Abstract
People have affection for confectionary products. Confectionery products are of two types, baker and sugar confectionary. Dark chocolates belong to sugar confectionery class. The present invention was carried out on the preparation of synbiotic dark chocolates. Synbiotics are food products that contain both prebiotics and probiotic microorganisms, wherein prebiotics encourage the growth of probiotics. The synbiotic dark chocolates were amended with flax seeds (Linum usitatissimum L.) as a prebiotic for LAB. Flax seed contains fiber and phenolic antioxidants which makes them prebiotic source. The isolated LAB culture, which was identified as Leuconostoc mesenteroides based on morphological, biochemical tests and MALDI-TOF, showed the properties of a probiotic culture viz., tolerance to sodium chloride, bile salt, pH, and temperature, sensitivity to antibiotics, nonhemolytic and production of hydrogen peroxide. Cytotoxic activity of the cell free supernatant was assessed against MDA MB 231 and neuroblastoma cell line. Probiotic strain showed 48% and 30% cytotoxicity against MDA MB 231 and neuroblastoma cell line. The synbiotic chocolate was found to have more antioxidant activity, i.e. 90 U/mL by DPPH assay and 200 (μg Trolox/mL) by FRAP assay. The synbiotic chocolate prepared will be beneficial for the gut health of the humans and will also have excellent nutritional value.
Keywords: Synbiotic, Probiotics, Antioxidant activity, Cytotoxicity, Cell line, Functional food
Introduction
The probiotics are live microorganisms which exert a beneficial effect on the health of the host when administered in adequate quantities (FAO/WHO 2002). The development of functional food is done using combination of both prebiotics and probiotics and is called “synbiotic food” (Araujo et al. 2009). LAB are considered as probiotic strains. Leuconostoc genus belongs to Firmicutes phylum, are Gram positive, heterofermentative microorganisms, and are present either in coccoid or rod-like shape. Leuconostoc sp. has been reported in the fermentation of food like sauerkraut, kimchi and cheese (Jung et al. 2014; Wang et al. 2008). Leuconostoc sp. are the bacteria that have significant roles in fermenting foods, such as cheese, kimchi and sauerkraut. Leuconostoc has the ability to contribute to other product characteristics, such as flavor, texture and nutritional content (Alegria et al. 2013). The LAB including Leuconostoc are recognized as safe (GRAS) hosts for microbial cell factory. Synbiotic affects the host beneficially by improving the survival and implantation of live microbial dietary supplements in the gastrointestinal tract. Flax seeds contain lignans, α-linolenic acid, and soluble dietary fiber or gum which help in colon cancer prevention and reduce the risk of cardiovascular disease (Hijova et al. 2011). In-vitro fermentation of flax seed results in production of high amounts of acetate and propionate (short-chain fatty acids) (Fodje et al. 2009). The combination of fermented flax seed with probiotics helps to enhance the health. Chocolates can be a suitable vehicle to introduce probiotics and prebiotics in the human diet.
The aim of this work was to formulate a synbiotic chocolate with an added probiotic strain, i.e. Leuconostoc mesenteroides.
Materials and methods
Isolation and identification of lactic acid bacteria
Homemade mango pickle was used as a source of LAB. The pickle (1%) was diluted in sterile saline and 100 μL was spread plate on sterile MRS agar medium. The plates were incubated at room temperature for 48 h in the anaerobic jar. The isolates were purified, characterized and identified based on the morphological, biochemical properties and MALDI-TOF (Koubek et al. 2012).
Determination of probiotics potential of isolated Leuconostoc sp.
The probiotics potential of isolated Leuconostoc sp. was carried by studying the tolerance to NaCl; bile salt; pH; sensitivity to temperature and the antibiotics (Pundir et al. 2013). The potential of isolated Leuconostoc sp. to produce lactic acid, hydrogen peroxide and diacetyl was determined as per the protocol of AOAC (1990).
Determination of antioxidant activity of isolated Leuconostoc sp.
Ferric reducing ability assay
Antioxidant activity of isolated Leuconostoc sp. and cell free supernatant was tested using ferric reducing ability (FRAP) assay (Benzie and Strain 1996). To 0.5 mL of intact cells and cell free supernatant, 2 mL of FRAP reagent [300 mM acetate buffer, pH 3.6] (acetate buffer pH 3.6 composition: 6.4 mL 2 M sodium acetate and 93.6 mL 2 M acetic acid); 10 mM 2, 4, 6-tri (2-pyridyl)-1,3,5-triazine (TPTZ) in 40 mM HCl; and 20 mM ferric chloride were added in the ratio 10:1:1 (v:v:v). The mixture was incubated for 30 min at room temperature in the dark and the absorbance was measured at 593 nm.
DPPH (1, 1-Diphenyl-2-picrylhydrazyl) radical scavenging activity assay
The scavenging effect of the cell free supernatant and isolated Leuconostoc sp. was determined using DPPH radical scavenging activity assay. To 0.5 mL of intact cells and cell free supernatant, 2 mL of freshly prepared DPPH solution (0.2 mM) was added. The mixture was vigorously shaken and kept for 30 min in the dark at room temperature. The control sample contained deionized water. The DPPH scavenging activity was monitored by determining the absorbance at 517 nm. The radical scavenging activity was quantified as units/mL (U/mL) by using the following formula (Gazi et al. 2007):
where Abc and Abc = absorbance of the control and test samples at 517 nm, respectively, V = volume (mL) of the sample.
Cell surface hydrophobicity
Microbial adherence to hydrocarbons (MATH)
The isolate was grown in MRS broth at 37 °C for 48 h. The broth was centrifuged at 13,000 rpm for 10 min. The pellet was collected; washed twice with phosphate buffered saline of pH 7.4 and resuspended in 3 mL of phosphate buffered saline. The cell suspension (5 mL) was added to 0.6 mL of n-hexadecane and mixed for 2 min. After an incubation period for 1 h at 37 °C, aqueous phase was removed and the absorbance was determined at 560 nm. Percentage of hydrophobicity (H%) was calculated using the equation:
The isolates with H% above 50% can be considered as hydrophobic according to Moreira (2005).
Salt aggregation test
The tests was performed as per the protocol of Jonsson and Wadstrom (1984). Bacterial cell suspension was prepared in 0.02 M sodium phosphate buffer (pH 6.8). Bacterial cell suspension (25 μL) was mixed in equal amount with various molar solutions of ammonium sulfate (0.02–4 mol/L). Lowest concentration of ammonium sulphate which showed visible aggregation was scored as the SAT hydrophobicity value.
In-vitro cytotoxicity assay
MTT assay (Sevda et al. 2015).
Leucononstoc mesenteroides were grown in MRS medium for 48 h at 37 °C in anaerobic condition. The cell culture was centrifuged at 5000 rpm for 30 min and supernatant was filtered by cellulose filter paper. The pH was adjusted to 7.0 by NaOH.
Cell line culture
The neuroblastoma and MDA-MB 232 cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM) F-12, including 10% fetal bovine serum (FBS), 1% (v/v) l-glutamine, 1% (v/v) penicillin–streptomycin solution and 7.5% NaHCO3. The cells were maintained at 37 °C in a humidified atmosphere of 5% CO2 and 95% air and sub cultivated with 70–80% confluence.
Cytotoxicity test
Cell viability were determined by MTT assay. The neuroblastoma and MDA-MB 232 cell lines were incubated in 96 well plate and cultured for 24 h. The cell free supernatant of different concentrations were added into cultured medium. Non-inoculated DMEM medium was used as a negative control. The cell lines were further incubated for 24 h. After incubation, the cell viability was determined by colorimetric MTT assay. MTT solution was added to each well and incubated at 37 °C for 24 h. After incubation, DMSO was added to dissolve the blue colored crystals and absorbance was noted at 570 nm using the microplate reader.
Formulation of synbiotic dark chocolate and its antioxidant properties
The synbiotic chocolate was prepared by incorporating sterile flax seed. The sterilized flax seed (6 g) were inoculated with 109 cfu/mL of LAB, incubated for 4 days and added to 125 g of ready to use compound [Morde Food Pvt. Ltd (FCCI), India]. Melted chocolate was molded in the container and kept at 4 °C and 30 °C. The count of Leuconostoc sp. in chocolate was determined after every 15 days and bacterial viability was calculated according to the formula:
N = log CFU/g after a certain period of storage. N0 = log CFU/g after product preparation.
Antioxidant activity of flax seeds, dark chocolate and synbiotic chocolate was determined using FRAP and DPPH assay.
Results and discussion
Morphological and biochemical properties of the isolates
Total five isolates were obtained. The isolate No. 3 was selected for further study based on morphological and biochemical characteristics and probiotic potential. The isolate No. 3 was Gram positive rod; catalase negative; oxidase and indole positive. The isolate was found to be nonhemolytic and also ferment glucose and lactose.
MALDI TOF of Leuconostoc mesenteroides
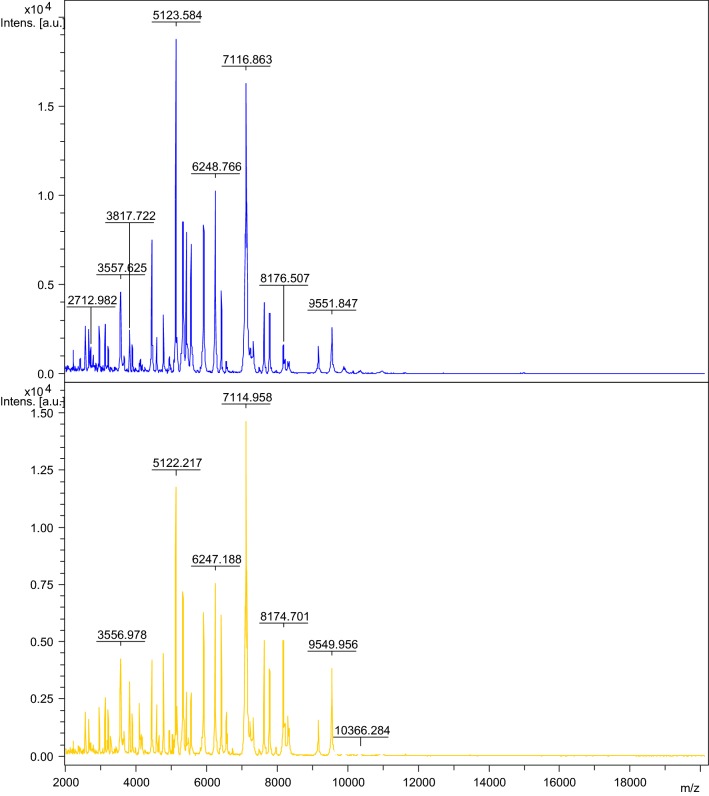
The highest intensity peak in Leu. mesenteroides appeared at m/z 5123 Da. The species-specific peak masses of Leuconostoc mesenteroides are 5123; 6248 and 7116. BioTyper log (score) ≥ 2.0 indicates secure genus identification. The MALDI TOF of Leu. mesenteroides is shown in Fig. 1.
Fig. 1.
MALDI-TOF of Leuconostoc mesenteroides
Probiotics potential of Leu. mesenteroides
Leuconostoc mesenteroides showed good tolerance for the NaCl concentration (1–7%); bile salt (0.5–2%), and growth was observed in the MRS broth with pH 3.0–7.0. The isolate showed good growth in the temperature range 25–40 °C. The LAB was found sensitive to all antibiotics, except Augmentin and Furazolidone. LAB was found to produce 26.75 mg of hydrogen peroxide. The cell free supernatant was found to contain lactic acid. Strains with hydrophobicity values below 40% are hydrophilic (Grajek et al. 2016). In our study, Leu. mesenteroides was found to have 7% hydrophobicity to n-hexadecane, which suggests that the strain is hydrophilic. SAT hydrophobicity value of Leu. mesenteroides was found to be 1 mol/L.
Antioxidant activity of Leuconostoc sp.
Leuconostoc mesenteroides was found to have more antioxidant activity as compared to its cell free supernatant. Antioxidant activity by DPPH method was found to be 60 and 15 U/mL for intact bacteria and its cell free supernatant, whereas antioxidant activity by FRAP assay was 120 and 22 μg trolox/mL for intact bacteria and its cell free supernatant.
Cytotoxicity assay
The MDA-MB-231 cell line is an epithelial, human breast cancer cell line. Neuroblastoma (NB) cell lines are transformed, neural crest derived cells. In-vitro cytotoxicity was assessed using MTT reagent which is based on blue formazan product formation due to reduction of MTT by mitochondrial dehydrogenase, which indicates the normal function of mitochondria and cell viability (Lau et al. 2004).
MTT assay using MDA-MB 231 cell line
The MTT assay using MDA-MB 231 cell line is shown in Table 1. The strongest effect of cell free supernatant was observed at 50 μL/mL after 24 h of incubation where 48% inhibition was observed (Table 1). IC 50 is the concentration of the L. mesenteroides cell free supernatant to cause 50% reduction in the cell viability. In case of L. mesenteroides, the IC50 of the cell free supernatant on the MDA-MB 231 cell line was found to be 5%.
Table 1.
MTT assay using MDA-MB 231 and Neuroblastoma cell lines
| A570 (MDA-MB 231 cell line) | A570 (Neuroblastoma Cell line) | Cytotoxicity (%) ± SD (MDA-MB 231 cell line) | Cytotoxicity (%) ± SD (Neuroblastoma cell line) | |
|---|---|---|---|---|
| Control | 0.636 | 0.506 | 0 ± 0.010 | 0 ± 0.006 |
| Cell free supernatant (μL/mL) | ||||
| 0.005 | 0.555 | 0.498 | 12.735 ± 0.007 | 1.645 ± 0.006 |
| 0.05 | 0.432 | 0.475 | 32.023 ± 0.001 | 6.122 ± 0.003 |
| 0.5 | 0.393 | 0.421 | 38.155 ± 0.004 | 16.721 ± 0.006 |
| 5.0 | 0.374 | 0.405 | 41.142 ± 0.01 | 20.01 ± 0.006 |
| 50.0 | 0.329 | 0.355 | 48.270 ± 0.003 | 29.75 ± 0.006 |
MTT assay using neuroblastoma cell line
The MTT assay using Neuroblastoma cell line is shown in Table 1. In these result, 50 μL/mL showed highest inhibition of 30%. In case of cytotoxicity of cell free supernatant of Leuconostoc against neuroblastoma cell line, IC50 value is more than 5% which showed that the strain will be less effective for antiproliferative activity in neuroblastoma cell line. The cell free supernatant showed more inhibition on MDA-MB cell line at concentration of 50 μL/mL after incubation of 24 h. According to these results, the cell free supernatant can inhibit 48% and 30% of breast cancer and neural diseases respectively.
There is a report on in-vitro cytotoxic activity of probiotic bacterial cell extracts against Caco-2 and HRT-18 colorectal cancer cells (Awaisheh et al. 2016). There is also a report on antioxidant activity of Lactobacillus pentosus ITA23 and Lb. acidipiscis ITA44 using FRAP method, where the intact cells and cell-free extracts of the two Lactobacillus strains showed more than 135 and less than 50 μg trolox/mL of antioxidant activity, respectively (Shokryazdan et al. 2018). Study has been done on Leuconostoc mesenteroides NRRL B-1149 as probiotic and its dextran with anticancer properties (Shukla et al. 2014).
Formulation of synbiotic dark chocolate and its antioxidant properties
Leu. mesenteroides ATCC 8293 was investigated for its macromolecular composition and it was found to be 29.7% protein, 7.9% lipid, 24.4% polysaccharide, 2.9% DNA, and 7.4% RNA. The amino acid content in Leuconostoc mesenteroides ATCC 8293 has been reported to have large amounts of lysine, glutamic acid, alanine, and leucine (Bang et al. 2017). Soluble dietary fiber (mucilage) of flax seeds has found to increase the growth of lactic acid bacteria in fermented dairy product (HadiNezhad et al. 2013). Flax seed serve as functional ingredient in variety of food products due to its content viz., dietary fiber, lignans, omega-3 and omega-6 essential fatty acids, phytosterols, vitamins and minerals (Teh et al. 2014). In-vitro fermentation of flax seed has shown presence of high amounts of acetate and propionate (short-chain fatty acids) (Fodje et al. 2009). The prebiotic content (dietary fiber) of flax seed makes them supporting medium for the growth of Leuconostoc mesenteroides. From our results, we conclude the fermented flex seed is suitable as prebiotic. Formulated synbiotic dark chocolates were checked for the viability of probiotic culture. Probiotic culture was found to be viable for six months in synbiotic chocolates when stored at 4 °C (Fig. 2), while viability of the Leu. mesenteroides decreased after 15 days in synbiotic dark chocolates when stored at 30 °C (Fig. 3), which is in comparison to the study done by Yao et al. 2008. Therefore, the viability of L. mesenteroides was good at 4 °C.
Fig. 2.
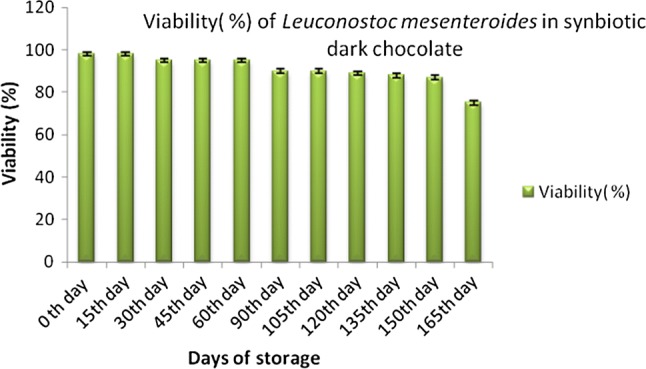
Viability (%) of Leuconostoc mesenteroides in synbiotic dark chocolate stored at 4 °C
Fig. 3.
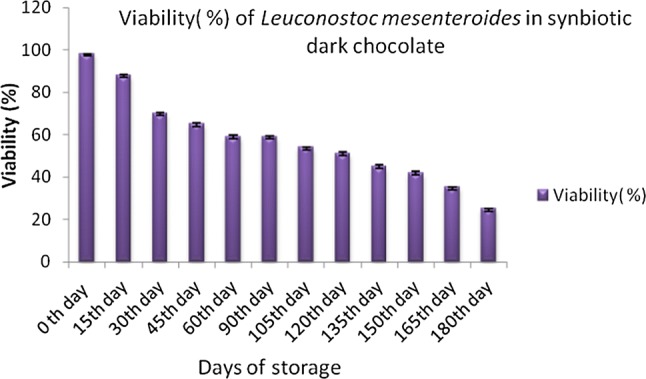
Viability (%) of Leuconostoc mesenteroides in synbiotic dark chocolate stored at 30 °C
The given growth medium and chocolate components does not inhibit or stop growth of probiotic bacteria. Live form of bacteria can be injected into the intestine of person by using this synbiotic chocolate and main motive of probiotic can be achieved. The DPPH radical scavenging activity of flax seeds, dark chocolate and synbiotic chocolate was found to be 69, 85 and 90 U/mL, respectively. Antioxidant activity by FRAP assay was found to be 150, 180 and 200 μg trolox/mL. Antioxidant activity of flax seeds is due to phenolic acids, flavonoids, phenylpropanoids and tannins (Kasote 2013). Dark chocolate is enriched with phenolics compounds due to its 67% of cocoa liquor (Da Silva-Madeiros et al. 2015). Synbiotic chocolates was found to have more antioxidant activity due to the combination of natural antioxidant flax seeds, dark chocolate and Leucononstoc mesenteriodes.
There is a report on probiotic chocolate prepared using yoghurt powder (Chetana et al. 2013). Also, study is done on preparation of dark chocolate incorporating probiotic Lb. plantarum isolated from cocoa beans (Foong et al. 2013). There is a report on sensory quality and volatile profile of dark chocolate enriched with encapsulated probiotic Lb. plantarum (Mirkovic et al. 2018).
Conclusion
Chocolate is a preferred choice by people of all ages and it serves as a promising bioactive compound carrier for prebiotic and probiotic.The work presented in this paper, is related with formulation of synbiotic chocolates using fermented flax seed as a prebiotic and probiotic Leuconostoc mesenteroides. Nutritional enrichment of food product is the need of hour, which can be accomplished by the consumption of functional foods enriched with dietary fibers. High antioxidant activity of synbiotic chocolates compared to its components, suggests its health beneficiary effects after its consumption. In-vitro fermentation of flax seeds has more nutritional values as compared to the original. Further, in vivo studies needs to be carried out to check the effect of these chocolates on the gut microflora. Functional foods will have more market value based on its human benefits. It can be concluded that, the current research will be beneficial for the food industry related with functional food.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alegria A, Delgado S, Florez AB, Mayo B. Identification, typing, and functional characterization of Leuconostoc spp. strains from traditional, starter-free cheeses. Dairy Sci Technol. 2013;93:657–673. doi: 10.1007/s13594-013-0128-3. [DOI] [Google Scholar]
- AOAC (1990) Association of official analytical chemists. Official methods of analysis, 15th edn. Gaithersburg, USA
- Araujo EA, Carvalho AF, Eleana SL, Furtado MM, Moraes CA. Production of cottage-like synbiotic cheese and study of probiotic cells survival when exposed to different stress levels. Pesqui Agropecu Trop. 2009;39:111–118. [Google Scholar]
- Awaisheh SS, Obeidat MM, Al-Tamimi HJ, Assaf AM, El-Qudah JM, Al-khazaleh JM, Rahahleh RJ. In-vitro cytotoxic activity of probiotic bacterial cell extracts against Caco-2 and HRT-18 colorectal cancer cells. Milk Sci Int. 2016;69:27–31. [Google Scholar]
- Bang J, Li L, Seong H, Kwon Y, Jeong E, Lee D, Han N. Macromolecular and elemental composition analyses of Leuconostoc mesenteroides ATCC 8293 cultured in a chemostat. J Microbiol Biotechnol. 2017;27:939–942. doi: 10.4014/jmb.1612.12038. [DOI] [PubMed] [Google Scholar]
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Chetana R, Reddy S, Negi P. Preparation and properties of probiotic chocolates using yoghurt powder. Food Nutr Sci. 2013;4:276–281. [Google Scholar]
- Da Silva-Madeiros N, Koslowsky-Marder R, Farias Wohlenberg M, Funchal C, Dani C. Total phenolic content and antioxidant activity of different types of chocolate, milk, semisweet, dark, and soy, in cerebral cortex, hippocampus, and cerebellum of wistar rats. Biochem Res Int. 2015;2015:1–9. doi: 10.1155/2015/294659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO (2002) Guidelines for the evaluation of probiotics in food. Report of a joint Food and Agriculture Organization (FAO) of the United Nations/World Health Organization (WHO) working group on drafting guidelines for the evaluation for the probiotics in food. London, Pntario, Canada
- Fodje AL, Chang PR, Leterme P. In-vitro bile acid binding and short chain fatty acid profile of flax fiber and ethanol co-products. J Med Food. 2009;12:1065–1073. doi: 10.1089/jmf.2008.0242. [DOI] [PubMed] [Google Scholar]
- Foong YJ, Lee ST, Ramli N, Tan YN, Ayob MK. Incorporation of potential probiotic Lactobacillus plantarum isolated from fermented cocoa beans into dark chocolate: bacterial viability and physicochemical properties analysis. J Food Qual. 2013;36:164–171. doi: 10.1111/jfq.12028. [DOI] [Google Scholar]
- Gazi MR, Yokota M, Tanaka Y, Kanda S, Itabashi H. Effects of protozoa on the antioxidant activity in the ruminal fluid and blood plasma of cattle. Anim Sci J. 2007;78:34–40. doi: 10.1111/j.1740-0929.2006.00401.x. [DOI] [Google Scholar]
- Grajek K, Sip A, Foksowicz-Flaczyk J, Dobrowolska A, Wita A. Adhesive and hydrophobic properties of the selected LAB isolated from gastrointestinal tract of farming animals. Acta Biochim Pol. 2016;63:311–314. doi: 10.18388/abp.2015_1128. [DOI] [PubMed] [Google Scholar]
- HadiNezhad M, Duc C, Han N, Hosseinian F. Flaxseed soluble dietary fiber enhances lactic acid bacterial survival and growth in kefir and possesses high antioxidant capacity. J Food Res. 2013;2:152–163. doi: 10.5539/jfr.v2n5p152. [DOI] [Google Scholar]
- Hijova E, Chmelarova A, Bomba A. Improved efficacy of prebiotic by flaxseed oil and horse chestnut in experimental colon cancer. Bratisl Med J. 2011;112:161–164. [PubMed] [Google Scholar]
- Jonsson P, Wadstrom T. Cell surface hydrophobicity of Staphylococcus aureus measured by the salt aggregation test (SAT) Curr Microbiol. 1984;10:203–209. doi: 10.1007/BF01627256. [DOI] [Google Scholar]
- Jung JY, Lee SH, Jeon CO. Kimchi microflora: history, current status, and perspectives for industrial kimchi production. Appl Microbiol Biotechnol. 2014;98:2385–2393. doi: 10.1007/s00253-014-5513-1. [DOI] [PubMed] [Google Scholar]
- Kasote DM. Flaxseed phenolics as natural antioxidants. Int Food Res J. 2013;20:27–34. [Google Scholar]
- Koubek J, Uhlik O, Jecna K, Junkova P, Vrkoslavova J, Lipov J, Kurzawova V, Macek T, Mackova M. Whole-cell MALDI TOF: rapid screening method in environmental microbiology. Int Biodeterior Biodegrad. 2012;69:82–86. doi: 10.1016/j.ibiod.2011.12.007. [DOI] [Google Scholar]
- Lau CB, Ho CY, Kim CF, Leung KN, Fung KP, Tse TF, Chan HH. Cytotoxic activities of Coriolus versicolor (Yunzhi) extract on human leukemia and lymphoma cells by induction of apoptosis. Life Sci. 2004;75:797–808. doi: 10.1016/j.lfs.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Mirkovic M, Seratlic S, Kilcawley K, Mannion D, Mirkovic N, Radulovic Z. The sensory quality and volatile profile of dark chocolate enriched with encapsulated probiotic Lactobacillus plantarum bacteria. Sensors. 2018;18:2570. doi: 10.3390/s18082570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira JS (2005) Isolamento, Identificaçao Molecular e Seleçao de Linhagens Probioticas de LactobacilosAviarios para uso como Veículo de uma Vacina Oral Contra Eimeriose. 69 f. Monografia (Graduaçao em Ciencias Biologicas)—Universidade Federal de Minas Gerais, Belo Horizonte
- Pundir RK, Rana S, Kashyap N, Kaur A. Probiotic potential of lactic acid bacteria isolated from food samples—an in vitro study. J Appl Pharm Sci. 2013;3:85–93. [Google Scholar]
- Sevda ER, Koparal AT, Kivanc M. Cytotoxic effects of various lactic acid bacteria on Caco-2 cells. Turk J Biol. 2015;39:23–30. doi: 10.3906/biy-1402-62. [DOI] [Google Scholar]
- Shokryazdan P, Faseleh JM, Fatemeh B, Zulkifli I, Boo LJ. Antiproliferation effects and antioxidant activity of two new Lactobacillus strains. Braz J Food Technol. 2018;21:1–8. [Google Scholar]
- Shukla R, Iliev I, Goyal A. Leuconostoc mesenteroides NRRL B-1149 as probiotic and its dextran with anticancer properties. J Biosci Biotechnol. 2014;3:79–87. [Google Scholar]
- Teh S, Bekhit A, Carne A, Birch J. Effect of the defatting process, acid and alkali extraction on the physico-chemical and functional properties of hemp, flax and canola seed cake protein isolates. J Food Meas Charact. 2014;8:92–104. doi: 10.1007/s11694-013-9168-x. [DOI] [Google Scholar]
- Wang Y, Li A, Jiang X, Zhang H, Mehmood K, Zhang L, Jiang J, Waqas M, Iqbal M, Li J. Probiotic potential of Leuconostoc pseudomesenteroides and Lactobacillus strains isolated from yaks. Front Microbiol. 2008;9:1–10. doi: 10.3389/fmicb.2018.02987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao A, Bera F, Franz C, Holzapfel W, Thonart P. Survival rate analysis of freeze-dried lactic acid bacteria using the Arrhenius and z-value models. J Food Protect. 2008;71:431–434. doi: 10.4315/0362-028X-71.2.431. [DOI] [PubMed] [Google Scholar]
- Zyzelewicz D, Motyl I, Nebesny E, Budryn G, Krysiak W, Rosicka-Kaczmarek J, Libudzisz Z. Chapter 12 Probiotic confectionery products—preparation and properties. In: Rigobelo E, editor. Probiotics. London: IntechOpen; 2012. [Google Scholar]