Abstract
This study investigated the formation of angiotensin-converting enzyme (ACE) inhibitory peptides from koro kratok beans tempe during gastrointestinal digestion. The absorption of bioactive peptides was also investigated in this study. Koro kratok was fermented by commercial culture including Rhizopus oligosporus for 48 h. Gastrointestinal digestion was simulated sequentially by hydrolysis of tempe protein extract with pepsin and pancreatin for 240 min. The peptide content, degree of hydrolysis, molecular weight distribution, and ACE inhibitory activity were analyzed. The absorption of ACE inhibitory peptides was evaluated using the inverted gut sac of Sprague Dawley rats. Results showed that some amino acids, such as Arg, Lys, Asp, Glu, Phe, and Leu, were predominantly found in tempe. After the hydrolysis process, cooked tempe exhibited the highest ACE inhibitory activity (90.05%). Although the ACE inhibitory activity of nonfermented koro kratok was lower than that of tempe, the increase in its inhibitory activity was too large (23.03%). The ACE inhibitory peptides from tempe showed a predominance of peptides with a molecular weight of < 1 kDa and could inhibit ACE activity by 84.34%. The majority of ACE inhibitory peptides from tempe was absorbed in the jejunum and exhibited an ACE inhibitory activity of 81.59%. Based on these results, it can be concluded that the fermentation and boiling process of koro kratok beans improved the release of ACE inhibitory peptides during the gastrointestinal digestion process and had an impact on its absorption.
Keywords: Tempe, ACE inhibitory activity, Digestion, Absorption
Introduction
Koro kratok (Phaseolus lunatus L.) is included in the genus Phaseolus. In some countries, koro kratok is known as lima beans. This plant adapts well in dry land and hence it can be easily cultivated in tropical areas. The protein and carbohydrate contents in koro kratok have been reported to be quite high at 24.6 and 57.3 g/100 g, respectively. However, its fat (2.8 g/100 g) and fiber (6.8 g/100 g) contents are low (Jayalaxmi et al. 2016). Furthermore, koro kratok contains large amounts of the amino acids Lys, Phe, Tyr, Leu, Asp, Glu, Ser, and Arg (Guerrero et al. 2012).
Koro kratok can be recommended as a functional food because of its high nutritional value. Tempe is a fermented soybean product that is widely consumed in Indonesia. Tempe is fermented by Rhizopus sp. Some other legumes such as jack bean, pigeon pea, and velvet bean can also be used in preparing tempe. Fermentation can improve the nutritional value of tempe by decreasing the antinutrient content and the formation of bioactive compounds (Astuti et al. 2000). Proteolysis causes the release of bioactive peptides. Peptides obtained from soybean tempe have been shown to exhibit antioxidant, antimicrobial, hypocholesterol, anticancer, and antihypertensive effects (Sanjukta and Rai 2016).
Bioactive peptides that can inhibit the activity of angiotensin-converting enzyme (ACE) can potentially be used as antihypertensive agents. The role of ACE is to convert angiotensin I to angiotensin II (vasoconstrictor), the compound that causes hypertension (Vital et al. 2015; Lee and Hur 2017). Molecular weight (MW) and amino acid sequences are known to influence the activity of ACE inhibitory peptides. In general, ACE inhibitory peptides have an MW < 1000 Da (Campos et al. 2011). Studies have also demonstrated that the presence of hydrophobic and charged amino acid residues affects ACE inhibitory activity (Gu and Wu 2013; Aluko et al. 2015). In addition, the activity of ACE inhibition on koro kratok hydrolysate has earlier been investigated by Uco et al. (2009) and Magana et al. (2015). These researchers demonstrated that koro kratok hydrolysate could inhibit the activity of ACE by approximately 0.056–0.610 mg/mL. Nevertheless, there is still a limitation in studies related to a bioactive peptide obtained from koro kratok fermented products.
Bioavailability is one of the biggest challenges in the utilization of bioactive peptides. The digestion and absorption processes of tempe have an impact on the physiological function of the body. Bioactive peptides must be absorbed by the small intestine before entering the blood circulation to exhibit the ability of ACE inhibitory activity.
Digestive enzymes can degrade the peptides. Due to the process of digestion, the bioactivity of peptides can possibly be improved or reduced in the gastrointestinal tract. In an earlier study, Pihlanto et al. (2000) reported that hydrolysis of whey protein with digestive enzymes elevated the ACE inhibitory activity. On the other hand, another study showed that heat treatment also influenced the ACE inhibitory activity (Akillioglu and Karakaya 2009).
Till date, there is a scarcity of information regarding the stability of the activity of bioactive peptides obtained from koro kratok in the gastrointestinal tract. Therefore, this study was conducted to investigate the phenomenon that occurs in tempe during the process of digestion. In vitro gastrointestinal simulation was conducted by hydrolysis of protein from tempe with pepsin and pancreatin. Then, the digested peptides were characterized and tested for bioavailability. A novel aspect of this study was the use of the everted gut sac method that comprised three parts of the intestine (duodenum, jejunum, and ileum) to understand the absorption of bioactive peptides of tempe.
Furthermore, the changes in ACE inhibitory activity in tempe after the digestion process were evaluated in this study. Characterization and absorption study of the produced peptides, which are potential ACE inhibitors, were also performed.
Materials and methods
Koro kratok (P. lunatus L.) beans were collected from Madura, East Java, Indonesia. A commercial inoculum, “Raprima,” containing R. oligosporus was used for preparing koro kratok tempe. Reagents used during in vitro hydrolysis and analysis included ortho-phaldehyde, NaOH, 37% HCl, Na2CO3, KNaC4H4O6·4H2O, CuSO4·5H2O, methanol, NaCl, folin, β-mercaptoetanol (Merck), pepsin (EC. 3.4.23.1) from porcine gastric mucosa (Sigma-Aldrich), pancreatin (EC. 232-468-9) from porcine pancreas (Sigma-Aldrich), dialysis tube MWCO 1, 3.5, and 14 kDa (Spectrum Lab), ACE (EC. 3.4.15.1) from rabbit (Sigma-Aldrich), hippuryl-histidine-leucine (Sigma-Aldrich), and standard l-leucine (Sigma-Aldrich).
Preparation of koro kratok tempe
Koro kratok beans were washed and sorted, after which they were boiled for 30 min and then soaked in water for 12 h. The beans were peeled off and then soaked again for 24 h. Next, they were boiled for 15 min and cooled to room temperature. The boiled beans (NF, nonfermented) were inoculated with 0.2% inoculum and incubated for 48 h at room temperature to produce tempe (RT, raw tempe). A sample of cooked tempe (CT) was prepared by boiling raw tempe in water at 95 °C for 15 min.
The samples were lyophilized and powdered. The sample powder was macerated in water (1:5) at 30 °C for 60 min. This solution was centrifuged at 20,000×g for 15 min. The resulting supernatant was termed as the peptide extract, which was lyophilized and stored at − 25 °C until use.
In vitro gastrointestinal simulation
The simulation of gastrointestinal digestion process was done according to the method described by Minekus et al. (2014) with slight modification. The extract samples were hydrolyzed sequentially with pepsin and pancreatin. Samples (5 mg/mL of protein) were set to pH 3.0 with the addition of 1 M HCl and preincubation for 5 min. This was followed by addition of pepsin (2000 U/mL). Then, the solution was incubated at 37 °C for 120 min. The reaction was stopped by adding 0.9 M NaHCO3 (pH 5.3). Hydrolysis with pancreatin (100 U/mL) was started when the pH of the sample reached 7.5. The pH of the samples was adjusted by adding 2 M NaOH. The samples were then incubated at 37 °C for 120 min. The hydrolysis reaction was stopped by heating in boiling water for 10 min. The resulting hydrolysates were centrifuged at 8000×g for 10 min at 4 °C. The obtained supernatant was stored at − 25 °C for further analysis.
Dialysis
A dialysis membrane was used to fractionate the peptides based on their molecular size. Hydrolysates with the highest activity were dialyzed gradually using three dialysis tubing with an MW cutoff (MWCO) of 1, 3.5, and 14 kDa. The peptides were divided into four fractions based on the MW distribution. Fraction I was peptide with MW < 1 kDa. Fraction II was peptide with MW 1–3.5 kDa. Fraction III was peptide with MW 3.5–14 kDa. Fraction IV was peptide with MW > 14 kDa. The dialysis tubing was filled with 10 mL hydrolysate, placed in 90 mL distillated water, and then stirred at 4 °C for 12 h. Peptides with MW smaller than the MWCO passed freely through the membrane to diffuse into the dialysate. Peptides with a larger MWCO that were retained on the sample side of the membrane were dialyzed again. The dialysate was collected and dried by lyophilization. The lyophilized powder was stored at − 25 °C for further analysis.
Absorption study using everted gut sac
The absorption of peptides was analyzed according to the method described by Amenta et al. (2018) with slight modification. A total of 15 adult male Sprague Dawley rats (weighing ± 250 g and aged 11–12 weeks) were purchased from the Center for Food and Nutrition Studies, Universitas Gadjah Mada, Yogyakarta, Indonesia. The rats were fasted for 20–24 h, after which they were euthanized by intramuscular ketamine injection (180 mg/kg) in the thighs. Dissection was done by making a midline incision in the abdomen so that the ileocecum could be visualized. The small intestine was collected and washed with 0.9% NaCl solution. The intestine was divided into three segments (duodenum, jejunum, and ileum), and each segment was cut at a length of 10 cm. The reversing process of the small intestine was performed rapidly in 0.9% NaCl solution. The two tips of the intestine were bonded and filled with 1 mL of 0.9% NaCl solution (serosa). Then, the small intestine was soaked in a tube containing a peptide solution (mucosal) for 120 min. All parts of the small intestine were submerged in the mucosa fluid and stirred continuously. Furthermore, the intestine was aerated with O2 (100 bubbles/min) at a constant temperature of 37 °C. The serosa sample was centrifuged at 13,500×g for 15 min, and the obtained supernatant was analyzed for peptide concentration and ACE inhibitory activity.
Analysis method
Proximate analysis
The proximate (moisture, ash, protein, and fat content) of tempe was determined using the AOAC procedure (2005).
Amino acid composition
Amino acids were quantified by liquid chromatography-mass spectrometry (LC–MS) analysis (The Water™ Xero™ TQD®, Milford, USA). This procedure was conducted as described by Chang et al. (1989). The tempe was hydrolyzed with 6 N HCl in an autoclave at 110 °C for 12 h. The sample solution was neutralized by adding 6 N NaOH and diluted with distilled water. The solution was filtered using a 0.22-µm syringe filter. Then, the sample was injected at a volume of 2 µL. Amino acids were identified using the reversed-phase (C18) column and mass spectrometry detectors. The mobile phase consisted of the following two eluents:
A: 0.1% pentadecafluorooctanoic acid:water/CH3CN with 0.1% formic acid (99.5:0.5).
B: 0.1% PDFOA:water/CH3CN with 0.1% formic acid (10:90).
The flow rate was set at 0.6 mL/min and was done with a gradient mode. Eluent A-B (90:10, v/v) run time 5 min. Eluent A-B (50:50, v/v) run time 0.5 min. Eluent A-B (90:10, v/v) run time 2 min.
Peptide content
Determination of peptide concentration was based on the method described by Church et al. (1983). OPA reagent was prepared by mixing 25 mL of 100 mM sodium tetraborate, 2.5 mL of 20% sodium dodecyl sulfate, and 1.1 mL OPA (40 mg OPA dissolved in 1 mL methanol and 100 mL β-mercaptoethanol) in 21.4 mL distilled water. A sample of 20 µL was reacted with 1 mL OPA reagent. The mixture was incubated at room temperature for 2 min, and its absorbance was measured at a wavelength of 340 nm. In the measurement of the peptide content, tripton was used as the standard. The peptide content was calculated using a standard linear regression curve with concentration variations of 0, 0.25, 0.50, 0.75, 1.0, 1.25, and 1.50 mg/mL.
Degree of hydrolysis (DH)
The DH was determined using the method described by Lin et al. (2017) with modification. Koro kratok powder was hydrolyzed with 8 M HCl (1:10 w/v) and incubated at 110 °C for 24 h. The sample was neutralized with 8 M NaOH and distilled water was added to make the total volume to 10 mL. The mixture was then filtered and the peptide concentration was analyzed using the OPA reagent. The DH was calculated using the following formula:
where (NH2)tx is the number of free amino acids at x min, (NH2)t0 is the number of free amino acids at 0 min, and (NH2)total is the total number of free amino acids.
ACE inhibitory activity assay
The ACE inhibitory activity was evaluated by measuring the rate of formation of hippuric acid from hippuryl-l-histidyl-l-leucine (Cushman and Cheung 1971). A total of 50 µL sample solution (1 mg/mL of protein) and 50 µL ACE solution (25 mU/mL) were preincubated at 37 °C for 10 min, and then 50 µL of the substrate (Hip-His-Leu 8 mM in 50 mM HEPES buffer pH 8.3 containing 300 mM NaCl) was added. The solution was incubated at 37 °C for 30 min. The reaction was stopped by the addition of 200 µL of 1 M HCl. The solution was extracted by adding 1.5 mL ethyl acetate and then centrifuged at 4000× g for 15 min at 4 °C. The organic phase was collected and evaporated. Then, the sample was dissolved in 3 mL distilled water, and the absorbance was measured at a wavelength of 228 nm using a UV–Vis spectrophotometer. The ACE inhibitory activity was determined using the following formula:
where A is the absorbance of the sample without inhibitor, B is the absorbance of the sample with ACE and inhibitor, and C is the absorbance of the blank.
Statistical analysis
The average results of three replications are described in this study. Data were processed in the IBM SPSS 20 software using one-way analysis of variance (ANOVA) with a 95% significance level. A follow-up test was conducted using the Duncan’s multiple range test (DMRT) in case there were significant differences.
Results and discussion
Proximate and amino acid composition of tempe
Tempe is one of the fermented products that has a white compact solid texture and high nutrient content. The moisture content was 63.47%, the ash content was 0.48%, the protein content was 28.06%, and the fat content was 1.37%. The protein content of koro kratok tempe was higher than that of tempe prepared from jack bean but was lower than that of tempe prepared from soybean (Andriati et al., 2018; Omosebi and Otunola, 2013).
The amino acid composition of raw tempe is shown in Table 1. Arginine was the most dominant amino acid found in the tempe. Amino acids with negatively charged side chains such as Asp and Glu were found in higher concentrations than amino acids with hydrophobic side chains. Udenigwe et al. (2012) had evaluated the activity of ACE inhibition and renin inhibition on flaxseed protein. The arginine concentration in the cationic hydrolysate fraction was found to be approximately 31.23%. The ACE inhibitory activity by the cationic hydrolysate fraction was 80% in 1 mg/mL. This confirmed that the high content of arginine in peptides could exhibit ACE inhibitory activity.
Table 1.
Amino acid content of raw tempe
| Amino acids | Content (g/100 g protein) | |
|---|---|---|
| Hydrophobic | Ala | 0.61 |
| Val | 0.64 | |
| Leu | 1.03 | |
| Ile | 0.53 | |
| Pro | 0.55 | |
| Phe | 0.72 | |
| Met | 0.10 | |
| 4.18 | ||
| Hydrophilic | ||
| Negative charge | Glu | 1.23 |
| Asp | 1.12 | |
| Positive charge | Arg | 5.62 |
| Lys | 0.78 | |
| His | 0.48 | |
| Neutral charge | Ser | 0.78 |
| Thr | 0.56 | |
| Gly | 0.47 | |
| Tyr | 0.25 | |
| Cys | 0.03 | |
| 11.32 | ||
According to Li and Aluko (2010), peptides containing large quantities of positively charged amino acid residues could exhibit high ACE inhibitory activity. The positively charged amino acid residues in the peptide would interact with Cl− ions, which are present on the ACE binding side, which would disrupt the substrate hydrolysis activity.
Peptides containing the amino acid residues Asp and Glu are known to chelate Zn, which is an ACE cofactor. Binding of Zn by peptides could reduce the bioavailability of Zn, which could thereby decrease the catalytic ability of ACE (Aluko et al. 2015). ACE inhibitory peptides are generally characterized by the presence of hydrophobic amino acids. The concentrations of leucine and phenylalanine in tempe were high enough to generate potential ACE inhibitory peptides. The high availability of the amino acids Arg, Asp, Glu, Leu, and Phe in tempe enables the formation of ACE inhibitory peptides.
Hydrolysis process during gastrointestinal digestion simulation
The sequential hydrolysis of tempe with pepsin and pancreatin for 240 min produced shorter chain peptides. The concentration of peptides increased with time. The DH was related to the number of peptides released during the hydrolysis process. The DH increased significantly compared to that at the initial time point (Fig. 1). The changes in DH from 0 to 120 min were not highly significant. The DH increased sharply at 150 min (average ~ 31.55%), but after 150 min, there was only a slight increase in DH with time. The DH values after the hydrolysis process with pepsin and pancreatin were 38.20% in nonfermented beans, 56.30% in raw tempe, and 43.23% in cooked tempe (CT). In the nonfermented beans, these values were slightly higher and similar to those reported by Magana et al. (2015) using koro kratok ungerminated beans (DH 32.16%). These results revealed that both the fermentation and the boiling process effectively supported protein hydrolysis with pepsin and pancreatin, thereby increasing its DH values.
Fig. 1.

Degree of hydrolysis during gastrointestinal digestion. NF: nonfermented beans. RT: raw tempe. CT: cooked tempe
The higher the DH value, the greater the concentration of the peptide produced. The DH of the samples obtained during hydrolysis with pepsin were lower than the DH values when hydrolyzing with pancreatin. Each enzyme had a different specificity so that it would affect the released peptide and the DH value. A high DH indicated that pancreatin, which consists of several digestive enzymes, had a broader specificity than pepsin, so that it is more efficient in producing small peptides.
Pepsin is an endopeptidase that specifically cuts peptide bonds containing residues of Phe, Leu, and Glu. Pepsin cannot cut peptide bonds containing the amino acid residues Val, Ala, and Gly. Pancreatin is a mixture of endopeptidases and exopeptidases, which includes trypsin, chymotrypsin, elastase, and carboxypeptidase. These enzymes have different substrate specificity and cutting patterns, so that the hydrolysis that occurs becomes more active. Therefore, it allows for the production of more peptides (Vital et al. 2015).
The peptide concentration of nonfermented beans after the hydrolysis with pepsin and pancreatin was 20.905 mg/mL, whereas those of raw tempe and CT were 38.807 and 28.033 mg/mL, respectively. These results were similar to those reported by Gibbs et al. (2004) who demonstrated that fermented products (natto and tempe) generated more amino acids and peptides than nonfermented soybean.
The heat generated in food processing could affect the formation of peptides during the digestion process. The process of heating causes changes in the tertiary and quaternary structure of the protein. This leads to reorientation of peptide bonds and exposure of the cutting sites so that the proteins become more susceptible to attack by enzymes and allow the increase of peptide release (Pihlanto et al. 2000; Vital et al. 2015). However, in this study, the results showed that the peptide concentration of CT was lower than that of raw tempe. This was possible because some peptides were extracted into the water during the boiling process.
ACE inhibitory activity
The peptides found in koro kratok have been shown to possess the ability to inhibit ACE activity. The ACE inhibitory activities of the three samples differed significantly both before and after the digestion process (Fig. 2). The ACE inhibitory activity in raw tempe was higher than that in nonfermented beans but lower than that in CT. This showed that the fermentation and the boiling process conducted before being consumed could increase the availability of ACE inhibitory peptides in the food product.
Fig. 2.
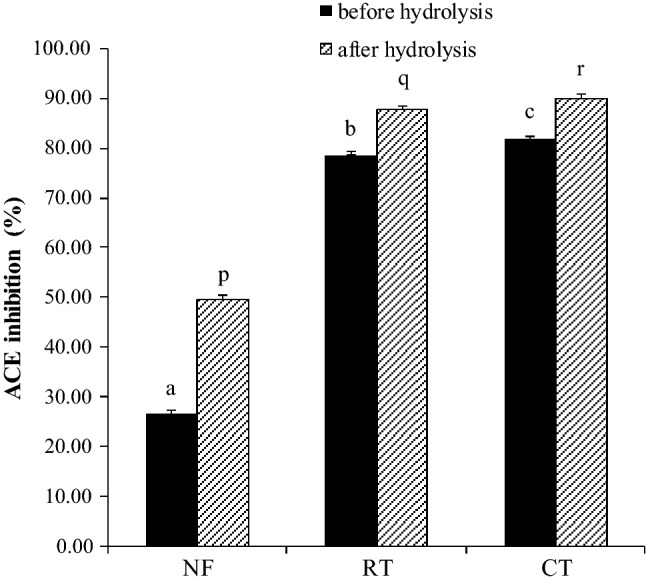
ACE inhibitory activity of hydrolysates. NF: nonfermented beans. RT: raw tempe. CT: cooked tempe
After the hydrolysis with pepsin and pancreatin, there was an increase in the inhibitory activity. The increase in the nonfermented beans was 23.03% (from 26.39 to 49.42%), whereas those in raw tempe were 9.32% (from 78.62 to 87.94%) and 8.25% (from 81.80 to 90.05%) in CT. The increase in the ACE inhibitory activity confirmed that ACE inhibitory peptides could be formed during the digestion process due to the hydrolysis of proteins and polypeptides by pepsin and pancreatin.
Proteolysis that occurred during the fermentation process of tempe resulted in the increase of small peptides that potentially as ACE inhibitors. In the fermentation process, proteolysis was relatively moderate so that further hydrolysis with pepsin and -pancreatin would generate more small bioactive peptides to elevate the ACE inhibitory activity. In a previous study, Wang et al. (2015) reported that the ACE inhibitory activity of douchi could be increased after hydrolysis with pepsin, trypsin, and chymotrypsin.
The heat generated during the boiling process had an impact in increasing the ACE inhibitory activity. The process of denaturation allows the release of ACE inhibitory peptides. Similarly, Akillioglu and Karakaya (2009) reported that heat treatment of pinto (P. vulgaris L.) beans and green lentil beans (Lens culinaris L.) for 15 min before the digestion process significantly increased the ACE inhibitory activity.
The inhibitory activities observed in this study were higher than cowpea inhibitory activities reported by Ancona et al. (2014). However, the effect of boiling process on the ACE inhibitory activity in this study was not highly significant. This was probably due to the formation of a new disulfide bridge. Heat denaturation exposed the SH or SS groups and an exchange reaction occurred between the denatured proteins to form a new strong enough bond. Studies have reported that the formation of disulfide bridges could reduce the release of peptides (Davis and Williams 1998; Tang 2008).
MW distribution
CT had the highest ACE inhibitory activity. CT was dialyzed to identify the MW of the formed peptides. The peptides formed were dominated by a peptide fraction (approximately 73.56%) with an MW < 1 kDa. The peptide fraction with an MW of 1–3 kDa comprised approximately 13.91%. The peptide fraction with an MW of 3.5–14 kDa comprised 4.21%, and that with an MW > 14 kDa constituted 8.32%. This confirmed that hydrolysis with pepsin and pancreatin could produce several short-chain peptides. This result was similar to that reported by Capriotti et al. (2015). The simulation of gastrointestinal digestion of soybeans and soy milk released peptides comprising 5–49 amino acid residues (MW 613–4932 Da).
ACE inhibition depended on the MW of the peptide. Regarding the characteristics of the ACE inhibitory peptides in general, they had a small MW. Results of the ACE inhibitory activity by the peptide fraction are presented in Table 2. The highest activity of 84.34% (IC50 0.587 mg/mL) was detected in the peptide fraction with an MW < 1 kDa, and the lowest activity of 79.68% (IC50 0.706 mg/mL) was found in the peptide fraction with an MW > 14 kDa. The peptide fraction with MW < 1 kDa and 1–3.5 kDa provided activities that were not significantly different. This indicated that the ability of the peptides with MW < 3.5 kDa to inhibit ACE activity was better than that of peptides with MW > 3.5 kDa. A similar result was reported by Campos et al. (2011) using peptides from cowpea hydrolysates with an MW < 1 kDa that exhibited the highest inhibitory activity. The smaller the size of the peptide, the easier it was to access the active side of ACE to interact. The position of the active side of ACE cavity was hidden and was blocked by three α-helix chains of the N terminal, so that it would limit access to large-sized substrates or inhibitors (Natesh et al. 2003).
Table 2.
ACE inhibitory activity of peptide fractions of CT
| Peptide fraction (kDa) | ACE inhibition (%) |
|---|---|
| < 1 | 84. 34 ± 0.9721c |
| 1–3.5 | 84.13 ± 0.6350c |
| 3.5–14 | 81.59 ± 0.6350a |
| > 14 | 79.68 ± 0.6350b |
CT: cooked tempe
Different letters indicate a significant difference between fractions (P < 0.05)
The statistical test DMRT was applied
Study of absorption
Absorption study of ACE inhibitory peptides was conducted to determine the number of peptides that could be absorbed and the ACE inhibitory activity exhibited after the absorption process in each segment of the intestine. Leucine standard was used as a positive control in this study. The majority of the peptides from tempe could be absorbed in the jejunum. Leucine was the most absorbed in the ileum. In a previous study, Matthews et al. (1971) reported that the capacity of peptide and amino acid absorption varied depending on the type of the intestinal segment. In the proximal small intestine (duodenum and jejunum), there was absorption of the l-methionyl-l-methionine peptide, whereas in the distal portion of the small intestine (ileum), the amino acid l-methionine was absorbed.
As shown in Fig. 3, heat treatment during the boiling process of tempe affected the absorption of peptides in the intestinal segments. In the jejunum, it affected in increasing the absorption by approximately 6.46% (from 42.97 to 49.43%). In the ileum, it decreased the absorption by 6.73% (from 26.20 to 16.47%). Based on these data, it appears that the sample consisted of various types of peptides so that it could influence the absorption. Xu et al. (2019) reported that bioavailability of peptides was influenced by various peptide properties such as size, MW, amino acid sequence, and resistance of peptides to enzymatic degradation.
Fig. 3.
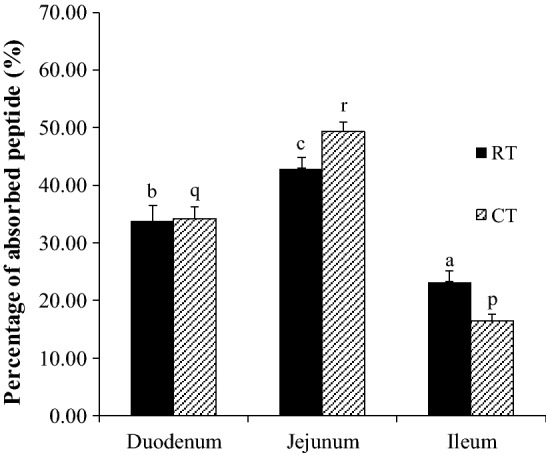
The effect of boiling process of tempe on the percentage of peptides absorbed in the small intestinal segments. RT: raw tempe. CT: cooked tempe. Different letters indicate a significant difference between intestinal segments (P < 0.05). The statistical test DMRT was applied
Heat treatment also changed the protein conformation structure during the boiling process and the pH condition in the gastrointestinal tract. The structure of the protein was unfolded and the nonpolar amino acids were exposed to the surface. This was more effective for proteases in the small intestine to hydrolyze the substrate so that it could produce short-chain peptides and amino acids that were easily absorbed in high amounts (Bax et al. 2013).
The high number of short-chain peptides in CT caused most of the peptides to be absorbed more quickly by the small intestine. This was confirmed by the increase in the absorption percentage of peptides in the jejunum. Few peptides that are not absorbed in the jejunum could be absorbed in the ileum. This was characterized by the decrease in the absorption percentage of peptides in the ileum.
Successful absorption of peptides indicated they were resistant to peptidases, which are present in the brush border membrane and in the cytoplasm. The difference in the concentration of absorbed peptides in each intestinal segment was influenced by the length of the peptide chain. Oligopeptides are hydrolyzed by aminopeptidase and produce shorter peptides. Short-chain peptides (di- and tri-peptides) are more easily absorbed than long-chain (penta- or hexa-peptide) peptides. This was because short-chain peptides passed through the epithelial cells of the small intestinal at a significantly higher rate than long-chain peptides (Chun et al. 1996).
Bioactive peptides contained in food are expected to still possess high bioactivity after being absorbed by the small intestine to exhibit the functional effects on the target organ. The ACE inhibitory activity exerted by the absorbed peptides in this study is depicted in Fig. 4. The highest ACE inhibitory activity in raw tempe was exhibited by the peptides absorbed in the ileum (76.71%), whereas that in CT was exhibited by the peptides absorbed in the jejunum (81.59%). These results indicated that some of the absorbed peptides could inhibit ACE activity. The high inhibitory activity showed that the presence of ACE inhibitory peptides is quite significant.
Fig. 4.

ACE inhibitory activity of absorbed peptides. RT: raw tempe. CT: cooked tempe. Different letters indicate a statistical difference (P < 0.05). The statistical test DMRT was applied
The absorption of ACE inhibitory peptides from raw tempe in the duodenum and jejunum was relatively low. Although the amount of peptides absorbed was large, the inhibitory activity exhibited was weak. The majority of ACE inhibitory peptides in raw tempe tend to be absorbed in the ileum. However, the process of boiling of tempe had enhanced the absorption capacity of ACE inhibitory peptides in each segment of the intestine. The increases in ACE inhibitory activity were 5.93% in the duodenum, 8.26% in the jejunum, and 2.97% in the ileum. This confirmed that the boiling process generated more ACE inhibitory peptides so that the number of bioactive peptides absorbed was more significant and had an impact on increasing the ACE inhibitory activity in each intestinal segment.
The least number of peptides were absorbed in the ileum, which further decreased after the boiling process. However, the inhibitory activity in the ileum was greater than that in the duodenum, which could possibly be due to the presence of activity of large peptides (MW > 14 kDa) that were resistant to peptidase.
The ACE inhibitory activity decreased by 9.39% compared to that before the absorption process. This was probably due to the fact that several peptides were not resistant to membrane hydrolysis and intracellular hydrolysis, so that not all peptides were absorbed in the form of short peptides. Some peptides were hydrolyzed into free amino acids and absorbed by the small intestine.
ACE inhibitory peptides with a low MW could pass through the small intestinal epithelial cells and enter the blood circulation through the process of active transport mediated by PepT1. The transport activity mediated by PepT1 depends on the proton gradient between the intestinal lumen (pH 5.5–6.0) and the epithelial cells (pH 7.0). Peptides and protons in the intestinal lumen approach the binding side of PepT1 to enable binding. The PepT1 binding site is specific. PepT1 has a high affinity for short-chain, neutral, and hydrophobic peptides. In addition, peptides and protons are released into the intracellular environment. The proton gradient is maintained through the Na+/K+ exchange. Protons are transported out of the cell and exchanged with Na+ on the brush border membrane when the excess concentration of Na+ in the cell is removed from the cell through the Na+/K+ ATPase pump in the basolateral (Gilbert et al. 2008; Xu et al. 2019).
Conclusion
Tempe prepared from koro kratok has the potential to be used as a functional food. The fermentation and boiling process increased the availability of ACE inhibitors in the food products. Peptides could be formed during the process of digestion and have an impact on increasing the ACE inhibitory activity. The highest inhibitory activity of 90.05% was detected in CT. The ACE inhibitory peptides from tempe were dominated by peptides with MW < 1 kDa and could inhibit ACE activity by 84.34%. Most of the ACE inhibitory peptides from tempe were absorbed in the jejunum and exhibited an ACE inhibitory activity of 81.59%. Further in vivo investigation is needed to better illustrate the biological effects of consuming tempe, as the simulation of gastrointestinal digestion done in this study only predicts the phenomena that occur in the gastrointestinal tract.
Acknowledgements
This study was financially supported by the Final Project Recognition Program (Program Rekognisi Tugas Akhir) 2019 from the Directorate of Research, Universitas Gadjah Mada (Grant No. 3392/UNI/DITLIT/DIT-LIT/LT/2019). The authors also thank Marta T. Handayani for the technical assistance provided in this study. The authors would like to thank Enago (www.enago.com) for the English language review.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Akillioglu HG, Karakaya S. Effects of heat treatment and in vitro digestion on the angiotensin converting enzyme inhibitory activity of some legume species. Eur Food Res Technol. 2009;229:915–921. doi: 10.1007/s00217-009-1133-x. [DOI] [Google Scholar]
- Aluko RE, Girgih AT, He R, Malomo S, Li H, Offengenden M, Wu J. Structural and functional characterization of yellow field pea seed (Pisum sativum L.) protein-derived antihypertensive peptides. Food Res Int. 2015;77:10–16. doi: 10.1016/j.foodres.2015.03.029. [DOI] [Google Scholar]
- Amenta F, Buccioni M, Ben DD, Lambertucci C, Navia AM, Ngnintedem MAN, Ricciutelli M, Spinaci A, Volpini R, Marucci G. Ex-vivo absorption study of lysine R-lipoate salt, a new pharmaceutical form of R-ALA. Eur J Pharm Sci. 2018;118:200–207. doi: 10.1016/j.ejps.2018.03.025. [DOI] [PubMed] [Google Scholar]
- Ancona DB, Espinoza TS, Ruiz JR, Campos MS, Guerrero LC. Enzymatic hydrolysis of hard-to-cook bean (Phaseolus vulgaris L.) protein concentrates and its effects on biological and functional properties. Int J Food Sci Tech. 2014;49:2–8. doi: 10.1111/ijfs.12267. [DOI] [Google Scholar]
- Andriati N, Anggrahini S, Setyaningsih W, Sofiana I, Pusparasi DA, Mossberg F. Physicochemical characterization of Jack Bean (Canavalia ensiformis) tempeh. Food Res. 2018;2(5):481–485. doi: 10.26656/fr.2017.2(5).300. [DOI] [Google Scholar]
- AOAC (2005) Official methods of analysis of the Association of Official Analytical Chemists, 14th edn. Washington DC
- Astuti M, Melial A, Dalais FS, Wahlqvist ML. Tempe, a nutritious and healthy food from Indonesia. Asia Pasific J Clin Nutr. 2000;9(4):322–325. doi: 10.1046/j.1440-6047.2000.00176.x. [DOI] [PubMed] [Google Scholar]
- Bax ML, Buffiere C, Hafnaoui N, Gaudichon C, Auzeloux IS, Dardevet D, Lhoutellier VS, Remond D. Effects of meat cooking, and of ingested amount, on protein digestion speed and entry of residual proteins into the colon: a study in Minipigs. PLoS ONE. 2013;8(4):1–7. doi: 10.1371/journal.pone.0061252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos MRS, Guerrero LAC, Ancona DAB. Purification of angiotensin I-converting enzyme inhibitory peptides from a cowpea (Vigna unguiculata) enzymatic hydrolysate. Process Biochem. 2011;46:864–872. doi: 10.1016/j.procbio.2010.12.008. [DOI] [Google Scholar]
- Capriotti AL, Caruso G, Cavaliere C, Samperi R, Ventura S, Chiozzi RZ, Lagana A. Identification of potential bioactive peptides generated by simulated gastrointestinal digestion of soybean seeds and soy milk proteins. J Food Compos Anal. 2015;44:205–213. doi: 10.1016/j.jfca.2015.08.007. [DOI] [Google Scholar]
- Chang KC, Skauge LH, Satterlee LD. Analysis of amino acids in soy isolates and navy beans using precolumn derivatization with phenylisothiocyanate and reversed-phase high performance liquid chromatography. J Food Sci. 1989;54:756–759. doi: 10.1111/j.1365-2621.1989.tb04699.x. [DOI] [Google Scholar]
- Chun H, Sasaki M, Fujiyama Y, Bamba T. Effect of peptide chain length on absorption and intact transport of hydrolyzed soybean peptide in rat intestinal everted sac. J Clin Biochem Nutr. 1996;21:131–140. doi: 10.3164/jcbn.21.131. [DOI] [Google Scholar]
- Church FC, Swaisgood HE, Porter GH, Catignani GL. Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J Dairy Sci. 1983;66:1219–1227. doi: 10.3168/jds.S0022-0302(83)81926-2. [DOI] [Google Scholar]
- Cushman DW, Cheung HS. Spectrophotometric assay and properties of the angiotensin converting enzymes from rabit lung. Biochem Pharmacol. 1971;20:1637–1648. doi: 10.1016/0006-2952(71)90292-9. [DOI] [PubMed] [Google Scholar]
- Davis PJ, Williams SC. Protein modification by thermal processing. Allergy. 1998;53:102–105. doi: 10.1111/j.1398-9995.1998.tb04975.x. [DOI] [PubMed] [Google Scholar]
- Gibbs BF, Zougman A, Masse R, Mulligan C. Production and characterization of bioactive peptides from soy hydrolysate and soy-fermented food. Food Res Int. 2004;37:123–131. doi: 10.1016/j.foodres.2003.09.010. [DOI] [Google Scholar]
- Gilbert ER, Wong EA, Webb KE. Peptide absorption and utilization: implications for animal nutrition and health. J Anim Sci. 2008;86:2135–2155. doi: 10.2527/jas.2007-0826. [DOI] [PubMed] [Google Scholar]
- Gu Y, Wu J. LC–MS/MS coupled with QSAR modeling in characterizing of angiotensin I-converting enzyme inhibitory peptides from soybean proteins. Food Chem. 2013;141:2682–2690. doi: 10.1016/j.foodchem.2013.04.064. [DOI] [PubMed] [Google Scholar]
- Guerrero LC, Magaña MD, Ayala AM, Ortiz GD, Ancona DB. Lima bean (Phaseolus lunatus) protein hydrolysates with ACE-I inhibitory activity. FNS. 2012;3:511–521. doi: 10.4236/fns.2012.34072. [DOI] [Google Scholar]
- Jayalaxmi B, Vijayalakshmi D, Ravindra U, Revenna ML, Chandru R. Effect of different processing methods on proximate, mineral and antinutrient content of lima bean (Phaseolus lunatus) seeds. Legume Res. 2016;39(4):543–549. [Google Scholar]
- Lee SY, Hur SJ. Antihypertensive peptides from animal products, marine organisms, and plants. Food Chem. 2017;228:506–517. doi: 10.1016/j.foodchem.2017.02.039. [DOI] [PubMed] [Google Scholar]
- Li H, Aluko RE. Identification and inhibitory properties of multifunctional peptides from pea protein hydrolysate. J Agric Food Chem. 2010;58:11471–11476. doi: 10.1021/jf102538g. [DOI] [PubMed] [Google Scholar]
- Lin HC, Alashi AM, Aluko RE, Pan BS, Chang YW. Antihypertensive properties of tilapia (Oreochromis spp.) frame and skin enzymatic protein hydrolysates. Food Nutr Res. 2017;61:1–11. doi: 10.1080/16546628.2017.1391666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magana MD, Campos MS, Ortiz GD, Ancona DB, Guerrero LC. ACE-I inhibitory properties of hydrolysates from germinated and ungerminated Phaseolus lunatus proteins. Food Sci Technol. 2015;35(1):167–174. doi: 10.1590/1678-457X.6551. [DOI] [Google Scholar]
- Matthews DM, Crampton RF, Lis MT. Sites of maximal intestinal absorptive capacity for amino acids and peptides: evidence for an independent peptide uptake system or system. J Clin Pathol. 1971;24:882–883. doi: 10.1136/jcp.24.9.882-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minekus M, Alminger M, Alvito P, Balance S, Bohn T, Bourlieu C, Carrière F, et al. A standardized static in vitro digestion method suitable for food—an international consensus. Food Funct. 2014;5(6):1113–1124. doi: 10.1039/C3FO60702J. [DOI] [PubMed] [Google Scholar]
- Natesh R, Schwager SLU, Sturrock ED, Acharya KR. Crystal structure of the human angiotensin-converting enzyme–lisinopril complex. Nature. 2003;42:551–554. doi: 10.1038/nature01370. [DOI] [PubMed] [Google Scholar]
- Omosebi MO, Otunola ET. Prelimenary studies on tempeh flour produced from three different rhizopus species. Int J Biotechnol Food Sci. 2013;1(5):90–96. [Google Scholar]
- Pihlanto LA, Koskinen P, Piilola K, Tupasela T, Korhonen H. Angiotensin I-converting enzyme inhibitory properties of whey protein digest: concentration and characterization of active peptides. J Dairy Res. 2000;67(1):53–64. doi: 10.1017/S0022029999003982. [DOI] [PubMed] [Google Scholar]
- Sanjukta S, Rai AK. Production of bioactive peptides during soybean fermentation and their potential health benefits. Trends Food Sci Tech. 2016;5:1–10. doi: 10.1016/j.tifs.2016.01.010. [DOI] [Google Scholar]
- Tang C. Thermal denaturation and gelation of vicilin-rich protein isolates from three Phaseolus legumes: a comparative study. LWT - Food Sci Technol. 2008;41:1380–1388. doi: 10.1016/j.lwt.2007.08.025. [DOI] [Google Scholar]
- Uco JT, Guerrero LC, Ayala AM, Ortız GD, Ancona DB. Angiotensin-I converting enzyme inhibitory and antioxidant activities of protein hydrolysates from Phaseolus lunatus and Phaseolus vulgaris seeds. LWT - Food Sci Technol. 2009;42:1597–1604. doi: 10.1016/j.lwt.2009.06.006. [DOI] [Google Scholar]
- Udenigwe CC, Adebivi AP, Doyen A, Li H, Bazinet L, Aluko RE. Low molecular weight flaxseed protein-derived arginine-containing peptides reduced blood pressure of spontaneously hypertensive rats faster than amino acid form of arginine and native flaxseed protein. Food Chem. 2012;132:468–475. doi: 10.1016/j.foodchem.2011.11.024. [DOI] [PubMed] [Google Scholar]
- Vital DAL, Mojica L, Mejía EG, Mendoza S, Pina GL. Biological potential of protein hydrolysates and peptides from common bean (Phaseolus vulgaris L.): a review. Food Res Int. 2015;76:39–50. doi: 10.1016/j.foodres.2014.11.024. [DOI] [Google Scholar]
- Wang Y, Li F, Chen M, Li Z, Liu W, Wang C. Angiotensin I-converting enzyme inhibitory activities of Chinese traditional soy-fermented Douchi and soypaste: effects of processing and stimulated gastrointestinal digestion. Int J Food Prop. 2015;18:934–944. doi: 10.1080/10942912.2014.913180. [DOI] [Google Scholar]
- Xu Q, Hong H, Wu J, Yan X. Bioavailability of bioactive peptides derived from food proteins across the intestinal epithelial membrane: A review. Trends Food Sci Tech. 2019;86:399–411. doi: 10.1016/j.tifs.2019.02.050. [DOI] [Google Scholar]


