Dear Editor,
Over one million people worldwide have been infected with Coronavirus disease 2019 (COVID-19). Up to 12% of them may require intensive care unit admission and 75% of them may need mechanical ventilation [1,2]. A significant proportion of COVID-19 patients suffer from chronic obstructive lung disease, asthma or other pulmonary disorders, which are associated with an increased risk of severe infection requiring respiratory assistance [3,4].
Due to concerns regarding the aerosol spread and medical team contamination, guidelines recommend using closed ventilation circuit and mandates extreme safety precautions whenever interruption of the ventilator's tubing continuity is undertaken [5]. Bronchodilators delivery in either intubated or non-invasively ventilated patients is usually done using standard nebulizers or metered-dose inhalers, which have an equal effect on lung function [6]. Nevertheless, using both devices in a COVID-19 patient might be hazardous, as it requires the opening of the ventilator circuit. Frequent bronchodilators administration may be necessary, requiring multiple health care- patient interaction, which we aim to minimize.
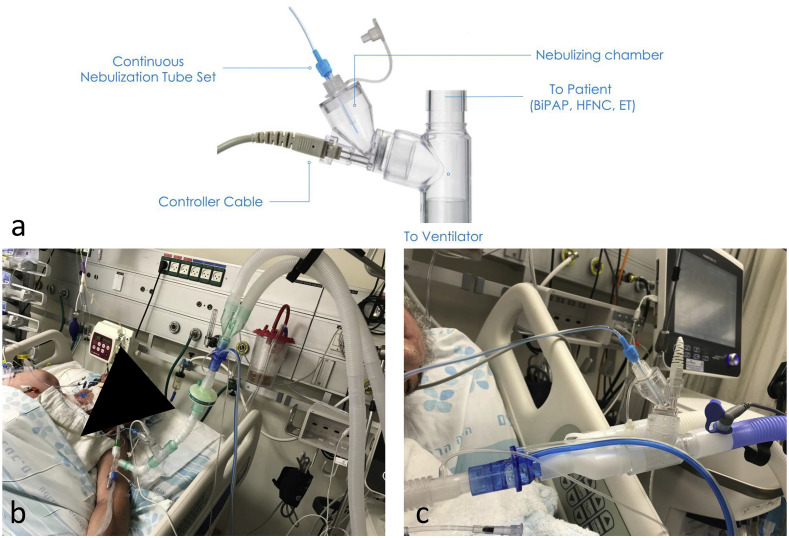
In order to mitigate these hazards, we successfully use continuous inhalation by Aeroneb Solo (Aerogen©, Galway, Ireland) in invasively and non-invasively ventilated COVID-19 patients with COPD and asthma (Fig. 1 ). The system is composed of a syringe mounted on a pump that continuously delivers medication to the nebulizing reservoir, isolated from the breathing circuit, where an electric powered ultrasonic mesh vibrating at ultra-high rate transforms the drug into an aerosol that enters the ventilator circuit. This closed system requires minimal staff handling and no circuit opening, thereby reducing workload and increasing safety. To our knowledge, this is the first report of administering bronchodilators safely and efficiently to COVID-19 ventilated patients.
Fig. 1.
a. Illustration of the Aerogen Solo continuous inhalation setup. b. The nebulizer is connected to the ventilator circuit distal to the HME filter. An antiviral filter can be placed at the exit of the inspiratory limb. c. The nebulizer is placed on the inspiratory limb just proximal to the Y piece. The HME filter is connected to the inspiratory tubing at the ventilator exit site.
HME - Heat and Moisture Exchanger.
Declarations of Competing Interest
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Phua J., Weng L., Ling L., Egi M., Lim C.-M., Divatia J.V. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;0 doi: 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K. Covid-19 in critically ill patients in the seattle region — case series. N Engl J Med. 2020 doi: 10.1056/NEJMoa2004500. (NEJMoa2004500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lippi G., Henry B.M. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19) Respir Med. 2020;0:105941. doi: 10.1016/j.rmed.2020.105941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W., Liang W., Zhao Y., Liang H., Chen Z., Li Y. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J. 2020;2000547 doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook T.M., El-Boghdadly K., McGuire B., McNarry A.F., Patel A., Higgs A. Consensus guidelines for managing the airway in patients with COVID-19. Anaesthesia. 2020 doi: 10.1111/anae.15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duarte A.G. Inhaled bronchodilator administration during mechanical ventilation. Respir Care. 2004;49:623–634. [PubMed] [Google Scholar]