Abstract
The human body needs an antioxidant-rich diet that comes from foods, beverages, and herbal products to support the physiological antioxidant systems. Thus, the development of an analytical tool for a simple assay of the total antioxidant capacity (TAC) of the rich antioxidant samples is crucial. The current work demonstrates a simple colorimetric assay of TAC of the herbal extract on the paper microzone plate (PµZP) that was constructed in the 70-well of patterned paper using the screen printing technique. The PµZP was constructed by immobilizing DPPH (2,2-diphenyl-1-picrylhydrazyl) onto 70-well of PµZP as a sensing zone for colorimetric detection. The purple-sensing zone exhibited a good response to gallic acid (GA), by producing slightly gray to pale yellow color that can be captured using a scanner and then analyzed using the ImageJ program. The paper-based sensor showed a linear response toward GA at 0.05–0.6 mM (r = 0.9895), reproducible response (RSD < 4%), and accurate measurement with 91 to 106% recovery for measuring TAC of herbal extract, presented as mM gallic acid equivalent and showed a good agreement with the standard DPPH method. The results suggested that the proposed method can be applied for simple TAC measurement in the herbal extract.
Keywords: Paper microzone plate, Paper-based sensor, Colorimetric, DPPH, Total antioxidant capacity, Herbal extract
Introduction
Antioxidants contained in food, beverages, and herbal products may be particularly critical for protection from the diseases associated with reactive oxygen species (ROS) (Halliwell 1996). ROS associated diseases appear owing to the oxidative damage of macromolecules, such as proteins, DNA, and lipids, that might be related to the development of heart and blood vessel disease, the nervous system degeneration, and cancer. Naturally, ROS are produced in the human physiology system. However, since the antioxidant defense systems are not strong enough to neutralize and tackle ROS completely. Therefore, our body needs an antioxidant-rich diet that comes from foods, beverages, and herbal products. In this context, the development of an analytical tool for a simple assay of TAC of the diet that rich with antioxidants content is crucial.
Currently, many antioxidant methods are based on the spectrophotometric, such as 1,1′-DPPH (diphenyl-2-picrylhydrazyl), DMPD (N,N-dimethyl-p-phenylendiamine), ABTS (2,2′-azinobis-3-ethylbenzothiazoline-6-sulfonic acid), and metal complex, e.g. CUPRAC (cupric-neocuproine) and FRAP (ferric-tripyridyltriazine), are generally employed to determine activity of antioxidant of natural and synthetic antioxidant, including for measuring total antioxidant capacity (TAC) of our diet. The DPPH is one of the popular assays used to determine the TAC of foods, beverages, and herbal products (Musa et al. 2013). Firstly, the DPPH assay was proposed by Blois (1958), and then further developed by others for antioxidant assay of various compounds and measuring TAC of various food, beverage and herbal samples (Bondet et al. 1997; Sánchez-Moreno et al. 1998). Briefly, the DPPH method is based on the reduction of radical compound (DPPH) by an antioxidant, e.g. a herbal extract that makes the radical change color, where this even can be analyzed spectrophotometrically at 515–520 nm (Molyneux 2004). This radical is stable so that it does not need to be prepared freshly prior to the analysis, like in other radical scavenging assays (Bobo-García et al. 2015).
In the standard DPPH method, a large volume (1.0–5.0 ml) of freshly prepared reagent solution in a cuvette is needed (Seo et al. 2014; Nirmal and Panichayupakaranant 2015; Nisar et al. 2015; Pękal and Pyrzynska 2015; Bener et al. 2016; Dolinsky et al. 2016). In order to reduce the reagent used, a microwell plate has been proposed in this assay (Lee et al. 1998). Here, it was found that no significantly different in terms of analytical characteristics between cuvette and microwell-based methods within intra-lab validation (Bobo-García et al. 2015). Then, the latter method is developed by others as high throughput screening (HTS) for antioxidant capacity (Cheng et al. 2006; Abderrahim et al. 2013; Musa et al. 2013; Condezo-Hoyos et al. 2015). In order to create a simpler and faster method as HTS, the microwell-based method was further developed for DPPH in dry reagent format (Musa et al. 2013). The dry-reagent assay exhibited a good correlation with the DPPH microwell-based assay for various food samples (e.g. green tea, banana, pink guava, and honeydew). Furthermore, in order to make this method suitable for field analysis and low-cost assay, the paper-based sensors have been proposed (Nuchtavorn and Macka 2016; Sirivibulkovit et al. 2018).
The paper-based sensor is simple, low-cost, and easy to produce as well as needs a low reagent or sample volume. The paper-based sensor color change as an antioxidant response can be scanned or captured using a flatbed scanner or camera. The paper-based antioxidant sensors based on DPPH were successfully developed (Nuchtavorn and Macka 2016; Sirivibulkovit et al. 2018). Lately, other methods (e.g. ABTS, CUPRAC, and Folin–Ciocalteu) were impregnated separately on the paper-based devices that enabled for determination of TAC and total phenolic content simultaneously (Puangbanlang et al. 2019). The paper microzone plate (PµZP) as a sensing platform was first introducing by Carrilho et al. (2009) using a 96-microzone plate and fabricated using the photolithography technique. Then, they were developed by others for the detection of total phenolic content (Aid et al. 2015), tumor markers (Wang et al. 2012), foodborne pathogens (Jokerst et al. 2012), etc. By considering the DPPH assay could also be performed on the 96-microwell plate (Musa et al. 2013; Condezo-Hoyos et al. 2015), it is open-up the potential that the DPPH assay could be employed using the PµZP platform.
In this context, a simple and low-cost sensing platform for TAC determination in herbal extracts by immobilizing DPPH onto the microzone sensing area of PµZP has been developed. Practically, it has a simpler fabrication compare to the previous paper-based sensor based on DPPH (Nuchtavorn and Macka 2016; Sirivibulkovit et al. 2018) since, in this work, the screen printing technique has been used to create 70-well of microzone areas of PµZP using rubber-based ink. Furthermore, the scanometric method has been used for colorimetric TAC assay in our recent work (Hidayat et al. 2019) that create simpler, faster, and a low-cost assay of TAC. As far as PµZP as a concern, our work might be the first DPPH method using the PµZP platform. The PµZP was successfully used for the TAC assay of herbal extracts, and the results have a good correlation with the standard DPPH method.
Materials and methods
Chemicals
Gallic acid (GA) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were obtained from Sigma-Aldrich (USA). Methanol was purchased from Merck (UK). The GA stock solutions were prepared in methanol and working solutions were prepared daily by dilution of the stock solution using distilled water. All chemicals used were analytical grade.
Herbal samples
Herbal samples used in this study, such as West Indian elm leaf (Guazuma ulmifolia), red and white guava leaf (Psidium guajava), stonebreaker leaf (Phyllanthus niruri), and green tea leaf (Camellia sinensis) were obtained from Materia Medika, Batu, Indonesia and submitted as voucher specimens (MM 1901–1905) at Pharmacognosy Laboratory, Faculty of Pharmacy, University of Jember, Indonesia. Each of the herbal leaves was dried, ground, and filtered via #100 sieves. Plant powder (0.2 g) was sonicated (Elmasonic S180H, Germany) using a 10 ml solvent for 10 min. Methanol was employed as an extraction solvent for herbal leaf samples. Then, they were filtered using Whatman filter paper #1 (Merk, UK) and the extract solutions were placed in glass tubes in stored a refrigerator. For standard DPPH assay, all herbal extracts were diluted 100 times using methanol.
The PµZP fabrication
The PµZP (paper microzone plates) as the paper-based sensor was fabricated by patterning paper sheets (Whatman filter paper No 1, Merck UK) using a screen-printed technique according to our recent work (Hidayat et al. 2019). Using this fabrication technique, several numbers of microzones or well plate shapes could be printed based on the desired purposes. In this case, the microzone as the antioxidant sensor was constructed by immobilizing DPPH reagent (3 µl), followed by the drying process for 10 min at ambient condition and the result can be given in Fig. 1. Finally, the PµZP was kept in the container at 4 °C before it is used.
Fig. 1.
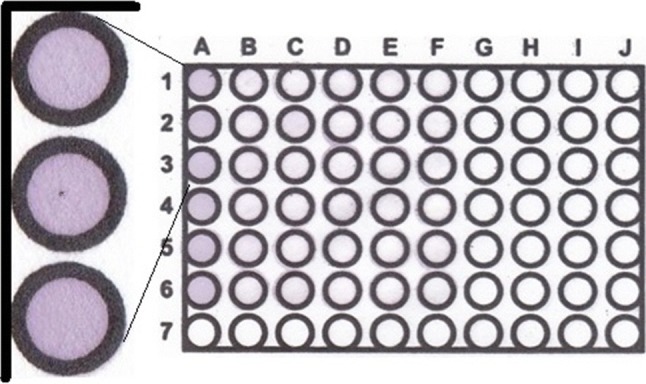
The PµZP with violet color microzones that acting as the antioxidant sensor
TAC determination
The general procedure can be described as follows: GA as a standard antioxidant or sample solution is taken 3 µl using a pipette and introduce into the microzone plate as a sensing zone. Then, a flatbed scanner (CanoScan, LIDE 110, Japan) is used to scan the microzone color change (300 dpi resolution in a color mode). The scanned images color intensity (JPEG format) was then measured using the ImageJ to calculate the mean value of R (red), G (green), B (blue), or the combined color as RGB (red–green–blue). For quantitative measurement, each color value of standard or herbal extract was subtracted with the blank values. TAC of the herbal sample was calculated using the constructed calibration curve of GA. All of the measurements were conducted in triplicate.
TAC assay using the standard DPPH method
The standard DPPH method was performed using the UV/Vis spectrophotometric method for assaying antioxidant capacity of herbal extracts using GA equivalent, as reported elsewhere (Lu et al. 2014). Here, the spectrophotometer was used with absorbance at 515 nm to make a calibration curve of GA as a gallic acid equivalent in mM (mM GAE). The TAC of the herbal samples were calculated using this calibration curve. All of the measurements were conducted in triplicate. Then the TAC value of all herbal samples measured by the PµZP and the standard DPPH methods were compared using the t test. In order to evaluate the correlation of the obtained TAC value of both methods, the analysis of correlation (r) was also used.
Results and discussion
Sensing mechanism
Here, radicals DPPH is employed as the basic sensing scheme for colorimetric detection of the antioxidant activity (Sánchez-Moreno et al. 1998). Owing to DPPH has been most frequently used for the assay of antioxidant activity of phytochemicals, e.g. polyphenols and flavonoids (Musa et al. 2013). Here, the chromogen radical (DPPH·) that is purple color is reduced to produce pale yellow of hydrazine (DPPH-H) by antioxidants, such as GA. In this case, the reduction of DPPH· by GA as a hydrogen-donating compound or antioxidant is detected colorimetrically via its color change to the pale yellow. Here, there is no required for the radicals regeneration in situ, which creates the antioxidant sensor simpler and easy to use the method. Moreover, for the determination of this reduced product (DPPH-H) quantitatively, the scanometric method was applied that used a common flatbed scanner and free color image analysis that makes this method even cheaper.
Color value selection
In order to obtain the optimum response of the PµZP toward antioxidants in term of color value, the different color response as R, G, B, and RGB, were evaluated and compared. Here, the optimum PµZP response was determined by calculating the color intensity change as an antioxidant sensor with the introduction of GA in a serial concentration (0.1–0.5 mM). Here, it was found that the blue color value (∆ Mean Blue) gave the highest response and showed the best correlation coefficient (r) among others. Based on this value, ∆ Mean Blue was selected and used for further measurements.
Reagent optimization
Here, the DPPH was used as a colorimetric reagent in a paper-based sensor as previously reported (Nuchtavorn and Macka 2016; Sirivibulkovit et al. 2018). Here, in order to develop the PµZP for the TAC assay, the DPPH was immobilized onto the surface of the microzone plate. Therefore, the concentration of DPPH needs to be optimized. In this case, various concentrations (2, 4, 5 and 7 mM) of DPPH immobilized and tested to obtain the best response toward antioxidants. Three microliters of each DPPH concentration was immobilized onto different microzone plates to produce the antioxidant sensor. Here, a serial concentration of GA (0.1–0.5 mM) was introduced to each DPPH concentration as the sensing zone. The constructed calibration curves presented as ∆ Mean Blue. Here, it was found that 5 mM of DPPH exhibits the highest response toward GA, in terms of its coefficient correlation (r) and slope. Therefore, the DPPH concentration at 5 mM was chosen as the sensor reagent.
Response time
The response time of the PµZP as an antioxidant sensor toward a GA solution (0.2 mM) presented as mean blue (Δ mean B) was found at 12 min, where the response was relatively constant. Based on this response time (12), the color change of the sensing zone in the PµZP toward serial concentrations of GA is given in Fig. 2a. Here, the violet sensing zone changed into slightly gray, after the addition of the GA solution. While the pale yellow was found at the GA concentration of 15 mM forward.
Fig. 2.
a The calibration curve of GA constructed by the PµZP at 12 min (n = 3). b The typical color change of antioxidant sensor after green tea extract addition (n = 9). A1–A3 = blank, B1–J1 = green tea extracts, and B2–J3 = empty micro zones (no added reagent)
Linearity and detection limit
Although the microzone color change can be easily viewed by the nude eye, for the quantitative measurements, they need to be calculated using the scanometric method as reported elsewhere (Hidayat et al. 2016). The PµZP has a linear response toward GA in the range of 0.05–0.6 mM (r = 0.995) (Fig. 2a). The sensitivity of the PµZP response based on the calibration slope was found to be 24.912 Δ mean Blue/mM GA (α = 0.05, n = 3). While the limit of detection calculated from the three times of the standard deviation of the blank was found to be 0.035 mM.
Reproducibility and accuracy
The reproducibility of the PµZP response in the TAC assay of herbal extracts was conducted according to Yuwono and Indrayanto (2005). Here, TAC of the green tea extract was assayed in several measurements (n = 9) as depicted in Fig. 2b. It was found that the relative standard deviation (RSD) values of the nine replicate measurements were 3.737%, indicating the PµZP has good reproducibility of TAC measurement in the herbal extracts. While the recovery study as the PµZP response accuracy in the TAC assay of the herbal extract (green tea), was conducted by spiked individually with GA to give 30, 45, and 60% of the initial TAC value of the extracts. Based on this study, the TAC recovery of the extract was found between 91 and 106%, indicated that the PµZP has good accuracy as an antioxidant sensor.
Stability of sensor
The PµZP stability was studied in two storage temperatures, i.e. at room (25 °C) and chiller (4 °C) condition by stored the PµZP in a sealed container separately at these two conditions. Here, the PµZP response was observed to decrease more than 15% after 5 h at room temperature. While at a chiller condition, the PµZP response decreases more than 15% was found in 3 days. The PµZP stability was less than the paper-based DPPH antioxidant sensor (Sirivibulkovit et al. 2018). This short stability might be due to the immobilized DPPH employed in this case was stored without plastic lamination as in the previous sensor, that might increase vaporization of the immobilized DPPH along with the effect of ambient condition. In this case, an attempt to laminate the PµZP with a plastic layer was carried out, and it could extend the stability up-to 5 days when it stored in the chiller condition.
Application in herbal samples
The comparison results of TAC values between the PµZP and the standard DPPH method are given in Table 1. Here, the TAC values of the herbal extracts resulted using the PµZP are similar to the result by the standard DPPH method. Hence, these results demonstrated that the PµZP was similar to the standard DPPH method in measuring TAC of herbal extracts. Furthermore, the resulted TAC values of both methods were in good agreement (r = 0.9887). Here, the PµZP was showed to be simpler, faster and cheaper than the standard DPPH method, or even with the microplate-based DPPH method (Krylova et al. 2016) in terms of method and cost, and also with more recently the paper-based sensor based on DPPH (Puangbanlang et al. 2019). The PµZP is also suitable for the field analysis as HTS for the TAC assay, and it is compatible to be used in the remote area or low resource-setting. Moreover, it can be used directly with a smartphone camera coupled with a color analysis application, such as Color Lab or Color Grap, that also freely available.
Table 1.
The comparison results of the TAC of the herbal extracts obtained by the PµZP and the standard DPPH method (n = 3, α = 0.05)
| Sample extracts | Sensor | UV–Vis | Sig. value (p) |
|---|---|---|---|
| West Indian elm leaf | 0.307 ± 0.039 | 0.335 ± 0.019 | 0.069 |
| Red guava leaf | 0.374 ± 0.125 | 0.417 ± 0.017 | 0.075 |
| White guava leaf | 0.427 ± 0.019 | 0.542 ± 0.051 | 0.144 |
| Stonebreaker leaf | 0.327 ± 0.058 | 0.352 ± 0.041 | 0.113 |
| Green tea leaf | 0.126 ± 0.026 | 0.113 ± 0.011 | 0.143 |
Conclusion
The PµZP as a paper-based antioxidant sensor for TAC assay of herbal extract combined with the scanometric method has been developed based on immobilized DPPH. The response time of the PµZP was found in 12 min that is faster compared to the standard DPPH method, and the TAC values of the herbal extracts measured by both methods were in good agreement. In PµZP as the antioxidant sensor, the reagent and sample need were reduced drastically, including the low-cost for performing the TAC assay.
Acknowledgements
This work was supported by The Applied Research Grand, DRPM, The Ministry of Research, Technology and Higher Education, Republic of Indonesia (Grant Number 175/SP2H/LT/DRPM/2019).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abderrahim F, Arribas SM, Carmen Gonzalez M, Condezo-Hoyos L. Rapid high-throughput assay to assess scavenging capacity index using DPPH. Food Chem. 2013;141:788–794. doi: 10.1016/j.foodchem.2013.04.055. [DOI] [PubMed] [Google Scholar]
- Aid T, Kaljurand M, Vaher M. Colorimetric determination of total phenolic contents in ionic liquid extracts by paper microzones and digital camera. Anal Methods. 2015;7:3193–3199. doi: 10.1039/C5AY00194C. [DOI] [Google Scholar]
- Bener M, Özyürek M, Güçlü K, Apak R. Optimization of microwave-assisted extraction of curcumin from Curcuma longa L. (Turmeric) and evaluation of antioxidant activity in multi-test systems. Rec Nat Prod. 2016;5:542–554. [Google Scholar]
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Bobo-García G, Davidov-Pardo G, Arroqui C, et al. Intra-laboratory validation of microplate methods for total phenolic content and antioxidant activity on polyphenolic extracts, and comparison with conventional spectrophotometric methods. J Sci Food Agric. 2015;95:204–209. doi: 10.1002/jsfa.6706. [DOI] [PubMed] [Google Scholar]
- Bondet V, Brand-Williams W, Berset C. Kinetics and mechanisms of antioxidant activity using the DPPH· free radical method. LWT Food Sci Technol. 1997;30:609–615. doi: 10.1006/fstl.1997.0240. [DOI] [Google Scholar]
- Carrilho E, Phillips ST, Vella SJ, et al. Paper microzone plates. Anal Chem. 2009;81:5990–5998. doi: 10.1021/ac900847g. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Moore J, Yu L. High-throughput relative DPPH radical scavenging capacity assay. J Agric Food Chem. 2006;54:7429–7436. doi: 10.1021/jf0611668. [DOI] [PubMed] [Google Scholar]
- Condezo-Hoyos L, Abderrahim F, Arriba SM, Carmen González M. A novel, micro, rapid and direct assay to assess total antioxidant capacity of solid foods. Talanta. 2015;138:108–116. doi: 10.1016/j.talanta.2015.01.043. [DOI] [PubMed] [Google Scholar]
- Dolinsky M, Agostinho C, Ribeiro D, et al. Effect of different cooking methods on the polyphenol concentration and antioxidant capacity of selected vegetables. J Culin Sci Technol. 2016;14:1–12. doi: 10.1080/15428052.2015.1058203. [DOI] [Google Scholar]
- Halliwell B. Commentary oxidative stress, nutrition and health. Experimental strategies for optimization of nutritional antioxidant intake in humans. Free Radic Res. 1996;25:57–74. doi: 10.3109/10715769609145656. [DOI] [PubMed] [Google Scholar]
- Hidayat MA, Jannah F, Kuswandi B. Development of paper based sensor for the determination of total phenolic content in green tea beverages. Agric Agric Sci Procedia. 2016;9:424–430. doi: 10.1016/j.aaspro.2016.02.159. [DOI] [Google Scholar]
- Hidayat MA, Chassana RI, Ningsih IY, et al. The CUPRAC-paper microzone plates as a simple and rapid method for total antioxidant capacity determination of plant extract. Eur Food Res Technol. 2019;245:2063–2070. doi: 10.1007/s00217-019-03312-1. [DOI] [Google Scholar]
- Jokerst JC, Adkins JA, Bisha B, et al. Development of a paper-based analytical device for colorimetric detection of select foodborne pathogens. Anal Chem. 2012;84:2900–2907. doi: 10.1021/ac203466y. [DOI] [PubMed] [Google Scholar]
- Krylova E, Gavrilenko N, Saranchina N, Gavrilenko M. Novel colorimetric sensor for cupric reducing antioxidant capacity (CUPRAC) measurement. Procedia Eng. 2016;168:355–358. doi: 10.1016/j.proeng.2016.11.120. [DOI] [Google Scholar]
- Lee SK, Mbwambo ZH, Chung H, et al. Evaluation of the antioxidant potential of natural products. Comb Chem High Throughput Screen. 1998;1:35–46. [PubMed] [Google Scholar]
- Lu Y, Shipton FN, Khoo TJ, Wiart C. Antioxidant Activity determination of citronellal and crude extracts of Cymbopogon citratus by 3 different methods. Pharmacol Pharm. 2014;05:395–400. doi: 10.4236/pp.2014.54047. [DOI] [Google Scholar]
- Molyneux P. The use of the stable free radical diphenylpicryl-hydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J Sci Technol. 2004;26:211–219. doi: 10.1287/isre.6.2.144. [DOI] [Google Scholar]
- Musa KH, Abdullah A, Kuswandi B, Hidayat MA. A novel high throughput method based on the DPPH dry reagent array for determination of antioxidant activity. Food Chem. 2013;141:4102–4106. doi: 10.1016/j.foodchem.2013.06.112. [DOI] [PubMed] [Google Scholar]
- Nirmal NP, Panichayupakaranant P. Antioxidant, antibacterial, and anti-inflammatory activities of standardized brazilin-rich Caesalpinia sappan extract. Pharm Biol. 2015;53:1339–1343. doi: 10.3109/13880209.2014.982295. [DOI] [PubMed] [Google Scholar]
- Nisar T, Iqbal M, Raza A, et al. Estimation of total phenolics and free radical scavenging of turmeric (Curcuma longa) Am J Agric Environ Sci. 2015;15:1272–1277. doi: 10.5829/idosi.aejaes.2015.15.7.9527. [DOI] [Google Scholar]
- Nuchtavorn N, Macka M. A novel highly flexible, simple, rapid and low-cost fabrication tool for paper-based microfluidic devices (μPADs) using technical drawing pens and in-house formulated aqueous inks. Anal Chim Acta. 2016;919:70–77. doi: 10.1016/j.aca.2016.03.018. [DOI] [PubMed] [Google Scholar]
- Pękal A, Pyrzynska K. Effect of pH and metal ions on DPPH radical scavenging activity of tea. Int J Food Sci Nutr. 2015;66:58–62. doi: 10.3109/09637486.2014.959899. [DOI] [PubMed] [Google Scholar]
- Puangbanlang C, Sirivibulkovit K, Nacapricha D, Sameenoi Y. A paper-based device for simultaneous determination of antioxidant activity and total phenolic content in food samples. Talanta. 2019;198:542–549. doi: 10.1016/j.talanta.2019.02.048. [DOI] [PubMed] [Google Scholar]
- Sánchez-Moreno C, Larrauri JA, Saura-Calixto F. A procedure to measure the antiradical efficiency of polyphenols. J Sci Food Agric. 1998;76:270–276. doi: 10.1002/(SICI)1097-0010(199802)76:2<270::AID-JSFA945>3.0.CO;2-9. [DOI] [Google Scholar]
- Seo J, Lee S, Elam ML, et al. Study to find the best extraction solvent for use with guava leaves (Psidium guajava L.) for high antioxidant efficacy. Food Sci Nutr. 2014;2:174–180. doi: 10.1002/fsn3.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirivibulkovit K, Nouanthavong S, Sameenoi Y. Paper-based DPPH assay for antioxidant activity analysis. Anal Sci. 2018;34:795–800. doi: 10.2116/analsci.18P014. [DOI] [PubMed] [Google Scholar]
- Wang S, Ge L, Song X, et al. Paper-based chemiluminescence ELISA: Lab-on-paper based on chitosan modified paper device and wax-screen-printing. Biosens Bioelectron. 2012;31:212–218. doi: 10.1016/j.bios.2011.10.019. [DOI] [PubMed] [Google Scholar]
- Yuwono M, Indrayanto G. Validation of chromatographic methods of analysis. In: Brittain H, editor. Profiles of drug substances, excipients and related methodology. Cambridge: Academic Press; 2005. pp. 243–259. [DOI] [PubMed] [Google Scholar]



