Abstract
ATP-binding cassette (ABC) transporters constitute a superfamily of 48 structurally similar membrane transporters that mediate the ATP-dependent cellular export of a plethora of endogenous and xenobiotic substances. Importantly, genetic variants in ABC genes that affect gene function have clinically important effects on drug disposition and can be predictors of the risk of adverse drug reactions and efficacy of chemotherapeutics, calcium channel blockers, and protease inhibitors. Furthermore, loss-of-function of ABC transporters is associated with a variety of congenital disorders. Despite their clinical importance, information about the frequencies and global distribution of functionally relevant ABC variants is limited and little is known about the overall genetic complexity of this important gene family. Here, we systematically mapped the genetic landscape of the entire human ABC superfamily using Next-Generation Sequencing data from 138,632 individuals across seven major populations. Overall, we identified 62,793 exonic variants, 98.5% of which were rare. By integrating five computational prediction algorithms with structural mapping approaches using experimentally determined crystal structures, we found that the functional ABC variability is extensive and highly population-specific. Every individual harbored between 9.3 and 13.9 deleterious ABC variants, 76% of which were found only in a single population. Carrier rates of pathogenic variants in ABC transporter genes associated with autosomal recessive congenital diseases, such as cystic fibrosis or pseudoxanthoma elasticum, closely mirrored the corresponding population-specific disease prevalence, thus providing a novel resource for rare disease epidemiology. Combined, we provide the most comprehensive, systematic, and consolidated overview of ethnogeographic ABC transporter variability with important implications for personalized medicine, clinical genetics, and precision public health.
Electronic supplementary material
The online version of this article (10.1007/s00439-020-02150-6) contains supplementary material, which is available to authorized users.
Introduction
ATP-binding cassette (ABC) transporters are a superfamily of membrane proteins that, in humans, comprise 48 genes. ABC transporters catalyse the translocation of a wide spectrum of endogenous substrates across biological membranes, including amino acids, sugars, nucleosides, vitamins, lipids, bile acids, leukotrienes, prostaglandins, uric acid, antioxidants, as well as a multitude of natural toxins (Liang et al. 2015). In addition, ABC transporters mediate the export of a plethora of drug substrates, including calcium channel blockers, HIV protease inhibitors, vinca alkaloids, topoisomerase inhibitors, methotrexate, anthracyclines, and taxanes, into the extracellular space and are thus key modulators of drug resistance, particularly in oncology (Robey et al. 2018). Hence, ABC transporters are of specific clinical and regulatory interest for their involvement in drug–drug interactions (König et al. 2013; Marquez and Van Bambeke 2011; Zhang et al. 2018).
Genetic variants in ABC transporters contribute to the inter-individual variability in the risk of adverse drug reactions and treatment efficacy, and are key modulators of drug resistance. Arguably, the most studied are polymorphisms in ABCB1 (encoding MDR1, P-gp), which have been associated with methotrexate clearance (Kim et al. 2012a), response to antiretroviral protease inhibitors (Coelho et al. 2013), as well as with pharmacokinetics, response, and toxicity of imatinib (Dulucq et al. 2008; Ma et al. 2017). Similarly, variants in ABCG2 (encoding BCRP) were reproducibly associated with exposure and response to statins (Bailey et al. 2010; Chasman et al. 2012; Hu et al. 2011) and allopurinol (Roberts et al. 2017; Wen et al. 2015). In addition to their pharmacogenetic importance, genetic variation in 21 ABC transporters can cause congenital diseases, the most common of which is cystic fibrosis (OMIM 219700) caused by variants in ABCC7 (CFTR).
Importantly, while many studies have provided critical data about the clinical importance of ABC polymorphisms (Bosch et al. 2005; Fukushima-Uesaka et al. 2007; Honjo et al. 2002; Leschziner et al. 2006; Pramanik et al. 2014; Saito et al. 2002; Słomka et al. 2015), information about their population frequencies is limited and mostly derived from relatively small, heterogeneous cohorts. Furthermore, most studies only interrogated a few selected candidate variants and did not map the entire landscape of rare genetic variability that is characteristic for pharmacogenes (Bush et al. 2016; Fujikura et al. 2015; Gordon et al. 2014; Ingelman-Sundberg et al. 2018; Kozyra et al. 2017; Wright et al. 2018; Zhou and Lauschke 2018). Importantly, the increasing prevalence of Next-Generation Sequencing (NGS) projects on a population scale allows for the first time to systematically parse the inter-individual and inter-population variability in ABC transporter superfamily.
In the current study, we systematically parsed the inter-individual and inter-population variability in the ABC transporter superfamily by analyzing whole-exome and whole-genome sequencing (WES and WGS, respectively) data from 138,632 individuals across seven major human populations. Using this large data set, we provide frequencies of 51 ABC variants and haplotypes frequencies with demonstrated clinical relevance. In addition to these well-characterized variations, we identified 62,793 exonic variants, the vast majority of which were rare and have not been characterized. Computational analyses using five partly orthogonal algorithms predicted that 19,309 of these (31%) resulted in functional alterations of the respective transporter protein. To substantiate these estimates, we mapped the identified genetic variability onto experimentally determined or homology-modeled transporter structures and found multiple amino acid exchanges in residues important for substrate binding and transporter function. The present study constitutes the most comprehensive analysis of genetic variation in the ABC superfamily published to date and the identified genetic complexity might have important implications for the evaluation of drug transporter variability during drug development and the personalized prediction of drug disposition, response, and toxicity.
Methods
Data collection and definitions
Single-nucleotide variant (SNV) and indel frequency data across 48 human ABC transporters were collected from WES and WGS data from 138,632 individuals (12,020 Africans, 17,210 Latinos, 5076 Ashkenazi Jews, 9435 East Asians, 15,391 South Asians, 12,897 Finns, 63,369 non-Finnish Europeans, and 3234 from other ethnic groups) acquired from the Genome Aggregation Database (Lek et al. 2016). Variants with MAF < 1% or MAF < 0.1% were defined as rare and very rare, respectively. Copy-number variation (CNV) data were extracted from the Exome Aggregation Consortium database using genomic information from 59,451 individuals and analyzed as previously described (Santos et al. 2018). Linkage disequilibria were computed by leveraging linkage from the 1000 Genomes Project using LDLink (Machiela and Chanock 2015). The Online Mendelian Inheritance in Man (OMIM) database was used to identify ABC genes associated with Mendelian disease, as well as their mode of inheritance (Amberger et al. 2015). One-way ANOVA was used to compare the difference between variant number across ABC subfamilies.
Variant effect predictions
To predict the functional consequences of missense variants, we used a panel of computational algorithms that analyze sequence conservation, as well as variant effects on physicochemical amino acid properties, solvent accessibility, and structural features. Specifically, we selected SIFT (Ng and Henikoff 2001), Polyphen2 (Adzhubei et al. 2010), MutationAssessor (Reva et al. 2011), VEST3 (Carter et al. 2013), and Eigen (Ionita-Laza et al. 2016), as they showed the best predictive performance in three independent benchmarking data sets (Li et al. 2018a). Variants were categorized as deleterious when the ≥ 50% of algorithms predicted effects on transporter function. In addition, all frameshifts, in-frame deletions or insertions, start-lost, stop-gained, or canonical splice site variants were regarded as putatively deleterious. For Mendelian disease analyses, ClinVar (Landrum et al. 2014) was used to remove benign variants from disease-associated ABC genes.
Structural analysis
We analyzed the impact of genetic variation on ABC transporter structures for the entire ABCA, ABCB, and ABCC transporter families (35 proteins in total). Experimentally determined crystal structures were available for 18 ABC transporter proteins and were extracted from PDB (Berman et al. 2000) and the available literature. The remaining 16 transporter structures were modeled based on homology using Phyre2 (Kelley et al. 2015). The structure of ABCA13 could not be modeled reliably and was thus excluded. PyMOL (version 2.1.1) was used to map the genetic variability data onto the corresponding transporter structures.
Results
Genetic variability of the human ABC transporter superfamily
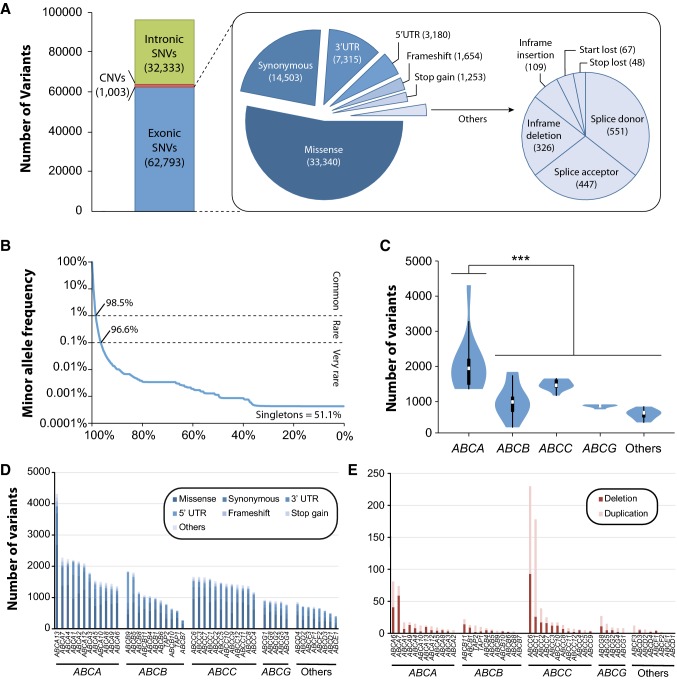
We systematically analyzed the genetic variability profiles of all 48 members of the human ABC transporter gene superfamily using NGS data from 138,632 individuals. In total, we identified 62,793 variants in exons, the majority of which were missense (n = 33,340; 53%), followed by synonymous (n = 14,503; 23%) and UTR variations (n = 10,495; 17%; Fig. 1a). Importantly, the vast majority of variations (n = 61,876; 98.5%) were rare with minor allele frequencies (MAF) < 1%, whereas only 917 (1.5%) variations were common (Fig. 1b). In addition, we found 1003 deletions or duplications spanning at least one ABC exon, jointly referred to as CNVs, as well as 32,333 intronic variants. The latter were, however, not systematically covered and thus excluded from further analyses.
Fig. 1.
Overview of the genetic germline variability in the human ABC transporter family. a In total 62,793 exonic variants and 1003 copy-number variations (CNVs) were identified across all 48 human ABC genes in 138,632 individuals. b The vast majority of exonic ABC variants were rare with 98.5% occurring in less than 1% of alleles worldwide. In addition, 51.1% of all variants were only found in a single individual. cABCA genes harbour significantly more variations than members of other ABC subfamilies (p = 0.002; ANOVA). These differences were mostly related to gene length (compare Supplementary Figure 1). d Stacked column plot depicting the number of variants across variants classes for all 48 ABC genes. e The number of CNVs that affect at least one exon are shown
Notably, the number of genetic variations differed considerably between ABC subfamilies and genes. Overall, the number of variants in the ABCA family of lipid transporters was significantly higher than in other ABC subfamilies (p = 0.002; fold difference = 1.9; Fig. 1c). Of all members of the human ABC superfamily of genes, the lipid transporters ABCA13 (n = 4310), ABCA7 (n = 274), and ABCA4 (n = 2224) harbored the highest number of variants, whereas > 10-fold less variations were found in ABCD1 (n = 496), ABCE1 (n = 407), and ABCB7 (n = 271; Fig. 1d). However, when the number variants were normalized by gene length, no significant differences were identified between the subfamilies (Supplementary Figure 1A). In contrast, variability varied more than sevenfold between different ABC genes with ABCB9 (n = 802.4 variants/kb) and ABCB8 (n = 537.4 variants/kb) being most polymorphic, whereas ABCB7 was most invariant (n = 120.1 variants/kb; Supplementary Figure 1B). To directly compare the evolutionary constraint, we compared the observed number of missense and loss-of-function variants in ABC genes with the expected numbers based on the genetic background variability. Missense variations in ABCC9, ABCA2, and ABCE1 were most depleted, whereas, surprisingly, CFTR was least conserved and harbored 30% more missense variations than expected by chance (Supplementary Figure 2A; Supplementary Table 1). Based on genetic constraints on loss-of-function variations, 4 genes, including ABCA2 and ABCE1, as well as ABCB7 and ABCD1 were considered as haploinsufficient, whereas little constraint on loss-of-function variations was detected in the remaining 44 ABC transporters (Supplementary Figure 2B; Supplementary Table 1).
In addition to SNVs, 46 of the 48 ABC transporter genes (96%) harbored CNVs, in which multiple exons up to the entire were deleted or duplicated (Fig. 1e). Overall, most CNVs were detected for ABCC6 (230 CNVs), ABCC1 (178 CNVs), and ABCA6 (81 CNVs), whereas no CNVs were identified in ABCB7 and ABCD1. While these CNVs are very likely to result in functional alterations, all deletions and duplications were found to be very rare with minor allele frequencies < 0.1%.
Worldwide frequencies of human ABC transporter polymorphisms with putative clinical relevance
Next, we systematically analyzed the global and population-specific frequencies of clinically important variants in ABC transporters linked to drug response or ADR risk. Specifically, we considered all variants as putatively clinically relevant for which an association with drug-response phenotypes or related traits, such as overall or disease-specific survival upon chemotherapy, have been reported. In ABCB1, we assessed the population frequencies of 10 SNPs (Table 1). The missense variant rs2032582 and the synonymous polymorphisms rs1045642 constitute arguably the most extensively studied ABCB1 variants and have been associated with risk of adverse reactions upon fluoropyrimidine therapy (Gonzalez-Haba et al. 2010) as well as toxicity to taxanes (Kim et al. 2012b) and anthracyclines (Ji et al. 2012; Wu et al. 2012). These variants are in strong linkage disequilibrium (Horinouchi et al. 2002) and have been shown to be associated with altered mRNA levels and protein folding (Cascorbi 2006). Rs2032582 constitutes a triallelic variant of amino acid position 893 with the reference sequence encoding an alanine and variants giving rise to a serine or threonine, respectively (Supplementary Figure 3). Ala893 is the predominant allele in Africans and East Asians, whereas in South Asians, Ser893 is most abundant (frequency 60.9% compared to 34.8% for Ala893). Thr893 is less prevalent ranging in frequencies between 0.4% in Africans and 13.3% in East Asians. Further ABCB1 variants of clinical relevance are the missense variants rs2229109 and rs9282564, which are associated with increased risk of relapse of acute lymphoblastic leukemia (Gregers et al. 2015) and paclitaxel toxicity (Bergmann et al. 2012), respectively. Both variants are most frequently found in Europeans (MAF = 4.3% and 10.8%) and least prevalent in Africans (MAF = 0.7% and 1.6%) and East Asians (MAF = 0 and < 0.1%). Linkage analyses revealed one haplotype block of four SNPs (rs1128503, rs4148737, rs12720066 and rs1045642) with moderate-linkage disequilibrium, which could have potentially important implications for clinical associations of these variants (Supplementary Figure 4A).
Table 1.
Population-specific frequencies of clinically important ABCB1 (MDR1; P-gp) variants
| Variant | Type | Minor allele frequencies (in %) | Clinical association of the minor allele | Effect or statistic | References | Sample size | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EUR | AFR | EAS | SAS | AMR | AJ | ||||||
| rs2032582 (triallelic) | Missense (A893S or A893T) | A: 54.7; S: 41.8; T: 3.5 | A: 91.8; S: 7.8; T: 0.4 | A: 47.8; S: 38.9; T: 13.3 | A: 34.8; S: 60.9; T: 4.3 | A: 54.6; S: 40; T: 5.4 | A: 62.4; S: 35; T: 2.6 | Major molecular response to imatinib in chronic myeloid leukemia | 78% of carriers achieved major molecular response versus 47.1% of non-carriers | Dulucq et al. (2008) | 86 |
| Toxicity of taxanes and platinum compounds in ovarian cancer patients | OR = 3.1 and 9.7 for hematological and gastrointestinal toxicity, respectively | Kim et al. (2009b) | 108 | ||||||||
| PFS in gastric cancer patients treated with paclitaxel | HR = 2.6 | Chang et al. (2010) | 43 | ||||||||
| OS in in metastatic colorectal cancer patients receiving first-line FOLFIRI treatment | 12-month survival of 78% in variant carriers compared to 70% in controls | De Mattia et al. (2013) | 250 | ||||||||
| Toxicity of induction chemotherapy (idarubicin plus cytarabine) in acute myeloid leukemia | OR = 2.9 and 5.1 for hepatic and renal ADRs, respectively | Megías-Vericat et al. (2017) | 221 | ||||||||
| Decreased response to modafinil in narcolepsy patients | OR = 0.28 | Moresco et al. (2016) | 107 | ||||||||
| rs1128503 | Synonymous | 43.3 | 18.9 | 64.3 | 60.7 | 49.1 | 36.7 | Toxicity of capecitabine in colorectal cancer patients | OR = 4.3 and 5.3 for neutropenia and HFS, respectively | Gonzalez-Haba et al. (2010) | 54 |
| Major molecular response to imatinib in chronic myeloid leukemia | 85% of hom carriers achieved major molecular response versus 47.7% | Dulucq et al. (2008) | 85 | ||||||||
| Toxicity of gefitinib in advanced NSCLC patients | OR = 15.8 and 10.8 for skin rash and diarrhea, respectively | Ma et al. (2017) | 59 | ||||||||
| Toxicity of induction chemotherapy (idarubicin plus cytarabine) in acute myeloid leukemia | OR = 6.9 and 3.8 for hepatic and renal ADRs, respectively | Megías-Vericat et al. (2017) | 225 | ||||||||
| Decreased response to FEC breast cancer chemotherapy | OR = 4.6 | Chaturvedi et al. (2013) | 100 | ||||||||
| Decreased toxicity to FEC breast cancer chemotherapy | OR = 1.9 for grade 2–4 toxicity | Chaturvedi et al. (2013) | 200 | ||||||||
| Decreased response to modafinil in narcolepsy patients | OR = 0.31 | Moresco et al. (2016) | 107 | ||||||||
| rs2229109 | Missense (S400N) | 4.3 | 0.7 | 0 | 1.5 | 1.7 | 2.8 | Increased risk of relapse of acute lymphoblastic leukemia patients to chemotherapy | OR = 2.9 of carriers vs. controls | Gregers et al. (2015) | 518 |
| rs1045642 | Synonymous | 53.4 | 20 | 36.7 | 39.5 | 45.4 | 35.6 | Bone marrow toxicity during doxorubicin, vincristine and prednisolone induction therapy | p = 0.01 (control vs. het) and p < 0.0001 (control vs. hom) | Gregers et al. (2015) | 517 |
| Increased exposure and toxicity of methotrexate in acute lymphoblastic leukemia or non- Hodgkin lymphoma patients | OR = 2.5 and 8.6 of carriers for plasma levels and hepatic toxicity, respectively | Suthandiram et al. (2014) | 71 | ||||||||
| PFS in gastric cancer patients treated with paclitaxel | HR = 4.6 | Chang et al. (2010) | 43 | ||||||||
| Protective effect on arthralgia upon anastrozole therapy in postmenopausal breast cancer patients | OR = 0.3 | Gervasini et al. (2017) | 78 | ||||||||
| Increased response to modafinil in narcolepsy patients | OR = 0.21 when comparing hom vs het carriers | Moresco et al. (2016) | 107 | ||||||||
| rs9282564 | Missense (N21D) | 10.8 | 1.6 | < 0.1 | 2.4 | 2.9 | 2.4 | Associated with toxicity of paclitaxel and carboplatin therapy in ovarian cancer patients in exploratory analysis | p = 0.03 | Bergmann et al. (2012) | 92 |
| Decreased serum tacrolimus levels after kidney transplantation | p = 0.001 | Hu et al. (2018) | 163 | ||||||||
| rs3213619 | 5′ UTR | 4.0 | 8.4 | 3.8 | N.A | 4.7 | 3.0 | Decreased risk of neuropathies in breast cancer patients treated with paclitaxel | OR = 0.47 | Abraham et al. (2014) | 1303 |
| Increased atenolol efficacy | p = 0.0002 | McDonough et al. (2013) | 768 | ||||||||
| rs12720066 | Intron | 5.5 | 1 | 0 | N.A | 3.5 | 5.2 | Decreased risk of irinotecan-induced neutropenia | β = 0.286 | Li et al. (2018b) | 78 |
| rs4148737 | Intron | 42.9 | 44.9 | 28.9 | N.A | 41.4 | 48.6 | Reduced OS of osteosarcoma patients after chemotherapy | HR = 3.7 per allele | Caronia et al. (2011) | 91 |
| rs3842 | 3′UTR | 13.6 | 16.9 | 26.2 | N.A | 14.2 | 17.5 | Increased clearance of efavirenz in HIV-1 patients | p = 0.001 | Mukonzo et al. (2014) | 99 |
| rs10267099 | Intron | 77.1 | 83.2 | 99.7 | N.A | 86.3 | 73.5 | Decreased response to atenolol | p = 0.006 | McDonough et al. (2013) | 768 |
PFS progression-free survival, OS overall survival, OR odds ratio, EUR Europeans, AFR Africans, EAS East Asians, SAS South Asians, AMR Latinos, AJ Ashkenazi Jews, N.A. not available
In the ABCC subfamily, we analyzed the population-specific frequencies of 25 SNVs that were correlated with chemotherapy outcomes or toxicity (Table 2). Interestingly, frequencies of risk variants for anthracycline-induced cardiotoxicity (ACT) were highly population-specific and differed > 100-fold between populations. The cardioprotective synonymous variant rs246221 in ABCC1 (Semsei et al. 2012) was most common with frequencies between 20.3% and 65.2% in South Asians and Africans, respectively. By contrast, East Asians did not harbor the risk variants rs8187710 (ABCC2) and rs45511401 (ABCC1), which are common in all other populations with frequencies up to 5.6% and 15.7%, respectively. Notably, rs45511401 is in linkage disequilibrium with the intronic ACT risk variant rs4148350 (R2 = 0.153; Supplementary Figure 4B), indicating that both associations might to some extent be traced back to the same genetic signal.
Table 2.
Population-specific frequencies of clinically important variants in genes of the ABCC subfamily
| Variant | Type | Minor allele frequencies (in %) | Clinical association of the minor allele | Effect or statistic | References | Sample size | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EUR | AFR | EAS | SAS | AMR | AJ | ||||||
| ABCC1 (MRP1) | |||||||||||
| rs45511401 | Missense (G671V) | 5.6 | 1.2 | < 0.1 | 1.6 | 1.7 | 3.3 | Increased risk of anthracycline-induced cardiotoxicity | OR = 3.6 | Wojnowski et al. (2005) | 42 |
| rs4148350 | Intron | 7.3 | 10.4 | 4.0 | N.A | 8.4 | 6.6 | Increased risk of anthracycline-induced cardiotoxicity | OR = 3.4 | Visscher et al. (2012) | 156 and 188 and 96 |
| Toxicity of induction chemotherapy (idarubicin plus cytarabine) in acute myeloid leukemia | OR = 5.3 for grade 3–4 hepatic toxicity | Megías-Vericat et al. (2017) | 225 | ||||||||
| rs246221 | Synonymous | 30.5 | 65.2 | 42.5 | 20.3 | 35.2 | 32.1 | Decreased risk of anthracycline-induced cardiotoxicity | Increased LVFS of homozygous carriers (40.7%) compared to het and controls (38.4%) | Semsei et al. (2012) | 164 |
| rs3743527 | 3′ UTR | 22.6 | 14.3 | 45.7 | N.A | 27.0 | 23.8 | Increased risk of anthracycline-induced cardiotoxicity | Decreased LVFS of homozygous carriers (34%) compared to het and controls (39.3%) | Semsei et al. (2012) | 168 |
| rs17501331 | Intron | 10.3 | 2.2 | 0 | N.A | 5.8 | 11.7 | Increased risk of irinotecan-induced neutropenia | β = − 0.295 | Li et al. (2018b) | 78 |
| rs212091 | 3′UTR | 14.9 | 13.0 | 25.4 | N.A | 10.2 | 9.7 | Virological failure of protease inhibitor regimens in HIV patients | OR = 4.4 | Coelho et al. (2013) | 87 |
| rs119774 | Intron | 6.8 | 1.6 | 0.3 | N.A | 4.4 | 6.9 | Increased response to montelukast in asthma | p = 0.004 when comparing hom vs het carriers | Lima et al. (2006) | 49 |
| rs2074087 | Intron | 84.4 | 81.5 | 82.5 | 68.2 | 78.9 | 77.0 | Increased risk of azathioprine-induced lymphopenia | OR = 3.4 | Lee et al. (2015) | 131 |
| ABCC2 (MRP2) | |||||||||||
| rs8187710 | Missense (C1515Y) | 5.6 | 15.7 | < 0.1 | 1.9 | 4.1 | 12.9 | Increased risk of anthracycline-induced cardiotoxicity | OR = 2.3 | Wojnowski et al. (2005) | 44 |
| rs3740065 | Intron | 9.7 | 21.6 | 34.1 | N.A | 14.0 | 17.2 | Response of patients with invasive breast cancer to tamoxifen monotherapy | HR = 10.6 | Kiyotani et al. (2010) | 282 |
| rs3740066 | Synonymous | 37 | 25.9 | 23 | 32.6 | 34.9 | 34.5 | Severe toxicity of irinotecan in NSCLC patients | OR = 5.6 | Han et al. (2009) | 107 |
| rs12762549 | Intergenic | 46.8 | 43.0 | 56.5 | N.A | 49.3 | 51.7 | Leukopenia risk upon docetaxel therapy | OR = 3.1 | Kiyotani et al. (2008) | 113 |
| Response of NSCLC patients to second line docetaxel therapy | OR = 7.3 | Szczyrek et al. (2017) | 52 | ||||||||
| rs717620 | 5′ UTR | 19.9 | 5.8 | 21.4 | 11.3 | 13.0 | 20.0 | Poor response and reduced OS of SCLC patients undergoing etoposide and/or platinum-based therapy | HR = 2.1 and 1.9 for response and OS, respectively | Campa et al. (2012) | 167 and 127 |
| rs17222723 | Missense (V1188E) | 5.6 | 6.0 | < 0.1 | 1.8 | 3.6 | 12.8 | Increased response of esophageal cancer patients to platinum-based therapy | OR = 0.21 of carriers compared to controls | Rumiato et al. (2016) | 116 |
| ABCC3 (MRP3, MOAT-D) | |||||||||||
| rs1051640 | Synonymous | 18.2 | 8.6 | 5.6 | 10.3 | 8.6 | 18.7 | Increased risk of cisplatin-induced hearing loss | OR = 1.8 | Pussegoda et al. (2013) | 247 |
| rs4148416 | Synonymous | 5.4 | 19.6 | 15.0 | 8.8 | 14.4 | 5.8 | Reduced OS of osteosarcoma patients after chemotherapy | HR = 8.1 per allele | Caronia et al. (2011) | 91 |
| Poor response to chemotherapy in osteosarcoma patients | OR = 3.8 | Yang et al. (2013) | 45 | ||||||||
| rs4148405 | Intron | 14 | 43.7 | 21.7 | N.A | 21.8 | 21 | Shorter disease-free survival in acute myeloid leukemia patients treated with cytarabine and etoposide | HR = 3.2 | Yee et al. (2013) | 153 |
| ABCC4 (MRP4, MOAT-B) | |||||||||||
| rs2274405 | Synonymous | 34.1 | 30.3 | 47.1 | 36.1 | 42.7 | 46.4 | Increased response of esophageal cancer patients to platinum-based therapy | OR = 0.56 and 0.15 of het and hom carriers, respectively, compared to controls | Rumiato et al. (2016) | 116 |
| ABCC5 (MRP5, MOAT-C) | |||||||||||
| rs3749438 | 3′ UTR | 36.1 | 26.2 | 40.6 | N.A | 28.4 | 39.7 | Increased risk of irinotecan-induced severe toxicity in metastatic colorectal cancer patients | OR = 1.9–2.1 | Chen et al. (2015a) | 452 and 322 |
| rs10937158 | Intron | 54.4 | 74.7 | 86.0 | N.A | 51.0 | 53.3 | Decreased risk of irinotecan-induced severe toxicity in metastatic colorectal cancer patients | OR = 0.4–0.45 | Chen et al. (2015a) | 328 and 448 |
| rs7627754 | Promoter | 11.4 | 36.0 | 34.9 | N.A | 18.5 | 7.6 | Increased risk of doxorubicin-induced cardiotoxicity | 8–12% reduction of ejection and shortening fractions of hom carriers | Krajinovic et al. (2016) | 251 |
| rs7636910 | Synonymous | 36.9 | 26.8 | 41.5 | 36.4 | 26.9 | 40.9 | Increased response and OS in pancreatic adenocarcinoma patients treated with gemcitabine-based chemoradiotherapy | OR = 1.7 | Tanaka et al. (2011) | 261 |
| ABCC6 (MRP6, MOAT-E) | |||||||||||
| rs2238472 | Missense (R1268Q) | 28.2 | 10.1 | 12.3 | 18.2 | 31.3 | 17.0 | Increased toxicity of docetaxel and thalidomide in castration-resistant prostate cancer patients | p = 0.006 | Deeken et al. (2009) | 47 |
| ABCC10 (MRP7) | |||||||||||
| rs2125739 | Missense (I948T) | 25.1 | 31.9 | 10.9 | 18.0 | 18.7 | 21.3 | Associated with nausea of paclitaxel and carboplatin therapy in ovarian cancer patients in exploratory analysis | p = 0.002 | Bergmann et al. (2012) | 92 |
| Increased OS in CRC patients who received oxaliplatin- based chemotherapy | OR = 0.56 | Kap et al. (2016) | 623 | ||||||||
| ABCC11 (MRP8) | |||||||||||
| rs17822931 | Missense (G180R) | 13 | 2.8 | 87 | 40.6 | 16.2 | 10.8 | Reduced MRP8 expression and increased disease-free survival upon nucleoside-based chemotherapy | p < 0.03 | Guo et al. (2009) and Uemura et al. (2010) | / |
CRC colorectal cancer, OR odds ratio, LVFS left-ventricular fraction shortening, OS overall survival, NSCLC non-small cell lung cancer, EUR Europeans, AFR Africans, EAS East Asians, SAS South Asians, AMR Latinos, AJ Ashkenazi Jews, N.A. not available
Multiple ABCC variants associated with irinotecan (rs3740066 in ABCC2, rs4148405 in ABCC3 as well as rs3749438 and rs10937158 in ABCC5) or taxane (rs12762549 in ABCC2 as well as rs2238472 and rs2125739 in ABCC6) toxicity or response were overall less population-specific and differed only by < 3-fold across populations with the exception of rs17501331 in ABCC1, which was not identified in East Asians (MAF = 0%) but reached frequencies of 11.7% and 10.3% in Ashkenazim and Europeans. By contrast, variants associated with response to platinum-based therapy differed substantially between ethnicities, including rs717620 (MAF between 21.4% in East Asians and 5.8% in Africans), rs17222723 (MAF between 12.8% in Ashkenazi Jews and < 0.1% in East Asians), and rs1051640 (MAF between 18.7% in Jews and 5.6% in East Asians). MRP8 encoded by ABCC11 is an export pump for nucleotide analogues (Oguri et al. 2007) and is associated with pemetrexed resistance (Uemura et al. 2010). The variant rs17822931 that results in proteasomal degradation of MRP8 (Toyoda et al. 2009) differs > 30-fold between populations with relatively low frequencies in Africans (MAF = 2.8%), whereas the variant constitutes the dominant genotype in East Asian populations (MAF = 87%).
The ABCG2 gene, encoding the BCRP transporter, harbors two important missense polymorphisms, which have been consistently implicated in response and toxicity of TKIs (Table 3). Rs2231142 results in increased risk of gefitinib toxicity (Cusatis et al. 2006) and increased rates of major molecular response to imatinib (Jiang et al. 2017). Similar effects on response and overall survival were found for rs2231142 (Chen et al. 2015b; Kim et al. 2009a), which is not linked with rs2231137 (Supplementary Figure 4C). Notably, both variants were most prevalent in East Asian and Latin Americans, whereas their frequencies were substantially lower in all other populations analyzed. Only a few associations of pharmacological or toxicological phenotypes with genetic variants in ABC transporters beyond ABCB1, ABCG2, and the ABCC subfamily have been presented to date (Supplementary Table 2).
Table 3.
Population-specific frequencies of clinically important variants in ABCG2 (BCRP)
| Variant | Type | Minor allele frequencies (in %) | Clinical association of the minor allele | Effect or statistic | Reference | Sample size | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EUR | AFR | EAS | SAS | AMR | AJ | ||||||
| rs2231135 | 5′ UTR | 7.0 | 2.0 | 0 | N.A | 4.3 | 4.0 | Increased risk of mucositis in osteosarcoma patients treated with high-dose methotrexate | OR = 2.5 for grade 2–3 mucositis in carriers compared to control | Jabeen et al. (2015) | 57 |
| rs2231142 | Missense (Q141K) | 10.4 | 2.7 | 30.7 | 9.3 | 22.6 | 6.6 | Increased diflomotecan exposure | Plasma levels increased threefold in het carriers compared to controls | Sparreboom et al. (2004) | 22 |
| Gefitinib toxicity in NSCLC patients | OR = 5.7 for dose-limiting diarrhea | Cusatis et al. (2006) | 124 | ||||||||
| Higher rate of major molecular response to imatinib therapy (meta analysis) | OR = 0.65 | Jiang et al. (2017) | 2184 | ||||||||
| Increased PFS of advanced stage ovarian cancer patients treated with platinum and taxane-based chemotherapy | 22.7 months PFS in carriers versus 16.8 months in controls | Tian et al. (2012) | 506 | ||||||||
| Decreased response of allopurinol | p = 3.4 × 10–7 | Wen et al. (2015) | 2027 | ||||||||
| rs2231137 | Missense (V12M) | 4.1 | 6.6 | 32.8 | 14.0 | 23.7 | 10.5 | Severe toxicity of irinotecan in NSCLC patients | OR = 5.1 | Han et al. (2009) | 107 |
| Improved response to imatinib therapy in chronic myeloid leukemia patients | OR = 0.64 for complete cytogenetic response in carriers compared to controls | Kim et al. (2009a) | 229 | ||||||||
| Longer OS in NSCLC patients receiving TKI therapy | 31 months OS in carriers versus 18 months in controls | Chen et al. (2015b) | 70 | ||||||||
| Improved treatment outcomes in acute myeloid leukemia patients receiving cytarabine or anthracyclines | HR = 0.44 for OS | Hampras et al. (2010) | 261 | ||||||||
| Increased toxicity in acute myeloid leukemia patients receiving cytarabine or anthracyclines | OR = 8.4 | Hampras et al. (2010) | 261 | ||||||||
| rs7699188 | Intron | 15.6 | 44.1 | 7.6 | N.A | 13.2 | 23.1 | Toxicity in in metastatic colorectal cancer patients receiving first-line FOLFIRI treatment | OR = 7.3 | De Mattia et al. (2013) | 250 |
| rs3109823 | Intron | 71.8 | 44 | 78.8 | N.A | 80.6 | 83.4 | Improved response and OS of SCLC patients undergoing etoposide and/or platinum-based therapy | OR = 0.3 and 0.6 for response and OS, respectively | Campa et al. (2012) | 171 |
| rs13120400 | Intron | 30.5 | 6.2 | 0 | N.A | 14.7 | 17.4 | Increased blood concentration of deferasirox | OR = 4.1 | Allegra et al. (2016) | Not reported |
| rs2199936 | Intron | 89.4 | 87.6 | 67.1 | N.A | 80.9 | 92.8 | Decreased Increased response to rosuvastatin | Effect of + 5.2 mg/dl | Chasman et al. (2012) | 6989 |
| rs4148155 | Intron | 10.5 | 2.3 | 32.8 | N.A | 18.6 | 6.6 | Increased response to allopurinol | p = 7.89 × 10–9 | Brackman et al. (2019) | 4446 |
PFS progression-free survival, OR odds ratio, NSCLC non-small cell lung cancer, SCLC small cell lung cancer, TKI tyrosine kinase inhibitor, OS overall survival, EUR Europeans, AFR Africans, EAS East Asians, SAS South Asians, AMR Latinos, AJ Ashkenazi Jews, N.A. not available
Functional consequences of rare genetic variation in human ABC transporters
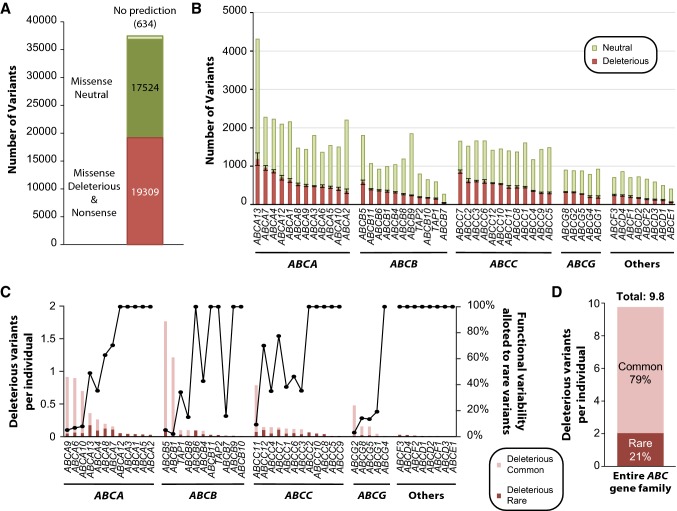
Next, we aimed to estimate the functional importance of rare ABC variations for which no experimental analyses or clinical association data were available. To this end, we used five partly orthogonal algorithms to predict the functional consequences. Of all 37,467 variants affecting the amino acid sequence of the encoded polypeptide, 19,309 variants (51.5%) were predicted to result in functional alterations of the respective ABC transporter (Fig. 2a; see methods). While functional effects can comprise both, variations that result in increased or decreased transporter function, previous studies showed that computational algorithms are significantly better at predicting loss-of-function effects compared to gain-of-function effects (Flanagan et al. 2010). We thus refer to variants with putative functional impacts as “deleterious” throughout this manuscript; however, we would like to alert the reader that the inclusion of some variants that result in increased transporter function cannot be excluded. Most deleterious variants were found in ABCA13 (n = 1183), ABCA7 (n = 953), and ABCA4 (n = 865), whereas ABCE1 (n = 60) and ABCB7 (n = 43) harbored least (Fig. 2b). The multi-drug resistance transporters ABCB1 (n = 344), ABCC1 (n = 453), and ABCG2 (n = 315) harbored medium numbers of variants with functional consequences.
Fig. 2.
ABC transporter genes harbor a plethora of genetic variants with functional consequences, many of which are rare. a In total, 37,467 variants affected the amino acid sequence of the corresponding gene product (missense and frameshift variants, variants that resulted in gain of a stop or loss of a start codon or that affected splice sites) of which 19,309 were predicted to result in functional consequences. b The number of deleterious and functionally neutral variants differs drastically between ABC transporter genes. Error bars indicate standard error of the mean (SEM) across five computational algorithms (see methods for details). c The average number of deleterious variants per ABC transporter are shown per individual (stacked columns; left ordinate). Note that the relative importance of rare genetic variations with frequencies < 1% differs substantially between genes (indicated by black dots; right ordinate). Calculations consider a diploid human genome. d Overall, each individual was found to harbour on average 9.8 genetic variations in the ABC transporter superfamily that affect transporter function. Rare variants accounted for 21% of this genetically encoded functional variability
Notably, only 14.8% (30 of 203) of common ABC missense variants with MAF > 1% were putatively deleterious, compared to 45.7% (15,152 of 33,137) for rare variations. The burden of functional genetic variability differed drastically between genes with an average diploid human genome harboring on average 1.8 and 1.2 variants with functional effects in ABCB5 and ABCB1, respectively, whereas 29 transporters were highly conserved with < 0.1 functional variants per individual genome (Fig. 2c). In some transporters, including ABCB1 and ABCG2, rare variations explained less than 10% of the genetically encoded functional variability. In contrast, rare variants are estimated to account for all variants with functional consequences in half (24 out of 48) of all human ABC transporter genes. Interestingly, the fraction of genetically encoded functional variability correlated significantly with the genetic constraint on the respective genes (r = 0.4; p = 0.005), suggesting that high evolutionary pressure tends to select against common variations that alter ABC transporter function. Overall, each individual was found to harbour 9.8 variants in the ABC gene family that entail functional alterations, of which 21% were attributed to by rare genetic variants (Fig. 2d).
Genetic ABC transporter variability is highly population-specific
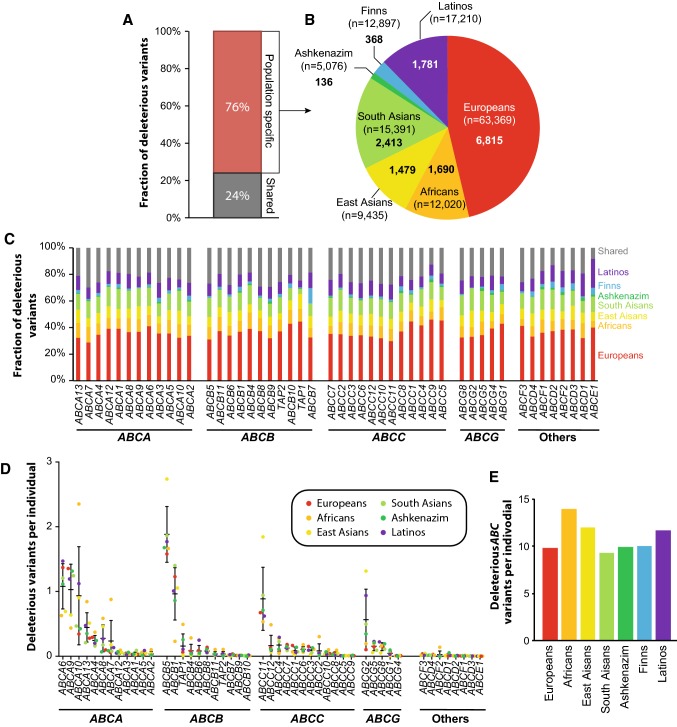
The genetic landscape of the ABC transporter superfamily differed considerably between human populations. Of the putatively deleterious variants, only 24% were shared between two or more ethnicities, whereas 76% were population-specific (Fig. 3a). Most population-specific variants were found in Europeans (6815), whereas least were found in Ashkenazim (136). These differences are likely, at least in part, due to the unequal distribution of available sequencing data and the differences in genetic heterogeneity between the populations (Fig. 3b). The ratios of population-specific variants differed between ABC genes from 70% in ABCA7 to 92% in ABCE1, whereas only 0.3% of variants were shared between all seven populations (Fig. 3c).
Fig. 3.
The genetically encoded functional variability of ABC transporters is highly population-specific. a The majority of genetic variations (76%) with putative functional impacts on ABC transporter function are population-specific. b Most of these population-specific variations were identified in Europeans. Numbers in bold indicate the total number of identified population-specific variations, while numbers in brackets denote the number of sequenced individuals for the respective population. c Stacked column plot showing the fraction of putatively functional variants specific to Europeans (red), Africans (orange), East Asians (yellow), South Asians (light green), Ashkenazi Jews (dark green), Finns (blue), and Latinos (purple). The fraction of variations that are found in at least two populations are shown in grey. d The number of ABC variants with functional consequences per individual is shown across populations. e Column plot depicting the functional ABC transporter variability when all putatively deleterious ABC transporter variants are aggregated. Note that African individuals harbour most functionally relevant ABC variants per individual, whereas functional variability in South Asians was overall lowest
The observed population specificity is estimated to translate into inter-ethnic differences in ABC transporter function. The largest differences in variants with putative functional impacts across populations were identified for ABCA10 where Africans harbor 2.4 putatively functional variations per individual compared to 0.3 in Europeans (Fig. 3d). Similar differences were observed for the breast cancer risk gene ABCC11 (1.8 in East Asians compared to 0.5 in Africans), as well as the multi-drug resistance genes ABCB1 (1.4 in South Asians compared to 0.2 in Africans) and ABCG2 (1.3 in East Asians compared to 0.1 in Europeans). In contrast, inter-ethnic variability in ABCC1 was less pronounced (0.16 in Europeans compared to 0.02 in East Asians). Overall, across the entire ABC transporter family Africans harbored most variations with putative functional impacts (13.9 deleterious variants per individual), whereas least variations were observed in South Asians (9.3 deleterious variants per individual; Fig. 3e).
Structural consequences of genetic ABC variability
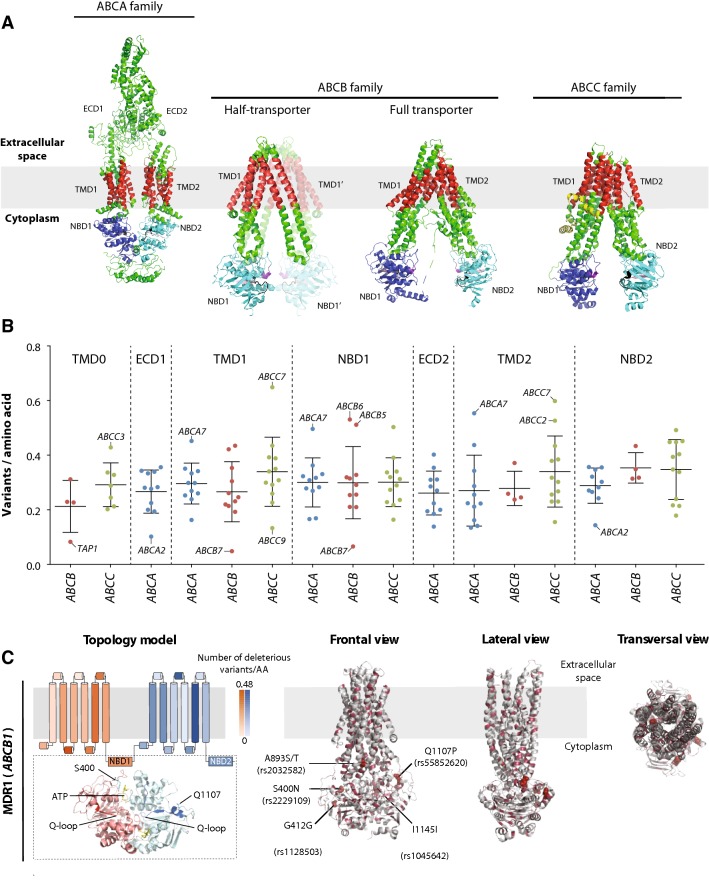
Next, we characterized the distribution of genetic variability across ABC transporter domains by mapping the identified genetic variants onto the tertiary structures of the respective. We used experimentally determined crystal structures for all transporters of the ABCA, ABCB, and ABCC families for which such information was available (n = 18), while the remaining 16 structures were predicted using homology modeling. Typical ABC transporters consist of two α-helix transmembrane domains (TMDs) and two cytoplasmic nucleotide-binding domains (NBDs) that catalyse ATP hydrolysis (Fig. 4a). In addition to this backbone, some transporters have additional domains. ABCA transporters have two large extracellular domains (ECDs), while transporters of the ABCB and ABCC subfamilies contain an additional N-terminal TMD0 domain with unclear functional relevance. Furthermore, seven ABC genes of the ABCB subfamily encode only half-transporters (one NBD and one TMD domain) that require homo- or heterodimerization for transporter activity.
Fig. 4.
Structural analysis of putatively deleterious genetic variants of ABC transporter superfamily. a Illustration of the tertiary structures of ABCA, ABCB, and ABCC transporters. As representative examples, the structures of ABCA1 (PDB identifier 5XJY), ABCB10 (ABCB half transporter; PDB identifier 4AYT), ABCB11 (BSEP; ABCB full transporter), and ABCC7 (CFTR; PDB identifier 5UAK) are shown. Transmembrane domains (TMDs) are shown in red, nucleotide-binding domains (NBDs) are depicted in blue and turquoise, Walker motifs are colored in salmon and the N-terminal Lasso motif is depicted in yellow. b Overview of the genetically encoded structural variability stratified by ABC subfamily and domain. c Schematic topology models as well as 3D protein structures of MDR1 encoded by ABCB1. Different domains in the topology models are shaded based on the identified number of deleterious variants per amino acid in the respective domain. MDR1 constitutes two pseudo-symmetrical TMDs and NBDs encoded in a single polypeptide, colored in orange and blue, respectively. Detailed 3D structure of key protein domains with functionally relevant variants (sticks in cyan or magenta) and substrates (sticks in yellow) are shown as insets under the topology model. In the 3D model, all putatively deleterious variants with MAF > 0.1% are shown as light red spheres, whereas the corresponding part of the secondary structure motif is highlighted in salmon in case of variants with MAF < 0.1%. Note that N21D localizes to the lasso motif for which no crystallographic data were available and the variant is thus not shown. ECD extracellular domain, TMD transmembrane domain, NBD nucleotide-binding domain
When stratifying by domains, we found that genetic variability differed substantially between transporters (Fig. 4b). The lowest numbers of variants per residue were found in the TMD0 domains of ABCB transporters with 0.21 variants/amino acid. In contrast, the NBD2 domains of ABCB and ABCC transporters are more variable (0.35 variants/amino acid). For individual genes, the TMD1 (0.05 variants/amino acid) and NBD1 domains (0.07 variants/amino acid) of ABCB7 were most conserved, while the TMD1 and TMD2 domains of ABCC7 (0.65 variants/amino acid) and ABCA7 (0.56 variants/amino acid), respectively, were > 10-fold more variable.
Finally, we aimed to corroborate our computational variant predictions using structural mapping approaches by focussing on the pharmacogenetically most important ABC transporter, MDR1 (also known as P-gp; encoded by ABCB1), for which high-resolution crystal structures are available (Kim and Chen 2018) (Fig. 4c). The clinically important missense variation A893S/T is located in the second intracellular loop of TMD2, which interacts with NBD1, and is necessary for structural stability. The S400N polymorphism is localized directly adjacent to the critical tyrosine at position 401, which coordinates the ATP in its binding pocket in NBD1 by direct van-der-Waals interactions with the adenine of the bound ATP molecule. Q1107P resides within the NBD2 Q-loop, which is necessary for ATPase activity and stabilizes the NBD dimer. No common variants were identified in any transmembrane helix or extracellular domain. However, we found a variety of rare variations in structurally important residues, including variants at the catalytic glutamate residue 556, which is required for ATP hydrolysis (Sauna et al. 2002), as well as various amino acid exchanges in the functionally critical NBD1 and NBD2 Q-loops (Zolnerciks et al. 2014).
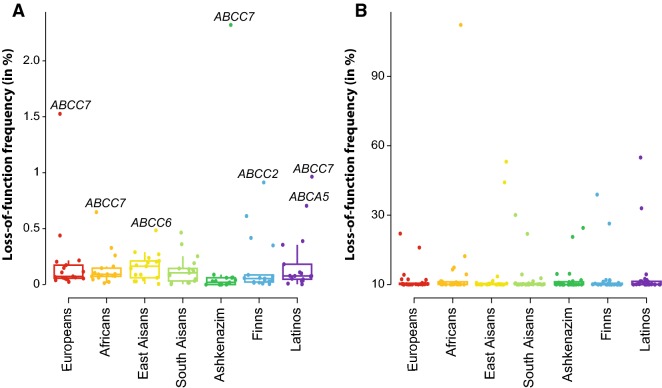
Ethnogeographic distribution of pathogenic ABC alleles can inform about Mendelian disease epidemiology
We previously showed that the frequency of loss-of-function variants in SLC transporter genes implicated in recessive Mendelian disorders are suitable proxies to estimate population-specific disease risk (Schaller and Lauschke 2019). Here, we analyzed whether similar associations could be identified for ABC transporter genes. To this end, we comparatively analyzed the frequencies of loss-of-function variants, defined as frameshifts, start-lost or stop-gain variations or variants that affected critical splice site residues, in ABC transporter genes with or without implication in hereditary disease (Fig. 5).
Fig. 5.
Genetic variability in ABC genes associated with genetic disorders can inform about population-specific disease risk. The gene-wise aggregated frequencies of loss-of-function (LoF) variants (frameshifts, start-lost, stop-gain, and splice site variants) are shown for ABC genes with known associations with congenital diseases (a) as well as for non-disease-associated genes (b)
Overall, 17 of 48 ABC genes are linked to autosomal recessive Mendelian disorders (Supplementary Table 3). Reduced CFTR (ABCC7) function is associated with cystic fibrosis (CF; OMIM 219700). We calculated homozygosity frequencies for ABCC7 loss-of-function variants of 1 in 1850 and 1 in 4300 in Ashkenazim and European individuals, whereas frequencies in individuals of Africans and Asian ancestry were 1 in 24,000 and < 1 in 40,000, respectively. Impaired function variants in ABCC6 are associated with pseudoxanthoma elasticum (PXE; OMIM 264800). In our data set, we find the highest aggregated ABCC6 loss-of-function frequency in individuals of East Asian ancestry (0.5%), resulting in estimates of affected individuals of 1 in 42,530. Similarly, high carrier rates were identified in Europeans (0.4%; 1 in 52,000) and Finns (0.4%; 1 in 82,000), whereas risk allele prevalence was significantly lower in all other populations. Congenital generalized hypertrichosis (OMIM 135400) is a rare disease with varying presentations and comorbidities that is speculated to be, at least in part, caused by loss of ABCA5 function (DeStefano et al. 2014). While global prevalence rates have, to our knowledge, not been reported, the disease was originally described in individuals of Mexican ancestry (Pavone et al. 2015), aligning with our finding of highest ABCA5 loss-of-function frequencies in Latino populations (0.7%; 1 in 20,500).
In conclusion, these data provide an overview of the frequency of ABC loss-of-function variants in the general population that can be used to estimate population-specific Mendelian disease risk, thus providing valuable information for epidemiological rare disease research and clinical geneticists.
Discussion
The ABC superfamily of transporters is of importance for drug response and toxicity, and genetic rare disease research. ABC transporters translocate a wide spectrum of endogenous substrates and medications. Consequently, identification of ABC transporters that interact with a drug candidate constitutes a critical step in drug discovery and development (Benadiba and Maor 2016; Yee et al. 2018). Previous clinical studies implicated genetic germline polymorphisms in at least 12 ABC genes with risk of adverse drug reactions or altered chemotherapy efficacy (Tables 1, 2, 3 and Supplementary Table 2). In addition, genetic variations in 21 ABC genes are causative for Mendelian disorders. Therefore, understanding the genetic landscape of ABC transporters constitutes a potentially important area for the personalization of oncological therapy and risk allele epidemiological study of relevant Mendelian diseases.
In this study, we detected a total of 62,793 exonic variants, the vast majority (98.5%) of which are rare and functionally poorly understood. In addition to these single-nucleotide variants and indels, we identified 1003 ABC alleles in which at least one exon was deleted or duplicated. Notably, somatic ABC gene CNVs have been implicated in acquired drug resistance. Studies of drug-resistant cell lines derived from human neoplasms identified amplifications of at least 13 ABC transporter genes, including ABCB1, ABCC1 and ABCC4 (Yasui et al. 2004). Conversely, deletions of the multi-drug resistance transporters predicted response to neoadjuvant therapy in breast cancer patients (Litviakov et al. 2016). Notably, while drug resistance is primarily characterized by somatic amplification events, the majority of CNVs in our data set were deletions and it will be interesting to observe whether patients with germline deletions of pharmacologically important drug transporters are predisposed to favorable therapeutic responses using drugs, which are substrates of the deleted transporter.
There is an increasing body of evidence describing differences in drug response, ADRs and clinical outcomes from chemotherapy based on genetic differences between ethnic groups (Phan et al. 2011). For instance, Caucasian colon cancer patients were at significantly higher risk to develop diarrhea, nausea, vomiting, and stomatitis during adjuvant 5-fluorouracil-based chemotherapy compared to African Americans (McCollum et al. 2002). Moreover, the risk of dose-limiting ADRs due to taxanes or platinum therapy was significantly lower in Caucasian lung cancer patients compared to patients of Asian descent, whereas response rates consistently showed inverse correlations (Gandara et al. 2009; Lara et al. 2009, 2010). This variability is likely to be at least in part caused by differences in the allelic distribution for genes involved in the disposition of the respective chemotherapeutics.
Mounting evidence suggests that the targeted interrogation of candidate pharmacogenetic polymorphisms is not sufficient to accurately predict the drug response of a given patient (Lauschke and Ingelman-Sundberg 2016, 2018). Importantly, our previous data indicate that variant burden rather than allele status of specific ABC variants is a predictor of clinical outcomes, thus corroborating that NGS-based approaches can add value to personalized cancer prognostics (Xiao et al. 2020). One plausible interpretation of this observation is that multiple ABC variants with individually small-effect sizes act modulate bioavailability of orally administered substrates and/or intra-tumoral drug concentrations in concert, thereby impacting treatment efficacy. These findings have important implications for cancer pharmacogenomics and incentivize studies into the underlying mechanisms.
Interestingly, mapping of clinically impactful variants onto the 3D structure of MDR1 revealed a preferential localization in NBDs. Generally, the NBDs in MDR1 are highly conserved compared to the substrate-binding domains, indicating that NBDs might be more sensitive to functional alterations, whereas impacts of variations in the substrate-binding domain or translocation channel seem to be less pronounced (Wolf et al. 2011). The two synonymous variants indicated here (G412G and I1145I), although not resulting in amino acid exchange, have been suggested to affect transporter function by disrupting the cotranslational folding process via introduction of rare codons (Kimchi-Sarfaty et al. 2007). The triallelic variation at position A893, which localizes to a less conserved transmembrane helix, has not been reported to affect transporter function in vitro (Kimchi-Sarfaty et al. 2002). Thus, functional effects associated with this variant might be due to the strong linkage with G412G and I1145I (Fung and Gottesman 2009).
Overall, we found that the ABC transporter superfamily was highly population-specific and inter-ethnic variability is commensurate with other genetically diverse pharmacogene families, including CYPs (Zhou et al. 2017), SLCOs (Zhang and Lauschke 2019) and UGTs (Kaniwa et al. 2005). Overall, 74.9% of all variants that were predicted to affect the functionality of the respective ABC transporter were specific to a single population and the overall load of functional genetic variability differed considerable between the analyzed populations. Inter-ethnic variability was furthermore reflected in differences in population-specific prevalence of ABC-associated Mendelian diseases with autosomal recessive inheritance. For instance, frequencies of CF are around 1 in 2500–3500 newborns of Caucasian ancestry, whereas only 1 in 17,000 and 1 in 31,000 children of African and Asian ancestry are affected, which closely aligns with predictions based on loss-of-function carrier rates (1 in 1850 in Europeans, 1 in 24,000 in Africans, and < 1 in 40,000 in East Asians). Similarly, PXE has been reported to have a prevalence around 1 in 50,000 Dutch individuals (Kranenburg et al. 2019), compared to our estimates of 1 in 52,000 in Europeans based on ABCC6 loss-of-function allele frequencies. Interestingly, ABCC6 was also the ABC gene that was found to harbour most CNVs, which is aligned with the previous studies describing genomic deletions in this locus in PXE patients (Costrop et al. 2010; Katona et al. 2005). Combined, these data suggest that population-scale sequencing data provide an important tool to predict Mendelian ABC disease risk. Notably, however, this approach is only suitable for diseases in which heterozygous loss of gene function is phenotypically silent, thus excluding autosomal dominant or X-linked modes of inheritance. Taken together, our analyses revealed striking ethnogeographic differences in ABC variability profiles that might explain at least part of the observed variability in chemotherapy response and incidence of Mendelian disorders between populations. Furthermore, the population-scale genomic data set presented here promises to provide a powerful resource for the evaluation of genetic ABC disease epidemiology.
In summary, we comprehensively profiled the genetic variability of the human ABC transporter superfamily and revealed a surprising extent of rare and population-specific variations. Computational evaluations of the functional impacts of these variants indicate that these variants contribute considerably to the variability in ABC transporter function with potentially important consequences for chemotherapeutic treatment regimens. Thus, these data incentivize the consideration of sequencing-based genotypes for patient stratification, particularly in the current era of clinical trial globalization. Furthermore, we expect that a deeper understanding of the functional consequences of ABC transporter variability might be useful to improve public health strategies and flag patients at risk of not responding appropriately to treatment with ABC substrates.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1: Genetic variability of ABC transporters after normalization for gene length. A, No significant differences were identified for the number of variations per gene length between ABC subfamilies (p>0.05). B, Stacked column plot depicting the genetic variability of all 48 human ABC transporters normalized by gene length (PDF 239 kb)
Supplementary Figure 2: Evolutionary constraints in the human ABC gene superfamily. A, The evolutionary constraint of missense variations is shown for all 48 human ABC genes. Higher Z scores indicate a depletion of missense variations within the respective gene compared to the genetic background variation, whereas scores <0 indicate that the gene is less constraint. B, The probability of being loss-of-function intolerant (pLI) is plotted for each human ABC gene. Note that only 4 genes are considered haploinsufficient, whereas the remaining 44 ABC genes are not depleted of their expected loss-of-function variation. Numerical conservation values and confidence intervals can be found in Supplementary Table 1. Constraint information was calculated and provided by (Lek et al. 2016) (PDF 366 kb)
Supplementary Figure 3: Inter-ethnic differences of the clinically important triallelic ABCB1 variant rs2032582. Frequencies of the different nucleotide and amino acid variations for the rs2032582 polymorphism are depicted for six worldwide populations (PDF 345 kb)
Supplementary Figure 4: Linkage disequilibrium and haplotype type structure of ABCB1, ABCC1 and ABCG2 loci. Linkage disequilibrium (LD) maps are shown for clinically important variants in ABCB1 (A), ABCC1 (B) and ABCG2 (C) are shown. LD is depicted as correlation between variant pairs (R2). Two weak haplotype blocks were identified for ABCB1 and ABCC1 (indicated by black frames), whereas the analysed variations in ABCG2 were only in very weak LD (PDF 389 kb)
Supplementary Table 1: Overview of evolutionary constraint scores in the human ABC transporter family. o/e indicates the ratio of observed to expected variations. Values in brackets indicate the 90% confidence interval. pLI indicates the probability of being loss-of-function intolerant. Z scores indicate the deviation of observed counts from the expected variant number (XLSX 12 kb)
Supplementary Table 3: Overview of Mendelian disease associations and their respective mode of inheritance for all human ABC genes (XLSX 15 kb)
Acknowledgements
Open access funding provided by Karolinska Institute. The work was supported by the Swedish Research Council (Grant agreement numbers: 2016-01153 and 2016-01154), by the Strategic Research Programme in Diabetes at Karolinska Institutet, and by the China Scholarship Council (Grant number: 201600160066) and Horizon 2020 Framework Programme (Grant no. 668353). The authors thank the Exome Aggregation Consortium and all contributing groups for sharing their data, which was instrumental for this work.
Compliance with ethical standards
Conflict of interest
VML is co-founder and shareholder of HepaPredict AB. QX and YZ have no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abraham JE, Guo Q, Dorling L, Tyrer J, Ingle S, Hardy R, Vallier A-L, Hiller L, Burns R, Jones L, Bowden SJ, Dunn JA, Poole CJ, Caldas C, Pharoah PPD, Earl HM. Replication of genetic polymorphisms reported to be associated with taxane-related sensory neuropathy in patients with early breast cancer treated with Paclitaxel. Clin Cancer Res. 2014;20:2466–2475. doi: 10.1158/1078-0432.CCR-13-3232. [DOI] [PubMed] [Google Scholar]
- Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegra S, Cusato J, De Francia S, Massano D, Piga A, D’Avolio A. Deferasirox AUC efficacy cutoff and role of pharmacogenetics. Eur J Clin Pharmacol. 2016;72:1155–1157. doi: 10.1007/s00228-016-2070-9. [DOI] [PubMed] [Google Scholar]
- Amberger JS, Bocchini CA, Schiettecatte F, Scott AF, Hamosh A. OMIM.org: online Mendelian Inheritance in Man (OMIM®), an online catalog of human genes and genetic disorders. Nucleic Acids Res. 2015;43:D789–D798. doi: 10.1093/nar/gku1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey KM, Romaine SPR, Jackson BM, Farrin AJ, Efthymiou M, Barth JH, Copeland J, McCormack T, Whitehead A, Flather MD, Samani NJ, Nixon J, Hall AS, Balmforth AJ, SPACE ROCKET Trial Group Hepatic metabolism and transporter gene variants enhance response to rosuvastatin in patients with acute myocardial infarction: the GEOSTAT-1 Study. Circ Cardiovasc Genet. 2010;3:276–285. doi: 10.1161/CIRCGENETICS.109.898502. [DOI] [PubMed] [Google Scholar]
- Benadiba M, Maor Y. Importance of ABC transporters in drug development. Curr Pharm Des. 2016;22:5817–5829. doi: 10.2174/1381612822666160810. [DOI] [PubMed] [Google Scholar]
- Bergmann TK, Brasch-Andersen C, Gréen H, Mirza MR, Skougaard K, Wihl J, Keldsen N, Damkier P, Peterson C, Vach W, Brøsen K. Impact of ABCB1 variants on neutrophil depression: a pharmacogenomic study of paclitaxel in 92 women with ovarian cancer. Basic Clin Pharmacol Toxicol. 2012;110:199–204. doi: 10.1111/j.1742-7843.2011.00802.x. [DOI] [PubMed] [Google Scholar]
- Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch TM, Kjellberg LM, Bouwers A, Koeleman BPC, Schellens JHM, Beijnen JH, Smits PHM, Meijerman I. Detection of single nucleotide polymorphisms in the ABCG2 gene in a Dutch population. Am J Pharmacogenom. 2005;5:123–131. doi: 10.2165/00129785-200505020-00005. [DOI] [PubMed] [Google Scholar]
- Brackman DJ, Yee SW, Enogieru OJ, Shaffer C, Ranatunga D, Denny JC, Wei WQ, Kamatani Y, Kubo M, Roden DM, Jorgenson E, Giacomini KM. Genome-wide association and functional studies reveal novel pharmacological mechanisms for allopurinol. Clin Pharmacol Ther. 2019;106:623–631. doi: 10.1002/cpt.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush WS, Crosslin DR, Owusu-Obeng A, Wallace J, Almoguera B, Basford MA, Bielinski SJ, Carrell DS, Connolly JJ, Crawford D, Doheny KF, Gallego CJ, Gordon AS, Keating B, Kirby J, Kitchner T, Manzi S, Mejia AR, Pan V, Perry CL, Peterson JF, Prows CA, Ralston J, Scott SA, Scrol A, Smith M, Stallings SC, Veldhuizen T, Wolf W, Volpi S, Wiley K, Li R, Manolio T, Bottinger E, Brilliant MH, Carey D, Chisholm RL, Chute CG, Haines JL, Hakonarson H, Harley JB, Holm IA, Kullo IJ, Jarvik GP, Larson EB, McCarty CA, Williams MS, Denny JC, Rasmussen-Torvik LJ, Roden DM, Ritchie MD. Genetic variation among 82 pharmacogenes: the PGRNseq data from the eMERGE network. Clin Pharmacol Ther. 2016;100:160–169. doi: 10.1002/cpt.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campa D, Müller P, Edler L, Knoefel L, Barale R, Heussel CP, Thomas M, Canzian F, Risch A. A comprehensive study of polymorphisms in ABCB1, ABCC2 and ABCG2 and lung cancer chemotherapy response and prognosis. Int J Cancer. 2012;131:2920–2928. doi: 10.1002/ijc.27567. [DOI] [PubMed] [Google Scholar]
- Caronia D, Patiño-Garcia A, Peréz-Martínez A, Pita G, Moreno LT, Zalacain-Díez M, Molina B, Colmenero I, Sierrasesúmaga L, Benitez J, Gonzalez-Neira A. Effect of ABCB1 and ABCC3 polymorphisms on osteosarcoma survival after chemotherapy: a pharmacogenetic study. PLoS ONE. 2011;6:e26091-6. doi: 10.1371/journal.pone.0026091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter H, Douville C, Stenson PD, Cooper DN, Karchin R. Identifying Mendelian disease genes with the variant effect scoring tool. BMC Genom. 2013;14(Suppl 3):S3. doi: 10.1186/1471-2164-14-S3-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascorbi I. Role of pharmacogenetics of ATP-binding cassette transporters in the pharmacokinetics of drugs. Pharmacol Ther. 2006;112:457–473. doi: 10.1016/j.pharmthera.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Chang H, Rha SY, Jeung H-C, Im CK, Noh SH, Kim JJ, Chung HC. Association of the ABCB1 3435C%3eT polymorphism and treatment outcomes in advanced gastric cancer patients treated with paclitaxel-based chemotherapy. Oncol Rep. 2010;23:271–278. doi: 10.3892/or_00000633. [DOI] [PubMed] [Google Scholar]
- Chasman DI, Giulianini F, MacFadyen J, Barratt BJ, Nyberg F, Ridker PM. Genetic determinants of statin-induced low-density lipoprotein cholesterol reduction: the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial. Circ Cardiovasc Genet. 2012;5:257–264. doi: 10.1161/CIRCGENETICS.111.961144. [DOI] [PubMed] [Google Scholar]
- Chaturvedi P, Tulsyan S, Agarwal G, Lal P, Agarwal S, Mittal RD, Mittal B. Influence of ABCB1 genetic variants in breast cancer treatment outcomes. Cancer Epidemiol. 2013;37:754–761. doi: 10.1016/j.canep.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Chen S, Villeneuve L, Jonker D, Couture F, Laverdière I, Cecchin E, Innocenti F, Toffoli G, Lévesque É, Guillemette C. ABCC5 and ABCG1 polymorphisms predict irinotecan-induced severe toxicity in metastatic colorectal cancer patients. Pharmacogenet Genom. 2015;25:573–583. doi: 10.1097/FPC.0000000000000168. [DOI] [PubMed] [Google Scholar]
- Chen X, Chen D, Yang S, Ma R, Pan Y, Li X, Ma S. Impact of ABCG2 polymorphisms on the clinical outcome of TKIs therapy in Chinese advanced non-small-cell lung cancer patients. Cancer Cell Int. 2015;15:43. doi: 10.1186/s12935-015-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho AVC, Silva SPS, de Alencar LCA, Stocco G, Crovella S, Brandão LAC, Guimarães RL. ABCB1 and ABCC1 variants associated with virological failure of first-line protease inhibitors antiretroviral regimens in Northeast Brazil patients. J Clin Pharmacol. 2013;53:1286–1293. doi: 10.1002/jcph.165. [DOI] [PubMed] [Google Scholar]
- Costrop LMF, Vanakker OOM, Van Laer L, Le Saux O, Martin L, Chassaing N, Guerra D, Pasquali-Ronchetti I, Coucke PJ, De Paepe A. Novel deletions causing pseudoxanthoma elasticum underscore the genomic instability of the ABCC6 region. J Hum Genet. 2010;55:112–117. doi: 10.1038/jhg.2009.132. [DOI] [PubMed] [Google Scholar]
- Cusatis G, Gregorc V, Li J, Spreafico A, Ingersoll RG, Verweij J, Ludovini V, Villa E, Hidalgo M, Sparreboom A, Baker SD. Pharmacogenetics of ABCG2 and adverse reactions to gefitinib. J Natl Cancer Inst. 2006;98:1739–1742. doi: 10.1093/jnci/djj469. [DOI] [PubMed] [Google Scholar]
- De Mattia E, Toffoli G, Polesel J, D’Andrea M, Corona G, Zagonel V, Buonadonna A, Dreussi E, Cecchin E. Pharmacogenetics of ABC and SLC transporters in metastatic colorectal cancer patients receiving first-line FOLFIRI treatment. Pharmacogenet Genom. 2013;23:549–557. doi: 10.1097/fpc.0b013e328364b6cf. [DOI] [PubMed] [Google Scholar]
- Deeken JF, Cormier T, Price DK, Sissung TM, Steinberg SM, Tran K, Liewehr DJ, Dahut WL, Miao X, Figg WD. A pharmacogenetic study of docetaxel and thalidomide in patients with castration-resistant prostate cancer using the DMET genotyping platform. Pharmacogenom J. 2009;10:191–199. doi: 10.1038/tpj.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeStefano GM, Kurban M, Anyane-Yeboa K, Dall’Armi C, Di Paolo G, Feenstra H, Silverberg N, Rohena L, López-Cepeda LD, Jobanputra V, Fantauzzo KA, Kiuru M, Tadin-Strapps M, Sobrino A, Vitebsky A, Warburton D, Levy B, Salas-Alanis JC, Christiano AM. Mutations in the cholesterol transporter gene ABCA5 are associated with excessive hair overgrowth. PLoS Genet. 2014;10:e1004333. doi: 10.1371/journal.pgen.1004333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulucq S, Bouchet S, Turcq B, Lippert E, Etienne G, Reiffers J, Molimard M, Krajinovic M, Mahon F-X. Multidrug resistance gene (MDR1) polymorphisms are associated with major molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood. 2008;112:2024–2027. doi: 10.1182/blood-2008-03-147744. [DOI] [PubMed] [Google Scholar]
- Flanagan SE, Patch A-M, Ellard S. Using SIFT and PolyPhen to predict loss-of-function and gain-of-function mutations. Genet Test Mol Biomark. 2010;14:533–537. doi: 10.1089/gtmb.2010.0036. [DOI] [PubMed] [Google Scholar]
- Fujikura K, Ingelman-Sundberg M, Lauschke VM. Genetic variation in the human cytochrome P450 supergene family. Pharmacogenet Genom. 2015;25:584–594. doi: 10.1097/FPC.0000000000000172. [DOI] [PubMed] [Google Scholar]
- Fukushima-Uesaka H, Saito Y, Tohkin M, Maekawa K, Hasegawa R, Kawamoto M, Kamatani N, Suzuki K, Yanagawa T, Kajio H, Kuzuya N, Yasuda K, Sawada J-i. Genetic variations and haplotype structures of the ABC transporter gene ABCC1 in a Japanese population. Drug Metab Pharmacokinet. 2007;22:48–60. doi: 10.2133/dmpk.22.48. [DOI] [PubMed] [Google Scholar]
- Fung KL, Gottesman MM. A synonymous polymorphism in a common MDR1 (ABCB1) haplotype shapes protein function. Biochem Biophys Acta. 2009;1794:860–871. doi: 10.1016/j.bbapap.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandara DR, Kawaguchi T, Crowley J, Moon J, Furuse K, Kawahara M, Teramukai S, Ohe Y, Kubota K, Williamson SK, Gautschi O, Lenz H-J, McLeod HL, Lara PN, Coltman CA, Fukuoka M, Saijo N, Fukushima M, Mack PC. Japanese–US common-arm analysis of paclitaxel plus carboplatin in advanced non-small-cell lung cancer: a model for assessing population-related pharmacogenomics. J Clin Oncol. 2009;27:3540–3546. doi: 10.1200/JCO.2008.20.8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervasini G, Jara C, Olier C, Romero N, Martínez R, Carrillo JA. Polymorphisms in ABCB1 and CYP19A1 genes affect anastrozole plasma concentrations and clinical outcomes in postmenopausal breast cancer patients. Br J Clin Pharmacol. 2017;83:562–571. doi: 10.1111/bcp.13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Haba E, García MI, Cortejoso L, López-Lillo C, Barrueco N, García-Alfonso P, Alvarez S, Jiménez JL, Martín ML, Muñóz-Fernández MA, Sanjurjo M, López-Fernández LA. ABCB1 gene polymorphisms are associated with adverse reactions in fluoropyrimidine-treated colorectal cancer patients. Pharmacogenomics. 2010;11:1715–1723. doi: 10.2217/pgs.10.159. [DOI] [PubMed] [Google Scholar]
- Gordon AS, Tabor HK, Johnson AD, Snively BM, Assimes TL, Auer PL, Ioannidis JPA, Peters U, Robinson JG, Sucheston LE, Wang D, Sotoodehnia N, Rotter JI, Psaty BM, Jackson RD, Herrington DM, O’Donnell CJ, Reiner AP, Rich SS, Rieder MJ, Bamshad MJ, Nickerson DA, Project NGES Quantifying rare, deleterious variation in 12 human cytochrome P450 drug-metabolism genes in a large-scale exome dataset. Hum Mol Genet. 2014;23:1957–1963. doi: 10.1093/hmg/ddt588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregers J, Gréen H, Christensen IJ, Dalhoff K, Schroeder H, Carlsen N, Rosthoej S, Lausen B, Schmiegelow K, Peterson C. Polymorphisms in the ABCB1 gene and effect on outcome and toxicity in childhood acute lymphoblastic leukemia. Pharmacogenom J. 2015;15:372–379. doi: 10.1038/tpj.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Köck K, Ritter CA, Chen Z-S, Grube M, Jedlitschky G, Illmer T, Ayres M, Beck JF, Siegmund W, Ehninger G, Gandhi V, Kroemer HK, Kruh GD, Schaich M. Expression of ABCC-type nucleotide exporters in blasts of adult acute myeloid leukemia: relation to long-term survival. Clin Cancer Res. 2009;15:1762–1769. doi: 10.1158/1078-0432.CCR-08-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampras SS, Sucheston L, Weiss J, Baer MR, Zirpoli G, Singh PK, Wetzler M, Chennamaneni R, Blanco JG, Ford L, Moysich KB. Genetic polymorphisms of ATP-binding cassette (ABC) proteins, overall survival and drug toxicity in patients with acute myeloid leukemia. Int J Mol Epidemiol Genet. 2010;1:201–207. [PMC free article] [PubMed] [Google Scholar]
- Han J-Y, Lim H-S, Park YH, Lee SY, Lee JS. Integrated pharmacogenetic prediction of irinotecan pharmacokinetics and toxicity in patients with advanced non-small cell lung cancer. Lung Cancer. 2009;63:115–120. doi: 10.1016/j.lungcan.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Honjo Y, Morisaki K, Huff LM, Robey RW, Hung J, Dean M, Bates SE. Single-nucleotide polymorphism (SNP) analysis in the ABC half-transporter ABCG2 (MXR/BCRP/ABCP1) Cancer Biol Ther. 2002;1:696–702. doi: 10.4161/cbt.322. [DOI] [PubMed] [Google Scholar]
- Horinouchi M, Sakaeda T, Nakamura T, Morita Y, Tamura T, Aoyama N, Kasuga M, Okumura K. Significant genetic linkage of MDR1 polymorphisms at positions 3435 and 2677: functional relevance to pharmacokinetics of digoxin. Pharm Res. 2002;19:1581–1585. doi: 10.1023/A:1020433422259. [DOI] [PubMed] [Google Scholar]
- Hu M, To KKW, Mak VWL, Tomlinson B. The ABCG2 transporter and its relations with the pharmacokinetics, drug interaction and lipid-lowering effects of statins. Expert Opin Drug Metab Toxicol. 2011;7:49–62. doi: 10.1517/17425255.2011.538383. [DOI] [PubMed] [Google Scholar]
- Hu R, Barratt DT, Coller JK, Sallustio BC, Somogyi AA. CYP3A5*3 and ABCB1 61A%3eG significantly influence dose-adjusted trough blood tacrolimus concentrations in the first three months post-kidney transplantation. Basic Clin Pharmacol Toxicol. 2018;123:320–326. doi: 10.1111/bcpt.13016. [DOI] [PubMed] [Google Scholar]
- Ingelman-Sundberg M, Mkrtchian S, Zhou Y, Lauschke VM. Integrating rare genetic variants into pharmacogenetic drug response predictions. Hum Genom. 2018;12:26. doi: 10.1186/s40246-018-0157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionita-Laza I, McCallum K, Xu B, Buxbaum JD. A spectral approach integrating functional genomic annotations for coding and noncoding variants. Nat Genet. 2016;48:214–220. doi: 10.1038/ng.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabeen S, Holmboe L, Alnæs GIG, Andersen AM, Hall KS, Kristensen VN. Impact of genetic variants of RFC1, DHFR and MTHFR in osteosarcoma patients treated with high-dose methotrexate. Pharmacogenom J. 2015;15:385–390. doi: 10.1038/tpj.2015.11. [DOI] [PubMed] [Google Scholar]
- Ji M, Tang J, Zhao J, Xu B, Qin J, Lu J. Polymorphisms in genes involved in drug detoxification and clinical outcomes of anthracycline-based neoadjuvant chemotherapy in Chinese Han breast cancer patients. Cancer Biol Ther. 2012;13:264–271. doi: 10.4161/cbt.18920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z-P, Zhao X-L, Takahashi N, Angelini S, Dubashi B, Sun L, Xu P. Trough concentration and ABCG2 polymorphism are better to predict imatinib response in chronic myeloid leukemia: a meta-analysis. Pharmacogenomics. 2017;18:35–56. doi: 10.2217/pgs-2016-0103. [DOI] [PubMed] [Google Scholar]
- Kaniwa N, Kurose K, Jinno H, Tanaka-Kagawa T, Saito Y, Saeki M, Sawada J-i, Tohkin M, Hasegawa R. Racial variability in haplotype frequencies of UGT1A1 and glucuronidation activity of a novel single nucleotide polymorphism 686C%3eT (P229L) found in an African-American. Drug Metab Dispos. 2005;33:458–465. doi: 10.1124/dmd.104.001800. [DOI] [PubMed] [Google Scholar]
- Kap EJ, Seibold P, Scherer D, Habermann N, Balavarca Y, Jansen L, Zucknick M, Becker N, Hoffmeister M, Ulrich A, Benner A, Ulrich CM, Burwinkel B, Brenner H, Chang-Claude J. SNPs in transporter and metabolizing genes as predictive markers for oxaliplatin treatment in colorectal cancer patients. Int J Cancer. 2016;138:2993–3001. doi: 10.1002/ijc.30026. [DOI] [PubMed] [Google Scholar]
- Katona E, Aslanidis C, Remenyik E, Csikós M, Kárpáti S, Paragh G, Schmitz G. Identification of a novel deletion in the ABCC6 gene leading to Pseudoxanthoma elasticum. J Dermatol Sci. 2005;40:115–121. doi: 10.1016/j.jdermsci.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJE. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DHD, Sriharsha L, Xu W, Kamel-Reid S, Liu X, Siminovitch K, Messner HA, Lipton JH. Clinical relevance of a pharmacogenetic approach using multiple candidate genes to predict response and resistance to imatinib therapy in chronic myeloid leukemia. Clin Cancer Res. 2009;15:4750–4758. doi: 10.1158/1078-0432.CCR-09-0145. [DOI] [PubMed] [Google Scholar]
- Kim HS, Kim M-K, Chung HH, Kim JW, Park NH, Song YS, Kang SB. Genetic polymorphisms affecting clinical outcomes in epithelial ovarian cancer patients treated with taxanes and platinum compounds: a Korean population-based study. Gynecol Oncol. 2009;113:264–269. doi: 10.1016/j.ygyno.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Kim I-W, Yun H-y, Choi B, Han N, Park S-Y, Lee ES, Oh JM. ABCB1 C3435T genetic polymorphism on population pharmacokinetics of methotrexate after hematopoietic stem cell transplantation in Korean patients: a prospective analysis. Clin Ther. 2012;34:1816–1826. doi: 10.1016/j.clinthera.2012.06.022. [DOI] [PubMed] [Google Scholar]
- Kim K-p, Ahn J-H, Kim S-B, Jung KH, Yoon DH, Lee JS, Ahn S-H. Prospective evaluation of the drug-metabolizing enzyme polymorphisms and toxicity profile of docetaxel in Korean patients with operable lymph node-positive breast cancer receiving adjuvant chemotherapy. Cancer Chemother Pharmacol. 2012;69:1221–1227. doi: 10.1007/s00280-011-1816-4. [DOI] [PubMed] [Google Scholar]
- Kim Y, Chen J. Molecular structure of human P-glycoprotein in the ATP-bound, outward-facing conformation. Science. 2018;359:915–919. doi: 10.1126/science.aar7389. [DOI] [PubMed] [Google Scholar]
- Kimchi-Sarfaty C, Gribar JJ, Gottesman MM. Functional characterization of coding polymorphisms in the human MDR1 gene using a vaccinia virus expression system. Mol Pharmacol. 2002;62:1–6. doi: 10.1124/mol.62.1.1. [DOI] [PubMed] [Google Scholar]
- Kimchi-Sarfaty C, Oh JM, Kim I-W, Sauna ZE, Calcagno AM, Ambudkar SV, Gottesman MM. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science. 2007;315:525–528. doi: 10.1126/science.1135308. [DOI] [PubMed] [Google Scholar]
- Kiyotani K, Mushiroda T, Imamura CK, Hosono N, Tsunoda T, Kubo M, Tanigawara Y, Flockhart DA, Desta Z, Skaar TC, Aki F, Hirata K, Takatsuka Y, Okazaki M, Ohsumi S, Yamakawa T, Sasa M, Nakamura Y, Zembutsu H. Significant effect of polymorphisms in CYP2D6 and ABCC2 on clinical outcomes of adjuvant tamoxifen therapy for breast cancer patients. J Clin Oncol. 2010;28:1287–1293. doi: 10.1200/JCO.2009.25.7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyotani K, Mushiroda T, Kubo M, Zembutsu H, Sugiyama Y, Nakamura Y. Association of genetic polymorphisms in SLCO1B3 and ABCC2 with docetaxel-induced leukopenia. Cancer Sci. 2008;99:967–972. doi: 10.1111/j.1349-7006.2008.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König J, Müller F, Fromm MF. Transporters and drug–drug interactions: important determinants of drug disposition and effects. Pharmacol Rev. 2013;65:944–966. doi: 10.1124/pr.113.007518. [DOI] [PubMed] [Google Scholar]
- Kozyra M, Ingelman-Sundberg M, Lauschke VM. Rare genetic variants in cellular transporters, metabolic enzymes, and nuclear receptors can be important determinants of interindividual differences in drug response. Genet Med. 2017;19:20–29. doi: 10.1038/gim.2016.33. [DOI] [PubMed] [Google Scholar]
- Krajinovic M, Elbared J, Drouin S, Bertout L, Rezgui A, Ansari M, Raboisson M-J, Lipshultz SE, Silverman LB, Sallan SE, Neuberg DS, Kutok JL, Laverdiere C, Sinnett D, Andelfinger G. Polymorphisms of ABCC5 and NOS3 genes influence doxorubicin cardiotoxicity in survivors of childhood acute lymphoblastic leukemia. Pharmacogenom J. 2016;16:530–535. doi: 10.1038/tpj.2015.63. [DOI] [PubMed] [Google Scholar]
- Kranenburg G, Baas AF, de Jong PA, Asselbergs FW, Visseren FLJ, Spiering W, SMART Study-Group The prevalence of pseudoxanthoma elasticum: revised estimations based on genotyping in a high vascular risk cohort. Eur J Med Genet. 2019;62:90–92. doi: 10.1016/j.ejmg.2018.05.020. [DOI] [PubMed] [Google Scholar]
- Landrum MJ, Lee JM, Riley GR, Jang W, Rubinstein WS, Church DM, Maglott DR. ClinVar: public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res. 2014;42:D980–D985. doi: 10.1093/nar/gkt1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara PN, Chansky K, Shibata T, Fukuda H, Tamura T, Crowley J, Redman MW, Natale R, Saijo N, Gandara DR. Common arm comparative outcomes analysis of phase 3 trials of cisplatin + irinotecan versus cisplatin + etoposide in extensive stage small cell lung cancer: final patient-level results from Japan Clinical Oncology Group 9511 and Southwest Oncology Group 0124. Cancer. 2010;116:5710–5715. doi: 10.1002/cncr.25532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara PN, Natale R, Crowley J, Lenz H-J, Redman MW, Carleton JE, Jett J, Langer CJ, Kuebler JP, Dakhil SR, Chansky K, Gandara DR. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol. 2009;27:2530–2535. doi: 10.1200/JCO.2008.20.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauschke VM, Ingelman-Sundberg M. Precision medicine and rare genetic variants. Trends Pharmacol Sci. 2016;37:85–86. doi: 10.1016/j.tips.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Lauschke VM, Ingelman-Sundberg M. How to consider rare genetic variants in personalized drug therapy. Clin Pharmacol Ther. 2018;103:745–748. doi: 10.1002/cpt.976. [DOI] [PubMed] [Google Scholar]
- Lee M-N, Kang B, Choi SY, Kim MJ, Woo SY, Kim J-W, Choe YH, Lee S-Y. Impact of genetic polymorphisms on 6-thioguanine nucleotide levels and toxicity in pediatric patients with IBD treated with azathioprine. Inflamm Bowel Dis. 2015;21:2897–2908. doi: 10.1097/MIB.0000000000000570. [DOI] [PubMed] [Google Scholar]
- Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, O’Donnell-Luria AH, Ware JS, Hill AJ, Cummings BB, Tukiainen T, Birnbaum DP, Kosmicki JA, Duncan LE, Estrada K, Zhao F, Zou J, Pierce-Hoffman E, Berghout J, Cooper DN, Deflaux N, DePristo M, Do R, Flannick J, Fromer M, Gauthier L, Goldstein J, Gupta N, Howrigan D, Kiezun A, Kurki MI, Moonshine AL, Natarajan P, Orozco L, Peloso GM, Poplin R, Rivas MA, Ruano-Rubio V, Rose SA, Ruderfer DM, Shakir K, Stenson PD, Stevens C, Thomas BP, Tiao G, Tusie-Luna MT, Weisburd B, Won H-H, Yu D, Altshuler DM, Ardissino D, Boehnke M, Danesh J, Donnelly S, Elosua R, Florez JC, Gabriel SB, Getz G, Glatt SJ, Hultman CM, Kathiresan S, Laakso M, McCarroll S, McCarthy MI, McGovern D, McPherson R, Neale BM, Palotie A, Purcell SM, Saleheen D, Scharf JM, Sklar P, Sullivan PF, Tuomilehto J, Tsuang MT, Watkins HC, Wilson JG, Daly MJ, MacArthur DG, Exome Aggregation Consortium Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leschziner G, Zabaneh D, Pirmohamed M, Owen A, Rogers J, Coffey AJ, Balding DJ, Bentley DB, Johnson MR. Exon sequencing and high resolution haplotype analysis of ABC transporter genes implicated in drug resistance. Pharmacogenet Genom. 2006;16:439–450. doi: 10.1097/01.fpc.0000197467.21964.67. [DOI] [PubMed] [Google Scholar]
- Li J, Zhao T, Zhang Y, Zhang K, Shi L, Chen Y, Wang X, Sun Z. Performance evaluation of pathogenicity-computation methods for missense variants. Nucleic Acids Res. 2018;46:7793–7804. doi: 10.1093/nar/gky678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Seiser EL, Baldwin RM, Ramírez J, Ratain MJ, Innocenti F, Kroetz DL. ABC transporter polymorphisms are associated with irinotecan pharmacokinetics and neutropenia. Pharmacogenom J. 2018;18:35–42. doi: 10.1038/tpj.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Li S, Chen L. The physiological role of drug transporters. Protein Cell. 2015;6:334–350. doi: 10.1007/s13238-015-0148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima JJ, Zhang S, Grant A, Shao L, Tantisira KG, Allayee H, Wang J, Sylvester J, Holbrook J, Wise R, Weiss ST, Barnes K. Influence of leukotriene pathway polymorphisms on response to montelukast in asthma. Am J Respir Crit Care Med. 2006;173:379–385. doi: 10.1164/rccm.200509-1412OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litviakov NV, Cherdyntseva NV, Tsyganov MM, Slonimskaya EM, Ibragimova MK, Kazantseva PV, Kzhyshkowska J, Choinzonov EL. Deletions of multidrug resistance gene loci in breast cancer leads to the down-regulation of its expression and predict tumor response to neoadjuvant chemotherapy. Oncotarget. 2016;7:7829–7841. doi: 10.18632/oncotarget.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Xin S, Huang M, Yang Y, Zhu C, Zhao H, Zhang Y, Chen L, Zhao Y, Li J, Zhuang W, Zhu X, Zhang L, Wang X. Determinants of Gefitinib toxicity in advanced non-small cell lung cancer (NSCLC): a pharmacogenomic study of metabolic enzymes and transporters. Pharmacogenom J. 2017;17:325–330. doi: 10.1038/tpj.2016.31. [DOI] [PubMed] [Google Scholar]
- Machiela MJ, Chanock SJ. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics. 2015;31:3555–3557. doi: 10.1093/bioinformatics/btv402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez B, Van Bambeke F. ABC multidrug transporters: target for modulation of drug pharmacokinetics and drug-drug interactions. Curr Drug Targets. 2011;12:600–620. doi: 10.2174/138945011795378504. [DOI] [PubMed] [Google Scholar]
- McCollum AD, Catalano PJ, Haller DG, Mayer RJ, Macdonald JS, Benson AB, Fuchs CS. Outcomes and toxicity in african-american and caucasian patients in a randomized adjuvant chemotherapy trial for colon cancer. J Natl Cancer Inst. 2002;94:1160–1167. doi: 10.1093/jnci/94.15.1160. [DOI] [PubMed] [Google Scholar]
- McDonough CW, Gillis NK, Alsultan A, Chang S-W, Kawaguchi-Suzuki M, Lang JE, Shahin MHA, Buford TW, El Rouby NM, Sá ACC, Langaee TY, Gums JG, Chapman AB, Cooper-DeHoff RM, Turner ST, Gong Y, Johnson JA. Atenolol induced HDL-C change in the pharmacogenomic evaluation of antihypertensive responses (PEAR) study. PLoS ONE. 2013;8:e76984. doi: 10.1371/journal.pone.0076984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megías-Vericat JE, Montesinos P, Herrero MJ, Moscardó F, Bosó V, Rojas L, Martínez-Cuadrón D, Hervás D, Boluda B, García-Robles A, Rodríguez-Veiga R, Martín-Cerezuela M, Cervera J, Sendra L, Sanz J, Miguel A, Lorenzo I, Poveda JL, Sanz MÁ, Aliño SF. Impact of ABC single nucleotide polymorphisms upon the efficacy and toxicity of induction chemotherapy in acute myeloid leukemia. Leuk Lymphoma. 2017;58:1197–1206. doi: 10.1080/10428194.2016.1231405. [DOI] [PubMed] [Google Scholar]
- Moresco M, Riccardi LN, Pizza F, Zenesini C, Caporali L, Plazzi G, Pelotti S. Pharmacogenetics and treatment response in narcolepsy type 1: relevance of the polymorphisms of the drug transporter gene ABCB1. Clin Neuropharmacol. 2016;39:18–23. doi: 10.1097/WNF.0000000000000119. [DOI] [PubMed] [Google Scholar]
- Mukonzo JK, Owen JS, Ogwal-Okeng J, Kuteesa RB, Nanzigu S, Sewankambo N, Thabane L, Gustafsson LL, Ross C, Aklillu E. Pharmacogenetic-based efavirenz dose modification: suggestions for an African population and the different CYP2B6 genotypes. PLoS ONE. 2014;9:e86919. doi: 10.1371/journal.pone.0086919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng PC, Henikoff S. Predicting deleterious amino acid substitutions. Genome Res. 2001;11:863–874. doi: 10.1101/gr.176601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguri T, Bessho Y, Achiwa H, Ozasa H, Maeno K, Maeda H, Sato S, Ueda R. MRP8/ABCC11 directly confers resistance to 5-fluorouracil. Mol Cancer Ther. 2007;6:122–127. doi: 10.1158/1535-7163.MCT-06-0529. [DOI] [PubMed] [Google Scholar]
- Pavone P, Praticò AD, Falsaperla R, Ruggieri M, Zollino M, Corsello G, Neri G. Congenital generalized hypertrichosis: the skin as a clue to complex malformation syndromes. Ital J Pediatr. 2015;41:55. doi: 10.1186/s13052-015-0161-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan VH, Tan C, Rittau A, Xu H, McLachlan AJ, Clarke SJ. An update on ethnic differences in drug metabolism and toxicity from anti-cancer drugs. Expert Opin Drug Metab Toxicol. 2011;7:1395–1410. doi: 10.1517/17425255.2011.624513. [DOI] [PubMed] [Google Scholar]
- Pramanik S, Surendran ST, Devi S, Krishnamurthi K, Chakrabarti T. Frequency and genotype distribution of ABCB1 gene polymorphisms among Maharashtrian population of Central India. Xenobiotica. 2014;44:579–582. doi: 10.3109/00498254.2013.866300. [DOI] [PubMed] [Google Scholar]
- Pussegoda K, Ross CJ, Visscher H, Yazdanpanah M, Brooks B, Rassekh SR, Zada YF, Dubé M-P, Carleton BC, Hayden MR. Replication of TPMT and ABCC3 genetic variants highly associated with cisplatin-induced hearing loss in children. Clin Pharmacol Ther. 2013;94:243–251. doi: 10.1038/clpt.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reva B, Antipin Y, Sander C. Predicting the functional impact of protein mutations: application to cancer genomics. Nucleic Acids Res. 2011;39:e118. doi: 10.1093/nar/gkr407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RL, Wallace MC, Phipps-Green AJ, Topless R, Drake JM, Tan P, Dalbeth N, Merriman TR, Stamp LK. ABCG2 loss-of-function polymorphism predicts poor response to allopurinol in patients with gout. Pharmacogenom J. 2017;17:201–203. doi: 10.1038/tpj.2015.101. [DOI] [PubMed] [Google Scholar]
- Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer. 2018;18:452–464. doi: 10.1038/s41568-018-0005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumiato E, Boldrin E, Malacrida S, Battaglia G, Bocus P, Castoro C, Cagol M, Chiarion-Sileni V, Ruol A, Amadori A, Saggioro D. A germline predictive signature of response to platinum chemotherapy in esophageal cancer. Transl Res. 2016;171:29–37.e1. doi: 10.1016/j.trsl.2015.12.011. [DOI] [PubMed] [Google Scholar]
- Saito S, Iida A, Sekine A, Miura Y, Ogawa C, Kawauchi S, Higuchi S, Nakamura Y. Identification of 779 genetic variations in eight genes encoding members of the ATP-binding cassette, subfamily C (ABCC/MRP/CFTR. J Hum Genet. 2002;47:147–171. doi: 10.1007/s100380200018. [DOI] [PubMed] [Google Scholar]
- Santos M, Niemi M, Hiratsuka M, Kumondai M, Ingelman-Sundberg M, Lauschke VM, Rodríguez-Antona C. Novel copy-number variations in pharmacogenes contribute to interindividual differences in drug pharmacokinetics. Genet Med. 2018;20:622–629. doi: 10.1038/gim.2017.156. [DOI] [PubMed] [Google Scholar]
- Sauna ZE, Müller M, Peng X-H, Ambudkar SV. Importance of the conserved walker B glutamate residues, 556 and 1201, for the completion of the catalytic cycle of ATP hydrolysis by human P-glycoprotein (ABCB1) Biochemistry. 2002;41:13989–14000. doi: 10.1021/bi026626e. [DOI] [PubMed] [Google Scholar]
- Schaller L, Lauschke VM. The genetic landscape of the human solute carrier (SLC) transporter superfamily. Hum Genet. 2019;7:248–319. doi: 10.1007/s00439-019-02081-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semsei AF, Erdelyi DJ, Ungvari I, Csagoly E, Hegyi MZ, Kiszel PS, Lautner Csorba O, Szabolcs J, Masat P, Fekete G, Falus A, Szalai C, Kovacs GT. ABCC1 polymorphisms in anthracycline-induced cardiotoxicity in childhood acute lymphoblastic leukaemia. Cell Biol Int. 2012;36:79–86. doi: 10.1042/CBI20110264. [DOI] [PubMed] [Google Scholar]
- Słomka M, Sobalska-Kwapis M, Korycka-Machała M, Bartosz G, Dziadek J, Strapagiel D. Genetic variation of the ABC transporter gene ABCC1 (multidrug resistance protein 1-MRP1) in the Polish population. BMC Genet. 2015;16:114. doi: 10.1186/s12863-015-0271-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparreboom A, Gelderblom H, Marsh S, Ahluwalia R, Obach R, Principe P, Twelves C, Verweij J, McLeod HL. Diflomotecan pharmacokinetics in relation to ABCG2 421C%3eA genotype. Clin Pharmacol Ther. 2004;76:38–44. doi: 10.1016/j.clpt.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Suthandiram S, Gan G-G, Zain SM, Bee P-C, Lian L-H, Chang K-M, Ong T-C, Mohamed Z. Effect of polymorphisms within methotrexate pathway genes on methotrexate toxicity and plasma levels in adults with hematological malignancies. Pharmacogenomics. 2014;15:1479–1494. doi: 10.2217/pgs.14.97. [DOI] [PubMed] [Google Scholar]
- Szczyrek M, Mlak R, Krawczyk P, Wojas-Krawczyk K, Powrózek T, Szudy-Szczyrek A, Zwolak A, Daniluk J, Milanowski J. Polymorphisms of genes encoding multidrug resistance proteins as a predictive factor for second-line docetaxel therapy in advanced non-small cell lung cancer. Pathol Oncol Res. 2017;23:607–614. doi: 10.1007/s12253-016-0156-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Okazaki T, Suzuki H, Abbruzzese JL, Li D. Association of multi-drug resistance gene polymorphisms with pancreatic cancer outcome. Cancer. 2011;117:744–751. doi: 10.1002/cncr.25510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C, Ambrosone CB, Darcy KM, Krivak TC, Armstrong DK, Bookman MA, Davis W, Zhao H, Moysich K, Gallion H, DeLoia JA. Common variants in ABCB1, ABCC2 and ABCG2 genes and clinical outcomes among women with advanced stage ovarian cancer treated with platinum and taxane-based chemotherapy: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;124:575–581. doi: 10.1016/j.ygyno.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda Y, Sakurai A, Mitani Y, Nakashima M, Yoshiura K-i, Nakagawa H, Sakai Y, Ota I, Lezhava A, Hayashizaki Y, Niikawa N, Ishikawa T. Earwax, osmidrosis, and breast cancer: why does one SNP (538G%3eA) in the human ABC transporter ABCC11 gene determine earwax type? FASEB J. 2009;23:2001–2013. doi: 10.1096/fj.09-129098. [DOI] [PubMed] [Google Scholar]
- Uemura T, Oguri T, Ozasa H, Takakuwa O, Miyazaki M, Maeno K, Sato S, Ueda R. ABCC11/MRP8 confers pemetrexed resistance in lung cancer. Cancer Sci. 2010;101:2404–2410. doi: 10.1111/j.1349-7006.2010.01690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher H, Ross CJD, Rassekh SR, Barhdadi A, Dubé M-P, Al-Saloos H, Sandor GS, Caron HN, van Dalen EC, Kremer LC, van der Pal HJ, Brown AMK, Rogers PC, Phillips MS, Rieder MJ, Carleton BC, Hayden MR, Canadian Pharmacogenomics Network for Drug Safety Consortium Pharmacogenomic prediction of anthracycline-induced cardiotoxicity in children. J Clin Oncol. 2012;30:1422–1428. doi: 10.1200/JCO.2010.34.3467. [DOI] [PubMed] [Google Scholar]
- Wen CC, Yee SW, Liang X, Hoffmann TJ, Kvale MN, Banda Y, Jorgenson E, Schaefer C, Risch N, Giacomini KM. Genome-wide association study identifies ABCG2 (BCRP) as an allopurinol transporter and a determinant of drug response. Clin Pharmacol Ther. 2015;97:518–525. doi: 10.1002/cpt.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojnowski L, Kulle B, Schirmer M, Schlüter G, Schmidt A, Rosenberger A, Vonhof S, Bickeböller H, Toliat MR, Suk E-K, Tzvetkov M, Kruger A, Seifert S, Kloess M, Hahn H, Loeffler M, Nürnberg P, Pfreundschuh M, Trümper L, Brockmöller J, Hasenfuss G. NAD(P)H oxidase and multidrug resistance protein genetic polymorphisms are associated with doxorubicin-induced cardiotoxicity. Circulation. 2005;112:3754–3762. doi: 10.1161/CIRCULATIONAHA.105.576850. [DOI] [PubMed] [Google Scholar]
- Wolf SJ, Bachtiar M, Wang J, Sim TS, Chong SS, Lee CGL. An update on ABCB1 pharmacogenetics: insights from a 3D model into the location and evolutionary conservation of residues corresponding to SNPs associated with drug pharmacokinetics. Pharmacogenom J. 2011;11:315–325. doi: 10.1038/tpj.2011.16. [DOI] [PubMed] [Google Scholar]
- Wright GEB, Carleton B, Hayden MR, Ross CJD. The global spectrum of protein-coding pharmacogenomic diversity. Pharmacogenom J. 2018;18:187–195. doi: 10.1038/tpj.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Kang H, Liu Y, Tong W, Liu D, Yang X, Lian M, Yao W, Zhao H, Huang D, Sha X, Wang E, Wei M. Roles of ABCB1 gene polymorphisms and haplotype in susceptibility to breast carcinoma risk and clinical outcomes. J Cancer Res Clin Oncol. 2012;138:1449–1462. doi: 10.1007/s00432-012-1209-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Q, Zhou Y, Winter S, Büttner F, Schaeffeler E, Schwab M, Lauschke VM. Germline variant burden in multidrug resistance transporters is a therapy-specific predictor of survival in breast cancer patients. Int J Cancer. 2020 doi: 10.1002/ijc.32898. [DOI] [PubMed] [Google Scholar]
- Yang J, Wang Z-G, Cai H-Q, Li Y-C, Xu Y-L. Effect of variation of ABCB1 and ABCC3 genotypes on the survival of bone tumor cases after chemotherapy. Asian Pac J Cancer Prev. 2013;14:4595–4598. doi: 10.7314/apjcp.2013.14.8.4595. [DOI] [PubMed] [Google Scholar]
- Yasui K, Mihara S, Zhao C, Okamoto H, Saito-Ohara F, Tomida A, Funato T, Yokomizo A, Naito S, Imoto I, Tsuruo T, Inazawa J. Alteration in copy numbers of genes as a mechanism for acquired drug resistance. Can Res. 2004;64:1403–1410. doi: 10.1158/0008-5472.can-3263-2. [DOI] [PubMed] [Google Scholar]
- Yee SW, Brackman DJ, Ennis EA, Sugiyama Y, Kamdem LK, Blanchard R, Galetin A, Zhang L, Giacomini KM. Influence of transporter polymorphisms on drug disposition and response: a perspective from the International Transporter Consortium. Clin Pharmacol Ther. 2018;104:803–817. doi: 10.1002/cpt.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee SW, Mefford JA, Singh N, Percival M-E, Stecula A, Yang K, Witte JS, Takahashi A, Kubo M, Matsuda K, Giacomini KM, Andreadis C. Impact of polymorphisms in drug pathway genes on disease-free survival in adults with acute myeloid leukemia. J Hum Genet. 2013;58:353–361. doi: 10.1038/jhg.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Lauschke VM. Genetic variability and population diversity of the human SLCO (OATP) transporter family. Pharmacol Res. 2019;139:550–559. doi: 10.1016/j.phrs.2018.10.017. [DOI] [PubMed] [Google Scholar]
- Zhang L, Huang S-M, Reynolds K, Madabushi R, Zineh I. Transporters in drug development: scientific and regulatory considerations. Clin Pharmacol Ther. 2018;104:793–796. doi: 10.1002/cpt.1214. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Ingelman-Sundberg M, Lauschke VM. Worldwide distribution of cytochrome P450 alleles: a meta-analysis of population-scale sequencing projects. Clin Pharmacol Ther. 2017;102:688–700. doi: 10.1002/cpt.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Lauschke VM. Comprehensive overview of the pharmacogenetic diversity in Ashkenazi Jews. J Med Genet. 2018;55:617–627. doi: 10.1136/jmedgenet-2018-105429. [DOI] [PubMed] [Google Scholar]
- Zolnerciks JK, Akkaya BG, Snippe M, Chiba P, Seelig A, Linton KJ. The Q loops of the human multidrug resistance transporter ABCB1 are necessary to couple drug binding to the ATP catalytic cycle. FASEB J. 2014;28:4335–4346. doi: 10.1096/fj.13-245639. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Genetic variability of ABC transporters after normalization for gene length. A, No significant differences were identified for the number of variations per gene length between ABC subfamilies (p>0.05). B, Stacked column plot depicting the genetic variability of all 48 human ABC transporters normalized by gene length (PDF 239 kb)
Supplementary Figure 2: Evolutionary constraints in the human ABC gene superfamily. A, The evolutionary constraint of missense variations is shown for all 48 human ABC genes. Higher Z scores indicate a depletion of missense variations within the respective gene compared to the genetic background variation, whereas scores <0 indicate that the gene is less constraint. B, The probability of being loss-of-function intolerant (pLI) is plotted for each human ABC gene. Note that only 4 genes are considered haploinsufficient, whereas the remaining 44 ABC genes are not depleted of their expected loss-of-function variation. Numerical conservation values and confidence intervals can be found in Supplementary Table 1. Constraint information was calculated and provided by (Lek et al. 2016) (PDF 366 kb)
Supplementary Figure 3: Inter-ethnic differences of the clinically important triallelic ABCB1 variant rs2032582. Frequencies of the different nucleotide and amino acid variations for the rs2032582 polymorphism are depicted for six worldwide populations (PDF 345 kb)
Supplementary Figure 4: Linkage disequilibrium and haplotype type structure of ABCB1, ABCC1 and ABCG2 loci. Linkage disequilibrium (LD) maps are shown for clinically important variants in ABCB1 (A), ABCC1 (B) and ABCG2 (C) are shown. LD is depicted as correlation between variant pairs (R2). Two weak haplotype blocks were identified for ABCB1 and ABCC1 (indicated by black frames), whereas the analysed variations in ABCG2 were only in very weak LD (PDF 389 kb)
Supplementary Table 1: Overview of evolutionary constraint scores in the human ABC transporter family. o/e indicates the ratio of observed to expected variations. Values in brackets indicate the 90% confidence interval. pLI indicates the probability of being loss-of-function intolerant. Z scores indicate the deviation of observed counts from the expected variant number (XLSX 12 kb)
Supplementary Table 3: Overview of Mendelian disease associations and their respective mode of inheritance for all human ABC genes (XLSX 15 kb)