Abstract
Effect of faba bean enzymatic protein extraction process parameters were investigated at different ultrasound powers (200, 300 and 400 W), sonication times (15, 25 and 35 min), enzyme dosages (0.15, 0.3 and 0.45%) and enzyme treatment times (15, 25 and 35 min). Physico-functional characterization of the protein samples through solubility, zeta potential, color, fat adsorption, emulsifying and foaming capacity measurements suggested faba protein as a promising substituent to egg yolk powder in reduced fat mayonnaise formulation. Reduced-fat mayonnaise formulation containing different faba protein/egg yolk powder ratios were prepared and characterized through rheological analyses as well as emulsion stability, texture, color and microstructural evaluations. The formulations containing equal compositions of faba bean protein and egg yolk powder (0.375%) and also the one with 0.5% faba bean protein and 0.25% egg yolk powder were finally suggested to substitute the conventional formulation.
Keywords: Mayonnaise, Faba protein, Viscosity, Texture, Emulsifying, Particle size, Physicochemical properties
Introduction
Over the past years, food technology has been attracted to legumes due to their high contents of fibers, proteins, minerals and bioactive components beneficial to improve nutritional characteristics of food products. Evidences indicating reduced risk of diabetes, cardiovascular disease and certain cancers upon consumption of legumes have been reported yet (Coda et al. 2017). Grain legumes providing twice protein content as in cereals have the required potential to be a promising substitutes for meat as a protein-rich food ingredient (Rosa-Sibakov et al. 2016). Vicia faba, a species in the fabaceae family, is well known as a kind of nutritious legume in Middle East countries. Most of the 4 million tons/year production of the annual plant comes from China, Ethiopia, Australia, France and Egypt. Faba bean respectively contains 20–41% and 51–68% of seed dry matter protein and carbohydrates besides fiber (5–5.8%), vitamin B and minerals (Hendawey and Younes 2013). It is competitable with soya bean in amino acids content (such as lysine) excluding tryptophan, methionine and cysteine. Fat and antinutrients (such as raffinose and stachyose) in faba bean is also lower compared to soya (Zee et al. 1988).
Viscosity, solubility, hydration, emulsifying, foaming and gelling properties of faba bean protein, especially the protein isolate, have been experimentally shown promising results in food applications (Jiang et al. 2016). Cepeda et al. (1998) found higher oil/water emulsification capacity, foamability and foam stability in faba protein flour compared to green mung, pea and beans flour. Multari et al. (2015) believe that structural and functional characteristics of isolate/concentrate bean proteins including faba bean is strongly affected by the preparation, extraction and drying procedures. Hence, right selection of technologies and circumstances for extraction of protein could be determining in the final nutritional characteristics of the food product. Regarding the physicochemical and structural properties (i.e. solubility, hydrophobicity, molecular weight, isoelectric point,…) of proteins; there is a wide variety of protein extraction and fractionation methods which are required to be optimized improving the compatibilization features in proteins’ application as well as minimizing the denaturation and proteolysis of protein (Martínez-Maqueda et al. 2013).
Ultrasound followed by controlled enzymatic hydrolysis has been suggested to improve extraction process yielding optimized protein characteristics. Martínez-Velasco et al. (2018) observed desirable progress in surface activity, extraction/fractionation yield, particle size, and structural change in protein molecules at high-intensity ultrasound conditions. Jiang et al. (2014) compared the effects of low-frequency (20 kHz) ultrasonication applied at various powers and for different durations on the functional/structural properties of black bean protein isolate dispersions. They detected changes in the tertiary structure of proteins after ultrasonic treatment through fluorescence emission spectra where the particle size reached to a minimum at 24 min. On the other hands, soya protein extraction yield and expenses have been reported to decrease significantly using ultrasound method generating higher concentrations of protein free amino groups at short durations compared to conventional long heating method (Mu et al. 2010). Ultrasonic treatment has also been reported to intensify the enzymatic hydrolysis of soya protein besides the improvements granting to the rheological behavior, solubility, protein isolate gel forming capability, surface interactions and soya protein concentrate emulsifying capacity (Chen et al. 2011).
Protein hydrolysis using proteases of diverse sources is another process suggested to reduce the allergic response of food products, improve functional characteristics, produce bioactive peptides and promote digestion. Lu et al. (2016) investigated alkaline protease enzyme hydrolysis followed by subcritical water treatment and observed improvements in physicochemical and emulsifying characteristics of soya protein. Nivala et al. (2017) observed that with increasing transglutaminase and tyrosinase enzymes’ concentration, solubility of oat and faba bean protein isolates decreased along with the increases in the crosslinking ability and foaming properties of the treated protein isolates; whereas colloidal stability did not change significantly. do Evangelho et al. (2017) used pepsin and alcalase to produce black bean protein hydrolysates and claimed that the pepsin-treated bean protein hydrolysates presented higher degree of hydrolysis than the alcalase-treated protein hydrolysates where alcalase treating resulted in higher surface hydrophobicities. High pressure alcalase treatment of kidney bean protein isolate was also studied resulting promoted antioxidant activities, higher degree of hydrolysis and production of desired bioactive peptides.
Mayonnaise is a semi-solid oil in water emulsion of vegetable oil combined with vinegar, egg yolk and other flavoring ingredients whose stabilization is dominantly provided by egg yolk presenting high phospholipid/protein emulsifying capacity. However, egg yolk has unfavorable high cholesterol contents limiting its use in commercial products turning to low-cholesterol mayonnaise presenting similar characteristics. Substitution of egg yolk or introduction of other appropriate emulsifiers besides this important ingredient in the mayonnaise formulation would reduce cholesterol, total fat content and also production expenses as well as microbial stability benefits. On the other hands, improved rheological properties have been demonstrated, thereupon. Within the several investigations published in recent years (Ghoush et al. 2008; Herald et al. 2009; Alu’datt et al. 2017), egg yolk has been substituted by diverse protein isolates and concentrates including wheat, soya, peas, white beans, whey and so on; either showing significant improvements in the emulsion stability, texture, viscosity, rheology or maintaining the primary characteristics. Regarding to the major availability of faba bean in several countries, in this research, controlled enzyme hydrolysis along with ultrasound technique was utilized in order to promote extraction yield, create added value and improve functional characteristics of faba bean protein which could be substituted with egg yolk powder in reduced-fat mayonnaise formulation.
Experimental
Materials
Faba bean, Alkaline protease: Alcalase 2.4L FG (Novozymes, Bagsværd, Denmark), sodium hydroxide, hydrogen chloride, phosphate buffer and sodium dodecyl sulfate (Merck, Darmstadt, Germany), egg yolk powder (Sanovo, Odense, Denmark), pre-gelatinized modified potato starch (E1422, cold swell 3681, Brande, Denmark), xanthan gum, guar gum, citric acid, potassium sorbate, sodium benzoate (Foodchem, Shanghai, China) and mustard powder (G.S. Dunn, Ontario, Canada) were used in the mayonnaise formulation investigated in this research. Vinegar (11%), sugar, salt and soy oil were supplied from the R&D department of local Food Industries.
Methods
Proximate analysis
Faba bean grains were manually cleaned, dehulled and milled into flour after drying. Sieved flour (Mesh size: 60) was characterized on protein, carbohydrate, fat, ash and fiber content according to the standard method approved by American association of cereal chemists (AACC 2000).
Extraction and preparation of faba bean protein
100 g of faba bean flour was dispersed into distilled water at a flour to water ratio of 1:10 and exposed to ultrasonic waves (Toseeh Fanavari Mafoghe sot, model: UP400A) with 200, 300 and 400 W power for 15, 25 and 30 min. Alcalase enzyme (2.4 LFG) with 0.15, 0.3 and 0.45 ratios was then introduced to the beaker for 15, 25 and 35 min. Sodium hydroxide solution (1 N) was used to reach pH of 9 and the suspension was then centrifuged (Behsan, model: B4/100) for 20 min under 4000 rpm. The supernatant was leached when hydrochloric acid (0.1 N) was introduced to obtain pH = 4.5; after 1 h pH was set to 7 with sodium hydroxide solution (2 N) and freeze dried (Gazlin, model: GTFD-20) kept at − 18 °C (Lu et al. 2016; Nivala et al. 2017).
Experimental design
Experimental design of samples with different independant variables (i.e. ultrasound power and duration and enzyme treatment time) as well as statistical analyses were performed using MINITAB software (release 17, Taguchi method) with error probability value of p < 0.05 to optimize the response variables recorded in the characterization section. Nine samples with different ultrasound power/sonication time/enzyme dosage/enzyme treatment time including FBP1(300/35/0.15/25), FBP2(300/25/0.45/15). FBP3(400/35/0.3/15), FBP4(200/15/0.15/15), FBP5(300/15/0.3/35), FBP6(200/25/0.3/25), FBP7(200/35/0.45/35), FBP8(400/25/0.15/35) and FBP9(400/15/0.45/25) were accordingly prepared and characterized. Parametric ANOVA and non-parametric Kruskal–Wallis were adhered in case of normal and abnormal data series distribution, respectively to detect the significant differences. Tukeys pair-wise comparison was also followed to analyze the significance of average results (Golchoobi et al. 2016; Karim et al. 2017).
Protein characterization
Protein solubility measurements were obtained in pH values from 2 to 10 according to Boye et al. (2010) method. 100 g of extracted protein was dispersed into 10 ml water and desired pH was reached using sodium hydroxide (1 N) and hydrochloric acid (1 N). After two subsequent steps of stirring (30 min) and centrifugation (30 min), solubility was calculated on the basis of the protein content in supernatant to that of primary sample.
Fat adsorption capacity 0.5 g of the extracted protein was shaked for 1 min with 3 ml corn oil in a graduated cylinder. Supernatant was weighed out of centrifuged mater (4000 g, 30 min). Calculation was conducted according to (Lin et al. 1974), in duplicate:
| 1 |
Water holding capacity (WHC) Mixture of 0.5 g faba bean protein and 10 ml distilled water was centrifuged after keeping intact for 80 min (Du et al. 2018). Free water volume was measured and WHC was calculated:
| 2 |
Emulsifying capacity According to Boye et al. (2010), 1.5 ml corn oil was introduced to 4.5 ml of 0.5 V % protein solution in 0.01 M phosphate buffer (pH = 7) and mixed with ULTRA-TURRAX homogenizer (IKA, Germany, Model: T25) at 10,000 rpm for 2 min. Absorption capacity of diluted emulsion was determined at 500 nm wavelength using UV–visible spectrophotometer (Unico, USA, Model: uv-2100). Emulsion ability and emulsion stability indexes (EAI and ESI) were then calculated according to:
| 3 |
| 4 |
where A0 and A10 are the absorption content at start point and 10th minute, respectively (ΔA = A0 − A10; Δt = 10 min).
Foaming capacity 50 ml of extracted protein solution (20 mg/ml) was stirred (10,000 rpm, 1 min) in a graduated cylinder through homogenizer (Kaushik et al. 2016) Foaming capacity and Foam stability was calculated based on the recorded volumes:
| 5 |
| 6 |
where V1, V2 and V3 are protein solution volume before homogenization, protein solution volume after homogenization and protein solution foam volume after 10 min of homogenization at ambient, respectively.
Zeta potential 0.05 w/w % protein solution in 0.01 M phosphate buffer (pH = 7) was analyzed with Zetasizer (Nano-ZS, Malvern, UK).
Color measurements Color parameters (i.e. L*, a* and b*) of extracted protein/mayonnaise samples were obtained by Hunter lab color measurement system (Model: Color flex No45/0, Ruston, USA), in duplicate.
Mayonnaise samples preparation
Mayonnaise samples were prepared with sugar (4.8%), oil (40%), vinegar (11%), citric acid (0.1%), salt (1.5%), mustard (0.4%), potassium sorbate (0.01%), sodium benzoate (0.06%), pre-gelatinated modified starch (2%), xanthan (0.2%), guar (0.05%), faba bean protein (0–0.75%), egg yolk powder (0–0.75) and water at laboratory vacuum (VMH-Lab, Arkan Felez) condition using homogenizer mixer. Faba bean protein (0–0.75%) and egg yolk powder (0–0.75%) were considered as independant variables of this research work in reduced-fat mayonnaise formulation (Golchoobi et al. 2016) Five mayonnaise samples with different faba protein/egg yolk powder composition ratios including RP1(0/75), RP2(0.25/0.5), RP3(0.375/0.375), RP4(0.5/0.25), and RP5(0.75/0) were prepared.
Mayonnaise samples characterization
Emulsion stability Evaluation of physical stability of mayonnaise samples was conducted according to the method conducted by Golchoobi et al. (2016).Ten grams of sauce sample was filled in a falcon tube and heated to 80 °C in a water bath for 30 min centrifugation (4200 g) was then conducted for 15 min. After a week, the emulsion stability was determined on the basis of the emulsified layer weight and total sample weight:
| 7 |
Rheological characterization Rheological behavior of the mayonnaise samples was investigated using a 25 mm parallel-plate (Gap = 1 mm) MCR501 rheometer (Paar Physica, Anton Paar GmbH, Graz, Austria) at 25 °C under nitrogen atmosphere, in duplicate. Flow characterization of mayonnaise samples were studied at shear rate of 0.001–100 s−1 fitted to Carreau model:
| 8 |
In which , , , and n are known as relaxation time (s), zero shear viscosity (Pa s), infinite shear viscosity (Pa s), shear rate (s−1), flow behavior index (–), respectively.
Viscoelastic experiments including strain sweep (strain range of 0.01–100% at fixed frequency of 1 Hz) and frequency sweep (frequency range of 0.01–100 Hz at 0.5% strain) were conducted 1 week after samples preparation. Storage modulus (G′), loss modulus (G″) and complex viscosity () were recorded as a function of frequency (Alimi et al. 2013), thereupon (Mirzaei et al. 2018).
Particle size analyses Particle size distribution at 25 °C was performed using Mastersizer 2000 (Malvern Instrument Ltd, Malvern, UK) equipped to quartz cell and laser beam with λ = 634 nm. Surface-weight (d4,3) and volume-weight (d3,2) mean particle sizes as well as span were recorded for the samples diluted with dodecyl sulphate, in duplicate (Golchoobi et al. 2016):
| 9 |
| 10 |
Texture profile analyses Texture analyzer (Model: Texture Pro CT V1.6, Brookfield Engineering Labs Inc., Middleboro, MA, USA) equipped to #TA10 cylindrical texture probe kit was utilized (10 kg load cell at 25 °C) to measure firmness, adhesiveness and cohesiveness of the prepared samples at both target values of 1 and 4 mm/s, in duplicate (Mirzaei et al. 2018).
Optical microscopy Microstructural evaluation of mayonnaise samples were performed using Carl Zeiss optical microscope (Jenaver, Germany).
Results and discussion
Faba bean protein characterization
Proximate analysis on the faba bean flour prepared here resulted in protein, carbohydrate, fat, ash and fiber contents equal to 29.73%, 58%, 1.5%, 3% and 7%, respectively.
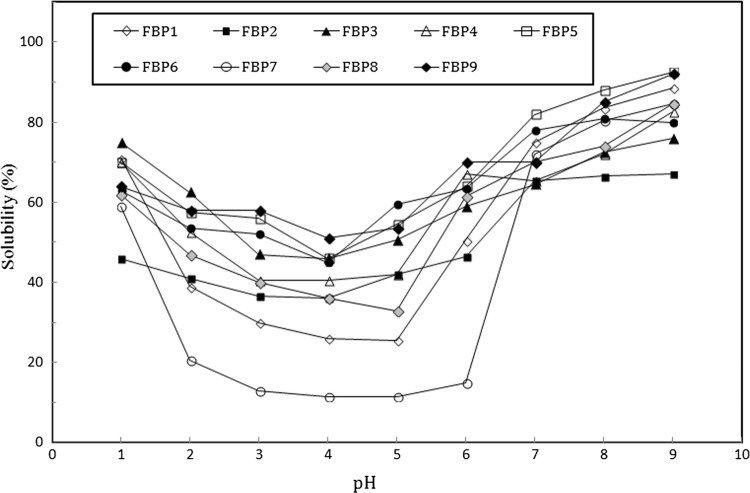
Solubility Solubility of a protein is, in fact, its main functional characteristic since would inevitably affect the gel formation, foaming and emulsifying capacity. Protein solubility can also alter the taste, texture and nutritional value of food (Du et al. 2018). Figure 1 compares solubility of nine faba bean extracted protein samples at pH values between 2 and 10. All samples showed their highest solubility at 2–3 and 7–10 pH values where solubility decreases at pH range of 4, 5 and 6. This is in accordance with the similar observations studying pea, chickpea and lentil protein concentrates using ultra filtration and isoelectric precipitation (Boye et al. 2010) as well as green mung (Du et al. 2018), pinto beans and soya protein isolates (Tan et al. 2014). At isoelectric point, protein is electrically neutral since the net charge is zero. Hydrophobic interactions between proteins is much larger than the hydrophilic repulsion force and hydration generated by the remaining charge; therefore, the least solubility, aggregation and coagulation is expected to happen at the isoelectric point (Constantinides and Adu-Amankwa 1980; Eromosele et al. 2008).
Fig. 1.
Faba bean protein solubility at pH range 2–10
Results show that solubility of the samples undergone by ultrasound and partial enzymatic hydrolysis has significantly grown compared to others (p < 0.05). this is in consistence with the previous results indicating that ultrasonication increases the protein solubility. Increasing the intensity and duration of ultrasound waves would also promote the solubility which is attributed to the partial expansion of protein molecules supporting the interactions between protein and water molecules. The resulting balance in the exposure of hydrophobic/hydrophilic groups to the aqueous media increases the protein solubility (Jiang et al. 2014). Arzeni et al. (2012) also believes that ultrasonication would overally decrease the size of aggregates which would, in turn, promote protein/water interactions leading to the more protein solubility.
Fat adsorption capacity Fat adsorption mechanism is governed by its physical binding with protein constituents and the inherent tendency of non-polar protein chains to bond with fat. Fat adsorption capacity affects the texture, taste and structure of foods and is a useful indication of whether proteins can adsorb and keep oil. In Fig. 2a, a significant difference of 95% is observed in fat adsorption capacity of faba bean protein extracts; as FBP1 (prepared under 200 W ultrasound power for 15 min where 0.15% enzyme hydrolysis reaction was 15 min) has the highest value as 415.3% and FBP4 (prepared under 200 W ultrasound power for 35 min where 0.15% enzyme hydrolysis reaction was 25 min) showed 174.4% fat adsorption capacity as the least of all. Regarding that the fat adsorption capacity of faba bean protein extracted without ultrasound and enzyme treatments were recorded to be 224%. Comparing the results revealed that ultrasonic homogenization along with the enzymatic hydrolysis of protein has had a positive effect on the improvement of fat adsorption capacity. While, Abdel-Aal et al. (1986) reported 76.6% fat adsorption capacity for faba bean protein concentrate extracted with isoelectric method.
Fig. 2.
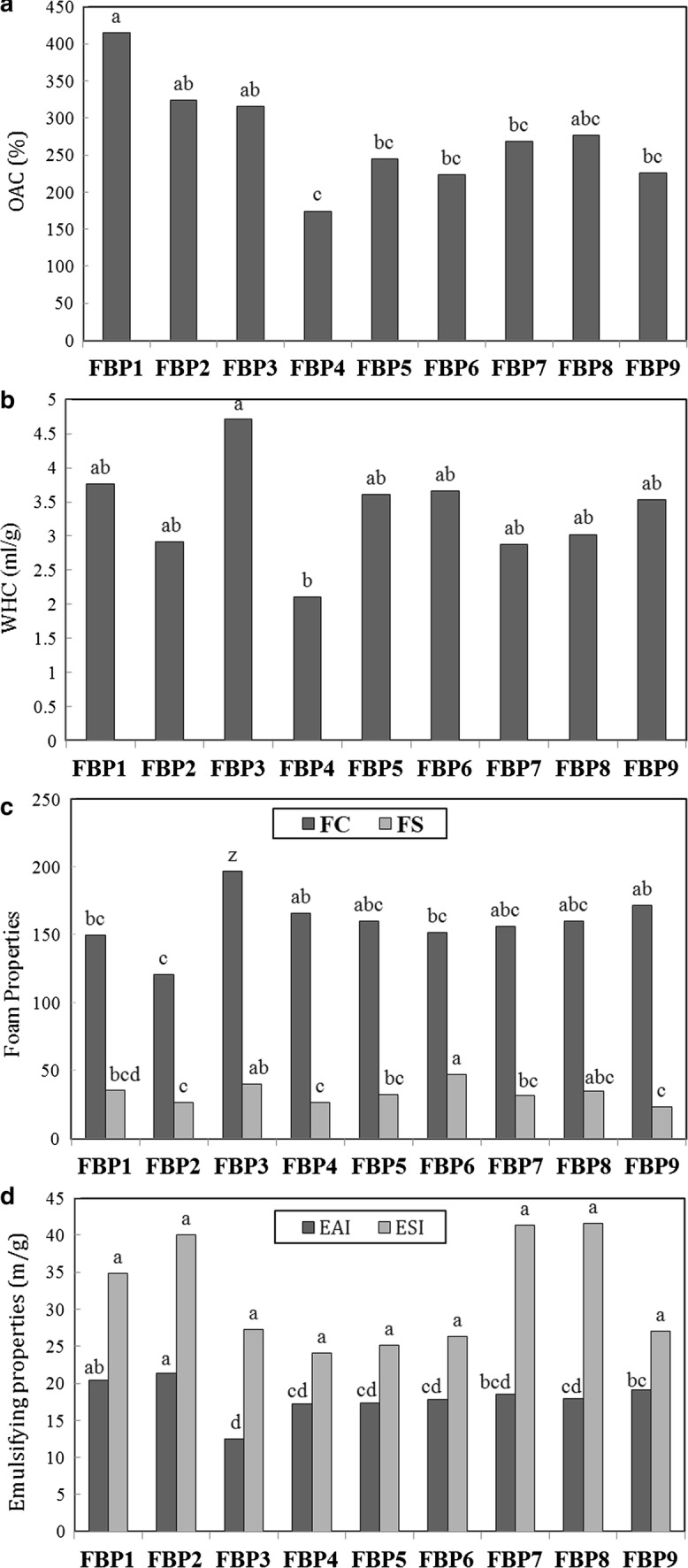
Characteristics of faba bean protein samples a oil adsoption capacity, b water holding capacity, c emulsifying properties and d foaming properties
Water holding capacity Water holding capacity for faba bean protein isolates extracted with ultrasound and enzymatic hydrolysis was measured to be between 2.1 and 4.7 g/g (see Fig. 2b). Comparing the results with the water holding capacity of faba bean protein extracted without ultrasound and enzyme treatments as 2.07 g/g and also the similar research data published for pea, chickpea and lentil protein extracted through ultrafiltration and isoelectric precipitation technique (Boye et al. 2010), one can clearly presume the progress obtained here. Increased ultrasound power and duration as well as enzymatic hydrolysis reaction time was also observed to promote WHC. The highest and least values were recorded for FBP3 and FBP4, respectively where significant difference (p < 0.05) was detected between the measurements. It is known that water holding capacity of a protein could be affected by amino acid composition, protein compatibility and surface polarity to hydrophobicity ratio (Seena and Sridhar 2005).
Emulsifying capacity Faba protein emulsifying capacity was evaluated on the basis of Emulsion ability (EAI) and emulsion stability indexes (ESI). First corresponds to the protein capacity in the formation of an emulsion where the latter provides an indication to the stability of emulsion formed within a specific duration (Okezie and Bello 1988). Results depicted in Fig. 2c show a significant difference between different faba bean samples prepared here (p < 0.05). EAI values were recorded to be between 12.50 m2/g (for FBP3) to 21.42 m2/g (FBP2) compared to the protein extracted without ultrasound and enzymatic hydrolysis reaction as 16.92 m2/g. EAI was also observed to improve with increased ultrasound power and duration as well as enzymatic hydrolysis reaction time. Emulsion stabilities laying between 21.43 min (for FBP4) to 41.60 (for FBP8 presenting the highest value) did not show significant differences (p > 0.05). The least emulsion stability belonged to the untreated sample. It is worth noting that the proteins with lower solubilities do not function as strong emulsifiers; they would either fail forming an emulsion or undergo early coagulation phenomena. Higher levels of solubility facilitates faster penetration of protein chains to the oil–water interfacial phases promoting emulsifying capacity of protein (Deep Singh et al. 2008). do Evangelho et al. (2017) observed that black bean alcalase enzyme hydrolisate protein was much stable than pepsin-induced extracts since the latter contained larger oil droplets. Generation of finer particles at the presence of alcalase-treated protein could be ascribed to the stronger hydrophobicity of produced molecules promoting interactions between protein chains and the emulsion oil droplets in the interfacial layers. According to Sikorski et al. (Sikorski 2001), emulsifying capacity of legums is strongly affected by molecular size, surface hydrophilic groups, network charge and molecular flexibility; however, the last two are most determining among the mentioned physicochemical parameters.
Foaming capacity significant difference was observed in the foaming capacity and foam stability of faba bean protein samples prepared here (p < 0.05). FBP3 and FBP2, respectively presented highest and least values measured here as 197 and 121% (see Fig. 2d); compared to that of 165% for the untreated sample. In fact, results indicate that increased ultrasound power could partially increase foaming capacity whereas introduction of alcalase enzyme reduced the foaming phenomena. Formed foam was as stable as 23.12% and 46.84% for FBP9 and FBP6, respectively showing the highest and least measured values compared to 55.07% corresponding to the untreated sample. This reveals that ultrasonication and partial hydrolysis, both, have had a reverse effect on the foam stability index. This is in accordance with the observations reported by Nivala et al. (2017) where the un-stable foam formed in faba bean protein isolate emulsion did not last for one night and transglutaminase/tyrosynase enzymatic treatment, even, did reduce the foaming capacity. They ascribed the inefficiency of enzymes to the reduced absorption of water/air interfacial layers due to the structural inflexibility. Increasing molecular weight of soluble proteins, such as faba bean protein isolate at neutral pH, has been shown to prevent effective absorption in air/water interface layers. On the other hands, Martínez-Velasco et al. (2018) detected that ultrasound-treated faba bean protein (258.3%) had a significantly higher foaming capacity compared to untreated protein (145.8%) while showed less syneresis along with finer bobbles demonstrating more homogeneity/stability. Sathe and Salunkhe (1981) believes that foaming capacity is directly proportional to the protein molecules’ flexibility that could reduce the interfacial tension. Legume-extracted protein molecules are generally hemispherical; thus presenting less foam formation capacity. Comparing the measurements conducted here, one can conclude that ultrasound power, unlike the enzymatic treatment, could improve foaming capacity.
Zeta potential Protein solution zeta potential is usually positive, if the number of positive charged amino acids are more than negatives. According to the data in Table 1, all faba bean protein samples extracted at different processing parameters showed negative zeta potential values, indicating the domination of negatively charged amino acid moieties in the emulsion media. Although the absolute value of measured zeta potential has increased upon ultrasound treatment but at medium and high ultrasound powers the negative charge steps down. This is important since the solubility, aggregation, coagulation and surface functionality of the protein chains are governed by their effective surface charges. Accordingly, ultrasonication at high and medium intensities as well as medium to high protease enzyme dosages might increase the negative surface charge of protein molecules. This was closely conforming with the results reported by Jiang et al. (2014). They observed that medium intensity ultrasonication increased negative surface charge on black bean protein isolate, strengthened interparticle electrostatic repulsion, disrupted existing protein aggregates, inhibited further aggregation and supported the stability of protein dispersions consequently improving protein solubility. The zeta potential value measured here for untreated faba bean protein extract were the maximum among all samples (− 11.80); whereas alkaline hydrolyzed faba bean protein extracted by Johnston et al. (Johnston et al. 2015) was − 46.4.
Table 1.
Color parameters and zeta potential measurements for faba protein samples extracted at different processing parameters
| Sample | Zeta potential (mV) | L* | b* | a* |
|---|---|---|---|---|
| FBP1 | − 16.55 ± 0.9a | 62.548 ± 1.2a | 25.814 ± 1.9ab | − 6.626 ± 2.2abcd |
| FBP2 | − 22.80 ± 0.2c | 30.862 ± 2.7f | 17.046 ± 2.0c | − 5.120 ± 1.6abc |
| FBP3 | − 23.55 ± 0.6c | 47.236 ± 2.9cd | 25.176 ± 3.3ab | − 9.722 ± 2.4cd |
| FBP4 | − 18.15 ± 0.4a | 53.852 ± 1.1b | 22.650 ± 5.2bc | − 2.454 ± 2.0a |
| FBP5 | − 21.30 ± 0.8bc | 37.970 ± 4.8e | 17.594 ± 2.7c | − 3.206 ± 1.6a |
| FBP6 | − 22.35 ± 0.6c | 43.288 ± 4.8de | 22.322 ± 3.2bc | − 8.692 ± 1.7bcd |
| FBP7 | − 17.95 ± 0.4a | 52.760 ± 0.6bc | 25.632 ± 5.2ab | − 10.226 ± 3.3d |
| FBP8 | − 18.50 ± 0.7a | 52.574 ± 1.5bc | 23.256 ± 3.2abc | − 4.752 ± 2.1ab |
| FBP9 | − 18.90 ± 0.8ab | 43.932 ± 1.3d | 22.548 ± 3.6bc | − 5.970 ± 2.6abcd |
Mean ± SD values followed by the same letter in each column are not significant at p ≤ 0.05 by ANOVA and Tukey’s test
Color analyses Color indexes including L*, a*, b* respectively indicating lightness, redness and yellowness were measured for all faba bean protein samples extracted at different processing conditions (see Table 1). Analysis of variance considering 95% confidence (p < 0.05), did not show significant differences, as FBP1 and FBP2 respectively presented the lightest and darkest samples. In fact, lightness and redness of protein samples are affected by the enzyme dosage used in faba bean flour treatment process. Accordingly, FBP1, FBP4 and FBP8 prepared at lower enzyme dosages turn to light reddish. This was confirmed by the lightness and redness qualities of untreated faba bean protein, i.e. 71.54 and − 1.84.
Processing optimization
Optimized independent variables were determined by Taguchi method on the basis of measured response variables i.e. OAC, WHC, EAI, ESI, FC, FS and Z potential. Software analysis of data in order to select the optimized treatment condition resulted in ultrasound power, ultrasonication time, enzyme dosage and hydrolysis reaction duration of 400 W, 35 min, 0.3% and 25 min, respectively. Selected sample was then prepared and characterized. Results showed acceptable accord between predicted/measured values for OAC (332.16/390.33), WHC (5.12/3.42), EAI (23.50/20.5), ESI (45.57/46.19), FC (44.41/40), FS (197/170) and Z potential (− 25.41/− 23).
Treated mayonnaise characterization
Emulsion stability According to Nikzade et al. (2012), emulsion stability test is performed in order to evaluate the emulsion against flocculation, coalescence and creaming undesirable phenomena. Table 2 summarizes the results on mayonnaise samples containing faba bean protein. All samples showed acceptable high stabilities indicating the formation of thick protein film surrounding oil droplets dispersed within the emulsion which prevents/postpones flocculation and coalescence by reducing the tensions in oil/water interfacial layer consequently resulting in a stable emulsion with finer oil droplets (Herald et al. 2009). Ghoush et al. (2008) as well as Herald et al. (2009) and Alu’datt et al. (2017) reported similar results on stability of emulsions investigating wheat protein and carrageenan, whey protein isolate/concentrate and gum mixtures also faba bean, lupin and pea proteins as substituents of egg yolk in mayonnaise formulation. Moreover, fat content is a determining factor on emulsion stability since restricted mobility of oil droplets at high packing density conditions in high fat real mayonnaise would further expose the emulsion to instability phenomena (Mun et al. 2009); where low or reduced-fat mayonnaises, as studied here, provide the appropriate condition where emulsifiers could substitute egg yolk by reducing the surface tension in the interfacial layer beside hydrocolloids mixtures forming a strong highly viscous gel network in aqueous phase. These results also confirm the results reported for zeta potential and emulsifying capacity whereas the highest value measured for both mentioned parameters belongs to the treated protein samples prepared at higher ultrasound powers and enzyme dosage levels (in optimized protein) in which higher levels of solubility and consequently faster penetration of protein chains to the oil–water interfacial phases has promoted emulsion stability in mayonnaise samples (Nikzade et al. 2012; Golchoobi et al. 2016).
Table 2.
Different characteristics of mayonnaise samples
| Characteristics | RP1 | RP2 | RP3 | RP4 | RP5 |
|---|---|---|---|---|---|
| Stability | |||||
| Emulsion stability (%) | 100 ± 0.00a | 100 ± 0.00a | 100 ± 0.00a | 100 ± 0.00a | 98.77 ± 0.01b |
| Texture | |||||
| Firmness (N) | 1.11 ± 0.01b | 1.02 ± 0.03b | 1.53 ± 0.04a | 1.22 ± 0.02ab | 0.77 ± 0.10c |
| Adhesiveness (J) | 0.51 ± 0.01b | 0.51 ± 0.01b | 0.51 ± 0.01b | 0.61 ± 0.01a | 0.41 ± 0.02c |
| Cohesiveness (–) | 0.74 ± 0.01b | 0.70 ± 0.01bc | 0.88 ± 0.02a | 0.67 ± 0.02bc | 0.65 ± 0.02c |
| Carreau parameters | |||||
| η0 (Pa s) | 9292.5 ± 2.1b | 8436.0 ± 1.4d | 10,471.5 ± 2.1a | 9255.0 ± 1.4c | 6002.0 ± 1.4e |
| η∞ (Pa s) | 1.21 ± 0.01a | 1.01 ± 0.01a | 1.04 ± 0.01a | 1.04 ± 0.01a | 1.86 ± 0.01a |
| λ (s) | 124.96 ± 0.01e | 128.18 ± 0.01c | 126.51 ± 0.01d | 135.35 ± 0.01b | 158.28 ± 0.01a |
| n (–) | 0.45 ± 0.01a | 0.45 ± 0.01a | 0.45 ± 0.01a | 0.45 ± 0.01a | 0.42 ± 0.01a |
| R2 | 0.99 ± 0.01a | 0.99 ± 0.01a | 0.99 ± 0.01a | 0.98 ± 0.01a | 0.98 ± 0.01a |
| Color | |||||
| L* | 80.01 ± 0.8a | 74.97 ± 1.1b | 72.10 ± 0.8c | 72.34 ± 2.6bc | 77.93 ± 1.2a |
| b* | 13.81 ± 0.5a | 14.80 ± 1.1a | 13.77 ± 1.5a | 15.36 ± 0.5a | 13.63 ± 0.9a |
| a* | − 4.37 ± 0.6b | − 2.34 ± 0.5a | − 1.40 ± 1.0a | − 1.71 ± 1.3a | − 3.06 ± 0.9ab |
| Particle size distribution parameters | |||||
| Span | 9.08 ± 0.03a | 9.63 ± 0.29a | 5.82 ± 0.10bc | 5.07 ± 0.24c | 6.75 ± 0.34b |
| d[4,3] | 16.61 ± 0.48b | 41.11 ± 0.05a | 9.85 ± 0.14e | 12.16 ± 0.12d | 13.74 ± 0.15c |
| d[3,2] | 3.52 ± 0.14ab | 3.94 ± 0.07a | 3.04 ± 0.14b | 3.81 ± 0.19a | 3.66 ± 0.11a |
Mean ± SD values followed by the same letter in each column are not significant at p ≤ 0.05 by ANOVA and Tukey’s test
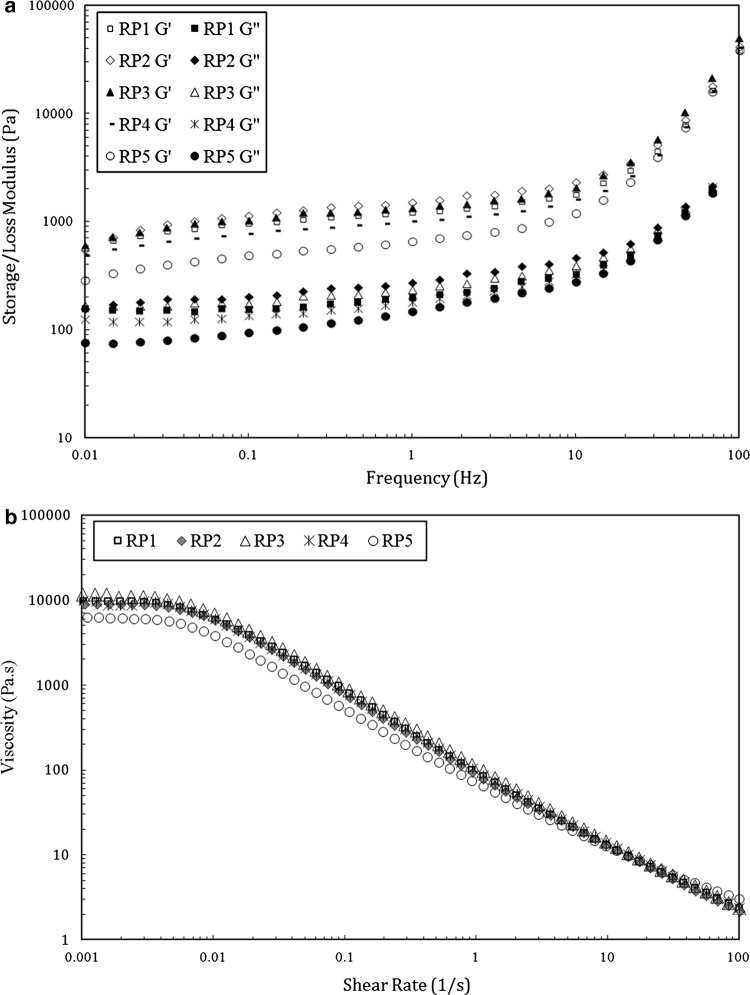
Rheological measurements Flow behavior-Fig. 3a demonstrates apparent viscosity versus shear rate. Viscosity shows a reducing trend with shear rate in all samples indicating the pseudoplastic shear thining behavior of mayonnaise samples. This behavior is the result of the structural destruction implied by the shear in thick emulsions where aggregation of particles forms a three dimensional network composed of oil droplets. Shear, in fact, deforms the aggregated oil droplets which would consequently reduce the viscosity (Nikzade et al. 2012).
Fig. 3.
Rheological characteriostics of mayonnaise samples a apparent viscosity versus shear rate, b storage and loss modulus versus frequency
Fitting the flow diagram of treated mayonnaise samples to Carreau rheological model (Table 2), estimates lower zero shear rate viscosities (η0) for all samples, except PR3, compared to PR1 conventional mayonnaise formulation. Equal composition of faba bean protein and egg yolk powder has resulted in a remarkable increase in zero shear rate viscosity. Moreover, PR5 presented the highest infinitive shear rate viscosity. Relaxation times greater than zero confirms the non-Newtonian characteristics of prepared samples where flow behavior index (n) values near unity are indicative of shear thinning nature of the mayonnaise samples (Mirzaei et al. 2018).
Viscoelastic properties Fig. 3b depicts storage and loss modulus versus frequency for all mayonnaise samples. G′ and G″ are observed to be frequency dependant and G′ > G″ indicating elastic dominant behavior corresponded to weak gels. Similar trends have also been reported before (Mancini et al. 2002; Mun et al. 2009).
Storage and loss modulus of samples containing 0–0.75% faba protein and egg yolk are consistently similar indicating equal viscoelastic characteristics. Regarding the low-frequency behaviors recorded, all samples, except RP5, present physical stability at long storage conditions. Sample RP3, containing equal compositions of egg yolk and faba protein, shows the highest modulus at frequencies above 50 Hz which reveals the short-term emulsion stability of this sample at higher shear rates confirmed by flow behavior and emulsion stability results reported above.
Texture properties Effect of different composition ratios of faba bean protein/egg yolk powder on the texture of mayonnaise samples has been summarized in Table 2. Firmness and cohesiveness depicted a significant difference (p < 0.05). Sample RP5 showed the least Firmness and cohesiveness indexes (0.77 and 0.65, respectively); whereas RP3 and RP4 measured to be 1.53 and 1.22, as the highest values of firmness among other. Liu et al. (2007), Golchoobi et al. (2016) and Maani et al. (2017) also reported similar behavior implying the influence of oil dispersed droplet on the texture characteristics of mayonnaise emulsions, as finer dispersed emulsions present higher physical stability, monodisperstity and elastic properties. These results are in accordance with the rheological analysis as well as particle size distribution and optical microscopy, reported here.
Adhesiveness also showed significant differences with introduction of faba bean protein to the reduced-fat mayonnaise formulations studied here (p < 0.05); as RP2 and RP3 did not significantly differ with RP1 (p > 0.05) and RP5 presented the least adhesiveness.
Particle size distribution Span, d[3,2] and d[4,3] values summarized in Table 2 had a significant difference (p < 0.05). d[3,2] and d[4,3] in RP4 and RP3 were the least among other samples indicating their finer average particle size; where wider particle size distribution in curve (i.e. span) corresponding to these samples suggested further oil droplet monodispersity. Equal compositions of egg yolk powder and faba bean protein has resulted in the finest mean particle diameters which could be ascribed to the favorable emulsifying capacity and surface functionality of faba protein molecules besides egg yolk phospholipids and proteins which would reduce the surface tensions forming a flexible protein film around dispersed oil droplets preventing their coalescence and flocculation. These results do confirm Microstructural observations and emulsion stability analysis reported here. Larger contact area between finer oil droplets would also intensify the frictional forces against emulsion free flow under shear which would increase the viscosity. The leading rheological characteristics observed for RP3 and RP4 were also verified in viscoelastic measurement results (Liu et al. 2007; Golchoobi et al. 2016).
Color characteristics Table 2 compares the color indexes of mayonnaise samples containing different compositions, showing significant differences between the conventional formulation and those containing faba bean protein besides egg yolk (p < 0.05), except b*. Highest lightness was presented by RP1 with no faba bean protein but no significant difference is detected with RP5 containing 0.75% faba protein and no egg yolk (p > 0.05). Increasing faba bean protein composition has not altered yellowness of mayonnaise samples; it can be concluded that introduction of this protein has not had negative effects on the color characteristics of sauce.
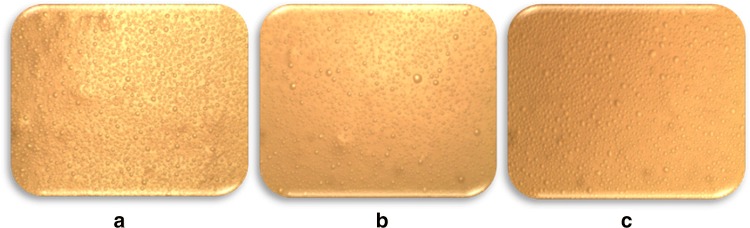
Optical microscopy Microstructure of reduced-fat mayonnaise samples RP1, RP3 and RP5 containing treated faba protein was observed through optical microscope (Fig. 4). Emulsifier type and concentration, particle size, oil concentration and aquouse phase viscosity are shown to alter the microstructural characteristics (Mun et al. 2009). Finer particle average size and higher monodispersity levels were obviously detected in RP3 confirming particle distribution analyses results. Oil droplets’ size in RP1 with no faba bean protein is larger compared to RP5. Improved monodispersity at the presence of faba bean protein is also indicated by span results.
Fig. 4.
Optical microscope photographs of a RP1, b RP3 and c RP5
Conclusion
Faba bean protein extraction was performed under different ultrasonication and enzymatic hydrolysis processing parameters. Results revealed improved functional properties including emulsifying capacity, foaming capacity, water holding capacity and water/oil adsorption. Ultrasound power, ultrasonication time, enzyme dosage and hydrolysis reaction duration was optimized to 400 W, 35 min, 0.3% and 25 min, considering the determining functional characteristics using Taguchi experimental design method. Selected protein extract was substituted with egg yolk in reduced-fat mayonnaise formulation. Significant difference was detected in the physical emulsion stability, viscosity, viscoelastic properties at different shear rates, texture, dispersed particle size, monodispersity and microstructure of the winning samples, namely RP3 containing equal compositions of faba bean protein and egg yolk powder (0.375%), and RP4 (0.5% faba bean protein and 0.25% egg yolk powder) compared to the conventional formulation (RP1).
Acknowledgements
R&D department of Behrouz Food Industries Company (Tehran, Iran) is gratefully acknowledged for the technical and financial supports.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- AACC . Approved method 56-30. 10. St. Paul: American Association of Cereal Chemists Incorporation; 2000. [Google Scholar]
- Abdel-Aal ESM, Shehata AA, El-Mahdy AR, Youssef MM. Extractability and functional properties of some legume proteins isolated by three different methods. J Sci Food Agric. 1986;37:553–559. [Google Scholar]
- Alimi M, Mizani M, Naderi G, Shokoohi S. Effect of inulin formulation on the microstructure and viscoelastic properties of low-fat mayonnaise containing modified starch. J Appl Polym Sci. 2013;130:801–809. [Google Scholar]
- Alu’datt MH, Rababah T, Alhamad MN, Ereifej K, Gammoh S, Kubow S, Tawalbeh D. Preparation of mayonnaise from extracted plant protein isolates of chickpea, broad bean and lupin flour: chemical, physiochemical, nutritional and therapeutic properties. J Food Sci Technol. 2017;54:1395–1405. doi: 10.1007/s13197-017-2551-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzeni C, Martínez K, Zema P, Arias A, Pérez O, Pilosof A. Comparative study of high intensity ultrasound effects on food proteins functionality. J Food Eng. 2012;108:463–472. [Google Scholar]
- Boye J, Aksay S, Roufik S, Ribéreau S, Mondor M, Farnworth E, Rajamohamed S. Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Res Int. 2010;43:537–546. [Google Scholar]
- Cepeda E, Villaran M, Aranguiz N. Functional properties of faba bean (Vicia faba) protein flour dried by spray drying and freeze drying. J Food Eng. 1998;36:303–310. [Google Scholar]
- Chen L, Chen J, Ren J, Zhao M. Effects of ultrasound pretreatment on the enzymatic hydrolysis of soy protein isolates and on the emulsifying properties of hydrolysates. J Agric Food Chem. 2011;59:2600–2609. doi: 10.1021/jf103771x. [DOI] [PubMed] [Google Scholar]
- Coda R, Varis J, Verni M, Rizzello CG, Katina K. Improvement of the protein quality of wheat bread through faba bean sourdough addition. LWT Food Sci Technol. 2017;82:296–302. [Google Scholar]
- Constantinides A, Adu-Amankwa B. Enzymatic modification of vegetable protein: mechanism, kinetics, and production of soluble and partially soluble protein in a batch reactor. Biotechnol Bioeng. 1980;22:1543–1565. [Google Scholar]
- Deep Singh G, Wani AA, Kaur D, Sogi DS. Characterisation and functional properties of proteins of some Indian chickpea (Cicer arietinum) cultivars. J Sci Food Agric. 2008;88:778–786. [Google Scholar]
- do Evangelho JA, Vanier NL, Pinto VZ, De Berrios JJ, Dias ARG, da Rosa Zavareze E. Black bean (Phaseolus vulgaris L.) protein hydrolysates: physicochemical and functional properties. Food Chem. 2017;214:460–467. doi: 10.1016/j.foodchem.2016.07.046. [DOI] [PubMed] [Google Scholar]
- Du M, Xie J, Gong B, Xu X, Tang W, Li X, Li C, Xie M. Extraction, physicochemical characteristics and functional properties of mung bean protein. Food Hydrocoll. 2018;76:131–140. [Google Scholar]
- Eromosele C, Arogundade L, Eromosele I, Ademuyiwa O. Extractability of African yam bean (Sphenostylis stenocarpa) protein in acid, salt and alkaline aqueous media. Food Hydrocoll. 2008;22:1622–1628. [Google Scholar]
- Ghoush MA, Samhouri M, Al-Holy M, Herald T. Formulation and fuzzy modeling of emulsion stability and viscosity of a gum–protein emulsifier in a model mayonnaise system. J Food Eng. 2008;84:348–357. [Google Scholar]
- Golchoobi L, Alimi M, Shokoohi S, Yousefi H. Interaction between nanofibrillated cellulose with guar gum and carboxy methyl cellulose in low-fat mayonnaise. J Texture Stud. 2016;47:403–412. [Google Scholar]
- Hendawey M, Younes A. Biochemical evaluation of some faba bean cultivars under rainfed conditions at El-Sheikh Zuwayid. Ann Agric Sci. 2013;58:183–193. [Google Scholar]
- Herald TJ, Abugoush M, Aramouni F. Physical and sensory properties of egg yolk and egg yolk substitutes in a model mayonnaise system. J Texture Stud. 2009;40:692–709. [Google Scholar]
- Jiang L, Wang J, Li Y, Wang Z, Liang J, Wang R, Chen Y, Ma W, Qi B, Zhang M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res Int. 2014;62:595–601. [Google Scholar]
- Jiang Z-q, Pulkkinen M, Wang Y-j, Lampi A-M, Stoddard FL, Salovaara H, Piironen V, Sontag-Strohm T. Faba bean flavour and technological property improvement by thermal pre-treatments. LWT Food Sci Technol. 2016;68:295–305. [Google Scholar]
- Johnston SP, Nickerson MT, Low NH. The physicochemical properties of legume protein isolates and their ability to stabilize oil-in-water emulsions with and without genipin. J Food Sci Technol. 2015;52:4135–4145. doi: 10.1007/s13197-014-1523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim M, Alimi M, Shokoohi S, Fazeli F. Effect of long-chain inulin and modified starch on the physicochemical and rheological properties of doogh (Iranian yogurt drink) Acta Aliment. 2017;46:51–60. [Google Scholar]
- Kaushik P, Dowling K, McKnight S, Barrow CJ, Wang B, Adhikari B. Preparation, characterization and functional properties of flax seed protein isolate. Food Chem. 2016;197:212–220. doi: 10.1016/j.foodchem.2015.09.106. [DOI] [PubMed] [Google Scholar]
- Lin MJ-Y, Humbert E, Sosulski F. Certain functional properties of sunflower meal products. J Food Sci. 1974;39:368–370. [Google Scholar]
- Liu H, Xu X, Guo SD. Rheological, texture and sensory properties of low-fat mayonnaise with different fat mimetics. LWT Food Sci Technol. 2007;40:946–954. [Google Scholar]
- Lu W, Chen X-W, Wang J-M, Yang X-Q, Qi J-R. Enzyme-assisted subcritical water extraction and characterization of soy protein from heat-denatured meal. J Food Eng. 2016;169:250–258. [Google Scholar]
- Maani B, Alimi M, Shokoohi S, Fazeli F. Substitution of modified starch with hydrogen peroxide-modified rice bran in salad dressing formulation: physicochemical, texture, rheological and sensory properties. J Texture Stud. 2017;48:205–214. doi: 10.1111/jtxs.12229. [DOI] [PubMed] [Google Scholar]
- Mancini F, Montanari L, Peressini D, Fantozzi P. Influence of alginate concentration and molecular weight on functional properties of mayonnaise. LWT Food Sci Technol. 2002;35:517–525. [Google Scholar]
- Martínez-Maqueda D, Hernández-Ledesma B, Amigo L, Miralles B, Gómez-Ruiz JÁ. Extraction/fractionation techniques for proteins and peptides and protein digestion. In: Toldrá F, Nollet L, editors. Proteomics in foods. Berlin: Springer; 2013. pp. 21–50. [Google Scholar]
- Martínez-Velasco A, Lobato-Calleros C, Hernández-Rodríguez BE, Román-Guerrero A, Alvarez-Ramirez J, Vernon-Carter EJ. High intensity ultrasound treatment of faba bean (Vicia faba L.) protein: effect on surface properties, foaming ability and structural changes. Ultrason Sonochem. 2018;44:97–105. doi: 10.1016/j.ultsonch.2018.02.007. [DOI] [PubMed] [Google Scholar]
- Mirzaei M, Alimi M, Shokoohi S, Golchoobi L. Synergistic interactions between konjac-mannan and xanthan/tragacanth gums in tomato ketchup: physical, rheological, and textural properties. J Texture Stud. 2018;49:586–594. doi: 10.1111/jtxs.12359. [DOI] [PubMed] [Google Scholar]
- Mu L, Zhao M, Yang B, Zhao H, Cui C, Zhao Q. Effect of ultrasonic treatment on the graft reaction between soy protein isolate and gum acacia and on the physicochemical properties of conjugates. J Agric Food Chem. 2010;58:4494–4499. doi: 10.1021/jf904109d. [DOI] [PubMed] [Google Scholar]
- Multari S, Stewart D, Russell WR. Potential of fava bean as future protein supply to partially replace meat intake in the human diet. Compr Rev Food Sci Food Saf. 2015;14:511–522. [Google Scholar]
- Mun S, Kim Y-L, Kang C-G, Park K-H, Shim J-Y, Kim Y-R. Development of reduced-fat mayonnaise using 4αGTase-modified rice starch and xanthan gum. Int J Biol Macromol. 2009;44:400–407. doi: 10.1016/j.ijbiomac.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Nikzade V, Tehrani MM, Saadatmand-Tarzjan M. Optimization of low-cholesterol–low-fat mayonnaise formulation: effect of using soy milk and some stabilizer by a mixture design approach. Food Hydrocoll. 2012;28:344–352. [Google Scholar]
- Nivala O, Mäkinen OE, Kruus K, Nordlund E, Ercili-Cura D. Structuring colloidal oat and faba bean protein particles via enzymatic modification. Food Chem. 2017;231:87–95. doi: 10.1016/j.foodchem.2017.03.114. [DOI] [PubMed] [Google Scholar]
- Okezie BO, Bello A. Physicochemical and functional properties of winged bean flour and isolate compared with soy isolate. J Food Sci. 1988;53:450–454. [Google Scholar]
- Rosa-Sibakov N, Heiniö R-L, Cassan D, Holopainen-Mantila U, Micard V, Lantto R, Sozer N. Effect of bioprocessing and fractionation on the structural, textural and sensory properties of gluten-free faba bean pasta. LWT Food Sci Technol. 2016;67:27–36. [Google Scholar]
- Sathe S, Salunkhe D. Functional properties of the great northern bean (Phaseolus vulgaris L.) proteins: emulsion, foaming, viscosity, and gelation properties. J Food Sci. 1981;46:71–81. [Google Scholar]
- Seena S, Sridhar K. Physiochemical, functional and cooking properties of Canavalia. J Food Chem. 2005;32:406–412. [Google Scholar]
- Sikorski ZE. Functional properties of proteins in food systems. In: Sikorski ZE, editor. Chemical and functional properties of food proteins. Florida: CRC Press; 2001. pp. 113–135. [Google Scholar]
- Tan E-S, Ying-Yuan N, Gan C-Y. A comparative study of physicochemical characteristics and functionalities of pinto bean protein isolate (PBPI) against the soybean protein isolate (SPI) after the extraction optimisation. Food Chem. 2014;152:447–455. doi: 10.1016/j.foodchem.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Zee JA, Boudreau A, Bourgeois M, Breton R. Chemical composition and nutritional quality of faba bean (Vicia faba L. Minor) based tofu. J Food Sci. 1988;53:1772–1774. [Google Scholar]