Abstract
Ziziphus mucronata is an important multi-purpose plant species that has been used in African traditional medicine for ages in the treatment of various devastating human and animal infections. The current paper is aimed at providing an overview of uses, toxicology, pharmacological properties and phytochemistry of Z. mucronata. The information used in the current work was retrieved using various search engines, including Pubmed, Science Direct, Google Scholar, Scielo, SciFinder and Scopus. The key words used included Ziziphus mucronata, secondary metabolites, chemistry, biological activity and pharmacology, anti-inflammatory, antimicrobial, antifungal, antiviral, ethnobotanical survey, medicinal uses, safety, toxicology and other related words. Out of the 46 infections which the plant species is used to treat, the most common uses includes sexually transmitted infections, skin infections, diarrhoea and dysentery, respiratory and chest complaints and gynaecological complaints (citations ≥6). Pharmacologically, the plant species exhibited a potential antimicrobial activity yielding a minimum inhibitory concentration of <1 mg/ml against important pathogens which includes Mycobacterium tuberculosis, Moraxella catarrhalis, Staphylococcus aureus, Escherichia coli, Propionibacterium acnes, Candida albicans, Cryptoccoos neoformans amongst other microorganisms. Furthermore, the extracts and compounds from Z mucronata revealed potent antiviral, antioxidant, anti-inflammatory and other activities in vitro. Phytochemically, cyclo-peptide alkaloids (commonly called mucronines) dominates and in conjunction with triterpenes, flavonoids, phenolic acids and anthocyanins. Besides these compounds, the plant species exhibited the presence of important in minerals. These phytoconstituents may well explain the reported biological activities. Although the extracts revealed no cytotoxic effect to Vero cells, further toxicological characteristics of the plant species still needs to be explored. There is also a need to carry out the comprehensive safety profiles of the plant species, including heavy metal detection. Although the plant species revealed important biological activities, which includes antimicrobial, antiviral, anti-diabetic, anti-inflammatory, anti-oxidant, anti-plasmodial, anthelmintic, and anti-anaemic activity in vitro, further research is needed to explore the in vivo studies, other compounds responsible for such activities and the mechanisms of action thereof. Such activities validates the use of the plant species in traditional medicine. The data on the possible use of the plant species in the treatment of diarrhoea, sexually transmitted infections, skin related and gynaecological complaints are scant and still needs to be explored and validated both in vitro and in vivo. Furthermore, the anticancer and anthelmintic activity of the plant species also needs to be explored.
Keywords: Toxicity, Toxicology, Antimicrobial, Antioxidant, Ethnomedicinal uses, Antidiabetic., Cyclopeptides, Anthelmintic, Antiplasmodial, Ziziphus mucronate, Chemistry, Food science, Agricultural science, Biological sciences, Veterinary medicine
Toxicity; Toxicology; Antimicrobial; Antioxidant; Ethnomedicinal uses; Antidiabetic.; Cyclopeptides; Anthelmintic; Antiplasmodial; Ziziphus mucronate, Chemistry; Food science; Agricultural science; Biological sciences; Veterinary medicine.
1. Introduction
Southern Africa is blessed with variety of medicinal plants species which are used in the treatment and management of various pathogenic infections that threaten human and animal health. Ziziphus mucronata Willd. is an important multi-purpose member of family Rhamnaceae. The genus Ziziphus comprise of approximately 135 plant species, which appear as spiny shrubs or trees, mostly found in Indo-Malayan arid region, while few others are found in Africa, Australia, America and subcontinent of South Asia [1, 2].
Biologically, Ziziphus species are known to possess various important pharmacological activities including antimicrobial [3, 4, 5, 6, 7, 8], antioxidant and anti-inflammatory properties [9, 10, 11, 12, 13, 14, 15], antidiabetic, anti-malarial and anthelmintic properties [16, 17, 18, 19, 20], anticancer, antiulcer, analgesic, sedative and antipyretic effects [21, 22, 23] amongst other important activities. Furthermore, the members of the genus are known to produce variety of fruits which are fleshy and edible thereby serving as possible foodstuffs from the wild [24]. Furthermore, the species are generally not toxic and mostly safe for both human and animal consumption [25]. Phytochemically, members of the genus are known to possess a large number of cyclopeptide alkaloids, flavonoids, tannins, saponins, terpenoids, fatty acids, sterols and a wide variety of phenolic compounds [26, 27, 28, 29, 30, 31, 32, 33, 34]. The current paper is aimed at comprehensively reviewing the indigenous ethnobotanical uses, phytochemical profile, pharmacological studies and toxicological effects of Ziziphus mucronata, an enormously important food and medicinal plant.
2. Materials and methods
The information reported in the current paper was collected from a literature search - using various computerized databases such as ScienceDirect, Scopus, Scielo, PubMed and GoogleScholar. Additional information was further retrieved from various academic dissertations, theses and general botanical books. Key words such as Ziziphus mucronata, ethno-medicinal uses, survey, ethno-pharmacological aspects, biological activity, antimicrobial activity, anti-bacterial, anti-fungal, pharmacological properties, cytotoxicity, toxicology, anti-cancer, phytochemical components, phytochemistry, anti-inflammatory, antioxidant properties, anti-diabetic, anti-malarial, pesticidal effect, anti-parasitic, anthelmintic, anti-convulsant and insecticidal effect were used. The data was collected with help from Library staff at the University of South Africa (Florida Campus).
3. Botanical profile and taxonomy of Ziziphus mucronata
3.1. Taxonomy
“Ziziphus” is a Latin name which means thorn and “mucronata” refers to the pointed leaves of this species [35]. Ziziphus mucronata, South African tree list number is 447 and is a small shrub to medium-sized tree with the erect or decumbent stem which is greyish with some fissure like structures on the outside (Figure 1A) and a dense spreading crown. The leaves are ovate to broadly ovate, glossy dark green above and the lower surface slightly hairy and possess three veins (Figure 1B and 1C). The fruit is a sub-globose drupe, almost spherical in shape, shiny reddish to brownish in colour when ripe (Figure 1 B and 1C). The base of the leaves is markedly asymmetric and the margin finely toothed (serrate). The stipules possess spines, one hooked, the other straight. Flowers are in axillary clusters and are small, yellowish green (Figure 1D). In South Africa, flowering is from October to April and fruiting from February to September.
Figure 1.
Different plant parts of Ziziphus mucronata (stem bark, 1A), (Leaves, thorns, green and ripe fruits, 1B &1C), (Leaves and flower, 1D). (Photos taken by Ofentse Mongalo).
3.2. History
Historically, the plant species possess two distinct subspecies i.e Ziziphus mucronata Willd. subsp. rhodesica R.B. Drumm. Commonly found in Zambia, Zimbabwe, Botswana, Tanzania and Zambia and Ziziphus mucronata Willd. subsp. mucronata commonly found in South Africa. The subspecies name rhodesica refers to the republic of Rhodesia which is currently known as Zimbabwe, while mucronata is derived from the word “mucronate” which means with a short narrow point or having abruptly projected point, referring to the leaves of the plant species which are narrowly pointed compared to those of rhodesica subspecies which looks much lesser pointed.
3.3. Distribution, conservation status and common names of Ziziphus mucronata
The mostly used common names of the plant species in south Africa includes “buffalo-thorn” in English, “blinkblaarwag-‘n-bietjie” in Afrikaans, “mokgalo” in Sepedi and Tswana, “umphafa” in IsiXhosa, “umlahlankosi” in IsiZulu [36]. In Zimbabwe the Shona tribes call the species by a common name “Chinanga”, “Mwichechete” “Muchecheni” or “Omusheshete”, while called “Mukhalu” by VhaVenda tribe and “Umpasamala”, “Umlahlankosi” and “Umphafa” in both IsiNdebele, iSiZulu and iSixhosa. In Sepedi, the the word mokgalo refers to bitterness, due to the bitterness of the fruit which is believed to cause excessive cough when consumed in larger quantities.
In South Africa, Ziziphus mucronata is distributed all over the country; Limpopo, Mpumalanga, Kwazulu-Natal, Eastern Cape, Northern Cape, North West, Gauteng and Free State Province; except in the Western Cape Province (Figure 2). In other African countries, the plant species is found in Angola, Botswana, Eritrea, Ethiopia, Ghana, Kenya, Lesotho, Mozambique, Zambia, Namibia, Niger, Senegal, Somalia, South Africa, Sudan, Swaziland, Tanzania, Uganda and Zimbabwe and grows in all types of soil and standing intense heat and cold equally well [37].
Figure 2.
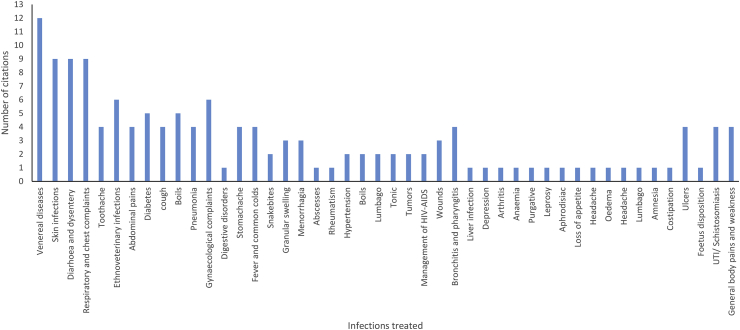
Number of citations vs the variety of infections that Z. mucronata may be used to treat.
Although the plant species is abundantly available in South Africa and is of least concern conservation wise [38, 39, 40, 41]. However, the use of stem bark and roots for medicinal plants have a negative impact on conservation status as removal is detrimental to plants.
4. Indigenous medicinal uses of Ziziphus mucronata
Ziziphus mucronata is an important medicinal plant species used for various ailments in Africa [42]. Sources from the literature (citation ≥6), have shown that the plant species s mostly used in the treatment of sexually transmitted infections, skin, respiratory or chest complaints, ethnoveterinary, gynaecological, diarrhoea and dysentery (Figure 2). The indigenous medicinal uses of the plant species are shown in Table1. The general consensus between countries and across the different African ethnic groups is that the different parts of the plant species, especially roots and stem bark, are used in the treatment and management of infections which includes various types of devastating sexually transmitted infections, sores and wounds treatment, management of possible opportunistic infections associated with HIV-AIDS, respiratory related infections, infertility in partners who could not conceive, gastrointestinal complaints and less often in treating depression and diabetes (Table 1). Contrarily, other authors reviews reported some leaves from other plant species to be prevalently used in the treatment of infections [43], while other corroborate the current study [44, 45]. However, the use of stem bark and roots for medicinal purposes have a devastating effect on health of plant species as it promotes ring barking and removal of roots which may lead to loss of species and later the distinction [46]. The plant species is also used in the treatment of ethno-veterinary infections, particularly in goats and cattle [47, 48].
Table 1.
Indigenous medicinal uses of Ziziphus mucronata.
| Country | Plant part used | Medicinal uses | Reference(s) |
|---|---|---|---|
| South Africa | Roots | Menorrhagia, infertility if women, tooth ache, scrofula, sexually transmitted infections including gonorrhoea and chlamydia. Roots are also used to treat diarrhoea, snake bites, body pains, chest problems and dysentery in humans. | [51, 52, 61, 62, 63, 64, 65] |
| Stem barks | Fever, dysentery, rheumatism, respiratory and chest ailments including cough, snake bites, body pains and tuberculosis. | [63, 64, 65, 66, 67] | |
| Stem barks | Ethno-veterinary purposes, ring worm. | [48, 67, 69] | |
| Roots | Ethno-veterinary purposes. | [47] | |
| Leaves | Tuberculosis and diarrhoea in humans. | [59, 60] | |
| Whole plant | Sores, boils, swelling and diabetes | [36, 62, 68] | |
| Botswana | Stem bark | Tooth ache | [56] |
| Zimbabwe | Roots | Abdominal pains, infertility in women, bilharzia, cholera, bladder infections and wounds. | [50, 70, 71, 72] |
| Mozambique | Roots | Stomach-ache, muscular pains and constipation | [73] |
| Leaves | Abdominal pains, infertility in women and wounds. | [72] | |
| Tanzania | Roots | Tonic, colds, lumbago, tumours, foetus disposition, aphrodisiac, stomach-ache, chest pains and hypertension. | [74, 75, 76] |
| Kenya | Stem bark | Abdominal pains, diarrhoea, common colds and cough. | [57] |
| Roots | Boils, skin infections and pulmonary related infections. | [77] | |
| Bukina Faso | Fruits | Relieves pain and inflammation. | [49] |
| Roots | Tooth ache and other oral infections. | [49] | |
| Mali | Roots | Urinary tract infections, psychological problems and intestinal infections | [55] |
| Zambia | Stem barks | Treatment of sexually transmitted infections (STIs) including syphilis, gonorrhoea and chlamydia. | [54] |
| Leaves | Ulcers and gonorrhoea. | [78] | |
| Roots | Management of HIV-AIDS related infections including pneumonia and STIs such as syphilis, gonorrhoea and chlamydia | [79] | |
| Whole plant | Arthritis, chest pains, digestive disorders, weakness, liver complaints, obesity, urinary troubles, diabetes, skin infections, loss of appetite, fever, pharyngitis, bronchitis, anaemia, diarrhoea and insomnia | [80] | |
| Nigeria | Leaves | Depression. | [81] |
| Whole plant | Mellitus diabetes | [82, 83] | |
| Namibia | Whole plant | Gonorrhoea, skin allergy, rush and sore fingers. | [72] |
| Roots | Ethno-veterinary purposes. Roots are also used to treat diarrhoea with some stools in the blood, tuberculosis, vomiting and stomach ulcers. | [53, 84] | |
| Angola | Roots | Urogenital infections, sore throat, mouth ulcers and diarrhoea. | [85] |
| Benin | Roots | Oedema | [86] |
| Senegal | Whole plant | Sexually related infections in humans, diabetes, dysentery and leprosy. | [87] |
4.1. Roots
The roots are boiled with salt and used to wash the mouth, when treating toothache [49]. The crushed roots are immersed in water overnight to treat infertility in women while powdered roots are applied directly on open wounds to facilitate healing in humans [50], while macerated roots are also drunk for treating rheumatism, fever and related infections [51, 52]. Elsewhere, a decoction of the roots is drunk to treat diarrhoea with blood in the stools and excessive coughing until the infections subsides [53].
4.2. Stem bark
The powdered plant material from the stem bark is applied to wounds. The cooked stem bark is drunk to treat chlamydia and other sexually transmitted infections [54]. A decoction of stem bark combined with fruits of Xylopia aethiopica (Dunal) A. Rich., stem bark of Ficus platyphylla Delile synonym Ficus platyphylla var. pubescens Aubrév., root of Ximenia americana L., aerial parts of unnamed Peritrophe spp and unidentified part of Stylosanthes spp is drunk twice a day for three days to cure urinary tract infections (UTIs) and intestinal infections (II) [55]. Elsewhere, the stem bark is boiled in water and the resulting liquid is strained and used as mouth wash and treats toothache [56]. Although the use of a larger number of medicinal plants species makes it a bit difficult when introspecting the possible active compounds, it is believed to alleviate the possible antimicrobial resistance that may arise when a similar medication is given to a patient at a later date, particularly in Western medicine. Elsewhere, the stem bark is chewed raw and or dried for treatment of cough and colds and alternatively crushed with addition of little water to treat abdominal pains and diarrhoea in infants [57]. The powdered stem bark is soaked into cold water and the resulting pulp is used a dressing for wounds in cattle and goats [48].
4.3. Leaves
The ground leaves are soaked into hot water overnight and drunk to cure diarrhoea in cattle and goats, administered as 1L per animal [58]. Leaves are ground, boiled in water and taken three times a day to cure diarrhoea in humans until the infection subsides [59]. The tender juvenile leaves of about 400 g are immersed in 2L of hot water and allowed to steep, and the liquid s drunk by adult patients until mild tuberculosis subsides [60].
4.4. Whole plant
Warm infusions of the root, bark and leaves are taken orally as tea which is also used as a medicine for diabetes and the decoctions are used topically to treat sores, boils and swelling [36]. The whole plant is immersed in water and the resulting liquid is drunk three times a day to treat arthritis, chest pains, digestive disorders, general body weakness, liver complaints and related diseases, obesity, urinary tract infections, mellitus diabetes, skin infections, loss of appetite, fever, pharyngitis, bronchitis, anaemia, diarrhoea and insomnia [80].
5. Toxicological studies of Ziziphus mucronata
5.1. Brine Shrimp Lethality (BSL) assay
The aqueous extract of the roots was investigated for toxicity using the Brine Shrimp Lethality (BSL) assay, at concentrations ranging from 15.6 to 1000 μg/ml and revealed 60 % mortality rate at 250, 500 and 1000 μg/ml at 24 h incubation period [88]. Elsewhere, the methanol extracts of roots and leaves revealed an LD50 of 1180 and 4560 μg/ml respectively in a BSL assay [89]. In a BSL assay, the dichloromethane and hexane extracts of the leaves revealed LC50 value of >200 μg/ml, while ethanol, methanol and aqueous extracts revealed LC50 > 1000 μg/ml [90]. The BSL assay is not a good method in determining the toxicity of plant extracts as the surviving nauplii may be counted a number of times in different quadrants of the petri dish, doesn't provide the mechanism of action of plant species and always warrants further cytotoxicity studies [91].
5.2. In vivo studies
The aqueous stem bark extract was evaluated for toxicity against Wistar rats orally, at a concentration of 250, 500 and 1000 mg/kg daily for a period of 10 days [92]. By extrapolation, the LD50 was found to be >5000 g/kg and no physical toxicity was observed on both extracts administered and control rats. The in vivo studies shows that the plant species is relatively not toxic at low concentrations. However, these results are important as traditional medicine involves the use of water as a solvent for extracting active plant components.
5.3. Cytotoxicity studies
The ethanol extracts from stem bark revealed an LC50 value of 118.2 μg/ml against monkey kidney Vero cells [93, 94], while 1:1 methanol: dichloromethane extract from the leaves revealed LC50 values of 100 and 220 μg/ml against bovine dermis and Vero cells respectively [95]. According to the American National Cancer Institute (NCI), the criteria of cytotoxicity for crude extracts is an LC50 < 30 μg/mL after an exposure time of 72 h in any in vitro microplate or MTT related assay [96]. These may well suggest that the extract did not reveal any toxicity to the cell line used. However, there is a great need to further explore cytotoxicity of the extracts and isolated compounds from Z. mucronata as it is frequently used in African traditional medicine to treat and manage various human and animal pathogenic infections.
5.4. The presence of heavy metals
The seeds were reported to possess copper content of 2.67 ppm [97], which is still below the limits set by World Health Organisation [98, 99] which refer to 40 ppm as of toxic level. However, Jaishankar et al [100], reports that copper may also have some devastating effects on important essential human and animal body parts including liver, kidney and heart and may result in devastating effects which may include adverse side effects, morbidity and possible mortality. These may well explain the need to carry put the comprehensive heavy metal assessment of the plant species from various localities within Southern Africa.
6. Phytochemical profile of Ziziphus mucronata
The 50 % methanol extracts of both the fresh and stored leaves, ethyl acetate and methanol extracts of roots revealed the presence of considerable amount of total phenolic contents, total flavonoids contents, quercetin contents, terpenoids, quinones and alkaloids [101, 102, 103, 104, 105] while the dried leaves revealed the presence of high energy, proteins and condensed tannin [106]. Besides containing higher dry matter digestibility, crude protein content, fibre and tannin contents, the seeds of the plant species revealed the presence of important minerals such as zinc, magnesium, calcium, iron, potassium, sodium and phosphorus which are needed to enhance growth and development of both plants and animals [97].
The identified compounds from Z. mucronata shows that the plant species possess a wide variety of compounds (Table 2) dominated by peptide alkaloids which may well explain the biological activity reported in the current review work. The GC-MS spectra of n-butanol fraction from the ethanol extract of the roots revealed the presence of catechol, ethyl-α-D-glucopyranoside, 2,4-Diisopropenyl-1-methyl-1-vinyl-cyclohexane, 2-methoxyhydroquinone, phthalic acid mono-(2-ethyl-hexyl) ester, 2,6-dimethoxyphenol and pyrogallol [107]. Mucronine A, Mucronine B, Mucronine C, Mucronine D, Mucronine E, Mucronine F, Mucronine G, Mucronine J, Abyssenine A, Abyssenine B and Abyssenine C are amongst such peptide's alkaloids [108, 109, 110, 111, 112]. Most isolated compounds from the plant species are the cyclopeptide alkaloids commonly known as mucronines and abyssenines [111].
Table 2.
Classes of compounds isolated from Ziziphus mucronata.
| Classes | Plant part | Compounds | Reference(s) |
|---|---|---|---|
| Phenolic acids | Stem bark | Pyrogallol, gallic acid, caffeic acid, | [107, 113] |
| Flavonoids | Stem bark | procatechin, catechin, epicatechin, rutin, and taxifolin | [107, 113] |
| Peptide alkaloids | Whole roots and root barks | Mucronine A, Mucronine B, Mucronine C, Mucronine D, Mucronine E, Mucronine F, Mucronine G and Mucronine J. | [108, 109, 111, 112] |
| Root bark | Abyssenine A, Abyssenine B, Abyssenine C. | [110, 112] | |
| Triterpenes | Zizyberanalinic acid | [115] (Moloto, 2004) | |
| Important Minerals | Seeds | Zinc, potassium, iron, Magnesium, calciumsodium and phosphorus. | [97] |
Elsewhere, Zininga et al [113] reported the presence of considerable amounts of caffeic acid, gallic acid, rutin, epicatechin, taxifolin and catechin from the various fractions of the ethanol extract from the stem bark. These compounds are of vital importance as have broad pharmacological activities reported, manufactured as compounds by pharmaceutical companies and used as control drugs in most laboratory experimental setups [114]. Elsewhere, the other authors have reported the presence of zizyberanalinic acid and 2, 3-dihyroxyl-up-20-en-28-oic acid [115], while [1-Cyano-2-(3-methoxy-phenyl)-vinyl]-phosphonic acid diethyl ester, 2-(2-Chlorobezylidene)- cyclohexanone and 2,3,4,Trimethoxy-6,8-dioxa-bicyclo[3.2.1] octane were also identified using GC-MS [107].
7. Pharmacological activities of Ziziphus mucronata
Various pharmacological activities of the plant species, particularly crude extracts and isolated compounds are reported in Table 3. Most of the pharmacologically reported studies includes antimicrobial and antioxidant activity.
Table 3.
Pharmacological activity of extracts, fractions and isolated compounds from Ziziphus mucronata.
| Activity investigated | Tested plant material | Model used | Tested doses | Control(s) used | Activity and notable results | Experimental evidence | References used |
|---|---|---|---|---|---|---|---|
| Antibacterial activity | Leaves, twigs and stem bark, Petroleum ether, dichloromethane, ethanol and ethyl acetate extracts. | Micro-dilution assay | 50 mg/ml diluted | None reported | The petroleum ether extract of the stem bark revealed MIC value of 0.4 mg/ml against Pseudomonas aeruginosa. | Positive evidence, dose dependence. | [134] |
| Stem bark and leaves, Aqueous, hexane and methanol extracts. | Micro-dilution assay | 100 mg/ml serially diluted | Positive control: Neomycin | Methanol extract of the leaf revealed a lowest MIC value of 0.2 mg/ml against Staphylococcus aureus. | Positive evidence, dose dependence. | [135] | |
| Leaves, aqueous and 1:1 (methanol: dichloromethane) extracts. | Micro-dilution assay | 32 mg/ml serially diluted | Positive control: Ciprofloxacin | The 1:1 (methanol: dichloromethane) extract revealed a MIC values of 0.01 mg/ml against Brevibacterium agri and 1.0 mg/ml against Escherichia coli, Propionibacterium acnes and Staphylococcus epidirmidis. | Positive evidence, dose dependence. | [138, 139] | |
| Leaves, Hexane, dichloromethane, chloroform, ethyl acetate, ethanol and methanol extracts. | Micro-dilution assay | 10 mg/ml diluted | Positive control: None reported | Hexane and chloroform extracts revealed MIC values of 0.21 and 0.27 mg/ml against Staphylococcus aureus. | Positive evidence, dose dependence. | [140] | |
| Leaves, Acetone extracts. | Micro-dilution assay | 10 mg/ml diluted | Positive control: Ampicillin | The extract revealed a MIC value of 0.53 mg/ml against both Escherichia coli and Staphylococcus aureus. | Positive evidence, dose dependence. | [141, 142] | |
| Stem bark, Dichloromethane and 90 % methanol extracts. | Micro-dilution assay | 20 mg/ml serially diluted | Positive control: Neomycin | The 90 % methanol extract revealed MIC value of 1.25 mg/ml against Pseudomonas aeruginosa. | Positive evidence, dose dependence. | [149] | |
| Leaves, Acetone extract. | Agar well diffusion assay | 20 mg/ml | Positive control: Neomycin | Acetone and methanol extracts of the leaves revealed a positive inhibition against Escherichia coli, Klebsiella pneumonia and strains of Staphylococcus aureus | Positive evidence. | [119] | |
| Leaves and roots, Methanol extracts. | Agar well diffusion and Micro-dilution assays | 10 mg/ml | Positive controls: Ampicillin, amoxicillin, tetramycin and gentamycin. | The methanol extract from both leaves and roots revealed a zone of inhibition of 3.25 and 5.0 mm against Escherichia coli and Streptococcus Group A strain respectively. Roots extract exhibited a MIC value of 2.5 mg/ml against Staphylococcus aureus. | Positive evidence, dose dependence. | [120] | |
| Roots and leaves, Aqueous extracts. | Disc diffusion assay and Micro-dilution method. | 10 mg/ml | Positive control: Amoxyllin, tetramycin and gentamycin. | Aqueous extract of the root exhibited zone of inhibition of 5.0 mm against Streptococcus Group A, while the leaf extract revealed MIC value of 1.25 mg/ml against similar bacterial strain. | Positive evidence, dose dependence. | [125] | |
| Stem bark and leaves, Aqueous and 1:1 methanol: dichloromethane | Micro-dilution method. | A concentration of 64 mg/ml serially diluted. | Positive control: Ciprofloxacin. | Aqueous extract from the stem bark revealed a MIC of 1.0 mg/ml against both Staphylococcus aureus, Pseudomonas aeruginosa and Brevibacterium agri. | Positive evidence, dose dependence. | [143] | |
| Root bark, Aqueous extract | Disc diffusion and Time-log kill methods. | Concentrations ranging from 200 to 1000 μg/ml. | Positive control: None reported. | The extract did not inhibit the growth of both Staphylococcus aureus and Pseudomonas aeruginosa at highest concentration tested. | Inconclusive evidence, not dose dependent. | [122] | |
| Stem bark and leaves, Aqueous and 1:1 methanol: dichloromethane | Micro-dilution method. | A concentration of 32 mg/ml serially diluted. | Positive control: Ciprofloxacin. | Organic extracts from the leaves revealed a MIC value of 1.0 mg/ml against three bacterial strains such as Streptococcus sanguis, Porphyromonas gingivalis and Fusobacterium nucleatum. | Positive evidence, dose dependence. | [145] | |
| Leaves, Ethyl acetate and chloroform extracts. | Disc Diffusion method. | A concentration of 2.5 mg/disc. | Positive control: Erythromycin | No activity observed | Negative evidence, not dose dependent | [121] | |
| Leaves and stem bark, Aqueous and 80 % methanol extracts. | Disc diffusion method | A concentration of 100 mg residue/ml. | Positive control: Neomycin. | Methanol extract of the leaves at 1 mg/ml revealed antibacterial activity of 0.30 relative control drug at 200-500 μg/ml. | Positive evidence, Not dose dependent. | [118] | |
| Stem bark, Aqueous extract. | Micro-dilution method. | Maximum concentration of 4 mg/ml serially diluted. | Positive control: Gentamycin sulphate, vancomycin hydrochloride, penicillin g, imipinem, ampicillin and chloramphenicol. | The extract did not reveal any inhibition at highest concentration tested against clinical strains of Enterococcus faecalis, Streptococcus agalactiae, Salmonella enterica, Klebsiella pneumonia and Escherichia coli. | Inconclusive evidence, not dose dependence | [150] | |
| Roots, Stem bark and leaves, 1:1 methanol:acetone extracts | Micro-dilution method. | A concentration of 64 mg/ml serially diluted. | Positive control: Coprofloxacin. Negative control: DMSO |
The leaves revealed a MIC value of 0.5 mg/ml against Moraxella catarrhalis. | Positive evidence, dose dependence | [190] | |
| Roots, Aqueous extract | Micro-dilution method. | A concentration of 50 mg/ml serially diluted. | Positive control: Coprofloxacin. | Aqueous extract revealed a MIC value of 0.19 against Staphylococcus aureus at 15 minutes boiling time and 1.56 mg/ml against Neisseria gonorrhoea at 0, 15 and 20 minutes boiling time intervals. | Positive evidence, dose dependence | [137] | |
| Leaves, ethanol, dichloromethane, hexane, aqueous and methanol extracts. | Micro-dilution method. | A concentration of 50 mg/ml serially diluted. | Positive control: Gentamycin and streptomycin. | The dichloromethane extract revealed a MIC value of 0.08 and 0.13 mg/ml against methicillin resistant Staphylococcus aureus (MRSA) and Clostridium tetani respectively. | Positive evidence, dose dependence | [90] | |
| Anti-mycobacterial activity | Leaves, acetone extract | Micro-dilution method. | 10 mg/ml serially diluted | Positive control: Rifampicin and isoniazid | The extract revealed a MIC value of 0.156 mg/ml against Mycobacterium tuberculosis (pathogenic strain TB8104) and 2.5 mg/ml against Mycobacterium fortuitum and Mycobacterium aurum. | Positive evidence, dose dependence. | [144] |
| Stem bark, Aqueous, ethanol, methanol and acetone extracts. | Micro-dilution method. | A highest concentration of 100 μg/ml serially diluted. | Positive control: Rifampicin, ethambutol, streptomycin and isoniazid | Acetone extract from stem bark exhibited MIC value of 50 μg/ml against two different Mycobacterium strains. | Positive evidence, dose dependence. | [136] | |
| Leaves, ethanol, dichloromethane, hexane, aqueous and methanol extracts. | Micro-dilution method. | A concentration of 50 mg/ml serially diluted. | Positive control: Gentamycin and streptomycin. | Dichloromethane extract exhibited a MIC value of <0.156 mg/ml against Mycobacterium terrae. | Positive evidence, dose dependence | [90] | |
| Stem bark, Aqueous extract. | Micro-dilution method. | A highest concentration of 500 μg/ml. | Positive control: Rifampicin and ethambutol dihydrochloride. | The extract revealed MIC value of >500 μg/ml against pathogenic Mycobacterium tuberculosis H37 strain. | Inconclusive evidence, dose dependence. | [150] | |
| Antifungal activity | Stem bark and leaves, Aqueous and 1:1 methanol: dichloromethane | Micro-dilution method. | A concentration of 64 mg/ml serially diluted. | Positive control: Amphotericin B. | The aqueous extract of the stem revealed a MIC value of 2.0 mg/ml against Trichophyton rubrum. | Positive evidence, dose dependence. | [143] |
| Roots and leaves, Aqueous extracts. | Disc diffusion assay and Micro-dilution method. | 10 mg/ml | Positive control: Amphotericin B. | The aqueous extract of roots exhibited zone of inhibition of 2.75 and 3.75 mm against Candida albicans and Aspergillus niger respectively, while aqueous extract of leaves exhibited 2.75 mm against both fungal stains. | Positive evidence. | [125] | |
| Roots, methanol extract. | Agar diffusion | 1 g extract diluted with media | Positive control: Ketoconazole | The extract revealed a zone of inhibition of 14.45 mm against Aspergillus fumigatus after 11 days incubation period. | Positive evidence. | [124] | |
| Stem bark, Acetone extract. | Agar diffusion assay | Not specified | Positive control: Nystatin and Flucytosine. | The extract revealed zone of inhibition of 15 mm against Cryptococcus neoformans. | Positive evidence. | [127] | |
| Leaves, Ethanol, aqueous and boiled aqueous extracts. | Cup Plate method | 100 mg/ml diluted 1:10, 1:100 and 1:500. | Negative control: Plates containing PDA. | Boiled aqueous extract revealed a stronger inhibition of 30 to 40 mm against Aspergillus flavus and Aspergillus glaucus. | Positive evidence. | [123] | |
| Stem bark, Hexane extract. | Agar diffusion assay | 2.5 to 20 % diluted with water | Positive control: 20 % fluconazole. Negative control: Distilled water. |
A 20 % hexane extract exhibited a zone of inhibition of 6.0 and 7.5 mm against Cryptococcus neoformans and Candida albicans respectively. | Positive evidence. | [126] | |
| Leaves, ethanol, dichloromethane, hexane, aqueous and methanol extracts. | Micro-dilution method. | A concentration of 50 mg/ml serially diluted. | Positive control: Gentamycin and streptomycin. | Hexane extract revealed a MIC value of 1.25 mg/ml against Candida albicans. | Positive evidence, dose dependence | [90] | |
| Leaves, Aqueous and (1:1) Methanol: dichloromethane extracts. | Micro-dilution assay | 32 mg.ml serially diluted. | Positive control: Amphotericin B. | The organic extract revealed a MIC value of 0.1 mg/ml against both Candida albicans and Trichophyton mentangrophytes. | Positive evidence, dose dependence. | [138, 139] | |
| Stem bark and leaves, Aqueous and 1:1 methanol: dichloromethane | Micro-dilution method. | A concentration of 32 mg/ml serially diluted. | Positive control: Amphotericin B. | Organic extract from the leaves revealed a MIC value of 1.0 mg/ml against Candida krusei. | Positive evidence, dose dependence. | [145] | |
| Stem bark and leaves, Aqueous and 1:1 methanol: dichloromethane | Micro-dilution method. | A concentration of 64 mg/ml serially diluted. | Positive control: Amphotericin B. | The organic leaf extract revealed a MIC value of 2.0 mg/ml against Trichophyton mentagrophytes. | Positive evidence, dose dependence. | [143] | |
| Roots, Stem bark and leaves, 1:1 methanol: acetone extracts | Micro-dilution method. | A concentration of 64 mg/ml serially diluted. | Positive control: Amphotericin B. | The leaves extract revealed a MIC value of 1.0 and 4.0 mg/ml against Cryptococccus neoformans and Candida albicans respectively. | Positive evidence, dose dependence | [130] | |
|
Fruit, Aqueous extract and fractions FZ1 (Water) FZ2 (95:5 H2O: acetonitrile) FZ3(90:10 H2O: acetonitrile) FZ4 (85:15 H2O: acetonitrile) FZ5 (10:90 H2O: acetonitrile). |
Direct contact of extract and spores in a test tube. | Fractions were tested at 1mg.ml | Positive control: Catechin and rutin. Negative control: Distilled water. |
Fractions FZ2 and FZ3 revealed a 57.14 % inhibition of spores from Puccinia arachidis and 50 % inhibition of Phaeoisariopsis personata. | Positive evidence. | [151] | |
| Anti-viral activity | Leaves, Aqueous and methanol extracts. | DNA polymerase and RNase H activities of HIV-1 RT. | 400 μg/ml serially diluted. | Positive control: DNA aptamer (ODN 93) | The methanol and aqueous extracts revealed LC50 values of 75 and 81.5 μg/ml against RNase H and RDDP respectively. | Positive evidence, dose dependence. | [153] |
| Stem bark, Aqueous extract. | Reverse transcriptase assay. | Two concentrations of 50 and 100 μg/ml | Positive control: Nevirapine. |
Both concentrations revealed less than 20 % inhibition of enzyme. | Inconclusive evidence, Not dose dependent | [150] | |
| Antioxidant activity | Roots, Methanol and ethyl acetate extracts. | ABTS and DPPH assays. | Concentrations ranging from 0.007 to 1.25 mg/ml. | Positive control: Trolox. | The methanol extract exhibited IC50 value of 19 and 29 μg/ml against ABTS and DPPH respectively. | Positive evidence, dose dependence. | [102] |
| Stem bark, roots and leaves, Aqueous, ethyl acetate and ethanol extracts. | DPPH, Hydroxyl radical and Nitric oxide radical assays. | Concentrations ranging from 15 to 240 μg/ml. |
Positive control: Trolox, gallic acid and ascorbic acid. | Ethanol extract from the leaves, roots and stem bark exhibited IC50 values of 1.68; 1.38 and 1.99 μg/ml against DPPH free radical. | Positive evidence, dose dependence. | [194] | |
| Stem bark, Acetone, ethanol and aqueous extracts. | DPPH, ABTS and FRAP. | Concentrations ranging from 0.02 to 0.1 mg/ml. | Positive control: BHT, rutin and ascorbic acid. | Ethanol and acetone extracts revealed potent IC50 values of 31 and 32 μg/ml against ABTS free radical, while BHT exhibited 34 μg/ml. | Positive evidence, dose dependence. | [193] | |
| Leaves, ethanol and methanol extracts. | DPPH assay. | 100 to 600 μg/ml. | Positive control: BHT. | The methanol extract revealed IC50 value of 45.19 μg/ml against DPPH. | Positive evidence, dose dependence. | [90] | |
| Fruits, Methanol, hexane, (1:1) methanol: chloroform, (1:1) hexane: chloroform extracts. | DPPH and ABTS assays. | Concentrations ranging from 2.5 to 100 μg/ml. | Positive control: Gallic acid and ascorbic acid. | The methanol extract revealed IC50 value of 7.21 μg/ml against ABTS free radical. | Positive evidence, dose dependence. | [195] | |
| Stem bark, Aqueous and methanol extracts. | DPPH and ABTS assays. | Concentrations ranging from 0.1 to 300 μg/ml. | Positive control: Trolox |
The methanol and aqueous extracts revealed IC50 values of 11.18 and 20.78 μg/ml against ABTS respectively, while methanol extract revealed 89.81 μg/ml against DPPH. | Positive evidence, dose dependence. | [167] | |
| Leaves, Freshly collected, stored for 12 years and 16 years old extracts. The solvent used was 50 % methanol. | DPPH, β-Carotene assays. | Concentrations of 100 and 200 μg/ml serially diluted in DPPH and β-Carotene assay respectively. | Positive control: BHT and ascorbic acid. Negative control: 50 % methanol. |
The aqueous extract of the 16 years old stored plant material revealed an IC50 value of 18.1 μg/ml against DPPH. | Positive evidence, dose dependence. | [103] | |
| Fruits, leaves and stem bark, Acetone extracts. | DPPH and metal chelating activity. | Concentration of 100 μg/ml serially diluted. | Positive control: Trolox, EDTA and ascorbic acid. | Acetone extract of the stem bark revealed IC50 value of 15.27 and 55.67 μg/ml against DPPH and Metal chelation activity respectively. | Positive evidence, dose dependence. | [160] | |
| Anti-inflammatory and anti-neurodegenerative effect | Roots, Methanol and ethyl acetate extracts. | Ache assay, micro plate | Concentrations ranging from 0.007 to 0.16 mg/ml. | Positive control: Galantamine. | The ethyl acetate extract revealed IC50 value of 1 μg/ml. | Positive evidence, dose dependence. | [102] |
| Stem bark, Dichloromethane and 90 % methanol extracts. | COX-1 and COX-2 assays. | A single concentration of 250 μg/ml. | Positive control: Indomethacin. | The 90 % methanol extract revealed 66.5 and 66.2 % against COX-1 and COX-2 respectively. | Positive evidence, Not dose dependent. | [149] | |
| Leaves, Ethanol and aqueous extracts. | Cyclooxygenase assay. | A single concentration of 20 mg residue/ml. | Positive control: Indomethacin. | Ethanol extract of the leaves revealed 87 % inhibition of cyclooxygenase. | Positive evidence, Not dose dependent. | [207] | |
| Stem bark, Aqueous extract. | In Vitro against RAW 264.7 cells and Nitric oxide production. | Two concentrations of 50 and 100 μg/ml | Positive control: Aminoguanidine. | The extracts did not inhibit nitric oxide production and induce the activation of macrophages. | Positive evidence. | [150] | |
| Anti-diabetic activity | Roots,n-butanol fraction. | Direct ingestion, In vivo against Sprague-Dawley 9SD rats. | Two concentrations, 150 ad 300 mg/kg. | Positive control: None reported | At 300 mg/kg, the fraction revealed a clear reduction in blood glucose, improved glucose tolerance ability and higher serum insulin. | Positive evidence. Not dose dependent. | [169] |
| Stem bark, Aqueous and methanol extracts. | In vitro α-amylase and α-glucosidase, Glucose uptake test using HepG2, RIN-m5F and C2C12 cell lines in vitro. | Three concentrations of 15.6, 31.2 and 62.5 μg/ml were investigated | Positive control: Acarbose. | All the extracts revealed IC50 values > 62.5 μg/ml in both α-amylase and α-glucosidase. Further, the extracts resulted in higher glucose uptake in C2C12 cells only. | Positive evidence, dose dependence | [167, 168] | |
| Roots, Four solvent fractions (from ethanol extract) such as aqueous, butanol, ethyl acetate and dichloromethane. | In vitro α-amylase and α-glucosidase | Four concentrations of 30, 60, 90, 120 and 240 μg/ml. | Positive control: Acarbose. | Butanol, aqueous, ethyl acetate and dichloromethane fractions revealed IC50 values of 1.41, 4.38, 23.6 and 43.91 μg/ml against α-glucosidase while control revealed 55.59 μg/ml. | Positive evidence, dose dependence | [107] | |
| Histopathologic and hepatoprotective effect | Leaves, 70% methanol extract. | Dimethoate induced liver damage in rats. Rats fed with extract. |
100, 200 and 300 mg/kg extracts. | Negative Control: Rats fed with dimethoate and water instead of extract. | The extract resulted in a significant decline serum marker enzymes SGOT, SGPT and ALP. The extract further resulted in less necrosis, limited loss of cell boundaries and reduced loss of intact hepatocytes. | Positive evidence, dose dependence | [195, 224] |
| Roots,n-butanol fraction obtained from ethanol extract. | Direct ingestion, In vivo against Sprague-Dawley 9SD rats. | Two concentrations, 150 ad 300 mg/kg. | Negative Control: Rats fed with water instead of extract. | The fraction resulted in less or no effect on the renal and hepatic damage caused by streptomycin induced diabetes. | Positive evidence, Not dose dependent. | [169] | |
| Anthelmintic activity | Twigs, fruits and wood, (1:1) methanol: dichloromethane and aqueous extracts. | Direct contact. | A single dose of 1 mg/ml. | Positive control: Levamisole. | Organic extract from twigs revealed average % worm death of 69.47 against Caenorhabditis elegans. | Positive evidence. Not dose dependent. | [189] |
| Stem bark, Aqueous and methanol extracts. | Larval mortality and egg hatch inhibition assays. | Concentration ranging from 0.06 to 4 mg/ml. | Positive control: Albendazole. | Methanol extract revealed LC50 value of 3.9 and 2.65 mg/ml against Haemonchus contortus in egg hatch inhibition and larval mortality assay respectively. | Positive evidence, Dose dependence. | [184, 185] | |
| Leaves + Stem bark, root bark, root kernel, Aqueous extracts | Direct contact | A concentration of 200 mg/ml diluted. | Positive control: None reported. | The root bark extract revealed an effect on cestodes of Hymenolepis diminuta after 1- and 24-hours incubation period at 1.6 and 0.002 mg/ml. | Positive evidence, Dose dependence. | [188] | |
| Anti-plasmodial activity | Stem bark, Acetone extract. | Flow cytometry assay. | Concentration of 100 mg/ml serially diluted. | Positive control: Chloroquine. | Acetone extract revealed LC50 value of 4.13 μg/ml against Plasmodium falciparum. | Positive evidence, Dose dependence. | [158] |
| Fruits, leaves and stem bark, Acetone extracts. | In Vitro anti-plasmodial bioassay. | Concentration of 100 mg/ml serially diluted. | Positive control: Qiunine and chloroquine. | The acetone extract of fruit revealed LC50 value of 67.18 μg/ml Plasmodium spp. | Positive evidence, Dose dependence. | [160] | |
| Leaves and roots, Aqueous and methanol extracts. | In Vitro anti-plasmodial bioassay. | A concentration of 100 μg/ml, serially diluted. | Positive control: None used. | Both aqueous and methanol extracts revealed LC50 values >20 μg/ml against Plasmodium falciparum 3D7A strain. | Positive evidence, Dose dependence. | [104] | |
| Leaves, Dichloromethane extract. | pLDH assay | Concentrations ranging from 0.2 to 100 μg/ml. | Positive control: Chloroquine diphosphate | The extract revealed LC50 value of 12.0 μg/ml against chloroquine sensitive strain D10 of Plasmodium falciparum. | Positive evidence, Dose dependence | [159] | |
| Leaves, Aqueous and methanol extracts. | In Vitro anti-plasmodial bioassay. | A concentration of 100 μg/ml, serially diluted. | Positive control: Chloroquine phosphate and artemisinin. | Aqueous extract of the leaves revealed anti-plasmodial activity of >100 μg/ml against Plasmodium falciparum. | Positive evidence, dose dependence. | [161] | |
| Stem bark, Ethanol extract, and fractions from column, ZMF1 (70:30 hexane: ethyl acetate), ZMF2 and ZMF3 (90:10 ethyl acetate: methanol), ZMF4 (70:30 ethyl acetate: methanol), ZMF 5 (30: 70 ethyl acetate: methanol ) | pLDH assay for growth of P. falciparum, calorimetric method for effect of plant materials on basal Hsp70 ATPase activity, and MDH activity for effect of plant materials on chaperone function of PfHsp70-1 and PfHsp70-z. | Concentrations ranging from 0 to 50 μg/ml. | Positive control: Chloroquine for in vitro studies. | Fractions and extract revealed potent inhibition of both PfHsp70-1 and PfHsp70-z yielding IC50s ranging from 3.1 to 9.3 μg/ml and 3.2 to 13.8 μg/ml. Further, crude ethanol extract, ZMF2 and ZMF5 revealed IC50 values of 7.4, 6.4 and 19.9 μg/ml respectively against Plasmodium falciparum D37 parasite in vitro. | Positive evidence, dose dependence. | [113] | |
| Anti-sickling effect (Anti-anaemic effect) | Stem bark and roots, Ethanol and aqueous extracts. | Emmel test, In vitro study. | Different concentrations of 20 to 160 μg/ml. | Positive control: None reported. | Ethanol extracts of both stem bark and roots revealed > 70 % anti-sickling effect in the Emmel test. | Positive evidence, Dose dependence. | [222] |
| Anticancer activity | Roots, Methanol extract. | Naphthol blue black assay | Two concentrations of 10 and 100 μg/ml. | Negative control: Cells and DMSO. | The extract revealed 50 to 75 % inhibition of HeLa, HT29 and A431 cells at 100 μg/ml. | Positive evidence, Not dose dependent. | [75] |
| Leaves, Ethanol and dichloromethane extracts. | MTT assay and APOPercantage TM assay against HeLa, Caco-2, Chinese harmster ovary cells, KMST-6, H157 and H4 cells. | 0 to 1000 μg/ml. | Negative control: Cells only without extract. | Dichloromethane extract revealed LC50 value of 126.73 μg/ml against H4 cell line. Dichloromethane, methanol and ethanol extracts revealed selective inhibition of Caco-2. | Positive evidence, Dose dependence. | [90] | |
| Mutagenicty/Genotoxicity | Stem bark, Dichloromethane and 90 % methanol extracts. | Ames test. | 50, 500 and 5000 μg/ml concentrations of plant extracts. | Positive control: 4-Nitoquinoline-N-oxide (4NQO) | The extracts were found to be mutagenic against strain TA98. | Positive evidence, Dose dependence. | [149] |
| Leaves, Dichloromethane and 90 % methanol extracts. | VITOTOX test, Ames test in TA98 and TA100 strains with and without metabolic activation. | 0.05, 0.5 and 5.0 mg/ml plant extracts. | The positive controls used: 4- Nitroquinoline 1-oxide (4-NQO) (for TA98 and TA100 without S9) and benzo[a] pyrene (B-[a]-P) (for TA98 and TA100 with S9). | The 90% methanol extract showed a mutagenic effect for TA98 in the presence of S9 mix alone. | Positive evidence, Dose dependence. | [226] | |
| Roots and Twigs, Dichloromethane and 90 % methanol extracts. | VITOTOX test Ames test in TA98 and TA100 strains with and without metabolic activation. | 0.05, 0.5 and 5.0 mg/ml plant extracts | The positive controls used: 4- Nitroquinoline 1-oxide (4-NQO) (for TA98 and TA100 without S9) and benzo[a] pyrene (B-[a]-P) (for TA98 and TA100 with S9). | The 90% methanol extracts showed a mutagenic effect in strain TA98. | Positive evidence, Dose dependence. | [227] | |
| Roots, leaves and Twigs, Dichloromethane and methanol extracts. | Ames, Umu-C and VITOTOX® tests, and with the micronucleus test and alkaline comet assay in human white blood cells in the absence of S9 only. | 0.05, 0.5 and 5.0 mg/ml plant extracts | The positive controls used: 4- Nitroquinoline 1-oxide (4-NQO) (for TA98 and TA100 without S9) and benzo[a] pyrene (B-[a]-P) (for TA98 and TA100 with S9). | The extracts did not reveal any mutagenicity. | Positive evidence, Dose dependence. | [228] | |
| Leaves, fresh and long term stored methanol plant extracts | Ames test in TA98, TA100 and TA1535 strains in the absence of metabolic activation. | 0.05, 0.5 and 5.0 mg/ml plant extracts. | The positive controls used: 4- Nitroquinoline 1-oxide | The plant extracts of both fresh and long-term stored materials did not show any mutagenic effect against the three tester bacterial strains. | Positive evidence, Dose dependence. | [229] |
7.1. Antifungal, antibacterial and anti-mycobacterial activity
Virulent microorganism, one way or the other, develop some resistance to common antibiotics mostly used in developing and much poorer countries. These resistances may be due to development of genes that resist the entry of different drugs into the microbial cell, thereby allowing pathogenic microbes to grow and result in devastating effects even if a potent drug is administered to a patient. According to Brown and Wright [116], from year 2000 until now, the discovery of antibiotics is much slower compared to the discovery of antibiotic resistance microorganisms. Some authors propose the use of antibiotics in conjunction with medicinal plants extracts as an alternative to curb problems associated with antimicrobial resistance [117]. These resistances warrant a thoroughly comprehensive search of new antibiotics, mostly of plant origin, to ease pressure on currently available drugs in various pharmaceutical set ups world-wide.
Ziziphus mucronata is one of such plants and have been assessed for antimicrobial activity using both disc diffusion or agar diffusion cup plate and micro dilution assays [118, 119, 120, 121, 122, 123, 124, 125, 126, 127]. These methods, disc diffusion or agar diffusion cup plate, are only scientifically used as starting point for determining the antimicrobial effect of a wide variety of plant species and therefore not easy to compare the results from various authors. These methods are generally dependent on a number of factors which may include the diffusion rate of the plant extract, size of the disc, type of agar medium used, concentrations of both the extracts and the inoculum, droplets arising from pouring a hot agar into the sterile petri dishes tampering with the concentration of the extract and other factors [128]. For that reason, it is difficult to compare the results of such antimicrobial assays.
Micro-dilution assay has also been used to determination of antimicrobial activity of plant materials from Ziziphus mucronata. According to Rios and Recio [129] and Suliman [130], the antimicrobial activity of plant extracts can be divided into three categories such as good or potent (MIC≤1 mg/ml), moderate activity (1.1 up to 6 mg/ml) and poor inhibitor of microbes (>6 mg/ml) antimicrobial activity. However, the consensus is that the antimicrobial activity of ≤1 is considered active against pathogens and worth introspecting for individual compounds with potent antimicrobial potential [131, 132, 133]. Various Z. mucronata extracts revealed a MIC values of less or equal to 1 mg/ml against pathogens such as Brevibacterium agri, Staphylococcus aureus, Pseudomonas aeruginosa, Moraxella catarrhalis, Streptococcus sanguis, Porphyromonas gingivalis, Fusobacterium nucleatum, Mycobacterium tuberculosis, Escherichia coli, Propionibacterium acnes, Staphylococcus epidirmidis Candida albicans, Cryptococcus neoformans and Trichophyton mentangrophytes [133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145].
Cryptococcus neoformans, Candida krusei, Staphylococcus aureus, Moraxella catarrhalis and Candida albicans are important pathogens that mainly infect the skin [146] and mostly prevalent in immunocompromised individuals, while P. acnes infects the skin resulting in various types of pimples. The data observed may well suggests that the plant species maybe used in the treatment of opportunistic infections associated with HIV, skin infections, wounds and TB. Furthermore, the in vivo (clinical studies) are essential in validating such claims scientifically. There is also a need to identify the individual active compounds from leaves, roots and stem bark that may account for these reported bioactivities. Furthermore, there is a need to further investigate the antimicrobial activity of extracts (different polarities) of the plant species against organisms belonging to the traditional sphere of causative agents of sexually transmitted infections like Trichomonas vaginalis, Oligella and Mycoplasma species and Neisseria gonorrhoea. Although Erasmus [137] reported MIC value of 1.56 mg/ml, there is a possibility of not marking the MIC correctly due to the use of haemoglobin and INT which may both give a similar reddish colour to growth of microorganisms which reduce the visual reagent into formazan, a red dye. Over and above that, a single report on the aqueous extract is not sufficient enough to validate the obtained data. It should be noted that TB, caused by pathogenic Mycobacterium species, is one of the top 10 causes of death worldwide with 1.7 million people reported dead only in 2006 [147]. The disease is further compounded by co-infection with HIV-AIDS, which allows for the reactivation of TB germ as the disease progresses.
Although the acetone extracts from both roots and leaves revealed potent anti-mycobacterial activity, with MIC values much less than 1 mg/ml, against M. tuberculosis and M. smegmatis [136, 144], other authors reported the ethanol extracts of the stem bark to be inactive against such Mycobacterium strains [93, 94]. These may well be explained as inconclusive evidence. Besides the differences in solvents used, one may suspect the active plant materials to be embedded mostly in roots and leaves. However, such compounds still need to be identified and compared scientifically. It is important to note that due to the cell wall composition of Mycobacterium species, their character of being able to survive many years in different habitats and develop multi drug resistant (MDR) and extremely drug resistant (XDR) genes makes TB very difficult to treat. Although there are current drugs in the market, the resistance of bacterium is still of greater concern to human life as it results in high mortality rates world-wide. However, it is promising that Dzoyem et al [144], reported the acetone extract from leaves to exhibit selectivity index of 382.1 against M. tuberculosis. This means the extract can be diluted 382 times and still inhibit the growth of the bacterium. Further work needs to be carried out to explore whether the extracts are bactericidal or bacteriostatic and these may provide the possible mechanisms of actions of such medicinal plant extracts.
Oljuyigbe and Afolayan [148], investigated the antimicrobial activity of ethanol extract from the stem bark in conjunction with antibiotics such as chloramphenicol, nalixidic acid, ciprofloxacin, tetracycline, amoxicillin and kanamycin. The interaction showed that the combinations resulted in a synergistic effect against the selected pathogenic strains. These synergistic effects mean the combinations would be more important in treating the multidrug resistant pathogens than the use of a single drug or a plant material. It should also be noted that traditional medicine may also use a combination of plant species in treating pathogenic and infectious diseases without side-effects and multi-resistant reports. Generally, the organic extracts inhibited the growth of microorganisms better than aqueous extracts which are more relevant in traditional medicine. Freeze drying might be a main factor as most scientists freeze the extract at extremely low temperatures and evaporate using technological tools. Elsewhere, Luseba et al [149] and Sigidi et al [150], reported the moderate to weaker antimicrobial activity. The 90 % methanol extracts of the stem bark exhibited MIC value of 1.25 mg/ml against Pseudomonas aeruginosa while aqueos extract of the stem bark revealed inactivity against various clinical isolates of Enterococcus faecalis, Streptococcus agalactiae, Salmonella enterica, Klebsiella pneumonia and Escherichia coli.
The effect of boiling intervals on the activity and efficacy of Z. mucronata individually and in combination with other medicinal plants was evaluated by Erasmus [137]. The aqueous extracts individually and in combination with other plants revealed better antimicrobial activity at boiling time intervals of 10 and 15 min. It is important to notice that the increased boiling period may be likely to increase the antimicrobial affect of medicinal plants.
The inhibitory effect of Z. mucronata extracts against mycotoxigenic fungi was also investigated against various Furasium and Aspergillus species [95]. Organic (1:1 methanol: dichloromethane) extracts of the leaves revealed potent MIC values 0.01, 0.20 and 0.78 mg/ml against Furasium graminareum, Aspergillus ochraceous and Aspergillus flavus respectively after 48 h incubation period. These fungal strains are known to produce various mycotoxins which are not only harmful to humans and animals but also negatively impact the production of agricultural products such as rice, grapes, dried vine fruits, wine, and coffee, maize and others. Elsewhere, Köìta et al [151], reported various fractions from the fruits to inhibit spore formation of Puccinia arachidis up to 57 %. These reports warrant the need to explore the possibility of the plant species in inhibition the growth of mycotoxigenic fungal strains. Mode of action is also of paramount importance.
7.2. Antiviral activity
HIV remains the major incurable disease worldwide. According to the global HIV and AIDS statistics, since the start of the pandemic, an estimated 78 million people have become infected with HIV. In 2013, there were 1.3 million (1.1 million–1.6 million) AIDS deaths in the top 30 countries representing 87% of global AIDS deaths and the highest mortality rate was found in central Africa [152]. As the disease progresses, a huge amount of opportunistic infections arises and most health setups are only concerned about management of such infections as the disease itself is incurable. The anti-HIV activity of leaves and stem bark extracts were studied by Bessong et al [153] and Sigidi et al [150]. The methanol and aqueous extracts revealed LC50 values of 75 and 81.5 μg/ml against RNase H and RDDP respectively, while aqueous extract of stem bark revealed less than 20 % inhibition of Reverse transcriptase enzyme.
According to Helfer et al [154], a potent inhibitor of HIV related enzymes needs to be able to interfere with the expression of early viral protein's Tat and Rev, interfere with the release of virions and yield the lowest LC50 almost relative to ±8 μg/ml while other authors recommend LC50 value of 0.04 μg/ml from an isolated compound [155]. Judging by these standards, the aqueous extract form Z. mucronata revealed a potential anti-HIV activity in vitro. However, the clinical trials are essential in validating the use of the plant species in the treatment and management of HIV-AIDS. It should be noted that traditional medicine involves use of aqueous extracts, not isolated compounds and organic extracts. Furthermore, the use of plant species in combination might result in a synergistic effect and may be safer for use with less or no side effects at traditional medicine is perceived to.
Although the 80 % methanol extract from leaves revealed 75 % inhibition of both Polio virus at high concentration, it is of lesser health concern as Polio vaccines are in place in most countries and given at a young age to control poliomyelitis which is a communicable disease [156].
7.3. Anti-malarial activity
Malaria is a life-threatening disease caused by Plasmidum species, particularly Plasmodium falciparum, and infects many individuals world-wide, resulting in approximately 445 000 in 2016 only [157]. The disease is more prevalent in Africa than any other continent in the entire world. The in vitro anti-plasmodial activity of different Ziziphus mucronata extracts is well documented in the literature [104, 113, 158, 159, 160, 161]. According to Memvanga et al. [162], the guidelines of World Health Organization and basic criteria for anti-plasmodial drug discovery classifies the activities of extracts as of high or pronounced activity (LC50 ≤ 5 μg/ml), good or promising activity (11–50 μg/ml) and weak activity (51–100 μg/ml), while a pure compound is defined as highly active when its LC50 is ≤ 1 μg/ml. Z. mucronata (ZMF2) and (ZMF5) fractions from the ethanol stem bark revealed potent activity, yielding 3.1 and 3.2 μg/ml against P. falciparum in PfHsp70-1 and PfHsp70-z proteins respectively [113]. These studies suggest that the use of the plant species in the treatment of malaria and related infections needs to be validated in vivo. Further, the plant extracts may be responsible for inhibition of proteins-related to growth of Plasmodium species.
7.4. Anti-diabetic activity
Over one-third of adults worldwide are overweight, 40% hypertensive, and approximately 700 million people diabetic with impaired glucose tolerance [163]. It is projected that the infections are more prevalent in South East Asian region and more likely to increase to threshold from 2003 to 2030, mainly due to obesity, high blood pressure, smoking, excessive physical inactivity, diet (saturated fats and trans-fatty acids) and other socioeconomic factors [164, 165]. Diabetic patients are frequently admitted in hospitals mainly due to their low immunity levels resulting in major skin, mucous membrane, nail and soft tissue infections which may result in devastating effects to human life [166].
The in vitro and in vivo studies of antidiabetic effects of extracts from Ziziphus mucronata have been studied [107, 167, 168, 169]. In the in vitro studies, the n-butanol, aqueous, ethyl acetate and dichloromethane fractions from the ethanol roots extract revealed IC50 values of 1.41, 4.38, 23.6 and 43.91 μg/ml against α-glucosidase while control drug acarbose exhibited 55.59 μg/ml. According to Kumar et al [170], an IC50 value of 100 μg/ml is potent and worth investigating for an in vivo study, possible phytochemicals involved and mechanism of actions involved. Other authors recommend an IC50 value better than the commonly used anti-diabetic control drug acarbose to be important for such purposes [171, 172, 173, 174]. These extracts are likely to inhibit the last step in carbohydrate digestion, namely the conversion of disaccharide to monosaccharide (glucose) and a consequent decrease in the rate of entry of glucose into the systemic circulation [175]. The phytochemicals mostly involved in inhibition of such enzymes are commonly dietary and natural polyphenols [176, 177, 178, 179]. In the In vivo studies, the n-butanol fraction from roots revealed a clear reduction in blood glucose, improved glucose tolerance ability and higher serum insulin [169]. Although these studies validate the use of the plant species in African traditional medicine, treating mellitus diabetes, there are no identified compounds responsible for such activities. Furthermore, the mechanisms of action of such extracts remains unknown.
7.5. Antiparasitic activity
The global food security will require the production of more food using resources including land more efficiently. However, it important to consider the role of parasites in agriculture as the possible threats to food security, production and quality. The resistance of nematodes that infects ruminants is becoming more common, with some organisations incorporating the use of dual anthelmintic compounds with broad spectrum and different mechanisms of action on helminths with the intention of reducing resistance and the treatment regime [180]. Haemonchus contortus is a gastrointestinal parasite that infects sheep and goats and have a devastating effect on sheep production, thereby resulting in negative financial effect on breeders [181]. A relatively huge number of African medicinal plants have been indigenously and pharmacologically implicated in the treatment of anthelmintic infections [182, 183]. The methanol and aqueous extracts from the stem were investigated for anthelminthic activity using larval mortality (LM) and egg hatch inhibition (EHI) assays against [184, 185]. Methanol extract revealed LC50 value of 3.9 and 2.65 mg/ml against Haemonchus contortus in EHI and LM assays respectively while aqueous extract revealed 7.5 and 14.7 mg/ml against similar organism in the LM and EHI respectively. Although the methanol extract revealed activity against H. contortus, the mechanism(s) of section still needs to be explored. However, the tannins, as reported in Phytochemistry section are suspected to be responsible for such activities [186, 187]. Elsewhere, the root bark extract revealed an effect on cestodes of Hymenolepis diminuta after 1- and 24-hours incubation period at 1.6 and 0.002 mg/ml, while the organic extract from twigs revealed average % worm death of 69.47 against Caenorhabditis elegans at 1 mg/ml [188, 189].
Entamoeba histolytica is a pathogenic amoeba causing amoebiasis in humans and common in homosexual who are sexually active [190]. It is important to note that the disease is asymptomatic. Although McGaw et al [191], reported LC50 value of >5 mg/ml against E. histolica, there is a need to explore the anthelmintic activity of various plant part of the plant species against these parasite and other common parasites.
7.6. Antioxidant activity
The Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) including peroxides, hydroxyl radicals, super-oxides, and nitrous oxide, generated in the living organisms by cellular metabolism, are known to play a vital role in oxidative cellular damage and various stresses resulting in variety of diseases [192]. Although free radicals are known to cause diseases, they can be controlled and quenched by antioxidant ability of various foods and some important secondary metabolites from plants, particularly medicinal plants. The antioxidant activity of extracts from Z. mucronata are well documented [90, 102, 167, 193, 194, 195]. The most commonly used method is DPPH assay which involves the ability of a plant extract to reduce a purplish stable radical at 517 nm spectrophotometrically [196]. Over and above that, the method is highly reproducible, sensitive and time efficient [197].
According to Sahidi and Zhong [198], the effectiveness of the antioxidants is generally influenced by several factors, including their structural features, concentration, temperature, type of oxidation substrate and physical state of the system as well as presence of pro-oxidants and synergists. The plant extracts with potent antioxidant activity should reveal an IC50 value of 20.8 μg/ml or less in at least three different assays [199] and are likely to act as reducing agents, hydrogen donor and quenchers of singlet oxygen [200]. The ethanol extract from the leaves, roots and stem bark exhibited IC50 values of 1.68; 1.38 and 1.99 μg/ml against DPPH free radical, while the acetone extract of the stem bark revealed IC50 value of 15.27 μg/ml in a similar assay [160, 192]. Furthermore, the ethyl acetate extract of both roots and stem bark exhibited IC50 values of 1.36 and 1.07 μg/ml in a hydroxyl radical scavenging activity assay [192], while methanol extract from the roots exhibited IC50 value of 19 μg/ml against ABTS free radical [102]. Although the extracts from Z. mucronata revealed a potent antioxidant activity in vitro mostly using chemical based assays, there is a need to perform in vivo and cellular model studies [201, 202]. It is important to notice that the in vitro models may not always translate into in vivo models.
7.7. Anti-inflammatory and anti-neurodegenerative effect
Inflammation is generally associated with several neurodegenerative diseases which may include Alzheimer's disease, Parkinson's disease, neurotropic viral infections, stroke, paraneoplastic disorders, traumatic brain injury and multiple sclerosis [203]. Usually, inflammation may be caused by pathogenic microorganisms which invade the body and reside in the body tissues or circulate in the blood stream [204]. Cyclooxygenase is an enzyme that converts arachidonic acid into prostaglandin and exists in two forms, COX-1 and COX-2 [205]. Before these COX forms were discovered, a single dose concentration of extracts was evaluated for anti-inflammatory activity against prostaglandin and revealed inhibition ranging from 0 to 89 % [206, 207]. These results are not very important as the target extracts and isolated compounds are expected to selectively inhibit COX-2 [208]. COX-2 is generally absent in healthy tissue and is induced in migratory and other cells by pro-inflammatory agents, such as cytokines, mitogens and endotoxins under pathological conditions such as inflammation [209].
The 90 % methanol extract revealed 66.5 and 66.2 % against COX-1 and COX-2 respectively at 250 μg/ml [149]. According to these data, the extract equally inhibit COX-1 and COX-2 and likely to result in adverse side-effects in humans, including a reduction in mucosal blood flow and mucous secretion, delay in the healing of ulcers and a reduction in renal blood flow [210]. However, it is important to note that the data reports only a single concentration which is also high and no IC50 values reported. Therefore, there is a need to explore the possibility of extracts from different plant parts to selectively inhibit COX-2.
In the Acetylcholinesterase (AChE) assay, the aqueous methanol extract of the leaves stored for 16 and 12 years revealed 90.4 % and 84 % inhibition of AChE at 1.0 mg/ml, while the ethyl acetate extract of roots revealed IC50 value of 1 μg/ml [102, 103]. These may well indicate that the compounds responsible for AChE inhibition could survive for ages without losing activity. According to Kaufmann et al [211], the potent AChE inhibitors should possess an IC50 value of 2.5 μg/ml or less and is therefore more likely to exhibit higher-fold stronger than the already known AChE inhibitor called galantamine. Furthermore, the potent IC50 makes the extract a possible candidate for drugs to be used in the prevention of neurological inflammation and related diseases. However, there is a need to explore the in vivo studies which should also determine the cytotoxic effects of the extract(s). According to Mathew and Subramanian [212], there is an urgent need of finding new AChE inhibitor lead compounds with lower toxicity and higher central nervous system penetration. The mode of action of such inhibitors still needs to be explored. Elsewhere, the methanol extract of roots revealed IC50 value of >100 μg/ml against untreated SY-SY5Y cells, while the aqueous extract from stem bark did not inhibit nitric oxide production and but induced the activation of macrophages [150, 213]. It is important to note that the macrophages play a dual role in host defence through acting as the first line of defence by mounting an inflammatory response to antigen exposure and also act as antigen presenting cells and initiate the adaptive immune response [214].
7.8. Anticancer activity
Cancer result in the development of tumour cells surpassing the growth of normal human cells and ma infect breast, liver, stomach, cervices, brain and prostate. The disease may be genetically inherited and is more pronounced in Europe than other continents. According to Bray et al [215], between 2008 and 2030, the disease is likely to increase to about 75 % globally and will double in less developed countries. Although surgery, radiation, chemotherapy, immunotherapy, hormone therapy, or gene therapy have been used as forms of treatment, there is a need to discover plant based anti-cancer compounds with less or no side effects [216, 217].
The anticancer activity of extracts from Ziziphus mucronata have been investigated against various cancerous cell lines including HeLa, Caco-2, A431, Chinese harmster ovary cells (CHO), HT29 and H4 cells at different concentrations [75, 90]. The methanol extract of roots revealed 50–75 % inhibition of HeLa, HT29 and A431 cells at 100 μg/ml [75]. Although the results did not show LC50, authors elsewhere recommend that the 60–80 % inhibition of cancerous cells to be of interest for introspecting new compounds [218]. However, Mongalo et al [133], reported the extracts with anti-proliferative effect of 55 % inhibition at a concentration of 25 μg/ml to be more active, suggesting that Z. mucronata extracts revealed a moderate anti-cancer activity against HeLa, HT29 and A431.
The dichloromethane extract of the leaves revealed activity against the CHO, Caco-2, KMST-6, HeLa and H157 cell lines and revealed a LC50 value of 126.73 μg/ml against H4 cell line [90]. According to the American Cancer Institute, the potent anticancer extracts should record LC50 value of 20 μg/ml or less, and classified ≤100 and ≤200 μg/ml as god and moderate inhibitors respectively [219], suggesting that extracts from Z. mucronata have moderately inhibited various cancerous cell lines. However, there is a need to explore the anticancer activity of various extracts from the plant species for anti-cancer activity against other drug sensitive cell lines including MCF-7, CCRF-CEM, CEM-/ADR 5000 and other cervical cancer cell lines.
7.9. Anti-sickling effect
Sickle cell disease (SCD) is a genetic blood disorder characterized by decrease in the RBC's flexibility red blood cells (RBCs) that assume an abnormal rigid sickle shape and results in a risk of various complications [220]. Anaemia is form of SCD more prevalent in pregnant women and young pre-school children and is a major public health problem, particularly in Africa [221]. However other blood disorders also falls into SCD group. Ethanol extracts of both stem bark and roots revealed >70 % anti-sickling effect, while aqueous extract of stem bark exhibited similar effect in the Emmel test [101]. The study further identified the anthocyanins as the potential compounds responsible for such anti-sickling effect and the result is supported by other authors [222, 223]. The anti-sickling effect of the aqueous extract is important as traditional medicine involves the use of water as a solvent in preparing various decoctions which are mostly administered orally in treating various pathogenic human and animal infections. However, these in vitro studies need to be taken to the next level in vivo. According to Dash et al [220], the studies on anti-sickling effects of medicinal plants extracts and isolated compounds is scarce and needs to be elevated with immediate effect.
7.10. Histopathologic and hepatoprotective effect
Although the n-butanol fraction from the ethanol extract of the roots, at a maximum concentration of 300 mg/kg, revealed other important biological activities, it did not result in appreciable amelioration of the hepatic and renal damage which was caused by induced diabetes [169]. However, it is possible that the fraction might significantly improve hepatic and renal damage at threshold concentrations. Elsewhere, the methanol extract from fruits resulted in a significant decline serum marker enzymes SGOT, SGPT and ALP [195, 224]. Furthermore, the protective effect of the extract was highly pronounced, revealing reduced necrosis compared to dimethoate fed rats.
7.11. Anti-mutagenic effect
Mutation can be caused several factors including metabolic defects in most cellular systems thereby triggering morbidity and possible mortality in living organisms. Mutagens are not only involved in genotoxicity and carcinogenesis but also in the inception and pathogenesis of several diseases including chronic degenerative diseases including hepatic disorders, neurodegenerative disorders, cardiovascular disorders, diabetes, arthritis, chronic inflammation and in the process of ageing [225].
Various extracts from the roots, leaves, stem bark and twigs were investigated for anti-mutagenic effects using various assays [149, 226, 227, 228, 229]. Generally, only the 90 % methanol extracts of roots, stem abrk and leaves revealed some anti-mutagenic effects at a highest concentration of 5 mg/ml. However, the stored plant materials did not show any mutagenic effect in both TA98 and TA 100 strains [229]. The plant extracts indicating anti-mutagenicity is not necessarily an anti-carcinogen; however, it is an indication of possible candidates for such purposes [230]. Besides exhibiting mutagenic and carcinogenic effects, it is important to notice that natural anti-mutagens are known to possess beneficial effects which includes anti-inflammatory, anti-diabetic, hepato-protective, immunomodulatory, anti-rheumatic, antioxidant and other detoxifying effects [231]. The mutagenicity of the 90 % methanol extracts is not more relevant in traditional medicine as the system employs the use of tap water as a solvent for extracting active plant materials which alleviate pathogens responsible for various diseases in both animals and humans.
7.12. Other potential values of Ziziphus mucronata
Besides being used as a medicine and source for firewood, the fruits of the plant are used in wine and beer making, as an alcoholic beverage for quenching the thirst. Beverage products fermented from these fruits are amongst the traditional malted beers are nutritious and form an essential component of diet in African rural areas [232, 233, 234]. Although not sold in most rural areas, these beers can be traded to tourists and other road users in most parts of Limpopo Province and have potential like the world known Marula. These traditional beverages and foods which may include mageu are also used as a carrier for bitter medicinal plants, such as Cassia abbreviata Oliv. (Monepenepe-in Sepedi) and Securidaca longipedunculata Fresen. (Mupesu-in TshiVenda), mostly used by men as aphrodisiacs [235]. These is mostly practised by Pedi tribes in Limpopo Province around the Month of April after the Marula regime which is mostly in February. The fruits are also edible [236, 237], and have a bitter taste. It is believed that the bitter taste may result in cough when consumed in larger quantities by young children. The other authors report a bitter taste to improve appetite and treat digestive disorders in humans [238, 239]. The fleshy part of the fruit may also be dried, ground and made into porridge during the consecutive years of drought which are generally characterised by the use of yellow mealie meal as a staple food [240]. Further, the remaining fruit kernel may also be used to make rosary beads which may generate income [53].
In other tribes, the juvenile leaves of the plant species are used a leafy vegetable which is consumed as “Morogo” in the Eastern Cape Province of South Africa [241]. The wood from the plant species may also be used for firewood, craftwork and fencing purposes as it is more durable and resist harsh environmental conditions and termites [48, 242, 243, 244, 245]. According to Van Wyk [245], the plant species is amongst the most commercially important plants in Africa. Although the nutritional value or status of Z. mucronata is not well studied, it is important to note that the fruits bearing indigenous plant species have potential to curb malnutrition, improve household food security and general community health in Sub-Saharan Africa [246].
The stem bark and roots are traded at various muthi markets within the country and the entire Southern Africa generating income for plant sellers [56, 247]. The trading may well limit and compromise the quality, safety and efficacy of the plant material as it may involve poor packaging and drying [248]. Some of the honey products are believed to have origin from Ziziphus mucronata and have potent antimicrobial activities [249]. The plant species is also used for ornamental purposes, and its leaves are palatable to livestock [250]. The browsed leaves of the plant species are highly digestible and has been reported to possess higher crude protein content, acid detergent lignin, hemi cellulose and crude fibre which are essential in animals [251, 252].
8. Conclusions, recommendations and future studies
Ziziphus mucronta is a valuable medicinal, ornamental and food plant. Although different countries may use the plant species for different medicinal purposes, the general consensus is that the different plant parts may be used in the treatment of both human and animal infections relating to diarrhoea, general body pains, skin-related infections, chest and respiratory related and variety of sexually transmitted infections ranging from dropsy to gonorrhoea. Pharmacological reports validates the use of the plant species in the treatment of some infections, particularly skin related and pulmonary infections. However, there is still a need to explore the antimicrobial activity of the plant species against STI related microorganisms as the data is scarce or unavailable for most organisms except Candida albicans and a few other organisms which may result in opportunistic infections arising from HIV-AIDS when the individual becomes immunocompromised. The data on the possible use of the plant species in the treatment of diarrhoea, sexually transmitted infections, skin related and gynaecological complaints are scant and still needs to be explored and validated both in vitro and in vivo. Furthermore, the anticancer and anthelmintic activity of the plant species also needs to be explored. However, the data is of satisfactory standard on the validation of the plant species in the treatment of stomach ache and tuberculosis. Further, the mode of action of such plant extracts is still unknown.
Besides potent antimicrobial activity, the in vitro studies revealed that the plant species possess appreciable and potential antioxidant, anti-diabetic, anti-viral, anthelmintic, antimalarial and anti-inflammatory activities. However, there is a need to explore such activities in vivo as the in vitro results may not necessarily translate into in vivo. Furthermore, the anticancer activity of the plant species still needs to be explored as the reports in the literature shows little evidence while the ethno-medicinal uses shows the plant species to be prevalently used in the treatment of cancer. Anticancer activity against both drug sensitive and drug resistant cell lines including MCF-7, CCRF-CEM, CEM-/ADR 500, MES-SADX-5 and other cell lines is of paramount importance. Although the anthelmintic activity is well reported, there is a need to explore such activities against common pathogens that infect domestic animals that are known to limit agricultural productivity of meat. Although the anti-inflammatory of the plant extracts is reported, there is a need to explore Anti-inflammatory activity against COX-2 to compare if the extracts selectively inhibit COX-2, not COX-1.
Phytochemically, the cyclopeptide alkaloids known as mucronines and abyssenines are prevalently present within the plant species. However, the biological activities of most of such compounds are still not well documented in the literature. In our current work, we are exploring the compounds from both roots and leaves, using GC-MS instruments, with the intention of comparing and quantifying the possible phytochemicals within the plant species. The food value and other important parameters of both the fleshy ripe and green fruits are also investigated.
Toxicologically the plant species may be toxic only at higher dosages. However, data on the cytotoxic effect of the plant species against normal human cell lines and the presence of heavy metals is scanty and still needs to be explored and validated as the plant species is used in the treatment of various human and animal infections. We are also exploring the metalloids concentrations of the plant species from different localities within South Africa. Furthermore, there is a need to develop a standardised fingerprint of Z. mucronata for quality control as the plant species is widely used for the treatment of major pathogenic infections in humans and also serve as a source for food which may, one way or the other, improve food security, curb malnutrition and serve as a starting material for production of alcoholic beverages which are used to quench thirst.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by the National Research Foundation (NRF) South Africa (Grant Unique Number 94179, University of South Africa).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Mhaskar K.S., Blatter E., Caius J.F. Sri Satguru Publications, Indian Books Center; Delhi, India: 2000. Indian Medical Science Series. [Google Scholar]
- 2.Ara H., Hassan A., Khanam M. Taxonomic study of the genus Ziziphus mill. (Rhamnaceae) of Bangladesh. Bangladesh J. Plant Taxon. 2008;15:47–61. https://www.banglajol.info/index.php/BJPT/article/view/917/983 [Google Scholar]
- 3.Najafi S. Phytochemical screening and antibacterial activity of leaf extract of Ziziphus mauritiana Lam. Int. Res. J. Appl. Basic Scie. 2013;4:3274–3276. https://pdfs.semanticscholar.org/a11c/b4ef82ad2096a0ec7c531aa0048d28c6329b.pdf [Google Scholar]
- 4.Sameera N.S., Mandakini B.P. Investigations into the antibacterial activity of Ziziphus mauritiana Lam. And Ziziphus xylopyra (Retz.) Willd. Int. Food Res. J. 2015;22:849–853. http://www.ifrj.upm.edu.my/22%20(02)%202015/(56).pdf [Google Scholar]
- 5.Ali A.B., Almagboul A.Z., Mohammed O.M. Antimicrobial activity of fruits, leaves, seeds and stems extracts of Ziziphus spina –Christi. Arab. J. Med. Arom. Plant. 2015;1:94–107. https://revues.imist.ma/index.php?journal=AJMAP&page=article&op=view&pa [Google Scholar]
- 6.Eslami G., Hashemi G., Yazdi M.M.K., Benvidi M.E., Rad P.K., Moradi S.L., Fallah F., Dadashi M. Antibacterial effects of Zataria multiflora, Ziziphus, Chamomile and Myrtus communis Methanolic extracts on IMP-Type metallo- beta-lactamase producing Pseudomonas aeruginosa. Arch. Clin. Infect. Dis. 2016;11 e32413. [Google Scholar]
- 7.Ads E.N., Rajendrasozhan S., Hasan S.I., Sharawy S.M.S., Humaidi J.R. Phytochemical, antimicrobial and cytotoxic evaluation of Ziziphus spina-christ (L.) stem bark. Biomed. Res. 2017;28:6646–6653. http://www.biomedres.info/biomedical-research/phytochemical-antimicrobial- [Google Scholar]
- 8.Ghasham A.A., Muzaini A.A., Qureshi K.A., Elhassan G.O., Khan R.A., Farhana S.A., Hashmi S., El-Agamy E., Abdallah W.E. Phytochemical screening, antioxidant and antimicrobial activities of methanolic extract of Ziziphus mauritiana Lam. leaves collected from Unaizah, Saudi Arabia. Int. J. Pharmaceut. Res. Allied Sci. 2017;6:33–46. https://ijpras.com/vol6-iss3/IJPRAS-2017-6-3-33-46.pdf [Google Scholar]
- 9.Gao Q.-H., Wub P.-T., Liu J.-R., Wu C.-S., Parry J.W., Wang M. Physico- chemical properties and antioxidant capacity of different jujube (Ziziphus jujuba Mill.) cultivars grown in loess plateau of China. Sci. Hort. 2011;130:67–72. [Google Scholar]
- 10.Koley T.K., Kaur C., Nagal S., Walia S., Sarika S.J. Antioxidant activity and phenolic content in genotypes of Indian jujube (Zizyphus mauritiana Lamk.) Arab. J. Chem. 2016;9:S1044–S1052. [Google Scholar]
- 11.Choopani R., Sadr S., Kaveh S., Kaveh N., Dehghan S. Pharmacological treatment of catarrh in Iranian traditional medicine. J. Trad. Compl. Med. 2015;5:71–74. doi: 10.1016/j.jtcme.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siddiqui S.N., Patil S.N. Assessment of antioxidant and cytotoxic activities of extracts of some Ziziphus species with identification of bioactive components. Eur. J. Med. Plants. 2015;8:202–213. [Google Scholar]
- 13.Jeong S.J., Kim O.S., Yoo S.R., Seo C.S., Kim Y., Shin H.K. Anti-inflammatory and antioxidant activity of the traditional herbal formula Gwakhyangjeonggi-san via enhancement of heme oxygenase-1 expression in RAW264.7 macrophages. Mol. Med. Rep. 2016;13:4365–4371. doi: 10.3892/mmr.2016.5084. [DOI] [PubMed] [Google Scholar]
- 14.Afzal S., Batool M., Ch B.A., Ahmad A., Uzair M., Afzal K. Immunomodulatory, cytotoxicity, and antioxidant activities of roots of Ziziphus mauritiana. Phcog. Mag. 2017;13:S262–S265. doi: 10.4103/pm.pm_398_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang W., Wang Y., Jiang X., Sun Y., Zhao Z., Li S. Protective effect of flavonoids from Ziziphus jujuba cv. Jinsixiaozao against acetaminophen- induced liver injury by inhibiting oxidative stress and inflammation in mice. Molecules. 2017;22:17. doi: 10.3390/molecules22101781. 1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bachaya H.A., Iqbal Z., Khan M.N., Sindhu Z.-U.-D., Jabbar A. Anthelmintic activity of Ziziphus nummularia bark and Acacia nilotica fruit against Trichostrongylid nematodes of sheep. J. Ethnopharmacol. 2009;123:325–329. doi: 10.1016/j.jep.2009.02.043. [DOI] [PubMed] [Google Scholar]
- 17.Jarald E.E., Joshi S.B., Jain D.C. Antidiabetic activity of extracts and fraction of Ziziphus mauritiana. Pharm. Biol. 2009;47:328–334. [Google Scholar]
- 18.Madara A.A., Ajayi J.A., Salawu O.A., Tijani A.Y. Anti-malarial activity of ethanolic leaf extract of Piliostigma thonningii Schum. (Caesalpiniacea) in mice infected with Plasmodium berghei berghei. Afr. J. Biotechnol. 2010;9:3475–3480. http://www.academicjournals.org/AJB [Google Scholar]
- 19.Benammar C., Baghdad C., Belarbi M., Subramaniam S., Hichami A., Khan N.A. Antidiabetic and antioxidant activities of Ziziphus lotus .L aqueous extracts in Wistar rats. J. Nutr. Food Sci. 2014:8. S8:004. [Google Scholar]
- 20.Keerthi M., Venkateswararao P., Devi P.L.A., Kanaka G., Laxmi M., Venu P. Phytochemical screening and anti-helminthic activity on the fruits of Ziziphus jujube. Pharma Innov. J. 2016;5:107–109. [Google Scholar]
- 21.Hung C.-F., Hsu B.-Y., Chang S.-C., Chen B.-H. Antiproliferation of melanoma cells by polysaccharide isolated from Ziziphus jujube. Nutrition. 2012;28:98–105. doi: 10.1016/j.nut.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Hoshyar R., Mohaghegh Z., Torabi N., Abolghasemi A. Antitumor activity of aqueous extract of Ziziphus jujube fruit in breast cancer: an in vitro and in vivo study. Asian Pac. J. Reprod. 2015;4:116–122. [Google Scholar]
- 23.Saeed M.E.M., Abdelgadir H., Sugimoto Y., Khalid H.E., Efferth T. Cytotoxicity of 35 medicinal plants from Sudan towards sensitive and multidrug- resistant cancer cells. J. Ethnopharmacol. 2015;174:644–658. doi: 10.1016/j.jep.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Khan M.P.Z., Ahmad M., Zafar M., Sultana S., Ali M.I., Sun H. Ethnomedicinal uses of edible wild fruits (EWFs) in Swat valley, Northern Pakistan. J. Ethnopharmacol. 2015;173:191–203. doi: 10.1016/j.jep.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 25.Al-Saeedi A.H., Al-Ghafri M.T.H., Hossain M.A. Brine shrimp toxicity of various polarities leaves and fruits crude fractions of Ziziphus jujuba native to Oman and their antimicrobial potency. Sustain Chem. Pharm. 2017;5:122–126. [Google Scholar]
- 26.Said A., Huefner A., Tabl E.-E.A.A., Fawzy G. Phenolic compounds from seeds of Ziziphus spina-christi. IUFS J. Biol. 2011;70:39–43. http://dergipark.gov.tr/download/article-file/95621 [Google Scholar]
- 27.Kaleem W.A., Muhammad N., Khan H., Rauf A. Pharmacological and phytochemical studies of genus Ziziphus. Middle-east. J. Sci. Res. 2014;21:243–1263. [Google Scholar]
- 28.Mahajan R.T., Chopda M.Z. Phyto-Pharmacology of Ziziphus jujuba Mill- A plant review. Phcog. Rev. 2009;3:320–329. http://www.phcogrev.com/text.asp?2009/3/6/320/59530 [Google Scholar]
- 29.Ibrahim J.A., Egharevba H.O., Nnamdi R.A., Kunle O.F. Comparative pharmacognostic and phytochemical analysis of Ziziphus spina-christi (L.) Desf. And Ziziphus abyssinica Hochst. Ex A. Rich. Int. J. Pharmacog. Phytochem. Res. 2015;7:1160–1166. http://impactfactor.org/PDF/IJPPR/7/IJPPR,Vol7,Issue6,Article21.pdf [Google Scholar]
- 30.Singh N., Kaushik N.K., Mohanakrishnan D., Tiwari S.K., Sahal D. Antiplasmodial activity of medicinal plants from Chhotanagpur plateau, Jharkhand, India. J. Ethnopharmacol. 2015;165:152–162. doi: 10.1016/j.jep.2015.02.038. [DOI] [PubMed] [Google Scholar]
- 31.Ray S.D., Dewanjee S. Isolation of a new triterpene derivative and in vitro and in vivo anticancer activity of ethanolic extract from root bark of Zizyphus nummularia Aubrev. Nat. Prod. Res. 2015;29:1529–1536. doi: 10.1080/14786419.2014.983921. [DOI] [PubMed] [Google Scholar]
- 32.Elaloui M., Laamouri A., Ennajah A., Cerny M., Mathieu C., Vilarem G., Chaar H., Hasnaoui B. Phytoconstituents of leaf extracts of Ziziphus jujuba Mill. Plants harvested in Tunisia. Ind. Crop. Prod. 2016;83:133–139. [Google Scholar]
- 33.Ahmad R., Ahmad N., Naqvi A.A. “Ziziphus oxyphylla”: ethnobotanical, ethnopharmacological and phytochemical review. Biomed. Pharmacother. 2017;91:970–998. doi: 10.1016/j.biopha.2017.04.129. [DOI] [PubMed] [Google Scholar]
- 34.Modi A., Kumar S.J.V. Zizyphus xylopyrus (Retz.) Willd: a review of its folkloric, phytochemical and pharmacological perspectives. Asian Pac. J. Trop. Dis. 2014;4:S1–S6. [Google Scholar]
- 35.World Agroforestry 2010. http://www.worldagroforestry.org/Sites/TreeDBS/aft/docs/Ziziphus_mucronata.doc
- 36.Deutschländer M.S., Lall N., Van de Venter M. Plant species used in the treatment of diabetes by South African traditional healers: an inventory. Pharm. Biol. 2009;47:348–365. [Google Scholar]
- 37.World Agroforestry . 2009. Ziziphus mucronata Willd.http://www.worldagroforestry.org/treedb/AFTPDFS/Ziziphus_mucronata.PDF [Google Scholar]
- 38.Zietsman P.C., Van Wyk A.E., Botha F.C. Vegetative and reproductive phenology of Ziziphus mucronata subspecies mucronata (Rhamnaceae) South Afr. J. Bot. 1989;55:564–573. [Google Scholar]
- 39.Raimondo D., Von Staden L., Foden W., Victor J.E., Helme N.A., Turner R.C., Kamundi D.A., Manyama P.A. South African National Biodiversity Institute; Pretoria: 2009. Red List of South African Plants. Strelitzia 25. [Google Scholar]
- 40.Mathibela K.M. University of Limpopo; Polokwane, South Africa: 2013. An Investigation into Aspects of Medicinal Plant Use by Traditional Healers from Blouberg Mountain, Limpopo Province, South Africa. MSc Dissertaion. [Google Scholar]
- 41.Semenya S.S., Potgieter M.J., Erasmus L.J.C. Indigenous plant species used by Bapedi healers to treat sexually transmitted infections: their distribution, harvesting conservation and threats. South Afr. J. Bot. 2013;87:66–75. [Google Scholar]
- 42.Mokgolodi N.C., Hu Y., Shi L., Liu Y. Ziziphus mucronata: an underutilised medicinal plant in Africa. For. Stud. China. 2011;13:163–172. doi: 10.1007/s11632-011-0309-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kedai T.S., Khan A.A. Guyabano (annona muricata): a review of its traditional uses phytochemistry and pharmacology. Am. J. Res. Comm. 2011;2:247–268. [Google Scholar]
- 44.Mongalo N.I. Peltophorum africanum Sond [Mosetlha]: a review of its ethnomedicinal uses, toxicology, phytochemistry and pharmacological activities. J. Med. Plants Res. 2013;7:3484–3491. [Google Scholar]
- 45.Maroyi A. Review of ethnomedicinal uses, phytochemistry and pharmacological properties of Euclea natalensis A.DC. Molecules. 2017;22:2128. doi: 10.3390/molecules22122128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tshisikhawe M.P., Van Rooyen M.W. Population biology of Elaeodendron transvaalense Jacq. in the presence of harvesting. FYTON. 2013;82:303–311. [Google Scholar]
- 47.Van Der Merwe D., Swan G.E., Botha C.G. Use of ethno-veterinary medicinal plants in cattle by Setswana-speaking people in the Madikwe area of the North West Province of South Africa. J. S. Afr. Vet. Assoc. 2001;72:189–196. doi: 10.4102/jsava.v72i4.651. [DOI] [PubMed] [Google Scholar]
- 48.Magwede K., Van Wyk B.-E., Van Wyk A.N. An inventory of VhaVenda useful plants. South Afr. J. Bot. 2018 Article in Press. [Google Scholar]
- 49.Tapsoba H., Deschamps J.-P. Use of medicinal plants for the treatment of oral diseases in Bukina Faso. J. Ethnpharmacol. 2006;104:68–78. doi: 10.1016/j.jep.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 50.Maroyi A. An ethnobotanical survey of medicinal plants used by the people of Nhema communal area, Zimbabwe. J. Ethnopharmacol. 2011;136:347–354. doi: 10.1016/j.jep.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 51.Arnold H.J., Gulumian M. Pharmacopoeia of traditional medicine in Venda. J. Ethnopharmacol. 1984;12:35–74. doi: 10.1016/0378-8741(84)90086-2. [DOI] [PubMed] [Google Scholar]
- 52.Hutchings A., Scott A.H., Lewis G., Cunningham G. University of Natal Press; Pietermaritzburg, South Africa: 1996. Zulu Medicinal Plants: an Inventory. [Google Scholar]
- 53.Coates M. third ed. Vol. 821. Struik Publishers; Cape Town, RSA: 2002. (Palgrave, Keith Coates Palgrave Trees of Southern Africa). Third Impression. [Google Scholar]
- 54.Chinsembu K.C. Ethnobotanical study of the medicinal flora utilised by traditional healers in the management of sexually transmitted infections in Sesheke District, Western Province, Zambia. Braz. J. Pharmacog. 2016;26:268–274. [Google Scholar]
- 55.Bah S., Diallo D., Dembélé S., Paulsen B.S. Ethnopharmacological survey of plants used for the treatment of schistosomiasis in Niono District, Mali. J. Ethnopharmacol. 2006;105:387–399. doi: 10.1016/j.jep.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 56.Setshogo M.P., Mbereki C.M. Floristic diversity and uses of medicinal plants sold by vendors in Gaborone, Botswana. Afr. J. Plant Sci. Biotechnol. 2011;5:69–74. [Google Scholar]
- 57.Kigen G., Maritim A., Some F., Kibosia J., Rono H., Chepkwony S., Kipkore W., Wanjoh B. Ethnopharmacological survey of the medicinal plants used in Tindiret, Nandi County, Kenya. Afr. J. Tradit., Complementary Altern. Med. 2016;13:156–168. [Google Scholar]
- 58.Chinsembu K.C., Negumbo J., Likando M., Mbangu A. An ethnobotanical study of medicinal plants used to treat livestock disease in Onayena and Katima Mulilo, Namibia. South Afr. J. Bot. 2014;94:101–107. [Google Scholar]
- 59.Madikizela B., Ndhlala A.R., Finnie J.F., Van Staden J. Ethnopharmacological study of plants from Pondoland used against diarrhoea. J. Ethnopharmacol. 2014;141:61–71. doi: 10.1016/j.jep.2012.01.053. [DOI] [PubMed] [Google Scholar]
- 60.Lawal I.O., Grierson D.S., Afolayan A.J. Phytotherapeutic information on plants used for the treatment of tuberculosis in Eastern Cape Province, South Africa. Evid. Base. Compl. Alt. Med. 2014:11. doi: 10.1155/2014/735423. Article ID 735423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steenkamp V. Traditional herbal remedies used by South African women for gynaecological complaints. J. Ethnopharmacol. 2003;86:97–108. doi: 10.1016/s0378-8741(03)00053-9. [DOI] [PubMed] [Google Scholar]
- 62.Van Wyk B.-E., Van Oudtshoorn B., Gericke N. second ed. Briza Publications; Pretoria, South Africa: 2009. Medicinal Plants of South Africa. [Google Scholar]
- 63.Corrigan B.M., Van Wyk B.E., Geldenhuys C.J., Jardine J.M. Ethnobotanical plant uses in the Kwanibela peninsula, St Lucia, South Africa. S Afr.J. Bot. 2011;77:346–359. [Google Scholar]
- 64.Lall N., Kishore N. Are plants used for skin care in South Africa fully explored? J. Ethnopharmacol. 2014;153:61–84. doi: 10.1016/j.jep.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 65.Chauke M.A., Shai L.J., Mogale M.A., Tshisikhawe M.P., Mokgotho M.P. Medicinal plant use of villagers in the Mopani district, Limpopo Province, South Africa. Afr. J. Tradit., Complementary Altern. Med. 2015;12:9–26. [Google Scholar]
- 66.McGaw L.J., Lall N., Meyer J.J.M., Eloff J.N. Potential of South medicinal plants against Mycobacterium infections. J. Ethnopharmacol. 2008;119:482–500. doi: 10.1016/j.jep.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 67.Orwa C., Mutua A., Kindt R., Jamnadass R., Anthony S. 2009. Agroforestree Database: A Tree Reference and Selection Guide.htttp:www.worldagroforestry.org/sites/tredbs/treedatabases.asp Version 4.0. [Google Scholar]
- 68.De Wet H., Nciki S., Van Vuuren S.F. Medicinal plants used for the treatment of various skin disorders by a rural community in northern Maputaland, South Africa. J. Ethnobiol. Ethnomed. 2013;9:51. doi: 10.1186/1746-4269-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Magwede K., Tshisikhawe M.P., Bhat R.B. Ethnobotanical survey of medicinal plants used in treatment of ticks. Int. J.Exp. Biol. 2014;83:155–165. [Google Scholar]
- 70.Gelfand M., Mavi S., Drummond R.B., Ndemera B. Vol. 411. Mambe Press; 1985. The Traditional Medicinal Practitioner in Zimbabwe. Gweru Zimbabwe. [Google Scholar]
- 71.Maroyi A. Traditional use of medicinal plants in south-central Zimbabwe: review and perspective. J. Ethnobiol. Ethnomed. 2013;9:31. doi: 10.1186/1746-4269-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maroyi A., Chekhyoussef A. A comparative study of medicinal plants used in rural areas of Namibia and Zimbabwe. Indian J. Trad. Knowl. 2015;14:401–406. [Google Scholar]
- 73.Bruschi P., Morganti M., Mancini M., Signorini M.A. Traditional healers and laypeople: a qualitative and quantitative approach to local knowledge on medicinal plants in Muda (Mozambique) J. Ethnopharmacol. 2011;138:543–563. doi: 10.1016/j.jep.2011.09.055. [DOI] [PubMed] [Google Scholar]
- 74.Chettleborough J., Lumeta J., Magesa S. The Society for Environmental Exploration, UK and the University of Dar es Salaam; 2000. Community Use of Non-timber forest Products: A Case Study from the Kilombero Valley, Frontier Tanzania. Kilombera Valley Integrated Environmental Management Programme. [Google Scholar]
- 75.Kamuhabwa A., Nshimo C., De Witte P. Cytotoxicity of some medicinal plants extracts used in Tanzanian traditional medicine. J. Ethnopharmacol. 2000;70:143–149. doi: 10.1016/s0378-8741(99)00161-0. [DOI] [PubMed] [Google Scholar]
- 76.Augustino S., Hall J.B., Makonda F.B.S., Ishengoma R.C. Medicinal resources of the Miombo woodlands of Urumwa, Tanzania: plants and its uses. J. Med. Plants Res. 2011;5:6352–6372. [Google Scholar]
- 77.Maundu P.M., Ngugi G.W., Kabuye C.H.S. National Museums of Kenya (NMK); 1999. Traditional Food Plants of Kenya, Nairobi: Kenya Resource Centre for Indigenous Knowledge (KENRIK) [Google Scholar]
- 78.Adam J.G., Echard N., Lescot M. Plantes medicinales Hausa de L’Ader (Republique du Niger) J. Agric. Trop. Bot. Appl. 1972;19:259–399. [Google Scholar]
- 79.Chinsembu K.C. Ethnobotanical study of plants used in the management of HIV-AIDS-related diseases in Livingstone, Southern Province, Zambia. Evid. Base. Compl. Alt. Med. 2016:14. doi: 10.1155/2016/4238625. Article ID 4238625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Koeven E. Klause Hess Publishers; Namibia: 2001. Medicinal, Poisonous, and Edible Plants in Namibia. Windhoek (Namibia) [Google Scholar]
- 81.Ior L.D., Otimenyin S.O., Okwori V.A., Umar D.M., Azila J.J. Ethnobotanical survey of plants used in the management of mental illnesses in some selected local government areas of Plateau State, Nigeria. J. Pharmacogn. Phytotherapy. 2017;9:146–156. [Google Scholar]
- 82.Etuk E.U., Bello S.O., Isezuo S.A., Mohammed B.J. Ethnobotanical survey of medicinal plants used for the treatment of diabetes mellitus in the North West region of Nigeria. Asian J. Exp. Biol. Sci. 2010;1:55–59. [Google Scholar]
- 83.Onakpa M.M., Owoleke O.E. A survey of medicinal plants used for the management of diabetes mellitus in north central Nigeria. Int. J. Appl. Biol. Pharmaceut. Technol. 2010;1:5. [Google Scholar]
- 84.Cheikhyoussef A., Embashu W. Ethnobotanical knowledge on indigenous fruits in Ohangwena and Oshikoto regions in Northern Namibia. J. Ethnobiol. Ethnomed. 2013;9:34. doi: 10.1186/1746-4269-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bossard E. Vol. 1. Ministerio de Investigacao Cientifica Tropica; 1996. La Medicine Traditionelle au Centre et a 1’Ouest de 1’Angola; p. 531. [Google Scholar]
- 86.Adjanohoun E., Adjakidje V., Ahyi M.R.A., Ake L., Assi A., Akoegninou J., d Almeida F., Apovo K., Boukef M., Chadare G., Gusset K., Dramane J., Eyme J., Gassita N., Gbaguidi N., Goudote E., Guinko S., Houngnon P., Issa Lo A., Keita H.V., Kinoffo D.K. Vol. 895. Agence de Cooperation Culturelle et Technique (ACCT); Paris: 1989. Contribution aux Etudes Ethnobotaniques et Floristiques en Republique Populaire du Benin. [Google Scholar]
- 87.Kerharo J., Adam J.G. Vol. 1011. Vigot Freres; Paris: 1974. La Pharmacopee Senegalaise Traditionelle. Plantes Medicinales et toxiques. [Google Scholar]
- 88.Shawa I.T., Mponda J., Msefula C., Manda H., Gondwe M., Maliwichi- Nyirenda C. Brine shrimp lethality and phytochemical determination of Senna sanguinea and Ziziphus mucronata. J. Basic Appl. Res. 2015;1:82–88. [Google Scholar]
- 89.Munodawafa T., Moyo S., Chipurura B., Chagonda L. Brine shrimp lethality bioassay of some selected Zimbabwean medicinal plants. Int. J. Phytopharm. 2016;7:229–234. [Google Scholar]
- 90.Ilonga S.K. University of Namibia; Namibia: 2012. Anticancer, Antioxidant and Antimicrobial Screening of Extracts from Ziziphus mucronata, Heliotropium Ciliatum and Gnidia polycephala from the Oshikoto Region of Namibia. MSc Dissertation. [Google Scholar]
- 91.Naidu J., Ismail R., Sasidharan S. Acute oral toxicity and brine shrimp lethality of methanol extract of Mentha spicata L. (Lamiaceae) Trop. J. Pharmaceut. Res. 2014;13:101–107. [Google Scholar]
- 92.Zezi A.U., Abdoulaye B.B., Danjuma N.M., Aliyu I.S., Yaro A.H., Musa K.Y. The effects of aqueous stem bark of Ziziphus mucronata on rat kidney functional status after 10 days daily treatment. Int. J. Pharmaceut. Res. Innovat. 2012;5:1–6. [Google Scholar]
- 93.Mativandlela S.P.N., Meyer J.J.M., Hussein A.A., Houghton P.J., Hamilton C.J., Lall N. Activity against Mycobacterium smegmatis and M. tuberculosis by extract of South African medicinal plants. Phytother Res. 2008;22:841–845. doi: 10.1002/ptr.2378. [DOI] [PubMed] [Google Scholar]
- 94.Mativandlela S.P.N. University of Pretoria; Pretoria, South Africa: 2009. Anti-tuberculosis Activity of Flavonoids from Galenia Africana L. Var. Africana. PhD Thesis. [Google Scholar]
- 95.Mongalo N.I., Dikhoba P.M., Soyingbe S.O., Makhafola T.J. Antifungal, anti- oxidant activity and cytotoxicity of South African medicinal plants against mycotoxigenic fungi. Heliyon. 2018;4 doi: 10.1016/j.heliyon.2018.e00973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ogbole O.O., Segun P.A., Adeniji A.J. In vitro cytotoxic activity of medicinal plants from Nigeria ethnomedicine on Rhabdomyosarcoma cancer cell line and HPLC analysis of active extracts. BMC Compl. Alternative Med. 2017;17:494. doi: 10.1186/s12906-017-2005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aganga A.A., Mosase K.W. Tannin content, nutritive value and dry matter digestibility of Lonchocarpus capassa, Ziziphus mucronata, Sclerocarrya birrea, Kirkia acuminata and Rhus lancea seeds. Anim. Feed Sci. Technol. 2001;91:107–113. [Google Scholar]
- 98.Arévalo-Gardini E., Arévalo-Hernández C.O., Baligar V.C., He Z.L. Heavy metal accumulation in leaves and beans of cacao (Theobroma cacao L.) in major cacao growing regions in Peru. Sci. Total Environ. 2017;605–606:792–800. doi: 10.1016/j.scitotenv.2017.06.122. [DOI] [PubMed] [Google Scholar]
- 99.Lo Dico G.M., Galvano F., Dugo G., D'ascenzi C., Macaluso A., Vella A., Giuseppe Giangrosso G., Cammilleria G., Ferrantellia V. Toxic metal levels in cocoa powder and chocolate by ICP-MS method after microwave-assisted digestion. Food Chem. 2018;245:1163–1168. doi: 10.1016/j.foodchem.2017.11.052. [DOI] [PubMed] [Google Scholar]
- 100.Jaishankar M., Tseten T., Anbalagan N., Mathew B.B., Beeregowda K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscipl. Toxicol. 2014;7:60–72. doi: 10.2478/intox-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mpiana P.T., Mudogo V., Tshibangu D.S.T., Kitwa E.K., Kanangila A.B., Lumbu J.B.S., Ngbolua K.N., Atibu E.K., Kakule M.K. Antisickling activity of anthocyanins from Bombax pentadrum, Ficus capensis and Ziziphus mucronata: photodegradation effect. J. Ethnopharmacol. 2008;120:413–418. doi: 10.1016/j.jep.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 102.Adewusi E.A., Steenkamp V. In Vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from Southern Africa. Asian Pac. J. Trop. Med. 2011:829–835. doi: 10.1016/S1995-7645(11)60203-4. [DOI] [PubMed] [Google Scholar]
- 103.Amoo S.O., Aremu A.O., Moyo M., Van Staden J. Antioxidant and acetylcholinesterase-inhibitory properties of long-term stored medicinal plants. BMC Compl. Alternative Med. 2012;12:87. doi: 10.1186/1472-6882-12-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nafuka S.N., Mumbengegwi D.R. Phytochemical analysis and in vitro antiplasmodial activity of selected ethnomedicinal plants used to treat malaria and associated symptoms in Northern Namibia. Int. Sci. Technol. J. Namib. 2013;2:78–93. [Google Scholar]
- 105.Hussain J., Mabood F., Al-Harrasi A., Ali L., Rizvi T.S., Jabeen F., Gilani S.A., Shinwari S., Ahmad M., Alabri Z.K., Al Ghawi S.H.S. New robust sensitive fluorescence spectroscopy coupled with PLSR for estimation of quercetin in Ziziphus mucronata and Ziziphus sativa. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018;194:152–157. doi: 10.1016/j.saa.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 106.Mkhize N.R., Heitkonig M.I.A., Scoggings P.F., Hattas D., Dziba L.E., Prins H.H.T., De Boer W.F. Supplemental nutrients increase the consumption of chemically defended shrubs by free-ranging herbivores. Agric. Ecosyst. Environ. 2016;235:119–126. [Google Scholar]
- 107.Ibrahim M.A. University of KwaZulu-Natal; Durban, South Africa: 2013. The in Vitro and in Vivo Antioxidative and Anti-diabetic Effects of Some African Medicinal Plants and the Identification of Bioactive Compounds. PhD thesis. [Google Scholar]
- 108.Fehlhaber H.J., Uhlendorf S., Tshesche D.R. Alkaloids from Rhamnaceae. XII. Mucronine-A,-B, and-C, peptide alkaloids with a type of structure, isolated from Zizyphus mucronata Willd. Justus Liebigs Ann. Chem. 1972;759:195–207. doi: 10.1002/jlac.19727590116. [DOI] [PubMed] [Google Scholar]
- 109.Tschesche R., Elgamal M., Eckhardt G., Von Radloff M. Peptide alkaloids from Ziziphus mucronata. Phytochemistry. 1974;13 2328-2328. [Google Scholar]
- 110.Tschesche R.S., David R., Zerbes M., Von Radloff E.K., Eckhardt G. Alkaloide aus Rhamnaceen, XIX1) Mucronin-E,-F,-G und-H sowie Abyssenin- A,-B und-C, weitere 15 gliedrige. Justus Liebigs Ann. Chem. 1974:1915–1928. [Google Scholar]
- 111.Barboni L., Gariboldi P., Torregiani E., Verotta R. Cyclopeptide alkaloids from Ziziphus mucronata. Phytochemistry. 1994;35:1579–1582. [Google Scholar]
- 112.Auvin C., Lezenven F., Blond A., Augeven-Bour I., Pousset J.L., Bodo B., Mucronine J. A 14-membered cyclopeptide alkaloid from Ziziphus mucronata. J. Nat. Prod. 1996;59:676–678. doi: 10.1021/np9601972. [DOI] [PubMed] [Google Scholar]
- 113.Zininga T., Anokwuru C.P., Sigidi M.T., Tshisikhawe M.P., Ramaite I.D.I., N Traore A., Hoppe H., Shonhai A., Potgieter N. Extracts obtained from Pterocarpus angolensis D.C. and Ziziphus mucronata exhibit antiplasmodial activity and inhibit heat shock protein 70 (Hsp70) function. Molecules. 2017;22:13. doi: 10.3390/molecules22081224. 1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Legeay S., Rodier M., Fillon L., Faure S., Clere N. Epigallocatechin gallate: a Review of its beneficial properties to prevent metabolic syndrome. Nutrients. 2015;7:5443–5468. doi: 10.3390/nu7075230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moloto M.P. Medical University of Southern Africa; South Africa: 2004. Isolation and Characterization of Antibacterial, Anthelmintic, Antioxidant and Cytotoxic Compounds Present in Ziziphus mucronata. MSc Thesis. [Google Scholar]
- 116.Brown E.D., Wright G.D. Antibacterial drug discovery in the resistance era. Nature. 2016;529:336–343. doi: 10.1038/nature17042. [DOI] [PubMed] [Google Scholar]
- 117.Cai Y., Zhang Q., Fu Y. Effectiveness of Chinese herbal medicine combined with antibiotics for extensively drug-resistant Enterobacteria and non-fermentative bacteria infection: real-sife experience in a retrospective cohort. BioMed Res. Int. 2017:9. doi: 10.1155/2017/2897045. Article ID 2897045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rabe T., Van Staden J. Antibacterial activity of South African plants used for medicinal purposes. J. Ethnopharmacol. 1997;56:81–87. doi: 10.1016/s0378-8741(96)01515-2. [DOI] [PubMed] [Google Scholar]
- 119.Mthethwa N.S. University of Zululand; Richards Bay, South Africa: 2009. Antimicrobial Activity Testing of Traditionally Used Plants for Treating Wounds and Sores at Ongoye Area KwaZulu-Natal, South Africa. MSc Dissertation. [Google Scholar]
- 120.Munodawafa T. University of Zimbabwe; Zimbabwe: 2009. Screening of Some Traditional Medicinal Plants from Zimbabwe for Biological and Antimicrobial Activity. MPhil Thesis. [Google Scholar]
- 121.Nyila M.A., Leonard C.M., Hussein A.A., Lall N. Activity of South African medicinal plants against Listeria monocytogenes biofilms and isolation of bioactive compound from Acacia karoo. South Afr. J. Bot. 2012;78:220–227. [Google Scholar]
- 122.Shawa I.T., Msefula C., Mponda J., Maliwichi-Nyirenda C., Gondwe M. Antibacterial effects of aqueous extracts of Musa paradisiaca, Ziziphus mucronata and Senna sanguinea plants from Malawi. Int. J. Health Sci. Res. 2016;6:200–207. [Google Scholar]
- 123.Coopoosamy R.M., Naidoo K.K., Ndlazi N.J. Cultural importance and antibacterial activity of Ziziphus mucronata (Willd.) in the Umlazi community in Durban. Afr. J. Pharm. Pharmacol. 2011;5:1979–1982. [Google Scholar]
- 124.Adamu H.M., Obayeh O.J., Ibok N.U., Kafu S.E. Antifungal activity of extracts of some Cassia, Detarium and Ziziphus species against dermatophytes. Nat. Product. Radiance. 2006;5:357–360. [Google Scholar]
- 125.Munodawafa T., Chagonda L.S., Moyo R.S. Antimicrobial and phytochemical screening of some Zimbabwean medicinal plants. J. Biol. Act. Prod. Nat. 2013;3:323–330. [Google Scholar]
- 126.Manyarara T.E., Chifamba J., Tarugarira F.T. Antifungal Activity of Ziziphus mucronata and Erythrina abyssinica bark crude extracts on Cryptococcus neofomans and Candida albicans species. Br. J. Pharmaceut. Res. 2016;10:1–11. [Google Scholar]
- 127.Samie A., Tambani T., Harshfield E., Green E., Ramalivhana J.N., Bessong P.O. Antifungal activities of selected Venda medicinal plants against Candida albicans, Candida krusei and Cryptococcus neoformans isolated from South African AIDS patients. Afr. J. Biotechnol. 2010;9:2965–2976. [Google Scholar]
- 128.Mongalo N.I., McGaw L.J., Finnie J.F., Van Staden J. Securidaca longepedunculata Fres. A review of its ethnomedicinal uses, toxicology, phytochemistry and pharmacology. J. Ethnopharmacol. 2015;165:215–226. doi: 10.1016/j.jep.2015.02.041. [DOI] [PubMed] [Google Scholar]
- 129.Rios J.L., Recio M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005;100:80–84. doi: 10.1016/j.jep.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 130.Suliman A. University of Witwatersrand; South Africa: 2010. The Antimicrobial Activity and Chemical Profile of Traditional Medicinal Plants Indigenous to Southern Africa Used to Treat Respiratory Tract Infections. MSc Dissertation. [Google Scholar]
- 131.Perumal S., Mahmud R., Pillai S., Lee W.C., Ramanathan S. Antimicrobial activity and cytotoxicity evaluation of Euphorbia hirta (L.) extracts from Malaysia. APCBEE Proc. 2012;2:80–85. [Google Scholar]
- 132.Aderogba M.A., Ndhlala A.R., Rengasamy R.R., Van Staden J. Antimicrobial and selected in vitro enzyme inhibitory effects of leaf extracts, flavonols and índole alkaloids isolated from Croton menyharthii. Molecules. 2013;18:12633–12644. doi: 10.3390/molecules181012633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mongalo N.I., McGaw L.J., Segapelo T.V., Finnie J.F., Van Staden J. Ethnobotany, phytochemistry, toxicology and pharmacological properties of Terminalia serícea Burch ex DC (Combretaceae)- A review. J. Ethnopharmacol. 2016;194:789–802. doi: 10.1016/j.jep.2016.10.072. [DOI] [PubMed] [Google Scholar]
- 134.Legoabe L.J. University of North West, Potchefstroom Campus; Potchefstroom, South Africa: 2004. Isolation and Characterization of Antimicrobial Compounds from Antizoma angustifolia. MSc Dissertation. [Google Scholar]
- 135.McGaw L.J., Van Der Merwe D., Eloff J.N. In Vitro anthelmintic activity, antibacterial and cytotoxic effect of extracts from plants used in South African ethnoveterinary medicine. Vet. J. 2007;173:366–372. doi: 10.1016/j.tvjl.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 136.Green E., Samie A., Obi C.L., Bessong P.O., Ndip R.N. Inhibitory properties of selected South African medicinal plants against Mycobacterium tuberculosis. J. Ethnopharmacol. 2010;130:151–157. doi: 10.1016/j.jep.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 137.Erasmus L.J.C. University of Limpopo; Polokwane, RSA: 2014. Impact of Various Boiling Intervals on the Antimicrobial Efficacy and Phytochemical Profile of Selected Crude Medicinal Plants Extracts, Used by Bapedi Traditional Healers in the Treatment of Sexually Transmitted Infections. PhD Thesis. [Google Scholar]
- 138.Nciki S., Vuuren S., Van Eyk A., De Wet H. Plants used to treat skin diseases in northern Maputaland, South Africa: antimicrobial activity and in vitro permeability studies. Pharm. Biol. 2016;54:2420–2436. doi: 10.3109/13880209.2016.1158287. [DOI] [PubMed] [Google Scholar]
- 139.Nciki S. University of Witwatersrand; Johannesburg, South Africa: 2015. Validating the Traditional Use of Medicinal Plants in Maputaland to Treat Skin Diseases. MSc Dissertation. [Google Scholar]
- 140.Nemudzivhadi V., Masoko P. Antioxidant and antibacterial properties of Ziziphus mucronata and Ricinus communis leaves extracts. Afr. J. Tradit., Complementary Altern. Med. 2015;12:81–89. [Google Scholar]
- 141.Lekganyane M.A., Matsebatlela T.M., Howard R.L., Shai L.J., Masoko P. The phytochemical, antibacterial and antioxidant activity of five medicinal plants against the wound infecting bacteria. Afr. J. Biotechnol. 2011;11:13210–13219. [Google Scholar]
- 142.Lekganyane M.A. University of Limpopo; Polokwane, South Africa: 2015. Isolation and Characterization of Antibacterial Compounds from Five Selected Plants Used against Bacteria Which Infect Wounds. MSc Dissertation. [Google Scholar]
- 143.Mabona U., Viljoen A., Shikanga E., Marston A., Van Vuuren S. Antimicrobial activity of South African medicinal plants with dermatological relevance: from an ethnopharmacological screening approach to combination studies to isolation of bioactive compound. J. Ethnopharmacol. 2013;148:45–55. doi: 10.1016/j.jep.2013.03.056. [DOI] [PubMed] [Google Scholar]
- 144.Dzoyem J.P., Aro O.A., McGaw L.J., Eloff J.N. Anti-mycobacterial activity against different pathogens and selectivity index of fourteen medicinal plants used in South Africa to treat tuberculosis and respiratory infections. J. Ethnopharmacol. 2016;102:70–74. [Google Scholar]
- 145.Akhalwaya S., Van Vuuren S., Patel M. An in vitro investigation of indigenous South African medicinal plants used to treat oral infections. J. Ethnopharmacol. 2018;210:359–371. doi: 10.1016/j.jep.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 146.Mongalo N.I., McGaw L.J., Finnie J.F., Van Staden J. Pharmacological properties of extracts from six South African medicinal plants used to treat sexually transmitted infections (STIs) and related infections. South Afr. J. Bot. 2017;112:290–295. [Google Scholar]
- 147.WHO, World Health Organisation . 2018. Global Tuberculosis Report. Geneva, Switzerland. [Google Scholar]
- 148.Olajuyigbe O.O., Afolayan A.J. Evaluation of combination effects of ethanolic extract of Ziziphus mucronata Willd. subsp. mucronata and antibiotics against clinically important bacteria. Sci. World J. 2013:9. doi: 10.1155/2013/769594. Article ID 769594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Luseba D., Elgorashi E.E., Ntloedibe D.T., Van Staden J. Antibacterial, anti-inflammatory and mutagenic effects of some medicinal plants used in South Africa for the treatment of wounds and retained placenta in livestock. South Afr. J. Bot. 2007;73:378–383. [Google Scholar]
- 150.Sigidi M.T., Anokwuru C.P., Zininga T., Shonhai A., Ramaite I.D.I., Traore A.N., Potgieter N. Comparative in vitro cytotoxic, anti-inflammatory and antimicrobiological activities of two indigenous Venda medicinal plants. Transl. Med. Comm. 2016;1:9. [Google Scholar]
- 151.Köita K., Neya F.B., Opoku N., Baissa Y., Campa C., Sankara P. Phytochemical analysis of Ziziphus mucronata Willd. extract and screening for antifungal activity against peanut pathogens. Afr. J. Plant Sci. 2017;11:394–402. [Google Scholar]
- 152.Granich R., Gupta S., Hersh B., Williams B., Montaner J., Young B., Zuniga J.M. Trends in aids, deaths, new infections and ART coverage in the top 30 countries with the highest aids mortality burden; 1990–2013. PloS One. 2015;10 doi: 10.1371/journal.pone.0131353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bessong P.O., Obi C.L., Andréola M.-L., Rojas L.B., Pouységu L., Igumbor E., Meyer J.J.M., Quideau S., Litvak S. Evaluation of selected South African medicinal plants for inhibitory activities against human immunodeficiency type 1 reverse transcriptase and integrase. J. Ethnopharmacol. 2005;99:83–91. doi: 10.1016/j.jep.2005.01.056. [DOI] [PubMed] [Google Scholar]
- 154.Helfer M., Koppensteiner H., Schneider M., Rebensburg S., Forcisi S. The root extract of the medicinal plant Pelargonium sidoides is a Potent HIV-1 attachment inhibitor. PloS One. 2014;9 doi: 10.1371/journal.pone.0087487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang H.-J., Rumschlag-Booms E., Guan Y.-F., Wang D.-Y., Liu K.-L., Li W.-F., Nguyen V.H., Cuong N.M., Soejarto D.D., Fong H.H.S., Rong L. Potent inhibitor of drug-resistant HIV-1 strains identified from the medicinal plant Justicia gendarussa. J. Nat. Prod. 2017;80:1798–1807. doi: 10.1021/acs.jnatprod.7b00004. [DOI] [PubMed] [Google Scholar]
- 156.WHO, World Health Organisation . 2013. WHO Technical Working Group Meeting on Revision of the WHO Recommendations for the Production and Control of Inactivated Poliomyelitis Vaccines: TRS No. 910, Annex 2, Geneva, Switzerland. [Google Scholar]
- 157.WHO, World Health Organisation . 2017. Malaria World Report. Geneva, Switzerland. [Google Scholar]
- 158.Prozesky E.A., Meyer J.J.M., Louw E.I. In vitro antiplasmodial activity and cytotoxicity of ethnobotanically selected South African medicinal plants. J. Ethnopharmacol. 2001;76:239–245. doi: 10.1016/s0378-8741(01)00245-8. [DOI] [PubMed] [Google Scholar]
- 159.Clarkson C., Maharaj V.J., Crouch N.R., Grace O.M., Pillay P., Motsabisa M.G., Bhagwandin N., Smith P.J., Folb P.I. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J. Ethnopharmacol. 2004;92:177–191. doi: 10.1016/j.jep.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 160.Sibandze G.F. University of Witwatersrand; Johannesburg, South Africa: 2009. Pharmacological Activity of Swazi Medicinal Plants. MSc Dissertation. [Google Scholar]
- 161.Muthaura C.N., Keriko J.M., Mutai C., Yenesew A., Gathirwa A.W., Iringu B.N., Nyangacha R., Mungai G.M., Derese S. Antiplasmodial potential of traditional antimalarial phytotherapy remedies used by the Kwale community of the Kenyan coast. J. Ethnopharmacol. 2015;170:148–157. doi: 10.1016/j.jep.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 162.Memvanga P.B., Tona G.L., Mesia K., LusakibanzaManzo M., Cimanga R.K. Antimalarial activity of medicinal plants from the Democratic Republic of Congo: a review. J. Ethnopharmacol. 2015;169:76–98. doi: 10.1016/j.jep.2015.03.075. [DOI] [PubMed] [Google Scholar]
- 163.WHO, World Health Organisation, National Diabetes Statistics Report . 2017. Estimates of Diabetes and its Burden in the United States. Geneva, Switzerland. [Google Scholar]
- 164.Kapoor D., Bhardwaj A.K., Kumar D., Raina S.K. Prevalence of diabetes mellitus and its risk factors among permanently settled tribal individuals in tribal and urban areas in Northern State of Sub-Himalayan region of India. Int. J. Chron. Dis. 2014:9. doi: 10.1155/2014/380597. Article ID 380597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dasappa H., Fathima F.N., Prabhakar R., Sarin S. Prevalence of diabetes and pre-diabetes and assessments of their risk factors in urban slums of Bangalore. J. Fam. Med. Prim. Care. 2015;4:399–404. doi: 10.4103/2249-4863.161336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Peleg A.Y., Weerarathna T., McCarthy J.S., Davis T.M.E. Common infections in diabetes: pathogenesis, management and relationship to glycaemic control. Diabetes/Metab. Res. Rev. 2007;23:3–13. doi: 10.1002/dmrr.682. [DOI] [PubMed] [Google Scholar]
- 167.Mousinho N.M. University of Pretoria; South Africa: 2013. In Vitro Assessment of Antidiabetic Activity of Sclerocarrya birrea and Ziziphus mucronata. MSc Dissertation. [Google Scholar]
- 168.Mousinho N.M., Van Tonder J.J., Steenkamp V. In vitro anti-diabetic activity of Sclerocarya birrea and Ziziphus mucronata. Nat. Prod. Comm. 2013;8:1279–1284. [PubMed] [Google Scholar]
- 169.Ibrahim M.A., Islam M.S. Effects of n-butanol fraction of Ziziphus mucronata root ethanol extract on glucose homeostasis, serum insulin and other diabetes related parameters in a murine model for type 2 diabetes. Pharm. Biol. 2017;55:1. doi: 10.1080/13880209.2016.1242632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kumar B.S.A., Khan S., Saran G.S., Nandeesh R., Manjunath N.K. In Vitro antidiabetic activity of Nisamalaki churna. Sains Malays. 2013;42:625–628. [Google Scholar]
- 171.Mogale M.A., Lebelo S.L., Thovhogi N., De Freitas A.N., Shai L.J. Α- amylase and α-glucosidase inhibitory effects of Sclerocarya birrea [(A. Rich.) Hochst.] subspecies caffra (Sond) Kokwaro (anacardiaceae) stem-bark extracts. Afr. J. Biotechnol. 2011;10:15033–15039. [Google Scholar]
- 172.Gopinath S.M., Priyanka P., Dayananda K.S., Reddy J.M., Ashwini P.G.M., Nair D.V. In Vitro Inhibitory effect of polyherbal formulation on alpha- amylase. Int. J. Innov. Res. Sci. Eng. Technnol. 2013;2:4556–4566. [Google Scholar]
- 173.Shori A.B. Screening of antidiabetic and antioxidant activities of medicinal plants. J. Integr. Med. 2013;13:297–305. doi: 10.1016/S2095-4964(15)60193-5. [DOI] [PubMed] [Google Scholar]
- 174.Banerjee A., Maji B., Mukherjee S., Chaudhuri K., Seal T. In Vitro Antidiabetic and anti-oxidant activities of methanol extract of Tinospora sinensis. J. Appl. Biol. Biotechnol. 2017;5:61–67. [Google Scholar]
- 175.Mohamed E.A.H., Siddiqui M.J.A., Ang L.F., Sadikun A., Chan S.H., Tan S.C., Asmawi M.Z., Yam M.F. Potent α-glucosidase and α-amylase inhibitory activities of standardized 50% ethanolic extracts and sinensetin from Orthosiphon stamineus Benth as anti-diabetic mechanism. BMC Compl. Alternative Med. 2012;12:176. doi: 10.1186/1472-6882-12-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pandey K.B., Rizvi S.I. Role of red grape polyphenols as antidiabetic agents. Integr. Med. Res. 2014;3:119–125. doi: 10.1016/j.imr.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Li P.-H., Lin Y.-W., Lu W.-C., Hu J.-M., Huang D.-W. In Vitro Hypoglycemic activity of the phenolic compounds in Longan fruit (Dimocarpus longan var. Fen Ke) shell against α-glucosidase and β-galactosidase. Int. J. Food Prop. 2016;19:1786–1797. [Google Scholar]
- 178.Umeno A., Horie M., Murotomi K., Nakajima Y., Yoshida Y. Antioxidative and antidiabetic effects of natural polyphenols and isoflavones. Molecules. 2016;21:708. doi: 10.3390/molecules21060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Taslimi P., Gulçin I. Antidiabetic potential: in vitro inhibition effects of some natural phenolic compounds on α-glycosidase and α-amylase enzymes. J. Biochem. Mol. Toxicol. 2017;31 doi: 10.1002/jbt.21956. [DOI] [PubMed] [Google Scholar]
- 180.Geary T.G., Hosking B.C., Skuce P.J., Von Samson-Himmelstjerna G., Maeder S., Holdsworthf P., Pomroy W., Vercruysse J. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) Guideline: anthelmintic combination products targeting nematode infections of ruminants and horses. Vet. Parasitol. 2012;190:306–316. [Google Scholar]
- 181.Ferreira L.E., Castro P.M.N., Chagas A.C.S., França S.C., Beleboni R.O. In vitro anthelmintic activity of aqueous leaf extract of Annona muricata L. (Annonaceae) against Haemonchus contortus from sheep. Exp. Parasitol. 2013;134:327–332. doi: 10.1016/j.exppara.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 182.Aremu A.O., Finnie J.F., Van Staden J. Potential of South African medicinal plants used as anthelmintics -their efficacy, safety concerns and reappraisal of current screening methods. South Afr. J. Bot. 2012;82:34–150. [Google Scholar]
- 183.Kuma F., Birhanu T., Hirpa E. Advanced review on anthelmintic medicinal plants. Report. Opin. 2015;7:6–16. [Google Scholar]
- 184.Ngaradoum O., Kagira J.M., Karanja S.M., Kipyegon C., Maina N. In Vitro ovicidal and larvicidal activity of aqueous and methanolic extracts of Ziziphus mucronata barks against Haemonchus contortus. Eur. J. Exp. Biol. 2017;7:7. [Google Scholar]
- 185.Ngaradoum O. Pan African University Institute for basic Science, Technology and Innovation (PAUISTI); Nairobi, Kenya: 2017. Phytochemical Composition, Safety and in Vitro Anthelmintic Activity of Ziziphus mucronata Barks Extract against Haemonchus contortus. [Google Scholar]
- 186.Bizimenyera E.S., Githiori J.B., Eloff J.N., Swan G.E. In Vitro activity of Peltophorum africanum Sond. (Fabaceae) extracts on the egg hatching and larval development of the parasitic nematode Trichostrongylus colubriformis. Vet. Parasitol. 2006;142:336–343. doi: 10.1016/j.vetpar.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 187.Bizimenyera S.E., Meyer S., Naidoo V., Eloff J.N., Swan G.E. Efficacy of Peltophorum africanum Sond. (Fabaceae) extracts on Haemonchus contortus and Trichostrongylus colubriformis in sheep. J. Anim. Vet. Adv. 2008;7:364–371. [Google Scholar]
- 188.Mølgaard P., Nielsen S.B., Rasmussen D.E., Drummond R.B., Makaza N., Andreassen J. Anthelmintic screening of Zimbabwean medicinal plants traditionally used against schistosomiasis. J. Ethnopharmacol. 2001;74:257–264. doi: 10.1016/s0378-8741(00)00377-9. [DOI] [PubMed] [Google Scholar]
- 189.Waterman C., Smith R.A., Pontiggia L., DerMarderosian A. Anthelmintic activity of sub-Saharan African plants used in traditional medicine. J. Ethnopharmacol. 2010;127:755–759. doi: 10.1016/j.jep.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 190.Burnham W.R., Reeve R.S., Finch R.G. Entamoeba histolytica infection in male homosexuals. Microb. Infect. 1980;14:1428–1441. doi: 10.1136/gut.21.12.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.McGaw L.J., Jӓger A.K., Van Staden J. Antibacterial, anthelmintic and anti-amoebic activity in South African medicinal plants. J. Ethnopharmacol. 2000;72:247–263. doi: 10.1016/s0378-8741(00)00269-5. [DOI] [PubMed] [Google Scholar]
- 192.Mukherjee S., Pawar N., Kulkarni O., Nagarkar B., Thopte S., Bhujbal A., Pawar P. Evaluation of free-radical quenching properties of standard Ayurvedic formulation Vayasthapana Rasayana. BMC Compl. Alternative Med. 2011;11:38. doi: 10.1186/1472-6882-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Olajuyigbe O.O., Afolayan A.J. Phenolic content and antioxidant property of the bark extracts of Ziziphus mucronata Willd. subsp. mucronata Willd. BMC Compl. Alternative Med. 2011;11:130. doi: 10.1186/1472-6882-11-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ibrahim M.A., Koorbanally N.A., Kiplimo J.J., Islam M.S. Antioxidative activities of the various extracts of stem bark, root and leaves of Ziziphus mucronata (Rhamnaceae) in vitro. J. Med. Plants Res. 2012;6:4176–4184. [Google Scholar]
- 195.Kwape T.E., Chaturvedi P., Kamau M., Majinda R. Antioxidant and hepatoprotective activities of Ziziphus mucronata fruit extract against dimethoate-induced toxicity. J. Pharmacopuncture. 2013;16:21–29. doi: 10.3831/KPI.2013.16.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Garcia E.O., Oldoni T.L.C., De Alencar S.M., Reis A., Loguercio A.D., Grande R.H.M. Antioxidant activity by DPPH assay of potential solutions to be applied on bleached teeth. Brazil. Dent. J. 2012;23:22–27. doi: 10.1590/s0103-64402012000100004. [DOI] [PubMed] [Google Scholar]
- 197.Koleva I.I., Van Beek T.A., Linssen J.P.H., De Groot A., Evstatieva L.N. Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem. Anal. 2002;13:8–17. doi: 10.1002/pca.611. [DOI] [PubMed] [Google Scholar]
- 198.Sahidi F., Zhong Y. Measurement of antioxidant activity. J. Func. Foods. 2015;18:757–781. [Google Scholar]
- 199.Galvez M.A.C. Evaluation of DPPH free radical scavenging activity and phytochemical screening of selected folkloric medicinal plants in Tinoc, Ifugao, Cordillera Administrative region, Philippines. Int. J. Sci. Res. Publ. 2015;5:440–445. [Google Scholar]
- 200.Nimmi M.S., George P. Evaluation of the antioxidant potential of a newly developed polyherbal formulation for anti-obesity. Int. J. Pharm. Pharmaceut. Sci. 2012;4:505–510. [Google Scholar]
- 201.Alam M.N., Bristi N.J., Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharmaceut. J. 2013;21:143–152. doi: 10.1016/j.jsps.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.López-Alarcón C., Denicola A. Evaluating the antioxidant capacity of natural products: a review on chemical and cellular-based assays. Anal. Chim. Acta. 2013;763:1–10. doi: 10.1016/j.aca.2012.11.051. [DOI] [PubMed] [Google Scholar]
- 203.Glass G.K., Saijo K., Winner B., Marchetto M.C., Gage F.H. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Azab A., Nassar A., Azab A.N. Anti-inflammatory activity of natural products. Molecules. 2016;21:1321. doi: 10.3390/molecules21101321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Amor S., Puentes F., Baker D., Van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Jӓger A.K., Hutchings A., Van Staden J. Screening of Zulu medicinal plants for prostaglandin-synthesis inhibitors. J. Ethnopharmacol. 1996;52:95–100. doi: 10.1016/0378-8741(96)01395-5. [DOI] [PubMed] [Google Scholar]
- 207.Lindsey K., Jӓger A.K., Raidoo D.M., Van Staden J. Screening of plants used by Southern African traditional healers in the treatment of dysmenorrhoea for prostaglandin-synthesis inhibitors and uterine relaxing activity. J. Ethnopharmacol. 1999;64:9–14. doi: 10.1016/s0378-8741(98)00097-x. [DOI] [PubMed] [Google Scholar]
- 208.Shaikh R.U., Pund M.M., Gacche R.N. Evaluation of anti-inflammatory activity of selected medicinal plants used in Indian traditional medication system in vitro as well as in vivo. J. Trad. Compl. Med. 2016;6:355–361. doi: 10.1016/j.jtcme.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Taylor J.L.S., Van Staden J. COX-1 and COX-2 inhibitory activity in extracts prepared from Eucomis species, with further reference to extracts from E. autumnalis. South Afr. J. Bot. 2002;68:80–85. [Google Scholar]
- 210.Wallace J.L., Chin B.C. New generation NSAIDS: the benefits without the risks? Drugs Today. 1997;33:371–378. [Google Scholar]
- 211.Kaufmann D., Dogra A.K., Tahrani A., Herrmann F., Wink M. Extracts from traditional Chinese medicinal plants inhibit acetylcholinesterase, a known Alzheimer’s disease target. Molecules. 2016;21:1161. doi: 10.3390/molecules21091161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Mathew M., Subramanian S. In Vitro screening for anti-cholinesterase and antioxidant activity of methanolic extracts of Ayurvedic medicinal plants used for cognitive disorders. PloS One. 2014;9 doi: 10.1371/journal.pone.0086804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Adewusi E.A., Fouche G., Steenkamp V. Effect of four medicinal plants on Amyloid-β induced neurotoxicity in SH-SY5Y Cells. Afr. J. Tradit., Complementary Altern. Med. 2013;10:6–11. [PMC free article] [PubMed] [Google Scholar]
- 214.Tripathi S., Bruch D., Kittur D.S. Ginger extract inhibits LPS induced macrophage activation and function. BMC Compl. Alternative Med. 2008;8:1. doi: 10.1186/1472-6882-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Bray F., Jemal A., Grey N., Ferlay J., Forman D. Global cancer transitions according to the Human Development Index (2008–2030): a population- based study. Lancet Oncol. 2012;13:790–801. doi: 10.1016/S1470-2045(12)70211-5. [DOI] [PubMed] [Google Scholar]
- 216.Mangal M., Sagar P., Singh H., Raghava G.P.S., Agarwal S.M. NPACT: naturally occurring plant-based anti-cancer compound-activity-target database. Nucleic Acids Res. 2013;41:D1124–D1129. doi: 10.1093/nar/gks1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Fridlender M., Kapulnik Y., Koltai H. Plant-derived substances with anti- cancer activity: from folklore to practice. Front. Plant Sci. 2015;6:799. doi: 10.3389/fpls.2015.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Shanthi M.P., Bupesh G., Magesh S., Meenakurmari K., Saravanan K., Muthiah N.S. In vitro anticancer activity of Biophytum sensitivum whole plant extracts against cervical and liver cancer cell lines. Int. J. Pharmaceut. Sci. Res. 2016;7:5128–5135. [Google Scholar]
- 219.Vijayarathna S., Sasidharan S. Cytotoxicity of methanol extracts of Elaeis guineensis on MCF-7 and Vero cell lines. Asian Pac. J. Trop. Biomed. 2012;2:826–829. doi: 10.1016/S2221-1691(12)60237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Dash B.P., Archana Y., Satapathy N., Naik S.K. Search for anti-sickling agents from plants. Phcog. Rev. 2013;7:53–60. doi: 10.4103/0973-7847.112849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.WHO, World Health Organisation . 2008. Worldwide Prevalence of Anaemia 1993–2005 WHO Global Database on Anaemia. Geneva, Switzerland. [Google Scholar]
- 222.Mpiana P.T., Mudogo V., Tshibangu D.S.T., Shetonde O.M., Ngbolua K.N., Mbala M.B. In vitro antisickling activity of anthocyanins extract of a Congolese plant: Alchornea cordifilia. J. Med. Sci. 2007;7:1182–1186. [Google Scholar]
- 223.Mpiana P.T., Mudogo V., Tshibangu D.S.T., Ngbolua K.N., Tshilanda D.D., Atibu E.K. Antisickling activity of anthocyanins of Jatropha curcas L. In: Singh V.K., Govil J.N., editors. Chemistry and Medicinal Value. Studium Press LLC; Houston: 2009. pp. 83–90. [Google Scholar]
- 224.Ali M.I., Kumar M. A Recent update on hepatoprotective potential of herbal plant. Int. J. Environ. Sci. Technol. 2015;1:25–50. http://www.gyanvihar.org/researchjournals/4.pdf [Google Scholar]
- 225.Bhattacarya S. Natural anti-mutagens: a review. Res. J. Med. Plant. 2011;5:116–126. [Google Scholar]
- 226.Elgorashi E.E., Taylor J.L.S., Maes A., Van Staden J., De Kimpe N., Verschaeve L. Screening of medicinal plants used in South African traditional medicine for genotoxic effects. South Afr. J. Bot. 2003;143:195–207. doi: 10.1016/s0378-4274(03)00176-0. [DOI] [PubMed] [Google Scholar]
- 227.Elgorashi E.E., Taylor J.L.S., Maes A., Van Staden J., De Kimpe N., Verschaeve L. Screening of medicinal plants used in South African traditional medicine for genotoxic effects. Toxicol. Lett. 2003;143:195–207. doi: 10.1016/s0378-4274(03)00176-0. [DOI] [PubMed] [Google Scholar]
- 228.Verschaeve L., Van Staden J. Mutagenic and anti-mutagenic properties of extracts from South African traditional medicinal plants. J. Ethnopharmacol. 2008;119:575–587. doi: 10.1016/j.jep.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 229.Aremu O.A., Moyo M., Amoo S.O., Van Staden J. Mutagenic evaluation of 10 long term stored medicinal plants commonly used in South Africa. South Afr. J. Bot. 2013;87:95–98. [Google Scholar]
- 230.Ghazali R., Abdullah R., Ramli N., Rajab N.F., Ahmad-Kamal M.S., Yahya N.A. Mutagenic and antimutagenic activities of Mitragyna speciosa Korth extract using Ames test. J. Med. Plants Res. 2011;5:1345–1348. http://www.academicjournals.org/JMPR [Google Scholar]
- 231.Shenkel D.M., Pillai S.P., Telikepalli S., Mennon S.R., Pillai C.A., Mitsche L.A. Role of antimutagens/anticarcinogens in cancer prevention. Biofactors. 2000;12:113–121. doi: 10.1002/biof.5520120118. [DOI] [PubMed] [Google Scholar]
- 232.Van Wyk A.-E. The potential of South African plants in the development of new food and beverage products. South Afr. J. Bot. 2011;77:857–868. [Google Scholar]
- 233.Misihairabgwi J., Cheikhyoussef A. Traditionally fermented foods and beverages of Namibia. J. Ethics Foods. 2017;4:145–153. [Google Scholar]
- 234.Roulette C.J., Njau E.-F.A., Quinlan M.B., Quilan R.J., Call D.R. Medicinal foods and beverages among Maasai agro-pastoralists in northern Tanzania. J. Ethnopharmacol. 2018;216:191–202. doi: 10.1016/j.jep.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 235.Mongalo N.I., Mafoko B.J. Cassia abbreviata Oliv: a review of its Ethnomedicinal uses, Toxicology, Phytochemistry, possible propagation techniques and pharmacology. Afr. J. Pharm. Pharmacol. 2013;7:2901–2906. [Google Scholar]
- 236.Urso V., Signorini M.A., Tonini M., Bruschi P. Wild medicinal and food plants used by communities living in Mopane woodlands of southern Angola: results of an ethnobotanical field investigation. J. Ethnopharmacol. 2016;177:126–139. doi: 10.1016/j.jep.2015.11.041. [DOI] [PubMed] [Google Scholar]
- 237.Moteetee A., Moffett R.O., Seleteng-Kose L. A review of the ethnobotany of the basotho of Lesotho and the free state Province of South Africa (south Sotho) South Afr. J. Bot. 2018 [Google Scholar]
- 238.Olivier D.K. PhD Thesis, University of Johannesburg; South Africa: 2012. The Ethnobotany and Chemistry of South African Traditional Tonic Plants. [Google Scholar]
- 239.Olivier D.K., Van Wyk B.E. Bitterness values of traditional tonic plants of Southern Africa. J. Ethnopharmacol. 2013;147:676–679. doi: 10.1016/j.jep.2013.03.059. [DOI] [PubMed] [Google Scholar]
- 240.Venter F., Venter J.-A. Briza Publications; Pretoria: 2009. Making the Most of Indigenous Trees, Second Edition, Fourth Impression, Revised Edition. [Google Scholar]
- 241.Asowata-Ayodele A.M., Afolayan A.J., Otunola G.A. Ethnobotanical survey of culinary herbs and spices used in the traditional medicinal system of Nkonkobe Municipality, Eastern Cape, South Africa. South Afr. J. Bot. 2016;104:69–75. [Google Scholar]
- 242.Shackleton C.M., Guthrie G. J. Keirungi, J. Stewart fuelwood availability and use in the Richtersveld national park, South Africa. Koedoe. 2003;46:1–8. [Google Scholar]
- 243.Blignaut J., Moolman C. Quantifying the potential of restored natural capital to alleviate poverty and help conserve nature: a case study from South Africa. J. Nat. Conserv. 2006;14:237–248. [Google Scholar]
- 244.Muga M.O., Githiomi J.K., Chikamai B.N. Classification of Kenyan wood carving species using macroscopic and microscopic properties. Int. J. Appl. Sci. Technol. 2014;4:167–178. [Google Scholar]
- 245.Van Wyk B.-E. A review of commercially important African medicinal plants. J. Ethnopharmacol. 2015;176:118–134. doi: 10.1016/j.jep.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 246.Chivandi E., Mukonowenzou N., Nyakudya T., Erlwanger K.H. Potential of indigenous fruit bearing trees to curb malnutrition, improve food security, income and community health in Sb-Saharan Africa: a review. Food Res. Int. 2015;76:980–985. [Google Scholar]
- 247.Moeng T.E. University of Limpopo; South Africa: 2010. An Investigation into the Trade of Medicinal Plants by Muthi Shops and Street Vendors in the Limpopo Province, South Africa. MSc Dissertation. [Google Scholar]
- 248.Street R.A., Stirk W.A., Van Staden J. South African traditional medicinal plant trade-Challenges in regulating quality, safety and efficacy. J. Ethnopharmacol. 2008;119:705–710. doi: 10.1016/j.jep.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 249.Khan F., Kaehler J.H., Allsopp M., Van Vuuren S. Antimicrobial properties and isotope investigations of South African honey. J. Appl. Microbiol. 2014;117:366–379. doi: 10.1111/jam.12533. [DOI] [PubMed] [Google Scholar]
- 250.Osuga I.M., Wambui C.C., Abdulrazak S.A., Ichinohe T., Fujihara T. Evaluation of nutritive value and palatability by goats and sheep of selected browse foliages from semiarid area of Kenya. Anim. Sci. J. 2008;79:582–589. [Google Scholar]
- 251.Gemeda B.S., Hassen A. Effect of tannin and species variation on in vitro digestibility, gas, and methane production of tropical browse plants. Asian-Australas. J. Anim. Sci. 2015;28:188–199. doi: 10.5713/ajas.14.0325. PMC4283163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Mnisi C.M., Mlambo V. Effects of harvesting site on chemical composition and potential protein value of Acacia erioloba, A. nilotica and Ziziphus mucronata leaves for ruminants. J. Anim. Physiol. Anim. Nutr. 2017;10:994–1003. doi: 10.1111/jpn.12535. [DOI] [PubMed] [Google Scholar]