Abstract
With the aim of developing a fruit-based beverage in products which are severely damaged by heat, a high-intensity ultrasound treatment combined with moderate heat treatment (called thermosonication) was applied. A fruit smoothie (mango, jackfruit and rice milk) was thermosonicated applying a Box–Benhken model with amplitude (70, 77.5 or 85%), time (15, 20 or 25 min) and temperature (40, 47.5 or 55 °C) as independent variables. From the obtained samples, microbiological (aerobic mesophilic and Enterobacteriaceae), physicochemical (pH, soluble solids and cloud index) and enzymatic analysis (polyphenol oxidase and pectin methylesterase) were carried out. Aerobic mesophiles and Enterobacteria inactivation in thermosonicated samples were 4.55 Log CFU/mL and 3.85 Log CFU/mL, respectively in most of the treatments applied, being influenced by linear terms of amplitude and temperature (p < 0.001). The cloud index was influenced by time term (p < 0.0001); meanwhile, interaction of amplitude * temperature (p < 0.01) and quadratic of time presented significant effect (p < 0.001) on polyphenol oxidase activity. Further, amplitude term had a significant effect (p < 0.001) on the decrease on pectin methylesterase enzymatic activity. The optimal process condition was 77.5% amplitude, 20 min and 47.5 °C. Thermosonication probed to be effective to control both enzymatic activities in treatments with high amplitudes combined with moderated temperature treatments. Based on this, the use of thermosonication is a viable alternative for fruit-based beverage preservation, that may employ perishable regional natural products offering them an added value.
Keywords: Thermosonication, Fruit smoothie, Microbial inactivation, Enzymatic inactivation
Introduction
Changes in the consumer habits for maintaining a diet that promotes better health have increased the demand of functional foods that supply antioxidants, vitamins, and other nutritive and functional compounds (Ribeiro et al. 2019; Abdullah and Chin 2014). Fruit smoothies are considered as functional beverages, gaining acceptance and a rapid growth (Chatterjee et al. 2015). The term “smoothie” is given to a blended fruit beverage characterized by a pulpy consistency, containing one or more fruits, yogurt, cow’s milk or, vegetables milk (Morales de la Peña et al. 2018). Tropical fruits such as mango (Mangifera indica) and jackfruit (Artocarpus heterophyllus) have proven to be a good sources of vitamin C and B such as phenolic compounds, carotenoids, and others bioactive compounds (Devalaraja et al. 2011). The addition of tropical fruits to smoothie’s elaboration not only contributes to the functional aspect but also gives excellent sensory characteristics (exotic taste, color, flavor) (Morales de la Peña et al. 2018). Moreover, in places with over fruit production (Mexico is the fourth world position of mango) it is important to develop added value products to address the overproduction in order to improve the region income (Worldatlas 2017). In addition, the development of smoothies from fruits not highly commercially exploited such as jackfruit, which could be an alternative to generate products with added value. The addition of vegetable milk as a rice-based in a smoothie formulation provide flavonoid compounds as ferulic acid, γ-tocotrienol, and α-tocopherol (homologue of vitamin E) (Lin and Lai 2011) and facilitates their consumption by lactose intolerant population (Andrés et al. 2016). In the case of fruit-smoothies, these should be consumed shortly after preparation to avoid fruit particle precipitation due are highly sensitive products whose characteristics can be modified during storage (Morales de la Peña et al. 2018). Conventional pasteurization used in the beverage industry to control spoilage and pathogenic microorganism, is not an option for this kind of products since it would reduce “freshness”, affecting the nutritional properties and the main quality characteristics required by consumers (color, flavor, taste, etc.) (Khandpur and Gogate 2016). Therefore, the necessity to preserve the product with minor sensory and physicochemical changes gives an opportunity to implement an emerging technology. Among emerging technologies, ultrasound (US) is considered a ‘green’ technology due to its high efficiency, low instrumental requirements compared with other conventional processing techniques and its economically viable performance (Mason and Peters 2002). Regarding US application on fruit beverages, diverse ultrasound treatments have been applied (apple, cactus pear, blackberry, etc.) resulted in an increase in shelf life with minor changes in the processed product (Zafra-Rojas et al. 2013; Mohideen et al. 2015). In this sense, in a mango smoothie made with whole milk and soymilk, a sonicated product (100% amplitude, 20 min, 35 and 55 °C) with similar physicochemical (pH, titratable acidity and soluble solids content) and nutritional (carotenoid profile) characteristics than the fresh product was obtained (Morales de la Peña et al. 2018). Ultrasound can be successfully employed to fruit smoothie processing, valuing the improvements in quality and safety from time, temperature and US parameters. Therefore, the aim of this research work was to evaluate the combined effect of different parameters (US amplitude, treatment time and temperature) on the quality characteristics of a fruit smoothie.
Materials and methods
Fruit smoothie preparation
Mango (Manguifera indica L., cv Ataulfo) and jackfruit fruit (Artocarpus heterophylius L.) were obtained from local producers (Nayarit, Mexico) and a vegetable commercial rice beverage (Nature’s heart terrafertil®, USA) was obtained from a local supermarket. The smoothie was prepared mixing mango (24%), jackfruit (6%) and the rice beverage (70%). Fruits and rice beverage were blended in a homogenizer (Model LI-3 International®, Mexico) for 5 min.
Thermosonication treatment
Based on the US treatments employed by Morales de la Peña et al. (2018) with some modifications. Fruit smoothies were treated by a high intensity ultrasound equipment (VCX-1500, Sonics & Materials, Inc., Newtown, USA) at 1500 W using a 25-mm probe connected to an amplitude transformer (booster). Amplitude level was set at 70–85 amplitude levels with pulse length of 2 s on and 4 s off according to the experimental design (Table 1). A constant frequency of 20 kHz and the energy input was controlled by adjusting the amplitude of the sonication probe. During sonication (70–85%), samples (400 mL) were heated in the jacketed vessel during 15 or 25 min reaching an outlet temperature of 40 or 55 °C according to the experimental design (Table 1). Sample overheating was prevented by employing a heat exchanger (Cole Parmer, USA) through the treatment chamber.
Table 1.
Experimental design matrix for thermosonication treatments on fruit smoothie
| No. | Pattern | Amplitude (%) X1 |
Time (min) X2 |
Temperature (°C) X3 |
|---|---|---|---|---|
| 1 | +0+ | 85 | 20 | 55 |
| 2 | 000 | 77.5 | 20 | 47.5 |
| 3 | −+0 | 70 | 25 | 47.5 |
| 4 | +−0 | 85 | 15 | 47.5 |
| 5 | −−0 | 70 | 15 | 47.5 |
| 6 | 0−+ | 77.5 | 15 | 55 |
| 7 | 000 | 77.5 | 20 | 47.5 |
| 8 | 0−− | 77.5 | 15 | 40 |
| 9 | 000 | 77.5 | 20 | 47.5 |
| 10 | 0+− | 77.5 | 25 | 40 |
| 11 | +0− | 85 | 20 | 40 |
| 12 | 000 | 77.5 | 20 | 47.5 |
| 13 | 000 | 77.5 | 20 | 47.5 |
| 14 | −0− | 70 | 20 | 40 |
| 15 | −0+ | 70 | 20 | 55 |
| 16 | ++0 | 85 | 25 | 47.5 |
| 17 | 0++ | 77.5 | 25 | 55 |
pH and total soluble solids
pH was measured using a digital pH meter (Hanna, PH210, Rumania) and total soluble solids (°Brix) were analyzed using a refractometer (Trading Co., Brix/ATC FG-113, China) (AOAC 2005).
Microbiological analysis
Serial dilutions were prepared by mixing sterilized distilled peptone water followed by further decimal dilutions of samples (up to 103). One milliliter of each dilution was transferred into sterile petri dishes containing the corresponding medium. Aerobic mesophilic (AM) was measured in plate count agar incubated (LSI-3016ª Labtech, Korea) at 30 °C for 48 h. Enterobacteriaceae (EB) were determined in violet red bile glucose agar incubated at 37 °C for 24 h, and malt extract agar was used to assess the presence of yeast and molds after incubation for 72 h at 25 °C (Cruz et al. 2007). Typical colonies were counted, and the results were expressed as Log colony forming units per milliliter (Log CFU/mL) of sample.
Cloud index
Samples (5 mL) were centrifuged at 1026 g (V6500, Hamilton Bell, USA) for 10 min at room temperature. The supernatant absorbance was measured at 660 nm using a microplate reader (Power Wave XS UV-Biotek, USA) using distilled water as blank (Versteeg et al. 1980).
Pectin methylesterase and polyphenol oxidase activity
Pectin methyl esterase (PME) activity was evaluated by titration of the free carboxyl groups released at pH 7.5 for 30 min. A 5 mL aliquot was added to 50 mL of 1% citrus pectin solution containing NaCl 0.3 M. The pectin/fruit smoothie mixture was adjusted to pH 7.5 with NaOH 0.02 N. Once the pH 7.5 was reached, the consumption of NaOH during a 30 min was recorded (Rouse and Atkins 1955).
Polyphenol oxidase residual (PPO) activity was assessed spectrophotometrically (Agilent, Cary 3500, USA) at 25 °C using catechol as substrate and recording the increase in absorbance at 420 nm. One unit of PPO activity was defined as 0.001 DA420/min/mL (Cano et al. 1997). PME and PPO results were expressed as unit of enzymatic activity per milliliter (UEA/ml) of sample.
Experimental design
In the present study the optimization of the thermosonication conditions were performed using response surface methodology, where ultrasound amplitude (X1, 70–85%), treatment time (X2, 15–25 min) and processing temperature (X3, 40–55 °C) were selected as independent processing parameters on the dependent variables (pH, total soluble solids, AM, EB, yeast and molds, cloud index, PME and PPO activity). Box–Behnken design was used and the complete design consisted of seventeen combinations including five replicates of the center point (Table 1). Experimental data from the Box–Behnken design was analyzed using a response surface regression (JMP 7.0.2, SAS Institute Inc., 2007) fitted to a second-order polynomial model:
where Y is the predicted response, β0 the constant (intercept), βi the linear coefficient, βii the quadratic coefficient and βij is the cross-product coefficient. Xi and Xj are independent variables. The Design Expert software (SigmaPlot 12.0, Institute Inc.) was used to obtain the three-dimensional curves from the response surface analysis.
Results and discussion
pH and total soluble solids
All the response variables exhibited high correlation coefficients with the mathematical model as demonstrated by the R-square (R2) with values ≥ 0.90 and 0.93 for pH and total soluble solids, respectively. The pH and total soluble solids of thermosonicated fruit smoothie in all treated samples were in the range of 4.54–5.19 and 13.1–14.8, respectively. In the regression analysis, pH was influenced by the parameters of time (linear terms) and amplitude (quadratic terms) both (X2 and X1 * X1, respectively) at p < 0.01 and the interaction of amplitude and temperature (p < 0.05). Meanwhile, the soluble solid content was significantly influenced by all independent parameters evaluated (except to quadratic terms of amplitude) mainly quadratic terms of time (X2 * X2) at p < 0.001 (data not shown).
Microbiological analysis
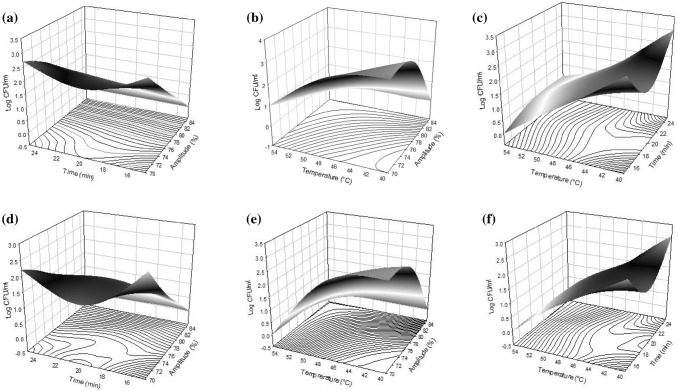
Thermosonication is a technique that reduces microbial population due to the synergic effect of cavitation and temperature on microorganism cell (Muñoz et al. 2012). In the present study, an AM count of 4.55 Log CFU/mL was observed in the fruit smoothie control and as it was expected, higher inactivation was achieved when the amplitude, temperature and time treatment increased, reached until a total inactivation in some treatments (Table 2). Many authors have evaluated the effect of thermosonication on microbial loads with important results. In this sense, Herceg et al. (2013) detected a complete AM inactivation in thermosonication treatments of 23% amplitude, 9 min at 55 °C in strawberry juice. In that case, the authors concluded that the thermal component in the treatment was the most significant for obtaining the complete AM inactivation. Further, some other authors presented studies of juices treated by thermosonication in a range of 10–20 °C with inactivation levels around 1 and 1.5 Log CFU/mL (Abid et al. 2014; Jabbar et al. 2014), which were below the results obtained in the present work. The differences between the inactivation observed may be explained by the treatment temperature applied, which was remarkably lower than the temperatures applied in the present study. A significant influence at p < 0.001 in linear term of amplitude (X1) and linear term of temperature (X3) at p < 0.01 was obtained for AM counts (Table 3). This indicates that increasing the amplitude or temperature, a decrease of AM was obtained (Fig. 1a–c).
Table 2.
Values of quality attributes for fruit smoothie thermosonicated at different amplitude, time and temperature according to experimental design
| Treatment | AMa | EBb | Cloud | PPOc | PMEd | ||
|---|---|---|---|---|---|---|---|
| Amplitude (%) | Time (min) | Temperature (°C) | |||||
| 85 | 20 | 55 | 0.0 | 0.0 | 0.57 | 4.1 | 0.08 |
| 77.5 | 20 | 47.5 | 1.7 | 1.7 | 0.25 | 4.73 | 0.12 |
| 70 | 25 | 47.5 | 2.7 | 2.2 | 1.87 | 6.3 | 0.11 |
| 85 | 15 | 47.5 | 0.0 | 0.0 | 0.13 | 5.6 | 0.09 |
| 70 | 15 | 47.5 | 2.9 | 2.8 | 0.26 | 7.05 | 0.12 |
| 77.5 | 15 | 55 | 0.0 | 0.0 | 1.30 | 6.1 | 0.13 |
| 77.5 | 20 | 47.5 | 0.9 | 1.4 | 0.22 | 4.84 | 0.12 |
| 77.5 | 15 | 40 | 2.9 | 2.5 | 0.07 | 7.24 | 0.12 |
| 77.5 | 20 | 47.5 | 1.7 | 1.8 | 0.23 | 4.86 | 0.13 |
| 77.5 | 25 | 40 | 3.0 | 2.6 | 1.94 | 5.87 | 0.13 |
| 85 | 20 | 40 | 0.0 | 0.0 | 0.85 | 6.2 | 0.11 |
| 77.5 | 20 | 47.5 | 1.7 | 1.3 | 0.19 | 4.85 | 0.12 |
| 77.5 | 20 | 47.5 | 1.8 | 1.6 | 0.21 | 4.85 | 0.11 |
| 70 | 20 | 40 | 3.2 | 2.9 | 0.46 | 4.25 | 0.12 |
| 70 | 20 | 55 | 1.0 | 0.0 | 0.27 | 4.95 | 0.15 |
| 85 | 25 | 47.5 | 0.0 | 0.0 | 1.83 | 5.8 | 0.09 |
| 77.5 | 25 | 55 | 0.5 | 0.0 | 1.80 | 6 | 0.14 |
aAerobic mesophiles (AM, Log CFU/mL)
bEnterobacteriaceae (EB, Log CFU/mL)
cPolyphenol oxidase activity (PPO, UEA/mL)
dPectin methylesterase activity (PME, UEA/mL)
Table 3.
Effects and respective significance levels (p) of amplitude, time and temperature on different quality attributes of fruit smoothie thermosonicated
| Coefficient | Aerobic mesophiles | Enterobacteria | Cloud | PPO | PME |
|---|---|---|---|---|---|
| Intercept | 1.598 | 1.570 | 0.224 | 4.826 | 0.124 |
| X1 | − 1.242b | − 0.998b | 0.065 | − 0.106 | − 0.017b |
| X2 | 0.0425 | − 0.061 | 0.709a | − 0.252 | 0.001 |
| X3 | − 0.960c | − 1.007b | 0.077 | − 0.301 | 0.003 |
| X1 * X2 | 0.045 | 0.157 | 0.021 | 0.237 | 0.003 |
| X1 * X3 | 0.550 | 0.725d | − 0.019 | − 0.700c | − 0.014c |
| X2 * X3 | 0.120 | − 0.035 | − 0.343d | 0.317 | 0.002 |
| X1 * X1 | − 0.366 | − 0.431 | 0.032 | − 0.033 | − 0.015c |
| X2 * X2 | 0.203 | 0.133 | 0.770b | 1.394b | − 0.0006 |
| X3 * X3 | − 0.181 | − 0.413 | 0.285 | 0.082 | 0.0106d |
| R-square | 0.90 | 0.93 | 0.93 | 0.90 | 0.90 |
Significance level: (a) p < 0.0001; (b) p < 0.001; (c) p < 0.01; (d) p < 0.05
Fig. 1.
Response surface plots of thermosonication on aerobic mesophiles, a amplitude (X1) and time (X2), b amplitude (X1) and temperature (X3), c time (X2) and temperature (X3). Response surface plots of thermosonication effect on Enterobacteriaceaed amplitude (X1) and time (X2), e amplitude (X1) and temperature (X3), and f time (X2) and temperature (X3)
Regarding EB, this microbial group tends to be less resistant to thermosonication than the AM group (Piyasena et al. 2003). The EB count was of 3.85 Log CFU/mL in control sample and there was a greater reduction in most of the applied treatments (Table 2) than AM group. Both, linear terms of amplitude (X1) and temperature (X3) significantly influenced at p < 0.001 (Table 3, Fig. 1d–f). Other research studies have shown the combined effect of amplitude and heat treatment for microbial inactivation with important results, which the heat treatment time was reduced by the application of ultrasound treatments (Guerrero et al. 2001; Huang et al. 2017). Further, in all the applied treatments, molds and yeast were inactivated (data not shown).
Cloud index
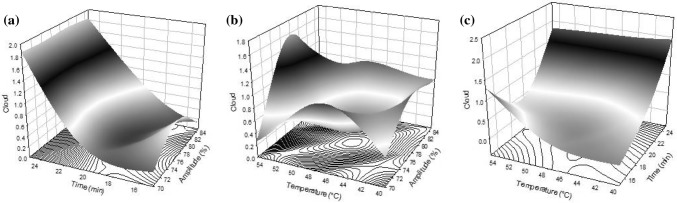
The cloud index is a parameter that reflects the particle stability on a matrix suspension. At the present study, high cloud values can be observed at 25 min (Table 2). Sound propagates through medium causing cavitation, during cavitation the formation, enlarge and implode of micro gas bubbles dissolved in the liquid by the compression and decompression of molecules take place and the bubble implosion generates an increase in temperature and pressure conditions (McClements 1995). The high cloud indexes could be attributed to time treatment applied since time seems to be an important parameter to occur the cavitation phenomenon. Regarding to regression coefficient (Table 3), cloud index was mainly influenced by linear terms of time (X2) (p < 0.0001) as well as time * temperature (p < 0.05). Figure 2a–c shows the effect of thermosonication conditions on cloud index; where high cloud indexes were observed at conditions of time and temperature elevated. Abid et al. (2013) demonstrated that sonication (25 kHz frequency, 20 °C) increased the cloud value of apple juice from 0.0563 up to 0.0780 and 0.0957 in treatments for 30, 60 and 90 min, respectively. The authors attributed that the increment to high-pressure gradient by cavitation during sonication treatment may cause the colloidal disintegration, dispersion and breakdown of macromolecules to smaller ones and make the juice properly homogenized and more consistent.
Fig. 2.
Response surface plots on cloud index of fruit smoothie thermosonicated a amplitude (X1) and time (X2), b amplitude (X1) and temperature (X3), c time (X2) and temperature (X3)
Pectin methylesterase and Polyphenol oxidase activity
Enzymes are sensitive to ultrasound processing being activate or inactivated. The changes in enzyme activity under ultrasonication might depend on the operational parameters of ultrasound, media properties, aminoacids composition and the conformational structure of the enzyme (Özbek and Ülgen 2000; Kadkhodaee and Povey 2008). Protein denaturation may be obtained by acoustic cavitation present in US treatments either by free radicals in sonolysis of water molecules or shear forces resulting from acoustic cavitation (Mason et al. 1994; Kadkhodaee and Povey 2008), so that selection of appropriate parameters can enhance enzymatic inactivation.
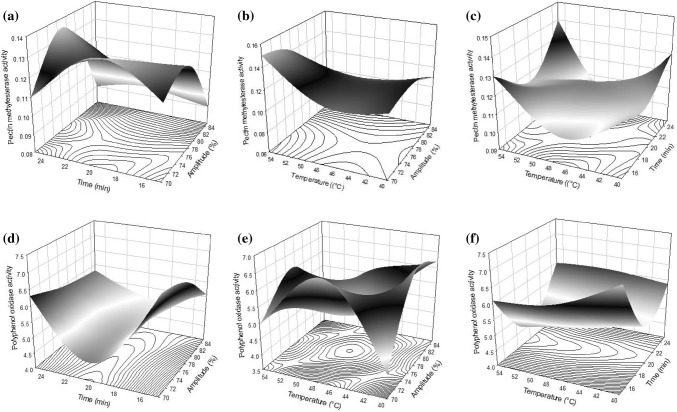
PME activity ranged from 0.08 to 0.15 (Table 2), amplitude was an important factor to consider for reaching PME inactivation. In fact, PME activity of fruit smoothie was significantly influenced (p < 0.001) by amplitude in linear term (X11) (Table 3). This is observed in Fig. 3a, in which, PME activity decreased by an increase in ultrasound amplitude. Moreover, the interaction among amplitude and temperature (X1 * X3) resulted significant (p < 0.01), PME activity increases from 72 to 80% of amplitude but after 80% PME activity decreases regardless the time treatment applied (Fig. 3b). Wang et al. (2012) reported that enzymatic activity enhanced with increasing ultrasonic power. They attributed the activity increment due to the rupture of hydrogen bonds or Van der Waals interactions by ultrasound, which bring the enzyme in active conformation. However, at high intensity ultrasound cavitation effects could cause great damage to polypeptide chains, leading inactivation of the enzyme (Şener et al. 2006). Besides, in treatments carried out at high amplitude and temperatures above 42 °C, PME activity decreases. Abid et al. (2014) observed a considerable PME inactivation in apple juice thermosonicated due to higher temperatures applied (60 °C). These authors concluded that the enzyme activity reduction was a temperature/time dependent manner. Further, Saeeduddin et al. (2015) observed an increasing trend for enzyme deactivation as the temperature treatment increase. Raviyan et al. (2005) reported an increase in PME inactivation in tomato juice sonicated in a temperature range of 50–72 °C compared to thermal treatment single. The authors commented that the increase in the enzyme inactivation depends on cavitation intensity applied as well as treatment temperature (Raviyan et al. 2005; Tiwari et al. 2009).
Fig. 3.
Response surface plots of pectin methylesterase activity of fruit smoothie thermosonicated a amplitude (X1) and time (X2), b amplitude (X1) and temperature (X3), and c time (X2) and temperature (X3). Response surface plots of d amplitude (X1) and time (X2), e amplitude (X1) and temperature (X3), f time (X2) and temperature (X3) on polyphenol oxidase activity of fruit smoothie thermosonicated
Regarding to PPO activity on fruit smoothie, values were between 4.1 and 7.24 (Table 2), PPO activity was influenced by the quadratic of time (X2 * X2) at p < 0.001 (Table 3). Figure 3d–f show the thermosonication effect on PPO activity where the lowest activity was observed in treatments ranged from 18 to 22 min. In concordance to this, it has been reported that prolonged exposure periods are necessary to inactivate PPO (Rithmanee and Intipunya 2012). However, after 24 min of thermosonication treatment PPO activity increased. In accordance to this behavior, Cheng et al. (2007) reported an increase in PPO activity in sonicated (35 kHz, 30 min) guava juice. They attributed the PPO activity increment to the production of a stabilized colloid system due to smaller particle size and higher phenolic compounds availability. Moreover, Fig. 3e shows that high temperatures did not have effect on PPO activity reduction. One explication could be that when the temperature increases, its vapor pressure also increases; this cushions the collapse of the bubbles and decreases the effect of cavitation (Mason and Peters 2002).
Cordeiro-Dias et al. (2015) reported a PPO reduction in soursop juice by sonication, independent of the processing time and power intensity employed. However, the lowest PPO activity values were obtained when higher processing time (> 8 min) and power intensity (> 330 W/cm2) were applied. More recently, Anaya-Esparza et al. (2017) reported a PPO reduction of 99% in soursop nectar by thermosonication (1.4 W/mL, 54 °C and 10 min), referring the reduction to an additive effect between cavitation and heat. Nevertheless, Illera-Gigante et al. (2018) worked with apple juice and reported that PPO residual activity decreases linearly with amplitude, obtaining the lowest residual activity at the maximum amplitude applied. This behavior has been attributed to an enzyme release usually bound to cell walls by the acoustic energy applied in the treatment (Baslar and Ertugay 2013).
In fact, enzyme damage by thermosonication is the result of thermal and mechanical effect of sonication due to microstreaming, where the thermosonication factors, single or in a combination have an important impact on enzyme activity (Vercet et al. 2002; Abid et al. 2014). Moreover, longer treatments increase the protein denaturation; hence, greater extent of enzymatic inactivation may be achieved in longer US treatments. Besides, physical stress due to bubble collapse may contribute to enzymatic inactivation (Ercan and Soysal, 2011). Other researchers mentioned that monomeric enzymes inactivation generally involves either enzyme defragmentation or enzyme formation-into aggregates. Whereas, polymeric enzymes tend to fragment into monomeric subunits, during ultrasonication (Mawson et al. 2011).
Finally, according the results of response surface regression, the optimal process condition was 77.5% amplitude, 20 min and 47.5 °C with values from the predicted profile of 1.48 Log CFU/mL of aerobic mesophiles, 1.57 Log CFU/mL of enterobacteria, 0.22 in cloud index, 4.82 and 0.12 of polyphenol oxidase and pectin methylesterase activity, respectively.
Conclusion
Fruit smoothies are novel products which are prepared by using mango and jackfruit with unique characteristics (taste, flavour, vitamins, antioxidants among others) that consumers appreciated. However, fresh fruit smoothies have short shelf-life, the effect of thermosonication on the physicochemical, enzymatic activity and safety characteristics of the mango and jackfruit smoothie was optimized to obtain high quality fruit smoothie. After thermosonication treatments, microorganism (AM and EB) loads and PME activity were reduced, where amplitude and temperature conditions had high influence on these parameters. Further, the PPO activity was influenced by time and amplitude conditions, meanwhile cloud index was affected by time and temperature parameters. Considering these results, US technology (treatment temperature, time and amplitude conditions) seems suitable to preserve or improve the smoothie safety and quality properties. However, more research is needed to determine the effect of thermosonication treatments on sensorial and functional properties of the fruit smoothie evaluated.
Acknowledgements
This study was possible thanks to the financial support from the PRODEP (Program for Professional Development Teaching, México) research project TEP-DCALI-2013-194. Author Chávez-Ocegueda was supported by a fellowship from the Consejo Nacional de Ciencia y Tecnología (CONACYT, Grant No. 279698). The authors have no conflict of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdullah N, Chin NL. Application of thermosonication treatment in processing and production of high quality and safe-to-drink fruit juices. Agric Agric Sci Procedia. 2014;2:320–327. doi: 10.1016/j.aaspro.2014.11.045. [DOI] [Google Scholar]
- Abid M, Saqib J, Tao W, Hashim M, Hu B, Lei S, Zhang X, Zeng X. Effect of ultrasound on different quality parameters of apple juice. Ultrason Sonochem. 2013;20:1182–1187. doi: 10.1016/j.ultsonch.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Abid M, Jabbar S, Hu B, Hashim MM, Wu T, Lei S, Khan MA, Zeng X. Thermosonication as a potential quality enhancement technique of apple juice. Ultrason Sonochem. 2014;21:984–990. doi: 10.1016/j.ultsonch.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Anaya-Esparza L, Velázquez-Estrada RM, Sayago-Ayerdi SG, Sánchez-Burgos JAM, Ramírez-Mares V, García-Magaña ML, Montalvo-González E. Effect of thermosonication on polyphenol oxidase inactivation and quality parameters of soursop nectar. LWT Food Sci Technol. 2017;75:545–551. doi: 10.1016/j.lwt.2016.10.002. [DOI] [Google Scholar]
- Andrés V, Villanueva MJ, Tenorio MD. Influence of high-pressure processing on microbial shelf life, sensory profile, soluble sugars, organic acids, and mineral content of milk-and soy-smoothies. LWT Food Sci Technol. 2016;65:98–105. doi: 10.1016/j.lwt.2015.07.066. [DOI] [Google Scholar]
- AOAC . Official methods of analysis. Arlington: Association of Official Analytical Chemists; 2005. [Google Scholar]
- Baslar M, Ertugay MF. The effect of ultrasound and photosonication treatment on polyphenoloxidase (PPO) activity, total phenolic component and colour of apple juice. Int J Food Sci Technol. 2013;48:886–892. doi: 10.1111/ijfs.12015. [DOI] [Google Scholar]
- Cano MP, Hernández A, De Ancos B. High pressure and temperature effects on enzyme inactivation in strawberry and orange products. J Food Sci. 1997;62:85–88. doi: 10.1111/j.1365-2621.1997.tb04373.x. [DOI] [Google Scholar]
- Chatterjee G, De Neve J, Dutta A, Das S (2015) Formulation and statistical evaluation of ready-to-drink whey-based orange beverage and its storage stability, Rev Mex Ing Quím 14:253–264. http://hdl.handle.net/1854/LU-7043912
- Cheng LH, Soh CY, Liew SC, Teh FF. Effects of sonication and carbonation on guava juice quality. Food Chem. 2007;104:1396–1401. doi: 10.1016/j.foodchem.2007.02.001. [DOI] [Google Scholar]
- Cordeiro-Dias DR, Pimenta Barros ZM, Oliveira de Carvalho CB, Araújo-Honorato F, Barbosa-Guerra N. Effect of sonication on soursop juice quality. LWT Food Sci Technol. 2015;62:883–889. doi: 10.1016/j.lwt.2014.09.043. [DOI] [Google Scholar]
- Cruz N, Capellas M, Hernández M, Trujillo A, Guamis B, Ferragut V. Ultra high-pressure homogenization of soymilk: microbiological, physicochemical and microstructural characteristics. Food Res Int. 2007;40:725–732. doi: 10.1016/j.foodres.2007.01.003. [DOI] [Google Scholar]
- Devalaraja S, Jain S, Yadav H. Exotic fruits as therapeutic complements for diabetes, obesity and metabolic syndrome. Food Res Int. 2011;44(7):1856–1865. doi: 10.1016/j.foodres.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercan SS, Soysal C. Effect of ultrasound and temperature on tomato peroxidase. Ultrason Sonochem. 2011;18:689–695. doi: 10.1016/j.ultsonch.2010.09.014. [DOI] [PubMed] [Google Scholar]
- Guerrero S, López-Malo A, Alzamora SM. Effect of ultrasound on the survival of Saccharomyces cerevisiae: influence of temperature, pH and amplitude. Innov Food Sci Emerg Technol. 2001;2:31–39. doi: 10.1016/S1466-8564(01)00020-0. [DOI] [Google Scholar]
- Herceg Z, Lelas V, Jambrak AR, Vukušić T, Levaj B. Influence of thermo-sonication on microbiological safety, color and anthocyanins content of strawberry juice. J Hyg Eng Des. 2013;1:26–37. [Google Scholar]
- Huang G, Chen S, Dai C, Sun L, Sun W, Tang Y. Effects of ultrasound on microbial growth and enzyme activity. Ultrason Sonochem. 2017;37:144–149. doi: 10.1016/j.ultsonch.2016.12.018. [DOI] [PubMed] [Google Scholar]
- Illera-Gigante AE, Sanz-Díes M, Benito-Román O, Varona-Fernández S, Beltrán-Calvo S, Melgosa-Gómez R, García-Solaesa Á. Effect of thermosonication batch treatment on enzyme inactivation kinetics and other quality parameters of cloudy apple juice. Innov Food Sci Emerg Technol. 2018;47:71–80. doi: 10.1016/j.ifset.2018.02.001. [DOI] [Google Scholar]
- Jabbar S, Abid M, Hu B, Wu T, Muhammad HM, Lei S. Quality of carrot juice as influenced by blanching and sonication treatments. LWT Food Sci Technol. 2014;55:16–21. doi: 10.1016/j.lwt.2013.09.007. [DOI] [Google Scholar]
- Kadkhodaee R, Povey MJW. Ultrasonic inactivation of Bacillus a-amylase. I. Effect of gas content and emitting face of probe. Ultrason Sonochem. 2008;15(2):133–142. doi: 10.1016/j.ultsonch.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Khandpur P, Gogate PR. Evaluation of ultrasound-based sterilization approaches in terms of shelf life and quality parameters of fruit and vegetable juices. Ultrason Sonochem. 2016;29:337–353. doi: 10.1016/j.ultsonch.2015.10.008. [DOI] [PubMed] [Google Scholar]
- Lin PY, Lai HM. Bioactive compounds in rice during grain development. Food Chem. 2011;127:86–93. doi: 10.1016/j.foodchem.2010.12.092. [DOI] [Google Scholar]
- Mason TJ, Peters D. Practical sonochemistry: power ultrasound uses and applications. 2. UK: Horwood Publishing Limited; 2002. [Google Scholar]
- Mason TJ, Lorimer JP, Baters DM, Zhao Y. Dosimetry in sonochemistry: the use of aqueous terephthalate ion as a fluorescence monitor. Ultrason Sonochem. 1994;1:91–95. doi: 10.1016/1350-4177(94)90004-3. [DOI] [Google Scholar]
- Mawson R, Gamage M, Terefe NS, Knoerzer K. Ultrasound in enzyme activation and inactivation. In: Feng H, Barbosa-Cánovas GV, Weiss J, editors. Ultrasound technologies for food and bioprocessing. New York: Springer; 2011. pp. 369–404. [Google Scholar]
- McClements DJ. Advances in the application of ultrasound in food analysis and processing. Trends Food Sci Technol. 1995;6:293–299. doi: 10.1016/S0924-2244(00)89139-6. [DOI] [Google Scholar]
- Mohideen FW, Solval JK, Li M, Zhang J, Chouljenko A, Chotiko A, Prudente AD, Bankston JD, Sathivel S. Effect of continuous ultra-sonication on microbial counts and physico-chemical properties of blueberry (Vaccinium corymbosum) juice. LWT Food Sci Technol. 2015;60:563–570. doi: 10.1016/j.lwt.2014.07.047. [DOI] [Google Scholar]
- Morales de la Peña M, Rosas-González C, Martín Belloso O, Welti-Chanes J (2018) Changes in bioactive compounds concentration and physicochemical properties of mango smoothies treated by ultrasound. Rev Mex Ing Quím 17(1):131–144. hdl.handle.net/10459.1/63119
- Muñoz A, Caminiti IM, Palgan I, Pataro G, Noci F, Morgan DJ, Cronin DA, Whyte P, Ferrari G, Lyng JG. Effects on Escherichia coli inactivation and quality attributes in apple juice treated by combinations of pulsed light and thermosonication. Food Res Int. 2012;45:299–305. doi: 10.1016/j.foodres.2011.08.020. [DOI] [Google Scholar]
- Özbek B, Ülgen K. The stability of enzymes after sonication. Process Biochem. 2000;35(9):1037–1043. doi: 10.1016/S0032-9592(00)00141-2. [DOI] [Google Scholar]
- Piyasena P, Mohareb E, McKellar RC. Inactivation of microbes using ultrasound: a review. Int J Food Microbiol. 2003;87(3):207–216. doi: 10.1016/S0168-1605(03)00075-8. [DOI] [PubMed] [Google Scholar]
- Raviyan P, Zhang Z, Feng H. Ultrasonication for tomato pectinmethylesterase inactivation: effect of cavitation intensity and temperature on inactivation. J Food Eng. 2005;70:189–196. doi: 10.1016/j.jfoodeng.2004.09.028. [DOI] [Google Scholar]
- Ribeiro LO, Santa Brígida AI, Sá DDG, Carvalho CF, et al. Effect of sonication on the quality attributes of juçara, banana and strawberry smoothie. J Food Sci Technol. 2019 doi: 10.1007/s13197-019-03998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rithmanee T, Intipunya P. Effects of high-power ultrasonic pretreatment on physicochemical quality and enzymatic activities of dried longan. J Agric Sci. 2012;4:299. doi: 10.5539/jas.v4n11p299. [DOI] [Google Scholar]
- Rouse AH, Atkins CD. Pectinesterase and pectinin commercial citrus juice as determined by methods used at the Citrus Experiment Station. Fla Agric Exp Sta Bull. 1955;570:1–19. [Google Scholar]
- Saeeduddin M, Abid M, Jabbar S, Wu T, Hashim MM, Awad FN, Hu B, Lei S, Zeng X. Quality assessment of pear juice under ultrasound and commercial pasteurization processing conditions. LWT Food Sci Technol. 2015;64:452–458. doi: 10.1016/j.lwt.2015.05.005. [DOI] [Google Scholar]
- Şener N, Apar DK, Özbek B. A modelling study on milk lactose hydrolysis and β-galactosidase stability under sonication. Process Biochem. 2006;41(7):1493–1500. doi: 10.1016/j.procbio.2006.02.008. [DOI] [Google Scholar]
- Tiwari BK, Muthukumarappan K, O’Donnell CP, Cullen PJ. Inactivation kinetics of pectin methylesterase and cloud retention in sonicated orange juice. Innov Food Sci Emerg Technol. 2009;10(2):166–171. doi: 10.1016/j.ifset.2008.11.006. [DOI] [Google Scholar]
- Vercet A, Sánchez C, Burgos J, Montañés L, Lopez Buesa P. The effects of manothermosonication on tomato pectic enzymes and tomato paste rheological properties. J Food Eng. 2002;53:273–278. doi: 10.1016/S0260-8774(01)00165-0. [DOI] [Google Scholar]
- Versteeg C, Rombouts FM, Spaansen CH, Pilnik W. Thermostability and orange juice cloud destabilizing properties of multiple pectinesterases from orange. J Food Sci. 1980;45:969–972. doi: 10.1111/j.1365-2621.1980.tb07489.x. [DOI] [Google Scholar]
- Wang Z, Lin X, Li P, Zhang J, Wang S, Ma H. Effects of low intensity ultrasound on cellulase pretreatment. Bioresour Technol. 2012;117:222–227. doi: 10.1016/j.biortech.2012.04.015. [DOI] [PubMed] [Google Scholar]
- Worldatlas (2017) The Top Mango Producing countries in the world. https://www.worldatlas.com/articles/the-top-mango-producing-countries-in-theworld.html
- Zafra-Rojas QY, Cruz-Cansino N, Ramírez-Moreno E, Delgado-Olivares L, Villanueva-Sánchez J, Alanís-García E. Effects of ultrasound treatment in purple cactus pear (Opuntia ficus-indica) juice. Ultrason Sonochem. 2013;20:1283–1288. doi: 10.1016/j.ultsonch.2013.01.021. [DOI] [PubMed] [Google Scholar]