Abstract
Betalains are pigments that have properties that benefit health, such as antioxidant, anticancer, and antimicrobial activity, and they also possess a high ability to provide color. However, these pigments, although used as colorants in certain foods, have not been able to be potentialized to diverse areas such as pharmacology, due to their instability to physicochemical factors such as high temperature, pH changes and high water activity. For this reason, different stabilization methods have been reported. The method that has presented best results for diversifying the use of betalains has been encapsulation. Encapsulation is a method of entrapment where the objective is to protect a compound utilizing more stable matrices from encapsulation technologies. This method has been employed to provide greater stability to betalains, using different matrices and encapsulation technologies. However, a review does not exist, to our knowledge, which analyzes the effect of matrices and encapsulation technologies on betalains stabilization. Therefore, the objective of this review article was to evaluate the different matrices and encapsulation techniques that have been employed to stabilize betalains, in order to arrive at specific conclusions concerning the effect of encapsulation on their stabilization and to propose new techniques and matrices that could promote their stabilization.
Keywords: Betalains, Encapsulation, Matrix, Stabilization, Effects
Introduction
Betalains are pigments found in fruits, flowers, roots, and leaves of plants (Gengatharan et al. 2015). They are synthesized from the amino acid tyrosine into two structural groups, betacyanins, and betaxanthins, it being the nature of the additional residue that determines the classification of the pigment (Gandía-Herrero and García-Carmona 2013). Betalains have interesting properties, such as high water solubility and color intensity, and they possess antioxidant and anticancer effects (Stintzing and Carle 2007).
However, one of the main problems that limit the application of betalains is their instability (Castellar et al. 2003). Due to the latter, investigations of betalains have focused on searching of ways to stabilize them and to increase their applications. Some of the stabilization methods that have been studied include the addition of antioxidants, chelating agents, and encapsulation (Herbach et al. 2006a, b; Azeredo 2009).
In recent years, encapsulation has been studied as a promising method for stabilizing betalains using different matrices (polysaccharides, proteins, and carbohydrates) and techniques (spray-drying, emulsions, ionic gelation, etc.) (Chong et al. 2014; Kaimainen et al. 2015a, b; Otálora et al. 2016). Encapsulation can be defined as a method of entrapment in which a compound is encapsulated by a more stable physical barrier, the compound localized in the center of the barrier that protects it. The encapsulated compound may be called core, active phase, or load; while the encapsulating agent usually is termed matrix, membrane, wall, coating, or capsule (Nedovic et al. 2011). Some researchers have evaluated the effect of different matrices and encapsulation techniques in the stabilization of betalains, allowing compilation of this information and reaching objective conclusions. The objective of this review was to propose best method of betalains encapsulation for their stabilization, evaluating the different matrices and encapsulation techniques that have been utilized, and to reach specific conclusions on the effect of encapsulation in the stabilization of betalains.
Overview and betalain properties
Betalains are hydrophilic vacuolar pigments that accumulate in the leaves, roots, stems, fruits, flowers, bracts, petioles, and seeds of plants of the order Caryophyllales. The only exception to this order are the families Caryophyllaceae and Molluginaceae, here the color is due to anthocyanins (Gandía-Herrero and García-Carmona 2013). Betalains in the 1950s were denominated nitrogenous anthocyanins; it was not until years later, that evidence that they comprised a set of different pigments from those of the anthocyanins was provided (Piattelli et al. 1964; Miller et al. 1968; Impellizzeri and Piattelli 1972). It was then that Mabry and Dreiding in (1968) coined the term “betalains”.
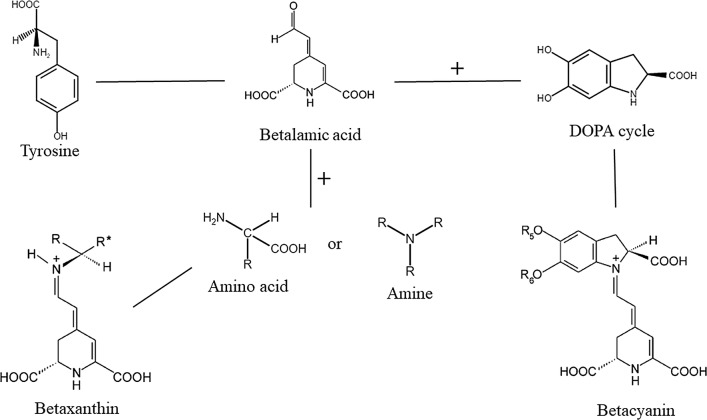
Betalains are naturally synthesized from the amino acid tyrosine into two principal structural groups: betacyanins and betaxanthins, betalamic acid their biosynthetic precursor. Betacyanins exhibit a red–violet color with an absorbance spectrum (λmax) of 541 ± 9 nm, and their basic structure consists of betalamic-acid condensation with the DihydrOxyPhenylAlanine cycle (the DOPA cycle). Additionally, betaxanthins show a yellow–orange color with an absorbance spectrum (λmax) of 471.5 ± 13.5 nm and their structure consists of the conjugation of betalamic acid with amino acids or amines (Fig. 1) (Strack et al. 2003; Khan et al. 2015). To date, about 70 betalains have been identified in nature, comprising approximately 50 betacyanins and 20 betaxanthins. They can be found in fruits and seeds such as beet (Beta vulgaris), amaranth (Amaranthus spp.), pear (Opuntia spp.), and pitaya (Stenocereus spp.), among others (Cai et al. 2005; Stintzing and Carle 2007).
Fig. 1.
Schematic diagram of the route to obtaining of betacyanins and betaxanthins
Investigations on betalains have been few, to our knowledge, compared with anthocyanins. This is due to their limited presence in nature. However, the study of betalains has increased over the past 15 years, generating more knowledge on their biosynthesis, structures, and properties. The importance of research on betalains lies mainly in their properties, such as water solubility, color intensity, and their antioxidant, antibacterial, and anticancer properties (Stintzing and Carle 2007; Cejudo-Bastante et al. 2014). Therefore, researchers in medicine and food areas have shown interest in studying betalains and in their potential use.
Betalain properties in food
At present, with the advancement of the study of betalains, their possible applications have been diversified. One of the main applications is their use as natural dyes. The use of natural dyes in food has grown, due to the alternative that it represent to the use of artificial dyes, which present harmful effects to health. Betalains are the most natural dyes used in food (Obón et al. 2009). The main source of betalains is red beets; however, the disadvantage of using betalains extracted from red beet is the earthy smell due to geosmin and pyrazines, which are undesirable when applied in food at high concentrations (Stintzing et al. 2000). Thus, novel sources of betalains are necessary for their application in foods. Some sources of betalains, which have encouraged food scientists to study them from a technological, and nutritional point of view include amaranth and cactus fruits, revealing great potential for employment as natural dyes (Cai et al. 2003; Moßhammer et al. 2005; Robert et al. 2015).
Another important contribution of betalains has been in the development of the packaging of food with natural polymers. These provide properties to food packaging, such as color, antioxidant capacity, stability in terms of photodegradation, and greater flexibility (Akhtar et al. 2012; Gutiérrez et al. 2016). Therefore, betalains, in addition to being used as dyes can favor the physicochemical properties of food packaging and provide added value. The relevance of betalains in foods and their properties has increased the interest of scientists to continue looking for ways to incorporate them into food and thus promote human health (Choo 2017).
Biological properties of betalains
Betalains present properties that promote health, primarily for their antimicrobial antioxidant, activity and anticancer (Vulić et al. 2013; Gandía-Herrero et al. 2016; Khan 2016a, b; Belhadj et al. 2017; Miguel 2018; Yong et al. 2018). Betalains show strong antioxidant activity, up to 7 times higher than vitamin C which is a very effective natural antioxidant, and up to 3–4 times higher than the ascorbic acid and catechin (Cai et al. 2003). The antioxidant activity of betalains has been studied from various sources such as fruits, leaves, flowers, and seeds. However, not all sources of betalains present the same antioxidant activity due, to the number and position of hydroxyl/imino groups and of the aglycones glycosylation in the structure of betalains (Cai et al. 2003).
Betalains due to their effect actively against free radicals can prevent the onset of cancer. The literature has reported its antiproliferative effect on human colon carcinoma, showing that betalains can stop and efficiently inhibit the cell cycle of cancer cell (Serra et al. 2013). Also, they have been tested in ovarian cancer cells, immortalized cells of cervical epithelium and cervical cancer cells, presenting a significant inhibitory effect of 40–60% (Zou et al. 2005). Another less studied property is the antimicrobial effect of betalains. It has been reported that betalains exhibit antimalarial properties, being capable of chelating the essential interior cations and transport block intracellular parasites (Hilou et al. 2006). Also, betalains have shown inhibitory effects against Gram-negative bacteria such as Pseudomonas aeruginosa and Salmonella typhimurium, among others, and Gram-positive bacteria, such as Staphylococcus aureus, Enterococcus faecalis, and Listeria monocytogenes (Čanadanović-Brunet et al. 2011; Tenore et al. 2012). Although it is known that betalains show antimicrobial effects, the literature has reported very little about its mechanism of inhibition, therefore, it can be a topic of opportunity for future research.
Despite the interesting food and medical properties that betalains exhibit, one of the main constraints that prevent their potential use has been their instability. Therefore, in recent years several studies have been search ways to stabilize them, and to increase their commercial applications taking advantage of benefits.
Betalain stability
Several factors affect the stability of betalains and have to be considered to ensure their properties. The stability of betalains is influenced by the presence of high temperature, pH < 3 or > 7, light, oxygen, and high water activity (StStintzing and Carle 2007; Cejudo-Bastante et al. 2014). In addition, it has been observed that the structure is also important in the stability of betalains. For example, the betacyanin and betaxanthin structural groups show different stabilities. Betacyanins exhibit higher temperature stability, acidic pH and are less prone to oxidation than betaxanthins, but betaxanthins show higher stability at pH 7, and hydrolytic enzymes. The higher stability of betacyanins about betaxanthins may be due, to the fact that some of them have a glycosylated structure, which has a high oxidation–reduction potential (Herbach et al. 2006a, b; Azeredo 2009).
The physicochemical factors that compromise the stability of betalains, on their own, do not greatly influence their degradation, except for water activity, which is an important factor due to hydrolytic reactions. Temperature, oxygen, light, and pH have been shown to act in synergy, favoring further degradation of betalains (Fig. 2) (Azeredo 2009; Reshmi et al. 2012).
Fig. 2.
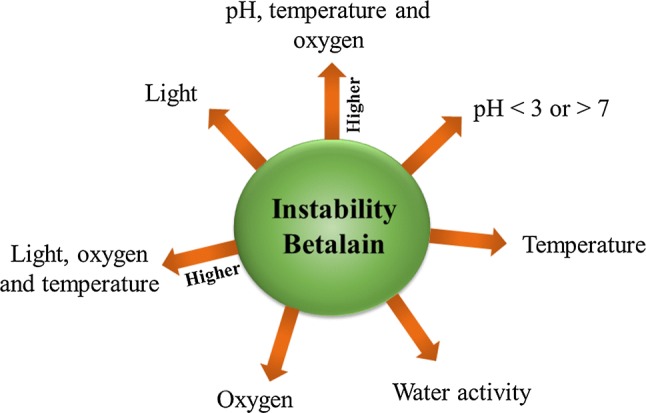
Physicochemical factors, alone and in combination, which provide instability to betalains
Due to the easy degradation of betalains, the objective-of-study in recent years has been based on finding ways to stabilize them in order to increase their commercial applications and to take advantage of their benefits. The literature has reported the use of additives, such as antioxidants, chelating agents and, in recent years, encapsulation, which represents a promising method for the stabilization of betalains.
Extraction methods of betalains
The extraction of betalains is the first step for its study, therefore, it is an important and crucial step in the final result. In the extraction of betalains, conventional and non-conventional methods have been used (Barba et al. 2017). The conventional methods are those that use solvents with and without thermal treatment such as maceration, hydrodistillation, and Soxhlet, while non-conventional methods are modern and ecological technologies with low energy consumption, some of these promising techniques are ultrasound-assisted extraction, microwave-assisted extraction, supercritical fluid assisted extraction, pulsed electric field assisted extraction and pressurized liquid extraction (Azmir et al. 2013; Soquetta et al. 2018). Conventional methods have been the most used in the extraction of betalains due they are simple methods and do not require sophisticated equipment, however, the extraction time is very long with low yields, and use high temperatures can degrade betalains (Tiwari and Cullen 2013; Celli and Brooks 2017). That is why in recent years, betalains extraction processes have been optimized using non-conventional methods. Laqui-Vilca et al. (2018) optimized the extraction of betalains from the quinoa husk by the ultrasound method and compared it with the maceration method, where they obtained by ultrasound 96.477 mg of betacyanins/100 g of fresh sample (FS) in 9.2 s and betaxanthins 201.01 mg/100 g of FS in 40 s, while in extraction by maceration, 30 min were required at room temperature to reach similar yields, demonstrating that the unconventional method was better for the extraction of betalains. Koubaa et al. (2016) they evaluated the potential of the previous treatments of ultrasounds and pulsed electric field to improve the extraction of betalains from the rind and pulp of the prickly pear, they performed a supplemental aqueous extraction. The results showed that the treatments used significantly improved the extraction of betalains, concluding that the pulsed electric field induces the permeability of the cellular wall without disintegrating the cellular tissue, which facilitates the recovery of intracellular compounds. Roriz et al. (2017) optimized the extraction of betacyanins from Gomphrena globose L. using microwave-assisted extraction and ultrasound where they reported a higher extraction yield using ultrasound with 46.9 ± 4.8 mg/g, validating that the ultrasound method is more suitable to obtain betacyanins from Gomphrena globose L. Thirugnanasambandham and Sivakumar (2017) optimized the extraction of betalains from the rind of the dragon fruit by the microwave method obtaining 9 mg/L of betalains in 8 min at 35 °C, they conclude that the result obtained shows the effectiveness of the microwave process in the extraction of betalains.
Some of the advantages and disadvantages of using these methods can be seen in Table 1, where the main disadvantage presented by conventional with respect to unconventional is time, temperature and the use of solvents. However, based on the above we can mention that although conventional methods may have limitations in the extraction of betalains, they are methods that can be combined with non-conventional methods to improve the extraction process without affecting the natural properties of betalains.
Table 1.
Advantages and disadvantages of conventional and non-conventional extraction methods
| Methods | Advantages | Disadvantages |
|---|---|---|
| Conventional | ||
| Maceration | Simple method and low cost | High extraction time, low yields and use of organic solvents |
| Soxhlet | Simple method | High energy consumption and use of organic solvents |
| Hydrodistillation | Organic solvents are not required | Hgh temperatures, high energy consumption and high extraction time |
| Non-conventional | ||
| Supercritical fluid | Extraction is rapid, selective and does not require additional cleaning | Temperature changes and specialized equipment |
| Pressurized liquid | Rapid extraction and reduced consumption of solvents | High temperatures and pressure |
| Ultrasound assisted | Low extraction time, low energy consumption and high yields | Use of organic solvents |
| Microwave assisted | It can be done with or without the addition of solvents | Specialized equipment |
| Pulsed electric field | Low extraction time and high yields | Use of solvents and energy consumption |
Stabilization of betalains
One of the different ways of stabilizing betalains is with the use of antioxidant compounds, especially ascorbic acid and isoascorbic acid. These antioxidants have demonstrated beneficial effects in crude extracts of betalains, such as in purified betalains (Khan and Giridhar 2014). Isoascorbic acid has exhibited greater potential in stabilizing betalains than ascorbic acid, due to its greater redox potential (Azeredo 2009). However, more study is required on the optimal amounts of ascorbic acid or isoascorbic acid for betalain stabilization, since there are discrepancies. Chelating agents have also shown a stabilizing effect on betalains. These act by partially neutralizing the electrophilic center (positively charged N) present in the structure of the betalains. Citric acid and EDTA (EthyleneDiamineTetraacetic Acid) are chelating agents that have been utilized to improve the stability of betalains, avoiding their oxidation and increasing their useful life (Herbach et al. 2006a, b). However, the use of citric acid at high concentrations can affect sensory characteristics in foods. Other methods of stabilization of betalains less studied and with great opportunity to develop new research can be seen in Table 2.
Table 2.
Other betalain stabilization methods: advantages and disadvantages
| Methods | Observations | Advantages/disadvantages | References |
|---|---|---|---|
| Complex formation (inorganic matrices such as γ-alumina) | Stabilize the pigment for more than twenty months | High stability / lower solubility and possible aggregate formation | Pérez-Ramírez et al. (2015), Lima et al. (2009), Molina et al. (2014) |
| Copigmentation (metals, phenols, alkaloids, and organic acids) | Promotes color retention for up to 6 months at 5 °C | Increase color intensity and stability / Low stability in purified pigments | Khan et al. (2015), Khan and Giridhar (2014), Trouillas et al. (2016) |
Another of the main methods for stabilizing betalains is encapsulation. Encapsulation has been a subject of investigation in recent years to stabilize, improve bioavailability, and facilitate the administration of betalains (Khan 2016a, b). One of the most important reasons for the use of encapsulation in betalains is to provide greater stability during processing and final products (Nedovic et al. 2011). Encapsulated betalains may present a storage stability of up to 6 months, stability that could not be obtained with other methods (Gandía-Herrero et al. 2013). This is due to the matrix that protects them from physicochemical factors that accelerate their degradation. Another advantage of encapsulation of betalains is that these latter can be added to liquid or solid substances without their degradation (Ravichandran et al. 2014; Pitalúa et al. 2010). Therefore, stability of betalains deriving from encapsulation comprises one of the most important objectives in their study today. However, the effect of the different encapsulation techniques and matrix types, which may be important factors in determining greater or less betalains stability, is also important.
Encapsulation of betalains
Encapsulation is a method of trapping solid, liquid, and gaseous materials in small capsules that can be of nanometric or micrometric size (Fang and Bhandari 2010). The contents of the capsule are isolated from the environment and their contents can be released at controlled rates for prolonged time periods by means of the action of pH, enzymes, temperature, among others (Augustin and Hemar 2009; Gouin 2004). This method is of interest to the pharmaceutical sector with respect to the release of drugs and vaccines. It is also of interest the food industry with respect to the addition of functional ingredients such as antioxidants, antimicrobials, and for controlling taste, texture, and color (Champagne and Fustier 2007; Vinceković et al. 2017).
Encapsulation is based on the protection of compounds sensitive to external factors, covering them with a more stable matrix such as polysaccharides, lipids, and proteins. These so-called core–shell systems are formed from the protective compound termed the core, and a shell or matrix that covers the compound (Fig. 3) (Janiszewska 2014).
Fig. 3.
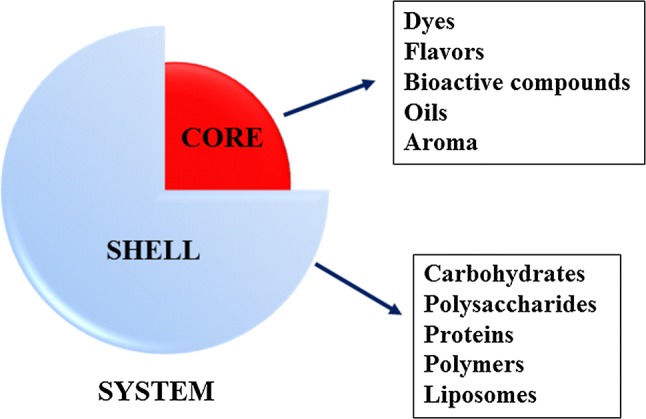
Schematic of the core–shell system with examples
Encapsulation is currently used to stabilize betalains. In the literature, several encapsulation and matrix technologies have been reported that have been used in the encapsulation of betalains. However, to our knowledge, there is not a review work that analyzes together the effect of matrices and encapsulation technologies on the stability of betalains.
Effect of the matrix and encapsulation technology on the stability of betalains
There are several factors that can contribute to the degradation of betalains into an encapsulated form. The main factors include the type of matrix, the technology of encapsulation, and the porosity of the matrix. This is because they are principally exposed to physicochemical factors mainly oxygen, that can easily enter the matrix and affect the betalains (Serris and Biliaderis 2001). The matrix is a physical barrier that is also frequently called a coating, capsule, membrane, or barrier (Fang and Bhandari 2010). These may be composed of carbohydrates, polysaccharides, lipids, proteins, and synthetic polymers, or may perhaps even be in combination, improving their barrier properties. Selection of the matrix is very important, since on it will depend the final application of the encapsulated compound and an efficient result (Chranioti et al. 2015). Currently, several matrices have been employed to encapsulate betalains as polysaccharides, proteins or in combination with polysaccharides. The reasons for this may be due to their ability to protect betalains from oxidation and the high solubility of the majority of polysaccharides (Gandía-Herrero et al. 2013; Robert et al. 2015; Chranioti et al. 2015; Otálora et al. 2016).
Maltodextrin is a polysaccharide obtained from the hydrolysis of starch. This polysaccharide is that which is most used as matrix to encapsulate betalains. In the literature, it has been reported that the use of maltodextrin, as an encapsulation matrix, increases the stability of betalains (Gandía-Herrero et al. 2013). Therefore, maltodextrin has been used alone and in combination with other polysaccharides. Ravichandran et al. (2014) reported the encapsulation of betalains from red beet. These authors employed maltodextrin and its combination with guar gum, gum Arabic, xanthan gum, and pectin. They found that encapsulation of betalains with maltodextrin and its combination with other polysaccharides increases their stability. Janiszewska et al. (2014) encapsulated beet juice employed maltodextrin and gum Arabic as matrix. These authors investigated the effect of the matrix on the stability of the pigments, demonstrating that gum Arabic possesses higher adsorption of water than maltodextrin, observing that the gum Arabic matrix degrades faster, exposing the pigment. These results are in agreement with the results obtained by Pitalua et al. (2010). These authors encapsulated betalains in gum Arabic by evaluating the matrix at different values of water activity, finding that the matrix dissolves in a short time period when it is exposed to high water activity. However, it has been found that when maltodextrin and gum Arabic are combined, they maintained their structural integrity in the presence of water, revealing their effectiveness for the encapsulation of pigments (Chranioti et al. 2015). Maltodextrin has also been combined with proteins. Castro-Muñoz et al. (2015) encapsulated tuna fruit juice utilizing a gelatin matrix, maltodextrin and a combination of gelatin/maltodextrin. The authors reported high hygroscopicity in the matrix; maltodextrin has a high capacity to absorb water of 72–83 g/100 g. Therefore, they mentioned that the properties of the matrix depend on the gelatin: maltodextrin ratio. These authors concluded that the compound can be used in food compounds. Robert et al. (2015) encapsulated betalains using soy proteins combined with maltodextrin or inulin, observing lower beta-lactam degradation when the matrix comprised a protein-polysaccharide. The maltodextrin-protein matrix was that with lower beta-lactam degradation compared with protein-inulin. In terms of this difference in betalain stability with respect to polysaccharide type the authors attributed this to the structural differences between both polysaccharides. In the literature, different betalain encapsulation technologies, such as spray-drying, freeze-drying, ionic gelation, and emulsions, have been reported (Vergara et al. 2014; Kaimainen et al. 2015a, b; Chranioti et al. 2015; Otálora et al. 2016). The most used technology for encapsulation of betalains is spray-drying, due to its low cost, simple processing, and its rapid obtaining of encapsulated betalains. However, the main disadvantage of spray-drying is that the matrix has to be soluble in solvents that can easily evaporate with temperature and that, due to the use of high temperatures, some bioactive compounds may degrade. Ravichandran et al. (2014) studied the effect of different matrices in the encapsulation of betalains by spray-drying and freeze-drying. These authors concluded that with the freeze-drying technique, higher loading and stabilization efficiency of betalains was obtained than with spray-drying. In addition, they mentioned that guar gum can be employed to encapsulate betalains with the freeze-drying technique, but that this is not suitable for spray-drying. There are other authors who also showed the limitations of the spray-drying technique (Gandía-Herrero et al. 2013). These authors encapsulated betalains in a matrix of maltodextrin and chitosan, revealing problems for the chitosan processing in this technique due to its viscosity and low solubility. Chranioti et al. (2015) reported that the dye of beet and saffron was encapsulated in matrices of maltodextrin, gum Arabic, modified starch, chitosan, and in combination, concluding that the technique is suitable for color stabilization. In Table 3 we can see an updated summary of the main encapsulation methods, matrices used and their effects on betalains stability.
Table 3.
Main techniques and betalains encapsulation matrices that have promoted its stabilization
| Encapsulation techniques | Matrices | Source of betalains /extraction method | Encapsulation efficiency | Stability after encapsulation | Applications | References |
|---|---|---|---|---|---|---|
| Hydration-sonication | Lecithin (nanoliposomes) | Betanin (red beet extract diluted with dextrin)/commercial | 80.35% | Improved the in vitro digestion stability of betanin | Diabetes treatment | Amjadi et al. (2019) |
| Spray dried | Cactus mucilage | Pulp and skin of Escontria chiotilla and Stenocereus queretaroensis /magnetic stirring and maceration | Information not presented | Betalains retention was more than 90% after three months of storage | Food coloring | Delia et al. (2019) |
| Ionic gelation | Calcium alginate | Betalain-rich extract of Opuntia ficus-indica fruit/maceration | Information not presented | After 30 days, the sample colour change | Natural colorant in gummy Candies | Otálora et al. (2019) |
| Gelation | Sodium alginate | Bougainvillea bracts (Bougainvillea spectabilis)/maceration | 88.63–89.82% | The encapsulation is efficient to protect and release the bioactive compounds from bougainvillea extracts | Bioactive pigments for applications in food | Orozco et al. (2019) |
| Ionotropic gelation | Ca(II)-alginate with sucrose and dextran | Stems/leaves of Beta vulgaris/maceration | 15–60% (depends on the formulation) | Good conservation of the antioxidant activity (up to 70%) | Antioxidant | Calvo et al. (2018) |
| Spray drying | Modified potato starch and commercial starch | Fruit of pitaya (Stenocereus pruinosus)/maceration | Information not presented | Modified starch-based microcapsules showed better potential pigmentation and greater stability during storage for 32 days at 4 °C in yogurt with pH 4.6 than commercial starch-based microcapsules | Pigmenting agent of yogurt | Vargas et al. (2018) |
| Hydration-sonication | Lecithin (nanoliposomes) | Betanin/commercial | 80.35% | Betanin stability decreases by approximately 10% for 60 days of storage | Gummy candies as a food model | Amjadi et al. (2018) |
| Spray dried | Maltodextrin, inulin, and whey protein isolate | Beetroot (Beta vulgaris L.)/juice was obtained with a food processing centrifuge | Information not presented | The use of whey protein isolate together with inulin achieved high stability | Food coloring | Do Carmo et al. (2018) |
| Spray drying and ionic gelation | maltodextrin-cactus cladode mucilage and sodium alginate | Orange pulp fruits Opuntia megacantha/maceration | Information not presented | Betaxanthins encapsulated with maltodextrin-cactus cladode mucilage by spray drying were more stable at 18 °C and 57% RH for 62 days | Natural colorant | Otálora et al. (2018) |
| Microchannel emulsification | Soybean oil | E162, red beetroots (Beta vulgaris, subsp. vulgaris) and Fresh beetroot juice/commercial, mechanic, mechanic | Information not presented | Betanin from different sources encapsulated in W/O/W emulsion showed to be temperature sensitive | Information not presented | Pagano et al. (2018) |
| Spray drying | Maltodextrin | Quinoa (Chenopodium quinoa Willd.)/mechanic | 100% | The oxygen consumption of the microparticles with betacyanin was higher when the temperature increased (80–90 °C) accompanied by a decrease in color intensity causing pigment degradation | Information not presented | Aguilar et al. (2018) |
| Freeze drying | Gum arabic and maltodextrin | Air parts of S. fruticosa | 86.50–92.30% | After the 8 weeks storage to 60 °C, 25.87% of the betalains was lost | Information not presented | Mohamed et al. (2018) |
| Freeze dried and spray dried | Combination of maltodextrin and xanthan gum | Red beetroot (Beta vulgaris L.)/mechanic | Information not presented | Stable for 7 days at different pH and dried by freeze dryer | Suggested as colorants for use in food products | Atigo et al. (2018) |
| Spray drying | Maltodextrin | Yellow pulp fruits of Opuntia ficus-indica | Information not presented | Excellent preservation in the dark, even after 28 days at 4 °C. However, the presence of light contributed to betaxanthin deterioration | Natural colorant in yogurt and soft-drink | Fernández et al. (2018) |
| Spray drying | Maltodextrin and pectin | Pitaya juice (Stenocereus griseus)/mechanic | Information not presented | The particles have critical values at a storage temperature of 25° C. Below these conditions, the particles can be stored while maintaining their stability | Functional foods as a colorant | García et al. (2017) |
| Spray drying | Maltodextrin and resistant maltodextrin | Pitaya juice (dragon fruit)/mechanic | Information not presented | Storage for 3 months at 4 °C, 25 °C and 40 °C exhibited higher betanin degradation in resistant maltodextrin at all temperatures with corresponding lower half-lives compared to maltodextrin | Natural colorant in sweets | Shaaruddin et al. (2017) |
| Spray drying | Maltodextrin | Red-violet fruits of Basella rubra L./ maceration | Information not presented | Stable after two years of storage without light at 4 °C | Use in food industry as a natural colourant | Kumar and Giridhar (2016) |
| Freeze drying | Soy protein | Beetroot pomace (Beta vulgaris L., cv. ‘Bicor’)/ultrasonic bath | 86.14% | Stability was reduced by 24% after three months of storage at 25 °C | Could be used in the pharmaceutical industry and as food additives | Tumbas Šaponjac et al. (2016) |
| Emulsion | Sunflower oil and whey protein isolate | Beetroot juice/Mechanic | 98–100% | The stability of the double emulsions prepared from beetroot juice, sunflower oil, and whey protein isolate is related to their high viscosity that prevents creaming and coalescence | Meat products | Eisinaite et al. (2016) |
| Freeze drying | Maltodextrin-gum Arabic and maltodextrin-pectin | Red dragon fruit (Hylocereus polyrhizus) peels | 90–95% | The encapsulation of betalains in carbohydrate matrices stabilizes them in addition to preserving and improving their biological activities | In vitro evaluation as an antioxidant, anti-inflammatory and antiangiogenic | Rodriguez et al. (2016) |
| Freeze drying | Maltodextrin (MD), gum Arabic (GA), gum Arabic-midified starch (GA-MS), modified starch-chitosan (MS-CH) and modified starch-maltodextrin-chitosan (MS-MD-CH) | Beetroot coloring extracts/beetroots extract were extracted with water in a commercial juice extractor | Information not presented | MD with GA proved to be effective agents for beetroot coloring extracts microencapsulation with a high half-life period, while that the incorporation study demonstrated higher stability for food model of low moisture such as chewing gum prepared with extracts encapsulated in GA–MS | Natural colorants in a chewing gum model system | Chranioti et al. (2015) |
| Spray drying | Gelatin and maltodextrin | Purple cactus pear fruits (Opuntia stricta) | 18.07% to 57.30% depending on the ratio of gelatin and maltodextrin | The use of the maltodextrin-gelatin complex generated directly microcapsules with better stability to the temperature greater than 200 °C which provides a good protection to the bioactive components | Information not presented | Castro et al. (2015) |
| Spray drying | Maltodextrin and cladode mucilage and maltodextrin | Purple fruits of Opuntia ficus-indica/maceration | Information not presented | The encapsulation of betalains in maltodextrin was more stable at 57% and 75% RH, with a half-life of 117.4 and 103.4 days | Natural colorant | Otálora et al. (2015) |
| Spray drying | Soybean protein isolate, maltodextrin, inulin and mixtures | Cactus pear fruits (O. ficus-indica)/Pressing | 99% | The protein and polysaccharide blends used as encapsulating agents for cactus pear pulp improved the polyphenol encapsulation and betalain stability at 60 °C as shown by the lower degradation rate constant | Food ingredients for functional foods | Robert et al. (2015) |
| Spray drying | Soluble fiber [(1 → 3) (1 → 4) β-d-glucan] | Juice of red cactus pear/Information not presented | Information not presented | The addition of encapsulated betalains to extrudates showed greater pigment retention at 80° C-100° C at a cutting speed of 225 rpm | Extruded products | Ruiz et al. (2015) |
| Spray dried | Capsul | Pear fruits (Opuntia ficus-indica)/Maceration and clarified by microfiltration and ultrafiltration | 98% | Microparticles with ultrafiltrated extract had better betanin stability 60 °C | Natural colourants for healthy foods | Vergara et al. (2014) |
| Spray drying and freeze drying | Maltodextrin, guar gum, gum Arabic, pectin and xanthangum with different concentration | Red beet roots/Maceration | Freeze drying results from showed higher recovery of betalains. Variation with xanthan gum showed increase up to 65% of betalains content than the control | Betalains with xanthan gum showed 21% more stability than the control (maltodextrin) Freeze drying encapsulation with xanthan showed a higher recovery of betalains by up to 1.3 times than spray drying encapsulation | Powdered food grade colorant | Ravichandran et al. (2014) |
| Spray drying | Maltodextrin, Arabic gum and a mixture of both | Beetroot juice/Information not presented | The highest content of pigments was observed for microcapsules obtained by the feed flux of the raw material 0.3 cm3/s and Arabic gum as a carrier | The gum arabic microcapsules with beet pigments were more stable compared to maltodextrin | Natural colorant | Janiszewska, (2014) |
| Spray drying | Maltodextrin and chitosan | Violet flowers of Lampranthus Productos and Beta vulgaris roots/Mechanical and membrane separated | Information not presented | Maltodextrin encapsulation strongly increased the stability of the pigment, which remained stable for months in the absence of light, at temperatures of − 20 and 4 °C | Food applications | Gandía-Herrero et al. (2013) |
| Spray drying | Gum Arabic | Beetroot juice/commercial juice extractor | Information not presented | The powder stored at aw < 0.521 to 30 °C presented the greatest stability | Antioxidant and as a red colorant | Pitalua et al. (2010) |
| Spray drying | Maltodextrin and inulin | Cactus pear fruits (Opuntia ficus-indica)/maceration | Information not presented | Indicaxanthins in all systems showed a slow degradation during storage at 60 °C and were more stable than betacyanins | Incorporation into functional foods | Saénz et al. (2009) |
| Spray drying | Dried glucose syrup | Fruit juice of Opuntia stricta/mechanic | Information not presented | The encapsulated dye stored at room temperature maintained 98% of its color after one month | Natural colorant | Obón et al. (2009) |
| Freeze drying | Pullulan and maltodextrin samples of different molecular weight | Beetroot pigment/commercial | Information not presented | The wall materials used for encapsulation of the pigment were effective in decreasing the rate of degradation, however the most stable among the three matrices was pullulan | Natural colorant | Serris and Biliaderis (2001) |
From the abovementioned findings, we can conclude that the matrix type exerts an important effect on the stability of betalains. Polysaccharides are those most used, showing great potential to increase the stability of betalains, mainly maltodextrin. However, the combination of other polysaccharides can provide different characteristics for the matrix and contribute greater protection for the betalains. Nonetheless, the combination demonstrating greatest stability for betalains is the protein-polysaccharide combination. The increase or decrease in the stability of betalains will be related to the type of polysaccharide used because, due to their structure, they possess different properties. The type of encapsulation technology also appears to exert an effect on the stability of betalains that, in our view, requires more detailed studies in this regard. However, although the spray-drying technique is the most commonly used today, this does not infer that it is the best technique for stabilizing betalains. In general, the greater or lesser stability of betalains will be influenced by the matrix, the encapsulation technology, and also by the storage conditions, mainly by water activity. The latter has displayed a problem in matrix stability, as in the betalains.
Future perspectives
Encapsulation for the stability of betalains will continue to be one of the current study objectives. The effect of the matrix on betalains stability should be further investigated, and future research should be directed toward the combination of protein–polysaccharides. These are a very interesting source of research, especially in the study of betalains stability. Proteins such as those of gluten have exhibited good barrier properties, and polysaccharides such as pullulan demonstrate greater stability than maltodextrin under storage conditions, which could favor the stability of betalains (Serris and Biliaderis 2001). The coaxial techniques of encapsulation could be used. This technique ensures that betalains will be found in the nucleus and not dispersed in the matrix, as in the majority of the aforementioned techniques. The technique that could offer this characteristic is that of coaxial electrospray, which, to date has not been used, to our knowledge, for the encapsulation of betalains.
Conclusion
The best way to stabilize and potentiate the use of betalains derives from their encapsulation. The matrix type exerts an effect on the stability of betalains, providing greater stability of the protein-polysaccharide combination; however, betalain stability will be influenced by the chemical structure of the polysaccharide and the protein’s physicochemical characteristics. These types of combinations require greater attention if one wishes to increase the stability of betalains. The use of novel encapsulation technologies with fewer conditions-for-use could diversify the employment of encapsulated betalains. In general, the best way to stabilize betalains is by the encapsulation method, utilizing polysaccharide-protein matrices by means of techniques such as freeze drying or techniques in which the betalains are found in the nucleus and not spread throughout the matrix.
Acknowledgements
The authors are grateful to the University of Sonora and to CONACYT (basic science Project Number 285445) for their support.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aguilar TS, Mamani NW, Espinoza SC, Basilio AJ, Condezo HL. Microencapsulated betacyanin from colored organic quinoa (Chenopodium quinoa Willd): optimization, physicochemical characterization and accelerated storage stability. J Sci of Food Agric. 2018;98:5873–5883. doi: 10.1002/jsfa.9152. [DOI] [PubMed] [Google Scholar]
- Akhtar MJ, Jacquot M, Jasniewski J, Jacquot C, Imran M, Jamshidian Desobry S. Antioxidant capacity and light-aging study of HPMC films functionalized with natural plant extract. Carbohydr Polym. 2012;89:1150–1158. doi: 10.1016/j.carbpol.2012.03.088. [DOI] [PubMed] [Google Scholar]
- Amjadi S, Ghorbani M, Hamishehkar H, Roufegarinejad L. Improvement in the stability of betanin by liposomal nanocarriers: its application in gummy candy as a food model. Food Chem. 2018;256:156–162. doi: 10.1016/j.foodchem.2018.02.114. [DOI] [PubMed] [Google Scholar]
- Amjadi S, Abbasi MM, Shokouhi B, Ghorbani M, Hamishehkar H. Enhancement of therapeutic efficacy of betanin for diabetes treatment by liposomal nanocarriers. J Funct Foods. 2019;59:119–128. [Google Scholar]
- Atigo JLD, Bergamasco RDC, Madrona GS. Effect of ph on the stability of red beet extract (Beta vulgaris l) microcapsules produced by spray drying or freeze drying. Food Sci Technol. 2018;38:72–77. [Google Scholar]
- Augustin MA, Hemar Y. Nano-and micro-structured assemblies for encapsulation of food ingredients. Chem Soc Rev. 2009;38:902–912. doi: 10.1039/b801739p. [DOI] [PubMed] [Google Scholar]
- Azeredo H. Betalains: properties, sources, applications, and stability–a review. Int J Food Sci Technol. 2009;44:2365–2376. [Google Scholar]
- Azmir J, Zaidul ISM, Rahman MM, Sharif KM, Mohamed A, Sahena F, Jahurul MHA, Ghafoor K, Norulaini NAN, Omar AKM. Techniques for extraction of bioactive compounds from plant materials: a review. J Food Eng. 2013;117:426–436. [Google Scholar]
- Barba FJ, Putnik P, Kovačević DB, Poojary MM, Roohinejad S, Lorenzo JM, Koubaa M. Impact of conventional and non-conventional processing on prickly pear (Opuntia spp) and their derived products: From preservation of beverages to valorization of by-products. Trends Food Sci Technol. 2017;67:260–270. [Google Scholar]
- Belhadj Slimen I, Najar T, Abderrabba M. Chemical and antioxidant properties of betalains. J Agric Food Chem. 2017;65:675–689. doi: 10.1021/acs.jafc.6b04208. [DOI] [PubMed] [Google Scholar]
- Cai Y, Sun M, Corke H. Antioxidant activity of betalains from plants of the Amaranthaceae. J Agric Food Chem. 2003;51:2288–2294. doi: 10.1021/jf030045u. [DOI] [PubMed] [Google Scholar]
- Cai YZ, Sun M, Corke H. Characterization and application of betalain pigments from plants of the Amaranthaceae. Trends Food Sci Technol. 2005;16:370–376. [Google Scholar]
- Calvo TRA, Perullini M, Santagapita PR. Encapsulation of betacyanins and polyphenols extracted from leaves and stems of beetroot in Ca(II)-alginate beads: a structural study. J Food Eng. 2018;235:32–40. [Google Scholar]
- Čanadanović-Brunet JM, Savatović SS, Ćetković GS, Vulić JJ, Djilas SM, Markov SL, Cvetković DD. Antioxidant and antimicrobial activities of beet root pomace extracts. Czech J Food Sci. 2011;29:575–585. [Google Scholar]
- Castellar R, Obón JM, Alacid M, Fernández-López JA. Color properties and stability of betacyanins from Opuntia fruits. J Agric Food Chem. 2003;51:2772–2776. doi: 10.1021/jf021045h. [DOI] [PubMed] [Google Scholar]
- Castro-Muñoz R, Barragán-Huerta BE, Yáñez-Fernández J. Use of gelatin-maltodextrin composite as an encapsulation support for clarified juice from purple cactus pear (Opuntia stricta) LWT-Food Sci Technol. 2015;62:242–248. [Google Scholar]
- Cejudo-Bastante MJ, Chaalal M, Louaileche H, Parrado J, Heredia FJ. Betalain profile, phenolic content, and color characterization of different parts and varieties of Opuntia ficus-indica. J Agric Food Chem. 2014;62:8491–8499. doi: 10.1021/jf502465g. [DOI] [PubMed] [Google Scholar]
- Celli GB, Brooks MSL. Impact of extraction and processing conditions on betalains and comparison of properties with anthocyanins—A current review. Food Res Int. 2017;100:501–509. doi: 10.1016/j.foodres.2016.08.034. [DOI] [PubMed] [Google Scholar]
- Champagne CP, Fustier P. Microencapsulation for the improved delivery of bioactive compounds into foods. Curr Opin Biotechnol. 2007;18:184–190. doi: 10.1016/j.copbio.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Chong PH, Yusof YA, Aziz MG, Nazli NM, Chin NL, Muhammad SS. Effects of spray drying conditions of microencapsulation of Amaranthus gangeticus extract on drying behaviour. Agric Agric Sci Procedia. 2014;2:33–42. [Google Scholar]
- Choo WS (2017) Betalains: application in functional foods. Bioact Food 1–28
- Chranioti C, Nikoloudaki A, Tzia C. Saffron and beetroot extracts encapsulated in maltodextrin, gum Arabic, modified starch and chitosan: incorporation in a chewing gum system. Carbohydr Polym. 2015;127:252–263. doi: 10.1016/j.carbpol.2015.03.049. [DOI] [PubMed] [Google Scholar]
- Delia SC, Chávez GM, Frank MLM, Araceli SGP, Irais AL, Franco AA. Spray drying microencapsulation of betalain rich extracts from Escontria chiotilla and Stenocereus queretaroensis fruits using cactus mucilage. Food Chem. 2019;272:715–722. doi: 10.1016/j.foodchem.2018.08.069. [DOI] [PubMed] [Google Scholar]
- do Carmo EL, Teodoro RAR, Félix PHC, de Barros Fernandes RV, de Oliveira ÉR, Veiga TRLA, Borges SV, Botrel DA. Stability of spray-dried beetroot extract using oligosaccharides and whey proteins. Food Chem. 2018;249:51–59. doi: 10.1016/j.foodchem.2017.12.076. [DOI] [PubMed] [Google Scholar]
- Eisinaite V, Juraite D, Schroën K, Leskauskaite D. Preparation of stable food-grade double emulsions with a hybrid premix membrane emulsification system. Food Chem. 2016;206:59–66. doi: 10.1016/j.foodchem.2016.03.046. [DOI] [PubMed] [Google Scholar]
- Fang Z, Bhandari B. Encapsulation of polyphenols–a review. Trends Food Sci Technol. 2010;21:510–523. [Google Scholar]
- Fernández LJA, Roca MJ, Angosto JM, Obón JM. Betaxanthin-rich extract from cactus pear fruits as yellow water-soluble colorant with potential application in foods. Plant Foods Human Nutr. 2018;73:146–153. doi: 10.1007/s11130-018-0664-3. [DOI] [PubMed] [Google Scholar]
- Gandía-Herrero F, García-Carmona F. Biosynthesis of betalains: yellow and violet plant pigments. Trends Plant Sci. 2013;18:334–343. doi: 10.1016/j.tplants.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Gandía-Herrero F, Cabanes J, Escribano J, García-Carmona F, Jiménez-Atiénzar M. Encapsulation of the most potent antioxidant betalains in edible matrixes as powders of different colors. J Agric Food Chem. 2013;61:4294–4302. doi: 10.1021/jf400337g. [DOI] [PubMed] [Google Scholar]
- Gandía-Herrero F, Escribano J, García-Carmona F. Biological activities of plant pigments betalains. Crit Rev Food Sci Nutr. 2016;56:937–945. doi: 10.1080/10408398.2012.740103. [DOI] [PubMed] [Google Scholar]
- García LKA, Méndez LLL, Rodríguez RJ, Campanella OH, Patel BK, Barriada BLG. Physical properties of spray dryed Stenocereus griseus pitaya juice powder. J Food Process Eng. 2017;40:12470. [Google Scholar]
- Gengatharan A, Dykes GA, Choo WS. Betalains: natural plant pigments with potential application in functional foods. LWT-Food Sci Technol. 2015;64:645–649. [Google Scholar]
- Gouin S. Microencapsulation: industrial appraisal of existing technologies and trends. Trends Food Sci Technol. 2004;15:330–347. [Google Scholar]
- Gutiérrez TJ, Guzmán R, Jaramillo CM, Famá L. Effect of beet flour on films made from biological macromolecules: native and modified plantain flour. Int J Biol Macromol. 2016;82:395–403. doi: 10.1016/j.ijbiomac.2015.10.020. [DOI] [PubMed] [Google Scholar]
- Herbach KM, Stintzing FC, Carle R. Betalain stability and degradation—structural and chromatic aspects. J Food Sci. 2006;71:R41–R50. [Google Scholar]
- Herbach KM, Rohe M, Stintzing FC, Carle R. Structural and chromatic stability of purple pitaya (Hylocereus polyrhizus [Weber] Britton & Rose) betacyanins as affected by the juice matrix and selected additives. Food Res Int. 2006;39:667–677. [Google Scholar]
- Hilou A, Nacoulma OG, Guiguemde TR. In vivo antimalarial activities of extracts from Amaranthus spinosus L and Boerhaavia erecta L in mice. J Ethnopharmacol. 2006;103:236–240. doi: 10.1016/j.jep.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Impellizzeri G, Piattelli M. Biosynthesis of indicaxanthin in Opuntia ficus-indica fruits. Phytochemistry. 1972;11:2499–2502. [Google Scholar]
- Janiszewska E. Microencapsulated beetroot juice as a potential source of betalain. Powder Technol. 2014;264:190–196. [Google Scholar]
- Kaimainen M, Laaksonen O, Järvenpää E, Sandell M, Huopalahti R. Consumer acceptance and stability of spray dried betanin in model juices. Food Chem. 2015;187:398–406. doi: 10.1016/j.foodchem.2015.04.064. [DOI] [PubMed] [Google Scholar]
- Kaimainen M, Marze S, Järvenpää E, Anton M, Huopalahti R. Encapsulation of betalain into w/o/w double emulsion and release during in vitro intestinal lipid digestion. LWT-Food Sci Technol. 2015;60:899–904. [Google Scholar]
- Khan MI. Stabilization of betalains: a review. Food Chem. 2016;197:1280–1285. doi: 10.1016/j.foodchem.2015.11.043. [DOI] [PubMed] [Google Scholar]
- Khan MI. Plant betalains: Safety, antioxidant activity, clinical efficacy, and bioavailability. Compr Rev Food Sci Food Saf. 2016;15:316–330. doi: 10.1111/1541-4337.12185. [DOI] [PubMed] [Google Scholar]
- Khan MI, Giridhar P. Enhanced chemical stability, chromatic properties and regeneration of betalains in Rivina humilis L berry juice. LWT-Food Sci Technol. 2014;58:649–657. [Google Scholar]
- Khan MI, Harsha PS, Chauhan AS, Vijayendra SVN, Asha MR, Giridhar P. Betalains rich Rivina humilis L berry extract as natural colorant in product (fruit spread and RTS beverage) development. J Food Sci Technol. 2015;52:1808–1813. doi: 10.1007/s13197-013-1175-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koubaa M, Barba FJ, Grimi N, Mhemdi H, Koubaa W, Boussetta N, Vorobiev E. Recovery of colorants from red prickly pear peels and pulps enhanced by pulsed electric field and ultrasound. Innov Food Sci Emerg Technol. 2016;37:336–344. [Google Scholar]
- Kumar SS, Giridhar P. Stabilization of bioactive betalain pigment from fruits of Basella rubra L. through maltodextrin encapsulation. Madridge J Food Technol. 2016;1:66–70. [Google Scholar]
- Laqui-Vilca C, Aguilar-Tuesta S, Mamani-Navarro W, Montano-Bustamante J, Condezo-Hoyos L. Ultrasound-assisted optimal extraction and thermal stability of betalains from colored quinoa (Chenopodium quinoa Willd) hulls. Ind Crops Prod. 2018;111:606–614. [Google Scholar]
- Lima E, Bosch P, Loera S, Ibarra IA, Laguna H, Lara V. Non-toxic hybrid pigments: Sequestering betanidin chromophores on inorganic matrices. Appl Clay Sci. 2009;42:478–482. [Google Scholar]
- Mabry TJ, Dreiding AS. The betalains: recent advances in phytochemistry. NY: Appleton-Centry-Crofts; 1968. [Google Scholar]
- Miguel M. Betalains in some species of the Amaranthaceae family: a review. Antioxidants. 2018;7:53. doi: 10.3390/antiox7040053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller HE, Rösler H, Wohlpart A, Wyler H, Wilcox ME, Frohofer H, et al. Biogenese der Betalaine Biotransformation von Dopa und Tyrosin in den Betalaminsäureteil des Betanins Vorläufige Mitteilung. Helv Chim Acta. 1968;51:1470–1474. doi: 10.1002/hlca.19680510634. [DOI] [PubMed] [Google Scholar]
- Mohamed EE, Iwamoto S, Yamauchi R. Optimization of betalain extraction from Salicornia fruticosa and its encapsulation. J Agroaliment Process Technol. 2018;24:1–8. [Google Scholar]
- Molina G, Hernández-Martínez A, Cortez-Valadez M, García-Hernández F, Estevez M. Effects of tetraethyl orthosilicate (TEOS) on the light and temperature stability of a pigment from Beta vulgaris and its potential food industry applications. Molecules. 2014;19:17985–18002. doi: 10.3390/molecules191117985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moßhammer MR, Stintzing FC, Carle R. Colour studies on fruit juice blends from Opuntia and Hylocereus cacti and betalain-containing model solutions derived therefrom. Food Res Int. 2005;38:975–981. [Google Scholar]
- Nedovic V, Kalusevic A, Manojlovic V, Levic S, Bugarski B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011;1:1806–1815. [Google Scholar]
- Obón JM, Castellar MR, Alacid M, Fernández-López JA. Production of a red–purple food colorant from Opuntia stricta fruits by spray drying and its application in food model systems. J Food Eng. 2009;90:471–479. [Google Scholar]
- Orozco VJ, Escobar RA, Buendía GL, García MC, Hernandez JC, Alvarez RJ. Evaluation of the protection and release rate of bougainvillea (Bougainvillea spectabilis) extracts encapsulated in alginate beads. J Dispers Sci Technol. 2019;40:1065–1074. [Google Scholar]
- Otálora MC, Carriazo JG, Iturriaga L, Nazareno MA, Osorio C. Microencapsulation of betalains obtained from cactus fruit (Opuntia ficus-indica) by spray drying using cactus cladode mucilage and maltodextrin as encapsulating agents. Food Chem. 2015;187:174–181. doi: 10.1016/j.foodchem.2015.04.090. [DOI] [PubMed] [Google Scholar]
- Otálora MC, Carriazo JG, Iturriaga L, Osorio C, Nazareno MA. Encapsulating betalains from Opuntia ficus-indica fruits by ionic gelation: Pigment chemical stability during storage of beads. Food Chem. 2016;202:373–382. doi: 10.1016/j.foodchem.2016.01.115. [DOI] [PubMed] [Google Scholar]
- Otálora MC, Carriazo JG, Osorio C, Nazareno MA. Encapsulation of cactus (Opuntia megacantha) betaxanthins by ionic gelation and spray drying: a comparative study. Food Res Int. 2018;111:423–430. doi: 10.1016/j.foodres.2018.05.058. [DOI] [PubMed] [Google Scholar]
- Otálora MC, de Jesús Barbosa H, Perilla JE, Osorio C, Nazareno MA. Encapsulated betalains (Opuntia ficus-indica) as natural colorants: case study: Gummy candies. LWT. 2019;103:222–227. [Google Scholar]
- Pagano APE, Khalid N, Kobayashi I, Nakajima M, Neves MA, Bastos EL. Microencapsulation of betanin in monodisperse W/O/W emulsions. Food Res Int. 2018;109:489–496. doi: 10.1016/j.foodres.2018.04.053. [DOI] [PubMed] [Google Scholar]
- Pérez-Ramírez E, Lima E, Guzmán A. Natural betalains supported on γ-alumina: a wide family of stable pigments. Dyes Pigments. 2015;120:161–168. [Google Scholar]
- Piattelli M, Minale L, Prota G. Isolation, structure and absolute configuration of indicaxanthin. Tetrahedron. 1964;20:2325–2329. [Google Scholar]
- Pitalua E, Jimenez M, Vernon-Carter EJ, Beristain CI. Antioxidative activity of microcapsules with beetroot juice using gum Arabic as wall material. Food Bioprod Process. 2010;88:253–258. [Google Scholar]
- Ravichandran K, Palaniraj R, Saw NMMT, Gabr AM, Ahmed AR, Knorr D, Smetanska I. Effects of different encapsulation agents and drying process on stability of betalains extract. J Food Sci Technol. 2014;51:2216–2221. doi: 10.1007/s13197-012-0728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshmi SK, Aravindhan KM, Suganyadavi P. The effect of light, temperature, pH on stability of betacyanin pigments in basella alba fruit. Asian J Pharm Clin Res. 2012;4:107–110. [Google Scholar]
- Robert P, Torres V, García P, Vergara C, Sáenz C. The encapsulation of purple cactus pear (Opuntia ficus-indica) pulp by using polysaccharide-proteins as encapsulating agents. LWT-Food Sci Technol. 2015;60:1039–1045. [Google Scholar]
- Rodriguez EB, Vidallon MLP, Mendoza DJR, Reyes CT. Health-promoting bioactivities of betalains from red dragon fruit (Hylocereus polyrhizus (Weber) Britton and Rose) peels as affected by carbohydrate encapsulation. J Sci Food Agric. 2016;96:4679–4689. doi: 10.1002/jsfa.7681. [DOI] [PubMed] [Google Scholar]
- Roriz CL, Barros L, Prieto MA, Barreiro MF, Morales P, Ferreira IC. Modern extraction techniques optimized to extract betacyanins from Gomphrena globosa L. Ind Crops Prod. 2017;105:29–40. [Google Scholar]
- Ruiz GM, Amaya GC, Quintero RA, Pérez CE, Ruiz AT, Báez GJ, Meléndez PC. Effect of extrusion cooking on bioactive compounds in encapsulated red cactus pear powder. Molecules. 2015;20:8875–8892. doi: 10.3390/molecules20058875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saénz C, Tapia S, Chávez J, Robert P. Microencapsulation by spray drying of bioactive compounds from cactus pear (Opuntia ficus-indica) Food Chem. 2009;114:616–622. [Google Scholar]
- Serra AT, Poejo J, Matias AA, Bronze MR, Duarte CM. Evaluation of Opuntia spp. derived products as antiproliferative agents in human colon cancer cell line (HT29) Food Res Int. 2013;54:892–901. [Google Scholar]
- Serris GS, Biliaderis CG. Degradation kinetics of beetroot pigment encapsulated in polymeric matrices. J Sci Food Agric. 2001;81:691–700. [Google Scholar]
- Shaaruddin S, Ghazali HM, Mirhosseini SH, Muhammad K. Stability of betanin in pitaya powder and confection as affected by resistant maltodextrin. LWT. 2017;84:129–134. [Google Scholar]
- Soquetta MB, Terra LDM, Bastos CP. Green technologies for the extraction of bioactive compounds in fruits and vegetables. CyTA-J Food. 2018;16:400–412. [Google Scholar]
- Stintzing FC, Carle R. Betalains–emerging prospects for food scientists. Trends Food Sci Technol. 2007;18:514–525. [Google Scholar]
- Stintzing FC, Schieber A, Carle R. Cactus pear-a promising component to functional food. Gemuese-und Kartoffelverarbeitung (Germany): Obst-; 2000. [Google Scholar]
- Strack D, Vogt T, Schliemann W. Recent advances in betalain research. Phytochemistry. 2003;62:247–269. doi: 10.1016/s0031-9422(02)00564-2. [DOI] [PubMed] [Google Scholar]
- Tenore GC, Novellino E, Basile A. Nutraceutical potential and antioxidant benefits of red pitaya (Hylocereus polyrhizus) extracts. J Funct Foods. 2012;4:129–136. [Google Scholar]
- Thirugnanasambandham K, Sivakumar V. Microwave assisted extraction process of betalain from dragon fruit and its antioxidant activities. J Saudi Soc Agric Sci. 2017;16:41–48. [Google Scholar]
- Tiwari BK, Cullen PJ (2013) Extraction of red beet pigments. In: Red beet biotechnology. Springer, Boston, pp 373–391
- Trouillas P, Sancho-Garcia JC, De Freitas V, Gierschner J, Otyepka M, Dangles O. Stabilizing and modulating color by copigmentation: insights from theory and experiment. Chem Rev. 2016;116:4937–4982. doi: 10.1021/acs.chemrev.5b00507. [DOI] [PubMed] [Google Scholar]
- Tumbas Šaponjac V, Čanadanović-Brunet J, Ćetković G, Jakišić M, Djilas S, Vulić J, Stajčić S. Encapsulation of beetroot pomace extract: RSM optimization, storage and gastrointestinal stability. Molecules. 2016;21:584. doi: 10.3390/molecules21050584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas CL, Valle GS, Martínez BF, Salinas MY, Lobato CC, Calvo LAD. Encapsulation and pigmenting potential of betalains of pitaya (Stenocereus pruinosus) fruit. J Food Sci Technol. 2018;55:2436–2445. doi: 10.1007/s13197-018-3161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara C, Saavedra J, Sáenz C, García P, Robert P. Microencapsulation of pulp and ultrafiltered cactus pear (Opuntia ficus-indica) extracts and betanin stability during storage. Food Chem. 2014;157:246–251. doi: 10.1016/j.foodchem.2014.02.037. [DOI] [PubMed] [Google Scholar]
- Vinceković M, Viskić M, Jurić S, Giacometti J, Kovačević DB, Putnik P, Donsi F, Barba FJ, Jambrak AR. Innovative technologies for encapsulation of Mediterranean plants extracts. Trends Food Sci Technol. 2017;69:1–12. [Google Scholar]
- Vulić JJ, Ćebović TN, Čanadanović VM, Ćetković GS, Djilas SM, Čanadanović-Brunet J, Velicanski AS, Cvetkovic DD, Tumbas VT. Antiradical, antimicrobial and cytotoxic activities of commercial beetroot pomace. Food Funct. 2013;4:713–721. doi: 10.1039/c3fo30315b. [DOI] [PubMed] [Google Scholar]
- Yong YY, Dykes G, Lee SM, Choo WS. Effect of refrigerated storage on betacyanin composition, antibacterial activity of red pitahaya (Hylocereus polyrhizus) and cytotoxicity evaluation of betacyanin rich extract on normal human cell lines. LWT. 2018;91:491–497. [Google Scholar]
- Zou DM, Brewer M, Garcia F, Feugang JM, Wang J, Zang Zou C. Cactus pear: a natural product in cancer chemoprevention. Nutr J. 2005;4:25. doi: 10.1186/1475-2891-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]