Abstract
Stimuli-responsive nanomaterials are especially interesting to enhance drug-delivery specificity for biomedical applications. With the aim to achieve a highly stable and inflammation specific drug release, we designed an reactive oxygen species (ROS)-responsive dextran-drug conjugate (Nap-Dex). By blending Nap-Dex with the acid-sensitive acetalated dextran polymer (Ac-Dex) we achieved a dual responsive nanoparticle with high specificity toward the inflammatory environment. The inflammatory environment not only has elevated ROS levels, but also a lower pH than healthy tissue, making pH and ROS highly suitable triggers to target inflammatory diseases. The anti-inflammatory COX-inhibitor naproxen was modified with an ROS-responsive phenylboronic acid and conjugated onto dextran. The dextran units were functionalized with up to 87% modified naproxen. This resulted in a complete drug release from the polymer within 20 min at 10 mM H2O2. The dual responsive nanoparticles reduced levels of the pro inflammatory cytokine IL-6 120 times more efficiently, and TNFα 6 times more efficiently than free naproxen from lipopolysaccharide (LPS)-activated macrophages. These additional anti-inflammatory effects were found to be mainly attributed to ROS scavenging effects. In addition, the model cargo fluorescein diacetate was released in an LPS-induced inflammatory response in vitro. We believe that drug-conjugation using phenylboronic acid can be applied to various drugs and dextran based materials for enhanced drug-efficacy, where this work demonstrates the significance of functionalized carbohydrates polymer-drug conjugates.
Keywords: Inflammation-responsive, ROS-responsive, acid-responsive, dual-responsive, polymer-drug conjugates, dextran
Graphical Abstract

1. Introduction
Stimuli-responsive nanoparticles (NP) have been coming into the spotlight due to their potential applications in various fields, especially in biomedical applications.1–15 These so-called ‘smart NP’ respond to stimuli in biological systems such as enzymes, biochemical molecules (ex; glucose, GSH, ATP), pH, and reactive oxygen species (ROS).16–18 Notably, pH and ROS are suitable triggers for materials targeting inflammatory diseases since it is known that the inflammatory environment includes increased ROS production and lower pH values than normal tissues due to increased metabolic activation.19–22
Similarly, incorporating stimuli-responsive linkers can provide controlled release of polymer-drug conjugates. Conjugating drugs to polymers has been proposed as a means to increase loading efficiency of encapsulated drugs and decrease leakage.23–25 In the past, polymer-drug conjugates reached clinical trials, however, they showed limited efficacy due to uncontrollable drug release, potentially due to non-stimuli responsive polymers (reviewed by Kopecek 2013).26 To enhance controlled release, stimuli-responsive linkers have recently been incorporated into the polymer-drug conjugates.27–32 However, few responsive linkers in general have been reported, especially for ROS-responsive conjugates.33–35
Phenylboronic acids (PBA)– the well-known Suzuki coupling reagent– react with ROS, resulting in the cleavage of covalent bond between the carbon and boron. This reaction is a quantitative reaction with high sensitivity and specificity towards H2O2 over other ROS.36–40
Since it consumes ROS during the reaction, it also acts as an ROS scavenger.41–42 PBA have previously been successfully used to modify the carbohydrate dextran into an ROS responsive polymer.43 Among carbohydrates, dextran is a widely used polymer for biomedical applications because it is FDA approved, biocompatible, and commercially available with low cost.44–46 In this regard, various modified dextran based polymers have been reported.43, 47–50 Of these modifications, the acetalated dextran (Ac-Dex) converts the hydrophilic dextran into a hydrophobic polymer by acetalating the diols on its backbone.51–53 Under acidic conditions, the acetals will hydrolyze and revert the polymer back to its hydrophilic state. Due to these properties, Ac-Dex has been incorporated into acid-responsive nanoparticles in which the hydrophobicity change allows for the release of the encapsulate.54–56.
Here, we designed an ROS-responsive dextran-drug conjugate, and blended it with a pH-responsive Ac-Dex to achieve a dual responsive nanoparticle (Figure 1). For this purpose, we modified the anti-inflammatory drug naproxen with the ROS-responsive PBA, and conjugated it onto dextran (Nap-Dex) for an inflammation responsive drug release. The PBA linker is cleavable by H2O2, and Nap-Dex releases conjugated naproxen with increasing polymer hydrophilicity. Conjugation efficiency and drug release from Nap-Dex were evaluated by GPC and UV absorption. We demonstrate controlled release of naproxen, superior anti-inflammatory efficacy of the NPs compared to free drug, as well as show model cargo release in response to inflammatory macrophage activation.
Figure 1.

Schematic illustration of nanoparticles and its degradation by stimuli.
2. Results and Discussion
2.1. Synthesis and Characterization of the Nap-Dex Polymer
First, naproxen was modified in order to conjugate it onto the dextran polymer (Nap-Dex). The carboxylic acid group on naproxen reacted with 4-(hydroxymethyl)phenylboronic acid pinacol ester by EDC coupling. After conjugation, the boronic ester group was oxidized using sodium periodate (NaIO4) and the naproxen prodrug (Scheme 1, compound 2, Figure S1–2) was obtained. This prodrug was conjugated onto dextran (9–11 kDa) by a coupling reaction with the boronic acid on the prodrug and the diol on dextran. However, according to a previous study, only 2.7 % of diols on dextran were modified by phenyl boronic acid because the reverse reaction occurs at a high rate.57 Considering that one of the greatest advantages of polymer-drug conjugates is high loading of drugs by chemical conjugation, a higher conjugation rate is required. Using an organic base increases the diol-boronic acid conjugation efficiency based on an equilibrium shift between the conjugation and cleavage.58–59 We used two different organic bases to improve conjugation efficacy. By adding 5 equivalents of triethylamine (TEA) to the synthesis of compound 2, the drug conjugation increased up to 33 % (33 per 100 glucose unit). The drug conjugation increased further up to 87 % when 5 equivalents of DBU were added. We speculate that because DBU is more miscible with the solvent, DMSO, the drug conjugation is more efficient under those conditions. The conjugation ratio was calculated using 1H-NMR or LC-MS by measuring the amount of conjugated and unconjugated prodrug.
Scheme 1.

Synthesis scheme of Nap-Dex and its H2O2-responsive cleavage. Reagent and conditions: a) naproxen, EDC, DMAP, DCM, 18 h, at r.t., 77 %. b) NaIO4, NH3OAc, Acetone/H2O, 18 h, r.t., 66 %. c) dextran, DBU, molecular sieves, DMSO, 18 h, 100 °C, 31 %.
The synthesized Nap-Dex was purified by dialysis against DMSO and ethyl acetate, and characterized by 1H-NMR and GPC. Compared to dextran, the molecular weight of Nap-Dex increased 1.4 times (Figure 2, A).
Figure 2.

A) and B) GPC signals of Nap-Dex (blue), dextran (black) and Nap-Dex incubated with 10 mM H2O2 (green). Samples were incubated for 24 hours before the measurement in 80 v% DMF and 20 v% aqueous solutions. C) Nap-Dex solution with/without 10 mM H2O2. Nap-Dex was dissolved in 50 v% DMSO and 50 v% H O and incubated for 3 hours at 37 ° Polymer concentration was 10 mg/ml and H2O2 concentration was adjusted to 0 mM or 10 mM. D) Turbidity change of the Nap-Dex solution measured by UV-Vis spectrometer. Nap-Dex solution (2 mg/ml in 50 v% DMSO and 50 v% aqueous) was incubated at 37 °C and the absorption at 750 nm was monitored by incubation time. E) LC-MS comparison between released molecules from Nap-Dex by H2O2 and pure naproxen. The Nap-Dex solution incubated with 10 mM H2O2 was filtrated using ultracentrifugal filtration and filtrated molecules were injected into LC-MS. F) Naproxen release from Nap-Dex by H2O2. The naproxen signal in LC-MS was monitored by incubation time. The release amount was calculated based on a calibration curve.
2.2. H2O2 reactivity of Nap-Dex
After the conjugation, we explored the H2O2 reactivity of Nap-Dex. Nap-Dex was incubated for 24 hours in a 10 mM H2O2 solution. The solution was analyzed via GPC and a UV absorption signal change was observed. UV absorption in the small molecular weight range (Rt: 18.5–27 min) increased significantly, suggesting that H2O2 cleaved the small molecules from the conjugated polymer (Figure 2, B). In addition, turbidity of the polymer solution changed remarkably after the incubation (Figure 2, C). The turbidity change was analyzed using UV spectroscopy by measuring absorbance at 750 nm. Absorption of the solution incubated with H2O2 drastically decreased within 20 min, while it maintained for 3 hours without H2O2 (Figure 2, D). The turbidity of the Nap-Dex solution with H2O2 decreased since hydrophilicity of the polymer increased by the H2O2-responsive drug release.
2.3. Naproxen release from Nap-Dex
For further confirmation of naproxen release, the released small molecules were analyzed by LC-MS and compared with pure naproxen. The Nap-Dex was incubated with 10 mM H2O2, then washed with methanol using ultracentrifugal filtration and injected into the LC-MS. Consequently; the released molecule had the exact same retention time (5.67 min) with pure naproxen (Figure 2, E). Furthermore, the mass of the released material (Mw, exp = 229.5) was identical with the measured mass of pure naproxen (Mw, exp = 229.5) and correlates well with the theoretical mass of naproxen (Mw, theory = 229.09). Based on these results, we concluded that naproxen was released from Nap-Dex after incubation in H2O2.
Naproxen released from Nap-Dex was analyzed by LC-MS at different incubation time points (-5, 0, 15, 30, 40, 60, 90 min). Figure 2, F shows that naproxen was completely released from the polymer within 20 min by 10 mM H2O2, while 14 % of drug released without H2O2. This may be due to hydrolysis of the esters between the drug and the linker. This confirms that H2O2 addition released the conjugated naproxen in a controlled manner. Moreover, the release of the naproxen profile corresponds well with the aforementioned turbidity change of the Nap-Dex solution.
The released small molecules observed using GPC were analyzed by NMR and verified to be naproxen. The Nap-Dex solution was incubated for 3 days with 50 mM H2O2. After the incubation time, the solution was lyophilized and the compounds were dissolved in d6-DMSO and analyzed by NMR. In Figure 3, a proton peak of the methoxyl group on naproxen before H2O2 addition (A-a), was a partially split broad peak at 3.79–3.82 ppm. On the other hand, the methoxyl group proton peak of naproxen after H2O2 addition (B-a), was a single peak at 3.81 ppm. We speculate that the peak was sharpened by H2O2 addition (B-a), because the naproxen was recovered as a small molecule from the polymer backbone. The speculation is supported by the LC-MS results, which shows the released small molecule having the same retention time and molecular mass as pure naproxen (Figure 2, E). Moreover, proton peaks of the linker largely shifted upon addition of H2O2 (Figure 3). The peak shift of the PBA linker confirms that the covalent bond between the linker and naproxen was cleaved. Although the ROS concentration is higher than the inflammatory environment, we conclude that the synthesized Nap-Dex polymer successfully released naproxen by naproxen linker cleavage in response to the H2O2.21
Figure 3.

A) NMR spectrum of Nap-Dex. B) NMR spectrum of Nap-Dex incubated with H2O2. Nap-Dex was dissolved in 50 v% DMSO and 50 v% 100 mM H2O2, and incubated for 3 days. Naproxen is labeled with small letters (green), and protons on the boronic ester linker are labeled with L1, L2, and L3 (L1’, L2’, and L3’ after cleavage). The protons on the dextran are labeled as D (blue).
2.4. NP formulation with Ac-Dex
After confirming drug release from the Nap-Dex, we formulated nanoparticles to co-deliver conjugated drug as well as a physically loaded cargo. Moreover, to achieve a dual-responsive system, we blended the Nap-Dex polymer with acid-responsive Ac-Dex during the particle formulation. Considering that the pH is slightly acidic in inflammation, blending acid-responsive polymer together with an ROS-responsive Nap-Dex would be a good strategy for anti-inflammatory drug delivery carriers. Nap-Dex and Ac-Dex blended nanoparticles (NPs) were formed by probe sonication using 5 mg of Nap-Dex and 5 mg of Ac-Dex. These dual-sensitive polymeric particles, which release chemically conjugated naproxen by H2O2 and physically loaded cargo by acids, was demonstrated to improve these functionalities for inflammation targeting (Figure 1). The hydrodynamic size of the formed particles was evaluated by dynamic light scattering (DLS) to be 260 nm (± 58 nm; Figure 4, A). The morphology of the particles, spherical nanoparticles, was observed by transmission electron microscopy (TEM; Figure 4, B).
Figure 4.

A) Size distribution of nanoparticles measured by DLS. B) Morphology of the nanoparticles observed by TEM.
The loading of the conjugated drug in the dual-responsive nanoparticles was calculated based on the conjugation ratio to be 27 wt%. This loading efficiency was more than 10 times higher compared with naproxen loaded nanoparticles such as lipid nanoparticles,60–61 magnetic nanoparticles,62 physically encapsulated polymeric nanoparticles,63 or polymeric prodrugs.64 However, the loading was lower compared to dextran-naproxen conjugate (68 wt% loading) utilizing an ester linkage between the hydroxyl group of dextran and carboxylate of naproxen.65
2.5. NP stability and pH-responsive NP size change
The stability of the NPs was monitored by DLS in varying pH and H2O2 concentrations. The NPs were incubated at pH 7.4 (with 0 mM and 10 mM H2O2) and at pH 5.0 (with 0 mM and 10 mM H2O2) for four days. The size of the NPs remained stable in pH 7.4 phosphate buffer for 4 days (Figure 5, A), while increased drastically at pH 5.0 within 24 hours. After 2 days of incubation at pH 5.0 the size was immeasurable due to the measurement limitation range. The increase of size can be explained by the hydration and dissociation of the particles by the hydrophilicity change of Ac-Dex, but also by the reverse reaction of the linker conjugation of Nap-Dex.66–67 Considering the H2O2-sensitivity of Nap-Dex, we expected a drastic disassembly of the NPs in a solution with 10 mM H2O2 at pH 7.4. However, the NP size remained stable at pH 7.4, even with 10 mM H2O2. The particles, incubated in 10 mM H2O2 for 24 hours, also maintained their spherical structures although polymeric aggregates were observed surrounding the particles. (see figure S3 for image and details). We assume that the reactivity decreased due to the hydrophobic core of the NPs, protecting Nap-Dex from H2O2 exposure, and partial release of naproxen from a surface of the nanoparticles was not enough to induce entire particle dissociation.
Figure 5.
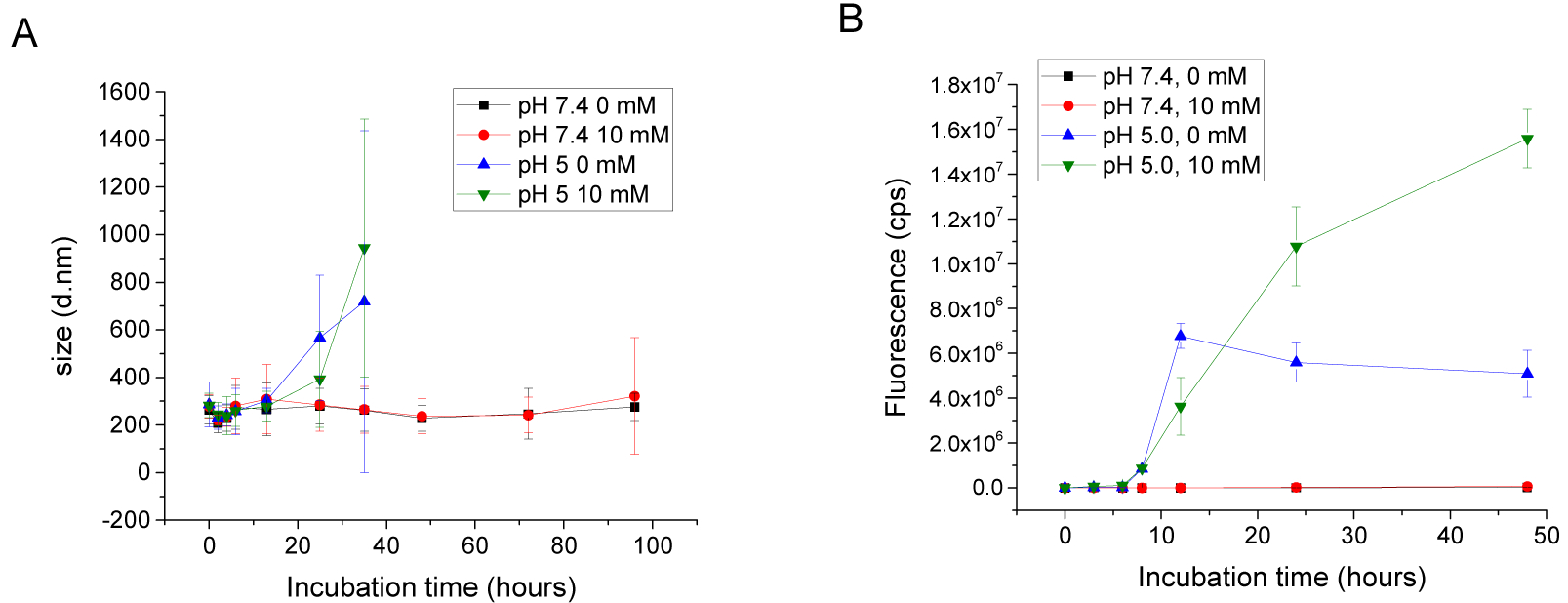
A) Size change of the nanoparticles by time, pH and H2O2 concentration. Black: pH 7.4 10 mM phosphate buffer with 0 mM H2O2. Red: pH 7.4 phosphate buffer with 10 mM H2O2. Blue: pH 5.0 10 mM acetate buffer with 0 mM H2O2. Green: pH 5.0 10 mM acetate buffer with 0 mM H2O2. (σ: calculated from obtained polydispersity index) B) Fluorescence signals of released rhodamine 6G from nanoparticles by stimuli and incubation time. Notations are same with A (n=3)
2.6. pH responsive cargo release
pH-responsive cargo release by particle dissociation was evaluated under the above conditions (pH 7.4 and pH 5.0 with 0 mM or 10 mM H2O2). Rhodamine 6G (an organic dye) was physically encapsulated into the NPs as a model cargo. The dye was dissolved in organic solutions (20 v% DMSO, 80 v% DCM) during the formulation process and 4 wt% of the dye was loaded. The rhodamine 6G loaded NPs were incubated in different buffer conditions and the released dye was separated by ultracentrifugal filtration. Fluorescence intensity of the collected dye was measured by a fluorescence spectrometer.
Figure 5, B shows the cargo release from the dual-responsive NPs after reacting to the stimuli. There was no release for 2 days at pH 7.4 with either 0 mM and 10 mM H2O2. This results correlate well with the particle size change under the same condition. On the other hand, under acidic conditions, the particle released its cargo after 9 hours, regardless of the presence of H2O2.
Interestingly, at pH 5.0, the cargo release was inhibited after 12 hours without H2O2. We assume that the release was suppressed since Nap-Dex remains hydrophobic, where some of the cargo remained in the NPs even after the Ac-Dex hydrolysis. Although de conjugation of the naproxen prodrug is preferred under acidic condition in Nap-Dex, we speculate that Nap-Dex is still partially hydrophobic considering the high conjugation ratio. To confirm it, we formed Nap-Dex nanoparticles without Ac-Dex and examined their stability at pH 5.0. The size of the particles measured by DLS maintained stable for 5 days at pH 5.0 (Figure S4).
Meanwhile, when the NPs were treated with both acid and H2O2 for 48 hours, the released fluorescent signal increased 2.5 times compared with the signals from the NPs in 0 mM H2O2 at pH 5.0. We believe that the cargo release increased due to the hydrophilicity change of both the Nap-Dex and Ac-Dex as a result of the stimuli. This result suggests that the cargo release from the dual-responsive NPs is more effective in inflammatory conditions, including both oxidative stress and acidosis.
2.7. Inflammation-triggered cargo release
Inflammation-triggered release was demonstrated in vitro by activating macrophages with lipopolysaccharide (LPS), a TLR4 agonist that induces the production of, amongst others, ROS.68 Due to enhanced cell activity after activation, pH also decreases in the microenvironment, creating an optimal inflammatory milieu to demonstrate drug release and anti-inflammatory efficacy from the dual-responsive NPs.
Fluorescein diacetate (FDA) was loaded into the nanoparticles as a model cargo to monitor inflammation-responsive cargo release. FDA is fluorescently inactive inside the NPs, but once it is released and internalized, cellular esterase converts FDA to fluorescent fluorescein.69–70 To confirm payload release from nanoparticles, LPS-activated or control (media) treated macrophages were incubated with particles containing FDA. Figure 6, A–B shows the fluorescence emission of fluorescein in macrophages, and LPS-activated cells resulted in a 1.3 times higher fluorescence intensity compared to media control (Figure 6, C). This demonstrates that the dual-responsive nanoparticles release cargo in an inflammatory environment.
Figure 6.

Triggered release of FDA from macrophages with intracellular conversion to fluorescein from NPs cultured with (A) LPS stimulation (5 Sg/ml) or (B) media control. Fluorescein fluorescence increase after LPS- initiated inflammation (C), quantifying A-B. Data is presented as mean ± SD; * p < 0.05.
2.8. ROS-responsive drug release in vitro
Most importantly, we evaluated inflammation-triggered efficacy of naproxen released from the NPs and verified the potential of the material as a drug-delivery vehicle. First, we confirmed that no toxicity was detected when tested in non-stimulated macrophages (Figure S5). Macrophages were then pre-activated with LPS and incubated with particles overnight. Pro-inflammatory cytokines were measured in the supernatants, and drug efficacy was verified by measuring reduced levels of the pro-inflammatory cytokines. An equivalent dose of the dual-responsive NPs were more efficient in reducing interleukin (IL)-6 levels than free naproxen (Figure 7, A). We also measured tumor necrosis factor alpha (TNFα), a pro-inflammatory cytokine reported to be less sensitive to cyclooxygenase (COX)-inhibitors such as naproxen.71 The NPs reduced TNFα to control levels (Figure 7, B), whereas the free drug by itself failed to reduce the cytokine. The increased levels of TNFα after treatment with free drug was concluded to be due to assay timing and dosing, as a longer incubation time with the free drug did not reproduce these increased results (results not shown).
Figure 7.

Macrophages were pre-activated (2 hours) with LPS (100 ng/ml, A and B), and then treatments were added overnight. IL-6 (A) and TNFα (B) were measured in the supernatant by enzyme linked immunosorbent assay (ELISA). C shows the quantification of reduced fluorescence of Amplex Red after 5 hours incubation with treatments and 150 SM H2O2 (without cells), measured 1h after assay stabilization. Data is presented as mean of triplicates assayed three times with similar results ± SD; * p < 0.05, *** p < 0.001
Instead, the increased efficacy indicated that the particles offer a synergistic anti-inflammatory effect with both naproxen and the H2O2-responsive PBA linker. We speculate that this increased efficacy could be due to the boronic ester acting as an ROS scavenger40–42. Considering that the anti-inflammatory drug is directly conjugated to the polymer, assaying the particles ROS scavenging ability in vitro would not distinguish the scavenging effect from the other anti-inflammatory effect that naproxen possess Thus we assayed the ROS scavenging capacity of the nanoparticles in a cuvette system, where the NPs scavenged H2O2 more efficiently than free drug (Figure 7, C). Interestingly, the Nap-Dex NPs were more efficient in ROS scavenging than its control Hybrid dextran (HyDex),48 a nanoparticle formed with OxiDex43 (boronic acid functionalized dextran similar to the Nap-Dex but without the naproxen) and AcDex at a 50:50 ratio similar to our NPs (Figure 7, C). We conclude that the Nap-Dex NPs has a higher H2O2 scavenging potential than the control particles due to a synergetic effect of the boronic ester and naproxen, and that Nap-dex potentially can scavenge 3 times more H2O2 than OxiDex, making it a more suitable treatment option for high ROS environments.
Together with Naproxen, the polymer also releases 4-hydroxybenzyl alcohol (HBA, see Scheme 1). HBA is known to play a role against oxidative stress related diseases such as stroke and cancer, and has shown to have anti-inflammatory and anti-angiogenic properties.72–73 Indeed, HBA has been designed to be released from polymers during the polymer’s hydrolytic degradation and used itself as an antioxidant.41, 74–76 We examined whether HBA or HyDex themselves could be attributed some of our drug-conjugated NPs extra anti-inflammatory effects at these doses. While we did see a decrease in IL-6 by both HBA and HyDex (Figure S6 a), interestingly, no effects were seen by either treatments when measuring TNFα (Figure S6, b), indicating another anti-inflammatory mechanism taking place. Taken together, conjugating naproxen to a ROS-responsive polymer nanoparticle created a treatment system with synergistic effects resulting in an overall increased anti-inflammatory efficacy compared to free naproxen itself.
3. Conclusion
We have designed and synthesized an ROS-responsive dextran-naproxen conjugate using a boronic acid linker. The drug was efficiently conjugated onto dextran and released by H2O2 in a controlled manner. To improve inflammation-sensitivity and controlled release of cargo, we formulated dual-responsive nanoparticles by blending Nap-Dex and Ac-Dex. We have successfully demonstrated an inflammation-responsive naproxen release and its efficacy. Interestingly, the dual-responsive NPs showed highly improved drug efficacy for both IL-6 and TNFα production as compared to free naproxen with an ROS scavenging effect. Additionally, we also examined a loaded cargo release from the nanoparticles triggered by inflammation in vitro. We believe that the drug conjugation through phenylboronic acid linkers can be applied to various dextran-based materials, and opens the door to further functionalization of carbohydrates.
4. Experimental Section
4.1. General methods and instrumentation
Unless otherwise noted all chemicals were purchased from Sigma Aldrich and used without further purification. Molecular sieves were obtained from Spectrum and Naproxen was acquired from MP Biomedicals. Purification of Synthesis products was performed by flash chromatography using Combiflash Rf 200 from Teledyne ISCO with RediSepRf normal phase column. Proton nuclear magnetic resonance (1H NMR) spectra were recorded using Varian 600 MHz with CDCl3 and DMSO-d6 as solvents. 13C NMRs were recorded using Varian NMR spectrometer at 150 MHz with each solvent. Chemical signals were reported as δ in parts per million (ppm). As a gel permeation chromatography (GPC), Agilent 1100 was utilized with PLgel Mixed D-D column. Exella E24 incubator shaker series by New brunswick scientific is used for particle incubation. Liquid chromatography & Mass spectrometry LCMS Agilent Technology. To measure fluorescence intensity, Horiba Fluorolog was utilized as a fluorescence spectrometer. Dynamic light scattering (DLS) using Malvern Zetasizer Nano ZS. Transmission electron microscopy (TEM, Tecnai FEI Spirit) for observing shape and morphology of the nanoparticles.
4.2. Synthesis
4.2.1. Synthesis of compound 1
4-Hydroxyphenylboronic acid pinacol ester was conjugated with naproxen. Naproxen (2.26 g, 1 eqv), 4-Hydroxyphenylboronic acid pinacol ester (2.3 g, 1 eqv.) and DMAP (1.44 g, 1.2 eqv.) were dissolved in anhydrous DCM (40 ml). After dissolving them completely, EDC (1.83 g, 1.2 eqv.) was added into the mixture. The reaction solution was agitated at r.t. After overnight reaction, the solution was diluted with DCM (260 ml) and washed using H2O (300 ml x3 times). Organic layer was collected and dried using MgSO4. The product was purified by column chromatography with ethylacetate and hexane (30:70). White solid was obtained as a product (3.38 g, yield: 77 %).
1H-NMR (600 MHz, CDCl3): δ 7.71 (d, J=7.8 Hz, 2H), 7.67 (t, J=9.1 Hz, 2H), 7.63 (s, 1H), 7.38 (dd, J= 8.4, 1.4 Hz, 1H), 7.21 (d, J= 7.9 Hz, 2H), 7.12 (dd, J= 9.0, 2.2 Hz, 1H), 7.09 (d, J= 2.1 Hz, 1H), 5.1 (q, J= 18.8, 12.8, 2H), 3.9 (s, 3H), 1.57 (d, J= 7.2 Hz, 3H), 1.31 (s, 12H)
13C-NMR (150 MHz, CDCl3): δ 174.6, 157.8, 139.2, 135.6, 135.1, 133.9, 129.5, 129.1, 127.3, 127.2, 126.5, 126.2, 119.2, 105.7, 84.0, 66.5, 55.5, 45.6, 25.0, 18.7
MS (ESI) m/z: [M+Na] + calcd for C27H31BO5Na, 469.22; found 469.1
4.2.2. Synthesis of compound 2
Compound 1 (2.4 g, 5.38 mmol), Sodium periodate (NaIO4) (3.45 g, 16.14 mmol) and Na4OAc (1.24 g, 16.14 mmol) were dissolved in 250 ml aceton and 125 ml water under stirring in an round bottom flask; stirring was continued overnight at room temperature. After removing the aceton under reduced pressure, the product was filtered and purified by a column chromatography with 20 % methanol 80 % DCM. The resulting product was precipitated into water and dried under vacuum (1.23 g, 66 %).
1H-NMR (600 MHz, d6-DMSO): δ 7.78 (d, J= 8.7 Hz, 2H), 7.72 (s, 2H), 7.77 (d, J=7.6 Hz, 1H), 7.4 (dd, J=3.2 Hz, 1.5 Hz, 1H), 7.3 (d, J=2.7 Hz, 1H), 7.19 (d, J=8.0 Hz, 2H), 7.15 (dd, J= 8.4Hz, 2.6 Hz, 1H), 5.12 (s, 2H), 4.00 (dd, J=7.1 Hz, 13.9 Hz, 2H), 3.87 (s, 3H), 3.83 (m, J= 13.1, 1H), 1.5 (d, J=7.6 Hz, 3H)
13C-NMR (150 MHz, d6-DMSO): δ 173.8, 157.2, 137.9, 135.6, 134.2, 133.3, 129.2, 128.4, 127.0, 126.5, 126.3, 125.7, 118.8, 105.7, 65.7, 55.2, 44.4, 18.4
MS (ESI) m/z: [M+2Na] + calcd for C21H20BO5Na2,409.12; found 409.1
4.2.3. Synthesis of Nap-Dex
Compound 2 (426 mg, 0.617 mmol) and Dextran (10 kDa, 200 mg, 0.617 mmol) were dissolved in 2 ml anhydrous DMSO in an oven dry 15 ml round bottom flask equipped with a stirring bar and molecular sieves. 1,2-diazabicyclo(5.4.0)undec-7-ene(1,5-5) (DBU) (1.29 ml, 4.32 mmol) was added to the solution and stirring was continued over night at room temperature. The Polymer was washed by dialysis using a membrane with MWCO of 3500 Da against DMSO and 1 % DBU. Then, the product was precipitated into ethyl acetate and dried overnight under vacuum (193.4 mg, 31 %).
4.3.1. Turbidity change observation
Turbidity change observation of Nap-Dex by H2O2 addition. Nap-Dex was dissolved in DMSO then diluted with same volume of 20 mM H2O2 solution or DI water. The final concentration of polymer was set to 10 mg/ml and H2O2 concentration was set to 0 mM or 10 mM. Each sample was incubated at 37 °C and absorption at 750 nm was measured using UV-Vis spectrometer after cooling down the sample temperature to r.t.
4.3.2. H2O2 responsive naproxen release
Release of small compound from the Nap-Dex was firstly observed by GPC. The Nap-Dex was dissolved in DMF and diluted with 50 mM H2O2 (DMF 80 v%, H2O2 (aq) 20 v%), therefore the final concentration was set to 4 mg/ml Nap-Dex and 10 mM H2O2 solution. The sample was incubated at 37°C for 24 hours. After the incubation time, the sample was injected into GPC and the UV absorption signal (at 280 nm) was compared with control (incubated Nap-Dex with 0 mM H2O2).
To analyze released molecules by H2O2, the released small molecules were collected and injected into LC-MS. The Nap-Dex (10 mg/ml) in 50 % DMSO, and 50 % H2O2 (0 mM or 10 mM) was incubated. At each time points, the sample was diluted by 80 % MeOH, and filtrated using centrifugal filtration (Amicon Ultra, 3 kDa, 13 krpm). The filtrated solution was injected into LC-MS.
The released materials were also analyzed by 1H-NMR. The Nap-Dex (10 mg/ml) in 50 % DMSO, and 50 % H2O2 (50 mM) was incubated for 3 days. Resulting solution was lyophilized to remove solution including H2O2. Obtained solids were dissolved in d6-DMSO for NMR measurement.
4.4.1. Particle formulation
Nanoparticles were formed using Nap-Dex and Ac-Dex by probe sonication. Nap-Dex (50 mg) was dissolved in DMSO (300 Sl), and Ac-Dex (50 mg) was dissolved in DCM (2.7 ml). Each solution was mixed well and added into in 1 w% PVA (aq) (60 ml). The resulting solution was sonicated in ice bath (5 min, 10 W, 2500 kJ). After the sonication, DCM was removed by vacuum. To remove PVA and DMSO, the particle solution was settle down using centrifuge (13 krpm, 15 min). Supernatant was removed and pallet was re dispersed in PBS. The washing was repeated 3 times.
4.4.2. Particle stability and degradation by stimuli
Nanoparticles were dissolved (10 Sg/ml) in each buffer, pH 7.4 phosphate buffer with 0 mM H2O2, pH 7.4 phosphate buffer with 10 mM H2O2, pH 5.0 acetate buffer with 0 mM H2O2, pH 5.0 acetate buffer with 10 mM H2O2, and incubated at 37 °C. Size and scattering intensity of the particles were measured by DLS (Zetasizer, nanoseries) at each time point
4.4.3. Cargo release by stimuli
The NPs solution was prepared in various buffer conditions (same with stability test condition). The NPs were incubated for 2 days at 37 °C. At each time points, 500 Sl of the NPs solution was washed using ultracentrifugal filtration (MWCO 10 kDa). Fluorescence intensity of the filtrated solution was measured by fluorescence spectrometry.
4.5. Cell in vitro studies
4.5.1. Inflammation-triggered FDA cargo release in macrophages
20 000 RAW 264.7 murine macrophages were grown as previously described and stimulated with LPS (5 Sg/mL) for 2 hours before NP addition. 2 hours after incubation with NPs, the media was washed and the release of FDA was measured by fluorescence measurement (λex= 495 nm, λem = 514 nm) using a plate reader (Molecular Devices SpectraMax M5) after a 4-hour incubation (determined by a time titration study), run in triplicates, and normalized to media background. To confirm release, fluorescence microscopy images of cells were acquired (Nikon TS100F).
4.5.2. Pro-inflammatory cytokine inhibition by Nap-Dex NP from macrophages in vitro
Raw 264.7 murine macrophages were activated by LPS (100 ng/ml) for 2 hours. Treatments were added at a final concentration of 5.2 mM: Free Naproxen, Nap-Dex (NPs) or media control. Cells were incubated over night before supernatants were collected. IL-6 and TNFα levels were measured using ELISA (Mouse IL-6 or TNFα DuoSet ELISA, R&D systems).
4.6. H2O2-quenching measured by Amplex Red
Nanoparticles and free drugs were dissolved at an equivalent of 19 mM Free Naproxen, NapDex (NP), hydroxybenzyl alcohol (HBA), or Hybrid dextran nanoparticles (HyDex) in Amplex Red Assay Buffer (control, Thermo Fisher). Particles were incubated for 5 hours at a final concentration of 150 SM H2O2 (determined by dose-, and time titration studies). Following manufacturers instructions, the Amplex Red reagents and HRP working solution was added to all samples and incubated for 30 minutes in the dark. The fluorescence was measured at ex530/em590. Background fluorescence was removed from all samples and a percentage of H2O2 quenching was calculated by dividing (sample reading/ control reading) ×100. The Assay was run in triplicate and repeated three times with similar results.
4.8. Statistical analysis
Data is presented as mean values ± SD unless otherwise indicated. Statistical analysis was performed using GraphPad Prism (GraphPad Software) version 7.0b. T-test (comparing two groups) or one-way analysis of variance (ANOVA) was used to compare independent groups; all groups were statistically compared followed by Tukey’s post hoc multiple comparisons test. P values <0.05 were considered statistically significant.
Supplementary Material
ACKNOWLEDGMENT
NMR data was acquired at the UCSD Skaggs School of Pharmacy and Pharmaceutical Sciences NMR Facility.
Funding Sources
The work was supported by the Air Force Office of Scientific Research (AFOSR) FA9550-15-1-0273, National Institutes of Health R01EY024134, and the Swedish Research Council’s International Postdoc Grant (AS: 2015-06470).
ABBREVIATIONS
- Ac-Dex
acetalated dextran
- COX
cyclooxygenase
- DLS
dynamic light scattering
- ELISA
enzyme linked immunosorbent assay
- FDA
fluorescein diacetate
- HBA
4-hydroxybenzyl alcohol
- IL-6
interleukin 6
- LPS
lipopolysaccharide
- Nap-Dex
naproxen conjugated to dextran through a phenylboronic acid linker
- NP
nanoparticles
- PBA
Phenylboronic acid
- ROS
reactive oxygen species
- TEA
triethylamine
- TEM
transmission electron microscopy
- TNFα
tumor necrosis factor alpha
Footnotes
Supporting Information.
The Supporting Information is available free of charge via the Internet at http://pubs.acs.org.
The Supporting Information consists of: Figure S1a 1H-NMR spectrum of Compound 1, Figure S1b 13C-NMR spectrum of Compound 1,Figure S2a 1H-NMR spectrum of Compound 2, Figure S2b 13C-NMR spectrum of Compound 2, Figure S3 TEM image of Nap-Dex/Ac-Dex NPs in 10 mM H2O2, Figure S4 Size measurements of Nap-Dex blended with PLGA at pH 5.0, Figure S5 Cell viability, Figure S6 ELISA measurements from LPS stimulated macrophages in vitro
Contributor Information
Naomi Hamelmann, Department of Biomolecular Nanotechnology, MESA+ Institute of Nanotechnology, Faculty of Science and Technology, University of Twente, P.O. Box 217, 7500 AE Enschede, The Netherlands.
Vincent Tran, Department of Chemistry and Biochemistry, University of California, San Diego, La Jolla, CA, 92093, USA.
Adah Almutairi, Center of Excellence in Nanomedicine, Skaggs School of Pharmacy and Pharmaceutical Sciences, Departments of NanoEngineering and Material Science and Engineering, University of California, San Diego, La Jolla, CA, 92093, USA.
REFERENCES
- 1.Ganta S; Devalapally H; Shahiwala A; Amiji M, A Review of Stimuli-Responsive Nanocarriers for Drug and Gene Delivery. J Control Release 2008, 126 (3), 187–204. [DOI] [PubMed] [Google Scholar]
- 2.Onaca O; Enea R; Hughes DW; Meier W, Stimuli-Responsive Polymersomes as Nanocarriers for Drug and Gene Delivery. Macromol Biosci 2009, 9 (2), 129–139. [DOI] [PubMed] [Google Scholar]
- 3.Meng FH; Zhong ZY; Feijen J, Stimuli-Responsive Polymersomes for Programmed Drug Delivery. Biomacromolecules 2009, 10 (2), 197–209. [DOI] [PubMed] [Google Scholar]
- 4.Stuart MAC; Huck WTS; Genzer J; Muller M; Ober C; Stamm M; Sukhorukov GB; Szleifer I; Tsukruk VV; Urban M; Winnik F; Zauscher S; Luzinov I; Minko S, Emerging Applications of Stimuli-Responsive Polymer Materials. Nat Mater 2010, 9 (2), 101–113. [DOI] [PubMed] [Google Scholar]
- 5.Motornov M; Roiter Y; Tokarev I; Minko S, Stimuli-Responsive Nanoparticles, Nanogels and Capsules for Integrated Multifunctional Intelligent Systems. Prog Polym Sci 2010, 35 (1–2), 174–211. [Google Scholar]
- 6.Liu F; Urban MW, Recent Advances and Challenges in Designing Stimuli-Responsive Polymers. Prog Polym Sci 2010, 35 (1-2), 3–23. [Google Scholar]
- 7.Esser-Kahn AP; Odom SA; Sottos NR; White SR; Moore JS, Triggered Release from Polymer Capsules. Macromolecules 2011, 44 (14), 5539–5553. [Google Scholar]
- 8.Hoffman AS, Stimuli-Responsive Polymers: Biomedical Applications and Challenges for Clinical Translation. Adv Drug Deliver Rev 2013, 65 (1), 10–16. [DOI] [PubMed] [Google Scholar]
- 9.Mura S; Nicolas J; Couvreur P, Stimuli-Responsive Nanocarriers for Drug Delivery. Nat Mater 2013, 12 (11), 991–1003. [DOI] [PubMed] [Google Scholar]
- 10.Chen HC; Liu DY; Guo ZJ, Endogenous Stimuli-Responsive Nanocarriers for Drug Delivery. Chem Lett 2016, 45 (3), 242–249. [Google Scholar]
- 11.Hatakeyama H, Recent Advances in Endogenous and Exogenous Stimuli-Responsive Nanocarriers for Drug Delivery and Therapeutics. Chem Pharm Bull 2017, 65 (7), 612–617. [DOI] [PubMed] [Google Scholar]
- 12.Zhou MX; Wen KK; Bi Y; Lu HR; Chen J; Hu Y; Chai ZF, The Application of Stimuli-responsive Nanocarriers for Targeted Drug Delivery. Curr Top Med Chem 2017, 17 (20), 2319–2334. [DOI] [PubMed] [Google Scholar]
- 13.Ma N; Li Y; Xu H; Wang Z; Zhang X, Dual Redox Responsive Assemblies Formed from Diselenide Block Copolymers. J Am Chem Soc 2010, 132 (2), 442–443. [DOI] [PubMed] [Google Scholar]
- 14.Xu H; Cao W; Zhang X, Selenium-Containing Polymers: Promising Biomaterials for Controlled Release and Enzyme Mimics. Acc Chem Res 2013, 46 (7), 1647–1658. [DOI] [PubMed] [Google Scholar]
- 15.Cao W; Gu Y; Li T; Xu H, Ultra-Sensitive ROS-Responsive Tellurium-Containing Polymers. Chem Commun (Camb) 2015, 51 (32), 7069–7071. [DOI] [PubMed] [Google Scholar]
- 16.Alarcon CDH; Pennadam S; Alexander C, Stimuli Responsive Polymers for Biomedical Applications. Chem Soc Rev 2005, 34 (3), 276–285. [DOI] [PubMed] [Google Scholar]
- 17.Lu Y; Sun WJ; Gu Z, Stimuli-Responsive Nanomaterials for Therapeutic Protein Delivery. J Control Release 2014, 194, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu MR; Du HL; Zhang WJ; Zhai GX, Internal Stimuli-Responsive Nanocarriers for Drug Delivery: Design Strategies and Applications. Mat Sci Eng C-Mater 2017, 71, 1267–1280. [DOI] [PubMed] [Google Scholar]
- 19.Chapple ILC, Reactive Oxygen Species and Antioxidants in Inflammatory Diseases. J Clin Periodontol 1997, 24 (5), 287–296. [DOI] [PubMed] [Google Scholar]
- 20.Apel K; Hirt H, Reactive Oxygen Species: Metabolism, Oxidative Stress, and Signal Transduction. Annu Rev Plant Biol 2004, 55, 373–399. [DOI] [PubMed] [Google Scholar]
- 21.Niethammer P; Grabher C; Look AT; Mitchison TJ, A Tissue-Scale Gradient of Hydrogen Peroxide Mediates Rapid Wound Detection in Zebrafish. Nature 2009, 459 (7249), 996–U123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joshi-Barr S; Lux CD; Mahmoud E; Almutairi A, Exploiting Oxidative Microenvironments in the Body as Triggers for Drug Delivery Systems. Antioxid Redox Sign 2014, 21 (5), 730–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vicent MJ; Duncan R, Polymer Conjugates: Nanosized Medicines for Treating Cancer. Trends Biotechnol 2006, 24 (1), 39–47. [DOI] [PubMed] [Google Scholar]
- 24.Luo C; Sun J; Sun BJ; He ZG, Prodrug-Based Nanoparticulate Drug Delivery Strategies for Cancer Therapy. Trends Pharmacol Sci 2014, 35 (11), 12–22. [DOI] [PubMed] [Google Scholar]
- 25.Pang X; Du HL; Zhang HQ; Zhai YJ; Zhai GX, Polymer-Drug Conjugates: Present State of Play and Future Perspectives. Drug Discov Today 2013, 18 (23-24), 1316–1322. [DOI] [PubMed] [Google Scholar]
- 26.Kopecek J, Polymer-drug conjugates: Origins, Progress to Date and Future Directions. Adv Drug Deliver Rev 2013, 65 (1), 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duncan R, The Dawning Era of Polymer Therapeutics. Nat Rev Drug Discov 2003, 2 (5), 347–360. [DOI] [PubMed] [Google Scholar]
- 28.Hoffman AS; Stayton PS, Conjugates of Stimuli-Responsive Polymers and Proteins. Prog Polym Sci 2007, 32 (8–9), 922–932. [Google Scholar]
- 29.Pang X; Jiang Y; Xiao QC; Leung AW; Hua HY; Xu CS, pH-Responsive Polymer-Drug Conjugates: Design and Progress. J Control Release 2016, 222, 116–129. [DOI] [PubMed] [Google Scholar]
- 30.Chang ML; Zhang F; Wei T; Zuo TT; Guan YY; Lin GM; Shao W, Smart Linkers in Polymer-Drug Conjugates for Tumor-Targeted Delivery. J Drug Target 2016, 24 (6), 475–491. [DOI] [PubMed] [Google Scholar]
- 31.Lv SX; Tang ZH; Zhang DW; Song WT; Li MQ; Lin J; Liu HY; Chen XS, Well-Defined Polymer-Drug Conjugate Engineered with Redox and pH-Sensitive Release Mechanism for Efficient Delivery of Paclitaxel. J Control Release 2014, 194, 220–227. [DOI] [PubMed] [Google Scholar]
- 32.Aguirre-Chagala YE; Santos JL; Huang YX; Herrera-Alonso M, Phenylboronic Acid-Installed Polycarbonates for the pH-Dependent Release of Diol-Containing Molecules. Acs Macro Lett 2014, 3 (12), 1249–1253. [DOI] [PubMed] [Google Scholar]
- 33.Saravanakumar G; Kim J; Kim WJ, Reactive-Oxygen-Species-Responsive Drug Delivery Systems: Promises and Challenges. Adv Sci 2017, 4 (1): 1600124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiu FY; Zhang M; Ji R; Du FS; Li ZC, Oxidation-Responsive Polymer-Drug Conjugates with a Phenylboronic Ester Linker. Macromol Rapid Comm 2015, 36 (22), 2012–2018. [DOI] [PubMed] [Google Scholar]
- 35.Kwon J; Kim J; Park S; Khang G; Kang PM; Lee D, Inflammation-Responsive Antioxidant Nanoparticles Based on a Polymeric Prodrug of Vanillin. Biomacromolecules 2013, 14 (5), 1618–1626. [DOI] [PubMed] [Google Scholar]
- 36.Kuivila HG, Electrophilic Displacement Reactions .3. Kinetics of the Reaction between Hydrogen Peroxide and Benzeneboronic Acid. J Am Chem Soc 1954, 76 (3), 870–874. [Google Scholar]
- 37.Kuivila HG; Armour AG, Electrophilic Displacement Reactions .9. Effects of Substituents on Rates of Reactions between Hydrogen Peroxide and Benzeneboronic Acid. J Am Chem Soc 1957, 79 (21), 5659–5662. [Google Scholar]
- 38.Miller EW; Albers AE; Pralle A; Isacoff EY; Chang CJ, Boronate-Based Fluorescent Probes for Imaging Cellular Hydrogen Peroxide. J Am Chem Soc 2005, 127 (47),16652–16659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee S; Stubelius A; Olejniczak J; Jang H; Huu VAN; Almutairi A, Chemical Amplification Accelerates Reactive Oxygen Species Triggered Polymeric Degradation. Biomater Sci 2017, 6 (1), 107–114. [DOI] [PubMed] [Google Scholar]
- 40.Guo Z; Shin I; Yoon J, Recognition and Sensing of Various Species Using Boronic Acid Derivatives. Chem Commun (Camb) 2012, 48 (48), 5956–5967. [DOI] [PubMed] [Google Scholar]
- 41.Lee D; Park S; Bae S; Jeong D; Park M; Kang C; Yoo W; Samad MA; Ke Q; Khang G; Kang PM, Hydrogen Peroxide-Activatable Antioxidant Prodrug as a Targeted Therapeutic Agent for Ischemia-Reperfusion Injury. Sci Rep-Uk 2015, 5,16592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang RX; Ahmed T; Li LY; Li J; Abbasi AZ; Wu XY, Design of Nanocarriers for Nanoscale Drug Delivery to Enhance Cancer Treatment using Hybrid Polymer and Lipid Building Blocks. Nanoscale 2017, 9 (4), 1334–1355. [DOI] [PubMed] [Google Scholar]
- 43.Broaders KE; Grandhe S; Frechet JMJ, A Biocompatible Oxidation-Triggered Carrier Polymer with Potential in Therapeutics. J Am Chem Soc 2011, 133 (4), 756–758. [DOI] [PubMed] [Google Scholar]
- 44.Mehvar R, Dextrans for Targeted and Sustained Delivery of Therapeutic and Imaging Agents. J Control Release 2000, 69 (1), 1–25. [DOI] [PubMed] [Google Scholar]
- 45.Ferreira LS; Gerecht S; Fuller J; Shieh HF; Vunjak-Novakovic G; Langer R, Bioactive Hydrogel Scaffolds for Controllable Vascular Differentiation of Human Embryonic Stem Cells. Biomaterials 2007, 28 (17), 2706–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun GM; Mao JJ, Engineering Dextran-Based Scaffolds for Drug Delivery and Tissue Repair. Nanomedicine-Uk 2012, 7 (11), 1771–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pramod PS; Shah R; Jayakannan M, Dual Stimuli Polysaccharide Nanovesicles for Conjugated and Physically Loaded Doxorubicin Delivery in Breast Cancer Cells. Nanoscale 2015, 7 (15), 6636–6652. [DOI] [PubMed] [Google Scholar]
- 48.Viger ML; Collet G; Lux J; Huu VAN; Guma M; Foucault-Collet A; Olejniczak J; Joshi-Barr S; Firestein GS; Almutairi A, Distinct ON/OFF Fluorescence Signals from Dual-Responsive Activatable Nanoprobes Allows Detection of Inflammation with Improved Contrast. Biomaterials 2017, 133, 119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Formica ML; Calles JA; Palma SD, Polysaccharide-Based Nanocarriers for Ocular Drug Delivery. Curr Pharm Design 2015, 21 (33), 4851–4868. [DOI] [PubMed] [Google Scholar]
- 50.Mitra S; Gaur U; Ghosh PC; Maitra AN, Tumour Targeted Delivery of Encapsulated Dextran-Doxorubicin Conjugate Using Chitosan Nanoparticles as Carrier. J Control Release 2001, 74 (1–3), 317–323. [DOI] [PubMed] [Google Scholar]
- 51.Bachelder EM; Beaudette TT; Broaders KE; Dashe J; Frechet JMJ, Acetal-Derivatized Dextran: An Acid-Responsive Biodegradable Material for Therapeutic Applications. J Am Chem Soc 2008, 130 (32), 10494–10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Broaders KE; Cohen JA; Beaudette TT; Bachelder EM; Frechet JMJ, Acetalated Dextran is a Chemically and Biologically Tunable Material for Particulate Immunotherapy. P Natl Acad Sci USA 2009, 106 (14), 5497–5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohen JA; Beaudette TT; Cohen JL; Brooders KE; Bachelder EM; Frechet JMJ, Acetal-Modified Dextran Microparticles with Controlled Degradation Kinetics and Surface Functionality for Gene Delivery in Phagocytic and Non-Phagocytic Cells. Adv Mater 2010, 22 (32), 3593–3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suarez S; Grover GN; Braden RL; Christman KL; Amutairi A, Tunable Protein Release from Acetalated Dextran Microparticles: A Platform for Delivery of Protein Therapeutics to the Heart Post-MI. Biomacromolecules 2013, 14 (11), 3927–3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cohen JL; Schubert S; Wich PR; Cui L; Cohen JA; Mynar JL; Frechet JMJ, Acid-Degradable Cationic Dextran Particles for the Delivery of siRNA Therapeutics. Bioconjugate Chem 2011, 22 (6), 1056–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bachelder EM; Beaudette TT; Broaders KE; Frechet JMJ; Albrecht MT; Mateczun AJ; Ainslie KM; Pesce JT; Keane-Myers AM, In Vitro Analysis of Acetalated Dextran Microparticles as a Potent Delivery Platform for Vaccine Adjuvants. Mol Pharmaceut 2010, 7 (3), 826–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li LX; Bai ZW; Levkin PA, Boronate-dextran: An Acid-Responsive Biodegradable Polymer for Drug Delivery. Biomaterials 2013, 34 (33), 8504–8510. [DOI] [PubMed] [Google Scholar]
- 58.Diemer SL; Kristensen M; Rasmussen B; Beeren SR; Pittelkow M, Simultaneous Disulfide and Boronic Acid Ester Exchange in Dynamic Combinatorial Libraries. Int J Mol Sci 2015, 16 (9), 21858–21872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yan J; Springsteen G; Deeter S; Wang BH, The Relationship among pK(a), pH, and Binding Constants in the Interactions between Boronic Acids and Diols - It is not as Simple as it Appears. Tetrahedron 2004, 60 (49), 11205–11209. [Google Scholar]
- 60.Pardeike J; Hommoss A; Muller RH, Lipid Nanoparticles (SLN, NLC) in Cosmetic and Pharmaceutical Dermal Products. Int J Pharmaceut 2009, 366 (1-2), 170–184. [DOI] [PubMed] [Google Scholar]
- 61.Puglia C; Blasi P; Rizza L; Schoubben A; Bonina F; Rossi C; Ricci M, Lipid Nanoparticles for Prolonged Topical Delivery: An in Vitro and in Vivo Investigation. Int J Pharmaceut 2008, 357 (1–2), 295–304. [DOI] [PubMed] [Google Scholar]
- 62.Thammawong C; Sreearunothai P; Petchsuk A; Tangboriboonrat P; Pimpha N; Opaprakasit P, Preparation and Characterizations of Naproxen-Loaded Magnetic Nanoparticles Coated with PLA-g-Chitosan Copolymer. J Nanopart Res 2012, 14 (8) 1046–1047. [Google Scholar]
- 63.Rodrigues MR; Lanzarini CM; Ricci E, Preparation, in Vitro Characterization and in Vivo Release of Naproxen Loaded in Poly-Caprolactone Nanoparticles. Pharm Dev Technol 2011, 16 (1), 12–21. [DOI] [PubMed] [Google Scholar]
- 64.Azori M; Pato J; Csakvari E; Tudos F, Polymeric Prodrugs .5. Dextran-Bound Antirheumatic Agent Naproxen. Makromol Chem 1986, 187 (9), 2073–2080. [Google Scholar]
- 65.Hornig S; Bunjes H; Heinze T, Preparation and Characterization of Nanoparticles based on Dextran-Drug Conjugates. J Colloid Interf Sci 2009, 338 (1), 56–62. [DOI] [PubMed] [Google Scholar]
- 66.Li YP; Xiao WW; Xiao K; Berti L; Luo JT; Tseng HP; Fung G; Lam KS, Well-Defined, Reversible Boronate Crosslinked Nanocarriers for Targeted Drug Delivery in Response to Acidic pH Values and cis-Diols. Angew Chem Int Edit 2012, 51 (12), 2864–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.He LH; Fullenkamp DE; Rivera JG; Messersmith PB, pH Responsive Self-Healing Hydrogels Formed by Boronate-Catechol Complexation. Chem Commun 2011, 47 (26), 7497–7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Griffiths HR; Gao D; Pararasa C, Redox Regulation in Metabolic Programming and Inflammation. Redox Biol 2017, 12, 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Coons AH; Kaplan MH, Localization of Antigen in Tissue Cells .2. Improvements in a Method for the Detection of Antigen by Means of Fluorescent Antibody. J Exp Med 1950, 91 (1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rotman B; Papermaster BW, Membrane Properties of Living Mammalian Cells as Studied by Enzymatic Hydrolysis of Fluorogenic Esters. P Natl Acad Sci USA 1966, 55 (1), 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hartman DA; Ochalski SJ; Carlson RP, The Effects of Antiinflammatory and Antiallergic Drugs on Cytokine Release after Stimulation of Human Whole-Blood by Lipopolysaccharide and Zymosan A. Inflamm Res 1995, 44 (7), 269–274. [DOI] [PubMed] [Google Scholar]
- 72.Lim EJ; Kang HJ; Jung HJ; Park EH, Anti-Angiogenic, Anti-Inflammatory and Anti Nociceptive Activity of 4 Hydroxybenzyl Alcohol. J Pharm Pharmacol 2007, 59 (9), 1235–1240. [DOI] [PubMed] [Google Scholar]
- 73.Laschke MW; Vorsterman van Oijen AE; Scheuer C; Menger MD, In Vitro and in Vivo Evaluation of the Anti-Angiogenic Actions of 4-Hydroxybenzyl Alcohol. Br J Pharmacol 2011, 163 (4), 835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim S; Park H; Song Y; Hong D; Kim O; Jo E; Khang G; Lee D, Reduction of Oxidative stress by p-Hydroxybenzyl Alcohol-Containing Biodegradable Polyoxalate Nanoparticulate Antioxidant. Biomaterials 2011, 32 (11), 3021–3029. [DOI] [PubMed] [Google Scholar]
- 75.Park H; Kim S; Kim S; Song Y; Seung K; Hong D; Khang G; Lee D, Antioxidant and Anti-Inflammatory Activities of Hydroxybenzyl Alcohol Releasing Biodegradable Polyoxalate Nanoparticles. Biomacromolecules 2010, 11 (8), 2103–2108. [DOI] [PubMed] [Google Scholar]
- 76.Lee D; Bae S; Ke Q; Lee J; Song B; Karumanchi SA; Khang G; Choi HS; Kang PM, Hydrogen Peroxide-Responsive Copolyoxalate Nanoparticles for Detection and Therapy of Ischemia-Reperfusion Injury. J Control Release 2013, 172 (3), 1102–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


