Abstract
The pro-health action of germinated lentils could be useful to be added with wheat flour in the production of box bread. In this work, we spectroscopically evaluate the germinated and non-germinated lentils, and use them at the concentrations of 5 and 10% for the production of box bread. The chemical and physical tests of the bread and its determination of phenolic acids and flavonoids (by HPLC) were also performed. As well as the evaluation of the quality of flour and dough used to produce the bread and the acceptance of the germinated lentil bread with a population of 20 people with diabetes or with diabetic relatives It is shown that: (1) The amplitude of photoacoustic signal obtained by photoacoustic spectroscopy is modified as a function of the percentage of germinated lentil (GL) flour (0, 5 or 10%) add to the bread; being higher the photoacoustic amplitude to higher concentration of GL in the absorption band of 300–425 nm, which is related to higher content of phenols and flavonoids. (2) The contents of phenolic acids (Sinapinic, β- resorcylic, Chlorogenic and Ferulic) and flavonoids (Quercetin and Isorhamnetin) tended to increase in the germinated lentil bread with 10% concentration of germinated lentil flour with respect to the control bread (0% GL). (3) The addition of germinated lentils flour to 5 and 10% into wheat flour to produce bread with higher hardness and less cohesiveness than bread based on wheat flour only. The Falling number indicate that there is no significant difference between the control sample and the 5% GL flour, while in the 10% GL flour there was a reduction of 21 s, with respect to the control. The effect of the germinated lentil flour percentage on the pasting properties of the flours was significant between the control and 10% GL flour. In general, the quality of the dough and flour are modified due to the addition of germinates lentils, and this affectation increases with the increase in the concentration of GL. (4) The bread added with germinated lentil has sensory acceptance with a group of people with diabetes and/or diabetic relatives in their attributes in general. The obtained results thus support the production of wheat bread with mixed germinated lentils flour, as a nutraceutical option for human consumption.
Keywords: Bread, Lentil sprout, Wheat flour, Secondary metabolites
Introducción
Lentils (Lens culinaris) are leguminous with high nutritional value, bioactive components, antioxidants, and other phytochemicals that render pro-health properties, Srivastava and Vasishtha (2012). The consumption of this popular legume has been increasing around the world, due to the emerging interest on functional food with nutraceutical value, Jahreis et al. (2016). Lentils are a rich and cheap source of proteins, Ladjal-Ettoumi et al. (2016) that are often used as a meat substitute, Jallinoja et al. (2016) and they can complement the intake of cereal proteins with several essential aminoacids, Migliozzi et al. (2015). The regular consumption of lentils is thus able to enrich the human nutrition as well as prevent and reduce the development of chronic diseases, such as cardio-vascular maladies, cancers, overweight and obesity, syndrome metabolic, and diabetes, Bouchenak and Lamri-Senhadji (2013). Lentils are therefore vital to improve the nutrition of people in developing countries specially, however their consumption is sometimes limited by the presence of some anti-nutritional constituents, such as saponins, which are natural compounds present in pulses having surface active properties, Ayet et al. (1997). High levels of saponins reduce the micronutrient bioavailability, but also have some beneficial effects on the human health, as reported in the literature, Liener (1994). In order to boost the lentils as a human food, the removal of their antinutrients are thus necessary, Ayet et al. (1997), which can be done by cooking, soaking, and sprouting, Ruiz et al. (1996). In fact, the sprout of lentils is an old method applied to improve and expand their nutritional and nutraceutical quality, López-Amorós et al. (2006) and could hence be used nowadays to increase their acceptance and consumption. The pro-health action of lentil sprouts and other plants is determined by their secondary metabolite content, including phenols, which are a group of compounds with antioxidant, anti-tumour, and anti-inflammatory capabilities, Haminiuk et al. (2012).
The incorporation of lentil sprouts into basic food, such as bread has the potential to provide functional, nutritional, and nutraceutical benefits in the diet of millions of people around the world, Devani et al. (2016). Wheat bread, as one of the most popular types of bread, Cauvain (2015), is an excellent source of energy and abundant in carbohydrates, but it usually contains a low quantity and quality of proteins, vitamins, minerals, and dietary fiber, Devani et al. (2016), Jezewska-Zychowicz (2016), as well as a high glycemic index, (Roberts 2000). Taking into account that the basic ingredients of bread are wheat flour, water, yeast and salt, Martin et al. (2004); the mixture of wheat flour with germinated lentils, D’Appolonia (1977) could thus improve the nutritional value of bread with a higher content of proteins and the amino acid lysine present in lentils, Savage (1991).
The goal of this work is to perform the optical characterization of germinated and non-germinated lentils along with the bread made with them. This is done by means of the photoacoustic spectroscopy (PAS) technique, which is a spectroscopic and non-destructive method, Bicanic (2011) that has been applied to study several foodstuffs, such as grains, fruits, vegetables, liquids, semiliquids, condiments, tortillas, flours, and legumes, (Hernández-Aguilar et al. 2019; Bicanic et al. 2010).
Materials and methods
Biological material
Lentil seeds (Lens culinaris Medik) were acquired in a local supermarket of the state of Mexico and their weight was of 5 g per 100 seeds. The seeds were washed using purified water and then left for 24 h inside a glass bottle with distilled water and covered with a cotton cloth. After this hydration and imbibition process, the seeds were washed again and the excess water was left to drain from the seeds during 10 min. Next, the germinated lentils were allowed to develop, washing them every 24 h for 5 days. The germinated lentils (GL) were then placed in kraft paper bags and dried in a forced air oven at 40 °C during 72 h. The dried GL were afterwards milled to powder by means of a T-Fal blender (550 W) for 3 min, to be used later in bread making. In the same way, Non-GL were also milled to powder and both, the Non-GL and GL were characterized through PAS.
PAS Experimental setup
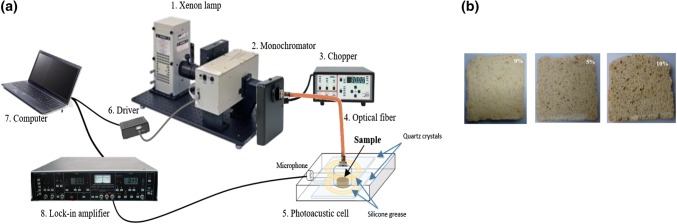
The experimental setup of PAS consists of a Xenon lamp used as the light excitation source, a monochromator applied to obtain a monochromatic light beam, and a mechanical chopper employed to modulate the light beam at the fixed frequency f = 17 Hz, as shown in Fig. 1a. The modulated beam was focused onto an optical fiber to guide it to the photoacoustic (PA) cell, whose signal was then detected by an electret microphone (photoacoustic sensor) connected to the cell through a fine channel (1 mm diameter). The PA signal was amplified by means of a lock-in amplifier (EG&G, Mod. 5210) before being recorded in a personal computer, as a function of beam wavelength. Experiments were performed for wavelengths between 250 to 700 nm and by placing the samples (GL and Non-GL powders) into the PA cell with a diameter of 6 mm. Three samples of bread (Fig. 1b) were prepared with the form of circular slides with a diameter of 5 mm, such that they do not obstruct the microphone sensor. In addition, an analysis of the first derivative of the photoacoustic signal was realized.
Fig. 1.
a Scheme of the PAS experimental setup used in our experiments and b bread made with 0, 5 and 10% of germinated lentils flour
Phytochemical screening on germinated and non-germinated lentils
The extraction of secondary metabolites was carried out from the germinated and non-germinated lentils that were dried and milled. The plant material was weighed and completely submerged into 10 ml of three different solvents (hexane, methanol, dichloromethane), during 72 h. The filtering and separation of water and solvent from the plant material was then carried out by means of a rotary evaporator. The identification of secondary metabolites in germinated and non-germinated lentils was made through the qualitative test proposed by Bhandary et al. (2012) and using these solvents to observe the presence or absence of different compounds. Each crude extracts obtained with hexane, methanol, dichloromethane was tested to recognize the presence of tannins, alkaloids, flavonoids, saponins and phenols.
Elaboration of bread
Three types of bread with 0% (B1), 5% (B2) and 10% (B3) of germinated lentils were elaborated with common ingredients of egg (one), salt (1.25 g), honey (5 ml), olive oil (75 ml), and yeast (11 g). The ingredients were mixed for 5 min, in a blender with a spiral hook. After its fermentation in warm water (30 °C), the yeast was incorporated into the mixture, which was then kneaded for 10 min and placed in a mold covered with a soaked cloth during 25 min. Next, the dough was again kneaded for 10 min and put in a greased mold for bread, where increased its volume during 20 min. Afterwards, each type of the three doughs was placed in different molds and simultaneously baked in a pre-heated electric oven (Black Decker) at 180 °C, for 45 min. After baking, the bread was allowed to cool down to room temperature during 24 h and subsequently cut it into slices (1.27 cm thick) through an electric knife (Hamilton beach type EK08, 121 V, 11 Hz).
Bread quality
Bread chemical analysis Chemical analysis was determined for germinated lentils flour and bread samples. Moisture content, ash, protein (N × 5.85), fat and fiber were evaluated according to AACC methods 44–15, 08–01, 46–13, 30–25 and 32–07, respectively (AACC 2000). Water activity (aw) was evaluated by an electronic hygrometer (Acqua Lab, MB45).
Bread physical analysis The specific volume of the bread was determined as bread volume divided by bread weight. Bread volume was determined by AACC rapeseed displacement method, 10–05 (AACC 2000), which was modified using linseed. After mechanically compacting the bread to exclude all empty spaces. Weights were measured using a 2-decimal precision scale. Bread porosity was evaluating by equation:
where apparent density was obtained as inverse of specific volumen and real density is solid density without empty spaces.
Color Bread crumbs were measured using a Minolta CR-300 colorimeter (Minolta, Osaka, Japan). The color parameters CIELAB (L*, a, and b, where L* = luminosity (100 = white, to 0 = black), the a = greenish-reddish, and the b* = yellowish–bluish.
Texture profile analysis and statistic analysis Bread texture analysis was performed using a Texture Analyzer Brookfield Model CT3 25 K USA. Bread samples were cut into cubes of 3 × 3 × 3 and it were kept in a polyethylene bag for 15 min, and then the texture profile analysis (TPA) of the bread was performed. The samples were measured to a 33% compression cycle using a TA General Probe Kit with a TA25/1000 test probe cylinder 50.8 mm diameter and 20 mm length at a speed of 1 mm/s. Two compression cycles were measured. Hence, it was possible to measure hardness, elasticity, cohesiveness and chewiness. Three replications were conducted at 24 ± 1 °C. The variance of the obtained data was analyzed with a generalized linear model (GLM) and the software SAS (2008 version). A comparison of the mean values of the low significant differences (LSD) was also done.
Mass and flour quality
Falling number El Falling number se realizó a las muestras de harina y se empleó una máquina de Falling number (Perten Falling number FN1310, Perten Instruments, Suiza). The method approved by AACC 56–81.03, AACC International (2010) was used. 7 g of sample were weighed and placed in the viscometer tube, which was mixed with 25 mL of distilled water. The sample was stirred with the viscometric tube until a homogeneous suspension was obtained. The tube is placed in a water bath and the device is activated and after 5 s the agitation begins automatically and after another 55 s the agitator is automatically released in its upper position and begins the descent due to its weight. The total time in seconds, elapsed from the moment the appliance activates until the agitator descends a certain distance is the Falling number.
Pasting properties The starch pasting profiles of nine water suspensions samples were analyzed using Anton Paar MCR 102 Rheometer, equipped with a starch cell using the methodology proposed by, Rincón-Londoño et al. (2016). The starch samples (3 g) were suspended in 18 mL of water. The suspension was heated over 5 min from 50 to 90 °C. Next, suspension was maintained at constant temperature of 90 °C for 5 min and finally the samples were cooled down to 50 °C for 5 min. The rotation speed of the system was 194 rpm.
Alveograph testing Viscoelastic behavior of dough was studied by the alveograph test, using an Alveolab Graph 2017 equipment (Chopin, France), following standard method, AACC (2000). The following alveograph parameters were automatically recorded by a computer software program: tenacity (P), dough extensibility (L), curve configuration ratio (P/L ratio), swelling index (G), elastisity index (I.e) and the strain work (W).
Rheological parameters Rheological parameters of the masa were measured by running oscillatory tests in a TA Instruments Rheometer, model RT 20 Haake, New Castle (USA) with a rough plate of 2.5 mm. A strain sweep test was performed to determine the linear viscoelastic region (LVR) in a range of 20 to 150 Pa at 25 °C. Frequency sweep, which increased from 0.1 to 30 Hz were evaluated to determine storage (elastic) modulus (G′) and loss (or viscous) modulus (G″) using the accompanying software.
Analysis of phenolic acids and flavonoids by HPLC
Extracts were obtained with 50 mg of dry and pulverized material in 1 mL of methanol of HPLC grade (Sigma-Aldrich number 36860) at 80%, incubated for 20 min in an ultrasound bath (BRANSON at Smithkline company 50/60 Hz, model B-220, USA), Meneses-Reyes et al. (2008). The crude extracts were centrifuged at 731 g (Centrifuge 5804 eppendorf, model 5804) for 10 min, Irakli et al. (2018). Supernatants were filtered with 25 mm diameter acrodiscs with nylon membrane and 0.45 mm pore size (Titan). These extracts were injected immediately for the analysis by HPLC of phenolic acids and flavonoids. The samples were analyzed in a Hewlett Packard® chromatograph mod. 1100 provided with diode array detector and an Agilent Technologies automatic injector model 1200. The column was a Hypersil ODS HP column of 125 mm length and 4 mm internal diameter, 5 μm particle size was used. The mobile phase was distilled water adjusted to pH 2.5 with trifluoroacetic acid (A) and acetonitrile (B), Bilia et al. (2001). The analysis was by gradient: T1 0.10 min (85% A) (15% B); T2 20 min (65% A) (35% B) and T3 25 min (65% A), (35% B), λ = 254, 280, 330 and 365 nm, Column temperature 30 °C and flow of 1 mL min−1. Calibration curves were performed for standards: the sinapinic acid, β- resorcylic acid, chlorogenic acid, ferulic acid, rosmarinic acid, protocatechuic acid, quercetin and Isorhamnetin (Sigma-Aldrich®).
The interpolations of all the extracts were calculated with ChemStation software © Agilent Technologies, Inc. 2004.
Sensory acceptability
Mexican consumers (20) who regularly consumed bread, were selected to participate in this study. Consumers participating mostly reside in the city and state of Mexico and between 25 and 70 years old. The test was conducted in the experimental classroom of the “ESIME, Zacatenco”. This classroom had lights illumination natural and fluorescent tube lamps. Each consumer was given an evaluation instrument (questionnaire) and received a set of three complete pieces of bread placed inside a plastic bag (low density polyethylene) hermetically sealed 1 h before carrying out the test. The bread was made 7 h before being placed in the bags. The questionnaire applied was divided into three parts: the first consisted of general data of each consumer, the second corresponded to the list of 11 attributes (color, porosity, sponginess, hardness, cohesiveness, aroma, stickiness, chewiness, flavor and healthy preferences and attributes in general; chosen to evaluate each of the breads using a hedonic scale, Laureati et al. (2012). The third section was about of asked direct questions related to their purchase decision on the proposed breads and their healthy preferences, as well as their health status of them and/or relatives mainly. Before the application of the questionnaire, the participants were informed about the purpose of the test, as well as the general indications about it for a better evaluation of each of the attributes of each bread. Additionally, the participants were provided with a bowl of water and other of coffee grains to be employed during the test.
Results and discussion
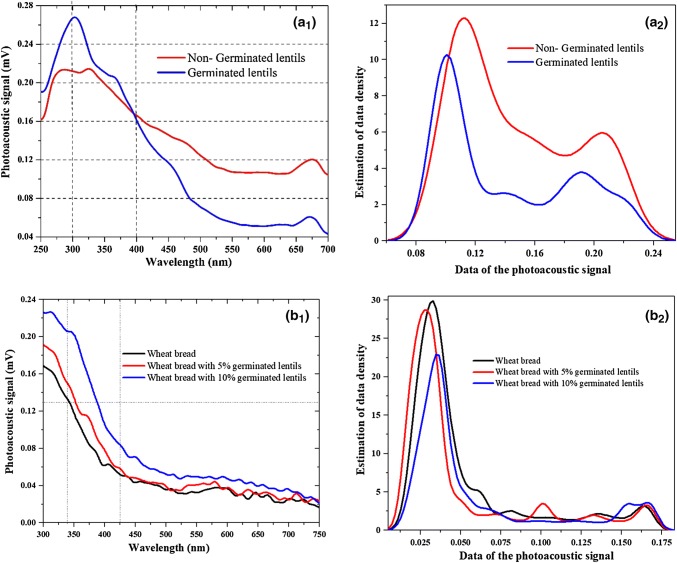
The amplitude values of the photoacoustic signal (PA) of germinated and non-germinated lentils are reported in Table 1 (section A), for the wavelengths of 480, 500, 550, 600 and 650 nm. Note that the amplitude of Non-GL is lower than the one of GL, in the range of 300–410 nm (Fig. 2a), such that the difference of both amplitudes take its maximum values within the interval of 300–350 nm. For the wavelength range of 410–700 nm, on the other hand, the amplitude of non-GL is higher than that of GL, such as their difference is maximum at 650 nm. At this latter wavelength, the amplitudes of the non-GL and GL were of 0.49 and 0.2, respectively, which represents a variation of 59%. Significant statistical differences were also found for the wavelengths of 480, 500, 550, 600 and 700 nm.
Table 1.
Comparison of the mean amplitude of the photoacoustic signal generated by A) non-germinated and germinated lentils as well as B) bread, for various wavelengths
| Material | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (A) Non-germinated and germinated lentils | ||||||||||
| (a) Non-GL | 1.03a | 0.89a | 0.76a | 0.67a | 0.59a | 0.54a | 0.47a | 0.46a | 0.49a | 0.40a |
| (b) GL | 1.2a | 0.93a | 0.71a | 0.51a | 0.36b | 0.31b | 0.24b | 0.22b | 0.2b | 0.16a |
| LSD (0.05%) | 2.68 | 0.90 | 0.94 | 0.74 | 0.07 | 0.02 | 0.01 | 0.03 | 0.15 | 0.25 |
| Mean | 1.12 | 0.91 | 0.74 | 0.59 | 0.47 | 0.42 | 0.35 | 0.34 | 0.36 | 0.28 |
| Significance | 0.5 ns | 0.63 ns | 0.6 ns | 0.22 ns | 0.01 | 0.006 | 0.002 | 0.007 | 0.02 | 0.05 |
| C.V. (%) | 18.81 | 7.79 | 10 | 9.91 | 1.27 | 0.52 | 0.26 | 0.81 | 3.36 | 7.07 |
| R2 | 0.43 | 0.81 | 0.66 | 0.89 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 |
| (B) Bread | ||||||||||
| (c) Control | 0.171b | 0.104c | 0.05a | 0.03a | 0.03a | 0.03a | 0.03a | 0.03a | 0.03a | 0.02a |
| (d) | 0.193b | 0.13b | 0.08b | 0.04a | 0.04a | 0.04a | 0.04a | 0.04a | 0.03a | 0.02a |
| (e) | 0.229a | 0.21a | 0.10c | 0.07a | 0.06a | 0.05a | 0.05a | 0.05a | 0.04a | 0.04a |
| LSD (0.05%) | 0.03 | 0.03 | 00 | 0.07 | 0.03 | 0.03 | 0.07 | 0.03 | 0.01 | 0.06 |
| Mean | 0.19 | 0.14 | 0.08 | 0.05 | 0.04 | 0.04 | 0.04 | 0.04 | 0.03 | 0.02 |
| Significance | 0.03 | 0.0001 | 0.0001 | 0.31 ns | 0.2 ns | 0.18 ns | 0.59 ns | 0.43 ns | 0.57 ns | 0.62 ns |
| C.V. (%) | 4.11 | 0 | 0 | 31.1 | 17.48 | 18.35 | 38.65 | 20.20 | 11.89 | 53.8 |
| R2 | 0.98 | 1 | 1 | 0.93 | 0.98 | 0.98 | 0.92 | 0.98 | 0.99 | 0.84 |
Means with the same letter (a, b, c) in a column are statistically equal (LSD (Lows significant differences, ))
Fig. 2.
Spectra of non-germinated and germinated lentils (a) and wheat bread with 0,5 and 10% of germinated lentils flour (b): a1 Photoacoustic signal a2 Estimation of data density, b1 Photoacoustic signal and b2 Analysis of the PA signal by Kernel
According to Fig. 2a1, the germinated lentils show a maximum absorption peak at 310 nm, while the non-germinated ones exhibit two peaks at 275 and 325 nm. This could be due to the increased content of secondary metabolites (i.e. phenols) that constitute the GL, Bartolomé et al. (1997) and typically generate spectral peaks for wavelengths between 250 and 265 nm and 280–300 nm, Amarowicz et al. (2003). This fact is confirmed by the phytochemical evaluation summarized in Table 2, which shows that the phenols had a minimal presence (+) in non-GL and a moderate one (++) in GL, when methanol was used as solvent. On the other hand, taking into account the presence of tryptophan usually shows up for wavelengths around 277 nm, Amarowicz et al. (2009). The PA signal in Fig. 2a1 indicates that the GL contains a bigger quantity of the essential amino acid than the non-GL. These results are consistent with previous works based on PAS, Dóka et al. (2017), Bergevin et al. (1995), Dóka et al. (2004) which reported absorbance peaks at the wavelengths of 275 and 278 nm for buckwheat and strawberries, respectively.
Table 2.
Identification of presence of secondary metabolites of germinated and non-germinated lentils
| Secondary metabolites | Hexane extract | Methanol extract | Dichloromethane Extract |
|---|---|---|---|
| Non-germinated lentils | |||
| Tannins | − | − | − |
| Alkaloids | − | − | − |
| Flavonoids | − | + | + |
| Saponins | − | ++ | + |
| Phenols | + | + | + |
| Germinated Lentils | |||
| Tannins | − | − | − |
| Alkaloids | − | − | − |
| Flavonoids | − | ++ | + |
| Saponins | − | + | − |
| Phenols | + | ++ | + |
+++ Abundant presence, ++ moderate presence, + minimum presence and − absence
The significant variations in the second (430–544 nm) and third (650–700 nm) absorption bands can be associated to the changes in the contents of saponins, Du et al. (2018) and green pigments, respectively. According to Table 2, the GL contain less saponins and more phenols and flavonoids than the non-GL.
The differences among the spectra of germinated and non-germinated lentils can be enhanced through the first and second derivatives of the PA signal. The intersections of the first derivative with the horizontal axis correspond to the absorption peaks, which are different for the GL (300, 565, 585, 625, 635, and 671 nm) and non-GL (285, 325, 561, 585, 600, 632 and 675 nm). On the other hand, the negative peaks at 310 and 370 nm of the second derivative are related to the absorption band (shoulder) of the PA signal. Figure 2a2 shows that the peak at 0.12 mV of the data density for the non-GL is shifted to 0.09 mV after germination. This indicates that, on average, the GL tends to generate a photoacoustic signal of less intensity. In addition, the probability density functions estimated by “Kernels” present a multimodal behavior, which points out that the data set of the PA signals come from different populations because of the complex and inhomogeneous nature of non-GL and GL with different components evidenced in the different absorbent bands. For non-GL, we have two bands and one shoulder, which correspond to phenols, flavonoids and saponins. On the other hand, for GL, there is two bands and two shoulders due to phenols, flavonoids, saponins and green pigment.
Other results in this research are related to the bread made with germinated lentils. Photoacoustic spectrum (Fig. 2b1) and the Kernel estimator (Fig. 2b2) of the wheat bread elaborated with 0 (B1), 5 (B2) and 10% (B3) of germinated lentils is shown in Fig. 2b. It is possible to observe how the level of the photoacoustic signal is modified as a function of the concentration of GL flour (0, 5 or 10%) and the wavelength. There is a maximum absorption band (at the beginning of the spectrum of each one of the photoacoustic signals of each bread), which tends to decrease with the increase of the wavelength (Fig. 2b1). Being higher the photoacoustic signal to higher concentration of GL; this increase is accentuated in the absorption band of 300–425 nm. Table 1 shows significant statistical differences in this absorption band, when is compared in 300, 350 and 400 nm. It is observed the comparison of mean amplitude of the photoacoustic signal at various wavelengths of the photoacoustic spectrum of bread (Section B), where means with different letter in a column are statistically different (LSD Lows significant differences, ).
Figure 2b1, shows maximum peak at 310 nm, where the photoacoustic signal of wheat bread added with GL is increased by 34% with respect to the signal obtained from wheat bread (0% GL). Bread spectra made with germinated lentils (Fig. 2b1) do not show the absorption band corresponding to green pigment that was observed in non-germinated lentil and germinated lentils spectra (Fig. 2a1). In the Fig. 2b2 is shown that in the case of the wheat bread there is a maximum in the region close to the value of 0.031 mV corresponding to the photoacoustic signal intensity, which indicates in this region the values occur with higher frequency. In the case of germinated lentils bread at 5 and 10%, it is possible to observe that the maximum occurs for a value of 0.026 and 0.035, respectively. In the density function of the PA signal a population is clearly distinguished, unlike of the density function of germinated and non-germinated lentils. This may be due to the bread baking process. So, in the elaboration of wheat bread with added germinated lentil, the bread components are modified.
The absorbent bands found, according to these results with significant statistical differences are basically in the band corresponding to phenols and flavonoids. It could be said that the elaboration of bread, with added germinated lentils (GL), improves its nutraceutical characteristics.
Chemical and physical analysis
Table 3 shows the parameters obtained from the chemical and physical analysis i.e. moisture (%), water activity, protein, fat, fiber, ashes, specific volume, porosity, color (L, a, b) and texture profile analysis (TPA). The results of the chemical analysis of the germinated lentil flour in percentage were, protein (23.51 ± 0.21), fat (3.40 ± 0.04), ashes (2.61 ± 0.16) and fiber (8.11 ± 0.22), which show a high value of protein and fiber content. Regarding the chemical analysis of bread, it is possible to see in Table 3, that the amount of protein in bread B3 increases with respect to B1 by 20% and the amount of fat and fiber decreases 30 and 34%, respectively. On the contrary, the ashes increase as the amount of germinated lentil added to the bread increases. Lentils are a rich source of proteins, Ladjal-Ettoumi et al. (2016) and they can complement the intake of cereal proteins, Migliozzi et al. (2015). In this case, it complements wheat for bread making. Bread being a food of daily consumption by most people, the population could enrich their diet by eating bread added with germinated lentil flour. Which, as noted, improves nutritional quality and also, by increasing fiber and decreasing fat, it is beneficial when the persons have health problems or in preventive stages of several sicks, Bouchenak and Lamri-Senhadji (2013).
Table 3.
Chemical, physical and quality analysis of bread and quality of dough and flour used in its preparation
| Test | Control bread | Lentil bread 5% | Lentil bread 10% |
|---|---|---|---|
| Moisture (%) | 36.80 ± 0.22a | 36.92 ± 0.23a | 35.95 ± 0.11b |
| Water activity | 0.924 ± 0.001a | 0.927 ± 0.002a | 0.919 ± 0.002b |
| Protein (%) | 10.51 ± 0.05b | 11.76 ± 0.15a | 12.59 ± 0.52a |
| Fat (%) | 12.16 ± 0.52a | 10.37 ± 0.94b | 8.60 ± 0.15b |
| Fiber (%) | 2.79 ± 0.10a | 3.05 ± 0.15a | 1.85 ± 0.12b |
| Ashes (%) | 0.89 ± 0.02a | 0.90 ± 0.06a | 0.96 ± 0.02a |
| Specific volume (mL/g) | 4.67 ± 0.19a | 4.59 ± 0.17a | 4.03 ± 0.11b |
| Porosity (%) | 27.91 ± 1.05b | 27.59 ± 0.21b | 31.67 ± 0.36a |
| Color L | 70.68 ± 1.75a | 68.66 ± 0.70ab | 62.21 ± 0.84b |
| a | − 0.88 ± 0.03c | − 0.65 ± 0.03b | − 0.35 ± 0.04a |
| b | 18.62 ± 0.88b | 17.55 ± 0.08b | 17.63 ± 0.18b |
| Hardness (N) | 3.20 ± 0.61c | 13.36 ± 1.03b | 25.93 ± 4.96a |
| Elasticity (mm) | 2.97 ± 0.23b | 2.54 ± 0.07b | 3.99 ± 0.45a |
| Cohesiveness | 0.78 ± 0.07a | 0.62 ± 0.02a | 0.59 ± 0.09a |
| Chewiness (mJ) | 7.33 ± 1.32c | 21.27 ± 1.52b | 57.17 ± 5.33a |
| FLOUR | 0%GL | 5% GL | 10% GL |
|---|---|---|---|
| Falling number (s) | 374 ± 15a | 379 ± 10a | 353 ± 12b |
| Viscosity maximun (mPa s) | 4087 ± 125a | 3965 ± 75a | 3752.5 ± 89 |
| Breakdown (mPa s) | 2680.5 ± 35a | 2501 ± 130b | 2240 ± 87c |
| Final viscosity (mPa s) | 4666.5 ± 101a | 4439 ± 78b | 4090 ± 86c |
| DOUGH (ALVEOGRAPH) | |||
| Tenacity (P) (mmH2O) | 95 ± 3.5a | 90 ± 4.3a | 84 ± 5.4b |
| Extensibility (L) (mm) | 98 ± 1.8a | 86 ± 2.6b | 68 ± 1.2c |
| Swelling Index (G) | 22 ± 0.7a | 20.6 ± 0.6b | 18.3 ± 0.4c |
| Strain work (10−4J) | 283.1 ± 7.1a | 238 ± 2.5b | 172.1 ± 5.4c |
| Elasticity index (%) | 51.7 ± 3.5a | 49.1 ± 5.7a | 40.6 ± 4.3b |
| Ration P/L | 0.970 ± 0.045a | 1.05 ± 0.089b | 1.24 ± 0.076c |
Mean ± standard deviation values followed of different letter in each column, are significantly different (ρ < 0.05)
The specific volume data are similar to those reported by Lim et al. (2011) who studied bread with turmeric, obtaining values of 5.2 to 4.0 mL/g. The lowest specific volume value was for bread with 10% (B3) germinated lentils because during the germination period the activity of proteases is limited (Marti et al. 2017) possibly due to the high activity of α-amylase that it could affect the gluten network. In relation to the porosity of the bread, it was found higher in the wheat bread added with 10% GL (B3), increasing with respect to the wheat bread (0% GL) of almost 14%.
On the other hand, the color of the breadcrumbs showed significant difference that were affected by the addition of germinated lentil flour. The value of L decreases significantly, the value of “a” increases and in “b” there is no significant difference. The changes may also be associated with an increase in Maillard reactions (Marti et al. 2018). As well as properly the color of the germinated lentil that is added. Color is one of the relevant attributes for consumer acceptance and deciding on its purchase (Herrera-Corredor et al. 2007).
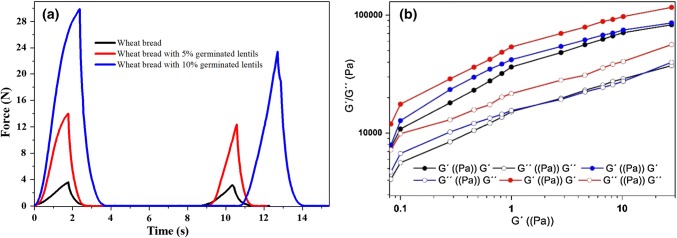
Regarding texture profile analysis (TPA) of the bread made, it was found that the bread added with 10% germinated lentils flour presented the highest values of strength in the two compression cycles (Fig. 3a).
Fig. 3.
a Force versus time from TPA analysis for three types of bread, b Rheological properties of dough with sprouted lentil
Table 3 shows the parameters obtained from the TPA and it is observed that for the cohesiveness there was no significant difference () between the different types of bread. On the contrary, hardness, elasticity and chewiness if they presented significant statistical difference (), being the bread added with 10% of flour of germinated lentils those that presented the highest values.
Schiraldi and Fessas (2000), reported that the hardness of bread is attributed to the matrix formed between amylose and amylopectin. However, Ghumman et al. (2016) indicated that germination results in the decomposition of high molecular weight proteins to low molecular weight proteins that have a large number of polar groups, which increases the water retention capacity, which can influence the hardness values obtained and chewiness of breads added with germinated lentils flour. Likewise, lentil sprouts increase in fiber content and decrease in fat, Chung et al. (2008). The addition of fiber in baked goods is a critical ingredient because although it is used to prolong the freshness of the bread given its water retention capacity, it can modify its volume, smoothness and firmness, Sangnark and Noomhorm (2004). The addition of excess fiber can modify the gluten network due to the interaction between gluten and fiber, Noort et al. (2010).
The highest elasticity was of the bread added with 10% germinated lentils flour, while between the bread (control) and the bread added with germinated lentils flour to 5% there was no significant difference.
According to what was reported by Hoseney (1994), during baking there are interactions between the gelatinized starch and the wheat flour gluten. The bread obtained with 10% of germinated lentils flour formed a crumb with greater hardness and elasticity, possibly due to the greater resistance of the swollen starch during the cooking process. However, the cohesive value was the lowest, probably originated by the dilution of the structure of the gluten (interaction between gluten and fibers), reducing the number of disulfide bonds that modify the structure of the gluten completely, forming a little mass structure cohesive.
The results indicate that adding germinated lentils flour in the two percentages 5 and 10%, to the wheat flour modifies the textural properties of the bread in comparison with the bread elaborated only of wheat flour. Bread added with germinated lentils is obtained with greater hardness and for the case of bread with 10%, less cohesive. However, breads elaborated from sprouted seed flours contain significant amounts of polyphenols, Caccialupi et al. (2010). Lentil and sprouted seeds are a good source of high quality starches and proteins, as well as fiber and low fat content. They have low glycemic index, slowly release the glucose in the blood, which transmits and ensures a more stable insulin response. For these reasons lentil sprouts are desirable for a population with diabetes and overweight, Chung et al. (2008).
Dough and flours quality
Falling number
The Falling number data shown in the Table 4 indicate that there is no significant difference between the control sample and the 5% GL flour, while in the 10% GL flour there was a reduction of 21 s, with respect to the control. This behavior is due to the mixture of GL flour that has a higher activity of α-amylase, which acts on the amylase breaking glucosidic bonds, causing changes in starch.
Table 4.
Concentration of phenolic acids and flavonoids in extracts of bread added with different percentages of germinated lentil (0, 5 and 10%)
| Phenolic acid (µg/mL) | B1 (Control) | B2 5% | B3 10% | DMS | R2 | Media |
|---|---|---|---|---|---|---|
| Sinapinic acid | 0.058a | 0.117a | 0.118a | 0.197 | 0.42 | 0.098 |
| β- resorcylic acid | 0.096a | 0.098a | 0.183a | 0.468 | 0.21 | 0.126 |
| Chlorogenic acid* | 0.009b | 0.013a | 0.011a | 0.003 | 0.88 | 0.011 |
| Ferulic acid* | 0.049b | 0.050a | 0.051a | 0.001 | 0.88 | 0.051 |
| Rosmarinic acid* | 0.094a | 0.095a | ND | 0.002 | 0.88 | 0.095 |
| Protocatechuic acid* | 0.385a | 0.386a | 0.380a | 0.012 | 0.62 | 0.384 |
| Flavonoids(µg/mL) | ||||||
| Quercetin* | ND | ND | 0.245a | 0.005 | 1 | 0.245 |
| Isorhamnetin* | ND | ND | 0.211a | 0.010 | 1 | 0.211 |
*There were significant differences between treatments according to the analysis of variance (p ≤ 0.05). The different letters indicate significant differences between the Tukey means test (α = 0.05, n = 9). ND: Not detected
Pasting properties
The effect of the germinated lentil flour percentage on the pasting properties of the flours was significant between the control and 10% GL flour. Maximum viscosity values decreased 8.5% between control flour and 10 GL %. The maximum viscosity indicates the ability of the starches to swell before physical decomposition (break down) (Ghumman et al. 2016). This behavior is attributed to the increase in germination that causes the degradation and de-branching of starch to simpler units (Ingbian and Adegoke 2007). The decrease in the maximum viscosity of the mixtures of wheat flour with lentil sprout flour could be related to the higher amylose content of lentil starch (35%) with respect to wheat starch, because it seems that starch interactions and proteins are modulated by the amylose to amylopectin ratio (Joshi et al. 2014). In the viscosity values of the break down if there was a significant difference between the three treatments. Between the control sample and the 10% GL there was a higher decrease in viscosity of 16%. Singh et al. (2014), reported that the breakdown is a measure of the ease with which swollen granules can detach during continuous heating and shearing. In the final viscosity, which is the ability for the material to form a viscous paste, the control sample had the highest values and the 10% GL flour decreased 12%. These data agree with those obtained in Falling number in which the 10% GL flour showed a higher drop and this is attributed to a higher activity of α-amylase.
Rheological parameters
The elastic (G′) and viscous (G″) modules are shown in Fig. 3b. In all cases the values of G′, predominates with the values of G″. Then the mass is predominantly considered a gel or solid; this behavior is considered a weak gel. Rubel et al. (2015) mention that high values of G′ indicate a higher character of a solid. The results show that the control and 5% GL mass have no significant difference. However, the 10% GL mass showed higher values of the modules. The addition of a higher percentage of germinated lentil flour alters the starch-gluten matrix, affecting the viscoelastic behavior of the dough and limiting its handling (Belghith et al. 2016).
Alveograph testing
The parameters evaluated in the alveograph showed significant difference by increasing the percentage of germinated flour. The dough strength or tenacity (P) It is indicative of the ability of the dough to retain gas (Belghith et al. 2016). The results (Table 3) show that there is no significant difference between the control and the 5% GL sample. The extensibility of the mass (L) is related to the handling of the mass. In this research, it is found that the values of L of the mass 10% GL is 30% lower than with the control sample. These parameters affect the P/L ratio that for this study the highest value was for 10% GL (1.24); which indicates that this mass has a higher tenacity than extensibility. This result justifies the data obtained in the elastic modules (Fig. 3b), because the highest values was also for 10% GL, which represents a mass of greater rigidity, which is not handling due to its low extensibility value. Deformation work values (W), swelling index and elasticity index decreased as the percentage of lentil sprouts increased. These results show us that there is a weakness in the gluten network, manifesting itself to a higher extent in sample 10% GL. One of the reasons is that germinated lentil proteins compete for water, delaying gluten hydration or it could also be that GL proteins interact with gluten proteins (Roccia et al. 2009).
Phenolic acids and flavonoids by HPLC
The phenolic acid most abundant in the bread control (without GL) was the Protocatechuic acid (0.385 µg/mL) followed by β- resorcylic acid (0.096 µg/mL) and Rosmarinic acid (0.094 µg/m). In the case of bread with a higher percentage of GL (10%), the most abundant were Protocatechuic acid (0.380 µg/mL) followed by Sinapinic acid (0.117 µg/mL) and β- resorcylic acid (0.098 µg/mL).
The contents of phenolic acids: Sinapinic and β- resorcylic in the bread B3, tended to increase more than 100 and 90%, respectively with respect to the control bread, without significant statistical differences. In relation to the content of Chlorogenic acid and Ferulic acid when comparing the three types of bread (0, 5 and 10% of GL), significant statistical differences were presented with an increase at 22 and 5%, respectively. Unlike phenolic acids of the type Rosmarinic and Protocatechuic, tended to decrease when compared with respect to the content found in the control samples.
In the case of flavonoids: Quercetin and Isorhamnetin in the control bread (without GL) were not found. They were only detected in the bread with the highest percentage of GL (B3), with the flavonoid most abundant Quercetin (0.245 µg/mL) followed by Isorhamnetin (0.211 µg/mL). Of this way, the obtained results support the production of wheat bread with mixed germinated lentils flour; as a nutraceutical option for human consumption due to its content of phenolic acids and flavonoids (see Table 4).
Bread sensory acceptability
The ANOVA results revealed significant (P < 0.05) differences among, the evaluations according to the hedonic scale in 6 attributes of bread sensory acceptability (Table 5): porosity, sponginess, hardness, cohesiveness, healthy option and general attributes. It can be seen, based on the attributes in general, the order of preference according to the scores was first the wheat bread added with 5% GL (B2), followed by the bread added 10% (B3) and finally the control wheat bread (0% of GL-B1).
Table 5.
Mean consumer scores for sensory acceptability of 3 breads (0, 5 and 10% of GL)
| Color | Porosity | Sponginess | Hardness | cohesiveness | Aroma | Stickiness | Chewiness | Flavor | Healthy option | General attributes | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0% | 7.13a | 7.53a | 7.06ba | 6.13b | 7.09a | 6.4a | 6.6a | 6.66a | 6.33a | 5.2b | 6.4b |
| 5% | 7.06a | 7.4a | 7.4a | 6.86ba | 6.5ab | 6.93a | 6.4a | 6.93a | 7.06a | 7.4a | 7.93a |
| 10% | 6.73a | 6.26b | 6.53b | 7.2a | 6.12b | 7.06a | 6.06a | 6.06a | 6.86a | 8.13a | 7.4a |
| LSD (0.05%) | 0.69 | 0.75 | 0.76 | 0.89 | 0.83 | 0.78 | 0.96 | 0.98 | 0.94 | 1.03 | 0.86 |
| Media | 6.97 | 7.06 | 7 | 6.73 | 6.57 | 6.8 | 6.35 | 6.55 | 6.75 | 6.93 | 7.24 |
| Significance | 0.45 ns | 0.0033* | 0.04* | 0.05* | 0.05* | 0.20 ns | 0.52 ns | 0.19 ns | 0.27 ns | 0.0001** | 0.004* |
| C.V. | 13.55 | 14.35 | 14.68 | 17.69 | 17.3 | 15.45 | 20.27 | 19.9 | 18.77 | 19.98 | 16.04 |
| R2 | 0.68 | 0.63 | 0.61 | 0.5 | 0.52 | 0.71 | 0.57 | 0.56 | 0.56 | 0.68 | 0.57 |
According to the information provided by the participants through the questionnaire; the majority have relatives with diabetes or hypertension, so they look for generally healthy foods; at least in this sample of participants in which the study was conducted of the sensory acceptance. In the score for the bread attribute that is considered healthier, the sample of participants choose, with the score most higher the wheat bread 10% GL, followed by the B2 bread (5% GL) and in the lowest score is the wheat bread of control (0% GL). It should be noted that all consumers (100%) surveyed replied that they would accept to consume the types of bread made with germinated lentil flour proposed in this research due to their nutritional properties, to that could be of lower glycemic index (for the information provided according to the literature scientific) and also for not having conservatives. Another attribute of interest to consumers surveyed in this study is the use of preservatives in the food. All participants prefer to consume bread without preservatives.
Sensory acceptability for porosity, hardness and cohesiveness attributes was evaluated in a higher score for wheat bread without germinated lentil flour (B1), with a score of 7.53, 7.06 and 7.09, for each one of these attributes. The bread with the lowest sensory acceptability according to these attributes is B3 (10% of GL) with a score obtained of 6.26, 6.53 and 6.12. It is important to point out that sensory acceptability decreased around 20% in these sensory properties of bread when germinated lentil flour is added in the elaboration of the bread. With respect to the flavor attribute, the trend of sensory acceptance of consumers, it is mostly for bread B2 (5% GL), since it tends to have the highest score (7.06), compared to the others, mainly control bread (0% GL-score 6.33). In this way, consumers in the sensory acceptance analysis prefer 10% more to bread with 5% GL, compared to control bread (0% GL). With respect to color of the bread, the consumers tend to have higher acceptability for B1 bread (0% GL- score 7.13) and the less acceptability for B3 bread (10% GL-score 6.73; being 5%, preferred B1 with respect to B3. It is possible to observe that consumers are looking for more nutritious products and that these could have less impact on the level of sugar in the blood. This is associated by the cases of diabetes of them or their relatives, according with the answers from of the questionnaire.
Conclusion
The addition of germinated and non-germinated lentils flours to the wheat flour has spectroscopically been evaluated and used for the production of box bread. It has been shown that: (1) The level of photoacoustic signal obtained by photoacoustic spectroscopy is modified as a function of the concentration of germinated lentil (GL) flour (0, 5 or 10%) in the bread; being higher the photoacoustic amplitude to higher concentration of GL in the absorption band of 300–425 nm, which is related to higher content of phenols and flavonoids. (2) The addition of germinated lentils flour with concentrations of 5 and 10% into wheat flour allows to produce bread with higher hardness and less cohesiveness than bread based on wheat flour only. (3) The contents of phenolic acids (Sinapinic, β- resorcylic, Chlorogenic acid and Ferulic acid) and flavonoids (Quercetin and Isorhamnetin) tended to increase in the germinated lentil bread with 10% concentration of germinated lentil flour with respect to the control bread (0% GL). (4) The addition of germinated lentils flour with concentrations of 5 and 10% into wheat flour to produce bread with higher hardness and less cohesiveness than bread based on wheat flour only. (5) The Falling number indicate that there is no significant difference between the control sample and the 5% GL flour, while in the 10% GL flour there was a reduction of 21 s, with respect to the control. The effect of the germinated lentil flour percentage on the pasting properties of the flours was significant between the control and 10% GL flour. (6) In general, the quality of the dough and flour are modified due to the addition of germinates lentils, and this affectation increases with the increase in the concentration of GL. (7) The bread added with germinated lentil has sensory acceptance with a group of people with diabetes and/or diabetic relatives in their attributes in general.
Acknowledgements
The authors are grateful for the support of the National Polytechnic Institute through the projects SIP, EDI, and COFFA. Claudia Hernandez-Aguilar thanks the collaboration of Cinvestav and Fes-Cuautitlán, through of Esther Ayala and I.A. Miriam Edith Fuentes Romero, for his assistance and support during the use of the infrastructure in the respective laboratories.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- AACC . Approved methods of the American Association of Cereal Chemists. Method 84–10 and 08–01. 10. St. Paul: American Association of Cereal Chemists; 2000. [Google Scholar]
- AACC International (2010) Approved methods of analysis. In: Determination of falling number, 11th edn. Approved November 2, 1972; Reapproved November 3, 1999. AACC International, St. Paul. 10.1094/AACCIntMethod-56-81.03
- Amarowicz R, Karamac M, Shahidi F. Antioxidant activity of phenolic fractions of lentil (Lens culinaris) J Food Lipids. 2003;10:1–10. [Google Scholar]
- Amarowicz R, Estrella I, Hernández T, Dueñas M, Troszyńska A, Kosińska A, Pegg R. Antioxidant activity of a red lentil extract and its fractions. Int J Mol Sci. 2009;10:5513–5527. doi: 10.3390/ijms10125513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayet G, Burbano C, Cuadrado C, Pedrosa MM, Robredo LM, Muzquiz M, de la Cuadra C, Castaño A, Osagie A. Effect of germination, under different environmental conditions, on saponins, phytic acid and tannins in lentils (Lens culinaris) J Sci Food Agric. 1997;74:273–279. [Google Scholar]
- Bartolomé B, Estrella I, Hernandez T. Changes in phenolic compounds in lentils (Lens culinaris) during germination and fermentation. Z Lebensm Unters Forsch A. 1997;205:290–294. [Google Scholar]
- Belghith L Fendri, Chaari Fatma, Maaloul Marwa, Kallel Fatma, Abdelkafi Lobna, Chaabouni Semia Ellouz, Ghribi-Aydi Dhouha. Wheat bread enrichment by pea and broad bean pods fibers: effect on dough rheology and bread quality. LWT Food Sci Technol. 2016;73:584–591. [Google Scholar]
- Bergevin M, N’Soukpoé-Kossi CN, Charlebois D, Leblanc RM, Willemot C. Assessment of strawberry maturity by photoacoustic spectroscopy. Appl Spectrosc Appl Spectrosc. 1995;49:397–399. [Google Scholar]
- Bhandary SK, Bhat SV, Sharmil PK, Bekal PM. Preliminary phytochemical screening of various extracts of Punica granatum peel, whole fruit and seeds. J Health Sci. 2012;2:34–38. [Google Scholar]
- Bicanic DD. On the photoacoustic, photothermal and colorimetric quantification of carotenoids and other phytonutrients in some food: a review. J Mol Struct. 2011;993:9–14. [Google Scholar]
- Bicanic D, Dimitrovski D, Luterotti S, Marković K, Van Twisk C, Buijnsters JG, Dóka O. Correlation of trans-lycopene measurements by the HPLC method with the optothermal and photoacoustic signals and the color readings of fresh tomato homogenates. Food Biophys. 2010;5:24–33. doi: 10.1007/s11483-009-9140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilia AR, Salvini D, Mazzi G. Characterization of calendula flower, milk-thistle fruit, and passion flower tinctures by HPLC-DAD and HPLC-MS. Chromatographia. 2001;53(34):210–215. [Google Scholar]
- Bouchenak M, Lamri-Senhadji M. Nutritional quality of legumes, and their role in cardiometabolic risk prevention: a review. J Med Food. 2013;16:185–198. doi: 10.1089/jmf.2011.0238. [DOI] [PubMed] [Google Scholar]
- Bread CS (2015) The product. In: Technology of breadmaking. Springer, Cham, pp 1–22
- Caccialupi P, Ceci LR, Siciliano RA, Pignone D, Clemente A, Sonnante G. Bowman-Birk inhibitors in lentil: heterologous expression, functional characterization and anti-proliferative properties in human colon cancer cells. Food Chem Food Chem. 2010;120:1058–1066. [Google Scholar]
- Chung HJ, Liu Q, Hoover R, Warkentin TD, Vandenberg A. In vitro starch digestibility, expected glycemic index, and thermal and pasting properties of flours from pea, lentil and chickpea cultivars. Food Chem. 2008;111:316–321. doi: 10.1016/j.foodchem.2008.03.062. [DOI] [PubMed] [Google Scholar]
- D’appolonia BL. Rheological and baking studies of legume-wheat flour blends. Cereal Chem. 1977;54:53–63. [Google Scholar]
- Devani BM, Jani BL, Kapopara MB, Vyas DM, Ningthoujam MD. Study on quality of white bread enriched with finger millet flour. J Agric Chem Environ Biotechnol. 2016;9:903. [Google Scholar]
- Dóka O, Bicanic DD, Dicko MH, Slingerland MA. Photoacoustic approach to direct determination of the total phenolic content in red sorghum flours. J Agric Food Chem. 2004;52:2133–2136. doi: 10.1021/jf030421a. [DOI] [PubMed] [Google Scholar]
- Dóka O, Brunori A, Schmidt R, Bicanic D, Végvári G. Rutin in buckwheat grain meal determined by UV photoacoustic spectroscopy and HPLC. Nova Biotechnol Chim. 2017;16:61–67. [Google Scholar]
- Du M, Guo S, Zhang J, Hu L, Li M. Quantitative analysis method of the tea saponin. Open J For. 2018;08:61–67. [Google Scholar]
- Ghumman A, Kaur A, Singh N. Impact of germination on flour, protein and starch characteristics of lentil (Lens culinari) and horsegram (Macrotyloma uniflorum L.) lines. LWT Food Sci Technol. 2016;65:137–144. [Google Scholar]
- Haminiuk CW, Maciel GM, Plata-Oviedo MS, Peralta RM. Phenolic compounds in fruits–an overview. Int J Food Sci Technol. 2012;47:2023–2044. [Google Scholar]
- Hernández-Aguilar C, Domínguez-Pacheco A, Cruz-Orea A, Ivanov R. Photoacoustic spectroscopy in the optical characterization of foodstuff: a review. J Spectrosc. 2019 doi: 10.1155/2019/5920948. [DOI] [Google Scholar]
- Herrera-Corredor JA, Saidu JEP, Khachatryan A, Prinyawiwatkul W, Carballo-Carballo A, Zepeda-Bautista R. Identifying drivers for consumer acceptance and purchase intent of corn tortilla. J Food Sci. 2007;72(9):S727–S731. doi: 10.1111/j.1750-3841.2007.00564.x. [DOI] [PubMed] [Google Scholar]
- Hoseney RC. Principles of Cereal science and technology. 3. United Kingdom: American association of cereal chemists, Inc.; 1994. pp. 203–206. [Google Scholar]
- Ingbian EK, Adegoke GO. Proximate compositions, pasting and rheological properties of mumu e a roasted maize meal. Int J Food Sci Technol. 2007;42:762e767. [Google Scholar]
- Irakli M, Chatzopoulou P, Ekateriniadou L. Optimization of ultrasound-assisted extraction of phenolic compounds: oleuropein, phenolic acids, phenolic alcohols and flavonoids from olive leaves and evaluation of its antioxidant activities. Ind Crops Prod. 2018;124:382–388. [Google Scholar]
- Jahreis G, Brese M, Leiterer M, Schaefer U, Boehm V. Legume flours: nutritionally important sources of protein and dietary fiber. Ernahrungs Umschau. 2016;63:36–42. [Google Scholar]
- Jallinoja P, Niva M, Latvala T. Future of sustainable eating? Examining the potential for expanding bean eating in a meat-eating culture. Futures. 2016;83:4–14. [Google Scholar]
- Jezewska-Zychowicz M. Impact of health and nutrition risks perception on the interest in pro-healthy food on the example of bread. Rocz Panstw Zakl Hig. 2016;67:1. [PubMed] [Google Scholar]
- Joshi M, Aldred P, Panozzo JF, Kasapis S, Adhikari B. Rheological and microstructural characteristics of lentil starch-lentil protein composite pastes and gels. Food Hydrocol. 2014;35:226–237. [Google Scholar]
- Ladjal-Ettoumi Y, Boudries H, Chibane M, Romero A. Pea, chickpea and lentil protein isolates: physicochemical characterization and emulsifying properties. Food Biophys. 2016;11:43–51. [Google Scholar]
- Laureati M, Giussani B, Pagliarini E. Sensory and hedonic perception of gluten-free bread: comparison between celiac and non-celiac subjects. Food Res Int. 2012;46:326e333. [Google Scholar]
- Liener IE. Implications of antinutritional components in soybean foods. Crit Rev Food Sci Nutr. 1994;34:31–67. doi: 10.1080/10408399409527649. [DOI] [PubMed] [Google Scholar]
- Lim Ho S, Park So H, Ghafoor Kashif, Hwang Sung Y, Park Jiyong. Quality and antioxidant properties of bread containing turmeric (Curcuma longa L.) cultivated in South Korea. Food Chem. 2011;124:1577–1582. [Google Scholar]
- López-Amorós ML, Hernández T, Estrella I. Effect of germination on legume phenolic compounds and their antioxidant activity. J Food Compos Anal. 2006;19:277–283. [Google Scholar]
- Marti A, Cardone G, Nicolodi A, Quaglia L, Pagani MA. Sprouted wheat as an alternative to conventional flour improvers in bread-making. LWT Food Sci Technol. 2017;80:230–236. [Google Scholar]
- Marti A, Cardone Gaetano, Pagani Maria Ambrogina, Casiraghi Maria Cristina. Flour from sprouted wheat as a new ingredient in bread-making. LWT Food Sci Technol. 2018;89:237–243. [Google Scholar]
- Martin PJ, Chin NL, Campbell GM. Aeration during bread dough mixing: iI. A population balance model of aeration. Food Bioprod Process. 2004;82:268–281. [Google Scholar]
- Meneses-Reyes JC, Soto-Hernández RM, Espinosa-Solares T, Ramírez-Guzmán ME. Optimización del proceso de extracción de flavonoides de flor de manzanilla (Matricaria recutita L.) Agrociencia. 2008;42(4):425–433. [Google Scholar]
- Migliozzi M, Thavarajah D, Thavarajah P, Nutrients Smith P. Lentil and kale: complementary nutrient-rich whole food sources to combat micronutrient and calorie malnutrition. Nutrients. 2015;7:9285–9298. doi: 10.3390/nu7115471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noort MWJ, Van Haaster D, Hemery Y, Schols HA, Hamer RJ. The effect of particle size of wheat bran fractions on bread quality e evidence for fibre protein interactions. J Cereal Sci. 2010;52:59–64. [Google Scholar]
- Rincón-Londoño N, Vega-Rojas N, Contreras-Padilla M, Acosta-Osorio A, Rodríguez-García ME. Analysis of the pasting profile in corn starch: structural, morphological, and thermal transformations. Int J Biol Macromol. 2016;91:106–114. doi: 10.1016/j.ijbiomac.2016.05.070. [DOI] [PubMed] [Google Scholar]
- Roberts SB. High–glycemic index foods, hunger, and obesity: is there a connection? Nutr Rev. 2000;58:163–169. doi: 10.1111/j.1753-4887.2000.tb01855.x. [DOI] [PubMed] [Google Scholar]
- Roccia P, Ribotta PD, Prez GT, León AE. Influence of soy protein on rheological properties and water retention capacity of wheat gluten. LWT Food Sci Technol. 2009;42(1):358–362. [Google Scholar]
- Rubel IA, Pérez EE, Manrique GD, Genovese DB. Fibre enrichment of wheat bread with Jerusalem artichoke inulin: effect on dough rheology and bread quality. Food struct. 2015;3:21–29. [Google Scholar]
- Ruiz RG, Price KR, Arthur AE, Rose ME, Rhodes MJ, Fenwick RG. Effect of soaking and cooking on the saponin content and composition of chickpeas (Cicer arietinum) and lentils (Lens culinaris) J Agric Food Chem. 1996;44:1526–1530. [Google Scholar]
- Sangnark A, Noomhorm A. Chemical, physical and baking properties of dietary fiber prepared from rice straw. Food Res Int. 2004;37:66–74. [Google Scholar]
- Savage GP. Lentils: a forgotten crop. Outlook Agric. 1991;20:109–112. [Google Scholar]
- Schiraldi A, Fessas D. Mechanism of staling. In: Pavinee C, Vodovotz Y, editors. Bred staling. New York: CRC Press Inc; 2000. pp. 2–10. [Google Scholar]
- Singh N, Paul P, Virdi SA, Kaur P, Mahajan G. Influence of early and delayed transplantation of paddy on physico-chemical, pasting, cooking, textural and protein characteristics of milled rice. Cereal Chem. 2014;91(4):389e397. [Google Scholar]
- Srivastava RP, Vasishtha H. Saponins and lectins of Indian chickpeas (Cicer arietinum) and lentils (Lens culinaris) Indian J Agric Biochem. 2012;25:44–47. [Google Scholar]