in the aqueous humor compared to VIGOMOX®, as control [35]. Moreover, the delivery of brinzolamide by the developed in situ gel prolonged the IOP lowering effect compared to standard solutions [18]. Gellan gum has also been combined with K-carrageenan to formulate an in situ gel for the delivery of econazole. Results showed higher precorneal retention of the drug compared to standard solutions [37].
2.3.2. Alginate-based in situ gelling systems
Alginate is a natural, anionic, hydrophilic polysaccharide isolated from brown seaweed. It is composed of β-D-mannuronic acid linked to R-L-guluronic acid units. Sodium alginate can interact with cations such as Ca2+ present in the tear film to form a gel upon contact with the cornea. Alginate is already used in clinic in different ophthalmic formulations such as MIKELAN LA® (Carteolol hydrochloride 1% or 2%, Otsuka Pharm Co., Ltd, Japan), MIKELUNA® (Carteolol hydrochloride 1%, Latanoprost 0.005%, Otsuka Pharm Co., Ltd, Japan), CARTEOL L.P. (Carteol 1% or 2%, Chauvin Laboratory, France). Alginate-based in situ gel was also used with cellulose HMPC as viscosifiers for the delivery of gatifloxacin, and showed better precorneal retention time than HMPC or alginate solutions alone [38]. Sodium alginate and gellan gum were also used with sodium carboxymethycellulose (NaCMC) to deliver gatifloxacin against induced bacterial keratitis on an infected rabbit model in vivo. It has been found that the in situ gel was more effective in the treatment of keratitis (redness, lacrimal secretion, mucoid discharge, response to ocular stimulus and swelling of eyelids) compared to conventional eye drops [36]. Interestingly, a mixture of gellan gum and alginate was used to form an in situ gel and showed a greater ability to retain the drug on the corneal surface than gellan gum or alginate in situ gel alone [39]. These results suggest that the combination of ion-sensitive polymers can improve the gelation properties, allowing increased precorneal retention of drugs on the corneal surface.
2.4. Multi-stimuli responsive gelling systems
In order to increase strength and gelation properties of gelling systems, combinations of different stimuli-responsive materials have been tested. For example, thermosensitive poloxamer® and ion-sensitive alginate were combined to formulate composite in situ gel for the delivery of pilocarpine to the anterior segment [40]. Drug release and pupil constriction were found to be higher for poloxamer®-alginate in situ gels compared with poloxamer or alginate gels alone (Fig. 3A–B). In another study, an in situ gel based on ion-sensitive xanthan gum and pH-sensitive Carbopol® has been formulated for the delivery of ofloxacin. Results demonstrated a significant increase in retention time with optimized concentrations of both polymers, compared with OCUFLOX®, a marketed ointment [41]. pH-sensitive chitosan was also combined with alginate to develop an in situ gel for sustained release of levofloxacin to the anterior segment. This formulation showed better therapeutic efficacy compared to standard eye drops [42].
Finally, a combination of chitosan with thermosensitive PNIPAAm was developed and assessed for the delivery of timolol maleate. [43]. In vivo studies demonstrated that drug release was higher and longer for this in situ gel compared with conventional eye drops (Fig. 3C). Moreover, the formulation had a higher IOP-lowering effect at all time points compared with the standard eye drops (Fig. 3D). All these studies prove that the development of multi stimuli-responsive materials improved gelation properties of in situ gels, providing higher precorneal drug retention on the ocular surface.
3. Nanoparticle-based drug delivery systems
One of the reasons for the low bioavailability of drugs after topical administration is the short retention time due to the rapid clearance of the ocular surface via tear film renewal, nasolacrimal drainage and biologic and enzymatic drug degradation. Therefore, microparticle- and nanoparticle-based systems have been used to increase the retention time of drugs on the ocular surface. Due to their functional groups and the surface charge, microparticles and nanoparticles (NPs) can closely interact with the mucin layer of the ocular surface to prolong the presence of drugs on the cornea [62]. Also, the encapsulation of drugs into NPs protects them from enzymatic degradation; thus, a lower concentration of drugs is required to reach the therapeutic effect, preventing side effects. Numerous lipidic and polymeric materials and/or a combination of them have been used to develop NPs that are able to deliver a variety of drugs to the anterior segment (Table 2).
Table 2.
Nanoparticles-based systems used for ophthalmic drug delivery.
| Drug model | Animal model | In vivo studies | In vivo results | Ref | |
|---|---|---|---|---|---|
|
Natural materials | |||||
| Chitosan | Ganciclovir | Rat | Determination of drug concentration in aqueous humor by HPLC. | AUC(0–∞) and Cmax values were found to be 4.69-fold and 2.7-fold higher, respectively, for NPs solution compared with aqueous solution. | [63] |
| Diclofenac | Rabbit | Eye irritation test (Draize test). Determination of drug concentration in aqueous humor by HPLC. | No sign of ocular irritation. AUC(0–720min) values were found to be 2.46-fold higher for NPs solution compared with commercial eye drops. Cmax was found to be similar for NPs solution and commercial eye drops. | [64] | |
| Cyclosporine A | Sheep | Determination of drug concentration in aqueous and vitreous humor by HPLC. | After 2h, drug concentration was found to be 40.70±1.0 and 35.60±2.50 ng/mL in aqueous and vitreous humors, respectively. After 72h, drug concentration was found to be 41.70±0.90 and 36.70±0.30 ng/mL in aqueous and vitreous humors, respectively. | [65] | |
| Cyclosporine A | Rabbit | Determination of drug concentration in ocular tissues by liquid scintillation counting. | Corneal and conjunctival drug levels were found 2- fold to 6-fold higher for NPs solution compared with standard aqueous solution. | [66] | |
| Indomethacin | Rabbit | Determination of drug concentration in aqueous humor by HPLC. | AUC and Cmax values were found 17-fold and 13-fold higher, respectively, for NPs solution compared with standard solution. | [67] | |
| Celocoxib | Rat | Determination of drug concentration in ocular tissues by HPLC. | AUC(0–24), AUC(0–∞) and Cmax values were found 4.8-fold to 27.7-fold higher for NPs solution compared with standard solution. | [68] | |
| Timolol | Rabbit | Precorneal retention by fluorescence imaging. Measurement of intraocular pressure. | After 1.5h, higher precorneal retention for NPs solution compared with standard eye drops. Maximal IOP lowering effect was observed at 4h with a value of 10.5±0.51 mmHg for NPs solution. Maximal IOP lowering effect was observed at 3h with a value of 6.8±0.35 mmHg for standard eye drops. | [69] | |
| Carteolol | Rabbit | Precorneal retention by gamma scintigraphy. Measurement of intraocular pressure by a Schiotz tonometer. | Standard solution showed a quick fall in radioactive counts on corneal surface with respect of time as compared to NPs suspension in 0.5h. Maximum IOP lowering effect was observed at 2h with a value of 18.04±0.697 mmHg for NPs solution. Maximum IOP lowering effect was observed at 1h with a value of 22.616±0.639 mmHg for NPs solution. | [70] | |
| Alginate-chitosan | Azelastine | Rat | Determination of drug efficacy by counting of scratching instances, by analysis of conjunctival hyperemia, edema and by eosinophil count. | Similar reduction in eye scratching behavior for NPs solution and Azelast®. Higher reduction of hyperemia and edema for NPs solution compared with Azelast®. Reduction of eosinophil count lasted 4h for Azelast® and 10h for NPs solution. | [71] |
| 5-Flourouracil | Rabbit | Determination of drug concentration in ocular tissues by HPLC. | AUC(0–8) and Cmax values were found 17-fold and 13-fold higher, respectively, for NPs solution compared with standard solution. | [72] | |
| Albumin | Pilocarpine | Rabbit | Measurement of intraocular pressure by a Schiotz tonometer. | AUC values were found to be 3.19-fold and 1.67-fold higher for 1%-drug NPs solution compared with 1%-drug and 4%- drug standard solution, respectively. | [73] |
| Albuminchitosan | Tetracaine | Rabbit | Determination of blink response after cotton swab stimuli. | No statistical difference of efficacy between NPs solution and standard solution. Duration of action was 4-fold higher for NPs solution compared with standard solution. | [74] |
| Atropine | Rabbit | Measurement of mydriasis by video recording and analysis. | AUC values were found to be at 10.67 for 0.66%-drug NPs solution and 10.02 for 1%- drug standard solution. Maximum effect (pupil-corneal ratio) was found to be at 0.630 for 0.66%-drug NPs solution and at 0.596 for 1%-drug standard solution. | [75] | |
| Gelatin | Timolol | Rabbit | Eye irritation test (Draize test). Measurement of intraocular pressure by a plunger load tonometer. | No sign of ocular irritation. AUC values were found to be 2.27-fold higher for NPs solutions compared with marketed eye drops. | [76] |
| Moxifloxacin | Rabbit | Eye irritation test. Assessment of antimicrobial efficacy by observation of clinical parameters. | No sign of ocular irritation. No difference in antimicrobial efficacy between NPs solution at a dose regime of twice a day and MOXIGRAM® at a dose regime of four times a day. NPs solution decreased secretion (discharge), redness and swelling faster when compared with MOXIGRAM®. | [77] | |
| HA-chitosan | Dexamethasone | Rabbit | Eye irritation test (Draize test). Determination of drug concentration in aqueous humor by HPLC. |
No sign of ocular irritation. AUC(0–∞) values were found to be 1.93-fold and 2.39-fold higher for chitosan NPs solution and chitosan-HA NPs solution, respectively, compared with standard solution. | [78] |
| Dorzolamide or Timolol | Rabbit | Eye irritation test (Draize test). Measurement of intraocular pressure by a Schiotz tonometer. | No sign of ocular irritation. IOP lowering effect peaked at 3h for marketed solution, at 4h and observed for up to 8h for chitosan NPs solution, and at 4h and observed for up to 12 h for chitosan-HA NPs solution. | [79] | |
| EC | Acetazolamide | Rabbit | Measurement of intraocular pressure by a tonometer. | Maximum IOP reduction was found 1.33-fold higher for NPs solution compared with standard solution. Mean time for IOP reducing effect was 6h for NPs solution and 5h for standard solution. | [80] |
|
Synthetic materials | |||||
| Eudragit® | Aceclofenac | Rabbit | Assessment of anti-inflammatory efficacy by observation of polymorphonuclear leucocyte (PMN) migration and lid closure. | PMN count in tears were found to be 1.57-fold and 1.18-fold lower for NPs solution and standard aqueous solution, respectively, compared with control eyes. | [81] |
| Aceclofenac | Rabbit | Assessment of anti-inflammatory efficacy by assessment of polymorphonuclear leucocyte (PMN) migration and lid closure. | PMN count in tears at 3h were found to be 1.66-fold and 1.28-fold lower for NPs solution and standard aqueous solution, respectively, compared with control eyes. | [82] | |
| Diclofenac | Rabbit | Assessment of anti-inflammatory efficacy by assessment of polymorphonuclear leucocyte (PMN) migration and lid closure. | Greater decrease of PMN count at all time points for NPs solution compared with standard aqueous solution. | [83] | |
| Ibuprofen | Rabbit | Eye irritation test (Draize test). Determination of drug concentration in ocular tissues by HPLC. | No sign of ocular irritation. 2h after instillation, drug concentrations were 1.54±0.06 μg/mL for NPs solution and 0.93±0.08 μg/mL for standard solution. | [84] | |
| Betaxolol | Rabbit | Eye irritation test (Draize test). Determination of drug concentration in tear fluid by HPLC. Measurement of intraocular pressure by an indentation tonometer. | NPs solution was found safer and less toxic than standard solution. Higher drug concentrations were found at all time points for NPs solution compared with standard solution. After 90 min, drug concentrations cannot be detected for standard solution, whereas drug concentrations were detected until 240 min for NPs solution. For standard solution, maximum IOP lowering effect was found at 30 min (5.04 mmHg) and the effect significantly declined after 60 min. For NPs solution, maximum IOP lowering effect was found at 120 min (4.89 mmHg). | [85] | |
| Acetozalamide | Rabbit | Measurement of intraocular pressure by a Riester tonometer. | For standard solution, maximal IOP lowering effect was observed at 2h with a ΔIOP value of 2.98±0.11 mmHg. After 6h, no IOP lowering effect was observed. For NPs solution, maximal IOP lowering effect was observed at 8h with a ΔIOP value of 5.32±0.07 mmHg. | [86] | |
| Brimonidine | Rabbit | Eye irritation test (Draize test). Measurement of intraocular pressure by a Schiotz tonometer. | No sign of ocular irritation. AUC(ΔIOP vs. t) values were found to be 3.55–6.98-fold higher for NPs solution, compared with IOBRIM®. | [87] | |
| Amphoterin B | Rabbit | Eye irritation test (Draize test). | No sign of ocular irritation. | [88] | |
| Azelastine | Rat | Assessment of eye scratching, hyperemia, edema and eosinophils in the conjunctiva. | No significant difference of eye scratching, hyperemia and edema between NPs solution and AZELAST®. Eosinophil counts were found lower at 6h and 10h for NPs solution compared with AZELAST®. | [89] | |
| Acetazolamide | Rabbit | Measurement of intraocular pressure by a tonometer. | Maximum IOP reduction was found 1.51-fold higher for NPs solution compared with standard solution. | [80] | |
| PLADextran- PBA | Cyclosporine A | Mice | Quantification of tear fluid production and fluorescein staining analysis after dry eye disease induction. Histopathology analysis. | Similar tear fluid production and fluorescein staining were observed for NPs instilled once a week compared with the conventional treatment (RESTASIS®) instilled three times a day. No sign of ocular irritation. | [90] |
| PLA-PMAPBA | Cyclosporine A | Rat | Slit lamp and OCT imaging examination. | No sign of ocular toxicity. | [91] |
| PLGA | Fluoromethalone | Pig | Eye irritation test (Draize test). Assessment of anti-inflammatory efficacy by scoring of clinical symptoms. Determination of drug concentration in ocular tissues by HPLC. | No sign of ocular irritation. Ocular inflammation was found significantly lower for NPs solution compared with ISOPTOFLUCON®. | [92] |
| Aceclofenac | Rabbit | Assessment of anti-inflammatory efficacy by observation of polymorphonuclear leucocyte (PMN) migration and lid closure. | PMN counts were found significantly lower for MPs solution, compared with standard aqueous solution. | [93] | |
| PLGA-PEG | Dorzolamide | Rabbit | Measurement of intraocular pressure by a tonometer. | Similar efficacy on IOP lowering between one drop of NPs and 4 drops of TRUSOPT®. | [94] |
| PCL | Cyclosporine A | Rabbit | Determination of drug concentration in tear fluid by liquid scintillation counting. | AUC values were significantly higher for NPs solution compared with oily control. | [95] |
| Indomethacin | Rabbit | Determination of drug concentration in tear fluid by liquid scintillation counting. | AUC(0–4h) and Cmax values were found to be 4-fold and 7-fold higher, respectively, for NPs solution compared with standard INDOCOLLYRE®. | [96] | |
|
Combination of natural and synthetic materials | |||||
| Chitosan- PLGA | Forskalin | Rabbit | Eye irritation test (infra-red camera). Assessment of precorneal retention by gamma scintigraphy. Measurement of intraocular pressure by a Schiotz tonometer. | No sign of ocular irritation. Precorneal retention was found significantly higher for NPs solution compared with standard solution. For standard solution, maximum IOP lowering effect was found at 1h (20.1±1.56 mmHg). For NPs solution, maximum IOP lowering effect was found at 8h (16.3±0.75 mmHg). | [97] |
| Fluocinolone | Rabbit | Eye irritation test (Draize test). Determination of drug concentration in tear fluid by HPLC. | No sign of ocular irritation. AUC(0–∞) and Cmax values were found to be 5.23-fold and 2.19-fold higher, respectively, for chitosan-PLGA NPs solution compared with PLGA NPs solution. | [98] | |
| Chitosan- PLA | Amphotericin B | Rabbit | Eye irritation test (Draize test). Determination of drug concentration in tear fluid by HPLC. Assessment of corneal permeation by fluorescein staining. | No sign of ocular irritation. AUC values were found 1.5-fold higher for NPs solution compared with standard solution. Higher permeation and retention effects were noted for NPs solution compared with fluorescein solution. | [99] |
| Chitosan- PEG | Resveratrol | Rabbit | Assessment of corneal permeation by fluorescein staining. Measurement of intraocular pressure by a tonometer. | Increased fluorescent signal at the inner site of the cornea for chitosan-PEG NPs solution compared to chitosan NPs. Chitosan-PEG NPs solution reduced IOP by 4.3±0.5 mmHg up to 8h. | [100] |
| Resveratrol and quercetin | Rabbit | Measurement of intraocular pressure by a tonometer. | Chitosan-PEG NPs solution reduced IOP by 5.5±0.5 mmHg up to 8h. | [101] | |
| Chitosan-PEG-PCL | Diclofenac | Rabbit | Eye irritation test (Draize test). Assessment of corneal permeation by Nile red staining. Determination of drug concentration in aqueous humor by HPLC. | No sign of ocular irritation. AUC(0–24h) and Cmax values were found 2.3-fold and 2.11-fold higher, respectively, for NPs solution compared with commercial eye drops. | [102] |
| Eduragit®-HA | Gatifloxacin and prednisolone | Rabbit | Determination of drug concentration in aqueous humor by HPLC. | AUC(0–24h) and Cmax values were found 1.77-fold and 1–76-fold higher, respectively, for NPs solution compared with commercial eye drops. | [103] |
|
Combination of polymers with lipidic vectors | |||||
| Chitosan | Methazolamide | Rabbit | Eye irritation test (Draize test). Measurement of intraocular pressure by a tonometer. | No sign of ocular irritation. AUC(0–8h) values were 237.8 mmHg for chitosan lipid NPs, 175.2 mmHg for Azopt®, 81.2 mmHg for lipid NPs and 49.9 mmHg for standard solution. | [104] |
| Dexamethasone | Rabbit | Eye irritation test (Draize test). Determination of drug concentration in aqueous humor by HPLC. | AUC(0–24h) and Cmax values were found 5.38-fold and 2.37-fold higher, respectively, for NPs solution compared with commercial eye drops. | [105] | |
| Timolol | Rabbit | Eye irritation test. Assessment of precorneal retention by gamma scintigraphy. Determination of drug concentration in tear fluid by HPLC. Measurement of intraocular pressure by a tonometer. | No sign of ocular irritation. Higher precorneal retention of chitosan-coated liposomes compared with standard eye drops and liposomes. AUC(0–∞) and Cmax values were found 1.72-fold and 2.67-fold higher, respectively, for chitosan-coated liposomes compared with uncoated liposomes. Maximum IOP was 19.67±1.11 mmHg for chitosan-coated liposomes and 23.80 ± 1.72 mmHg for standard eye drops. | [106] | |
| Amphotericin B | Rabbit | Eye irritation test (symptom scoring). Determination of drug concentration in tear fluid and aqueous humor by HPLC. | No sign of ocular irritation. Chitosan-lipid carriers had a significantly greater percentage activity remaining in the pre-corneal area after 30 min (71.7%) as compared with lipid carriers (54.1%) and standard eye drops (40.8%). AUC(0–∞) and Cmax values were found 1.99-fold and 1.27-fold higher, respectively, for chitosan lipid carriers compared with lipid carriers. | [107] | |
| Amphotericin B | Rabbit | Eye irritation test (Draize test). Determination of drug concentration in tear fluid by mass spectrophotometry. | No sign of ocular irritation. AUC(0–∞) values were found 2.05-fold higher, for chitosan/lecithin NPs compared with Fungizone®. | [108] | |
| Flurbiprofen | Rabbit | Eye irritation test (symptom scoring). Assessment of precorneal retention by gamma scintigraphy. | No sign of ocular irritation. AUC(0–10min) values for chitosan-coated liposomes were found to be 2.84-fold and 1.53-fold higher in the cornea-conjunctiva region compared with standard eye drop and uncoated liposomes, respectively. | [109] | |
| Flurbiprofen | Rabbit | Eye irritation test (symptom scoring). Assessment of precorneal retention by gamma scintigraphy. | No sign of ocular irritation. AUC(0–10min) values for chitosan-coated lipid carriers were found 4.66-fold and 1.70-fold higher in the cornea-conjunctiva region compared with standard eye drops and uncoated lipid carriers, respectively. | [110] | |
| Ofloxacin | Rabbit | Eye irritation test (Draize test). Assessment of precorneal retention by fluorescein staining. Determination of drug concentration in aqueous humor by HPLC. Assessment of anti-microbial efficacy by keratitis induction and symptoms scoring. | Precorneal retention time was observed during 40–60 min for chitosan lipid carriers and for 20–40 min for lipid carriers. Maximum drug concentration was found at 1h for commercial eye drops and at 4h for chitosan lipid carrier. After keratitis induction, significantly lower conjunctival redness and corneal opacity was observed with chitosan lipid nanocarrier treatment compared with commercial solution. | [111] | |
| Natamycin | Rabbit | Eye irritation test (Draize test). Determination of drug concentration in tear fluid by mass spectrophotometry. | No sign of ocular irritation. AUC(0–∞) values were found 1.47-fold higher, for chitosan/lecithin NPs compared with standard suspension. Clearance was significantly decreased (7.4-fold) for chitosan/lecithin NPs compared with standard suspension. | [112] | |
| Cyclosporin A | Rabbit | Determination of drug concentration in cornea, conjunctiva and sclera by HPLC. | Higher drug absorptions in cornea, conjunctiva and sclera for chitosan-coated liposomes compared with liposomes. | [113] | |
| Ciprofloxacin | Rabbit | Assessment of anti-microbial efficacy by bacterial conjunctivitis induction and symptoms scoring. | No significant difference of antimicrobial efficacy between chitosan-coated liposomes and Ciloxan®. | [114] | |
| Chitosan-HA | Moxifloxacin | Rabbit | Eye irritation test (Draize test). Determination of drug concentration in tear fluid by HPLC. | No sign of ocular irritation. AUC(0–∞) and Cmax values were found 6.74-fold and 3.17- fold higher, respectively for NPs solution compared with Vigamox®. | [115] |
| HA | Tacrolimus | Rabbit | Determination of drug concentration in aqueous humor by HPLC. | The relative bioavailability of HA-coated niosomes was 2.3-fold and 1.2-fold for that of suspension and non-coated niosomes, respectively. | [116] |
| Doxorubicin | Rabbit | Determination of drug concentration in aqueous humor. Assessment of drug permeation in cornea by laser scanning microscopy. | AUC and Cmax values were found 1.68-fold and 1.36-fold higher, respectively, for NPs solution compared with standard solution. Higher drug permeation was noted for NPs solution compared with standard solution. | [117] | |
| PEG-PCL | Diclofenac | Rabbit | Eye irritation test (Draize test). Determination of drug concentration in aqueous humor by HPLC. | No sign of ocular irritation. AUC(0–24h) and Cmax values were found 2.02-fold and 3.03-fold higher, respectively, for NPs solution compared with standard solution. | [118] |
| PEG-PLA | Cyclosporin A | Rabbit | Determination of drug concentration in aqueous humor and cornea by HPLC. | NPs solution exhibited 4.5-fold increase in retention effect on eyes compared with standard emulsions. | [119] |
Abbreviations: AUC= Area under the curve; Cmax = Maximal concentration; EC= Ethyl cellulose; HA= Hyaluronic acid; HPLC = High performance liquid chromatography; IOP= Intraocular pressure; MPs= Microparticles; NPs= Nanoparticles; PCL= Poly(epsilon-caprolactone); PEG= Polyethylene glycol; PLA= Polylactide; PBA = Phenylboronic; PMA = poly(methacrylic acid); PLGA= Poly(lactic-co-glycolic acid); PMN= Polymorphonuclear leucocyte ; ΔIOP= Intraocular pressure variation.
3.1. Polymeric nanoparticles
Due to recent advances in the fields of biomaterials and nanotechnology, new types of polymeric DDS have been developed. Both natural and synthetic polymers have largely been used to formulate NP-based systems for ocular drug delivery [4].
3.1.1. Naturally derived polymer-based nanoparticles
Natural polymers are generally considered more biocompatible and mucoadhesive compared to synthetic polymers, making them suitable for the formulation of NPs for ocular DDS. Among them, chitosan, alginate, albumin, gelatin and hyaluronic acid (HA) have shown promising in vivo results as NP-based DDS.
Chitosan.
As we described in section 1.2, chitosan has been used as an ingredient for in situ gel forming formulation, due to its ability to increase its viscosity at body pH. Its high mucoadhesiveness and permeability make chitosan an attractive candidate for the formulation of NPs for ocular DDS. The mucoadhesive nature of chitosan is mediated via electrostatic interactions, hydrogen bonds, and hydrophobic effects [120]. Chitosan is also considered as a paracellular permeability enhancer due to its ability to reversibly open the tight junctions between epithelial cells [121]. During the last decades, chitosan has been used widely to develop NPs-based DDS for the anterior segment. It showed promising results in the delivery of a variety of drugs, such as anti-inflammatory drugs (ganciclovir [63], diclofenac [64], cyclosporine A [65,66], indomethacin [67] and celocoxib [68]) and anti-glaucoma drugs (timolol [69] and carteolol [70]). A recent study compared the stability and pharmacokinetics of different types of polymer as nanocarriers to deliver celecoxib: chitosan, alginate and other synthetic polymers such as PCL, PLA and PLGA. Results demonstrated that chitosan NPs had the best in vitro stability and in vivo bioavailability in a rat model [68].
Alginate.
With its high molecular weight, alginate has mucoadhesive properties and thus represents a promising material to be used in ocular DDS. However, alginate has low stability and fast biodegradation, limiting its use for sustained drug release. Thereby, alginate was combined with chitosan to increase its stability. A study demonstrated that alginate-chitosan microspheres were able to prolong the retention time of azelastine in the cul-de-sac and to improve the therapeutic efficacy on in vivo using a rat model. Another study compared the effect of a chitosan coating of chitosan-alginate NPs for the delivery of 5-Flourouracil (5-FU). Interaction between corneal mucin layer and chitosan-alginate NPs was observed only with chitosan coating, resulting in higher bioavailability. A significantly higher level of 5-FU was found in aqueous solution of chitosan-alginate NPs compared to standard 5-FU solution [72].
Albumin.
Albumin is a natural globular protein, commonly found in egg or blood plasma. Pilocarpine nitrate was encapsulated in egg albumin microspheres and showed a higher miotic response and duration [73]. Albumin was also combined with chitosan to formulate tetracaine-loaded [74] and atropine-loaded [75] microspheres. These studies reported that microencapsulated tetracaine significantly increased the duration of action and effect of the drugs, compared to standard drug solution.
Gelatin.
Gelatin is a polymer derived from collagen, a natural constituent of the corneal tissue. Gelatin can interact with the negatively charged mucin layer due to the presence of positively charged amino groups in its structure. Moreover, the presence of arginine-glycine-aspartic acid sequence (RGD motif) provides cell adhesion properties [122]. Gelatin NPs have been formulated to successfully deliver timolol [76] and moxifloxacin [77] to the corneal surface. Moreover, it has been shown that gelatin NPs possessed good stability, effective lowering of the IOP, high drug bioavailability and lack to irritation [76].
Hyaluronic acid.
HA has not only been used as an ingredient for in situ gel forming formulation (as described in section 1.2) but has also been combined with other polymers to formulate NPs. For example, HA has been assessed as a coating of chitosan NPs in several studies. HA-coated chitosan NPs demonstrated a higher sustained release of dexamethasone compared to uncoated chitosan NPs, showing that the combination of HA with chitosan results in higher mucoadhesive properties by interacting with hyaluronan receptors on the corneal epithelia [78]. Moreover, HA-modified chitosan NPs allowed successful delivery of dorzolamide and timolol on an in vivo albino rabbit model. A significantly higher reduction of IOP was observed when compared to a standard drug formulation as well as unmodified chitosan NPs [79].
Overall, NPs-based systems using natural polymers showed high adhesive properties and good biocompatibility allowing significantly higher drug retention and permeation through ocular tissues without inducing toxicity. However, natural polymers are also known to be easily degraded and their production process are limiting by low batch-to-batch reproducibility [4].
3.1.2. Synthetic derived polymer-based nanoparticles
Compared to natural polymers, synthetic polymers are generally more stable due to lower biodegradability rates, providing a slower and sustained release of drugs. Furthermore, synthetic polymers are more suitable for modifications such that it allows adjustment of their chemical and biological properties, physicochemical state, degradability and mechanical strength, according to the final biomedical applications [123]. However, synthetic polymers are also considered as less mucoadhesive than natural polymers due to the lack of functional groups that are able to interact with the mucin layer, limiting their bioavailability [4]. Among the various synthetic polymers, Eudragit®, poly(lactic acid) (PLA), poly(lactic-co-glycolic) (PLGA) and polycaprolactone (PCL) showed particularly promising in vivo results for improving drug bioavailability and efficacy by topical administration.
Eudragit®.
Eudragit® is the trade name used for synthetic copolymers derived from esters of acrylic and methacrylic acid. Eudragit® polymers present great versatility according to the functional groups in the side-chain of the polymer. Eudragit® RS100 and RL100 polymers or a combination of them have been commonly used as ocular DDS due to their positive charge, which can increase its precorneal retention time by interacting with the negatively charged mucin layer.
These polymers have been used to successfully deliver a variety of drugs on the ocular surface including anti-inflammatory drugs (aceclofenac [81,82], diclofenac [83] and ibuprofen [84]), anti-glaucoma drugs (betaxolol [85], acetozalamide [86], brimonidine [87]), amphotericin B [88] and azelastine [89]. Recently, researchers developed a particularly interesting formulation based on Eudragit®/montmorillonite (Mt) microspheres to deliver betaxolol by topical administration. Drug release occurred in a 4-step process, allowing sustained release of betaxolol and thus longer bioavailability. In vitro studies showed an extended release duration of 12h with Eudragit®/Mt microspheres in comparison to standard betaxolol solution (2.5h) and only Eudragit® microspheres (5h). Moreover, in vivo Draize rabbit eye test demonstrated a lower toxicity of betaxolol loaded in Eudragit®/Mt microspheres compared to betaxolol in standard solution [85].
Poly(lactic acid) (PLA).
PLA is a hydrophobic polyester synthetized by ring-opening polymerization of lactide. It is FDA-approved and has been widely used for various biomedical applications [124]. However, the biodegradability rate of PLA is relatively low compared to other polymers [125], limiting its use for formulation of eye drops; therefore, it is usually grafted with other polymers to tailor its biodegradability [126].
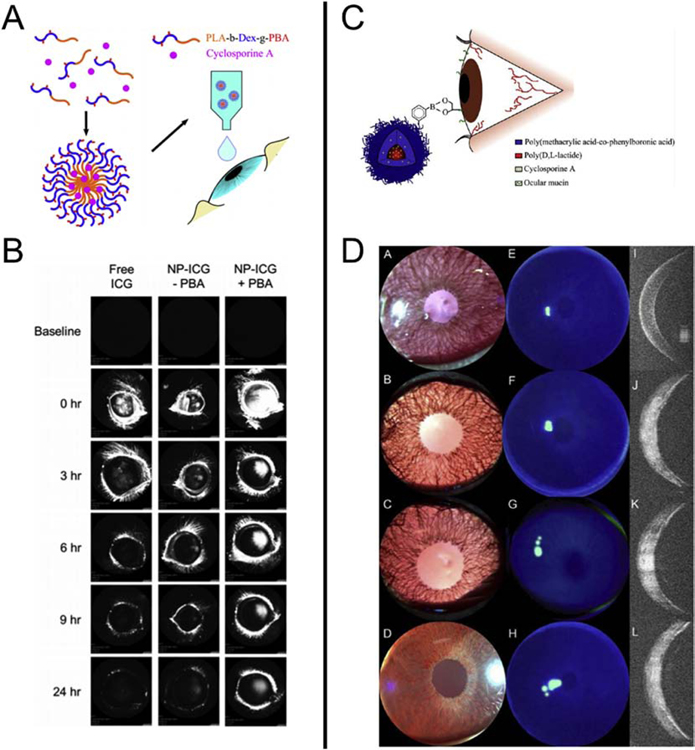
In a recent study, Liu et al. developed NPs composed of PLA, dextran and phenylboronic (PBA) for the delivery of cyclosporin A on the ocular surface (Fig 4A) [127]. PBA is a molecule able to form covalent linkage with cis-diol groups of carbohydrates of the mucin layer [90]. In vivo studies performed on mice demonstrated that the PBA coating of the PLA-dextran NPs increased the retention time of the NPs when compared to conventional eye drops (Fig. 4B). Remarkably, it has also been shown that once a week dosage of NPs had similar therapeutic effect to three times a day dosage of the marketed formulation RESTASIS®. Another study assessed the use of NPs composed of PLA grafted with poly(methacrylic acid) (PMA) and PBA for the delivery of cyclosporin A (Fig. 4C) [91]. Different ratios of PLA:PMA:PBA were used including LMP-0 (49.8:50.2:0), LMP-10 (51.3:46.7:3.8) and LMP-30 (58.1:35.2:10.4). Results showed that the addition of PBA increased the drug retention time without significant toxicity in an in vivo rat model (Fig. 4D).
Fig. 4. Increased drug retention by using poly(lactic acid) (PLA)-based nanoparticle systems.
(A) Schematic of PLA-Dextran NPs the for the delivery of cyclosporin A (From [127]). (B) Images of rabbit eyes treated with free indocyanine green (ICG), NP–ICG (− PBA), and NP–ICG (+ PBA) obtained with confocal scanning laser ophthalmoscopy, (λex = 795 nm and λem = 810 nm). (C) Schematic of PLA-PMA-PBA NPs the for the delivery of cyclosporin A. (D) Slit lamp, fluorescence, and OCT images for LMP-0 (A,E,I), LMP-10 (B,F,J), LMP-30 (C,G,K), and negative control (D,H,L).
Poly(lactic-co-glycolic acid) (PLGA).
PLGA is a FDA-approved synthetic copolymer of PLA and poly(glycolic acid) (PGA), known for its biodegradability and biocompatibility. Compared to PLA, the biodegradability rate of PLGA is relatively faster and its mechanical properties can be finely turned by modulating the PLA/PGA ratio. This polymer has particularly been used to formulate nanocarriers for topical ocular delivery.
PLGA NPs were developed for the delivery of fluoromethalone [92] and aceclofenac [93]. No significant cytotoxicity of PLGA NPs were found in vitro and in vivo [128]. Compared to a standard drug formulation, PLGA NPs increased drug bioavailability, providing better drug efficacy. However, unlike other polymers, PLGA is not mucoadhesive [4]; therefore, it was combined with polyethylene glycol (PEG), a synthetic polymer able to interact with the mucin layer [129], to formulate mucoadhesive microspheres. These microspheres were used to deliver dorzolamide to the eyes of rabbits and showed a 35% greater maximum IOP decrease, and 2-fold increase in the duration of the IOP decrease, compared to TRUSOPT®. Interestingly, it has been found that a single drop of PLGA-PEG microspheres had a similar efficacy compared to 4 drops of TRUSOPT® or 2 administrations of TRUSOPT® at a 4-h interval [94].
Polycaprolactone (PCL).
PCL is another synthetic biodegradable polyester, particularly used in tissue engineering and drug delivery systems for varied biomedical applications. PCL has been especially used as nanocarriers for the delivery of carteolol [130], cyclosporine A [95] and indomethacin [96] to the anterior segment. In particular, PCL NPs showed a more pronounced IOP decrease compared to commercial carteolol eye drops [130].
3.1.3. Combination of natural and synthetic polymers
As discussed previously, natural and synthetic polymers present specific advantages and disadvantages for ocular drug delivery. In order to gather the advantages of each source of polymers, some studies studied the combination of natural and synthetic polymers for the formulation of NPs-based DDS.
Chitosan-based combinations.
Synthetic polymers are generally not mucoadhesive, limiting the bioavailability on the corneal surface. In order to overcome this limitation, several formulations of synthetic polymers have been combined with chitosan, which has highly mucoadhesive properties. For example, chitosan was used as a coating for PLGA NPs and showed a sustained delivery of forskalin and thus, a greater IOP lowering effect and duration compared to standard forskalin solution. Chitosan-coated PLGA NPs have also been used to deliver fluocinolone to the anterior segment of rabbit eyes [98]. Results showed that chitosan coating increased and sustained drug release by PLGA NPs. Due to its hydrophilic properties, chitosan is not suitable to encapsulate hydrophobic drugs such as amphotericin B. Therefore, some researchers formulated amphiphilic NPs based on PLA-grafted-chitosan copolymer. An in vivo ocular pharmacokinetic study showed a prolonged precorneal retention time. Moreover, no sign of irritation was observed during the ocular irritation study [99]. Chitosan was also combined with PEG to formulate resveratrol-loaded NPs [100] and resveratrol and quercetin co-encapsulated NPs [101]. Both studies showed a sustained and enhanced reduction of IOP compared to standard drug solutions. Chitosan was also combined with PCL and PEG to formulate diclofenac-loaded nanosuspension [102]. In vivo pharmacokinetics studies showed enhanced precorneal retention time and penetration of the formulated nanosuspensions compared with commercial diclofenac eye drops.
Eudragit®-based combinations.
Eudragit®/ethylcellulose (EC) NPs were designed to combine the advantages of the mucoadhesiveness, the controlled-release properties of EC and the positive charges of Eudragit® that can interact with the negatively charged mucin layer [80]. This formulation was used to successfully deliver acetazolamide in normotensive rabbits. Results showed a greater IOP decrease and longer duration of the effect was displayed in normotensive rabbits compared with standard acetazolamide solution. Eudragit® NPs were also coated with HA in order to increase their mucoadhesiveness. However, no difference was observed for the simultaneous delivery of gatifloxacin and prednisolone, with or without HA coating [103].
3.2. Polymeric/lipidic nanoparticles
For the past decades, the use of lipidic vectors, such as liposomes or solid-lipid NPs, have widely been used for drug delivery in numerous biomedical applications, especially in ophthalmology. Due to its hydrophobicity, lipid carriers are suitable for encapsulation of hydrophobic drugs. Moreover, drugs encapsulated into lipophilic carriers can pass the corneal epithelial layer due to the solubilization of the carriers in the lipid cell membranes. However, lipidic formulations are known to be less stable and thus less suitable for sustained drug release. In recent years, addition of polymers to lipidic NPs formulations have raised special interest in order to increase the stability and mucoadhesiveness of NPs on the corneal surface.
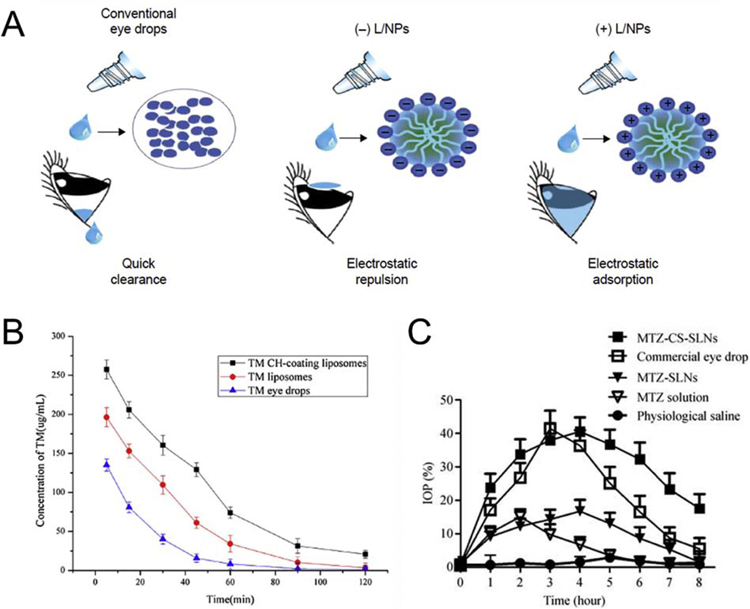
So far, chitosan is the polymer that is most combined with liposomes, micelles or solid lipidic NPs. Chitosan/lipidic NPs were formulated and showed an increased bioavailability and a sustained release of a variety of drugs, such as dexamethasone [105], timolol [106], amphotericin B [107,108], flurbiprofen [109,110], ofloxacin [111], natamycin [112], cyclosporin A [113], ciprofloxacin [114]. In a study by Ban et al., they compared the delivery of dexamethasone in three different vectors: standard aqueous solution, negatively charged lipidic NPs and positively charged lipidic NPs. Interestingly, an in vivo study on rabbit eyes showed an increase of dexamethasone permeation of 2.7-fold and 1.8-fold for chitosan-modified and unmodified lipidic NPs, respectively [105]. These results display the importance of the effect of NP surface charge on the drug release and bioavailability (Fig. 5A). More interestingly, chitosan/lipidic NPs loaded with anti-glaucoma drugs demonstrated an increased IOP lowering effect compared to standard drug formulations, showing the correlation between drug bioavailability and its efficacy (Fig. 5B–C) [104,106]. Moreover, no significant ocular irritation, damage or toxicity were observed by using chitosan/lipidic NPs [104,106–111].
Fig. 5. Increased drug retention and therapeutic effect using chitosan for the development of polymeric/lipidic NPs.
(A) Schematic illustration of differently charged lipidic NPs carriers containing dexamethasone (From [105]). (B) The concentration–time curves of timolol (TM) in rabbit tears following topical administration of TM eye drops and liposomes with or without chitosan (CH) (mean ± SD, n = 3) (From [106]). (C) Percentage decrease in intraocular pressure (IOP) after administration of methazolamide solution, methazolamide-SLNs (solid lipids NPs), methazolamide-chitosan-SLNs, commercial eye drops and physical saline solution. (mean ± SD, n = 6) (From [104]).
More recently, HA has also been combined with lipidic NPs to deliver moxifloxacin [115], tacrolimus [116] and doxorubicin [117]. Due to the ability of HA to target CD44 receptor on the corneal epithelial cells, these studies demonstrated an increase of drug bioavailability without significant toxicity.
Synthetic polymers have also been used to increase the stability and sustained release of drugs delivered by micelles. In particular, diclofenac was loaded in PEG-PCL micelles and showed a 2-fold increase of drug delivery in the aqueous humor of rabbit eyes compared with diclofenac PBS solution eye drops [118]. More recently, PEG-PLA micelles were formulated to deliver cyclosporine A in rabbit eyes, resulting a 4.5-fold increase of drug retention compared with 0.05% cyclosporine A emulsion [119].
4. Combination of several DDS
As previously described, in situ forming gels and NPs-based systems represent promising strategies for ocular DDS. In order to combine the efficacy of each of these systems, combination of NPs and in situ gels has been investigated (Table 5).
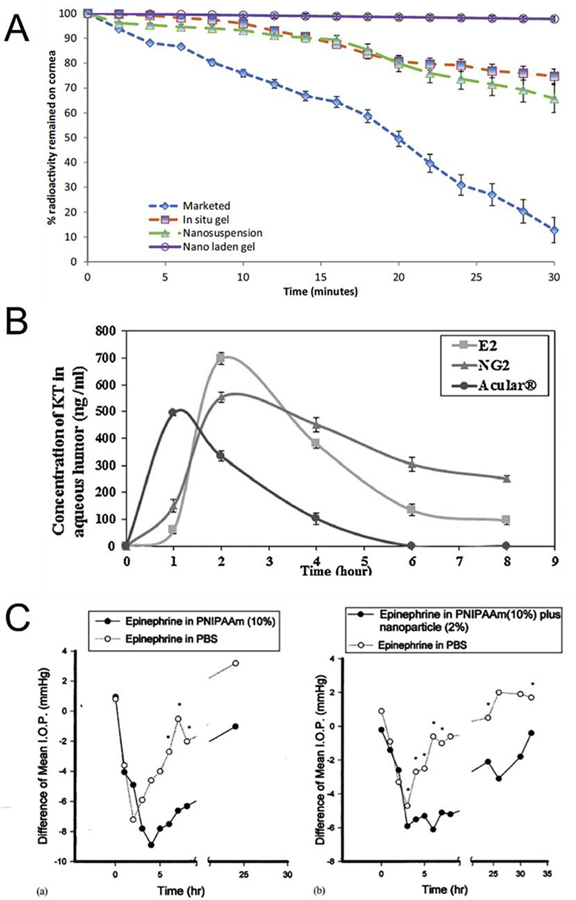
Different combinations of NPs and in situ gels were formulated and allowed a successful delivery of a variety of drugs, such as antimicrobial drugs (levofloxacin [131,134], vancomycin [139], fluconazole [140]), anti-glaucoma drugs (brimonidine [132], curcumin [135], dexamethasone [136], epinephrine [23], timolol [141]), anti-inflammatory drugs (keratolac [137], pranoprofen [138]) and 5-fluorouracil [133]. For example, chitosan NPs loaded in alginate/HPMC in situ gels increased precorneal retention time and limited the drainage via nasolacrimal conduct [131]. Similar results were obtained for the formulation of levofloxaxin-loaded PLGA NPs combined with chitosan in situ gels. It has been shown that drainage was faster for the marketed formulation, compared with in situ gel, NPs and NPs-gel (Fig. 6A) [134].
Fig. 6. Increased corneal retention and sustained release of drugs by combining in situ gels and NPs.
(A) Dynamic gamma scintigraphy study showing percentage radioactivity remaining on cornea with time (blue-diamond shape) marketed, (green triangle shape) chitosan in situ gel, (red-square shape) nanosuspension, (purple-circle shape) nanoparticle laden in situ gel (From [134]). (B) Concentration of ketarolac in aqueous humor of rabbit eyes with time from the nanodispersion (E2) and in situ gel incorporated with E2 (NG2) compared to Acular® eye drops (From [137]). (C) The difference of IOP between two eyes (i.e. IOP lowering effect) for (a) linear PNIPAAm eye drops; and (b) linear PNIPAAm and nanoparticles mixture eye drops (From [23]).
Also, several studies showed that the combination of NPs and in situ gels can improve drug permeation into ocular tissues and aqueous humor. Chitosan-HA NPs loaded in chitosan in situ gels exhibited a sustained delivery of 5-fluorouracil in rabbit aqueous humor compared to NPs or in situ gels only solution [133]. Improved drug penetration was also observed for albumin NPs loaded in poloxamer® gel [135], poloxamer NPs loaded in poloxamer® gel [136] and Eudragit® NPs loaded in poloxamer®/HPMC gel [137], PLGA-Eudragit® and PCL-Eudragit® loaded in Carbopol® gel [139] and liposomes loaded in HA gel [140]. Compared to NPs or in situ gels only, their combination allowed to avoid a burst release and sustain the drug delivery to the aqueous humor (Fig. 6B).
More interestingly, combination of NPs and in situ gels also provides a higher therapeutic effect. A formulation of PLGA NPs loaded in Carbopol® in situ gel have been developed for delivery of pranoprofen. This formulation was compared with OFTALAR®, a marketed eye drop, in an in vivo rabbit model of inflammation induced by arachidonic acid sodium. Results demonstrated a lower inflammation score with NPs-gel compared to the OFTALAR® [138]. Moreover, it has been shown that liposomes loaded in gellan gum in situ gels allowed an increase of the IOP lowering effect and duration of timolol compared with standard eye drops [141]. Similar results in IOP lowering effect were observed for chitosan and alginate NPs loaded in poloxamer gel [132] and PNIPAAm NPs loaded in PNIPAAm gel [23] (Fig. 6C).
5. Challenges for the commercial development of new ophthalmic drug delivery systems
Despite the high number of publications describing new ophthalmic DDS, relatively few products are finally commercialized. From bench to batch to market, numerous steps need to be achieved including preclinical and clinical development and pharmacovigilance. Regulations for commercialization of new ophthalmic DDS can vary according to the country. Here, we will describe the regulatory affairs of the three regions where most eye drops are currently commercialized: the United States, Europe and Japan.
5.1. Regulatory affairs
Eye drops are defined as medicinal products, if used through pharmaceutical, immunological or metabolic action, or as medical devices if used for cleaning, rinsing or hydrating [142]. Eye drops containing polymers (HA, cellulose derivatives or others) such as artificial tears are considered as medical devices because their action is limiting to hydrate the corneal surface. Due to the presence of active pharmaceutical drugs in the formulation, DDS-based eye drops are considered as medicinal products. To commercialize a new eye drop formulation, pharmaceutical companies must prove the efficacy and safety of their formulations by performing preclinical and clinical studies. After these processes, they can ask for marketing authorization to the relevant competent authority of the country where they want to sell the product, particularly the European Medicines Agency (EMA, Europe), the Food and Drug Administration (FDA, US) and the Ministry of Health, Labour and Welfare (Japan).
5.2. Production process and quality controls
One of the most challenging steps for commercialization of new DDS is the production of large-scale batches. These batches must be produced under Good Manufacturing Practices (GMPs), which are guidelines that ensure quality and reproducibility from batch-to-batch. The modification of manufacturing process between academic laboratories and industry can significantly affect the product characteristics and thus, its efficacy and safety [143]. The Pharmacopeia is a regulatory publication describing all criteria necessary for the manufacturing of medicinal products and the methods of analysis to guarantee quality controls. A complete description of the product must be provided according to the Pharmacopeia of the relevant country, including biological and chemical characterizations, manufacturing process, and quality controls. Biological and chemical criteria include product composition and physicochemical properties (appearance, color, pH, osmolarity, drug concentration, stability, sterility and purity). For NP-based systems, more specific criteria need to be assessed such as particle size/distribution, surface characterization (zeta potential, functionality and surface chemistry), morphology, drug loading, and drug encapsulation [144].
Particle size.
A particle size that exceed 10 µm cannot be absorbed by ocular tissues, nor eliminated through the nasolacrimal conduct, which can cause ocular irritability [145]. Thereby, the United States Pharmacopeia requires less than 50 particles superior or equal to 10 µm diameter per mL of solution.
Stability.
Stability tests are required to guarantee that the formulation presents the same properties and characteristics within specified limits and throughout its period of storage and use, that it possessed at the time of its manufacturing. Natural materials, such as chitosan or gelatin, are known to be less stable and more degradable than synthetic polymers [146], explaining the reason for relatively low use of natural materials in ophthalmic formulations. Maintaining the stability of nano-emulsions and nano-suspensions can also be particularly challenging due to the risk of aggregation and degradation of NPs [147].
Sterility.
Different techniques of sterilization can be used such as sterile filtration, autoclaving, irradiation or treatment with ethylene oxide and gas plasma. Each of these techniques has advantages and disadvantages that need to be considered according to the properties of the active and inactive ingredients of the formulation. Irradiation with γ-radiation, electron beam or X-rays are the techniques most used for ophthalmic preparations because no heat or chemicals are required [4,148]. Moreover, benzalkonium chloride (BAK) is widely used in multidose eye drops to maintain sterility between uses. However, several studies showed that BAK can have side effects for ocular tissues, resulting in complications such as dry eye, trabecular meshwork degeneration and ocular inflammation [149,150]. An alternative to BAK is the use of single-dose vials or multidose bottles fitted with an antimicrobial membrane.
Purity.
The different Pharmacopeia recommend that endotoxin limits cannot exceed 0.5 EU/mL for ophthalmic preparations. As described previously, natural polymers, such as chitosan or alginate, have particularly interesting properties for use in ophthalmic DDS. However, because they are extracted from natural sources, impurities such as endotoxins could be present and cause immunogenic reactions [151]. These impurities could explain why chitosan is not used in marketed formulations, despite its numerous advantages for ocular drug delivery.
5.3. Preclinical and clinical studies
The goal of preclinical and clinical studies is to assess the efficacy and safety of the formulation from pharmacology, pharmacokinetics and toxicology aspects. In the case of NP-based systems, preliminary in vitro tests are required including drug release, therapeutic activity, mechanism of action, cellular uptake and immunology [143]. It is usually recommended to perform preclinical studies on two different in vivo animal models, rodent and non-rodent. Rabbit animal models are the most frequently used for topical ophthalmic drugs and DDS, followed by dog and rat models. In preclinical studies, the formulation is applied on the animal eye and the adapted dosing and side effects are determined. Larger animal models have the advantage of closer anatomical and size proximity to human eyes, but are more expensive, and in the case of some species (e.g. rabbits, dogs) suffer from fewer reagents such as specific antibodies for pharmacodynamic studies. For eye drops, these pharmacokinetic studies are generally performed by quantification of the drug in plasma, tears and other ocular tissues, at different time points after instillation of the eye drop. Preclinical studies present limitations such as the low number of animals used and the short observation period. Furthermore, the difference in size and shape of ocular tissues, metabolic activity, and blinking rate between animal models and humans may also limit the extrapolation of such data to humans.
6. Concluding perspectives
Topical medications are the preferred method of drug delivery for numerous ocular disorders, including glaucoma which represents the third cause of blindness, with 105 million cases worldwide [152]. Despite its ease of use and relatively low cost compared to other treatments, the use of eye drops requires a strict dose regimen. Moreover, the high (albeit highly variable) concentrations that can be achieved in ocular tissues can cause side effects that range from relatively minor tolerability issues to significant toxicity side-effects such as the increasingly appreciated toxic effects of anti-glaucoma medications on the ocular surface epithelium. Poor tolerability profiles are usually associated with poor patient compliance, a major limiting factor for many topical medications.
Over the past few decades, a variety of DDS have been marketed for treatment of ocular conditions. In situ gelling systems are cost-effective, easy to produce, and generally biocompatible, making them good candidates for the development of ocular DDS. Beside its advantages, a limited number of in situ gels are currently in clinical use. In most of the studies detailed herein, significant improved therapeutic effects have been observed using gelling systems. Nevertheless, these improvements are usually not sufficient to significantly reduce the drug dose regimen. Among the marketed formulations containing gelling systems, similar side effects are observed compared with standard formulations. For these reasons, the development of new types of DDS shows special interests given the numerous published papers on these fields over the last decade.
In situ gels have shown their potential to increase the retention time of drugs on the ocular surface, thereby improving their therapeutic effect. Due to the sol-gel transition, the viscosity of in situ forming gels increases upon contact with the eye, limiting drug elimination via nasolacrimal drainage. Conversely, increase in viscosity can potentially also induce higher lacrimation that can accelerate drug elimination. Moreover, for some stimuli-responsive materials, the gelation efficiency is relatively weak. High concentrations of materials or a combination of several materials have been used that can increase their toxicity. Highly viscous gels can also induce visual blur, a limiting factor in their use.
Compared to in situ forming gels, NP-based systems have shown their ability to increase both drug retention and permeation through ocular tissues with limited increase of the formulation viscosity. Moreover, NP-based systems allow modification of the pharmacokinetics of drug release by prevention of a burst release effect of the drug, particularly of interest in cases of chronic diseases, such as glaucoma. Overall, cationic carriers showed better performance than anionic or non-ionic carriers, due to the electrostatic interactions with the negatively-charged mucin layer of the corneal surface. NP-based systems have also been incorporated in in situ forming gels and results showed better performance than NPs or gels alone. This trend of combining several DDS suggests that none of these systems alone seems to be efficient enough to achieve a significantly better performance.
Despite promising results, the biggest challenge will be to develop these DDS for clinical use. Despite their excellent adhesive and biocompatible properties, the use of natural polymers (especially from animal origin) in eye drops formulations considerably complicates the production process. For this reason, problems of stability, sterility and purity need to be anticipated at the very early stage of the product development. Therefore, more research and development need to be done in order to significantly improve methods of preparation and storage guaranteeing efficacy and safety of ocular DDS.
Table 3.
Drug delivery systems combining nanoparticles and in situ forming gels.
| NP types | In situ gels | Drug model | In vivo model | In vivo study | In vivo results | Ref |
|---|---|---|---|---|---|---|
| Chitosan | Alginate-HPMC | Levofloxacin | Rabbit | Precorneal retention by gamma scintigraphy. | NPs-gel retained for longer duration at corneal surface and negligible radioactivity was observed in other organs compared with aqueous solution. | [131] |
| Chitosan and alginate | Poloxamer | Brimonidine | Mice | Measurement of intraocular pressure by Tonolab tonometer. | For chitosan NPs, AUCtotal values were found 4.14-fold and 5.09-fold higher for in situ gel and NPs-gel, respectively, compared with ALPHAGAN P®. For alginate NPs, AUCtotal values were found 3.72-fold and 4.66-fold higher for in situ gel and NPs-gel, respectively, compared with ALPHAGAN P®. | [132] |
| Chitosan-HA | Chitosan | 5-fluorouracil | Rabbit | Determination of drug concentration in aqueous humor by HPLC. | AUC(0–8h) was found 3.5-fold higher for in situ gel compared to standard eye drops. In situ gel achieved a Cmax of approximatively 0.65 μg/mL followed by a slow decline. NPs-gel had a plateau (0.25–0.3 μg/mL) in the time interval of 0.5–7 hours. | [133] |
| PLGA | Chitosan | Levofloxacin | Rabbit | Drug retention on corneal surface by gamma scintigraphy | Marketed formulation reached into the systemic circulation via nasolacrimal drainage in 5h. Faster decline in radioactivity counts on the corneal surface for marketed formulation, compared with in situ gel, NPs and NPs-gel. | [134] |
| Albumin | Poloxamer | Curcumin | Rabbit | Eye irritation test (Draize test). Determination of drug concentration in aqueous humor by HPLC. | No sign of ocular irritation. Cmax was found to be 5.6-fold higher and AUC(0– 25h) 4.4-fold higher for NPs-gel compared to standard eye drop. | [135] |
| Poloxamer | Poloxamer | Dexamethasone | Rabbit | Eye irritation test (Draize test). Determination of drug concentration in aqueous humor by HPLC. | No sign of ocular irritation. AUC(0–12h) was found 2.8-fold and 2.86-fold higher for in situ gel and NPs-gel, respectively, compared with TOBRADEX®. Cmax was found to be 1.56-fold and 1.91-fold higher for in situ gel and NPs-gel, respectively, compared with TOBRADEX®. | [136] |
| Eudragit® | Poloxamer-HPMC | Keratolac | Rabbit | Eye irritation test (Draize test and winking method). Determination of drug concentration in aqueous humor by HPLC. | No sign of ocular irritation and no abnormal winking compared with simulated tear fluid. AUC(0–8h) was found to be 2.03-fold and 2.51-fold higher for NPs and NPs-gel, respectively, compared with ACULAR®. Cmax was found to be 1.41-fold and 1.20-fold higher for NPs and NPs-gel, respectively, compared with ACULAR®. | [137] |
| PLGA | Carbomer | Pranoprofen | Rabbit | Eye irritation test (Draize test). Anti-inflammatory efficacy assessment by inflammation symptom scoring. | No sign of ocular irritation. Better anti-inflammatory effica cy for NPs-gel compared to OFTALAR®. | [138] |
| PLGA-Eudragit ® or PCL-Eudragit ® | Carbomer | Vancomycin | Rabbit | Eye irritation test (Draize test). Determination of drug concentration in external ocular tissues by the disc diffusion. | No sign of ocular irritation. AUC(0.25–24h) was found 2.14-fold and 2.33-fold higher for PLGA-Eudragit NPs-gel and PCL-Eudragit NPs-gel, respectively, compared with in situ gel. Cmax was found 8.73-fold and 10.06-fold higher for PLGA-Eudragit NPs-gel and PCL-Eudragit NPs-gel, respectively, compared with in situ gel. | [139] |
| pNIPAAm | pNIPAAm | Epinephrine | Rabbit | Measurement of intraocular pressure by ophthalmic tonometer. | In situ gel decreased IOP for at least 32h with a minimum of 6.1 mmHg at 6h. Standard eye drop decreased IOP for 6h with a minimum of 4.7 mmHg. | [23] |
| Liposomes | HA | Fluconazole | Rabbit | Eye irritation test (Draize test). Determination of drug concentration in aqueous humor by HPLC. | No sign of ocular irritation. Drug concentration was found above MIC (minimum inhibitory concentration, 8 μg/mL) for 24h for NPs-gel and for 6h for standard drug solution. | [140] |
| Liposomes | Gellan gum | Timolol | Rabbit | Eye irritation test for a single and multiple dosing. Measurement of intraocular pressure by invagination tonometer. | No sign of ocular irritation. Liposomes-gel decreased IOP from 30–300 min and a minimum of 11.96±0.74 mm/Hg was observed at 1h. Standard eye drops decreased IOP from 30–180min, with a minimum of 13.61 mm/Hg at 2h. | [141] |
Abbreviations: AUC= Area Under the Curve; Cmax= Maximal concentration; HA= Hyaluronic acid; HPLC= High performance liquid chromatography; HPMC= Hydroxypropyl methyl cellulose; IOP= Intraocular pressure; NPs= Nanoparticles; PCL= Poly(epsilon-caprolactone); PLGA= Poly(lactic-co-glycolic acid); pNIPAAm = Poly(N-isopropylacrylamide)
Highlights.
Eye drops have limited efficacy due to the unique physio-anatomy of the eye
Advances in polymer science have led to development of new drug delivery systems
These systems show improved ocular drug penetration and retention in animal models
Increased bioavailability could reduce dose regimen and side effects for patients
Better manufacturing processes are essential for successful clinical translation
7. Acknowledgements
This work is supported by National Institutes of Health (NIH) (R01EB023052 and R01HL140618), Department of Defense Vision Research Program Technology/Therapeutic Development Award (W81XWH-18-1-0654) and Research to Prevent Blindness (RPB) Stein Innovation Award.
ABRREVIATIONS:
- ADME
Absorption, distribution, metabolism, and excretion
- AUC
Area under the curve
- BAK
Benzalkonium chloride
- Cmax
Maximum concentration
- CRO
Contract research organizations
- CTD
Common technical document
- DDS
Drug delivery system
- EB
Evans blue
- EMA
European medicines agency
- EC
Ethyl cellulose
- FDA
Food and drug administration
- GLP
Good laboratory practices
- GMPs
Good manufacturing practices
- HA
Hyaluronic acid
- HCl
Hydrochloride
- HEC
Hydroxyethyl cellulose
- HET-CAM
Hen’s egg-chorioallantoic membrane test
- HPLC
High performance liquid chromatography
- HPMC
Hydroxypropyl methyl cellulose
- IOP
Intraocular pressure
- LD50
Lethal Dose, 50%
- MC
Methylcellulose
- MDs
Medical devices
- MPs
Microparticles
- NaCMC
Sodium carboxymethycellulose
- NPs
Nanoparticles
- PBA
Phenylboronic
- PCL
Poly(epsilon-caprolactone)
- PEG
Polyethylene glycol
- PEO
Polyethylene oxide
- PET
Positron emission tomography
- PK
Pharmacokinetics
- PLA
Polylactide
- PLGA
Poly(lactic-co-glycolic acid)
- PMA
poly(methacrylic acid)
- PMN
Polymorphonuclear leucocyte
- pNIPAAm
Poly(N-isopropylacrylamide)
- PPO
Polypropylene oxide
- TA
Triamcinolone acetonide
- ΔIOP
Intraocular pressure variation
- 5-FU
5- FluoroUracil
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
8. References
- [1].Awwad S, Ahmed AHAM, Sharma G, Heng JS, Khaw PT, Brocchini S, et al. Principles of pharmacology in the eye. British Journal of Pharmacology 2017. 10.1111/bph.14024. [DOI] [PMC free article] [PubMed]
- [2].Gause S, Hsu K-H, Shafor C, Dixon P, Powell KC, Chauhan A. Mechanistic modeling of ophthalmic drug delivery to the anterior chamber by eye drops and contact lenses. Adv Colloid Interface Sci 2016;233:139–54. 10.1016/j.cis.2015.08.002. [DOI] [PubMed] [Google Scholar]
- [3].Ghate D, Edelhauser HF. Ocular drug delivery. Expert Opinion on Drug Delivery 2006;3:275–87. 10.1517/17425247.3.2.275. [DOI] [PubMed] [Google Scholar]
- [4].Imperiale JC, Acosta GB, Sosnik A. Polymer-based carriers for ophthalmic drug delivery. J Control Release 2018;285:106–41. 10.1016/j.jconrel.2018.06.031. [DOI] [PubMed] [Google Scholar]
- [5].Worakul N, Robinson JR. Ocular pharmacokinetics/pharmacodynamics. European Journal of Pharmaceutics and Biopharmaceutics 1997;44:71–83. 10.1016/S0939-6411(97)00064-7. [DOI] [Google Scholar]
- [6].Agrahari V, Mandal A, Agrahari V, Trinh HM, Joseph M, Ray A, et al. A comprehensive insight on ocular pharmacokinetics. Drug Deliv Transl Res 2016;6:735–54. 10.1007/s13346-016-0339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sears ML, editor. Pharmacology of the Eye. Berlin Heidelberg: Springer-Verlag; 1984. [Google Scholar]
- [8].Mitra AK, Anand BS, Duvvuri S. Drug Delivery to the Eye In: Fischbarg J, editor. Advances in Organ Biology, vol. 10, Elsevier; 2005, p. 307–51. 10.1016/S1569-2590(05)10012-3. [DOI] [Google Scholar]
- [9].Dastjerdi MH, Sadrai Z, Saban DR, Zhang Q, Dana R. Corneal Penetration of Topical and Subconjunctival Bevacizumab. Invest Ophthalmol Vis Sci 2011;52:8718–23. 10.1167/iovs.11-7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang T, Xiang CD, Gale D, Carreiro S, Wu EY, Zhang EY. Drug Transporter and Cytochrome P450 mRNA Expression in Human Ocular Barriers: Implications for Ocular Drug Disposition. Drug Metab Dispos 2008;36:1300–7. 10.1124/dmd.108.021121. [DOI] [PubMed] [Google Scholar]
- [11].Kölln C, Reichl S. mRNA expression of metabolic enzymes in human cornea, corneal cell lines, and hemicornea constructs. J Ocul Pharmacol Ther 2012;28:271–7. 10.1089/jop.2011.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Karla PK, Earla R, Boddu SH, Johnston TP, Pal D, Mitra A. Molecular expression and functional evidence of a drug efflux pump (BCRP) in human corneal epithelial cells. Curr Eye Res 2009;34:1–9. 10.1080/02713680802518251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ahmed I, Gokhale RD, Shah MV, Patton TF. Physicochemical determinants of drug diffusion across the conjunctiva, sclera, and cornea. Journal of Pharmaceutical Sciences 1987;76:583–6. 10.1002/jps.2600760802. [DOI] [PubMed] [Google Scholar]
- [14].Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Advanced Drug Delivery Reviews 2006;58:1131–5. 10.1016/j.addr.2006.07.027. [DOI] [PubMed] [Google Scholar]
- [15].Patel A, Cholkar K, Agrahari V, Mitra AK. Ocular drug delivery systems: An overview. World J Pharmacol 2013;2:47–64. 10.5497/wjp.v2.i2.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ludwig A. The use of mucoadhesive polymers in ocular drug delivery. Adv Drug Deliv Rev 2005;57:1595–639. 10.1016/j.addr.2005.07.005. [DOI] [PubMed] [Google Scholar]
- [17].Bayat N, Zhang Y, Falabella P, Menefee R, Whalen JJ, Humayun MS, et al. A reversible thermoresponsive sealant for temporary closure of ocular trauma. Sci Transl Med 2017;9 10.1126/scitranslmed.aan3879. [DOI] [PubMed] [Google Scholar]
- [18].Sun J, Zhou Z. A novel ocular delivery of brinzolamide based on gellan gum: in vitro and in vivo evaluation. Drug Des Devel Ther 2018;12:383–9. 10.2147/DDDT.S153405. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [19].Patel N, Nakrani H, Raval M, Sheth N. Development of loteprednol etabonate-loaded cationic nanoemulsified in-situ ophthalmic gel for sustained delivery and enhanced ocular bioavailability. Drug Deliv 2016;23:3712–23. 10.1080/10717544.2016.1223225. [DOI] [PubMed] [Google Scholar]
- [20].Qian Y, Wang F, Li R, Zhang Q, Xu Q. Preparation and evaluation of in situ gelling ophthalmic drug delivery system for methazolamide. Drug Dev Ind Pharm 2010;36:1340–7. 10.3109/03639041003801893. [DOI] [PubMed] [Google Scholar]
- [21].Zeng Y, Chen J, Li Y, Huang J, Huang Z, Huang Y, et al. Thermo-sensitive gel in glaucoma therapy for enhanced bioavailability: In vitro characterization, in vivo pharmacokinetics and pharmacodynamics study. Life Sciences 2018;212:80–6. 10.1016/j.lfs.2018.09.050. [DOI] [PubMed] [Google Scholar]
- [22].Mansour M, Mansour S, Mortada ND, Abd Elhady SS. Ocular poloxamer-based ciprofloxacin hydrochloride in situ forming gels. Drug Dev Ind Pharm 2008;34:744–52. 10.1080/03639040801926030. [DOI] [PubMed] [Google Scholar]
- [23].Hsiue G-H, Hsu S, Yang C-C, Lee S-H, Yang I-K. Preparation of controlled release ophthalmic drops, for glaucoma therapy using thermosensitive poly-N-isopropylacrylamide. Biomaterials 2002;23:457–62. [DOI] [PubMed] [Google Scholar]
- [24].Zhu M, Wang J, Li N. A novel thermo-sensitive hydrogel-based on poly(N-isopropylacrylamide)/hyaluronic acid of ketoconazole for ophthalmic delivery. Artif Cells Nanomed Biotechnol 2018;46:1282–7. 10.1080/21691401.2017.1368024. [DOI] [PubMed] [Google Scholar]
- [25].Wu Y, Yao J, Zhou J, Dahmani FZ. Enhanced and sustained topical ocular delivery of cyclosporine A in thermosensitive hyaluronic acid-based in situ forming microgels. Int J Nanomedicine 2013;8:3587–601. 10.2147/IJN.S47665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Miyazaki S, Suzuki S, Kawasaki N, Endo K, Takahashi A, Attwood D. In situ gelling xyloglucan formulations for sustained release ocular delivery of pilocarpine hydrochloride. International Journal of Pharmaceutics 2001;229:29–36. 10.1016/S0378-5173(01)00825-0. [DOI] [PubMed] [Google Scholar]
- [27].Chen X, Li X, Zhou Y, Wang X, Zhang Y, Fan Y, et al. Chitosan-based thermosensitive hydrogel as a promising ocular drug delivery system: preparation, characterization, and in vivo evaluation. J Biomater Appl 2012;27:391–402. 10.1177/0885328211406563. [DOI] [PubMed] [Google Scholar]
- [28].Song Y, Nagai N, Saijo S, Kaji H, Nishizawa M, Abe T. In situ formation of injectable chitosan-gelatin hydrogels through double crosslinking for sustained intraocular drug delivery. Materials Science and Engineering: C 2018;88:1–12. 10.1016/j.msec.2018.02.022. [DOI] [PubMed] [Google Scholar]
- [29].Cheng Y-H, Tsai T-H, Jhan Y-Y, Chiu AW, Tsai K-L, Chien C-S, et al. Thermosensitive chitosan-based hydrogel as a topical ocular drug delivery system of latanoprost for glaucoma treatment. Carbohydr Polym 2016;144:390–9. 10.1016/j.carbpol.2016.02.080. [DOI] [PubMed] [Google Scholar]
- [30].Wu C, Qi H, Chen W, Huang C, Su C, Li W, et al. Preparation and evaluation of a Carbopol/HPMC-based in situ gelling ophthalmic system for puerarin. Yakugaku Zasshi 2007;127:183–91. [DOI] [PubMed] [Google Scholar]
- [31].Wu H, Liu Z, Peng J, Li L, Li N, Li J, et al. Design and evaluation of baicalin-containing in situ pH-triggered gelling system for sustained ophthalmic drug delivery. International Journal of Pharmaceutics 2011;410:31–40. 10.1016/j.ijpharm.2011.03.007. [DOI] [PubMed] [Google Scholar]
- [32].Sultana Y, Aqil M, Ali A, Zafar S. Evaluation of carbopol-methyl cellulose based sustained-release ocular delivery system for pefloxacin mesylate using rabbit eye model. Pharm Dev Technol 2006;11:313–9. 10.1080/10837450600767698. [DOI] [PubMed] [Google Scholar]
- [33].Dubey A, Prabhu P. Formulation and evaluation of stimuli-sensitive hydrogels of timolol maleate and brimonidine tartrate for the treatment of glaucoma. International Journal of Pharmaceutical Investigation 2014;4:112–8. 10.4103/2230-973X.138340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gupta S, Vyas SP. Carbopol/Chitosan Based pH Triggered In Situ Gelling System for Ocular Delivery of Timolol Maleate. Sci Pharm 2010;78:959–76. 10.3797/scipharm.1001-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].El-Laithy HM, Nesseem DI, El-Adly AA, Shoukry M. Moxifloxacin-Gelrite in situ ophthalmic gelling system against photodynamic therapy for treatment of bacterial corneal inflammation. Arch Pharm Res 2011;34:1663–78. 10.1007/s12272-011-1011-5. [DOI] [PubMed] [Google Scholar]
- [36].Kesavan K, Kant S, Pandit JK. Therapeutic Effectiveness in the Treatment of Experimental Bacterial Keratitis with Ion-activated Mucoadhesive Hydrogel. Ocular Immunology and Inflammation 2015:1–4. 10.3109/09273948.2015.1005238. [DOI] [PubMed] [Google Scholar]
- [37].Díaz-Tomé V, Luaces-Rodríguez A, Silva-Rodríguez J, Blanco-Dorado S, García-Quintanilla L, Llovo-Taboada J, et al. Ophthalmic Econazole Hydrogels for the Treatment of Fungal Keratitis. J Pharm Sci 2018;107:1342–51. 10.1016/j.xphs.2017.12.028. [DOI] [PubMed] [Google Scholar]
- [38].Liu Z, Li J, Nie S, Liu H, Ding P, Pan W. Study of an alginate/HPMC-based in situ gelling ophthalmic delivery system for gatifloxacin. International Journal of Pharmaceutics 2006;315:12–7. 10.1016/j.ijpharm.2006.01.029. [DOI] [PubMed] [Google Scholar]
- [39].Liu Y, Liu J, Zhang X, Zhang R, Huang Y, Wu C. In Situ Gelling Gelrite/Alginate Formulations as Vehicles for Ophthalmic Drug Delivery. AAPS PharmSciTech 2010;11:610–20. 10.1208/s12249-010-9413-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lin H-R, Sung KC, Vong W-J. In Situ Gelling of Alginate/Pluronic Solutions for Ophthalmic Delivery of Pilocarpine. Biomacromolecules 2004;5:2358–65. 10.1021/bm0496965. [DOI] [PubMed] [Google Scholar]
- [41].Deshmukh PK, Gattani SG. In vitro and in vivo consideration of novel environmentally responsive ophthalmic drug delivery system. Pharm Dev Technol 2013;18:950–6. 10.3109/10837450.2011.644297. [DOI] [PubMed] [Google Scholar]
- [42].Aqil M, Khar R, Ali A, Bhatnagar A, Mittal G, Gupta H. An alternative in situ gel-formulation of levofloxacin eye drops for prolong ocular retention. Journal of Pharmacy and Bioallied Sciences 2015;7:9 10.4103/0975-7406.149810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Cao Y, Zhang C, Shen W, Cheng Z, Yu LL, Ping Q. Poly(N-isopropylacrylamide)-chitosan as thermosensitive in situ gel-forming system for ocular drug delivery. J Control Release 2007;120:186–94. 10.1016/j.jconrel.2007.05.009. [DOI] [PubMed] [Google Scholar]
- [44].Ward MA, Georgiou TK. Thermoresponsive Polymers for Biomedical Applications. Polymers 2011;3:1215–42. 10.3390/polym3031215. [DOI] [Google Scholar]
- [45].Hacker MC, Klouda L, Ma BB, Kretlow JD, Mikos AG. Synthesis and characterization of injectable, thermally and chemically gelable, amphiphilic poly(N-isopropylacrylamide)-based macromers. Biomacromolecules 2008;9:1558–70. 10.1021/bm8000414. [DOI] [PubMed] [Google Scholar]
- [46].Sponchioni M, Capasso Palmiero U, Moscatelli D. Thermo-responsive polymers: Applications of smart materials in drug delivery and tissue engineering. Mater Sci Eng C Mater Biol Appl 2019;102:589–605. 10.1016/j.msec.2019.04.069. [DOI] [PubMed] [Google Scholar]
- [47].Hørven I. Corneal Temperature in Normal Subjects and Arterial Occlusive Disease. Acta Ophthalmologica 1975;53:863–74. 10.1111/j.1755-3768.1975.tb00404.x. [DOI] [PubMed] [Google Scholar]
- [48].Girardin F, Orgül S, Erb C, Flammer J. Relationship Between Corneal Temperature and Finger Temperature. Arch Ophthalmol 1999;117:166–9. 10.1001/archopht.117.2.166. [DOI] [PubMed] [Google Scholar]
- [49].Almeida H, Amaral MH, Lobão P, Lobo JMS. Applications of poloxamers in ophthalmic pharmaceutical formulations: an overview. Expert Opinion on Drug Delivery 2013;10:1223–37. 10.1517/17425247.2013.796360. [DOI] [PubMed] [Google Scholar]
- [50].Edsman K, Carlfors J, Petersson R. Rheological evaluation of poloxamer as an in situ gel for ophthalmic use. Eur J Pharm Sci 1998;6:105–12. [DOI] [PubMed] [Google Scholar]
- [51].Jeong B, Lee DS, Shon J-I, Bae YH, Kim SW. Thermoreversible gelation of poly(ethylene oxide) biodegradable polyester block copolymers. Journal of Polymer Science Part A: Polymer Chemistry 1999. . [DOI]
- [52].Wu C, Zhou S. Light scattering study of spherical poly(N-isopropylacrylamide) microgels. Journal of Macromolecular Science, Part B 1997;36:345–55. 10.1080/00222349708212388. [DOI] [Google Scholar]
- [53].Ramanan RMK, Chellamuthu P, Tang L, Nguyen KT. Development of a Temperature-Sensitive Composite Hydrogel for Drug Delivery Applications. Biotechnology Progress 2006;22:118–25. 10.1021/bp0501367. [DOI] [PubMed] [Google Scholar]
- [54].Liu W, Huang Y, Liu H, Hu Y. Composite structure of temperature sensitive chitosan microgel and anomalous behavior in alcohol solutions. Journal of Colloid and Interface Science 2007;313:117–21. 10.1016/j.jcis.2007.04.022. [DOI] [PubMed] [Google Scholar]
- [55].Morra M. Engineering of biomaterials surfaces by hyaluronan. Biomacromolecules 2005;6:1205–23. 10.1021/bm049346i. [DOI] [PubMed] [Google Scholar]
- [56].Sakakibara CN, Sierakowski MR, Chassenieux C, Nicolai T, de Freitas RA. Xyloglucan gelation induced by enzymatic degalactosylation; kinetics and the effect of the molar mass. Carbohydrate Polymers 2017;174:517–23. 10.1016/j.carbpol.2017.06.118. [DOI] [PubMed] [Google Scholar]
- [57].Shirakawa M, Yamatoya K, Nishinari K. Tailoring of xyloglucan properties using an enzyme. Food Hydrocolloids 1998;12:25–8. 10.1016/S0268-005X(98)00052-6. [DOI] [Google Scholar]
- [58].Chenite A, Buschmann M, Wang D, Chaput C, Kandani N. Rheological characterisation of thermogelling chitosan/glycerol-phosphate solutions. Carbohydrate Polymers 2001;46:39–47. 10.1016/S0144-8617(00)00281-2. [DOI] [Google Scholar]
- [59].Gupta P, Vermani K, Garg S. Hydrogels: from controlled release to pH-responsive drug delivery. Drug Discovery Today 2002;7:569–79. 10.1016/S1359-6446(02)02255-9. [DOI] [PubMed] [Google Scholar]
- [60].Rathore KS, Nema RK. AN INSIGHT INTO OPHTHALMIC DRUG DELIVERY SYSTEM. 1 2009:1–5. 10.25004/IJPSDR.2009.010101. [DOI] [Google Scholar]
- [61].Braccini I, Pérez S. Molecular Basis of Ca2+-Induced Gelation in Alginates and Pectins: The Egg-Box Model Revisited. Biomacromolecules 2001;2:1089–96. 10.1021/bm010008g. [DOI] [PubMed] [Google Scholar]
- [62].Janagam DR, Wu L, Lowe TL. Nanoparticles for drug delivery to the anterior segment of the eye. Advanced Drug Delivery Reviews 2017;122:31–64. 10.1016/j.addr.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kapanigowda UG, Nagaraja SH, Ramaiah B, Boggarapu PR. Improved intraocular bioavailability of ganciclovir by mucoadhesive polymer based ocular microspheres: development and simulation process in Wistar rats. Daru 2015;23 10.1186/s40199-015-0132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Asasutjarit R, Theerachayanan T, Kewsuwan P, Veeranodha S, Fuongfuchat A, Ritthidej GC. Development and Evaluation of Diclofenac Sodium Loaded-N-Trimethyl Chitosan Nanoparticles for Ophthalmic Use. AAPS PharmSciTech 2015;16:1013–24. 10.1208/s12249-015-0290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Başaran E, Yenilmez E, Berkman MS, Büyükköroğlu G, Yazan Y. Chitosan nanoparticles for ocular delivery of cyclosporine A. J Microencapsul 2014;31:49–57. 10.3109/02652048.2013.805839. [DOI] [PubMed] [Google Scholar]
- [66].De Campos AM, Sánchez A, Alonso MJ. Chitosan nanoparticles: a new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to cyclosporin A. International Journal of Pharmaceutics 2001;224:159–68. 10.1016/S0378-5173(01)00760-8. [DOI] [PubMed] [Google Scholar]
- [67].Badawi AA, El-Laithy HM, El Qidra RK, El Mofty H, El dally M. Chitosan based nanocarriers for indomethacin ocular delivery. Archives of Pharmacal Research 2008;31:1040–9. 10.1007/s12272-001-1266-6. [DOI] [PubMed] [Google Scholar]
- [68].Ibrahim MM, Abd-Elgawad A-EH, Soliman OA-E, Jablonski MM. Stability and Ocular Pharmacokinetics of Celecoxib-Loaded Nanoparticles Topical Ophthalmic Formulations. J Pharm Sci 2016;105:3691–701. 10.1016/j.xphs.2016.09.019. [DOI] [PubMed] [Google Scholar]
- [69].Zhao R, Li J, Wang J, Yin Z, Zhu Y, Liu W. Development of Timolol-Loaded Galactosylated Chitosan Nanoparticles and Evaluation of Their Potential for Ocular Drug Delivery. AAPS PharmSciTech 2017;18:997–1008. 10.1208/s12249-016-0669-x. [DOI] [PubMed] [Google Scholar]
- [70].Ameeduzzafar, Ali J, Bhatnagar A, Kumar N, Ali A. Chitosan nanoparticles amplify the ocular hypotensive effect of cateolol in rabbits. International Journal of Biological Macromolecules 2014;65:479–91. 10.1016/j.ijbiomac.2014.02.002. [DOI] [PubMed] [Google Scholar]
- [71].Shinde UA, Shete JN, Nair HA, Singh KH. Design and characterization of chitosan-alginate microspheres for ocular delivery of azelastine. Pharmaceutical Development and Technology 2014;19:813–23. 10.3109/10837450.2013.836217. [DOI] [PubMed] [Google Scholar]
- [72].Nagarwal RC, Kumar R, Pandit JK. Chitosan coated sodium alginate–chitosan nanoparticles loaded with 5-FU for ocular delivery: In vitro characterization and in vivo study in rabbit eye. European Journal of Pharmaceutical Sciences 2012;47:678–85. 10.1016/j.ejps.2012.08.008. [DOI] [PubMed] [Google Scholar]
- [73].Rathod S, Deshpande SG. Albumin microspheres as an ocular delivery system for pilocarpine nitrate. Indian J Pharm Sci 2008;70:193–7. 10.4103/0250-474X.41454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Addo RT, Siddig A, Siwale R, Patel NJ, Akande J, Uddin AN, et al. Formulation, characterization and testing of tetracaine hydrochloride-loaded albumin-chitosan microparticles for ocular drug delivery. J Microencapsul 2010;27:95–104. 10.3109/02652040903010638. [DOI] [PubMed] [Google Scholar]
- [75].Addo RT, Yeboah KG, Siwale RC, Siddig A, Jones A, Ubale RV, et al. Formulation and Characterization of Atropine Sulfate in Albumin–Chitosan Microparticles for In Vivo Ocular Drug Delivery. Journal of Pharmaceutical Sciences 2015;104:1677–90. 10.1002/jps.24380. [DOI] [PubMed] [Google Scholar]
- [76].Shokry M, Hathout RM, Mansour S. Exploring gelatin nanoparticles as novel nanocarriers for Timolol Maleate: Augmented in-vivo efficacy and safe histological profile. International Journal of Pharmaceutics 2018;545:229–39. 10.1016/j.ijpharm.2018.04.059. [DOI] [PubMed] [Google Scholar]
- [77].Mahor A, Prajapati SK, Verma A, Gupta R, Iyer AK, Kesharwani P. Moxifloxacin loaded gelatin nanoparticles for ocular delivery: Formulation and in - vitro, in – vivo evaluation. Journal of Colloid and Interface Science 2016;483:132–8. 10.1016/j.jcis.2016.08.018. [DOI] [PubMed] [Google Scholar]
- [78].Kalam MA. The potential application of hyaluronic acid coated chitosan nanoparticles in ocular delivery of dexamethasone. International Journal of Biological Macromolecules 2016;89:559–68. 10.1016/j.ijbiomac.2016.05.016. [DOI] [PubMed] [Google Scholar]
- [79].Wadhwa S, Paliwal R, Paliwal SR, Vyas SP. Hyaluronic acid modified chitosan nanoparticles for effective management of glaucoma: development, characterization, and evaluation. J Drug Target 2010;18:292–302. 10.3109/10611860903450023. [DOI] [PubMed] [Google Scholar]
- [80].Quinteros DA, Ferreira LM, Schaffazick SR, Palma SD, Allemandi DA, Cruz L. Novel Polymeric Nanoparticles Intended for Ophthalmic Administration of Acetazolamide. J Pharm Sci 2016;105:3183–90. 10.1016/j.xphs.2016.06.023. [DOI] [PubMed] [Google Scholar]
- [81].Katara R, Sachdeva S, Majumdar DK. Design, characterization, and evaluation of aceclofenac-loaded Eudragit RS 100 nanoparticulate system for ocular delivery. Pharm Dev Technol 2019;24:368–79. 10.1080/10837450.2018.1486424. [DOI] [PubMed] [Google Scholar]
- [82].Katara R, Majumdar DK. Eudragit RL 100-based nanoparticulate system of aceclofenac for ocular delivery. Colloids Surf B Biointerfaces 2013;103:455–62. 10.1016/j.colsurfb.2012.10.056. [DOI] [PubMed] [Google Scholar]
- [83].Ahuja M, Dhake AS, Sharma SK, Majumdar DK. Diclofenac-loaded Eudragit S100 nanosuspension for ophthalmic delivery. J Microencapsul 2011;28:37–45. 10.3109/02652048.2010.523794. [DOI] [PubMed] [Google Scholar]
- [84].Pignatello R, Bucolo C, Ferrara P, Maltese A, Puleo A, Puglisi G. Eudragit RS100 nanosuspensions for the ophthalmic controlled delivery of ibuprofen. Eur J Pharm Sci 2002;16:53–61. [DOI] [PubMed] [Google Scholar]
- [85].Tian S, Li J, Tao Q, Zhao Y, Lv Z, Yang F, et al. Controlled drug delivery for glaucoma therapy using montmorillonite/Eudragit microspheres as an ion-exchange carrier. Int J Nanomedicine 2018;13:415–28. 10.2147/IJN.S146346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Verma P, Gupta RN, Jha AK, Pandey R. Development, in vitro and in vivo characterization of Eudragit RL 100 nanoparticles for improved ocular bioavailability of acetazolamide. Drug Deliv 2013;20:269–76. 10.3109/10717544.2013.834417. [DOI] [PubMed] [Google Scholar]
- [87].Bhagav P, Upadhyay H, Chandran S. Brimonidine Tartrate–Eudragit Long-Acting Nanoparticles: Formulation, Optimization, In Vitro and In Vivo Evaluation. AAPS PharmSciTech 2011;12:1087–101. 10.1208/s12249-011-9675-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Das S, Suresh PK. Nanosuspension: a new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to amphotericin B. Nanomedicine 2011;7:242–7. 10.1016/j.nano.2010.07.003. [DOI] [PubMed] [Google Scholar]
- [89].Shinde UA, Shete JN, Nair HA, Singh KH. Eudragit RL100 based microspheres for ocular administration of azelastine hydrochloride. J Microencapsul 2012;29:511–9. 10.3109/02652048.2012.665088. [DOI] [PubMed] [Google Scholar]
- [90].Liu S, Jones L, Gu FX. Development of mucoadhesive drug delivery system using phenylboronic acid functionalized poly(D,L-lactide)-b-dextran nanoparticles. Macromol Biosci 2012;12:1622–6. 10.1002/mabi.201200216. [DOI] [PubMed] [Google Scholar]
- [91].Prosperi-Porta G, Kedzior S, Muirhead B, Sheardown H. Phenylboronic-Acid-Based Polymeric Micelles for Mucoadhesive Anterior Segment Ocular Drug Delivery. Biomacromolecules 2016;17:1449–57. 10.1021/acs.biomac.6b00054. [DOI] [PubMed] [Google Scholar]
- [92].Gonzalez-Pizarro R, Silva-Abreu M, Calpena AC, Egea MA, Espina M, García ML. Development of fluorometholone-loaded PLGA nanoparticles for treatment of inflammatory disorders of anterior and posterior segments of the eye. International Journal of Pharmaceutics 2018;547:338–46. 10.1016/j.ijpharm.2018.05.050. [DOI] [PubMed] [Google Scholar]
- [93].Katara R, Sachdeva S, Majumdar DK. Enhancement of ocular efficacy of aceclofenac using biodegradable PLGA nanoparticles: formulation and characterization. Drug Delivery and Translational Research 2017;7:632–41. 10.1007/s13346-017-0416-1. [DOI] [PubMed] [Google Scholar]
- [94].Park CG, Kim YK, Kim S-N, Lee SH, Huh BK, Park M-A, et al. Enhanced ocular efficacy of topically-delivered dorzolamide with nanostructured mucoadhesive microparticles. International Journal of Pharmaceutics 2017;522:66–73. 10.1016/j.ijpharm.2017.02.035. [DOI] [PubMed] [Google Scholar]
- [95].Calvo P, Sánchez A, Martínez J, López MI, Calonge M, Pastor JC, et al. Polyester Nanocapsules as New Topical Ocular Delivery Systems for Cyclosporin A. Pharmaceutical Research 1996;13:311–5. 10.1023/A:1016015803611. [DOI] [PubMed] [Google Scholar]
- [96].CALVO P, ALONSO MJ, VILA-JATO JL, ROBINSON JR. Improved Ocular Bioavailability of Indomethacin by Novel Ocular Drug Carriers. Journal of Pharmacy and Pharmacology 1996;48:1147–52. 10.1111/j.2042-7158.1996.tb03911.x. [DOI] [PubMed] [Google Scholar]
- [97].Khan N, Ameeduzzafar, Khanna K, Bhatnagar A, Ahmad FJ, Ali A. Chitosan coated PLGA nanoparticles amplify the ocular hypotensive effect of forskolin: Statistical design, characterization and in vivo studies. International Journal of Biological Macromolecules 2018;116:648–63. 10.1016/j.ijbiomac.2018.04.122. [DOI] [PubMed] [Google Scholar]
- [98].Salama AH, Mahmoud AA, Kamel R. A Novel Method for Preparing Surface-Modified Fluocinolone Acetonide Loaded PLGA Nanoparticles for Ocular Use: In Vitro and In Vivo Evaluations. AAPS PharmSciTech 2016;17:1159–72. 10.1208/s12249-015-0448-0. [DOI] [PubMed] [Google Scholar]
- [99].Zhou W, Wang Y, Jian J, Song S. Self-aggregated nanoparticles based on amphiphilic poly(lactic acid)-grafted-chitosan copolymer for ocular delivery of amphotericin B. Int J Nanomedicine 2013;8:3715–28. 10.2147/IJN.S51186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Pandian S, Jeevanesan V, Ponnusamy C, Natesan S. RES-loaded pegylated CS NPs: for efficient ocular delivery. IET Nanobiotechnology 2017;11:32–9. 10.1049/iet-nbt.2016.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Natesan S, Pandian S, Ponnusamy C, Palanichamy R, Muthusamy S, Kandasamy R. Co-encapsulated resveratrol and quercetin in chitosan and peg modified chitosan nanoparticles: For efficient intra ocular pressure reduction. International Journal of Biological Macromolecules 2017;104:1837–45. 10.1016/j.ijbiomac.2017.04.117. [DOI] [PubMed] [Google Scholar]
- [102].Shi S, Zhang Z, Luo Z, Yu J, Liang R, Li X, et al. Chitosan grafted methoxy poly(ethylene glycol)-poly(ε-caprolactone) nanosuspension for ocular delivery of hydrophobic diclofenac. Sci Rep 2015;5 10.1038/srep11337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Ibrahim HK, El-Leithy IS, Makky AA. Mucoadhesive nanoparticles as carrier systems for prolonged ocular delivery of gatifloxacin/prednisolone bitherapy. Mol Pharm 2010;7:576–85. 10.1021/mp900279c. [DOI] [PubMed] [Google Scholar]
- [104].Wang F, Zhang M, Zhang D, Huang Y, Chen L, Jiang S, et al. Preparation, optimization, and characterization of chitosan-coated solid lipid nanoparticles for ocular drug delivery. J Biomed Res 2018;32:411–23. 10.7555/JBR.32.20160170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Ban J, Zhang Y, Huang X, Deng G, Hou D, Chen Y, et al. Corneal permeation properties of a charged lipid nanoparticle carrier containing dexamethasone. Int J Nanomedicine 2017;12:1329–39. 10.2147/IJN.S126199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Tan G, Yu S, Pan H, Li J, Liu D, Yuan K, et al. Bioadhesive chitosan-loaded liposomes: A more efficient and higher permeable ocular delivery platform for timolol maleate. International Journal of Biological Macromolecules 2017;94:355–63. 10.1016/j.ijbiomac.2016.10.035. [DOI] [PubMed] [Google Scholar]
- [107].Fu T, Yi J, Lv S, Zhang B. Ocular amphotericin B delivery by chitosan-modified nanostructured lipid carriers for fungal keratitis-targeted therapy. J Liposome Res 2017;27:228–33. 10.1080/08982104.2016.1224899. [DOI] [PubMed] [Google Scholar]
- [108].Chhonker YS, Prasad YD, Chandasana H, Vishvkarma A, Mitra K, Shukla PK, et al. Amphotericin-B entrapped lecithin/chitosan nanoparticles for prolonged ocular application. International Journal of Biological Macromolecules 2015;72:1451–8. 10.1016/j.ijbiomac.2014.10.014. [DOI] [PubMed] [Google Scholar]
- [109].Chen H, Pan H, Li P, Wang H, Wang X, Pan W, et al. The potential use of novel chitosan-coated deformable liposomes in an ocular drug delivery system. Colloids Surf B Biointerfaces 2016;143:455–62. 10.1016/j.colsurfb.2016.03.061. [DOI] [PubMed] [Google Scholar]
- [110].Luo Q, Zhao J, Zhang X, Pan W. Nanostructured lipid carrier (NLC) coated with Chitosan Oligosaccharides and its potential use in ocular drug delivery system. International Journal of Pharmaceutics 2011;403:185–91. 10.1016/j.ijpharm.2010.10.013. [DOI] [PubMed] [Google Scholar]
- [111].Üstündağ-Okur N, Gökçe EH, Bozbıyık Dİ, Eğrilmez S, Özer Ö, Ertan G. Preparation and in vitro–in vivo evaluation of ofloxacin loaded ophthalmic nano structured lipid carriers modified with chitosan oligosaccharide lactate for the treatment of bacterial keratitis. European Journal of Pharmaceutical Sciences 2014;63:204–15. 10.1016/j.ejps.2014.07.013. [DOI] [PubMed] [Google Scholar]
- [112].Bhatta RS, Chandasana H, Chhonker YS, Rathi C, Kumar D, Mitra K, et al. Mucoadhesive nanoparticles for prolonged ocular delivery of natamycin: In vitro and pharmacokinetics studies. International Journal of Pharmaceutics 2012;432:105–12. 10.1016/j.ijpharm.2012.04.060. [DOI] [PubMed] [Google Scholar]
- [113].Li N, Zhuang C-Y, Wang M, Sui C-G, Pan W-S. Low molecular weight chitosan-coated liposomes for ocular drug delivery: in vitro and in vivo studies. Drug Deliv 2012;19:28–35. 10.3109/10717544.2011.621994. [DOI] [PubMed] [Google Scholar]
- [114].Mehanna MM, Elmaradny HA, Samaha MW. Mucoadhesive liposomes as ocular delivery system: physical, microbiological, and in vivo assessment. Drug Dev Ind Pharm 2010;36:108–18. 10.3109/03639040903099751. [DOI] [PubMed] [Google Scholar]
- [115].Liu D, Lian Y, Fang Q, Liu L, Zhang J, Li J. Hyaluronic-acid-modified lipid-polymer hybrid nanoparticles as an efficient ocular delivery platform for moxifloxacin hydrochloride. Int J Biol Macromol 2018;116:1026–36. 10.1016/j.ijbiomac.2018.05.113. [DOI] [PubMed] [Google Scholar]
- [116].Zeng W, Li Q, Wan T, Liu C, Pan W, Wu Z, et al. Hyaluronic acid-coated niosomes facilitate tacrolimus ocular delivery: Mucoadhesion, precorneal retention, aqueous humor pharmacokinetics, and transcorneal permeability. Colloids Surf B Biointerfaces 2016;141:28–35. 10.1016/j.colsurfb.2016.01.014. [DOI] [PubMed] [Google Scholar]
- [117].Lin J, Wu H, Wang Y, Lin J, Chen Q, Zhu X. Preparation and ocular pharmacokinetics of hyaluronan acid-modified mucoadhesive liposomes. Drug Deliv 2016;23:1144–51. 10.3109/10717544.2014.991952. [DOI] [PubMed] [Google Scholar]
- [118].Li X, Zhang Z, Li J, Sun S, Weng Y, Chen H. Diclofenac/biodegradable polymer micelles for ocular applications. Nanoscale 2012;4:4667–73. 10.1039/C2NR30924F. [DOI] [PubMed] [Google Scholar]
- [119].Yu Y, Chen D, Li Y, Yang W, Tu J, Shen Y. Improving the topical ocular pharmacokinetics of lyophilized cyclosporine A-loaded micelles: formulation, in vitro and in vivo studies. Drug Delivery 2018;25:888–99. 10.1080/10717544.2018.1458923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Sogias IA, Williams AC, Khutoryanskiy VV. Why is Chitosan Mucoadhesive? Biomacromolecules 2008;9:1837–42. 10.1021/bm800276d. [DOI] [PubMed] [Google Scholar]
- [121].Thanou M, Verhoef JC, Junginger HE. Chitosan and its derivatives as intestinal absorption enhancers. Advanced Drug Delivery Reviews 2001;50:S91–101. 10.1016/S0169-409X(01)00180-6. [DOI] [PubMed] [Google Scholar]
- [122].Davidenko N, Schuster CF, Bax DV, Farndale RW, Hamaia S, Best SM, et al. Evaluation of cell binding to collagen and gelatin: a study of the effect of 2D and 3D architecture and surface chemistry. J Mater Sci Mater Med 2016;27:148 10.1007/s10856-016-5763-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Malikmammadov E, Tanir TE, Kiziltay A, Hasirci V, Hasirci N. PCL and PCL-based materials in biomedical applications. Journal of Biomaterials Science, Polymer Edition 2018;29:863–93. 10.1080/09205063.2017.1394711. [DOI] [PubMed] [Google Scholar]
- [124].Tyler B, Gullotti D, Mangraviti A, Utsuki T, Brem H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Advanced Drug Delivery Reviews 2016;107:163–75. 10.1016/j.addr.2016.06.018. [DOI] [PubMed] [Google Scholar]
- [125].Gentile P, Chiono V, Carmagnola I, Hatton PV. An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int J Mol Sci 2014;15:3640–59. 10.3390/ijms15033640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Liang H, Friedman JM, Nacharaju P. Fabrication of biodegradable PEG–PLA nanospheres for solubility, stabilization, and delivery of curcumin. Artificial Cells, Nanomedicine, and Biotechnology 2017;45:297–304. 10.3109/21691401.2016.1146736. [DOI] [PubMed] [Google Scholar]
- [127].Liu S, Dozois MD, Chang CN, Ahmad A, Ng DLT, Hileeto D, et al. Prolonged Ocular Retention of Mucoadhesive Nanoparticle Eye Drop Formulation Enables Treatment of Eye Diseases Using Significantly Reduced Dosage. Molecular Pharmaceutics 2016;13:2897–905. 10.1021/acs.molpharmaceut.6b00445. [DOI] [PubMed] [Google Scholar]
- [128].Zhang X-P, Sun J-G, Yao J, Shan K, Liu B-H, Yao M-D, et al. Effect of nanoencapsulation using poly (lactide-co-glycolide) (PLGA) on anti-angiogenic activity of bevacizumab for ocular angiogenesis therapy. Biomedicine & Pharmacotherapy 2018;107:1056–63. 10.1016/j.biopha.2018.08.092. [DOI] [PubMed] [Google Scholar]
- [129].Bin Choy Y, Park J-H, Prausnitz MR. Mucoadhesive Microparticles Engineered for Ophthalmic Drug Delivery. J Phys Chem Solids 2008;69:1533–6. 10.1016/j.jpcs.2007.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Marchal-Heussler L, Sirbat D, Hoffman M, Maincent P. Poly(ε-Caprolactone) Nanocapsules in Carteolol Ophthalmic Delivery. Pharmaceutical Research 1993;10:386–90. 10.1023/A:1018936205485. [DOI] [PubMed] [Google Scholar]
- [131].Ameeduzzafar, Imam SS, Abbas Bukhari SN, Ahmad J, Ali A. Formulation and optimization of levofloxacin loaded chitosan nanoparticle for ocular delivery: In-vitro characterization, ocular tolerance and antibacterial activity. International Journal of Biological Macromolecules 2018;108:650–9. 10.1016/j.ijbiomac.2017.11.170. [DOI] [PubMed] [Google Scholar]
- [132].Ibrahim MM, Abd-Elgawad A-EH, Soliman OA-E, Jablonski MM. Natural Bioadhesive Biodegradable Nanoparticle-Based Topical Ophthalmic Formulations for Management of Glaucoma. Translational Vision Science & Technology 2015;4:12 10.1167/tvst.4.3.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Fabiano A, Bizzarri R, Zambito Y. Thermosensitive hydrogel based on chitosan and its derivatives containing medicated nanoparticles for transcorneal administration of 5-fluorouracil. Int J Nanomedicine 2017;12:633–43. 10.2147/IJN.S121642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Gupta H, Aqil M, Khar RK, Ali A, Bhatnagar A, Mittal G. Nanoparticles laden in situ gel of levofloxacin for enhanced ocular retention. Drug Deliv 2013;20:306–9. 10.3109/10717544.2013.838712. [DOI] [PubMed] [Google Scholar]
- [135].Lou J, Hu W, Tian R, Zhang H, Jia Y, Zhang J, et al. Optimization and evaluation of a thermoresponsive ophthalmic in situ gel containing curcumin-loaded albumin nanoparticles. Int J Nanomedicine 2014;9:2517–25. 10.2147/IJN.S60270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Wen Y, Ban J, Mo Z, Zhang Y, An P, Liu L, et al. A potential nanoparticle-loaded in situ gel for enhanced and sustained ophthalmic delivery of dexamethasone. Nanotechnology 2018;29:425101 10.1088/1361-6528/aad7da. [DOI] [PubMed] [Google Scholar]
- [137].Morsi N, Ghorab D, Refai H, Teba H. Ketoroloac tromethamine loaded nanodispersion incorporated into thermosensitive in situ gel for prolonged ocular delivery. International Journal of Pharmaceutics 2016;506:57–67. 10.1016/j.ijpharm.2016.04.021. [DOI] [PubMed] [Google Scholar]
- [138].Abrego G, Alvarado H, Souto EB, Guevara B, Bellowa LH, Parra A, et al. Biopharmaceutical profile of pranoprofen-loaded PLGA nanoparticles containing hydrogels for ocular administration. European Journal of Pharmaceutics and Biopharmaceutics 2015;95:261–70. 10.1016/j.ejpb.2015.01.026. [DOI] [PubMed] [Google Scholar]
- [139].Yousry C, Elkheshen SA, El-Laithy HM, Essam T, Fahmy RH. Studying the influence of formulation and process variables on Vancomycin-loaded polymeric nanoparticles as potential carrier for enhanced ophthalmic delivery. Eur J Pharm Sci 2017;100:142–54. 10.1016/j.ejps.2017.01.013. [DOI] [PubMed] [Google Scholar]
- [140].Moustafa MA, Elnaggar YSR, El-Refaie WM, Abdallah OY. Hyalugel-integrated liposomes as a novel ocular nanosized delivery system of fluconazole with promising prolonged effect. Int J Pharm 2017;534:14–24. 10.1016/j.ijpharm.2017.10.007. [DOI] [PubMed] [Google Scholar]
- [141].Yu S, Wang Q-M, Wang X, Liu D, Zhang W, Ye T, et al. Liposome incorporated ion sensitive in situ gels for opthalmic delivery of timolol maleate. Int J Pharm 2015;480:128–36. 10.1016/j.ijpharm.2015.01.032. [DOI] [PubMed] [Google Scholar]
- [142].Racchi M, Govoni S, Lucchelli A, Capone L, Giovagnoni E. Insights into the definition of terms in European medical device regulation. Expert Review of Medical Devices 2016;13:907–17. 10.1080/17434440.2016.1224644. [DOI] [PubMed] [Google Scholar]
- [143].Ragelle H, Danhier F, Préat V, Langer R, Anderson DG. Nanoparticle-based drug delivery systems: a commercial and regulatory outlook as the field matures. Expert Opin Drug Deliv 2017;14:851–64. 10.1080/17425247.2016.1244187. [DOI] [PubMed] [Google Scholar]
- [144].Barenholz Y. Doxil®--the first FDA-approved nano-drug: lessons learned. J Control Release 2012;160:117–34. 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- [145].Diebold Y, Calonge M. Applications of nanoparticles in ophthalmology. Prog Retin Eye Res 2010;29:596–609. 10.1016/j.preteyeres.2010.08.002. [DOI] [PubMed] [Google Scholar]
- [146].Wu L, Zhang J, Watanabe W. Physical and chemical stability of drug nanoparticles. Advanced Drug Delivery Reviews 2011;63:456–69. 10.1016/j.addr.2011.02.001. [DOI] [PubMed] [Google Scholar]
- [147].Zhang W. Nanoparticle aggregation: principles and modeling. Adv Exp Med Biol 2014;811:19–43. 10.1007/978-94-017-8739-0_2. [DOI] [PubMed] [Google Scholar]
- [148].Vetten MA, Yah CS, Singh T, Gulumian M. Challenges facing sterilization and depyrogenation of nanoparticles: effects on structural stability and biomedical applications. Nanomedicine 2014;10:1391–9. 10.1016/j.nano.2014.03.017. [DOI] [PubMed] [Google Scholar]
- [149].Aguayo Bonniard A, Yeung JY, Chan CC, Birt CM. Ocular surface toxicity from glaucoma topical medications and associated preservatives such as benzalkonium chloride (BAK). Expert Opin Drug Metab Toxicol 2016:1–11. 10.1080/17425255.2016.1209481. [DOI] [PubMed] [Google Scholar]
- [150].Baudouin C, Denoyer A, Desbenoit N, Hamm G, Grise A. In vitro and in vivo experimental studies on trabecular meshwork degeneration induced by benzalkonium chloride (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc 2012;110:40–63. [PMC free article] [PubMed] [Google Scholar]
- [151].Bellich B, D’Agostino I, Semeraro S, Gamini A, Cesàro A. “The Good, the Bad and the Ugly” of Chitosans. Mar Drugs 2016;14 10.3390/md14050099. [DOI] [PMC free article] [PubMed]
- [152].WHO | Blindness 2: Major Causes Worldwide. WHO n.d https://www.who.int/mediacentre/factsheets/fs143/en/ (accessed March 21, 2019).