Abstract
The study was planned to optimise the extraction process of protein and pectin from pumpkin seeds and peels respectively. The extraction of protein and pectin was performed with three independent variables such as extraction temperature, extraction time and pH. The optimized process variables for protein extraction were 32.7 °C, 16.06 min, pH of 9.51 and yield at these optimized conditions was 70.31 ± 2.32%. However, for pectin extraction optimized conditions were 89.98 °C, 13 min, pH of 2.85 and yield was reported as 69.89 ± 2.90%. Further, protein and pectin were isolated at optimized condition. Isolated protein and pectin were utilized for developing the edible film. The protein and pectin were mixed in varying proportions i.e. 1:0, 1:1, 0:1 and film were casted by standard methods. Further, films mechanical and barrier properties were assessed and it was found in acceptable range (Tensile strength: 2.04–5.28 MPa; elongation: 13.13–14.37%; water vapour permeability: 3.24 × 10−6–6.24 × 10−6 g/Pa m h).
Keywords: Pumpkin seed protein, Pumpkin peel pectin, Extraction, Waste utilization, Edible film
Introduction
Pumpkins are gourd squashes which are grown commonly in tropical and sub-tropical countries (Kim et al. 2012) and it is grown and consumed as vegetables. This can also be incorporated into foods such as candies, bread and rice cake (Kim et al. 2012; Pongjanta et al. 2006). During processing of pumpkins large amount of waste/by-products generated in the form of seed and peel. The seeds of pumpkin plant contain nutrient-rich, with a high content of protein, peptides, dietary fiber and micro-nutrients (Wang and Ng 2003). Depending on the quality of pumpkin seeds, protein content varies from 24.5 to 36.0% (Quanhong and Caili 2005). The protein content in pumpkin seeds can be increased by removing the oil present in the seed. The protein content in an oil cake can be increased up to 60%, which makes it a valuable material for extraction of protein (Bucko et al. 2016). The alkaline solution is commonly used for extraction of protein by isoelectric precipitation.
During processing of pumpkin, its pectin rich peels are discarded as waste. Such pectin exhibits significant heterogeneity depending upon on its structure and composition (Gulfi et al. 2005). The properties of such pectin are affected by extraction condition and source of raw materials (Jun et al. 2006). The extraction of pectin is possible with acidic solution like hydrochloric, sulfuric or nitric acid. Pectin is extensively used in various food processing operations because of its gelling properties and is also used as stabilizer in jams, jellies etc. and also serves as thickening agents.
During extraction of protein and pectin, parameters like temperature, time, pH, solvent to meal ratio, type of solvent used affect the extractability (Wani et al. 2008). When interaction of many factors affects the responses, response surface methodology (RSM) can provide the desire information in a short period of time with lesser number of experiments (Rustom et al. 1991). RSM is suitable for optimizing the extraction condition and in future could be used in food industry due to simplicity (Lv et al. 2011).
Use of natural polymeric materials for developing edible films and coating is continuously increasing research interest of food packaging industries sustainable development and growth (Pavlath and Orts 2009). Edible films were developed by various authors from different protein and pectin sources. The protein source includes lentil (Bamdad et al. 2006), mung bean (Bourtoom 2008), soyabean (Chinma et al. 2015), brewer’s spent grain (Lee et al. 2015) and pectin source includes pomelo peel (Das et al. 2018), orange peel (Baron et al. 2017) and banana peels (Oliveira et al. 2017). So far, there is no work reported on using pumpkin seed protein and pumpkin peel pectin for development of edible films.
Therefore, the work was planned to study the influence of pH, temperature, and time on protein and pectin extraction from pumpkin seeds and peels respectively, and to formulate the edible film using extracted protein and pectin.
Materials and methods
Materials
The seeds and peel of matured (ripened) pumpkin with solid orange color were collected from Local market of Lunglei, Mizoram, India. All chemicals and solvent standards used in this study are analytical reagent grade and are purchased from Hi Media Laboratories (India).
Pre-treatment of pumpkin seeds and peels
The collected pumpkin peels and seeds were washed and dried at 45 °C for 48 h. The dried pumpkin seeds and peels were grinded using a grinder to make into fine powder particles of size < 500 µm. The fine powder particles were collected and kept in an air-tight container.
Sample preparation
The pumpkin seed powder was defatted using n-hexane (n-hexane: flour, 10:1, v/w), under continuous stirring and the solvent present in the seeds meal was removed. The defatted meal was dried in a tray drier (45 °C for 8 h) and stored at room temperature (Wani et al. 2008). The defatted seeds were used for extraction of protein. The pumpkin peel powder (50 g) was washed three times with ethanol to remove alcohol soluble component. First wash was for 5 min with 600 mL of boiling 70% v/v ethanol, second wash for 5 min with 600 mL boiling pure ethanol, third wash again for 5 min with 600 mL of absolute ethanol at room temperature and finally it was washed in 200 mL acetone (99%). Between washing, the materials were filtered through a muslin cloth. The alcohol insoluble residue (AIR) was dried at room temperature for 24 h (Oliveira et al. 2017). The protein content of defatted pumpkin seed were analysed according to the standard AOAC method (Latimer 2016). The pectin content of pumpkin peel was determined by following standard procedure (Ranganna 1986).
Experimental design
Response surface methodology was used to determine the effect of three independent variables on extraction of protein and pectin using central composite design (CCD) and Box–Behnken design (BBD) respectively and to find out the optimum conditions for same. The effect of independent variables x1 (temperature), x2 (extraction time) and x3 (pH) at three variation levels on the extraction process of protein from seed and pectin from peel was investigated. The ranges of independent variables are given below.
For extraction of protein:
For extraction of protein:
Extraction of protein and pectin
Pumpkin seed meal was used for protein with different levels of variables (temperature 30–60 °C, extraction time 10–30 min and pH was 7–10). The protein extraction was carried out with alkaline solution (Sodium Hydroxide) in water bath connected with stirrer. The solution was stirred continuously for required time (10–30 min) and then centrifuged in a cooling centrifuged at 1000 rpm for 15 min at 4 °C. The supernatant was filtered through a filter paper and the soluble protein content was determined using Lowry’s method (Wani et al. 2008). The amount of protein extracted from total protein available in pumpkin seed meal is referred as protein yield (in percentage). The total protein in pumpkin seed meal was estimated by multiplying the nitrogen content with a factor of 6.25. Protein extract were isolated by adding 0.1 N hydrochloric acid till it reach pH 5, followed by washing with water, the protein were finally collected by centrifugation (6500 rpm for 30 min at 4 °C and then dried (Osman and Simon-Sarkadi 1991).
The pectin was extracted from alcohol insoluble residue (AIR) with citric acid solution (AIR: citric acid solution ratio, 1:20 w/v), with three variables; viz. temperature (70–90 °C), extraction time (60–130 min) and pH of the citric acid solution (2–4 pH). The extraction was carried out in a water bath and then stirring (150 rpm) for selected period. After centrifugation (3000 rpm, 30 min, 10 °C), the supernatant was vacuum filtered, added with the same volume of absolute ethanol, and the pH was adjusted to 3.5 (pH of minimum pectin solubility) with KOH and HCl. The mixture was stirred for 30 min, left to precipitate at 4 °C for 12 h, and centrifuged (15 min, 4 °C, 3500 rpm). The precipitates (pellet) were collected, washed with ethanol 70% (v/v), centrifuged again (20 min, 4 °C, 3500 rpm), and dried at room temperature (23 ± 2 °C) for 24 h. It was then added with distilled water (pellet: water ratio, 1:3 w/v), stirred (20 min), had its pH adjusted to 7, and was again dried and milled to a fine powder by using a grain mill (Pereira et al. 2016). The amount of pectin extracted from total pectin available in pumpkin peel is referred as pectin yield.
Optimization of protein and pectin yield
The numerical optimization tool of RSM was used to invest optimum conditions for maximizing the yield of extract from pumpkin seed and pumpkin peel. The variables of pH, temperature and time were fitted using RSM for optimization process. The parameters used for optimization of the extraction are higher extraction yield, lesser time and temperature (Kanmani et al. 2014; Lv et al. 2011).
Development of film from isolated protein and pectin
The films were developed from isolated protein and pectin by mixing in three different proportions viz. 100:0 (F1), 50:50 (F2), 0:100 (F3). Accordingly, isolated protein and pectin were mixed with deionised water which was kept constant (5 g/100 mL) during the process. The protein solution was stirred at room temperature for 1 h to dissolve the protein and pectin solution was mixed at 90 °C for 1 h. Both the solutions were mixed together in equal proportion for making film from protein and pectin. Glycerol was added 40% (w/w) as a plasticizer. The solution was poured into a petri plate (80 mm diameter) and kept in a tray dryer for drying (50 ± 2 °C, 8 h). Calcium chloride (2% solution) was poured on the dried film which acts as a cross linking agent and drying was continued and the film was peeled off for further analysis (Gounga et al. 2007).
Calcium chloride (2%) was poured in the dried film which acts as a cross linking agent which helps to increase the strength of film.
Analysis of films
Films were evaluated with thickness measurement, water vapor permeability, swelling properties, solubility, mechanical properties, color and transparency. The average thickness of ten pieces of the film was measured by using a micro-meter (Alton M820-25, China), with a sensitivity of 0.01 nm. The WVP of films was determined using dish method as suggested by Jouki et al. (2013). Swelling properties were calculated as the amount of water absorbed by film when immersed in deionised water for 2 min. The solubility was calculated as the percentages of dry matter of the film solubilised after 24 h immersion in water (Borah et al. 2017). Tensile strength (TS) and elongation at break (EB) was measured using Kieffer Dough and Gluten Extensibility Rig (A/KIE) with the help of Texture Analyzer (TA-HDPlus, Stable Microsystems, UK) (Borah et al. 2017). Tensile strength was calculated by dividing the maximum force at break by the length and thickness of the film. Elongation at break was determined as percentage of change in length of film. Film color was measured using a Hunter Lab calorimeter (Ultrascan VIS, Hunter Lab. Inc., USA) with co-ordinates values (L*, a*, b*) of the film. The transparency of the film sample was measured at wavelength 560 nm using a UV–VIS spectrophotometer (Spectronic 20D+, Thermo Scientific, USA) (Ma et al. 2016).
Statistical analysis
Fitted models were evaluated for each response by means of multiple regressions. Analysis of variance (ANOVA) was used to find the model significant terms for each response. Model adequacies were checked by lack of fit test, coefficient of regression (R2), adjusted coefficient of regression (Adj. R2), predicted coefficient of regression (Pred. R2), adequacy precision and residual sum of squares. Extractions conditions were optimized using numerical optimization tool of RSM. Average values and standard deviation was calculated for film properties. Single factor ANOVA was used to determine the critical difference of means and variance among the different values. Duncan test was performed at a significant level of p ≤ 0.05 using SPSS software.
Results and discussion
Protein extraction from defatted pumpkin seed meal
The protein content of defatted pumpkin seed was found as 54 ± 1.00 g/100 g. The protein yield for each experimental run was calculated and the values were found in the range of 39.78–80.79%. The result of different experimental run of protein extraction using Faced Centred Composite Design (FCCD) which were analysed using Design Expert Software is shown in Table 1. The regression equations for protein yield in terms of coded factors are as follows:
| 1 |
Table 1.
Extraction yield of protein and pectin with different combinations of experimental conditions
| Run | Uncoded variable level for extrication of protein | Protein yield (%) | Uncoded variable level for extrication of pectin | Pectin yield (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Temp. (°C) | Time (min) | pH | Temp. (°C) | Time (min) | pH | |||
| 1 | 30 | 10 | 7 | 72.52 ± 2.19 | 70 | 95 | 4 | 27.67 ± 0.04 |
| 2 | 60 | 10 | 7 | 50.27 ± 1.24 | 70 | 60 | 3 | 34.15 ± 0.03 |
| 3 | 30 | 30 | 7 | 55.69 ± 1.45 | 70 | 130 | 3 | 34.34 ± 0.04 |
| 4 | 60 | 30 | 7 | 53.43 ± 1.86 | 70 | 95 | 2 | 48.82 ± 0.12 |
| 5 | 30 | 10 | 10 | 70.43 ± 0.20 | 80 | 60 | 2 | 38.15 ± 0.16 |
| 6 | 60 | 10 | 10 | 50.22 ± 1.03 | 80 | 95 | 3 | 46.57 ± 0.04 |
| 7 | 30 | 30 | 10 | 68.14 ± 1.20 | 80 | 95 | 3 | 38.53 ± 0.05 |
| 8 | 60 | 30 | 10 | 52.46 ± 1.19 | 80 | 95 | 3 | 45.85 ± 0.21 |
| 9 | 30 | 20 | 8.5 | 80.79 ± 0.87 | 80 | 130 | 4 | 40.88 ± 0.04 |
| 10 | 60 | 20 | 8.5 | 52.06 ± 0.55 | 80 | 95 | 3 | 50.33 ± 0.18 |
| 11 | 45 | 10 | 8.5 | 59.01 ± 1.66 | 80 | 60 | 4 | 24.95 ± 0.07 |
| 12 | 45 | 30 | 8.5 | 39.78 ± 1.61 | 80 | 95 | 3 | 49.25 ± 0.19 |
| 13 | 45 | 20 | 7 | 52.34 ± 2.41 | 80 | 130 | 2 | 44.05 ± 0.12 |
| 14 | 45 | 20 | 10 | 63.29 ± 0.63 | 90 | 130 | 3 | 74.54 ± 0.21 |
| 15 | 45 | 20 | 8.5 | 56.07 ± 0.87 | 90 | 95 | 4 | 55.05 ± 0.13 |
| 16 | 45 | 20 | 8.5 | 53.19 ± 2.13 | 90 | 95 | 2 | 66.45 ± 0.11 |
| 17 | 45 | 20 | 8.5 | 50.31 ± 1.48 | 90 | 60 | 3 | 52.81 ± 0.25 |
| 18 | 45 | 20 | 8.5 | 59.10 ± 1.65 | – | – | – | – |
| 19 | 45 | 20 | 8.5 | 54.99 ± 2.95 | – | – | – | – |
| 20 | 45 | 20 | 8.5 | 48.52 ± 1.03 | – | – | – | – |
All data are the mean ± SD of three replicates
The regression co-efficient (R2) was found out to be 0.81, which implies that the sample variation of 81.2% for extraction of protein were attributed to the independent variable, and only 18.8% of the total variation could not be explained by the model. Also the adj R2 is comparable to R2 (Table 2) and the difference is negligible indicating that the regression model is the best fitted model. According to the co-efficient of variation (CV) and adequate precision ratio the model is reliable and can be reproduced. The validity of the model is confirm by the non-significant lack of fit relative to pure error (p > 0.05). The overall result indicates that the model can be used in optimization of protein extraction from pumpkin seeds.
Table 2.
Analysis of variance (ANOVA) for fitted model of protein yield and pectin yield
| Source | Protein yield | Pectin yield | ||
|---|---|---|---|---|
| Sum of squares | p value | Sum of squares | p value | |
| Model | 1433.31 | 0.0110 | 2453.96 | 0.0012 |
| 794.42 | 0.0006 | 1348.30 | < 0.0001 | |
| 108.57 | 0.1001 | 239.29 | 0.0109 | |
| 41.17 | 0.2906 | 299.21 | 0.0064 | |
| 75.15 | 0.1626 | 116.07 | 0.0481 | |
| 16.19 | 0.5001 | 23.77 | 0.3152 | |
| 23.19 | 0.4220 | 25.13 | 0.3027 | |
| 283.19 | 0.0151 | 247.96 | 0.0101 | |
| 130.26 | 0.0753 | 97.81 | 0.0642 | |
| 6.50 | 0.6669 | 77.10 | 0.0924 | |
| Residual | 330.73 | 142.15 | – | |
| Lack of fit | 255.71 | 0.1023 | 56.71 | 0.5208 |
| R2 | 0.81 | 0.94 | ||
| Adj R2 | 0.64 | 0.87 | ||
| Pred R2 | − 0.39 | 0.59 | ||
| Adeq precision | 7.86 | 14.43 | ||
| C.V.% | 10.06 | 9.92 | ||
From ANOVA table (Table 2) it can be seen that the model and the linear parameter of temperature and the quadratic parameter of temperature are significant at p < 0.05 whereas, the linear parameter of time, pH and the interaction parameter of temperature and time, temperature and pH and also time and pH and the quadratic parameter of time and pH are insignificant (p > 0.05).
Effect of process variables on protein extraction
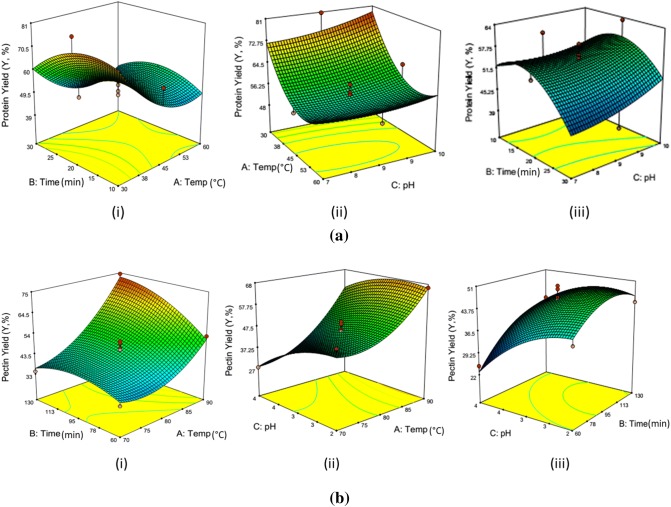
The process variable used for protein extraction was temperature (°C), time (min) and pH of NaOH solution. From Table 2 it is seen that among the independent variable temperature has the significant effect. The model equation (Eq. 1) shows that temperature and time have a negative effect on response whereas pH has a positive effect on response. This implies that with increase in temperature and time the protein yield decreased whereas with increase in pH level protein yield increased.
The effect of the independent variable and their mutual interaction on the protein yield during the extraction process was investigated by plotting a three dimensional response surface profile of multiple non-linear regression models (Fig. 1a). The response was plotted using Z-axis against two independent variables and other variables were kept at zero and response surface was generated.
Fig. 1.
a Protein yield as function of (i) temperature and time (ii) temperature and pH (iii) time and pH. b Pectin yield as function of (i) temperature and time (ii) temperature and pH (iii) time and pH
The decrease in protein yield with increase in time and temperature can be due to the protein denaturation at higher extraction temperature and time. Lv et al. (2011) also described that in extraction of protein from grape seed with the increase in temperature leads to denaturation of protein. However; with increase in pH level from pH 7 to pH 10 the protein yield slightly increases from 48 to 61% the slight increase in the protein yield could be due to increase protein solubility at higher pH level. As reported by Bucko et al. (2016), solubility of pumpkin protein increased as the pH of the solution is increased towards more alkaline or decreased towards more acidic conditions. Hou et al. (2017) reported that the increase in protein solubility at higher temperature is affected by change in thiol group and a disulfide bond of protein as well as surface hydrophobicity of protein.
Pectin extraction from pumpkin peels
The pectin content of pumpkin peel was found as 24.96 ± 0.24 g/100 g. The process variables used for extraction of pectin were temperature, time and pH of citric acid. The result of different experimental run of pectin extraction using Box Behnken Design (BBD) which were analysed using Design Expert Software is shown in Table 1. The final predictive equation in term of coded value (Eq. 2) was obtained as follow.
| 2 |
The regression co-efficient (R2) was found out to be 0.945, which indicates that the sample variation of 94.5% for pectin extraction were attributed to the independent variable, and only 5.5% of the total variation could not be explained by the model. Also the adj R2 is comparable to R2 (Table 2) and the difference is negligible indicating that the regression model is the best fitted model. According to the co-efficient of variation (CV) and adequate precision ratio the model is reliable and can be reproduced. The validity of the model is confirm by the non-significant lack of fit (p > 0.05). The overall result indicates that the model can be used in optimization of pectin extraction from pumpkin peels.
From ANOVA Table 2 it can be observed that the model and the linear parameter of temperature, time and pH, the interaction parameter of temperature and time and the quadratic parameter of temperature were significant at p < 0.05 whereas, the interaction parameter temperature and pH and the quadratic parameter of time and pH are insignificant (p > 0.05).
Effect of process variables on pectin extraction
The process variable used in this study was temperature (°C), time (min) and pH of citric acid solution. Among the independent variable temperature has the highest effect followed by pH and time (Table 2). It also observed from equation (Eq. 2) that temperature and time have a positive effect on response whereas pH has a negative effect on response. This implies that with increase in temperature and time the pectin yield increases whereas with increase in pH level pectin yield decreases. The increase in pectin content with increased in temperature may be due to more solubility and diffusivity of pectin into the solvent (Maran and Priya 2015; Jafari et al. 2017). The increase in pectin yield with increased in extraction temperature and time provides more reaction time for the mass transfer from the sample to the extracting solution. The similar interpretations were given by Jafari et al. (2017). The effect of the pH on the yield of pectin is depicted in Fig. 1(b). It is seen that extraction at lower pH increase the pectin yield. The increase in pectin yield at lower pH might be due to the reason that at lower pH value that is high acidic condition the insoluble pectin is hydrolysed into soluble pectin. Similar interpretations were given by Maran and Priya (2015).
Optimization of protein and pectin process
The numerical optimization technique was used based on the desirability approach for optimization of process variables. It was carried out for obtaining the maximum protein yield and total extraction of pectin. It provides optimum conditions for the protein yield from pumpkin seeds and the total extraction of pectin from pumpkin peels having the higher response values. The optimized process variables for protein extraction were 32.70 °C, 16.06 min, pH of 9.51 and predicted yield at these optimized conditions was 72.45%, which is in line with experimental value of 70.31 ± 2.32%. However, for pectin extraction optimized conditions were 89.98 °C, 13 min, pH of 2.85 and predicted yield was 72.82% which is closer to experimental value of 69.89 ± 2.90%.
Properties of film developed from isolated protein and pectin
The film produced from protein extracted and pectin extracted possess smooth surface (Fig. 2). This smoothness of the surface may be due to the smaller size of the particles. Lipid phase separation was not observed during drying of the film. The different properties of film are discussed below.
Fig. 2.
Protein based film (F1), protein–pectin based film (F2) and pectin based film (F3). Film diameter: 80 mm and film thickness F1: 0.12 ± 0.02 mm, F2: 0.18 ± 0.01 mm, F3: 0.20 ± 0.03 mm
Thickness and water vapor permeability of film
The thickness of film developed from protein (F1), pectin (F3) and combination of both materials (F2) were observed as 0.12 ± 0.02 mm, 0.18 ± 0.01 mm and 0.20 ± 0.03 mm. The diameters of all the films were 80 mm. Water vapor barrier properties of film were evaluated by water vapor permeability test. The WVP (g/Pa m h) of films F1, F2 and F3 was found as 3.24 × 10−6, 6.24 × 10−6 and 5.09 × 10−6 respectively (Table 3). The WVP values of films show significant difference to each other (p ≤ 0.05). The film developed using protein showed the lowest WVP values; this may be due to the degree of organization of the protein network. In case of pectin film probably presented less organization of the polymeric matrix and consequently less packing, hence WVP values were comparatively higher than protein based film (Saikia and Badwaik 2018).
Table 3.
Properties of film developed from isolated protein and their comparison with other films
| Film no | Materials (Protein:pectin) | Film thickness (mm) | WVP × 10−6 (g/Pa m h) | Swelling (%) | Solubility (%) | Tensile strength (MPa) | Elongation (%) | L* | a* | b* | Transparency (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | 100:0 | 0.12 ± 0.02a | 3.24 ± 0.03a | 31.94 ± 0.03a | 50.76 ± 0.17a | 2.04 ± 0.05a | 13.13 ± 0.32a | 67.68 ± 0.22c | − 53.86 ± 0.03a | − 26.02 ± 0.85a | 21.38 ± 0.10a |
| F2 | 50:50 | 0.20 ± 0.03a | 6.24 ± 0.03c | 45.99 ± 0.26c | 64.98 ± 0.92c | 2.52 ± 0.02b | 13.84 ± 0.24b | 65.67 ± 0.19b | − 52.49 ± 0.17b | − 19.59 ± 0.37b | 33.13 ± 0.04c |
| F3 | 0:100 | 0.18 ± 0.01a | 5.09 ± 0.07b | 43.56 ± 0.15b | 61.31 ± 0.12b | 5.28 ± 0.04c | 14.37 ± 0.36c | 64.60 ± 0.12a | − 51.43 ± 0.13c | − 16.83 ± 0.19c | 30.91 ± 0.20b |
All data are the mean ± SD of three replicates. Mean followed by different letters in the same column differs significantly (p ≤ 0.05)
Swelling and solubility of film
The swelling percentage of the films was reported as 31.94, 45.99 and 43.56% for F1, F2 and F3 films respectively (Table 3). The swelling properties of these films are high because of the nature of the film which is hygroscopic in nature. The solubility of the films F1, F2 and F3 were obtained as 50.76, 64.98 and 61.31% respectively. The addition of pectin in protein increased the swelling and solubility of the film significantly (p ≤ 0.05). Generally, higher solubility of the film indicates lower water resistance and it also dependant on concentration of plasticizer.
Mechanical properties
Tensile strength and percentage elongation of the film under tensile conditions were calculated. The tensile strength for each film was significant to each other (p ≤ 0.05) indicating pectin has the highest tensile strength comparing to the protein and a mixture of protein and pectin. The rise in tensile strength is attributed not only to the geometry and rigidity of the film, but also to the formation of a stiff continuous network of film linked through hydrogen bonding (Chaichi et al. 2017). The flexibility of the film was measures by elongation at break of film. The elongation percentage was highest for film F3 which is 14.37 ± 0.36%. These films showed significant difference with each other at p ≤ 0.05 (Table 3). This is due to the good interaction of the compound with plasticizer which leads to the effective transfer of applied stress through the polymer (Kanagaraj et al. 2007).
Film color and transparency
Three different films produced from protein (F1), protein–pectin combination (F2) and pectin (F3) varied in their color, which is mostly due to the color of protein and pectin from seeds and peels of pumpkin. Color values showed that, composite F3 has the lowest ‘L*’ value (64.60 ± 0.12) and highest for F1 (67.68 ± 0.22). The ‘L*’ values indicates the polymer is darker in color; however, ‘a*’ and ‘b*’ values indicating greenish color pattern of film. There is significant difference (p ≤ 0.05) between each sample. Transparency of film was checked at wavelengths of 560 nm. The film developed from protein, mixture of protein and pectin, and pectin was compared to one another. The transparency of the film was varying in different film and significant difference were observed among them (p ≤ 0.05).
Comparison of isolated protein–pectin based film with other plant protein and pectin based film
The film developed from isolated protein and pectin from pumpkin waste was compared with some other protein and pectin based film (Table 4). The solubility of protein film (F1) is comparatively much higher than the film developed from other protein sources. This may be due to different proportion of plasticizer used by other authors. Whereas, in case of pectin based film (F3), the solubility was less in comparison with pomelo pectin and pure pectin based film. The solubility of film had an inverse relation with elongation of film. The elongation was much more in case of pea protein based film. The elongation of pectin film is in line with values for pomelo peel pectin and pure pectin based film. The strength of all developed film is comparable with reported film strength of other plant materials. The tensile strength was higher than pumpkin oil cake protein isolate and pea protein based films. However, greater strength is reported for lentil protein, mung bean protein and soy protein based film. Overall the developed films are comparable with other protein and pectin based films.
Table 4.
Comparison of some properties of isolated protein–pectin based film with other films
| Properties | F1 (100:0) Protein:Pectin |
F2 (50:50) Protein:Pectin |
F3 (0:100) Protein:Pectin |
PuOC proteinA | Pea proteinB | Lentil proteinC | Mung bean proteinD | Soy proteinE | Pomelo peel pectinF | Pure pectinG |
|---|---|---|---|---|---|---|---|---|---|---|
| Solubility (%) | 50.76 ± 0.17a | 64.98 ± 0.92c | 61.31 ± 0.12b | 22.7–89.52 | 43.5 ± 4.0 | 38.75 ± 3.2 | 37.53–39.43 | 24.3 ± 0.13 | 100 ± 0.00 | 75.64 ± 2.56 |
| Tensile strength (MPa) | 2.04 ± 0.05a | 2.52 ± 0.02b | 5.28 ± 0.04c | 0.86–6.56 | 0.69 ± 0.07 | 4.24 ± 1.26 | 5.70–6.51 | 4.6 ± 0.2 | 159.367 g | 1.35 ± 0.21 |
| Elongation (%) | 13.13 ± 0.32a | 13.84 ± 0.24b | 14.37 ± 0.36c | 22.2–196.61 | 92.0 ± 21.5 | 58.22 ± 12.88 | 32.06–40.08 | 14.8 ± 1.7 | 10.80 ± 1.91 | 11.25 ± 1.24 |
F1, F2, F3 are film developed from isolated protein and pectin from pumpkin waste
APumpkin oil cake protein isolate films (Popović et al. 2012)
BPea-protein based edible films (Choi and Han 2001)
CLentil Protein based film (Bamdad et al. 2006)
DMung bean proteins based film (Bourtoom 2008)
ESoya protein based film (Soliman et al. 2007)
FPomelo peel pectin based film (Das et al. 2018)
GPur pectin based film (Saikia and Badwaik 2018)
Mean followed by letters a, b and c in the same column differs significantly (p ≤ 0.05)
Conclusion
Pumpkin processing wastes were explored for extracting important components useful in food products and food packaging. Protein and pectin were extracted successfully from pumpkin seed and pumpkin peel respectively. The extraction of protein and pectin was affected by process variables. The temperature has the significant effect on yield of protein and pectin followed by pH and time. The optimized process variables for protein extraction were 32.70 °C, 16.06 min, pH of 9.51 and yield at these optimized conditions was 70.31 ± 2.32%. However, for pectin extraction optimized conditions were 89.98 °C, 13 min, pH of 2.85 and yield was reported as 69.89 ± 2.90%. The methodology adopted for extraction is effective and experimental results were consistent to objectives underlined. The extracted protein and pectin was successfully used for film development. The mechanical and barrier properties of all films were good. However, solubility of film needs to be improved for acceptability. Film can also be casted with different proportion of protein and pectin for better understanding the findings. Such film can be used for application on food products like cut fruits, bakery products and sweets etc.
Acknowledgements
The equipment funded by Indian Council of Agricultural Research (ICAR), New Delhi (Agri. Engg. 27(23)/2015-AE) were helpful for carrying out this research. All authors are thankful to ICAR for proving this facility. Again authors are thankful to AICTE-NEQIP, DST-FIST and UGC-SAP for financial support for carrying out the work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bamdad F, Goli AH, Kadivar M. Preparation and characterization of proteinous film from lentil (Lens culinaris): edible film from lentil (Lens culinaris) Food Res Int. 2006;39:106–111. doi: 10.1016/j.foodres.2005.06.006. [DOI] [Google Scholar]
- Baron RD, Pérez LL, Salcedo JM, Córdoba LP, do Amaral Sobral PJ. Production and characterization of films based on blends of chitosan from blue crab (Callinectes sapidus) waste and pectin from Orange (Citrus sinensis Osbeck) peel. Int J Biol Macromol. 2017;98:676–683. doi: 10.1016/j.ijbiomac.2017.02.004. [DOI] [PubMed] [Google Scholar]
- Borah PP, Das P, Badwaik LS. Ultrasound treated potato peel and sweet lime pomace based biopolymer film development. Ultrason Sonochem. 2017;36:11–19. doi: 10.1016/j.ultsonch.2016.11.010. [DOI] [PubMed] [Google Scholar]
- Bourtoom T. Factors affecting the properties of edible film prepared from mung bean proteins. Int Food Res J. 2008;15:167–180. [Google Scholar]
- Bucko S, Katona J, Popovic L, Vastag Z, Petroviv L. Functional properties of pumpkin (Cucurbita pepo) seed protein isolate and hydrolysate. J Serb Chem Soc. 2016;81:35–46. doi: 10.2298/JSC150615081B. [DOI] [Google Scholar]
- Chaichi M, Hashemi M, Badii F, Mohammadi A. Preparation and characterization of a novel bionanocomposite edible film based on pectin and crystalline nanocellulose. Carbohydr Polym. 2017;157:167–175. doi: 10.1016/j.carbpol.2016.09.062. [DOI] [PubMed] [Google Scholar]
- Chinma CE, Ariahu CC, Alakali JS. Effect of temperature and relative humidity on the water vapour permeability and mechanical properties of cassava starch and soy protein concentrate based edible films. J Food Sci Technol. 2015;52:2380–2386. doi: 10.1007/s13197-013-1227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WS, Han JH. Physical and mechanical properties of pea-protein-based edible films. J Food Sci. 2001;66:319–322. doi: 10.1111/j.1365-2621.2001.tb11339.x. [DOI] [Google Scholar]
- Das P, Borah PP, Badwaik LS. Transformation of chicken feather keratin and pomelo peel pectin into biodegradable composite film. J Polym Environ. 2018;26:2120–2129. doi: 10.1007/s10924-017-1109-z. [DOI] [Google Scholar]
- Gounga ME, Xu SY, Wang Z. Whey protein isolate-based edible films as affected by protein concentration, glycerol ratio and pullulan addition in film formation. J Food Eng. 2007;83:521–530. doi: 10.1016/j.jfoodeng.2007.04.008. [DOI] [Google Scholar]
- Gulfi M, Arrigoni E, Amadò R. Influence of structure on in vitro fermentability of commercial pectins and partially hydrolysed pectin preparations. Carbohydr Polym. 2005;59(2):247–255. doi: 10.1016/j.carbpol.2004.09.018. [DOI] [Google Scholar]
- Hou F, Ding W, Qu W, Oladejo AO, Xiong F, Zhang W, Ma H. Alkali solution extraction of rice residue protein isolates: influence of alkali concentration on protein functional, structural properties and lysinoalanine formation. Food Chem. 2017;218:207–215. doi: 10.1016/j.foodchem.2016.09.064. [DOI] [PubMed] [Google Scholar]
- Jafari F, Khodaiyan F, Kiani H, Hosseini SS. Pectin from carrot pomace: optimization of extraction and physicochemical properties. Carbohydr Polym. 2017;157:1315–1322. doi: 10.1016/j.carbpol.2016.11.013. [DOI] [PubMed] [Google Scholar]
- Jouki M, Khazaei N, Ghasemlou M, HadiNezhad M. Effect of glycerol concentration on edible film production from cress seed carbohydrate gum. Carbohydr Polym. 2013;96:39–46. doi: 10.1016/j.carbpol.2013.03.077. [DOI] [PubMed] [Google Scholar]
- Jun HI, Lee CH, Song GS, Kim YS. Characterization of the pectic polysaccharides from pumpkin peel. LWT Food Sci Technol. 2006;39:554–561. doi: 10.1016/j.lwt.2005.03.004. [DOI] [Google Scholar]
- Kanagaraj S, Varanda FR, Zhil’tsova TV, Oliveira MS, Simões JA. Mechanical properties of high density polyethylene/carbon nanotube composites. Compos Sci Technol. 2007;67:3071–3077. doi: 10.1016/j.compscitech.2007.04.024. [DOI] [Google Scholar]
- Kanmani P, Dhivya E, Aravind J, Kumaresan K. Extraction and analysis of pectin from citrus peels: augmenting the yield from Citrus limon using statistical experimental design. Iran J Energy Environ. 2014;5:303–312. [Google Scholar]
- Kim MY, Kim EJ, Kim YN, Choi C, Lee BH. Comparison of the chemical compositions and nutritive values of various pumpkin (Cucurbitaceae) species and parts. Nutr Res Pract. 2012;6:21–27. doi: 10.4162/nrp.2012.6.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latimer G. Official methods of analysis of AOAC International. 20. Maryland: P.imprenta; 2016. [Google Scholar]
- Lee JH, Lee JH, Yang HJ, Song KB. Preparation and characterization of brewer’s spent grain protein-chitosan composite films. J Food Sci Technol. 2015;52:7549–7555. doi: 10.1007/s13197-015-1941-x. [DOI] [Google Scholar]
- Lv C, Jia X, Li M, Yang J, Zhao G. Optimization of extraction process of crude protein from grape seeds by RSM. Food Sci Technol Res. 2011;17:437–445. doi: 10.3136/fstr.17.437. [DOI] [Google Scholar]
- Ma Q, Hu D, Wang H, Wang L. Tara gum edible film incorporated with oleic acid. Food Hydrocoll. 2016;56:127–133. doi: 10.1016/j.foodhyd.2015.11.033. [DOI] [Google Scholar]
- Maran JP, Priya B. Ultrasound-assisted extraction of pectin from sisal waste. Carbohydr Polym. 2015;115:732–738. doi: 10.1016/j.carbpol.2014.07.058. [DOI] [PubMed] [Google Scholar]
- Oliveira TÍS, Rosa MF, Ridout MJ, Cross K, Brito ES, Silva LM, Mazzetto SE, Waldron KW, Azeredo HM. Bionanocomposite films based on polysaccharides from banana peels. Int J Biol Macromol. 2017;101:1–8. doi: 10.1016/j.ijbiomac.2017.03.068. [DOI] [PubMed] [Google Scholar]
- Osman MK, Simon-Sarkadi L. Extraction and isolation of protein from lupine (Lupinus termis L.) seeds. Period Polytech Chem Eng. 1991;35:65. [Google Scholar]
- Pavlath AE, Orts W. Edible films and coatings for food applications. New York, NY: Springer; 2009. Edible films and coatings: why, what, and how? pp. 1–23. [Google Scholar]
- Pereira PHF, Oliveira TÍS, Rosa MF, Cavalcante FL, Moates GK, Wellner N, Azeredo HM. Pectin extraction from pomegranate peels with citric acid. Int J Biol Macromol. 2016;88:373–379. doi: 10.1016/j.ijbiomac.2016.03.074. [DOI] [PubMed] [Google Scholar]
- Pongjanta J, Naulbunrang A, Kawngdang S, Manon T, Thepjaikat T. Utilization of pumpkin powder in bakery products. Songklanakarin J Sci Technol. 2006;28:71–79. [Google Scholar]
- Popović S, Peričin D, Vaštag Ž, Lazić V, Popović L. Pumpkin oil cake protein isolate films as potential gas barrier coating. J Food Eng. 2012;110:374–379. doi: 10.1016/j.jfoodeng.2011.12.035. [DOI] [Google Scholar]
- Quanhong L, Caili F. Application of response surface methodology for extraction optimization of germinated pumpkin seeds protein. Food Chem. 2005;92:701–706. doi: 10.1016/j.foodchem.2004.08.042. [DOI] [Google Scholar]
- Ranganna S. Handbook of analysis and quality control for fruit and vegetable products. New York: Tata McGraw-Hill Education; 1986. pp. 40–42. [Google Scholar]
- Rustom I, Ópez-leiva MHL, Nair BM. Optimization of extraction of peanut proteins with water by response surface methodology. J Food Sci. 1991;56(6):1660–1663. doi: 10.1111/j.1365-2621.1991.tb08665.x. [DOI] [Google Scholar]
- Saikia M, Badwaik LS. Characterization and antimicrobial property of casein, gelatin and pectin based active composite films. J Package Technol Res. 2018;2:233–242. doi: 10.1007/s41783-018-0044-3. [DOI] [Google Scholar]
- Soliman EA, Tawfik MS, El-Sayed H, Moharram YG. Preparation and characterization of soy protein based edible/biodegradable films. Am J Food Technol. 2007;2:462–476. doi: 10.3923/ajft.2007.462.476. [DOI] [Google Scholar]
- Wang HX, Ng TB. Isolation of cucurmoschin, a novel antifungal peptide abundant in arginine, glutamate and glycine residues from black pumpkin seeds. Peptides. 2003;24:969–972. doi: 10.1016/S0196-9781(03)00191-8. [DOI] [PubMed] [Google Scholar]
- Wani AA, Kaur D, Ahmed I, Sogi DS. Extraction optimization of watermelon seed protein using response surface methodology. LWT Food Sci Technol. 2008;41:1514–1520. doi: 10.1016/j.lwt.2007.10.001. [DOI] [Google Scholar]