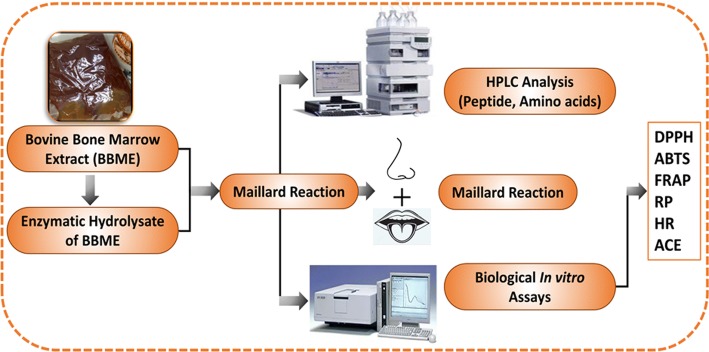
Abstract
Abstract
Maillard reaction and Maillard reaction products (MRPs) are commonly used in food industry, with various sensory and functional properties. Bovine bone marrow extract (BBME) was subjected to enzymatic hydrolysis, followed by the Maillard reaction to enhance its sensory attributes. The antioxidant and antihypertensive activities of BBME, its hydrolysate and their MRPs were assessed. (37) orthogonal experimental design was used to obtain the optimal reaction conditions for generation of MRPs with improved sensory characteristics. The optimal conditions were: processing temperature, 120 °C; processing time, 60 min; xylose concentration, 2.5% (w/v); cysteine concentration, 2%; Vitamin B1, 1.2%; Hydrolyzed vegetable protein, 6%; and sample to liquid ratio, 1:1. The total content of free amino acid in BBME decreased from 86.18 to 25.50 g L−1 in EH-BBME-MRPs. In addition, EH-BBME and EH-BBME-MRPs contained significantly higher amount of low molecular weight peptides (< 1000 Da; 47.2% and 21.84%, respectively) compared to other samples. They also exhibited enhanced antioxidant activities against DPPH, ABTS and hydroxyl free radicals, and presented improved FRAP activity and reducing power. EH-BBME-MRPs also exhibited higher antihypertensive activity compared to other samples. The results indicate that MRPs derived from BBME hydrolysate are promising components for improving food flavor and also provide health benefits.
Graphic abstract
Electronic supplementary material
The online version of this article (10.1007/s13197-019-04212-8) contains supplementary material, which is available to authorized users.
Keywords: Bovine bone marrow extract, Hydrolysate, Maillard reaction, Antioxidant activity, Free radical scavenging activity
Introduction
A high volume of waste byproducts is accumulated by meat industry annually, and a large proportion of which contains livestock bones. Beef bone marrow has been used to produce broth, soup and stocks, among which the stocks are utilized in sauces or gravies, meat and vegetable dishes, and soups (Rozi et al. 2018). Beef bone marrow is normally found in vertebrae, sternum, ribs and long bones. High-pressure steaming and concentration processes are widely used to produce bone marrow extract (also called “bone collagen”) from animal bones and are used as a natural flavor ingredient (Xu et al. 2018). Because of its unique flavor and high viscosity, the marrow extract from beef bones is often used as an ingredient to enhance the flavor of other low-value ingredients of foods such as nutritious sport foods (soup- or sauce-based) and pet foods. Bone marrow is a nutritious resource both for food and medicine, containing a variety of proteins, phosphorproteins, oil, chondroitin, mucopolysaccharides, vitamins, as well as minerals such as calcium, phosphorus, iron, zinc, copper, strontium and other health friendly substances. Accordingly, it exhibits numerous biological functions including antioxidant, immuno-modulatory, antimicrobial, antitumor and hypoglycemic activities (Rozi et al. 2018).
Enzymatic hydrolysis is often employed to transform low-value food ingredients into high-value foods, such as meat products. Viscosity of these foods can be reduced by cleaving peptide bonds in their protein to generate low molecular weight peptides and free amino acids. Production of meaty flavor from chicken and pig bones by enzymatic hydrolysis using Trypsase, Protamex, and Flavourzyme has also been reported (Sun et al. 2013). Furthermore, bioactive peptides have been produced using digestive enzymes, such as pepsin, chymotrypsin and trypsin; their contents in food materials can be manipulated by controlling protein hydrolysis.
The Maillard reaction (MR) is a non-enzymatic reaction between amino groups in amino acids, proteins or peptides and carbonyl groups in reducing sugars. A report has shown that flavor attributes that are produced by the senses of smell, taste and touch and are perceived with in the mouth, can be enhanced due to Maillard peptides (1000–5000 Da) (Ogasawara et al. 2006). Maillard’s reaction is used to enhance flavor and taste of the product, which impart in not only sensory but also health benefits (Morales and Jimenez-Perez 2001). In vivo experiments in rats after oral administration of MRPs have revealed reducing fat deposition and plasma leptin levels that in turn prevented weight gain (Yilmaz and Toledo 2005).
Foods such as coffee, malt, bread, cocoa and honey, which are widely consumed, are rich in melanoidins. These melanoidins have antimicrobial, prebiotic and antioxidant activities. Various studies have reported that MRPs have metal chelation ability and superoxide, hydrogen peroxide and hydroxyl radical scavenging activities (Hwang et al. 2011; Jing and Kitts 2002). In addition, a sugar “d-tagatose” found in MRPs of tuna backbone hydrolysates has been reported to improve 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging activity and reducing power, compared to d-fructose.
Angiotensin-I converting enzyme (ACE) inhibitors have been isolated from enzymatic digestion products of various foods, for example, chicken muscle, sardine muscle, corn gluten, milk, soy protein isolate, mung bean protein isolate, and wheat gluten (Wu et al. 2015). ACE plays an important physiological role in regulating blood pressure via kinin-kallikrein and the renin-angiotensin systems. ACE initially stimulates the transformation of angiotensin I into angiotensin II (a potent vasoconstrictor); after that, it deactivates bradykinin (a vasodilator). This dual function of ACE results in an increase of blood pressure and development of hypertension. Another study has also reported purification of ACE inhibitory peptides from porcine skeletal muscle proteins (Arihara et al. 2001). Hence, it is evident that food is a source of functional peptides, and their dietary intake may play roles in regulating blood pressure and overcoming hypertension.
Only few studies have investigated biological and antioxidant activities of MRPs derived from enzymatic hydrolysate of bovine bone marrow extract (BBME). However, to our knowledge, comparison of profiles of MRPs from hydrolysate of BBME with its original products has not been conducted. The objectives of this study were to determine the optimal processing conditions for MR of BBME hydrolysate, study the anti-oxidative activities of BBME, BBME hydrolysates and their MRPs, and to evaluate the changes in peptide distribution, sensory characteristics, free amino acids content, and antihypertensive activity in MRPs.
Materials and methods
Sample and reagents
Bovine bone marrow extract (BBME) was kindly provided by Dufengxuan Gusheng Co. Ltd. (Fushun, Liaoning, China). Potassium ferricyanide, trichloroacetic acid (TCA), hydrogen peroxide, 2,2-azino-bis(3-ethylbenzthiazoline)-6-sulfonic acid (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH), peroxidase, potassium and ferric chloride were purchased from Alfa Aesar (Shanghai, China). Enzymes including Flavourzyme® and Protamex® (500 MG) were obtained from Novozymes (Bagsvaerd, Denmark). Standard amino acids, fluorenylmethyl chloroformate (FMOC), o-phthaldialdehyde (OPA) and borate were supplied by Agilent Technologies (Palo Alto, USA). Oligopeptide standards (GASST, TEDVDVK, and CG, 95%) were purchased from Apeptides (Shanghai, China). Other standards, including aprotinin, glycine, bacitracin and cytochrome C, were obtained from Sigma-Aldrich (St. Louis, USA). All other chemicals were of analytical or HPLC grade and were either purchased from Thermo Fisher Scientific (Waltham, USA) or Sigma-Aldrich (Santa Clara, USA).
Preparation of BBME hydrolysates
The BBME hydrolysates were prepared by the enzymatic hydrolysis at 55 °C for 4 h in a water bath with continuous stirring. The enzymes used for hydrolysis were Protamex™ (47,500 U g−1) and Flavourzyme™ 500MG (25,000 U g−1). After the reaction was completed, the mixture was soaked in boiling water in a water bath for 10 min to deactivate enzyme; after that, it was cooled down to room temperature (25 ± 1 °C). The degree of hydrolysis (DH) was determined and calculated as the ratio of amino acids nitrogen to the total nitrogen in percentage (%). Formaldehyde titration method was used to quantify the nitrogen content in amino acid (AN) while total nitrogen (TN) content was determined following the method of Kjeldahl using a FOSS KJELTEC 8400 (Foss Co. Ltd, Denmark). The DH measured according to this method was 11.4%.
Optimization of processing for BBME Maillard reaction
The upper layer of BBME hydrolysate was used as the raw material to produce MRPs. Orthogonal experiments were designed according to earlier reported methods of Qinzhu et al. (2018). Orthogonal design L18 (37) having seven key factors affecting the Maillard reaction, including A (temperature), B (time), C (xylose), D (cysteine hydrochloride), E (Vitamin B1), F (hydrolyzed vegetable protein (HVP) and G (sample/liquid ratio) were examined. Each factor was varied at three different levels shown in Table 1. After attaining optimal conditions for Maillard reaction, 20 g of each sample and 20 mL of distilled water were mixed with xylose (1 g), thiamine (0.5 g), cysteine hydrochloride (0.8 g), and HVP (2.4 g). This mixture was incubated in a stainless high-pressure reactor (Parr Instrument Co., Moline, USA) and heated at 120 °C for about 60 min (allowing the thermal reaction to occur). The MRPs were immediately shifted to ice bath to stop further reaction and then stored in a refrigerator at 4 °C for consequent analysis.
Table 1.
Levels of important factors in BBME hydrolysate Maillard reaction products (MRPs) using orthogonal test
| Factors key | Factors | Level | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| A | Temperature (°C) | 100 | 110 | 120 |
| B | Time (min) | 60 | 70 | 80 |
| C | Xylose (%) | 1.5 | 2 | 2.5 |
| D | Cysteine (HCl) (%) | 1 | 1.5 | 2 |
| C | Vitamin B1 (%) | 1 | 1.2 | 1.4 |
| F | HVP (%) | 4 | 6 | 8 |
| G | Sample/liquid ratio | 1:0 | 1:1 | 1:2 |
HVP hydrolyzed vegetable protein
Determination of molecular weight distribution
The MW distribution of samples was determined by means of Size Exclusion Chromatography using Agilent HPLC-1100 system equipped with a TSK gel 2000 SWXL column (300 × 7.8, 5 µm) Tosoh Co., Tokyo, Japan. The separation was conducted at a flow rate of 0.5 mL/min using 20% acetonitrile, pH 3 (adjusted with aqueous formic acid) as a mobile phase. Diode array detector (DAD) was used having an absorbance–wavelength of 220 nm. The molecular weights of peptides were determined by a calibration curve constructed using various standards as follows: aprotinin, bacitracin, heptapeptide (TEDVDVK), pentapeptide (GASST), dipeptide (CG) and glycine, of which the molecular weights were 6512 Da, 1422 Da, 804 Da, 421 Da, 175 Da and 75 Da, respectively.
Quantification of free amino acids
Quantification of free amino acids in the samples were performed following an improved amino acids method using Agilent HPLC-1100 system equipped with ZORBAX Eclipse-AAA columns (Agilent Technologies, CA, USA) operated at the solvent flow rate of 1 mL/min. The injection volume was 0.5 µL, the column temperature was 40 °C and the DAD detection wavelength was 338 nm. Before injecting into the HPLC, the samples were treated with ophthalaldehyde and 3-mercaptopropionic acid (OPA), followed by 9-fluorenylmethylchloroformate in acetonitrile (FMOC). External standards at various concentrations were used to construct a calibration curve that was used for quantifying free amino acids, according to the method previously described by Henderson et al. (2000).
Sensory evaluation
Sensory descriptive test was performed on BBME-MRPs and EH-BBME-MRPs by eight trained panelists (five females and three males, aged 20–35 years) recruited from the Laboratory of Molecular Sensory Science, Beijing Technology and Business University, according to the method described by Xu et al. (2018) and Begum et al. (2019). The panelists were trained on various relevant aromas including: roasted aroma (roasted beef), sulfur odor (diluted standard and 2-methylthiophene), meaty aroma (boiled beef), burnt odor (grilled meat), caramel aroma (caramel syrup), fishy odor (fish soup) and sour odor (acetic acid). They were also trained on relevant tastes as follows: sour taste (20 mmol L−1 citric acid), sweet taste (50 mmol L−1 sucrose), umami taste (10 mmol L−1 of monosodium glutamate), kokumi and mellow tastes (10 g of diluted beef condensed soup) and bitter taste (1 mmol L−1 caffeine). Before the sensory evaluation of the samples, the reference solutions in different concentrations were tasted by the panelists and they were asked to memorize the intensities of relevant aromas and tastes. The intensities were already set by the panelists after mutual agreement. The evaluation was based on a ten-point intensity scale: weaker, 1–2; weak, 3–4; middle, 5–6; strong, 7–8; and stronger, 9–10. The scores were then averaged and rounded to the nearest integer.
Anti-oxidative activities
Determination of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity
The DPPH free radical scavenging activity of the samples was determined using the method of Wu et al. (2003) with slight modifications. In short, 1.5 mL of each sample was mixed with the same volume of 0.2 mM DPPH (preformed in 95% ethanol) and then stored at room temperature in darkness for 30 min. An absorbance at 517 nm was then measured using a Shimadzu UV-2401/2501 spectrophotometer (Molecular Devices, Japan). The DPPH radical scavenging activity was determined by the calibration curve prepared using Trolox. The values were expressed as μmol Trolox equivalents (TE)/mL.
The DPPH radical scavenging activity (%) was also calculated by the following equation:
| 1 |
where A1 is the absorbance of sample with DPPH solution, A2 is the sample solution without DPPH, A0 is the absorbance of solvent and DPPH.
Determination of ABTS radical scavenging activity
The scavenging activity of samples against ABTS radical was determined based on the method previously described by Re et al. (1999). Stock ABTS solution was prepared by mixing 7 mM ABTS with 2.45 mM potassium persulphate, and the mixture was stored at room temperature (25 ± 1 °C) for 16 h. The working ABTS solution (stable for 2 days) was prepared by diluting the stock solution with ethanol until the absorbance at 734 nm reached 0.7 ± 0.05. After the sample was diluted 20 times, 200 µL was withdrawn to mix with 2 mL of working ABTS solution. The mixture was vortexed and then incubated at room temperature (25 ± 1 °C) for 6 min to allow to the reaction to complete; and an absorbance at 734 nm was measured thereafter: The activity was expressed as µmol Trolox equivalent (TE)/mL and determined by the standard curve prepared by using Trolox (0–42 µmol/mL).
The ABTS radical scavenging activity (%) was calculated by the following equation:
| 2 |
Ferric reducing/antioxidant power (FRAP) assay
FRAP of the samples was performed using the FRAP kit (manufactured by Beyotime Biotechnology, China). Five μL of the diluted sample was added to 96-well microplates containing freshly prepared working FRAP solution (180 μL). The mixture was incubated at 37 °C for 3–5 min, and an absorbance at 593 nm of the mixture was then measured according to Shen et al. (2019). The FRAP values of the samples were determined using the standard curve and were expressed as mM Fe(II) equivalent/mL of sample.
Reducing power measurement
Reducing power of the samples, based on reduction of Fe3+ to Fe2+, was determined by the modified method described in Liu et al. (2012). Two milliliters of sample was mixed with 2 mL of 0.2 M phosphate buffer, pH 6.5 and 2 mL of 1% potassium ferricyanide. The mixture was incubated at 50 °C in water bath for 20 min. After cooling down to room temperature (25 ± 1 °C), the mixture was added with 10% TCA. Subsequently, 2 mL of the sample was mixed with 2 mL of distilled water and 400 μL of 0.1% FeCl3 in a test tube. After 10 min of incubation, the absorbance at 700 nm was measured using a Shimadzu UV-2401/2501 spectrophotometer and was expressed as ascorbic acid equivalent (AAE) µg/mL.
Hydroxyl radical scavenging activity (HRSA)
Determination of hydroxyl radical scavenging activity was carried out based on a previously described method of Saravanakumar et al. (2019) with slight modifications. One milliliter of FeSO4 solution (6 mM) was mixed with an equivalent volume of sample and H2O2 (6 mM) in a test tube and then vortexed. After that, the reaction was initiated by adding 1 mL of salicylic acid (6 mM) into the prepared mixture and was then incubated at room temperature (25 ± 1 °C) for 30 min. The absorbance at 510 nm of the mixture (A1), the solvent without sample (A0), and the sample solution without H2O2 (A2) were subsequently measured. The percentage inhibition (which reflects the radical scavenging activity) of the samples was calculated as follows:
| 3 |
Angiotensin-I converting enzyme (ACE) inhibitory activity
The assay of ACE inhibitory activity was performed according to the previously reported method of Hwang et al. (2011). Ten microliters of sample was mixed with 20 µL of NaCl-borate buffer (1 M), pH 8.2 containing 2.0 mU ACE-I at 37 °C for 10 min. After that, 30 µL of substrate (hippuryl histidyl leucine; 5 mM) was added to the mixture. After incubation at 37 °C for 1 h, the reaction was halted by adding 100 µL of HCl (1 M), after which was filtered through a 0.20-µm Millipore filter. Detection and quantification of the hippuric acid was carried out on an Agilent-1100 series HPLC consisting of an Agilent auto-sampler (ALS-1100) and a diode array detector (DAD) 1100; the injection volume was 10 µL. An SB C18 (250 × 4.6 mm) analytical column containing a packing material with 5 µm particle size was used, the flow rate was 0.8 mL/min. In the last step, the percent inhibition was calculated by the following equation:
| 4 |
where A1 indicate the hippuric acid with sample, A0 represents hippuric acid without sample.
Data analysis
All experiments were conducted in triplicate, and the data were presented as means ± standard deviations (SD). One-way ANOVA and Duncan’s multi-range test were carried out to determine the significant difference, using SPSS-17 (IBM, NY, USA) and Origin 9.1 software (OriginLab Corporation, Massachusetts, USA).
Results and discussion
Process optimization for Maillard reaction of BBME
Analysis of orthogonal experimental design
In the present study, the L18 (37) orthogonal design, made up of 18 test parameters, 7 factors and 3 levels, was used to optimize processing conditions for Maillard reaction. A (temperature), B (time), C (xylose), D (cysteine hydrochloride), E (Vitamin B1), F (hydrolyzed vegetable protein (HVP) and G (sample/liquid ratio) were examined as the processing factors and comprehensive score was employed as the evaluation indicators (Table 2). The influences of the processing variables over the comprehensive score was determined by range (R) analysis (Table 2) and ANOVA (S 1). Results of the range analysis showed that the influential orders of the seven factors to comprehensive score as A3B1C3D3E2F2G2 respectively. The Fisher’s F test used for the conformation of the confidence level either the factors have significant effect on experiment response or not. A large F-test mean showed the greater effect of the factor on experimental response. Variance analysis for the orthogonal experiment showed that effect of xylose concentration is significant (p < 0.05) while the other factors have not significant (p > 0.05). Optimum condition avail by the analysis test was finally confirmed by the validation experiment which show improved comprehensive score.
Table 2.
Analysis based on orthogonal design experiment
| Exp. no. | Temp (°C) (A) | Time (min) (B) | Xylose (%) (C) | Cys (HCl) (%) (D) | VB1 (%) (E) | HVP (%) (F) | Sample/liquid ratio (G) | Comprehensive score |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 31.2 |
| 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 41.2 |
| 3 | 1 | 3 | 3 | 3 | 3 | 3 | 3 | 37.5 |
| 4 | 2 | 1 | 1 | 2 | 2 | 3 | 3 | 36.32 |
| 5 | 2 | 2 | 2 | 3 | 3 | 1 | 1 | 37.32 |
| 6 | 2 | 3 | 3 | 1 | 1 | 2 | 2 | 30.7 |
| 7 | 3 | 1 | 2 | 1 | 3 | 2 | 3 | 43.07 |
| 8 | 3 | 2 | 3 | 2 | 1 | 3 | 1 | 31.84 |
| 9 | 3 | 3 | 1 | 3 | 2 | 1 | 2 | 41.73 |
| 10 | 1 | 1 | 3 | 3 | 2 | 2 | 1 | 40.35 |
| 11 | 1 | 2 | 1 | 1 | 3 | 3 | 2 | 34.48 |
| 12 | 1 | 3 | 2 | 2 | 1 | 1 | 3 | 38.13 |
| 13 | 2 | 1 | 2 | 3 | 1 | 3 | 2 | 45.72 |
| 14 | 2 | 2 | 3 | 1 | 2 | 1 | 3 | 36.36 |
| 15 | 2 | 3 | 1 | 2 | 3 | 2 | 1 | 36.15 |
| 16 | 3 | 1 | 3 | 2 | 3 | 1 | 2 | 37.15 |
| 17 | 3 | 2 | 1 | 3 | 1 | 2 | 3 | 42.8139 |
| 18 | 3 | 3 | 2 | 1 | 2 | 3 | 1 | 47.2 |
| K1 | 37.14 | 39 | 36.61 | 37.17 | 36.73 | 37.01 | 36.84 | |
| K2 | 36.59 | 37.3 | 35.67 | 36.32 | 40.5 | 39.54 | 38.52 | |
| K3 | 40.66 | 35 | 42.10 | 41 | 37.14 | 38.84 | 35.03 | |
| R | 4.07 | 4 | 6.43 | 4.68 | 3.77 | 2.53 | 3.49 | |
|
The impact of factors C > D > A > B > E > G > H Optimized conditions A3B1C3D3E2F2G2 | ||||||||
Comprehensive score = sensory score + taste nucleotide + amino acid score + soluble solid
K1, K2, K3 corresponding to level-1, level-2 and level-3, represent the sum of the comprehensive score
R = MaxKi–MinKi (I = 1, 2 or 3)
Molecular weight distribution (MWD) of peptide in hydrolysate and MRPs
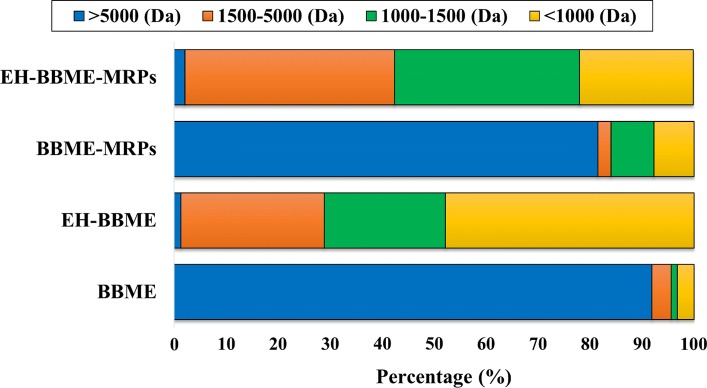
The enzymatic hydrolysis and the Maillard reaction are believed to change the MWD of peptides. The MWD of peptide in the samples are shown in Fig. 1. All samples were separated into four fractions: > 5000, 1500–5000, 1000–1500, and < 1000 Da. The low molecular weight (LMW) fraction (< 1000 Da) was dominant in BBME hydrolysate and its MRPs, representing 47.82% and 21.84% of the total peptide distribution, respectively. This is followed by the 1000–1500 Da fraction, which contributed to 23.29% and 35.61% of the total distribution, respectively. The > 5000 Da fraction had the lowest percentage in BBME hydrolysate, but highest in BBME with values of 1.37% and 91.87%, respectively. These findings suggest that EH-BBME contains high proportion of LMW peptides. In addition, Fig. 1 also displays the variation in MWD of peptides in BBME and its MRPs. Low amount of peptides with MW of < 1000 Da was observed in BBME and its MRPs (3.22% and 7.72%, respectively). In contrast, the peptides with MW of 1000–5000 Da, which are known as Maillard peptides (Eric et al. 2013), were significantly (p < 0.05) increased in BBME-MRPs and EH-BBME-MRPs by 10.78% and 75.85%, respectively, compared to those in BBME and EH-BBME (increased by 4.91% and 50.81%, respectively). The decrease in < 1000 Da peptides (LMW) and the increase in 1500–5000 Da peptides in MRPs can be ascribed to the cross-linking of small peptides, which may then act as active substances for the production of Maillard peptides during the reaction. These observations are in line with the previous studies, which have reported that the cross-linking of peptides could occur at the temperatures reached or slightly exceeded 110 °C (Liu et al. 2015).
Fig. 1.
Distribution of molecular weight of BBME, EH-BBME, BBME-MRPs and EH-BBME-MRPs. BBME bovine bone marrow extract, EH-BBME enzyme hydrolysate of BBME, BBME-MRPs Maillard reaction product of BBME, EH-BBME-MRPs Maillard reaction product of EH-BBME
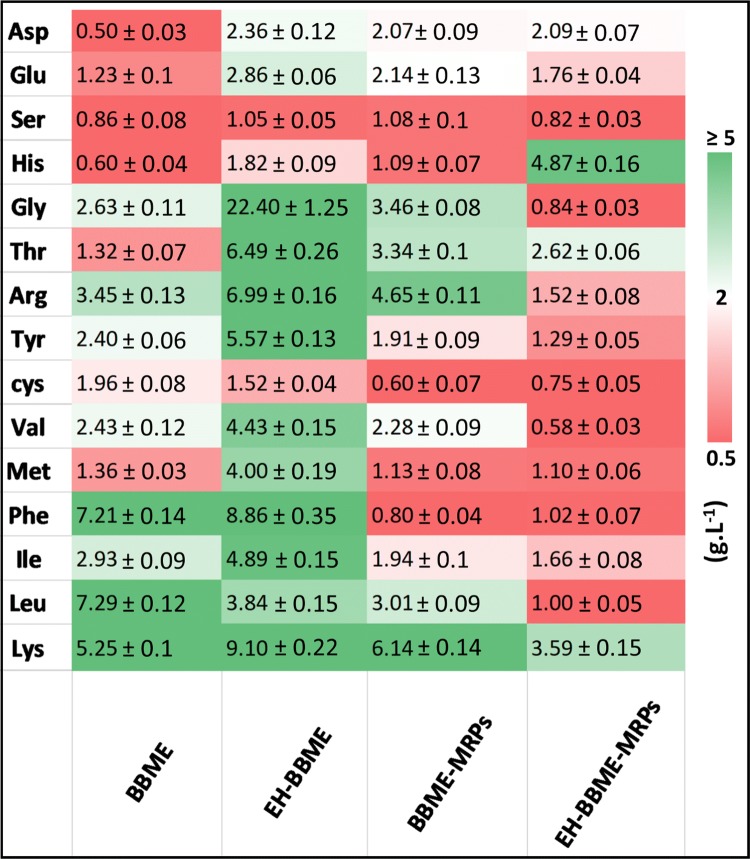
Variation in free amino acids content
Contents and compositions of free amino acids are linked to antioxidant activities and taste-related properties. The free amino acid profiles of BBME, EH-BBME, BBME-MRPs and EH-BBME-MRPs are shown in Fig. 2. The color intensity indicates the level of change in free amino acids contents with respect to the original sample (BBME). For instance, the color intensity in red–white–green color tone indicates that the amino acid contents are ≥ 5–2–0.5 g L−1, respectively. Compared with that in BBME, the free amino acid content in EH-BBME was increased (by 100%), while that in BBME-MRPs and EH-BBME-MRPs (obtained after Maillard reaction) was significantly decreased (by 14% and 70%, respectively). Similar results have been reported, in which the content of free amino acid was found to increased significantly (p ≤ 0.05) after hydrolysis and to decrease after Maillard reaction (Wei et al. 2018). As a result of the Maillard reaction, the total amino acid content reduced from 86.18 g L−1 (in EH-BBME) to 25.50 g L−1 (in EH-BBME-MRPs). This reduction can be attributed to flavor compounds, among others such as free amino acids, peptides and reducing sugars, generated by the Millard reaction, as has recently been described by Richarme et al. (2016).
Fig. 2.
The free amino acids distribution of BBME and their MRPs after enzyme treatment. The color scale presenting the intensity of change in free amino acids contents of each sample with respect to the original sample (BBME). For instance, the color intensity supplemented with the original values (red–white–green) shows the amino acid contents (≥ 5–2–0.5 g L−1, respectively). Values presented are the Mean ± SD (color figure online)
Eight out of the total seventeen amino acids analyzed, including Leu, Ile, Val, Phe, Ser, Met, His, and Arg, are bitter amino acids; among which three amino acids (Arg, Met, and His) are considered strong bitter amino acids. Amounts of Arg, Leu and Phe (bitter amino acids) decreased rapidly after the Maillard reaction (Fig. 2). Similar findings have been reported by Rabbani and Thornalley, who demonstrated that in the presence of xylose, Arg reacts most rapidly in the Maillard reaction (Rabbani and Thornalley 2014). As can be seen in Fig. 2, the contents of Arg, Leu, and Phe decreased from 6.99 ± 0.16, 3.84 ± 0.15 and 8.86 ± 0.35 g L−1 (in EH-BBME) to 1.52 ± 0.08, 1.00 ± 0.05 and 1.02 ± 0.07 g L−1 (EH-BBME-MRPs) after the Maillard reaction. Studies have also reported that bitter amino acids are highly reactive, thus are rapidly consumed by the Maillard reaction, resulting in MRPs that have low or no bitter taste (Liu et al. 2015).
Researchers have reported that the presences of some hydrophilic amino acids and high content of glutamic acid are responsible for glutamate-like taste (Schlichtherle-Cerny and Amadò 2002). BBME-MRPs contained higher concentration of glutamic acid (2.14 ± 0.13 g L−1); therefore, it exhibited stronger umami taste than EH-BBME-MRPs (1.76 ± 0.04 g L−1; Fig. 2). We also found that the enzymatic hydrolysis (EH-BBME) not only increased the umami taste (Glu) 2.86 ± 0.12 g L−1, but also contributed to the reduced bitter taste of MRPs (EH-BBME-MRPs); hence, the flavor of EH-BBME-MRPs was more desirable compared to that of BBME-MRPs.
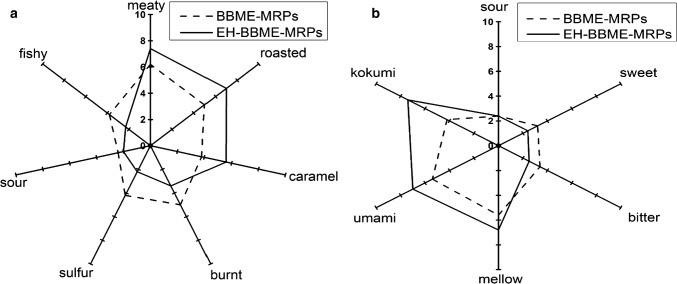
Sensory evaluation
A spider charts representing the comparison of aroma and taste profiles of EH-BBME-MRPs with those of BBME-MRPs are shown in Fig. 3a, b. According to Fig. 3a, EH-BBME-MRPs had higher meaty and caramel aromas but lower burnt, fishy and sour aromas compared with other samples. This result is in agreement with the previous finding, which has revealed that the meat flavor of food is dependent on the concentration of sulfur-containing compounds (Wang et al. 1996). The Maillard reaction is primarily used as food flavor enhancers to generate unique tastes and aromas during the thermal treatment.
Fig. 3.
Flavor profiles of MRPs by sensory evaluation. Each value represents the average taste intensity scored on a scale ranging from 0 to 10. a Sensory evaluation of aroma profile of MRPs of EH-BBME and BBME. b Sensory evaluation of taste profile of MRPs of EH-BBME and BBME
The taste profile also presented that EH-BBME-MRPs had higher of kokumi and umami scores, and lowest bitter and sour tastes compared with other samples (Fig. 3b). This indicates that the enzymatic hydrolysis can decrease bitterness in MRPs. Additionally, the bitter taste of EH-BBME-MRPs was lower than that of BBME-MRPs (non-hydrolyzed form), which may be due to the decreased content of bitter amino acid as they are utilized to generate new Maillard peptides by the Maillard reaction; as a result, the bitterness of MRPs was suppressed (Liu et al. 2012). These results indicates that EH-BBME-MRPs have better sensory characteristics (i.e., more pleasant meaty aroma and kokumi umami tastes) compared to BBME-MRPs. This is in accordance with the previous research, which has reported that the enzymatic hydrolysis can enhance the sensory characteristic of chicken soup (Kong et al. 2017). Similar findings have also been reported in a study by Xu et al., in which MRPs of various enzymatic hydrolysates were shown to significantly improve flavor characteristics (Xu et al. 2018).
Antioxidant activities of BBME, BBME hydrolysate and their MRPs
Antioxidants are essential for the human antioxidative defense system; they inhibit various reactive oxygen species (ROS), hypochlorous acid (HOCl), and other harmful free radicals. Therefore, we investigated the antioxidant activities of BBME, EH-BBME, BBME-MRPs and EH-BBME-MRPs, containing diverse amino acids and varying sized peptides.
DPPH and ABTS free radical scavenging activities
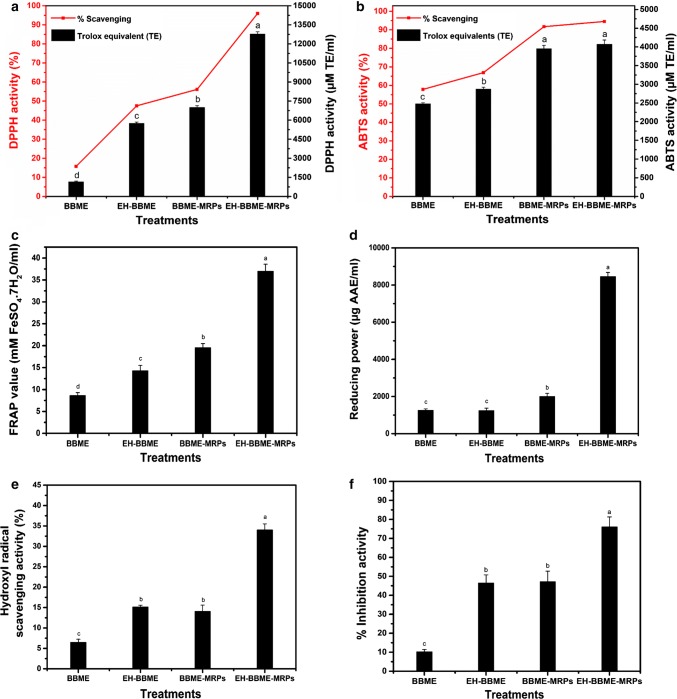
The abilities of the samples to scavenge DPPH and ABTS free radicals were determined. In scavenging DPPH free radicals, the antioxidants donate hydrogen atoms to form stable DPPH molecules (Matthäus 2002). The values of DPPH scavenging activity were presented as µM TE/mL and as percent (%). The DPPH scavenging activities of all samples were between 1153 ± 50.23 and 12,776.8 ± 177.58 µM TE/mL (Fig. 4a). The DPPH scavenging activities of EH-BBME-MRPs and BBME-MRPs were significantly higher than those of BBME and EH-BBME. It is apparent that the enzymatic treatment has an effect on the DPPH radical scavenging activity, which depends on the composition of amino acids and the molecular weight and structure of peptides (Su et al. 2011). Benjakul et al., have reported that the hydrogen donors may acts either as the intermediates or the final brown polymer, and the anti-DPPH radical activity may be due to sugar caramelization (Benjakul et al. 2005). Yilmaz and Toledo have reported that heating temperature and time (e.g. heating between 100 and 120 °C for varying times) can greatly influence the antioxidant activity of the water-soluble MRPs (Yilmaz and Toledo 2005). Other studies have also reported the DPPH-radical scavenging activity of xylose-lysine MRPs and reductone compounds (Yen and Hsieh 1995).
Fig. 4.
DPPH radical scavenging activity (a). ABTS radical scavenging activity (b). Data are presented as both percent (%) and µM TE/mL. Ferric reducing antioxidant power (FRAP) and expressed as mM FeSO4·7H2O/mL (c), reducing power (RP) is presented as Ascorbic acid equivalent (AAE) = µg of AAE/mL (d), hydroxyl radical scavenging activity of various samples (e), and ACE-I inhibitory activity of BBME, its hydrolysate and their Maillard reaction products are expressed as % inhibition of ACE (f). Error bars represent standard deviations from triplicate determinations. Different letters indicate significant differences (p < 0.05)
ABTS free radical scavenging activity of BBME, EH-BBME, BBME-MRPs and EH-BBME-MRPs were examined and the results are displayed in Fig. 4b. The values are expressed as µM TE/mL and as percent. The ABTS scavenging activity were found to be in a range of 2480 ± 25–4073.26 ± 110.5 µM Trolox/mL. The activity of EH-BBME-MRPS was significantly higher (4073.26 ± 110.5 µM Trolox/mL) than that of BBME and EH-BBME; and that of BBME and EH-BBME MRPs were not significantly different. Additionally, BBME had the lowest activity of 2480.69 ± 25 µM Trolox/mL. Higher ABTS radical scavenging activity of MRPs can be ascribed to higher amounts of MRPs and some newly formed peptides. Melanoidins (brown polymers resulted from heating peptides in the presence of reducing sugars) and free amino acids or peptides from MRPs have the ability to donate hydrogen atoms to ABTS free radicals; for this reason, they exhibit high antioxidant activity.
Ferric reducing/antioxidant power (FRAP) assay
FRAP is a rapid, sensitive, reproducible assay generally used to evaluate antioxidant capability in routine analysis. The increase in absorbance is related to the amount of iron that is reduced and is an indication that there is a high amount of antioxidants in the sample (Zou et al. 2011). The Fe3+-reduction capability of all samples can be ranked (from strongest to weakest) as follows: EH-BBME-MRPs (37.03 ± 1.6 mM FeSO4·7H2O equivalent/mL) > BBME-MRPs (25.33 ± 0.78 mM FeSO4·7H2O equivalent/mL) > EH-BBME (19.55 ± 0.92 mM FeSO4·7H2O equivalent/mL) > BBME (8.65 ± 0.65 mM FeSO4·7H2O equivalent/mL); which suggests that MRPs from hydrolysate have positive effect on reducing power (Fig. 4c). These results are in line with those of the previous studies, in which several antioxidant peptides derived from enzymatic hydrolysis from meat and milk protein were found to exhibit substantial FRAP activity (Bah et al. 2015).
Reducing power (RP)
In the presence of antioxidants, ferric chloride/ferricyanide complex is reduced to form ferrous ion (Fe2+), and the yellow color of the original sample is changed to the color shades between blue and green depending on the reducing power of the sample. This change can be monitored spectrophotometrically at 700 nm. As illustrated in Fig. 4d, EH-BBME-MRPs exhibited a significantly higher reducing power (8461.5 µg AAE/mL) than other samples, and the reducing power of BBME and its hydrolysate (EH-BBME) were not significantly different. It appears that samples with higher content of peptides may provide more hydrogen or electron donors, thereby contributing to higher reducing power. Similar results, in which MRPs derived from chicken were studied, have also been reported by Wu et al. (2005).
Hydroxyl radical scavenging activity (HRSA)
The hydroxyl radical scavenging activities of BBME, EH-BBME, BBME-MRPs and EH-BBME-MRPs are presented in Fig. 4e. Among all samples, the hydroxyl radical scavenging activity of EH-BBME-MRPs was highest (34%), followed by EH-BBME (15.15%) and BBME-MRPs (14.08%), and BBME (6.59%). Studies have shown that the hydroxyl radical scavenging activity of MRPs is due to the combined effects of reducing power, scavenging of active oxygen and donation of hydrogen atoms (Yen and Hsieh 1995). It is likely that the hydroxyl scavenging activity of EH-BBME-MRPs is due to their active hydroxyl substitution and hydrogen donation abilities. High molecular weight aromatic rings and hydroxyl groups and their intermediates are also essential for hydroxyl radical scavenging activity. Previous studies have also reported that MRPs derived from protein hydrolysates of Alaska Pollack frame and conger eel muscles have strong hydroxyl radical scavenging activity (Je et al. 2005).
Inhibitory activity against angiotensin-I converting enzyme (ACE)
In addition to regulating blood pressure, ACE maintains salt and fluid balance in mammals. ACE inhibitors may be useful as antihypertensive medicines. In this study, inhibitory effects of BBME, EH-BBME and their MRPs on ACE were investigated, and the values are expressed as percent inhibition (Fig. 4f). ACE inhibitory activity of BBME-MRPs was 47.14%, which is comparable to that of EH-BBME (46.45%). MRPs derived from the hydrolysates of BBME (EH-BBME-MRPs) had the highest ACE inhibition activity (76.11%), whereas the untreated sample (BBME) had the lowest activity (10.28%). ACE inhibitory activity was increased after enzymatic hydrolysis. According to the literature, a variety of protein sources exhibits ACE inhibitory activity after they are hydrolyzed with different proteases (Murray and FitzGerald 2007). Some researchers have proposed possible relationship between antihypertensive activity and melanoidins obtained from the Maillard reaction of foods, such as beer, sweet-wine and coffee (Rufián-Henares and Morales 2007). Nonetheless, further studies that evaluate the inhibition mechanism of ACE by MRPs should be carried out.
Conclusion
Improving flavor characteristics and functional features of BBME (a by-product from meat industry) were attempted through enzymatic hydrolysis and the Maillard reaction. Optimal conditions for BBME hydrolysate MRPs (EH-BBME-MRPs) were achieved using orthogonal array design. As a result the MRPs generated from EH-BBME were found to improve the meaty, roasted, caramel, kokumi, mellow, and umami flavors, while reduce the undesirable flavors, such as fishy, sulfur, sour and bitter. The results further revealed that EH-BBME-MRPs exhibited enhanced antioxidant activities against hydroxyl, DPPH, and ABTS free radicals, as well as higher FRAP activity, reducing power and ACE inhibitory activity. Therefore, it is concluded that the MRPs generated during Maillard reaction of BBME hydrolysate not only posture better flavor characteristics, but also depicted higher antioxidant and ACE inhibitory activities with respect to untreated BBME. Thus, it might be useful in formulation of foods providing health benefits. However, the structural characteristics of MRPs, their mechanism of generation, and their health-related functional properties need to be further explored.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Arihara K, Nakashima Y, Mukai T, Ishikawa S, Itoh M. Peptide inhibitors for angiotensin I-converting enzyme from enzymatic hydrolysates of porcine skeletal muscle proteins. Meat Sci. 2001;57(3):319–324. doi: 10.1016/s0309-1740(00)00108-x. [DOI] [PubMed] [Google Scholar]
- Bah CS, Bekhit AE-DA, Carne A, McConnell MA. Production of bioactive peptide hydrolysates from deer, sheep and pig plasma using plant and fungal protease preparations. Food Chem. 2015;176:54–63. doi: 10.1016/j.foodchem.2014.12.025. [DOI] [PubMed] [Google Scholar]
- Begum N, Raza A, Song H, Zhang Y, Zhang L, Liu P. Effect of thermal treatment on aroma generation from bovine bone marrow extract during enzymatic hydrolysis. J Food Process Preserv. 2019 [Google Scholar]
- Benjakul S, Lertittikul W, Bauer F. Antioxidant activity of Maillard reaction products from a porcine plasma protein–sugar model system. Food Chem. 2005;93(2):189–196. [Google Scholar]
- Eric K, Raymond LV, Huang M, Cheserek MJ, Hayat K, Savio ND, Amédée M, Zhang X. Sensory attributes and antioxidant capacity of Maillard reaction products derived from xylose, cysteine and sunflower protein hydrolysate model system. Food Res Int. 2013;54(2):1437–1447. [Google Scholar]
- Henderson JW, Ricker RD, Bidlingmeyer BA, Woodward C (2000) Rapid, accurate, sensitive, and reproducible HPLC analysis of amino acids. In: Amino acid analysis using Zorbax Eclipse-AAA columns and the 1100 HPLC, pp 1–10
- Hwang IG, Kim HY, Woo KS, Lee J, Jeong HS. Biological activities of Maillard reaction products (MRPs) in a sugar–amino acid model system. Food Chem. 2011;126(1):221–227. [Google Scholar]
- Je J-Y, Park P-J, Kim S-K. Antioxidant activity of a peptide isolated from Alaska pollack (Theragra chalcogramma) frame protein hydrolysate. Food Res Int. 2005;38(1):45–50. [Google Scholar]
- Jing H, Kitts D. Chemical and biochemical properties of casein–sugar Maillard reaction products. Food Chem Toxicol. 2002;40(7):1007–1015. doi: 10.1016/s0278-6915(02)00070-4. [DOI] [PubMed] [Google Scholar]
- Kong Y, Yang X, Ding Q, Zhang Y-Y, Sun B-G, Chen H-T, Sun Y. Comparison of non-volatile umami components in chicken soup and chicken enzymatic hydrolysate. Food Res Int. 2017;102:559–566. doi: 10.1016/j.foodres.2017.09.038. [DOI] [PubMed] [Google Scholar]
- Liu P, Huang M, Song S, Hayat K, Zhang X, Xia S, Jia C. Sensory characteristics and antioxidant activities of Maillard reaction products from soy protein hydrolysates with different molecular weight distribution. Food Bioprocess Technol. 2012;5(5):1775–1789. [Google Scholar]
- Liu J, Liu M, He C, Song H, Chen F. Effect of thermal treatment on the flavor generation from Maillard reaction of xylose and chicken peptide. LWT Food Sci Technol. 2015;64(1):316–325. [Google Scholar]
- Matthäus B. Antioxidant activity of extracts obtained from residues of different oilseeds. J Agric Food Chem. 2002;50(12):3444–3452. doi: 10.1021/jf011440s. [DOI] [PubMed] [Google Scholar]
- Morales FJ, Jiménez-Pérez S. Free radical scavenging capacity of Maillard reaction products as related to colour and fluorescence. Food Chem. 2001;72(1):119–125. [Google Scholar]
- Murray B, FitzGerald R. Angiotensin converting enzyme inhibitory peptides derived from food proteins: biochemistry, bioactivity and production. Curr Pharm Des. 2007;13(8):773–791. doi: 10.2174/138161207780363068. [DOI] [PubMed] [Google Scholar]
- Ogasawara M, Katsumata T, Egi M. Taste properties of Maillard-reaction products prepared from 1000 to 5000 Da peptide. Food Chem. 2006;99(3):600–604. [Google Scholar]
- Qinzhu Z, Yan-ling C, Dong-xiao S, Tian B, Yang Y, Shan H. Process optimization and anti-oxidative activity of peanut meal Maillard reaction products. LWT Food Sci Technol. 2018;97:573–580. [Google Scholar]
- Rabbani N, Thornalley PJ. Special edition of amino acids of selected papers from the eleventh international symposium on the Maillard reaction. Berlin: Springer; 2014. [DOI] [PubMed] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med. 1999;26(9–10):1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Richarme G, Marguet E, Forterre P, Ishino S, Ishino Y. DJ-1 family Maillard deglycases prevent acrylamide formation. Biochem Biophys Res Commun. 2016;478(3):1111–1116. doi: 10.1016/j.bbrc.2016.08.077. [DOI] [PubMed] [Google Scholar]
- Rozi P, Maimaiti P, Abuduwaili A, Wali A, Yili A, Akber Aisa H. Isolation and evaluation of bioactive protein and peptide from domestic animals’ bone marrow. Molecules. 2018;23(7):1673. doi: 10.3390/molecules23071673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufián-Henares JA, Morales FJ. Angiotensin-I converting enzyme inhibitory activity of coffee melanoidins. J Agric Food Chem. 2007;55(4):1480–1485. doi: 10.1021/jf062604d. [DOI] [PubMed] [Google Scholar]
- Saravanakumar A, Periyasamy P, Jang HT. In vitro assessment of three different artemisia species for their antioxidant and anti-fibrotic activity. Biocatal Agric Biotechnol. 2019;18:101040. [Google Scholar]
- Schlichtherle-Cerny H, Amadò R. Analysis of taste-active compounds in an enzymatic hydrolysate of deamidated wheat gluten. J Agric Food Chem. 2002;50(6):1515–1522. doi: 10.1021/jf010989o. [DOI] [PubMed] [Google Scholar]
- Shen Q, Jiang J, Wang M, Chen J, Liu D, Ye X, Hu Y. Volatile compounds and antioxidant properties of pickled and dried mustard as influenced by different cooking methods. J Food Process Preserv. 2019;43:e13918. [Google Scholar]
- Su G, Zheng L, Cui C, Yang B, Ren J, Zhao M. Characterization of antioxidant activity and volatile compounds of Maillard reaction products derived from different peptide fractions of peanut hydrolysate. Food Res Int. 2011;44(10):3250–3258. [Google Scholar]
- Sun H, Li X, Zhang C, Dong X, Li Y, Jia W. Comparison of volatile compounds in different Maillard reaction productions from chicken bone extract and its enzymatic hydrolysate. J Instrum Anal J Instrum Anal. 2013;32:661–667. [Google Scholar]
- Wang K, Maga J, Bechtel P. Taste properties and synergisms of beefy meaty peptide. J Food Sci. 1996;61(4):837–839. [Google Scholar]
- Wei C-K, Thakur K, Liu D-H, Zhang J-G, Wei Z-J. Enzymatic hydrolysis of flaxseed (Linum usitatissimum L.) protein and sensory characterization of Maillard reaction products. Food Chem. 2018;263:186–193. doi: 10.1016/j.foodchem.2018.04.120. [DOI] [PubMed] [Google Scholar]
- Wu H-C, Chen H-M, Shiau C-Y. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus) Food Res Int. 2003;36(9–10):949–957. [Google Scholar]
- Wu H-C, Pan BS, Chang C-L, Shiau C-Y. Low-molecular-weight peptides as related to antioxidant properties of chicken essence. Drug Food Anal. 2005;13(2):176–183. [Google Scholar]
- Wu S, Xuezhen F, Xiongdiao L, Yuanjin X, Dankui L. Purification and identification of angiotensin-I converting enzyme (ACE) inhibitory peptide from lizard fish (Saurida elongata) hydrolysate. J Funct Foods. 2015;13:295–299. [Google Scholar]
- Xu X, You M, Song H, Gong L, Pan W. Investigation of umami and kokumi taste-active components in bovine bone marrow extract produced during enzymatic hydrolysis and Maillard reaction. Int J Food Sci Technol. 2018;53(11):2465–2481. [Google Scholar]
- Yen GC, Hsieh PP. Antioxidative activity and scavenging effects on active oxygen of xylose-lysine Maillard reaction products. J Sci Food Agric. 1995;67(3):415–420. [Google Scholar]
- Yilmaz Y, Toledo R. Antioxidant activity of water-soluble Maillard reaction products. Food Chem. 2005;93(2):273–278. [Google Scholar]
- Zou Y, Chang SK, Gu Y, Qian SY. Antioxidant activity and phenolic compositions of lentil (Lens culinaris var. Morton) extract and its fractions. J Agric Food Chem. 2011;59(6):2268–2276. doi: 10.1021/jf104640k. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.