Abstract
Copper‐based antimicrobial compounds are widely and historically used to control plant diseases, such as late blight caused by Phytophthora infestans, which seriously affects the yield and quality of potato. We previously identified that copper ion (Cu2+) acts as an extremely sensitive elicitor to induce ethylene (ET)‐dependent immunity in Arabidopsis. Here, we found that Cu2+ induces the defence response to P. infestans in potato. Cu2+ suppresses the transcription of the abscisic acid (ABA) biosynthetic genes StABA1 and StNCED1, resulting in decreased ABA content. Treatment with ABA or inhibitor fluridone made potato more susceptible or resistance to late blight, respectively. In addition, potato with knockdown of StABA1 or StNCED1 showed greater resistance to late blight, suggesting that ABA negatively regulates potato resistance to P. infestans. Cu2+ also promotes the rapid biosynthesis of ET. Potato plants treated with 1‐aminocyclopropane‐1‐carboxylate showed enhanced resistance to late blight. Repressed expression of StEIN2 or StEIN3 resulted in enhanced transcription of StABA1 and StNCED1, accumulation of ABA and susceptibility to P. infestans. Consistently, StEIN3 directly binds to the promoter regions of StABA1 and StNCED1. Overall, we concluded that Cu2+ triggers the defence response to potato late blight by activating ET biosynthesis to inhibit the biosynthesis of ABA.
Keywords: ABA, ABA1, EIN3, ethylene, fungicide, NCED1, potato late blight
Cu2+‐mediated late blight resistance activates ET signalling, subsequently suppressing ABA biosynthesis. This recalls two tips when using copper‐based antimicrobial compounds: water in advance; avoid strong sunshine and drought.

1. INTRODUCTION
Potato is the fourth largest crop in the world. Late blight, caused by the oomycete pathogen Phytophthora infestans, is the most devastating disease in potato production and seriously affects the yield and quality of tubers, resulting in approximately $6.7 billion in losses annually (Kamoun et al., 2015). To control late blight, protective and systemic fungicides have been widely used, such as chlorothalonil, metalaxyl/mefenoxam, cymoxanil, and dimethomorph (Nowichi et al., 2012). As an undesirable effect, an increasing number of insensitive strains have emerged (Randall et al., 2014). Copper‐based antimicrobial compounds (CBACs) have been used as protectants for more than 150 years in controlling late blight and many other diseases (Lamichhane et al., 2018). Currently, they are still effective in organic potato production. However, with the long‐term application of CBACs, the problem of heavy metal toxicity to soil organisms arises, which needs to be solved by reducing usage.
CBACs have been widely used in agriculture since the late 19th century. For example, Bordeaux mixture (copper sulphate pentahydrate and lime mixture) was used on vines to combat downy mildew beginning in 1845. It is normally thought that CBACs have two tiers of function: to release Cu2+, which inhibits the activity of enzymes in fungal spores to prevent germination (Pscheidt and Ocamb, 1999), and to provide a protective barrier against plant pathogens by covering plant surfaces (Lamichhane et al., 2018). After long‐time application, numerous copper‐tolerant strains have been identified in the field; however, CBACs application still has the ability to manage copper‐tolerant strains and reduce disease severity (Strayer‐Scherer et al., 2018). The two‐tiered protective model is hardly adequate to explain the resistance of copper‐tolerant strains. In our previous study, we reported that Cu2+ not only has heavy metal toxicity to suppress pathogenic microbe growth but also acts as an elicitor to induce host defence responses at concentrations lower than 10 nM (Liu et al., 2015). Cu2+ activated the accumulation of reactive oxygen species (ROS), promoted callose deposition, and up‐regulated pathogenesis‐related gene expression, and Cu2+‐mediated resistance was dependent on the salicylic acid (SA) and ethylene (ET) signalling pathways in Arabidopsis (Liu et al., 2015). This phenomenon provides a third layer of protective function to CBACs that helps to explain the successful management of copper‐tolerant pathogens. It was also found that Cu2+ treatment specifically induced the early transcription of AtACS8, resulting in the production of ET, which is required in the Cu2+‐activated defence response (Zhang et al., 2018). However, the downstream signalling of ET to enhance the defence response remains unclear.
Many phytohormones, such as SA, jasmonic acid (JA) and ET, have been reported to be involved in regulating plant immunity to pathogens. The plant hormone abscisic acid (ABA) not only plays prominent roles in the plant response to abiotic stresses but also functions as a crucial regulator in the plant defence response to biotic stresses. ABA has been demonstrated to positively or negatively regulate host defences against bacterial, fungal, and oomycete pathogens (Ton et al., 2009; Cao et al., 2011). Furthermore, the multifaceted role of ABA in disease resistance is dependent on the specific host–pathogen combination and the timing of the plant defence response (Ton et al., 2009). For instance, ABA is required for microbe‐associated molecular pattern (MAMP)‐induced stomatal closure to inhibit bacterial invasion in Arabidopsis (Melotto et al., 2006). In contrast, it has also been reported that ABA can suppress MAMP‐induced callose deposition, which is a hallmark of basal defence (Clay et al., 2009). Furthermore, the bacterial pathogen Pseudomonas syringae pv. tomato (Pst) DC3000, using type III effectors, such as avrPtoB, strongly induced the biosynthesis of ABA. Exogenous application of ABA resulted in more susceptibility in Arabidopsis to Pst DC3000 (De Torres‐Zabala et al., 2007) and in soybean to oomycete Phytophthora megasperma (Ward et al., 1989). In addition, the ABA‐deficient tomato mutant sitiens is resistant to the fungus Botrytis cinerea (Audenaert et al., 2002). Interestingly, the fungal pathogens B. cinerea and Cercospora rosicola could synthesize and secrete ABA through the direct pathway to suppress plant defence (Neill et al., 1982; Hirai et al., 1986). Thus, ABA biosynthesis and signalling transduction play important roles in plant–pathogen interactions.
The biosynthesis of ABA is strictly controlled by plants. In higher plants, ABA biosynthesis occurs mainly through the indirect pathway, which starts from β‐carotene transferred to zeaxanthin by β‐carotene hydroxygenase (BCH). Then, zeaxanthin is converted into violaxanthin by zeaxanthin epoxidase (ZEP). Plants mutated in the zep gene show ABA deficiency in tobacco, tomato, and Arabidopsis (Duckham et al., 1991; Marin et al., 1996; Agrawal et al., 2001; Galpaz et al., 2008), so this mutation is named aba1. 9‐cis‐violaxanthin and 9′‐cis‐neoxanthin, which are converted from violaxanthin, are the substrates of 9‐cis‐epoxycarotenoid dioxygenase (NCED). NCED catalyses the oxidative cleavage of 9‐cis‐epoxycarotenoids to xanthoxin, which is the rate‐limiting step in ABA biosynthesis. Furthermore, xanthoxin is converted to abscisic aldehyde (ABA‐ld) and subsequently oxidized to ABA by aba deficient 2 and ABA‐ld oxidase (ABAO), respectively (Xiong et al., 2001). Because NCED3 is so important to catalyse the rate‐limiting step of the ABA biosynthetic pathway, its transcription is commonly induced to promote ABA biosynthesis and improve tolerance to abiotic stresses (Endo et al., 2008; Tan et al., 2010).
Previously, we demonstrated that Cu2+‐enhanced resistance to bacterial pathogens in Arabidopsis was dependent on the ET biosynthetic and signalling pathways (Liu et al., 2015; Zhang et al., 2018). However, although CBACs have been widely used to control late blight, the mechanisms of Cu2+‐mediated resistance to P. infestans remain unclear. In this study, we report that Cu2+ acted as an elicitor to activate potato plants resistance to P. infestans. Within 15 min, Cu2+ treatment rapidly promoted the production of ET, which suppressed the transcription of the ABA biosynthetic genes StABA1 and StNCED1, resulting in decreased levels of ABA. ABA negatively regulated the defence response to P. infestans in potato. Interestingly, Cu2+ activated ET to suppress ABA biosynthesis in Arabidopsis. Thus, we concluded that decreased ABA levels play important roles in the Cu2+‐mediated defence response in plants.
2. RESULTS
2.1. Cu2+ protects potato against P. infestans
Previously, we identified that Cu2+ activates defence responses and protects Arabidopsis plants from the bacterial pathogen Pst DC3000 (Liu et al., 2015). To test whether Cu2+ plays a similar role in other plants, we investigated the function of Cu2+ on the potato immunity system by testing its effects on the invasion of P. infestans. Compared with the control (10 μM MgSO4), the leaves of potato plants treated with CuSO4 (0.1, 1, or 10 μM) showed markedly decreased water‐soaked lesions caused by P. infestans isolate EC1 (Figure 1a). Consistently, the disease index was significantly reduced in Cu2+‐treated plants compared with the control (Figure 1b). To further confirm this result, we investigated the biomass level of pathogens in the potato leaves by quantifying the P. infestans‐specific PiO8 element. The PiO8 content in Cu2+‐treated plants was significantly lower than that in the control plants (Figure 1c). These data indicate that Cu2+ protected potato plants from the oomycete pathogen P. infestans. As a heavy metal, excess Cu2+ is toxic and may suppress the growth of P. infestans. To confirm the activator function of Cu2+ on potato plants, we investigated the effects of Cu2+ on the growth of EC1. When the concentration was lower than 25 μM, Cu2+ had no inhibition in the growth of the EC1 (Figure S1). In addition, we found that Cu2+ could promote the accumulation of ROS and callose deposition in leaves of potato (Figure 1d,e). These results show that Cu2+ acts an elicitor to activate the defence response in potato against P. infestans.
Figure 1.

Cu2+ enhances resistance to potato late blight caused by Phytophthora infestans. (a) Leaves treated with 0.1, 1, and 10 μM of CuSO4 4 hr prior to P. infestans inoculation. Images were photographed at 3 days post‐inoculation. Bar = 2 cm. (b) The disease index analysis for pretreatment with 0.1, 1, and 10 μM of CuSO4. Data represent the mean ± SD (n = 10). (c) The biomass level analysis of the potato leaves pretreated with 0.1, 1, and 10 μM of CuSO4. The level of PiO8 element and StEF1 were used to quantify the level of P. infestans and plant cells by quantitative PCR. Data represent the mean ± SD (n = 3). (d) Callose deposition in the leaves of potato plants at 12 hr treated with CuSO4 (10 μM) or control (CK, MgSO4). (e) Superoxide accumulation in the leaves at 2 hr treated with CuSO4 (10 μM) or control (MgSO4). The blue formazan precipitate indicates superoxide production. The asterisks in (b) and (c) indicate a significant difference compared with the control (t test; *p < .05; **p < .01). The experiments were repeated three times with similar results
2.2. RNA‐Seq revealed a role for Cu2+ in decreasing ABA biosynthesis
As previous described in Arabidopsis (Zhang et al., 2018), an RNA‐Seq analysis was performed in potato plants sprayed with CuSO4 for 0, 2, and 24 hr. Over 195.25 million reads were generated, and an average of 88.35% reads were mapped to the potato genome, representing a total of 25,953 genes expressed in all samples (Table S1). Compared with the control (0 hr), Cu2+ up‐regulated 839 and 659 genes and down‐regulated 1,002 and 615 genes at 2 and 24 hr, respectively (Figure S2a). Among these differentially expressed genes (DEGs), many genes were involved in metabolic process, cellular process, response to stimulus, biological regulation, and regulation of biological process. In addition, the KEGG analysis results showed that Cu2+ significantly affected the photosynthesis‐antenna proteins, plant hormone signal transduction, and linoleic acid metabolism (Figure S2b and Table S2).
Interestingly, genes associated with the biosynthesis, signal transduction, and catabolism pathway of the phytohormone ABA showed different expression patterns after CuSO4 treatment (Figure 2a). Compared with the control, Cu2+ treatment significantly decreased the transcript levels of StABA1 and StNCED1, which play important roles in the entry point and the limiting step of ABA synthesis (Figure 2a,c). To validate this result, we performed a quantitative reverse transcription PCR (RT‐qPCR) assay and found a similar result (Figure 2b). These results suggest that Cu2+ treatment decreased the transcription of genes associated with ABA biosynthesis in potato.
Figure 2.

Cu2+ suppresses the transcription of the abscicic acid (ABA) biosynthetic genes StABA1 and StNCED1 in potato. (a) Heatmap of genes involved in the ABA biosynthetic pathway in response to Cu2+. R1, R2, and R3 are the three independent biological replicates. (b) The quantitative reverse transcription PCR profiles of the genes StABA1 and StNCED1 in potato plants treated with CuSO4. StActin gene was used as an internal control. Data was from three biological replicates. The asterisks indicate a significant difference compared with the control (t test; *p < .05; **p < .01). (c) The KEGG pathway associated with ABA biosynthesis
2.3. Cu2+ suppresses ABA biosynthesis in potato
The repressed expression of StABA1 and StNCED1 suggests that Cu2+ regulates the synthesis of ABA in plants. To validate this hypothesis, we quantified the ABA content in potato plants by high‐performance liquid chromatography‐mass spectrometry/mass spectrometry (HPLC‐MS/MS) assay. The level of ABA was significantly lower than that of the control at 2 hr post‐CuSO4 treatment (hpt) (Figure 3a). It is confirmed that Cu2+ treatment suppresses ABA biosynthesis in potato plants. However, there was no significant difference in ABA levels between CuSO4‐treated plants and the control at 24 hpt, suggesting that the suppression of ABA biosynthesis is an early event after Cu2+ treatment.
Figure 3.

Cu2+ decreases the level of abscicic acid (ABA) in potato. (a) The ABA levels in potato leaves sprayed with 10 μM CuSO4. Data are the means ± SD from four independent biological replicates. (b) Cu2+ promotes the opening of stomata in the leaves. Images were photographed at 0, 1 and 3 hr post‐treatment (hpt). Bar = 100 μm. (c) Stomatal apertures were measured in the leaves treated with CuSO4 or control at 0, 1 and 3 hpt. Data are shown as the mean (n = 20 stomata) ± SEM. (d) Cu2+ promotes water loss. A comparison of the water loss in the leaves treated with CuSO4 or control (CK). Data are shown as the mean ± SD from three independent biological replicates. (e) Cu2+‐treated potato plants were more sensitive to drought stress. The potato plants were under drought stress for 1 week after being sprayed with MgSO4 or CuSO4. The asterisks indicate a significant difference compared with the control (t test; *p < .05)
Because Cu2+ treatment decreases the level of ABA, which is associated with stomatal movement in plants, we investigated the stomatal aperture in leaves treated with CuSO4 at 2 hpt. The results show that the stomatal aperture is significantly larger in Cu2+‐treated plants than in the control (Figure 3b,c), suggesting that Cu2+ treatment promotes stomatal opening in potato leaves. Moreover, a water loss assay identified that the leaves of Cu2+‐treated plants lost more water than the control plants (Figure 3d), which is consistent with the influence of Cu2+ treatment on stomatal movement. We also found that Cu2+‐treated potato plants were more sensitive to drought stress than the control (Figure 3e). Overall, these results suggest that Cu2+ suppresses the transcription of genes in the ABA biosynthetic pathway and decreases the ABA level in plants.
2.4. ABA negatively regulates potato defence to P. infestans
ABA is well known to suppress plant defence responses to bacteria, fungi, and oomycetes (Ward et al., 1989; Ton et al., 2009; Cao et al., 2011). Thus, we hypothesized that ABA negatively regulates the potato defence response to P. infestans. To examine this hypothesis, we pretreated potato plants with ABA and subsequently inoculated with P. infestans EC1. Compared with the control, ABA‐treated plants showed enhanced disease symptoms with larger water‐soaked lesion areas (Figure 4a) and higher disease indices (Figure 4b). We also examined the function of fluridone, an ABA biosynthesis inhibitor, in regulating the potato response to P. infestans. The fluridone‐treated leaves showed enhanced resistance to EC1 compared to control leaves (Figure 4a,b). To further confirm the regulation of ABA on potato defence to late blight, we knocked down the transcription of StABA1 and StNCED1 by virus‐induced gene silencing (VIGS) in potato (Figure S3). We observed that the staba1‐VIGS and stnced1‐VIGS plants were more resistant to EC1 than the control (Figure 4c,d). Moreover, we transiently expressed StABA1 or StNCED1 gene on potato plants (Figure S3) and inoculated with EC1 on detached leaves. Compared with the disease index for the control plants infiltrating with Agrobacterium tumefaciens GV3101 containing the empty vector (EV), StABA1‐ and StNCED1‐ovexpressing plants showed similar scores on spraying 10 μM MgSO4. However, copper‐mediated resistance to P. infestans was attenuated in both StABA1‐ and StNCED1‐ovexpressing plants (Figure 4e). Overall, we concluded that ABA negatively regulates potato defence responses to P. infestans and is required for Cu2+‐mediated late blight resistance.
Figure 4.
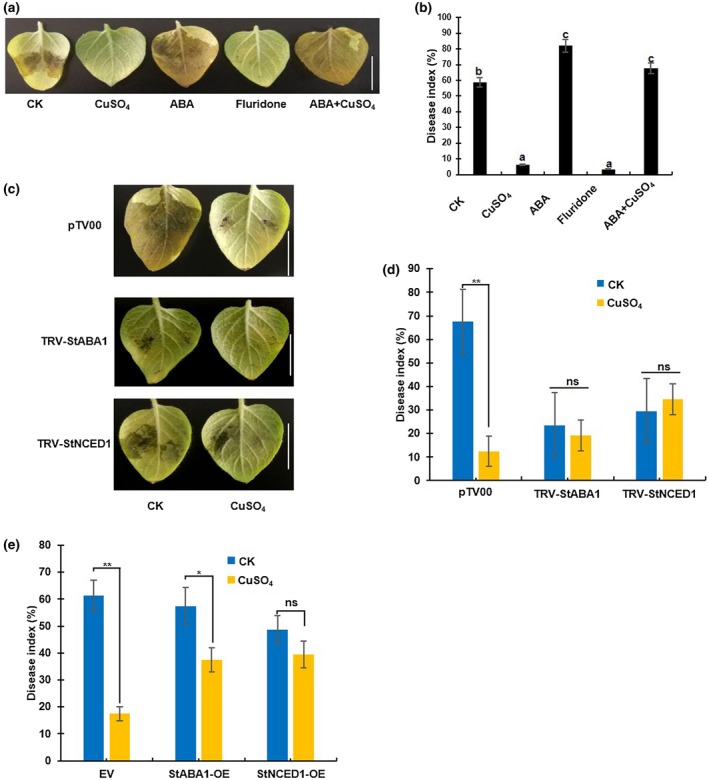
Abscicic acid (ABA) negatively regulates potato defence to Phytophthora infestans. (a) Leaves treated with ABA, CuSO4, and the ABA biosynthesis inhibitor fluridone or control (CK) 4 hr prior to P. infestans EC1 inoculation. The photograph was taken at 3 days post‐inoculation (dpi). Bar = 2 cm. (b) The disease index analysis results of the leaves shown in (a). Data represent the mean ± SD (n = 10). Different letters indicate statistically significant differences between treatments (t test; p < .05). (c) Leaves of VIGS‐StABA1, VIGS‐StNCED1, and control pTV00 potato plants infected with EC1. The photograph was taken at 3 dpi. Bar = 2 cm. (d) The disease index analysis results of the leaves shown in (c). Data represent the mean ± SD (n = 10). (e) The disease index of StABA1‐OE, StNCED1‐OE, and EV (empty vector) potato plants treated with control (MgSO4) or CuSO4 at 3 dpi. Data represent the mean ± SD (n = 6). The asterisks in (d) and (e) indicate a significant difference compared with the control (t test; *p < .05; **p < .01). The experiments were repeated three times with similar results
2.5. Cu2+ rapidly promotes ET biosynthesis in potato
We previously observed that Cu2+‐induced early expression of the AtACS8 gene to promote ET production in Arabidopsis (Zhang et al., 2018). However, among the DEGs in CuSO4‐treated potato plants, we did not identify any up‐regulated ACS genes. We suspect that Cu2+ activates more rapid ET production in potato than in Arabidopsis. To examine this possibility, the level of ET was tested in potato plants treated with CuSO4 within 2 hr. We observed that CuSO4 treatment promoted more ET in potato plants than in control plants. The level of ET increased to a peak at 0.25 hpt with an approximately 6.8‐fold higher level and then dropped to a similar level to that of the control at 1 hpt (Figure 5a). We also measured the transcription of ACS genes in potato plants after CuSO4 treatment by RT‐qPCR and found that several ACS genes were up‐regulated by Cu2+ (Figure 5b–i), suggesting that Cu2+ may induce transcription of potato ACS genes to promote ET signalling. To further confirm the roles of ET in immunity to P. infestans, we treated potato with ET precursor 1‐aminocyclopropane‐1‐carboxylate (ACC). Compared with the control treatment, ACC treatment decreased the disease symptom of EC1 on potato leaves, which is similar to that of CuSO4 treatment (Figure 5j). The disease index also showed a significant decrease in ACC‐ and CuSO4‐treated plants (Figure 5k). These results suggest that ET positively regulates potato late blight resistance. Thus, the process by which Cu2+ activates transcription of the ACS gene to promote ET production is conserved in Arabidopsis and potato.
Figure 5.

Cu2+ rapidly promoted ethylene (ET) production in potato. (a) ET was measured in a gas chromatograph equipped with a photoionization detector. (b)–(i) The transcript levels of the genes StACS3, StACS4, StACS5, StACSL, StERF1, StERF3, StEIN2, and StEIN3 in leaves sprayed with 10 μM CuSO4. Data are means ± SD (n = 3); mpt, min post‐treatment. StActin gene was used as a control to normalize expression levels. (j) Leaves of potato plants treated with CuSO4, ACC or control (CK) 4 hr prior to inoculation. The photograph was taken at 3 days post‐inoculation. Bar = 2 cm. (k) The disease index analysis results of the leaves shown in (j). Data represent the mean ± SD (n = 10). The asterisks in (a) and (k) indicate a significant difference compared with the control (t test; *p < .05; **p < .01). The experiments were repeated at least two times with similar results
2.6. ET negatively regulates ABA biosynthesis in potato
In Arabidopsis, the ET‐insensitive mutant ein2 produces higher levels of ABA and is more sensitive to ABA (Ghassemian et al., 2000), suggesting that ET negatively regulates the biosynthesis of ABA. We also detected the expression of StABA1 and StNCED1 in EIN2‐VIGS and EIN3‐VIGS potato plants. All silenced plants showed significantly higher expression of the genes StABA1 and StNCED1 than the control plants (Figure 6a). We also measured the level of ABA and ET in these VIGS plants and observed that the EIN2‐VIGS and EIN3‐VIGS plants contained approximately 3.73–4.41‐fold higher levels of ABA (Figure 6b), while the levels of ET in EIN2‐VIGS and EIN3‐VIGS plants was 60% that in the control (Figure S4). These results suggest that ET negatively regulates the biosynthesis of ABA in potato.
Figure 6.

Ethylene suppressed abscicic acid (ABA) biosynthesis to enhance late blight resistance in potato. (a) The transcript levels of StABA1 and StNCED1 in the leaves of VIGS‐StEIN2, VIGS‐StEIN3, and control pTV00 potato plants. StActin gene was used as a control to normalize expression levels. Data represent the mean ± SD (n = 3) from three independent biological replicates. (b) The distribution of ABA accumulation with the optimized immunofluorescence technique. The ABA concentration index was ABA/Alexa Fluor 555 fluorescence intensity as measured by ImageJ (mean ± SD, n ≥ 20). (c) Leaves of VIGS‐StEIN2, VIGS‐StEIN3, VIGS‐StEIN2 + VIGS‐StABA1, VIGS‐StEIN2 + VIGS‐StNCED1, and control pTV00 potato plants infected with EC1. The photograph was taken at 3 days post‐inoculation. Bar = 1 cm. (d) The disease index analysis results of the leaves shown in (c). Data represent the mean ± SD (n = 10). The asterisks indicate a significant difference compared with the control (t test; *p < .05; **p < .01). CK, control. The experiments were repeated at least two times with similar results
We also examined the transcript levels of AtABA1 and AtNCED3 in the Arabidopsis mutants ein2 and ein3 and found that both of these mutants expressed higher levels of AtABA1 and AtNCED3 than wild‐type plants. In addition, the level of ABA in the Arabidopsis mutants of atein2 and atein3 was significantly higher than that of wild‐type plants (Figure S5), suggesting that ET suppression of ABA biosynthesis is a conserved trait in Arabidopsis and potato.
To further confirm the role of ET signal transduction and ABA biosynthetic genes in Cu2+‐mediated resistance to late blight, we knocked down the transcription of StEIN2 StABA1 and StEIN2 StNCED1 using VIGS in potato plants. Compared with the control, the stein2‐VIGS and stein3‐VIGS plants were more susceptible to EC1, while stein2 staba1‐VIGS and stein2 stnced1‐VIGS plants were more resistant to EC 1 (Figure 6c,d). In addition, Cu2+ failed to enhance resistance to EC1 in these VIGS plants (Figure 6c,d), suggesting that the ET signalling pathway is required for Cu2+‐mediated late blight resistance. Because staba1‐VIGS and stnced1‐VIGS plants were more resistant to EC1 than the control (Figure 4), it was apparent that the ET signalling pathway is upstream of the ABA biosynthetic pathway. Overall, these results indicate that Cu2+ promotes ET production that inhibits the expression of the StABA1 and StNCED1 genes to suppress ABA biosynthesis in plants.
2.7. StEIN3 binds promoters of StABA1 and StNCED1
The transcription factor of EIN3 plays important roles in ET signalling transduction, which binds to the EIN3‐binding sites (EBSs) of the conserved sequence AYGWAYCT (Kosugi and Ohashi, 2000). There are 10 and 6 predicted EBSs in the promoters of StABA1 and StNCED1, respectively (Figure 7a). To find out whether StEIN3‐mediated suppression of StABA1 and StNCED1 is a direct or indirect interaction, a chromatin immunoprecipitation (ChIP) assay was performed using potato with transient expression of FLAG‐StEIN3 under the control of a cauliflower mosaic virus 35S promoter. In the StABA1 promoter, positions 3 (P3) and 4 (P4) showed significant enrichment with FLAG‐StEIN3, while no signal was found in the negative control (Figure 7b). In contrast, in the StNCED1 promoter detectable enrichment was observed in P1, P2, P3, and P4 in FLAG‐StEIN3‐expressing potato, but not in the potato treated with empty vector (Figure 7c). These results suggest that StEIN3 directly targets the EBSs and binds to the promoters of StABA1 and StNCED1.
Figure 7.

StEIN3 binds the promoters of StABA1 and StNCED1 in potato. (a) Scheme of the StABA1 and StNCED1 promoter. The arrows show the predicted EIN3 binding sites and the lines below show DNA fragments used for chromatin immunoprecipitation (ChIP)‐quantitative PCR experiments. Boxes are exons and the translation start site (ATG) is shown at position + 1. (b) Quantification of enriched DNA fragments in the promoter of StABA1 following ChIP from the FLAG‐StEIN3‐ or empty vector‐expressing potato. (c) Quantification of enriched DNA fragments in the promoter of StNCED1. Data represent the mean enrichment of the percentage of input chromatin. Error bars correspond to the mean ± SD (n = 3). (d) Electrophoretic mobility shift assay (EMSA) for StEIN3 binding to the promoter of StABA1 in vitro. (e) EMSA for StEIN3 binding to the promoter of StNCED1 in vitro. Data in (b) and (c) represent the mean enrichment of the percentage of input chromatin. Error bars correspond to the mean ± SD (n = 3). The asterisks in (b) and (c) indicate a significant difference compared with the control (t test; *p < .05; **p < .01). In (d) and (e), biotin‐labelled DNA fragments were incubated with maltose‐binding protein‐StEIN3 protein, and the free and bound DNA were separated in a polyacrylamide gel. Unlabelled DNA fragments were used as competitors. The experiments were repeated at least two times with similar results
To further confirm if StEIN3 directly binds to the promoter of gene StABA1 and StNCED1, DNA electrophoretic mobility shift assay (EMSA) was performed. A maltose‐binding protein (MBP)‐tagged StEIN3 (MBP‐StEIN3) protein was expressed and affinity purified from Escherichia coli. The MBP‐StEIN3 was identified to bind the biotin‐labelled P3 and P4 DNA fragments of StABA1 promoter (Figure 7d) and the P1 DNA fragment of StNCED1 promoter (Figure 7e). The binding was attenuated by each of the unlabelled competent fragments. Overall, these results demonstrate that StEIN3 can directly bind to the StABA1 and StNCED1 promoter both in vivo and in vitro.
3. DISCUSSION
As the first Cu2+‐based fungicide, Bordeaux mixture has been extensively used to protect crops, vegetables, fruit trees, and flowers against plant disease caused by bacteria, fungi, and oomycetes since 1845. Currently, CBACs, including Bordeaux mixture, have the sixth highest sales volume in the world. They are still effective in controlling plant diseases, even those caused by copper‐tolerant pathogens (Cha and Cooksey, 1991; Strayer‐Scherer et al., 2018). Hence, we hypothesized that Cu2+ not only has toxic activity but also induces defence responses in plants. We demonstrated that Cu2+ could also protect potato plants from oomycete pathogens (Figure 1) and protect Arabidopsis against bacterial pathogens (Liu et al., 2015). Our data suggest that Cu2+ acts as an elicitor to activate the defence response to bacteria and oomycete pathogens (Figure 8). These findings provide another mechanistic explanation for the widespread and long‐term use of CBACs in agriculture. Importantly, the long‐term and large‐scale use of CBACs has led to heavy metal pollution in soil and water. The concentration of Cu2+ in Bordeaux mixture is approximately 4 mM, while in our research a low level of Cu2+ (0.1 μM) could protect potato plants against P. infestans (Figure 1), suggesting that rational use of fungicides with lower Cu2+ concentrations should be effective to control disease and be friendly to the environment at the same time.
Figure 8.

A model of Cu2+‐mediated immunity to Phytophthora infestans in potato
ET is an important phytohormone that plays essential roles in plant defence responses. The production of ET could be found in Arabidopsis within 30 min after MAMP treatment (Zipfel et al., 2006). Here, we found that Cu2+‐induced transcription of ACS genes and ET production is a rapid event in potato, with a 1 hr duration time (Figure 5). Similarly, in CuSO4‐treated plants, the ABA level decreased at 2 hpt and recovered to a normal level at 24 hpt (Figure 3a). Based on these results, there should be feedback‐based regulation of ET biosynthesis after Cu2+ treatment. In Arabidopsis, Dong et al. (2016) screened ABI4 (ABA Insensitive 4) as a negative regulator of ET biosynthesis. ABI4 is a transcription factor that directly represses the transcription of AtACS4 and AtACS8. Thus, it might be that ABI4 or ABI4‐like protein feedback regulates the biosynthesis of ET after Cu2+ treatment in Arabidopsis and potato.
Interestingly, the ET signalling pathway was found to positively regulate the transcription of FLS2, which encodes the receptor for the flagellin‐derived flg22 peptide (Boutrot et al., 2010). In contrast, ET signalling was found to decrease plant resistance by negatively regulating the expression of the SID2 gene, which plays major roles in SA biosynthesis (Chen et al., 2009a). Here, we show that Cu2+ positively regulates the ET biosynthesis and signalling pathway to activate the defence response in Arabidopsis and potato. The ein2 and ein3 knockdown potato plants were more susceptible than the control (Figure 6), suggesting that ET signalling pathway positively regulates the late blight resistance. Moreover, in Arabidopsis, the etr1 and ein2 knockout mutants showed larger bacterial populations than the wild type and failed in the Cu2+‐mediated defence response (Liu et al., 2015). Our data suggest that the ET signalling pathway is required for Cu2+‐mediated defence response in plants.
The ET synthesis pathway contains three steps, and the conversion of S‐adenosylmethionine to ACC catalysed by ACC synthase (ACS) is the rate‐limiting step. It is induced by pathogen attack and environmental stimuli such as wounding, flooding, heavy metal stress, or ozone exposure (Moeder et al., 2002; Nie et al., 2002; Rao et al., 2002; Keunen et al., 2016). For example, ACS2 and ACS6 are required for ET induction by flg22 (Liu and Zhang, 2004; Guan et al., 2015) and the heavy metal chromium (Schellingen et al., 2014). In our previous study, Cu2+ promoted the transcription of the AtACS8 gene to induce ET production (Zhang et al., 2018). Here, we also found that Cu2+ up‐regulated StACS genes and promoted ET synthesis in potato within 15–30 min (Figure 5). Thus, it could be concluded that Cu2+ activates ET production in plants, and during this action ACS genes play essential roles. However, whether one or more StACS genes play a similar role to that of AtACS8 in potato requires further research.
ABA is a well‐known hormone involved in many abiotic stresses. For example, drought, salt, and cold stress could induce ABA biosynthesis, resulting in improved tolerance to stresses. In addition to abiotic stress, many fungi and bacterial pathogens have been found to increase ABA in plants. B. cinerea uses a direct pathway to synthesize ABA from mevalonic acid (MVA) (Neill et al., 1982), and Pst DC3000 invasion increases the level of ABA by inducing the transcription of AtNCED3, which catalyses the rate‐limiting step in the ABA biosynthetic pathway (Torres‐Zabala et al., 2014). Exogenous application of ABA enhances plant susceptibility, while ABA‐deficient aba1 mutants showed resistance to Plectosphaerella cucumerina (Sánchez‐Vallet et al., 2012) and Pst DC3000 (Thaler and Bostock, 2004). Moreover, ABA‐deficient sitiens tomato mutants showed enhanced resistance to B. cinerea (Audenaert et al., 2002; Asselbergh et al., 2007). The suppression of plant defence by ABA is associated with its function in attenuating callose deposition (Clay et al., 2009) and inhibition of SA synthesis (Audenaert et al., 2002; Mohr and Cahill, 2007) and JA/ET signal transduction (Anderson et al., 2004). Because there are many pathogens that use stomata to penetrate plant tissue, ABA is known to close stomata to increase plant preinvasive penetration resistance. Contrarily, in some interactions between plants and microbes, ABA plays positive roles by stimulating callose deposition (Flors et al., 2005). Here, we show that Cu2+ activated plant immunity by suppressing ABA synthesis through repressing the transcription of StABA1 and StNCED1 (Figure 2). Moreover, the Cu2+‐induced defence response could be inhibited by ABA application (Figure 4). Thus, our data can partially explain the broad effects of CBACs.
There are many examples of signalling crosstalk between biotic and abiotic stresses. Drought, salinity, and cold promote the level of ABA, which specifically activates the kinase activity of OsMAPK5, resulting in enhanced tolerance to above stresses. However, OsMAPK5 was also induced by the invasion of blast fungus and negatively regulated resistance to bacterial and fungal pathogens (Xiong and Yang, 2003). Exposure of Arabidopsis to high temperatures or drought leads to inhibition of the defence response to bacterial and fungal pathogens (Mohr and Cahill, 2007; Wang et al., 2009). By contrast, abiotic stress is also reported to enhance plant resistance to pathogens. For example, drought increases the endogenous level of ABA and suppresses invasion of B. cinerea and Oidium neolycopersici in tomato (Achuo et al., 2006). Although many abiotic stresses have been reported to promote ABA production, little is known about the inhibition of ABA biosynthesis. Here, we show that Cu2+ suppresses the biosynthesis of ABA dependent on ET signal transduction, leading to the opening of stomata and a sensitivity to drought stress in addition to enhancing the defence response to pathogens. Our findings provide an example of elicitor inhibition of ABA biosynthesis to activate the defence response in plants. Furthermore, this result suggests that prior watering is better at decreasing phytotoxicity by using CBACs on Cu2+‐sensitive plants.
The crosstalk between ET and ABA is complex. ET and ABA have antagonistic functions in controlling seed germination, seedling development, and stomatal movement. For example, when screening the ABA‐resistant seed germination phenotype, the ET‐insensitive mutants etr1 and ein2 showed enhanced seed dormancy and were more sensitive to ABA, while the constitutive ET response mutant ctr1 exhibited more resistance to ABA (Beaudoin et al., 2000). However, when testing the effects of ABA on the growth of roots, etr1 and ein2 showed more resistance to ABA and ET (Ghassemian et al., 2000). These results suggest that ET negatively regulates ABA function in seed germination and positively affects ABA function in seedling root development. It was found that the ein2 mutant had higher transcription of the ABA1 gene and accumulated more ABA (Ghassemian et al., 2000). Here, we found that StEIN2 and StEIN3 negatively regulated the expression of StABA1 and StNCED1 (Figure 6), indicating that ET signal transduction negatively regulates ABA biosynthesis in the Cu2+‐induced defence response. Furthermore, we found that StEIN3 directly binds StABA1 and StNCED1 promoters (Figure 7), suggesting that StEIN3 directly regulates the expression of StABA1 and StNCED1 in potato, so it is clear that ET also suppressed ABA biosynthesis in immunity response.
Suppression ABA biosynthesis may not be the only function of ET. The TRV‐EIN2/ABA1 and TRV‐EIN2/NCED1 plants (Figure 6d) showed a higher disease index than that of TRV‐ABA1 and TRV‐NCED1 (Figure 4c,d), suggesting that the ET signalling pathway plays more important roles in Cu2+‐mediated late blight resistance. It is possible that, except for suppressing ABA biosynthesis, ET activates other immunity responses. Because EIN3 is well known for its function to activate transcription with approximately 1,314 targets in the Arabidopsis genome (Chang et al., 2013), there may other genes be activated by or interacting with EIN3 to positively regulate plant immunity.
4. EXPERIMENTAL PROCEDURES
4.1. Strains and plasmids construction
The bacterial and P. infestans strains and plasmids used in this study are described in Table S3. The E. coli strains and A. tumefaciens GV3101 were cultured on Luria Bertani (LB) medium at 37 and 28 °C, respectively. The P. infestans isolate EC1 was cultured on rye B agar medium at 18 °C.
To construct the VIGS vectors, about 200‐bp fragments of gene StABA1, StNCED1, StEIN2, or StEIN3 were amplified using primers as described in Table S4 and inserted into the vector pTV00 (Ratcliff et al., 2001). To transiently express StABA1 or StNCED1 genes in potato, the coding sequence fragments were amplified by reverse transcription‐PCR and inserted into the vector pCXSN (Chen et al., 2009b). All plasmids were validated by DNA sequencing.
4.2. Plant materials and chemical treatments
Potato (Solanum tuberosum) cultivar Desirée plantlets were grown in nutrient substrate in a growth chamber with 12‐hr days (at 23 °C) and 12‐hr nights (at 21 °C) at 60%–75% relative humidity. CuSO4 or MgSO4 solubilized in deionized H2O supplemented with 0.05% (vol/vol) Tween 20 was sprayed on the potato plants. Pathogen inoculation was performed at 4 hpt, unless stated otherwise. ABA and ACC were solubilized in ethanol diluted in deionized H2O supplemented with 0.05% (vol/vol) Tween 20. The ABA solution (1 μM) and ACC solution (100 μM) were sprayed on the potato plants. The superoxide accumulation and callose deposition were analysed as described previously (Liu et al., 2015).
4.3. Virus‐induced gene silencing and transient expression in potato
Two‐ or 3‐week‐old potato plantlets were treated by vacuum infiltration of A. tumefaciens GV3101 (OD600 = 1) carrying pTRV1 and the various pTRV2 recombinants in a 1:1 ratio (Brigneti et al., 2004). The silencing level of potato genes were tested 21–24 days post‐agroinfiltration when the control pTV‐StPDS plants were showing signs of bleaching. An OD600 of 0.5 was adjusted for Agrobacterium‐mediated transient expression in the leaves of Desirée as described by Bhaskar et al. (2009).
4.4. P. infestans colonization assays
Two‐week‐old EC1 was flooded with sterile distilled water to collect sporangia. The spore suspension was incubated in an ice‐water bath for 2–3 hr. Sporangia were quantified using a haemocytometer and adjusted to 50,000/ml. Droplets (10 µl) were pipetted onto the surface of detached leaves, placed in sealed boxes with moist tissue to maintain humidity, and kept in weak light. Lesions were measured 3–5 days post‐inoculation (dpi) and scored as described previously (Wang et al., 2019). Disease indexes were analysed with about 30 leaves from 10 potato plants for each treatment. Disease indexes were measured at 5 dpi and scored as follows: 0, no visible infection; 1, <25% infection; 2, 26%–50% infection; 3, 51%–75% infection; 4, 76%–100% infection. Disease index (%) = /(× max ) × 100 (x i represent different scores and y i represent the number of leaves belong to different scores).
The biomass level analysis was performed as described by Wang et al. (2019). The leaves were harvested at 3–5 dpi and total DNA was extracted with cetyltrimethylammonium bromide solution. Then, the biomass level was measured by quantitative PCR (qPCR) with a P. infestans‐specific PiO8 element to quantify the biomass level of EC1 and the StEF1 gene was used to normalize the level of potato.
4.5. Hormone measurement
To measure the level of ET and ABA, potato plants were sprayed with 10 μM CuSO4 or MgSO4 supplemented with 0.05% (vol/vol) Tween 20. ET biosynthesis rates were measured by gas chromatography as described previously (Spollen et al., 2000; Zhang et al., 2018). For ABA, each of the 100 mg samples was prepared and quantified using an HPLC‐MS/MS system as reported by Xu et al. (2016). At least three biological replicates were analysed. ABA was also detected using immunocytochemistry in potato leaves as previously described (Ondzighi‐Assoume et al., 2016; Lu et al., 2019).
4.6. Water loss assay
Water loss assay was performed with 10 potato plants for each treatment as described previously (Li et al., 2018). About 30 leaves of potato plants treated with CuSO4 or MgSO4 were harvested. The weight of samples was measured every hour. The water loss was calculated with the following formula: water loss (%) = (fresh weight − dry weight)/fresh weight × 100.
4.7. Stomatal aperture measurement
The stomatal apertures on the leaves of potato plants treated with CuSO4 or MgSO4 were measured as reported by Melotto et al. (2006). Potato plants were kept under light for at least 3 hr and then treated with CuSO4 or MgSO4. Leaves were harvested at 0, 1, 2, and 3 hr post‐treatment. The stomata of random regions were observed under a Ni‐U fluorescence microscope (Nikon).
4.8. RNA extraction, reverse transcription PCR, qPCR
Leaves of potato plants were collected to extract total RNA using TRIzol reagent (Invitrogen). Using a ReverTra Ace qPCR RT Master Mix with gDNA Remover (TOYOBO), 1 μg of total RNA was used to synthesize the first‐strand cDNA described as the manufacturer's instructions. qPCR was performed with a QuantStudio 6 Flex Real‐Time PCR System (Life Technologies) using KOD SYBR qPCR Mix (TOYOBO) as described in the manufacturer's instructions. The primers used in RT‐qPCR are described in Table S4.
4.9. RNA‐Seq and data analysis
As previously described (Zhang et al., 2018), three biological replicates of RNA samples were collected from 4‐week‐old potato plants treated with 10 μM CuSO4 for 0, 2, or 24 hr. A total of nine libraries were constructed, and sequencing was performed with a BGISEQ‐500 by the Beijing Genomic Institution (http://www.genomics.org.cn, BGI, Shenzhen, China). Clean tags were mapped to the reference genome and genes that are available in the NCBI_SolTub_3.0 reference genome. For gene expression analysis, the matched reads were calculated and then normalized to reads per kb per million reads using RNA‐Seq by Expectation‐Maximization software. The significance of differential gene expression was confirmed with the BGI bioinformatics service using the combination of the absolute value of log2 ratio ≥ 1 and p ≤ 0.05 in this research.
4.10. Chromatin immunoprecipitation assay
For ChIP assay, the vector containing FLAG‐StEIN3 under the control of the CaMV 35S promoter was transiently expressed in potato using A. tumefaciens AGL1. The samples were harvested at 3 dpi and submerged in 1% formaldehyde and a vacuum applied for 10 min. The cross‐linking reaction was stopped by adding glycine to a final concentration of 0.125 M and applying a vacuum for an additional 5 min. Subsequently, the chromatin was isolated and sonicated as described previously (Li et al., 2018). Anti‐FLAG monoclonal antibody (Abways) and Co‐immunoprecipitation Kit (Thermo) were used for immunoprecipitation. The potato plants treated with empty vector were used as negative control. The primers used in ChIP‐qPCR are described in Table S4.
4.11. Electrophoretic mobility shift assay
The EMSA was performed as described previously (Li et al., 2018) with Chemiluminescent EMSA Kit (Beyotime). To express StEIN3 protein in E. coli BL21, the DNA fragment was amplified by PCR and inserted into the pMal‐c2X‐BamHI‐SalI‐cut vector. The protein MBP‐StEIN3 was purified using affinity chromatography. For probes, biotin‐labelled forward primer was used to amplify the DNA fragments by PCR. For competitor, DNA fragments were amplified by PCR using nonlabelled primers.
Supporting information
FIGURE S1 The effect of Cu2+ on the growth of Phytophthora infestans. (a) Growth of P. infestans EC1 in rye B medium containing different concentrations of CuSO4. Images were photographed at 7 days. (b) The diameter of EC1 colony cultured in rye B medium containing different concentrations of CuSO4. Data represent the mean ± SD (n = 3). The asterisks indicate a significant difference compared with the control (t test; *p < .05; **p < .01)
FIGURE S2 Gene expression regulated by Cu2+ in potato. (a) Numbers of genes up‐regulated and down‐regulated by more than 2‐fold at 2 and 24 hr post‐CuSO4 treatment (hpt). (b) Heatmap of genes up‐regulated and down‐regulated by more than 2‐fold at 2 and 24 hpt
FIGURE S3 The transcript levels of the targeted genes in virus‐induced gene silencing or transient expression in plants. StActin gene was used as a control to normalize expression levels of StABA1, StNCED1, StEIN2, and StEIN3. Data represent the mean ± SD (n = 3) from three independent biological replicates
FIGURE S4 The level of ethylene in stein2‐VIGS, stein3‐VIGS, and pTV00 potato plants after CuSO4 treatment. Ethylene was measured in a gas chromatograph equipped with a photoionization detector. The average rates of ethylene production are shown. The asterisks indicate a significant difference compared with the control (pTV00) (t test; *p < .05)
FIGURE S5 Ethylene negatively regulated the biosynthesis of abscisic acid (ABA) in Arabidopsis. (a) The transcript levels of AtABA1 and AtNCED3 in atein2, atein3 mutations and wild‐type plants. AtActin2 gene was used as a control to normalize expression levels. Data represent the mean ± SD from three independent biological replicates. The asterisks indicate a significant difference compared with the wild‐type plants (t test; *p < .05; **p < .01). (b) The distribution of ABA accumulation with the optimized immunofluorescence technique in the roots of atein2, atein3 mutations, and wild‐type plants. The ABA concentration index was ABA/Alexa Fluor 555 fluorescence intensity as measured by ImageJ (mean ± SD, n ≥ 20). Asterisks indicate statistically significant differences (t test; *p < .05)
TABLE S1 Summary of the sequencing result
TABLE S2 Differentially expressed genes identified at 2 and 24 hr post‐treatment
TABLE S3 Strains and plasmids used in this study
TABLE S4 List of oligonucleotide primers used in this study
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (31801722), the Shandong Provincial Key Research and Development Program (2019LYX007), and the China Postdoctoral Science Foundation (2017M612310). We are grateful to Professor Zhendong Tian (Huazhong Agricultural University) for providing P. infestans EC1. The authors declare that they have no competing interests. H.L. designed and performed RNA‐Seq and ChIP‐qPCR, analysed the data, and drafted the manuscript; X.X. performed most of the VIGS and inoculation experiments; Y.Y. performed most of the RT‐qPCR and ET experiments; M.X. helped to perform inoculation and RT‐qPCR experiments; L.C. helped to detect ABA by immunocytochemistry; X.M. helped to perform hormone quantification; B.Z. initiated the experiments on potato; X.D. provided laboratory management; Z.C. supervised the project, designed some of experiments, interpreted data, and revised the manuscript. All authors read and approved the final manuscript.
Liu H‐F, Xue X‐J, Yu Y, et al. Copper ions suppress abscisic acid biosynthesis to enhance defence against Phytophthora infestans in potato. Molecular Plant Pathology. 2020;21:636–651. 10.1111/mpp.12919
Hai‐Feng Liu, Xiao‐Jing Xue and Yue Yu contributed equally to this work.
Funding information
This work was supported by the National Natural Science Foundation of China (31801722), the Shandong Provincial Key Research and Development Program (2019LYX007), and the China Postdoctoral Science Foundation (2017M612310)
DATA AVAILABILITY STATEMENT
The RNA‐Seq original sequence data that support the findings of this study are openly available in the NCBI Sequence Read Archive at http://www.ncbi.nlm.nih.gov/sra, under the accession number SRR8791013.
REFERENCES
- Achuo, E.A. , Prinsen, E. and Hofte, M. (2006) Influence of drought, salt stress and abscisic acid on the resistance of tomato to Botrytis cinerea and Oidium neolycopersici . Plant Pathology, 55, 178–186. [Google Scholar]
- Agrawal, G.K. , Yamazaki, M. , Kobayashi, M. , Hirochika, R. , Miyao, A. and Hirochika, H. (2001) Screening of the rice viviparous mutants generated by endogenous retrotransposon Tos17 insertion tagging of a zeaxanthin epoxidase gene and a novel ostatc gene. Plant Physiology, 125, 1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, J.P. , Badruzsaufari, E. , Schenk, P.M. , Manners, J.M. , Desmond, O.J. , Ehlert, C. et al (2004) Antagonistic interaction between abscisic acid and jasmonate‐ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis . The Plant Cell, 16, 3460–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselbergh, B. , Curvers, K. , Franca, S.C. , Audenaert, K. , Vuylsteke, M. , Van Breusegem, F. et al (2007) Resistance to Botrytis cinerea in sitiens, an abscisic acid‐deficient tomato mutant, involves timely production of hydrogen peroxide and cell wall modifications in the epidermis. Plant Physiology, 144, 1863–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audenaert, K. , De Meyer, G.B. and Hofte, M.M. (2002) Abscisic acid determines basal susceptibility of tomato to Botrytis cinerea and suppresses salicylic acid‐dependent signaling mechanisms. Plant Physiology, 128, 491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudoin, N. , Serizet, C. , Gosti, F. and Giraudat, J. (2000) Interactions between abscisic acid and ethylene signaling cascades. The Plant Cell, 12, 1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar, P.B. , Venkateshwaran, M. , Wu, L. , Ané, J.M. and Jiang, J.M. (2009) Agrobacterium‐mediated transient gene expression and silencing: a rapid tool for functional gene assay in potato. PLoS ONE, 4, e5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrot, F. , Segonzac, C. , Chang, K.N. , Qiao, H. , Ecker, J.R. , Zipfel, C. et al (2010). Direct transcriptional control of the Arabidopsis immune receptor FLS2 by the ethylene‐dependent transcription factors EIN3 and EIL1. Proceedings of the National Academy of Sciences of the United States of America, 107, 14502–14507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigneti, G. , Martín‐Hernández, A.M. , Jin, H. , Chen, J. , Baulcombe, D.C. , Baker, B. et al (2004) Virus‐induced gene silencing in Solanum species. The Plant Journal, 39, 264–272. [DOI] [PubMed] [Google Scholar]
- Cao, F.Y. , Yoshioka, K. and Desveaux, D. (2011) The roles of ABA in plant–pathogen interactions. Journal of Plant Research, 124, 489–499. [DOI] [PubMed] [Google Scholar]
- Cha, J.S. and Cooksey, D.A. (1991) Copper resistance in Pseudomonas syringae mediated by periplasmic and outer membrane proteins. Proceedings of the National Academy of Sciences of the United States of America, 88, 8915–8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, K.N. , Zhong, S. , Weirauch, M.T. , Hon, G. , Pelizzola, M. , Li, H. et al (2013) Temporal transcriptional response to ethylene gas drives growth hormone cross‐regulation in Arabidopsis. eLife, 2, e00675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H. , Xue, L. , Chintamanani, S. , Germain, H. , Lin, H. , Cui, H. et al (2009a) Ethylene insensitive 3 and ethylene insensitive 3‐like 1 repress salicylic acid induction deficient 2 expression to negatively regulate plant innate immunity in Arabidopsis . The Plant Cell, 21, 2527–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S. , Songkumarn, P. , Liu, J. and Wang, G. (2009b) A versatile zero background T‐vector system for gene cloning and functional genomics. Plant Physiology, 150, 1111–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay, N.K. , Adio, A.M. , Denoux, C. , Jander, G. and Ausubel, F.M. (2009) Glucosinolate metabolites required for an Arabidopsis innate immune response. Science, 323, 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Torres‐Zabala, M. , Truman, W. , Bennett, M.H. , Lafforguel, G. , Mansfield, J.W. , Egea, P.R. et al (2007) Pseudomonas syringae pv. tomato hijacks the Arabidopsis abscisic acid signaling pathway to cause disease. EMBO Journal, 26, 1434–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, Z. , Yu, Y. , Li, S. , Wang, J. , Tang, S. and Huang, R. (2016) Abscisic acid antagonizes ethylene production through the ABI4‐mediated transcriptional repression of ACS4 and ACS8 in Arabidopsis . Molecular Plant, 9, 126–135. [DOI] [PubMed] [Google Scholar]
- Duckham, S.C. , Linforth, R.S.T. and Taylor, I.B. (1991) Abscisic‐acid‐deficient mutants at the aba gene locus of Arabidopsis thaliana are impaired in the epoxidation of zeaxanthin. Plant, Cell and Environment, 14, 601–606. [Google Scholar]
- Endo, A. , Sawada, Y. , Takahashi, H. , Okamoto, M. , Ikegami, K. , Koiwai, H. et al (2008) Drought induction of Arabidopsis 9‐cis‐epoxycarotenoid dioxygenase occurs in vascular parenchyma cells. Plant Physiology, 147, 1984–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flors, V. , Ton, J. , Jakab, G. and Mauch‐Mani, B. (2005) Abscisic acid and callose: team players in defence against pathogens? Journal of Phytopathology, 153, 377–383. [Google Scholar]
- Galpaz, N. , Wang, Q. , Menda, N. , Zamir, D. and Hirschberg, J. (2008) Abscisic acid deficiency in the tomato mutant high‐pigment 3 leading to increased plastid number and higher fruit lycopene content. The Plant Journal, 53, 717–730. [DOI] [PubMed] [Google Scholar]
- Ghassemian, M. , Nambara, E. , Cutler, S. , Kawaide, H. , Kamiya, Y. and McCourt, P. (2000) Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis . The Plant Cell, 12, 1117–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, R. , Su, J. , Meng, X. , Li, S. , Liu, Y. , Xu, J. et al (2015) Multilayered regulation of ethylene induction plays a positive role in Arabidopsis resistance against Pseudomonas syringae . Plant Physiology, 169, 299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai, N. , Okamoto, M. and Koshinizu, K. (1986) The 1′,4′‐trans‐diol of abscisic acid, a possible precursor of abscisic acid in Botrytis cinetea . Phytochemistry, 25, 1865–1868. [Google Scholar]
- Kamoun, S. , Furzer, O. , Jones, J.D. , Judelson, H.S. , Ali, G.S. , Dalio, R.J. et al (2015) The top 10 oomycete pathogens in molecular plant pathology. Molecular Plant Pathology, 16, 413–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keunen, E. , Schellingen, K. , Vangronsveld, J. and Cuypers, A. (2016) Ethylene and metal stress: small molecule, big impact. Frontiers in Plant Science, 7, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi, S. and Ohashi, Y. (2000) Cloning and DNA‐binding properties of a tobacco Ethylene‐Insensitive3 (EIN3) homolog. Nucleic Acids Research, 28, 960–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichhane, J.R. , Osdaghi, E. , Behlau, F. , Köhl, J. , Jones, J.B. and Aubertot, J.N. (2018) Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agronomy for Sustainable Development, 38, 28. [Google Scholar]
- Li, N. , Wei, S. , Chen, J. , Yang, F. , Kong, L. , Chen, C. et al (2018) OsASR2 regulates the expression of a defense‐related gene, Os2H16, by targeting the GT‐1 cis‐element. Plant Biotechnology Journal, 16, 771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H. , Zhang, B. , Wu, T. , Ding, Y. , Ding, X. and Chu, Z. (2015) Copper ion elicits defense response in Arabidopsis thaliana by activating salicylate‐ and ethylene‐dependent signaling pathways. Molecular Plant, 8, 1550–1553. [DOI] [PubMed] [Google Scholar]
- Liu, Y. and Zhang, S. (2004) Phosphorylation of 1‐aminocyclopropane‐1‐carboxylic acid synthase by MPK6, a stress‐responsive mitogen‐activated protein kinase, induces ethylene biosynthesis in Arabidopsis . The Plant Cell, 16, 3386–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, C. , Chen, M. , Liu, R. , Zhang, L. , Hou, X. , Liu, S. et al (2019) Abscisic acid regulates auxin distribution to mediate maize lateral root development under salt stress. Frontiers in Plant Science, 10, 716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin, E. , Nussaume, L. , Quesada, A. , Gonneau, M. , Sotta, B. , Hugueney, A.F. et al (1996) Molecular identification of zeaxanthin epoxidase of Nicotiana plumbaginifolia, a gene involved in abscisic acid biosynthesis and corresponding to the ABA locus of Arabidopsis thaliana . EMBO Journal, 15, 2331–2342. [PMC free article] [PubMed] [Google Scholar]
- Melotto, M. , Underwood, W. , Koczan, J. , Nomura, K. and He, S.Y. (2006) Plant stomata function in innate immunity against bacterial invasion. Cell, 126, 969–980. [DOI] [PubMed] [Google Scholar]
- Moeder, W. , Barry, C.S. , Tauriainen, A.A. , Betz, C. , Tuomainen, J. , Utriainen, M. et al (2002) Ethylene synthesis regulated by biphasic induction of 1‐aminocyclopropane‐1‐carboxylic acid synthase and 1‐aminocyclopropane‐1carboxylic acid oxidase genes is required for hydrogen peroxide accumulation and cell death in ozone‐exposed tomato. Plant Physiology, 130, 1918–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr, P.G. and Cahill, D.M. (2007) Suppression by ABA of salicylic acid and lignin accumulation and the expression of multiple genes, in Arabidopsis infected with Pseudomonas syringae pv. tomato . Functional & Integrative Genomics, 7, 181–191. [DOI] [PubMed] [Google Scholar]
- Neill, S.J. , Horgan, R. , Walton, D.C. and Lee, T.S. (1982) The biosynthesis of abscisic‐acid in Cercospora rosicola . Phytochemistry, 21, 61–65. [Google Scholar]
- Nie, X. , Singh, R.P. and Tai, G.C.C. (2002) Molecular characterization and expression analysis of 1‐aminocyclopropane‐1‐carboxylate oxidase homologs from potato under abiotic and biotic stresses. Genome, 45, 905–913. [DOI] [PubMed] [Google Scholar]
- Nowichi, M. , Foolad, M.R. , Nowakowska, M. and Kozik, E.Z. (2012) Potato and tomato late blight caused by Phytophthora infestans: an overview of pathology and resistance breeding. Plant Disease, 96, 4–17. [DOI] [PubMed] [Google Scholar]
- Ondzighi‐Assoume, C.A. , Chakraborty, S. and Harris, J.M. (2016) Environmental nitrate stimulates abscisic acid accumulation in Arabidopsis root tips by releasing it from inactive stores. The Plant Cell, 28, 729–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pscheidt, J.W. and Ocamb, C.M. (1999) Pacific Northwest Plant Disease Control Handbook. Corvallis, OR: Oregon State University. [Google Scholar]
- Randall, E. , Young, V. , Sierotzki, H. , Scalliet, G. , Birch, P.R.J. , Cooke, D.E.L. et al (2014) Sequence diversity in the large subunit of RNA polymerase I contributes to Mefenoxam insensitivity in Phytophthora infestans . Molecular Plant Pathology, 15, 664–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, M.V. , Lee, H.I. and Davis, K.R. (2002) Ozone‐induced ethylene production is dependent on salicylic acid, and both salicylic acid and ethylene act in concert to regulate ozone‐induced cell death. The Plant Journal, 32, 447–456. [DOI] [PubMed] [Google Scholar]
- Ratcliff, F. , Martinhernandez, A.M. and Baulcombe, D.C. (2001) Technical advance tobacco rattle virus as a vector for analysis of gene function by silencing. The Plant Journal, 25, 237–245. [DOI] [PubMed] [Google Scholar]
- Sánchez‐Vallet, A. , López, G. , Ramos, B. , Delgado‐Cerezo, M. , Riviere, M. , Llorente, F. et al (2012) Disruption of abscisic acid signaling constitutively activates Arabidopsis resistance to the necrotrophic fungus Plectosphaerella cucumerina . Plant Physiology, 160, 2109–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellingen, K. , Straeten, D.V.D. , Vandenbussche, F. , Prinsen, E. , Remans, T. , Vangronsveld, J. et al (2014) Cadmium‐induced ethylene production and responses in Arabidopsis thaliana rely on ACS2 and ACS6 gene expression. BMC Plant Biology, 14, 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spollen, W.G. , LeNoble, M.E. , Samuels, T.D. , Bernstein, N. and Sharp, R.E. (2000) Abscisic acid accumulation maintains maize primary root elongation at low water potentials by restricting ethylene production. Plant Physiology, 122, 967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strayer‐Scherer, A. , Liao, Y.Y. , Young, M. , Ritchie, L. , Vallad, G.E. , Santra, S. et al (2018) Advanced copper composites against copper‐tolerant Xanthomonas perforans and tomato bacterial spot. Phytopathology, 108, 196–205. [DOI] [PubMed] [Google Scholar]
- Tan, B.C. , Joseph, L.M. , Deng, W.T. , Liu, L. , Li, Q.B. , Cline, K. et al (2010) Molecular characterization of the Arabidopsis 9‐cis epoxycarotenoid dioxygenase gene family. The Plant Journal, 35, 44–56. [DOI] [PubMed] [Google Scholar]
- Thaler, J.S. and Bostock, R.M. (2004) Interactions between abscisic‐acid‐mediated responses and plant resistance to pathogens and insects. Ecology, 85, 48–58. [Google Scholar]
- Ton, J. , Flors, V. and Mauchmani, B. (2009) The multifaceted role of ABA in disease resistance. Trends in Plant Science, 14, 310–317. [DOI] [PubMed] [Google Scholar]
- Torres‐Zabala, M.D. , Truman, W. , Bennett, M.H. , Lafforgue, G. , Mansfield, J.W. , Egea, P.R. et al (2014) Pseudomonas syringae pv. tomato hijacks the Arabidopsis abscisic acid signalling pathway to cause disease. EMBO Journal, 26, 1434–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Gao, C. , Li, L. , Cao, W. , Dong, R. , Ding, X. et al (2019) Transgenic RXLR effector PITG_15718.2 suppresses immunity and reduces vegetative growth in potato. International Journal of Molecular Sciences, 20, 3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Bao, Z.L. , Zhu, Y. and Hua, J. (2009) Analysis of temperature modulation of plant defense against biotrophic microbes. Molecular Plant‐Microbe Interactions, 22, 498–506. [DOI] [PubMed] [Google Scholar]
- Ward, E.W. , Cahill, D.M. and Bhattacharyya, M.K. (1989) Abscisic acid suppression of phenylalanine ammonia‐lyase activity and mRNA, and resistance of soybeans to Phytophthora megasperma f. sp. glycinea . Plant Physiology, 91, 23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, L.M. , Ishitani, M. and Zhu, J.K. (2001) The Arabidopsis los5/aba3 locus encodes a molybdenum cofactor sulfurase and modulates cold stress‐ and osmotic stress‐responsive gene expression. The Plant Cell, 13, 2063–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, L.Z. and Yang, Y.N. (2003) Disease resistance and abiotic stress tolerance in rice are inversely modulated by an abscisic acid‐inducible mitogen‐activated protein kinase. The Plant Cell, 15, 745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Q. , Truong, T.T. , Barrero, J.M. , Jacobsen, J.V. , Hocart, C.H. and Gubler, F. (2016) A role for jasmonates in the release of dormancy by cold stratification in wheat. Journal of Experimental Botany, 67, 3497–3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, B. , Liu, H. , Ding, X. , Qiu, J. , Zhang, M. and Chu, Z. (2018) Arabidopsis thaliana ACS8 plays a crucial role in the early biosynthesis of ethylene elicited by Cu2+ ions. Journal of Cell Science, 131, jcs202424. [DOI] [PubMed] [Google Scholar]
- Zipfel, C. , Kunze, G. , Chinchilla, D. , Caniard, A. , Jones, J.D.G. , Boller, T. et al (2006) Perception of the bacterial MAMP EF‐Tu by the receptor EFR restricts Agrobacterium‐mediated transformation. Cell, 125, 749–760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 The effect of Cu2+ on the growth of Phytophthora infestans. (a) Growth of P. infestans EC1 in rye B medium containing different concentrations of CuSO4. Images were photographed at 7 days. (b) The diameter of EC1 colony cultured in rye B medium containing different concentrations of CuSO4. Data represent the mean ± SD (n = 3). The asterisks indicate a significant difference compared with the control (t test; *p < .05; **p < .01)
FIGURE S2 Gene expression regulated by Cu2+ in potato. (a) Numbers of genes up‐regulated and down‐regulated by more than 2‐fold at 2 and 24 hr post‐CuSO4 treatment (hpt). (b) Heatmap of genes up‐regulated and down‐regulated by more than 2‐fold at 2 and 24 hpt
FIGURE S3 The transcript levels of the targeted genes in virus‐induced gene silencing or transient expression in plants. StActin gene was used as a control to normalize expression levels of StABA1, StNCED1, StEIN2, and StEIN3. Data represent the mean ± SD (n = 3) from three independent biological replicates
FIGURE S4 The level of ethylene in stein2‐VIGS, stein3‐VIGS, and pTV00 potato plants after CuSO4 treatment. Ethylene was measured in a gas chromatograph equipped with a photoionization detector. The average rates of ethylene production are shown. The asterisks indicate a significant difference compared with the control (pTV00) (t test; *p < .05)
FIGURE S5 Ethylene negatively regulated the biosynthesis of abscisic acid (ABA) in Arabidopsis. (a) The transcript levels of AtABA1 and AtNCED3 in atein2, atein3 mutations and wild‐type plants. AtActin2 gene was used as a control to normalize expression levels. Data represent the mean ± SD from three independent biological replicates. The asterisks indicate a significant difference compared with the wild‐type plants (t test; *p < .05; **p < .01). (b) The distribution of ABA accumulation with the optimized immunofluorescence technique in the roots of atein2, atein3 mutations, and wild‐type plants. The ABA concentration index was ABA/Alexa Fluor 555 fluorescence intensity as measured by ImageJ (mean ± SD, n ≥ 20). Asterisks indicate statistically significant differences (t test; *p < .05)
TABLE S1 Summary of the sequencing result
TABLE S2 Differentially expressed genes identified at 2 and 24 hr post‐treatment
TABLE S3 Strains and plasmids used in this study
TABLE S4 List of oligonucleotide primers used in this study
Data Availability Statement
The RNA‐Seq original sequence data that support the findings of this study are openly available in the NCBI Sequence Read Archive at http://www.ncbi.nlm.nih.gov/sra, under the accession number SRR8791013.


