Abstract
The present study investigated the effect of processing on the nutritional, functional, organoleptic and rheological properties of millet and sprouted legumes beverage flavored with jaggery (B1) and buttermilk (B2) for its processing suitability. The millets, sprouted legumes, flavoured with jaggery (B1); and buttermilk and salt (B2), used influenced the suspension stability, nutritional, sensory quality and rheology of beverages. Millets imparted minerals and starch while sprouted legumes improved solubility and extractability of nutrients and also increased the levels of anti-oxidants and flavonoids. Buttermilk improved the stability, increased contents of proteins and minerals and imparted a light colour to the beverage. Jaggery was responsible for caramelized colour and flavor, improved consistency, psuedoplasticity and better organoleptic acceptability. The nutritional quality of B2 was higher in terms of iron (1.8 mg/100 g) and calcium (75 mg/100 g) with 90% antioxidant activity. Highest L* values were obtained for B2 indicating lighter color, whereas B1 was darker with lower L* values. Organoleptic evaluation showed higher acceptability (7.6) of B1 as compared to B2. Results of flow behavior indicated pseudo-plastic nature of beverages. Significant increase in viscosity was also observed with the increase in temperature (10, 25 and 45 °C) of the beverages. The flow curves of B1 produced the best fit applying the Power law model and for B2, Casson model was the best fit. Results of this study could be used in improvement of the process for making millet-based beverage, design of packaging system and also to predict the flow behavior of beverages during storage.
Keywords: Beverages, Rheology, Model fitting, Nutritional quality, Organoleptic evaluation
Introduction
In recent years, beverages with functional properties have become popular for those who want specific health benefits from their foods. The utilization of processed products using sorghum and finger-millets are now important because they contain resistant starch (Wadikar et al. 2007) proteins, minerals, vitamins and phytochemicals. Green-gram (Vigna radiata) and soybeans are excellent sources of protein, dietary fibre, vitamins and minerals. Sprouting increases protein and dietary fiber; reduces tannin and phytic acid and increases mineral bioavailability (Agrahar-Murugkar and Jha 2010) and are therefore incorporated in mixes to enhance nutrient content of diets. Coconut powder called a “functional food” because of its several health benefits like lowering bad cholesterol, keeping digestive tract healthy and maintaining blood sugar levels is rich in fiber, protein, and calcium (Ramaswamy 2014). Jaggery, a traditional sweetener made by concentrating sugarcane juice, is totally natural and serves as rich source of minerals especially iron. Buttermilk is an excellent source of minerals, vitamins, enzymes, and protein (Conway et al. 2014) with therapeutic potential (Conway et al. 2013) and has applications in a variety of foods such as drinks, nutritious bakery products, confectionaries etc. (Fuller et al. 2013).
It would be very interesting to study how factors like functionality of ingredients like millets, sprouted legumes, coconut, buttermilk, jaggery etc.; processing conditions; and interactions between colloidal ingredients (Cano-Ruiz and Richter 1998) influence the qualitative properties of beverages that are planned in the study.
Knowledge of rheology is essential to develop a beverage with an optimum consistency for a good mouth-feel. Beverages contain emulsions and dispersions, making product stability during storage an important factor in its commercial development and rheology contributes immensely in this area. Design of flow systems, product development, and mechanization of the beverage production process (Bhattacharya et al. 1999) use rheological properties like flowability. Flow behavior of a beverage is an important attribute in rheology which influences consumer preferences and determines the processing and quality control of fluid foods (Sopade and Kiaka 2001). Thus rheological properties lead to a refinement of process parameters for beverage development.
Use of ingredients like millets, sprouted legumes, buttermilk, coconut, jaggery etc. to develop a beverage; processing the ingredients to extract maximum nutritional, functional and organoleptic quality while maintaining stability is a challenge, which has been taken up in this study.
Keeping in mind the above challenges a study was planned with the objectives following objectives, (1) to investigate effect of ingredients and its processing on the suspension stability, nutritional, functional, organoleptic and rheological properties of beverages, (2) to analyze the shear behavior of beverages in terms of parameters defined by standard rheological models, and (3) to investigate the processing suitability and quality of a millet based beverage enriched with sprouted legumes and flavored with jaggery (sweet) and buttermilk (salty).
Materials and methods
Preparation of beverages
Raw materials
Sorghum (Jowar), finger-millet (Ragi), whole green-gram, whole soybean, dairy whitener (Banaskantha Dist. Co-operative Milk Producers’ Union Ltd., India), buttermilk (Sanchi- Madhya Pradesh Cooperative Dairy Federation, Bhopal, India), cumin seeds, salt, desiccated coconut and jaggery were procured from the local markets in Bhopal, India.
Pre-preparation of legumes flour
Soybean and green-gram were cleaned thoroughly and made free from dust, dirt, stubbles and foreign matter. For sprouting, cleaned seeds were surface sterilized with 0.1% (w/v) potassium permanganate solution, followed by soaking in distilled water for 4 h at room temperature (RT). The seeds were germinated in a programmable environmental test chamber (Remi Laboratory instruments, India) at 25 °C, 90% RH for 48–72 h for soybean (Agrahar-Murugkar and Jha 2009) and 24–48 h for green-gram (Agrahar-Murugkar et al. 2015). After sprouting, the seeds were dried in an oven overnight at 60 °C and then cooled to RT and powdered using analytical mill (Cole Parmar, Vernon, IL, USA) at high speed (10,590 G). Flours were then sieved through mesh of size 300 microns.
Beverages processing
The base ingredients for the beverages included sorghum and finger-millet (5.8% each), sprouted soy flour (1.1%), sprouted green-gram flour (0.7%), dairy whitener (1.6%) and desiccated coconut (1.8%). In addition, Beverage 1 (B1), contained jaggery (8.3%) and water (75%) while Beverage 2 (B2), contained butter-milk (67%), cumin and black salt (1% each) and water (15%).
Sorghum and finger-millets were cleaned, washed and soaked separately in water in the ratio of 1:2 (seeds to water) for 2 h at 30 °C. After soaking, excess water was drained and millets were coarsely wet-milled in a mixer (Spar mixer, Model SP-800A-C Spar food machinery Mfg. Co. Ltd., Taiwan, R.O.C) for 2 min at medium speed (2nd position) using the serrated blade followed by the addition of water (80 °C) and mixing for 2 min at medium speed. After wet milling of the millets, sprouted soybean flour, green-gram flour and desiccated coconut were added and mixed well into slurry. The slurry was then put into the grinding cum blanching unit of Soymilk preparation unit (fabricated at Central Institute of Agricultural Engineering, Bhopal, India). Water was then added (1:2; solids to water) in the unit and a pressure of 1 kg/cm2 (15 psi) was generated by infusing culinary steam at a pressure of 1–3 kg/cm2 (15 to 54 psi). Grinding cum blanching was carried out for 12 min at 80 °C. After the pressure from unit was released, the slurry was filtered with a muslin cloth and beverages were extracted. In case of B1, jaggery and milk powder were dissolved into the beverage and the final volume was made up with 75% water. In case of B2, buttermilk, cumin powder and salt was added and the final volume was made up with 15% water. The beverages were homogenized in colloidal mill and then stored in sterilized in bottles until further analysis.
Suspension stability
Suspension stability was determined according to a procedure modified from a method described by Priepke et al. (1980) by measuring the top to bottom ratios of total solids in beverages stored undisturbed in a 20 ml bottle for 5 days at 4 °C. For this portions (3 ml) were carefully withdrawn with a 5 ml syringe from the centre of the upper 1/3 of the bottle and from the lower 1/3 of the bottle. Samples from each 3 ml portion were analysed for total solids content using a 105 °C drying method.
Proximate analysis and phenolic, flavonoid content and anti-oxidant activity
The moisture, fat, protein, minerals, calcium, phosphorus and iron content of the beverages were estimated by standard methods of AOAC (2005).
For functional components, 0.5 g of moisture free sample was extracted in 6 ml 80% methanol overnight in centrifuge tubes. The samples were centrifuged at 10,000 rpm at 2 °C for 20 min. The supernatant was collected and used for analysis of functional components.
Phenolics were quantified by the Folin–Ciocalteu method of Singleton and Rossi (1965) as described by Siwela et al. (2010). Flavonoid content was determined using aluminium-chloride colorimetric method as described by Kiranmai et al. (2011). A standard calibration plot was generated using quercetin (10–50 µg/ml). Flavonoids were expressed as Quercetin equivalents (QE) mg/100 g sample. Stable 1, 1-diphenyl-2-picryl hydrazyl radical (DPPH) was used for determination of free radical-scavenging activity (RSA) of the samples (Lee et al. 2003). RSA of the sample was determined as follows:
where, A control = Absorbance of control at 517 nm and A sample = Absorbance of sample at 517 nm.
Color analysis of beverages
Beverages were measured for colour using lab scan XE spectro colorimeter (model no LX16244, Hunter Associate Laboratory Virginia, USA) in terms of CIE ‘L’ (lightness) ‘a’ (redness and greenness) and ‘b’ (yellowness and blueness), following the method of McGuire (1992). Before the test, the instrument was calibrated with standard black and white tiles. Samples from each batch were placed in a glass sample cup of 5.8 cm internal diameter and color coordinates were measured. An average of 10 readings was reported for each sample.
Organoleptic evaluation
A 9-point hedonic scale was used to determine the sensory acceptability of the beverages for taste, texture, flavor, color, appearance and OAA. Thirty panelists from staff and students of Central Institute of Agricultural Engineering, Bhopal, India who were non-smokers and did not consume alcohol evaluated the sensory attributes of the beverage. Both the samples were served in a bowl, in random order. The sensory evaluation took place in a sensory laboratory with individual booths. Each booth was provided with an incandescent light, a small basin and a tap to rinse the mouth between tastings. The attributes evaluated were colour, appearance, mouth feel, taste, flavor and overall acceptability. For each sample, panellists scored their liking of these characteristics using the nine point Hedonic scale. Average of 30 scores for each parameter is reported. The scores represented 1 = dislike extremely, 2 = dislike very much, 3 = dislike moderately, 4 = dislike slightly, 5 = neither like nor dislike, 6 = like slightly, 7 = like moderately, 8 = like very much and 9 = like extremely.
Rheological properties of beverages
Steady shear properties were obtained at selected temperatures of 10, 25 and 45 °C using a parallel-plate rheometer (Anton Paar, Germany: Physica MCR 51). Beverages were prepared just before the experiments. The gap between the plates was 1 mm. Shear rate, which was increased linearly from 0.798 to 150 s−1, was applied to the sample for 5 min (Yolacaner et al. 2008). Temperature was controlled by a circulating water-bath. Throughout the tests, shear rate-shear stress and shear rate-apparent viscosity data were collected. Data were fitted to Casson Eq. (1), Herschel–Bulkley model Eq. (2) and Power law rheological models Eq. (3):
| 1 |
Casson yield stress (σ0c) and Casson plastic viscosity (ηca) was determined as square of intercept (K0c) and slope (Kc) respectively (Rao 2014).
| 2 |
where σ is shear stress (Pa) and γ is shear rate (s−1). τ 0 is yield stress calculated from Casson Model (Eq. 1), KH is consistency index (Pa sn) and n H is flow behaviour index of the model (Rao 2014).
This non-linear model, with appropriate values of τ0, K and n, the model can be converted to power law model [Eq. (3)] or simple linear model.
| 3 |
where τ (Pa) is shear stress, K (Pa s) is consistency coefficient, γ is shear rate (s_1) and n is the dimensionless flow behaviour index.
Statistical analysis
The experiments were carried out in triplicate and results are presented as mean values with standard deviations. Different mean values were analyzed by one way analysis of variance (ANOVA) using SPSS16. A probability level of p < 0.05 was considered to be significant for all statistical procedures.
Results and discussion
Suspension stability
Stability of particles in the beverage in terms of suspension stability is shown in Table 1. Both the beverages showed stability as determined by top to bottom ratios of total solids of approximately 1.00. B2 showed a significantly (p < 0.5) higher suspension stability of 0.98 as compared to B1. This could be attributed to the presence of buttermilk which contains higher amount of protein and phospholipids with hydrophilic protein–lipid complexes like milk-fat globule membrane surrounding the fat globules in buttermilk. This stabilizes them against coalescence making them effective surface active agents resulting in good emulsifying properties.
Table 1.
Physiochemical, nutritional and functional quality of beverages
| Suspension stability | Nutritional quality | Functional quality | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Moisture % | Protein % | Fat % | Minerals % | Calcium (mg/100 g) | Iron (mg/100 g) | Phosphorus (mg/100 g) | Total phenolics (GAE1 mg/100 g) | Flavanoids (QE2 mg/100 g) | Antioxidants (% RSA3) | |
| B1 | ||||||||||
| 0.94b ± 0.01 | 89.1a ± 0.17 | 2.0b ± 0.14 | 1.0 ± 0.08 | 0.27b ± 0.01 | 51.2b ± 3.6 | 0.51b ± 0.15 | 27.9b ± 8.1 | 31.79a ± 3.70 | 1.53b ± 0.26 | 83.06b ± 0.98 |
| B2 | ||||||||||
| 0.98a ± 0.01 | 88.2b ± 0.33 | 2.9a ± 0.04 | 1.2 ± 0.03 | 0.98a ± 0.01 | 75.0a ± 5.25 | 1.84a ± 0.16 | 57.1a ± 12.8 | 34.13a ± 2.19 | 3.01a ± 1.04 | 90.22ab ± 3.25 |
Values are expressed as mean ± standard deviation (n = 9). Means having different letters as superscripts with in the same column differ significantly (n = 9) at p < 0.05
B1 sweet beverage, B2 salty beverage
1GAE gallic acid equivalent
2QE quercetin equivalent
3RSA radical scavenging activity
Nutritional and functional component of beverages
The nutritive value of the beverages (Table 1) studied showed that both beverages were good sources of minerals, phenolics flavonoids and anti-oxidants compared to the prevalent beverages available in the market today. Millets are known to contain good amounts of minerals as has been observed in our study as well. Thapa and Tamang (2004) have also reported higher calcium, iron and phosphorus content in Kodo ko jaanr, a traditional beverage prepared from finger-millets. Sprouting of legumes improves solubility of nutrients and thereby increases its extractability into the aqueous milieu. Apart from this, the availability of minerals improves as does the content of antioxidants, flavonoids and phenolics (Agrahar-Murugkar et al. 2015). Between the two beverages, B2 contained significantly (p < 0.5) higher protein, total minerals, calcium, iron and phosphorus compared to B1. Addition of buttermilk containing high value milk proteins improved the protein content (Nunes et al. 2009) of the beverage. Fermented milk products are excellent sources of essential minerals particularly, calcium and phosphorus (Gurr 1987), and addition of buttermilk resulted in the higher mineral content of B2. Results of functional components revealed significantly (p < 0.5) higher total flavanoids (3.0QE mg/100 g) and antioxidant content (90.2% RSA/100 g) in B2 in comparison to B1 (1.2 QE mg/100 g and 83.1% RSA/100 g) respectively. The phenolic content and antioxidant activity shows strong correlation in many studies (Agbor et al. 2011). Studies have showed that buttermilk was effective at producing an antioxidant effect by acting as a reducing agent to scavenge peroxide and hydroxyl radical, and sequestering both Fe2+ and Fe3+ (Wong and Kitts 2003).
Color analysis
Table 2 shows the color of the beverage samples, expressed in terms of tri-stimulus attributes, L*, a* and b* values. Lightness value (L*), associated with luminous intensity in B1 (57.3) was lower than B2 (73.6). The lower L* values of B1 could be due to added jaggery caramelization in the product, which is known to provide color and specific aromas to foods (Pintea 2008). L* value increases with increase in fat content and in our study as well as by Phillips et al. (1995). The a* and b* values of beverages were on the positive side, indicating that the product was slightly reddish-yellow.
Table 2.
Sensory and colour attributes of beverages
| Beverage | Hunter CIE colour | Sensory analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | Colour | Appearance | Mouth feel | Taste | Flavour | Overall acceptability | |
| B1 | 57.3b ± 0.10 | 5.1a ± 0.1 | 23.5a ± 0.88 | 7.1b ± 0.57 | 7.6 ± 0.97 | 7.7 ± 0.48 | 7.6a ± 0.70 | 7.5 ± 0.97 | 7.6a ± 0.70 |
| B2 | 73.6a ± 0.50 | 2.3b ± 0.04 | 11.7b ± 0.08 | 7.6a ± 0.52 | 7.8 ± 0.63 | 7.6 ± 0.52 | 7.3b ± 0.48 | 7.4 ± 0.97 | 7.1b ± 0.57 |
Values are expressed as mean ± standard deviation (n = 9). Means having different letters as superscripts with in the same column differ significantly (n = 10) at p < 0.05
B1 sweet beverage, B2 salty beverage
Organoleptic evaluation
Sensory evaluation offers the opportunity to obtain a complete analysis of the various properties of food as perceived by human sense. Table 2 shows the mean sensory scores of the beverages. Both of the beverages were acceptable to the consumers as indicated by the score above 6 for all the attributes. Use of jaggery and buttermilk as the differing ingredients in the nutritional beverage- B1 and B2 affected the sensory acceptability as indicated by the variation in the means sensory scores of beverages. There was no significant difference (p < 0.5) in the score for appearance, mouth-feel and flavour for the beverages developed. Colour, taste and overall acceptability of the beverages differed significantly (p < 0.05). B2, the lighter coloured beverage received a higher score as compared to B1 which was a light brown coloured beverage as can be seen by the lower L* value obtained due to jaggery. However, the overall acceptability of B1 was 7.6 and B2 was 7.1 indicating higher consumer acceptability of B1 despite being rated lower for colour. This is because of the higher scores being given to the taste and flavour attributes. Other studies have also reported higher consumer acceptance products with addition of coconut and coconut milk (Kolapo and Olubamiwa 2012). Comparing the results from rheological measurements and sensory evaluation, it was possible to verify that B1, which received higher acceptability by panellists, was the beverage with higher consistency index and higher pseudoplasticity, calculated by power law model, showing correlation between acceptability and consistency of the product as also reported by other researchers (Penna et al. 2001).
Rheological properties of beverages
Flow behavior and Viscosity
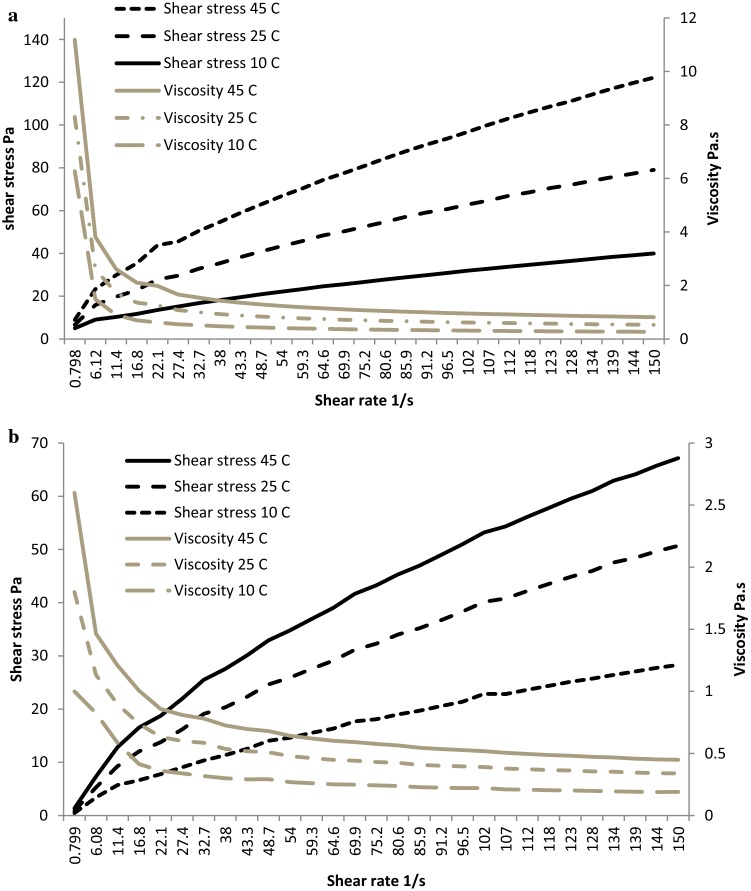
Apparent viscosity depends upon shear rate and temperature (Vitali and Rao 1984). Its variation at different shear rates and temperatures (10, 25 and 45 °C) are shown in Figs. 1 and 2. According to flow curves, the shear stress (Pa) increased with an increase in shear rate while apparent viscosity decreased in both of the beverages, which suggested pseudo-plastic or shear-thinning nature of beverages. Significant increase in viscosity was also observed with the increase in temperature of the beverages. This could be due to the combined result of stronger protein interactions with increase in temperature contributing in favour of higher viscosity, in opposition to the decrease in viscosity of water as temperature increased (Kristensen et al. 1997). The results suggested that B2 had lower viscosity and showed less shear thinning than B1. This might be correlated with heating which is responsible for breaking down starch granules during the cooking process, thus reducing viscosity without dilution with water while simultaneously enhancing the energy and nutrient density (Tizazu et al. 2010) as observed in terms of nutrient content of B2 (Table 1). Ingredients like finger-millet, soy flour, green-gram flour and milk powder might have influenced the rheological behaviour of the beverages. In polymeric and colloidal systems, structural changes in the continuous phase, as well as temperature sensitive interactions in the dispersed phase may result in increase or decrease of flow properties with temperature (Ferguson and Kembłowski 1991).
Fig. 1.
Flow curve showing shear rate versus shear stress and viscosity relationship of a sweet beverage (B1) at 10, 25 and 45 °C, b salty beverage (B2) at 10, 25 and 45 °C
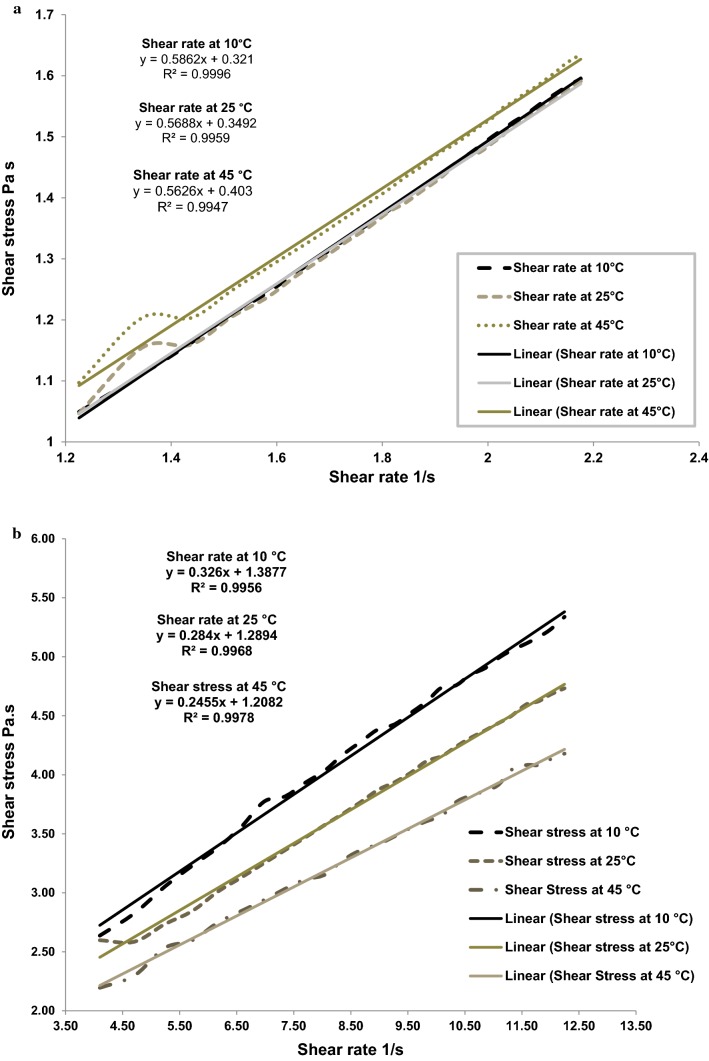
Fig. 2.
a Power model fitting for sweet beverage (B1) at 10, 25 and 45 °C. b Casson model fitting for salty beverage (B2) at 10, 25 and 45 °C
The shear stress (σ) versus shear rate (γ) data obtained for both beverages were fitted to Casson, Herschel Bukley and Power law models for beverages at 10, 25 and 45 °C. The evaluation of standard error and regression values of the fits determined the choice of rheological flow model. Casson (Eq. 1), Herschel Bukley (Eq. 2) and Power laws (Eq. 3) models are shown in Table 3. The flow curves of the full shear stress range of B1 produced the best fit applying the Power law model and for B2 flow curves Casson model was the best fit (Fig. 2). Most pseudo-plastic liquid foods like sweetened condensed milk, ice cream etc. follow the Ostwald de Waelle model commonly known as Power law model (Janhøj et al. 2008). Flow behavior index (nH) of beverages ranged from 0.56 to 0.65 which indicated shear-thinning (pseudo-plastic) behavior, which means that the apparent viscosity decreases as the shear rate increases. This might be due to alignment of microstructure with the flow direction and breaking up of the agglomerated particles as explained by Singh and Heldman 2009. Consistency index (KH) is related to the capacity of the sample for retaining air. B1 at 45 °C had significantly (p < 0.05) higher value for consistency index and lowest value for flow behavior index compared to B1 at 10 and 25 °C whereas B2 showed no significant differences (p < 0.05) between temperature’s for consistency index and flow behavior index. The estimates of K and n suggested that B2 was less viscous (lower K values) and showed less shear thinning (higher n values) than the B1. Kristensen et al. (1997) also reported lower viscosity and shear thinning behavior in buttermilk. Both of the beverages were found to have a slight thixotropic behavior. Fitting best to Casson model, B2 showed lowest viscosity (0.06) and yield stress (1.47) at 45 °C and highest viscosity (0.11) and yield stress (1.93) at 10 °C.
Table 3.
Parameters of Casson, Herschel Bukley and power law models for beverages at 10, 25 and 45 °C
| Models | Casson | Herschel Bukley | Power Law | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Beverages | Casson’s yield stress (Pa s) | Casson’s plastic viscosity | MSE | Consistency index (Pa sn) | Yield stress (Pa s) | Flow behavior index (n) | MSE | Consistency index (Pa sn) | Flow behavior index (n) | MSE |
| B1-10 °C | 2.81b | 0.15b | 0.83c | 0.26b | 7.96b | 0.97b | 1.12d | 2.07b | 0.59b | 0.32c |
| B1-25 °C | 2.78b | 0.15b | 0.99a | 0.29b | 7.99b | 0.94b | 0.80e | 2.09b | 0.59b | 0.18d |
| B1-45 °C | 3.25a | 0.16a | 0.93b | 0.36a | 8.73a | 0.93b | 2.55c | 2.63a | 0.56b | 0.33c |
| B2-10 °C | 1.93c | 0.11c | 0.17e | 0.10c | 5.38c | 1.14a | 4.80b | 1.10c | 0.65a | 0.06e |
| B2-25 °C | 1.66d | 0.08d | 0.05f | 0.09c | 4.24d | 1.0a | 2.38c | 1.30c | 0.62a | 2.69b |
| B2-45 °C | 1.47e | 0.06e | 0.10d | 0.07d | 3.80e | 1.15a | 8.83a | 1.13c | 0.61a | 9.21a |
Values are expressed as mean (n = 9). Means having different letters as superscripts with in the same column differ significantly (n = 9) at p < 0.05
B1 sweet beverage, B2 salty beverage
Conclusion
The ingredients in beverage making influenced the suspension stability, nutritional, sensory quality and rheology of beverages made using millets, sprouted legumes, flavoured with jaggery (B1); and buttermilk and salt (B2). Millets imparted minerals and starch while sprouted legumes improved solubility and extractability of nutrients and also increased the levels of anti-oxidants and flavonoids. Buttermilk improved the stability, increased contents of proteins and minerals and imparted a light colour to the beverage. Jaggery was responsible for caramelized colour and flavor, improved consistency, psuedoplasticity and better organoleptic acceptability. Shear stress (Pa) increased with the increase in shear rate while apparent viscosity decreased in both beverages, which suggested the pseudo-plastic or shear-thinning nature of beverages. Significant increase in viscosity was also observed with the increase in temperature of the beverages. B2 had the lower viscosity and showed less shear thinning than B1. The flow curves of the full shear stress range of B1 produced the best fit applying the Power law model and for B2 flow curves Casson model was the best fit.
The study shows that processing suitability is very high and the quality of a millet based beverage enriched with sprouted legumes and flavored with jaggery (sweet) and buttermilk (salty) in terms of nutrition, rheology and sensory acceptability is very good.
Results of this study could be used to improve the process for making millet-based beverage, design of packaging system and to predict the flow behavior of beverages during storage.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agbor GA, Moumbegna P, Oluwasola EO, Nwosu LU, Njoku RC, Kanu S, Abudei FA. Antioxidant capacity of some plants foods and beverages consumed in the eastern region of Nigeria. Afr J Trad Complement Altern Med. 2011;8(4):362–369. doi: 10.4314/ajtcam.v8i4.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrahar-Murugkar D, Jha K. Effect of sprouting on nutritional and functional characteristics of soybean (Glycine max L) J Food Sci Technol. 2009;46(3):240–243. doi: 10.1007/s13197-010-0082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrahar-Murugkar D, Jha K. Effect of drying on the nutritional and functional quality and electrophoretic pattern of soy flour from sprouted soybean (Glycine max) J Food Sci Technol. 2010;47(5):482–483. doi: 10.1007/s13197-010-0082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrahar-Murugkar D, Gulati P, Kotwaliwale N, Gupta C. Evaluation of nutritional, textural and particle size characteristics of dough and biscuits made from composite flours containing sprouted and malted ingredients. J Food Sci Technol. 2015;52(8):5129–5137. doi: 10.1007/s13197-014-1597-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC . Method 920.87. Official methods of analysis. Association of Official Analytical Chemists. 14. Arlington: AOAC; 2005. [Google Scholar]
- Bhattacharya S, Vasudha N, Krishna Murthy KS. Rheology of mustard paste: a controlled stress measurement. J Food Eng. 1999;41(3):187–191. [Google Scholar]
- Cano-Ruiz ME, Richter RL. Changes in physicochemical properties of retort-sterilized dairy beverages during storage. J Dairy Sci. 1998;81(8):2116–2123. doi: 10.3168/jds.S0022-0302(98)75787-X. [DOI] [PubMed] [Google Scholar]
- Conway V, Couture P, Richard C, Gauthier SF, Pouliot Y, Lamarche B. Impact of buttermilk consumption on plasma lipids and surrogate markers of cholesterol homeostasis in men and women. Nutr Metab Cardiovasc Dis. 2013;23(12):1255–1262. doi: 10.1016/j.numecd.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Conway V, Gauthier SF, Poulio Y. Buttermilk: much more than a source of milk phospholipids. Anim Front. 2014;4(2):44–51. [Google Scholar]
- Ferguson J, Kembłowski Z. Applied fluid rheology. Barking: Elsevier; 1991. [Google Scholar]
- Fuller KL, Kuhlenschmidt TB, Kuhlenschmidt MS, Jiménez-Flores R, Donovan SM. Milk fat globule membrane isolated from buttermilk or whey cream and their lipid components inhibit infectivity of rotavirus in vitro. J Dairy Sci. 2013;96(6):3488–3497. doi: 10.3168/jds.2012-6122. [DOI] [PubMed] [Google Scholar]
- Gurr MI. Nutritional aspects of fermented milk products. FEMS Microbiol Lett. 1987;46(3):337–342. [Google Scholar]
- Janhøj T, Frøst MB, Ipsen RH. Sensory and rheological characterization of acidified milk drinks. Food Hydrocoll. 2008;22(5):798–806. [Google Scholar]
- Kiranmai M, Kumar CM, Ibrahim M. Comparison of total flavanoid content of Azadirachta indica root bark extracts prepared by different methods of extraction. Res J Pharmacoll Biol Chem Sci. 2011;2:254–261. [Google Scholar]
- Kolapo AL, Olubamiwa AO. Effect of different concentrations of coconut milk on the chemical and sensory properties of soy-coconut milk based yoghurt. Food Public Health. 2012;2(4):85–91. [Google Scholar]
- Kristensen D, Jensen PY, Madsen F, Birdi KS. Rheology and surface tension of selected processed dairy fluids: influence of temperature. J Dairy Sci. 1997;80(10):2282–2290. [Google Scholar]
- Lee SE, Hwang HJ, Ha JS, Jeong H, Kim JH. Screening of medicinal plant extracts for antioxidant activity. Life Sci. 2003;73:167–179. doi: 10.1016/s0024-3205(03)00259-5. [DOI] [PubMed] [Google Scholar]
- McGuire RG. Reporting of objective color measurements. Horti Sci. 1992;27:1254–1255. [Google Scholar]
- Nunes MHB, Moore MM, Ryan LA, Arendt EK. Impact of emulsifiers on the quality and rheological properties of gluten free breads and batters. Eur Food Res Technol. 2009;228:633–642. [Google Scholar]
- Penna ALB, Sivieri K, Oliveira MN. Relation between quality and rheological properties of lactic beverages. J Food Eng. 2001;49(1):7–13. [Google Scholar]
- Phillips LG, McGrift ML, Barbano DM, Lawless HA. The influence of fat on the sensory properties, viscosity and color of low-fat milk. J Dairy Sci. 1995;78:1258–1266. [Google Scholar]
- Pintea AM. Food colorants derived from natural sources by processing. In: Socaciu C, editor. Food colorants: chemical and functional properties. Florida: CRC Press; 2008. pp. 329–346. [Google Scholar]
- Priepke PE, Wei LS, Nelson LS, Steinberg MP. Suspension stability of Illinois soybean beverage. J Food Sci. 1980;45:242–245. [Google Scholar]
- Ramaswamy L. Coconut flour: a low carbohydrate, gluten free flour. Int J Ayur Herb Med. 2014;4:1426–1436. [Google Scholar]
- Rao MA. Flow and functional models for rheological properties of fluid foods. In: Barbosa-Canovas GV, editor. Rheology of fluid, semisolid, and solid foods, food engineering series. 3. New York: CRC Press; 2014. pp. 27–57. [Google Scholar]
- Singh RP, Heldman DR. Introduction to food engineering. 4. USA: Elsevier; 2009. [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Siwela M, Taylor JR, de Milliano WA, Duodu KG. Influence of phenolics in finger millet on grain and malt fungal load, and malt quality. Food Chem. 2010;121:443–449. [Google Scholar]
- Sopade PA, Kiaka K. Rheology and microstructure of sago starch from Papua New Guinea. J Food Eng. 2001;50:47–57. [Google Scholar]
- Thapa S, Tamang JP. Product characterization of Kodo ko Jaanr: fermented finger millet beverage of the Himalayas. Food Microbiol. 2004;21(5):617–622. [Google Scholar]
- Tizazu S, Urga K, Abuye C, Retta N. Improvement of energy and nutrient density of sorghum-based complementary foods using germination. Afr J Food Agric Nutr Dev. 2010;10(8):2927–2942. [Google Scholar]
- Vitali AA, Rao MA. Flow properties of low-pulp concentrated orange juice: effect of temperature and concentration. J Food Sci. 1984;49(3):882–888. [Google Scholar]
- Wadikar DD, Premvalli RS, Satyanarayanswamy YS, Bawa AS. Lipid profile in finger-millet. J Food Sci Technol. 2007;44(1):79–81. [Google Scholar]
- Wong PYY, Kitts DD. Chemistry of buttermilk solid antioxidant activity. J Daiiry Sci. 2003;86(5):1541–1547. doi: 10.3168/jds.S0022-0302(03)73739-4. [DOI] [PubMed] [Google Scholar]
- Yolacaner E, Sumnu G, Sahin S. Rheological properties and quality of rice cakes formulated with different gums and an emulsifier blend. Food Hydrocolloids. 2008;22(2):305–312. [Google Scholar]