Abstract
Salting process is widely used in the process of meat products, whereas few studies have revealed the digestibility of actomyosin after salting treatment, which is closely related with the nutrition of meat. This work reported effect of salting on the structural change and digestibility of actomyosin before and after heat treatment. Actomyosin in 0.4 M and 0.8 M of NaCl had higher content of disulfide bonds, and actomyosin in 0.4 M NaCl showed the largest particle sizes before and after heat treatment. In addition, actomyosin in 0.6 M and 0.8 M of NaCl was oxidized more severely after heat treatment. Based on peptidomics analysis by using liquid chromatography tandem mass spectrometry (LC–MS/MS), actomyosin in 0.6 M was digested more easily, which was followed by sample in 0.8 M and 0.4 M of NaCl in descending order. The lowest digestibility of actomyosin in 0.4 M NaCl was related with its higher content of disulfide bond and severer aggregation behavior. The lower digestibility of actomyosin in 0.8 M NaCl should be related with the higher content of disulfide bonds and surface oxidation. These results highlight the crucial role of salting process in affecting the digestibility of meat protein.
Electronic supplementary material
The online version of this article (10.1007/s13197-019-04210-w) contains supplementary material, which is available to authorized users.
Keywords: Actomyosin, Salting, Structure, Digestibility, Peptidomics
Introduction
Salting process is widely used in meat products in drying, smoking and cooking, and this process contributes to the improvement of flavor, texture and shelf life of meat products (Aliño et al. 2010; Kumar et al. 2015). Salting denatures meat proteins and affects their water holding capacity and solubility (Si et al. 2015; Sharedeh et al. 2015; Nayak et al. 1996). In addition, formation of flavor compounds such as 2-dimethylacetaldehyde, 2-dimethylbutyraldehyde and 3-dimethylbutyraldehyde have been reported during salting process (Montel et al. 1996). Addition of NaCl alters the electrostatic, hydration, and water structuring effects of meat proteins, in which result in enhanced solubility (salting-in effect) or insolubility (salting-out) (Thorarinsdottir et al. 2011). When NaCl concentration increases from 0.3 to 1.0 M, Cl-ions will bind to the filaments and increase the electrostatic repulsive force between filaments, allowing the filament lattice to expand, which is termed as the salting-in of meat proteins (Offer and Trinick 1983). When the ionic strength increases above 1.0 M, the solubility of meat proteins begins to decrease, which have been generally ascribed to the exposure of hydrophobic residues and aggregation (Chen et al. 2015).
Structural changes in myofibril occurs during salting process. Si et al. (2015) reported decreases in the sulfhydryl content, surface hydrophobicity, α-helix, and β-turn of actomyosin along with the increase of salt concentration. Nguyen et al. (2011) found that the total content of sulfhydryl group of myofibrillar protein decreased and the content of disulfide bond increased in cod along with the increase of salt concentration. In addition, increased concentration of salt is proved to result in the depolarization of myofibrils, which increased surface hydrophobicity and induced aggregation of actomyosin (Thorarinsdottir et al. 2002). These structural changes of actomyosin induced by salting may change the accessibility of relative residues to digestive proteases during gastrointestinal digestion, which is rarely studied in previous work (Du et al. 2018a, b). Accordingly, the digestibility and peptide composition in the digests of actomyosin may be changed by salting, which will be studied in this work.
Although many studies have reported the digestibility of dietary proteins, most of them have focused on measuring degree of hydrolysis, showing SDS-PAGE image of digests. Detail information regarding the changes in peptide composition of digests was seldom revealed. Peptidomics method was used in this work to show the change in peptide composition of actomyosin that was treated with different NaCl concentration. The associations between the structural changes and digestibility changes of actomyosin was further established, aiming to explore the effect of salting process on the digestibility of meat proteins.
Materials and methods
Actomyosin preparation
Pork (longissimus dorsi) muscle were collected from the carcass of Duroc × (Landrace × Yorkshire) (DLY) crossbred pigs in Sushi meat products co. LTD in Huai’an city of China. Actomyosin was prepared from the muscle according to the method of Liu et al. (2008). Pork was homogenized (T 25 digital ULTRA-TURRAX ® Disperser, IKA, USA) in phosphate buffer (100 mM, pH 7.0) at 10,000 g for 5 min at 4 °C. The precipitate was dissolved in Weber–Edsall solution (0.6 M KCl, 0.04 M NaHCO3, 0.01 M Na2CO3, pH 7.2) and the previous centrifugation was repeated. The connective tissue was removed by filtering the supernatant through cheesecloth and washed with nine volumes of cold distilled water. The filtrate was centrifuged at 15,000×g for 10 min at 4 °C. The precipitate (actomyosin) was collected and lyophilized before being stored at − 20 °C.
Samples preparation and heating procedures
The actomyosin was dissolved in different NaCl solution (0.4 M, 0.6 M and 0.8 M) in Tris–HCl buffer (20 mM, pH 7.0) to obtain the final concentration of 5 mg/mL, all these samples were stand for 2 h before heat treatment. After that, 8 mL of each sample was heated at 70 °C for 30 min and cooled to around 4 °C immediately by ice-water bath before being stored at − 20 °C. Six replicates were applied for each group.
Reactive and total sulfhydryl contents in samples
The reactive sulfhydryl group (SH) content was determined according to the procedure of Beveridge et al. (1974). Actomyosin solution (100 μL, 5 mg/mL) was diluted with 1 mL Tris buffer (containing 6.9 g/L glycine and 1.2 g/L EDTA, pH 8.0). Then, 20 μL 5,5′-dithiobis-2-nitrobenzoic acid (D8130 Sigma Aldrich, Saint Louis, MO, 4 mg/mL in Tris buffer,) was mixed with diluted solution and incubated in a dark place at room temperature for 30 min. The absorbance of the incubated solution was measured at 412 nm and free SH content was calculated as follows:
where A412 was the absorbance at 412 nm; C was sample concentration in mg protein/mL; and D was dilution factor, 11.2. For the total sulfhydryl content, 100 μL actomyosin solution was diluted in 1 mL of Tris buffer (10.4 g Tris, 6.9 g glycine and 1.2 g EDTA per liter, pH 8.0) containing 8 M urea. The other steps are same as described above. Three replicates were applied for each sample.
Surface hydrophobicity (H0)
Surface hydrophobicity of actomyosin was determined using 1-anilino-8-napthalenesulfonate (ANS, A1028, Sigma Aldrich, Saint Louis, MO) method, according to Zhao et al. (2018). The actomyosin samples were diluted to 0.05, 0.125, 0.25, 0.375 and 0.5 mg/mL in 10 mmol/L PBS buffer. An aliquot of diluted samples (4 mL) was mixed with 20 μL 8 mM ANS and stand in dark for 10 min. Fluorescence intensity of the mixture was measured using a SpectraMax M2 (Molecular Devices Limited, USA) with an excitation of 374 nm and an emission of 485 nm. The fluorescent intensities were plotted against protein concentration, and the slope was calculated as the protein H0. Three replicates were applied for each sample.
Raman spectroscopy analysis
The actomyosin samples were loaded on glass slides and air-dried for 2 h at room temperature. Then Raman measurements were made on a LabRAM HR Evolution spectrometer (Horiba/Jobin, Yvon, Longjumeau, France) according to the method of Berhe et al. (2014) with minor modifications (the laser power was changed from 120 to 100 mW). Briefly, the slides were placed on a metal sheet under a microscope with a 50 × objective. The instrument was equipped with a 532 nm laser. The laser power on the samples was 100 mW. The spectra were acquired from 400 to 3200 cm−1 with 3 scans each and an exposure time of 20 s. Three replicates were applied for each sample.
Particle size measurement
Particle sizes were measured using an integrated-laser light scattering instrument (Mastersizer 3000, Malvern, Worcestershire, UK) as previously described by Cao et al. (2012). Actomyosin samples (5 mL) were injected in a sample chamber and homogenized by a mixing rotor. D4,3 and D3,2 represent the mean diameters in volume and in surface, respectively. Dx(10), Dx(50) and Dx(90) represent the particle sizes which is larger than 10%, 50% and 90% of the sample particles, respectively. Three replicates were applied for each sample.
X-ray photoelectron spectroscopy
The XPS measurement was performed on a PHI Quantera II spectrometer (Ulvac-Phi, Japan) using monochromatic Al Kα X-ray source according to the method of Debiemme-Chouvy et al. (2007). The high-resolution spectra were recorded by a 15.0 kV anode potential and 10 mA emission current. The photoelectron take-off angle of 45° was applied. The spectra of three elements (C1s, O1s and N1s) were recorded. The spectra were smoothed using a linear mode. The C1s was used as an internal energy reference whose binding energy peak was 284.5 eV. The compositions and higher solution peak fitting of C1s, O1s, and N1s were carried out using an XPS Peak 4.1 software provided by Roymond Kwok. All experiments were analyzed in six replicates. Three replicates were applied for each sample.
In vitro digestion
The gastrointestinal digestion process followed the method of Li et al. (2017) and Zhao (2017). Actomyosin (200 μg) was treated without or with DTT before digestion to test the influence of disulfide bond on protein digestibility. Each sample was digested with pepsin (P7012, Sigma-Aldrich, Shanghai) for 2 h at a ratio of 30:1 (protein/enzyme) and a pH of 2.5. After that NaOH was added to elevate the pH to 7.0, which was followed by addition of trypsin (Promega, Madison, WI, USA) at a ratio of 50: 1 (protein/enzyme) and a incubation for 4 h at 37 °C.
Liquid chromatography tandem mass spectrometry (LC–MS/MS)
The gastrointestinal digested products were desalted with a ZipTip C18 column (Millipore, Billerica, MA). The desalted peptides (10 μL) were loaded onto a C18 column (2 cm × 200 μm, 5 μm) before entering a C18 chromatographic column (75 μm × 100 mm, 3 μm). Peptides were separated by step-gradient elution with buffers A (0.1% formic acid in water) and B (0.1% formic acid in 84% acetonitrile) at a flow rate of 300 nL/min as follows: 97% A, 3% B (0–12 min), 72% A, 28% B (12–100 min), 45% A, 55% B (100–120 min), 2% A, 98% B (122–144 min) and 97% A, 3% B (144–160 min). Peptides were identified under a hybrid quadrupole orbitrap mass spectrometer equipped with a nano-electrospray ionization source (Thermo Fisher Scientific, Palo Alto, CA). The data-dependent mode was selected, and a scan cycle was initiated with a full-scan MS spectrum (from 300 to 1800 Da). MS/MS spectra were processed under the program of Proteome Discoverer-1.4 (Thermo Fisher Scientific) against Sus scrofa for pork (http://www.uniprot.org/). Data matching was performed in the Swiss-Prot database with a parent ion tolerance of 10 ppm and a false discovery rate (FDR) of 1%., and two missing cleavages were allowed. Both pepsin and trypsin were chosen in peptic/tryptic peptides search.
Statistical analysis
The significance level of sulfhydryl group contents, surface hydrophobicity and particle sizes were analyzed using one-way analysis of variance under a Duncan’s multiple-range test in the SAS software (version 9.2, 2016, SAS Institute Inc., USA). The means were considered significantly different if the P value was smaller than 0.05. PLS-DA analysis was applied by Ezinfo 3.0 (Waters, Milford, USA), and heat maps were made by Cluster 3.0 from Stanford University.
Results and discussion
Structure of actomyosin treated with different concentration of NaCl
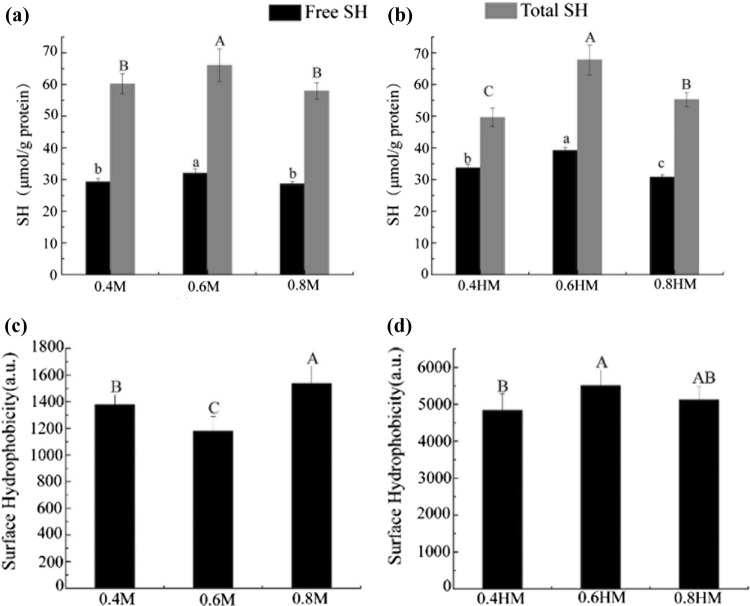
Reactive and total sulfhydryl groups: The reactive sulfhydryl indicates the exposed sulfhydryl groups that are accessible without denaturation by urea, which can reflect the changes in the microenvironments of sulfhydryl group. By contrast, the total sulfydryl reflects the loss of sulfydryl groups, which usually occurred during the formation of disulfide bonds and oxidation of sulfydryl groups. Salting and heat treatment can denature the protein, during which free sulfydryl groups may expose and combine into disulfide bonds (Bax et al. 2012). As shown in Fig. 1a, b, the reactive sulfhydryl group contents in actomyosin were the highest in sample in 0.6 M NaCl solution both before and after heating. This result indicates that more sulfhydryl groups were exposed when the filament expanded in actomyosin dissolved in 0.6 M NaCl. Normally, 0.3–1.0 M NaCl can induce a salting-in effect of myofibrillar proteins, this salting-in effect should be more significant in sample in 0.6 M NaCl than the other two samples (Offer and Trinick 1983). As salt concentration increased to 0.8 M, aggregation of actomyosin may had occurred more intensively than that in 0.6 M NaCl, and thus may be responsible for the lower content of reactive sulfhydryl group in sample dissolved in 0.8 M NaCl. Heating appeared to increase the reactive SH contents by unfolding the protein (Bax et al. 2012).
Fig. 1.
Change in sulfhydryl group and surface hydrophobicity of actomyosin in different salt concentrations before and after heat treatment. a, b Reactive and total sulfhydryl group content before and after heat treatment; c, d hydrophobicity before and after heat treatment. Different letters (a–c, A–C) in the same group indicate significant differences (P < 0.05)
Notably, the total sulfhydryl group contents in actomyosin are also the highest in sample in 0.6 M NaCl solution before and after heating. This result may indicate that fewer disulfide bonds formed or fewer oxidation occurred in this sample than sample in 0.4 and 0.8 M of NaCl. Disulfide bond and oxidation had been found to decrease the digestibility of protein (Hamaker et al. 1987; Hu et al. 2018). Therefore, these discrepancies in the content of total sulfhydryl group among actomyosins with different salting disposals could result in their different digestion behavior to some degree.
Hydrophobic residues: H0 values in Fig. 1c, d reflect the exposure of hydrophobic residues induced by salting and heat treatment. Much higher H0 values were observed after heat treatment, which indicate that much severer unfolding of actomyosin occurred during heat treatment. The surface hydrophobicity was the lowest in unheated actomyosin in 0.6 M NaCl solution (Fig. 1c, P < 0.05). By contrast, it was the highest after heat treatment of actomyosin in 0.6 M NaCl solution (Fig. 1d, P < 0.05), which is in accordance with its higher exposing extent of reactive sulfhydryl group (Fig. 1b).
As shown in Fig. 2, Raman spectrum can reflect the change in the polarity of microenvironment. The ratios of I−1760cm/I−11003cm and I−1850cm/I−1830cm are related to the exposed or buried status of tryptophan (Trp) and tyrosine (Tyr) residues, respectively (Li-Chan 1996). The ratio of I−1756cm/I−11003cm in actomyosin was the highest in 0.6 M NaCl solution (Fig. 2a), indicating that the Trp residues of actomyosin were more exposed in 0.6 M NaCl solution. By contrast, the highest value of I−1850cm/I−1830cm was found in sample in 0.8 M NaCl solution, demonstrating more exposed Tyr residues in this sample before heat treatment (Fig. 2a). After heat treatment, both the ratio of I−1756cm/I−11003cm and I−1850cm/I−1830cm were generally elevated, possibly due to unfolding behavior of actomyosin that occurred during heating (Bax et al. 2012). The ratio of I−1756cm/I−11003cm of actomyosin in 0.6 M NaCl solution was significantly lower than other twos, while the ratio of I−1850cm/I−1830cm was higher in 0.6 M NaCl solution (Fig. 3b). These results could indicate that Trp residues were less exposed, whereas the Tyr residues were more exposed in samples treated in 0.6 M NaCl solution after heat treatment. These complex results from H0 values and Raman spectroscopy indicate inconsistent changes in the microenvironments of hydrophobic residues of actomyosin, as induced by the difference in the concentration of NaCl. Some residues such as Phe, Tyr, Trp and Leu are important cleavage sites for pepsin (Dunn 2001; Zhao et al. 2017). Therefore, the higher H0 in the heat sample could resulted in their higher digestibility, which was confirmed by the following digestion analysis. In addition, discrepancies in the microenvironment among actomyosins with different salting disposals could result in their different digestion behavior to some degree.
Fig. 2.
Ratio of I−1760cm/I−11003cm and I−1850cm/I−1830cm of actomyosin in different salt concentrations before (a) and after (b) heat treatment, determined by Raman spectra. Different letters in the same group indicate significant differences (P < 0.05)
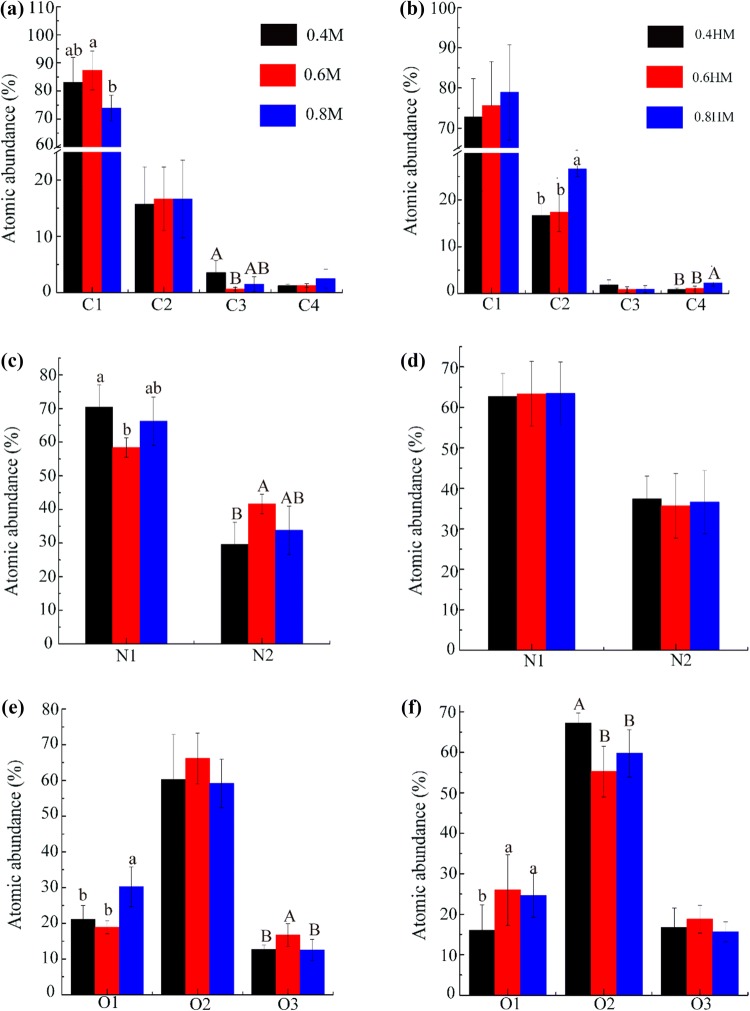
Fig. 3.
C1s, N1s, and O1s relative abundances calculated from XPS spectra of actomyosin in different salt concentrations before (a–c) and after (d–f) heat treatment. a, d Refer to abundance of C1: C–C, C–H (284.8 eV); C2:C–O and C–N (286.1 eV); C3: O–C–O, C=O (287.7 eV); C4: O=C–OH, O=C–OR (288.7 eV); b, e refer to abundance of N1: NH, NH2 (399.7 eV); N2: NH3 (401.3 eV); c, f refer to abundance of O1: O=C–OR, O=C–N (531.4 eV); O2: C–OH, C–O–C, C–O (532.5 eV); O3: O=C–OR (533.8 eV). Different letters in the same group indicate significant differences (P < 0.05)
Particle size: High salt concentration and heat treatment also induced aggregation of proteins which are mainly driven by hydrophobic interaction, formation of disulfide bonds and hydrogen bonds (Promeyrat et al. 2013). Table 1 showed the particle sizes of unheated actomyosin samples differed in NaCl concentration. The particle size of actomyosin was the highest in 0.4 M NaCl solution and was similar in 0.6 and 0.8 M NaCl before heat treatment. In heated samples, the particle sizes decreased along with increase in salt concentration. Higher iron strength increased the electrostatic repulsive force of protein and inhibited the heat-induced aggregation, which were widely reported in previous study (Brisson et al. 2007) Additionally, the aggregation behavior can change the digestibility of protein by burring some residues inside the aggregates, which may reduce the digestibility of protein to some extent (Zhao et al. 2017; Gilani et al. 2012). The bigger particle size of sample treated in 0.4 M of NaCl may resulted in its decreased digestibility, which was confirmed in the following digestion analysis.
Table 1.
Particle size of actomyosin in different salt concentrations before and after heating (mean ± standard deviation, n = 6)
| Salt concentration | Dx (10) (μm) | Dx (50) (μm) | Dx (90) (μm) | D4,3 (μm) | D3,2 (μm) |
|---|---|---|---|---|---|
| Before heat treatment | |||||
| 0.4 M | 13.48 ± 1.06a | 42.07 ± 3.85a | 118.08 ± 5.89a | 55.53 ± 3.41a | 29.81 ± 2.34a |
| 0.6 M | 5.98 ± 0.43c | 18.85 ± 3.27c | 98.97 ± 32.87ab | 50.29 ± 19.95ab | 12.40 ± 2.62c |
| 0.8 M | 9.74 ± 0.61b | 26.03 ± 2.25b | 77.18 ± 8.42b | 36.21 ± 3.62b | 20.30 ± 1.52b |
| After heat treatment | |||||
| 0.4 M | 21.23 ± 1.35a | 107.29 ± 24.27a | 206.50 ± 16.24a | 105.74 ± 7.77a | 54.52 ± 3.86a |
| 0.6 M | 14.57 ± 1.86b | 62.22 ± 9.73b | 152.46 ± 25.08b | 74.07 ± 6.48b | 35.90 ± 4.52b |
| 0.8 M | 12.18 ± 1.84c | 44.36 ± 9.82c | 121.71 ± 9.07c | 54.94 ± 6.40c | 28.36 ± 4.90c |
The different letters (a, b, c) in the same column indicated significant difference (P < 0.05)
Surface oxidation of actomyosin: XPS analysis of high resolution C1s revealed four main chemical components contributing to the spectra, namely C–C and C–H (C1), C–O and C–N, (C2), O–C–O, O=C–N and O=C (C3), and O=C–OH, O=C–OR (C4) (Bax et al. 2012). The abundance of C1 peak that corresponded to aliphatic lateral chains of amino acids was the highest in actomyosin dissolved in 0.6 M NaCl solution, whereas the abundance of C3 peak that corresponded to amide functions and esters was the lowest in this sample (Fig. 3a).
In Fig. 3b, N1 refers to uncharged nitrogen including amine (C–NH2) or amide (O=C–NH2 and O=C–NH-C), and N2 refers to positively charged N (C–NH3+) in protonated amine or quaternary ammonium functions in proteins (Sun et al. 2014). The abundance of N1 peak was the lowest, but the abundance of N2 peak was the highest in sample dissolved in 0.6 M. Higher N1 abundance and lower N2 abundance in actomyosin were observed in 0.4 M and 0.6 M NaCl solutions. This phenomenon might be associated with the loss of ε-NH3 groups, since the oxidative deamination of amino acid side chains might block the N-terminal amino acids by forming α-ketoacyl derivatives (Sun et al. 2014). The O1s peak was decomposed into three peaks. And the abundance of O1 peak that corresponded to O=C–OR, O=C–N in amide, ester, and carboxyl groups was the highest in 0.8 M NaCl solution. The abundance of O3 peak that corresponded to O=C–OR in ester functions was the highest in 0.6 M NaCl solution (Fig. 3c). These results indicate that more carbonyl groups were generated in actomyosin treated in 0.8 M NaCl before heat treatment.
In heated samples, the abundances of C2 and C4 peak that corresponded to amide functions and carbonyl groups increased along with the salt concentration increased (Fig. 3d). No significant difference was observed in N1 and N2 abundance (Fig. 3e). In contrast, the abundance of O1 in heated actomyosin sample was higher in 0.6 M and 0.8 M NaCl solutions. Reverse changes were observed in O2 (C–OH, C–O–C, C–O) that corresponded to amino side chains (Fig. 3f). These results suggest that more carbonyl groups could form in actomyosin in 0.6 M and 0.8 M NaCl solutions after heat treatment.
These different in the surface oxidation among samples treated with different NaCl may be in relation to the unfolding and aggregation behavior of actomyosin, which was reported in the heat treatment of actomyosin (Zhao et al. 2019). The higher oxidation extent of samples in 0.6 and 0.8 M NaCl could be related with their more exposed structure, as shown by the higher H0 value in Fig. 1c. In addition, the severer aggregation of sample in 0.4 M NaCl (Table 1) could buried more residues and protect them from being oxidized. Oxidation of a protein have been widely reported to change its digestibility, and elevated oxidation of residues was proved to be related with the decreased digestibility of proteins (Hu et al. 2018; Soladoye et al. 2015). Therefore, these changes in surface oxidation could influence their digestibility to some extent.
Peptide composition of the in vitro digests of actomyosin
Qualitative analysis of peptides: Peptidomics was applied to identify the peptide composition in digests. Differences in the peptide composition, as induced by salt concentration and heat treatment, were shown in Table 2. Twenty-four (from myosin), 8 (from tropomyosin), 4 (from actin) and 5 (from myosin binding protein C) peptides were identified in the digest of sample treated in 0.4 M NaCl before heat treatment. After heating, 53 (from myosin), 12 (from tropomyosin), 9 (from actin) and 14 (from myosin binding protein C) peptides were identified in the same sample. In addition, 137 and 69 peptides were identified from myosin in the heated sample treated in 0.6 M after and before the addition of DTT, respectively. According to these results, heat treatment and addition of DTT were generally shown to increase number of peptides in digest. What’s more, compared with sample treated in 0.4 M and 0.8 M of NaCl, more peptides were found in 0.6 M NaCl-treated sample. Therefore, sample treated in 0.6 M of NaCl could be more digestible than the other samples.
Table 2.
Counts of peptides in the digests of 4 actomyosin fractions (myosin, actin, tropomyosin, myosin binding protein C) after different salting treatments
| Samples | Number of identified peptides in digests | |||
|---|---|---|---|---|
| myosin | tropomyosin | actin | Myosin binding protein C | |
| Before heat treatment | ||||
| 0.4 M (without DTT) | 24 | 8 | 4 | 5 |
| 0.6 M (without DTT) | 59 | 12 | 9 | 9 |
| 0.8 M (without DTT) | 28 | 10 | 6 | 8 |
| 0.4 M (with DTT) | 25 | 8 | 4 | 10 |
| 0.6 M (with DTT) | 53 | 17 | 9 | 15 |
| 0.8 M (with DTT) | 49 | 13 | 8 | 10 |
| After heat treatment | ||||
| 0.4 M (without DTT) | 53 | 12 | 9 | 14 |
| 0.6 M (without DTT) | 69 | 12 | 10 | 18 |
| 0.8 M (without DTT) | 60 | 15 | 9 | 14 |
| 0.4 M (with DTT) | 94 | 10 | 10 | 23 |
| 0.6 M (with DTT) | 137 | 19 | 14 | 33 |
| 0.8 M (with DTT) | 99 | 14 | 13 | 24 |
The peptide composition was further analyzed using partial least squares discriminant analysis (PLS-DA) and the results was shown in Fig. 4. The digests of actomyosin in 0.6 M NaCl solution were well separated from other groups. In addition, the digests of the DTT-treated (Fig. 4b) samples is similar to those of untreated samples (Fig. 4a). These results indicated that digests of actomyosin in 0.6 M NaCl was significantly different from those in other two groups of samples before heat treatment. After heat treatment, the digests of actomyosin in 0.6 M NaCl were still well separated from other samples without addition of DTT (Fig. 4c). However, the digests products of actomyosin in 0.6 M and 0.4 M NaCl were more like each other after DTT treatment (Fig. 4d). These results further confirm the important roles of disulfide bonds in affecting the digestibility of actomyosin, which were also observed in the study of Guo et al. (2007). These results corresponded to those in Table 2, which suggested that salts concentration, formation of disulfide bonds and heat treatment affected the digestibility of actomyosin. Heating and addition of DTT should have damaged the native structure of actomyosin and exposed more cleavage sites for pepsin and trypsin.
Fig. 4.
PLS-DA plots of the pepsin/trypsin digests of actomyosin in different salt concentrations before (a, b) and after (c, d) heat treatment. a, c Refer to digests of actomyosin without DTT treatment; b, d refer to digests of actomyosin DTT treatment
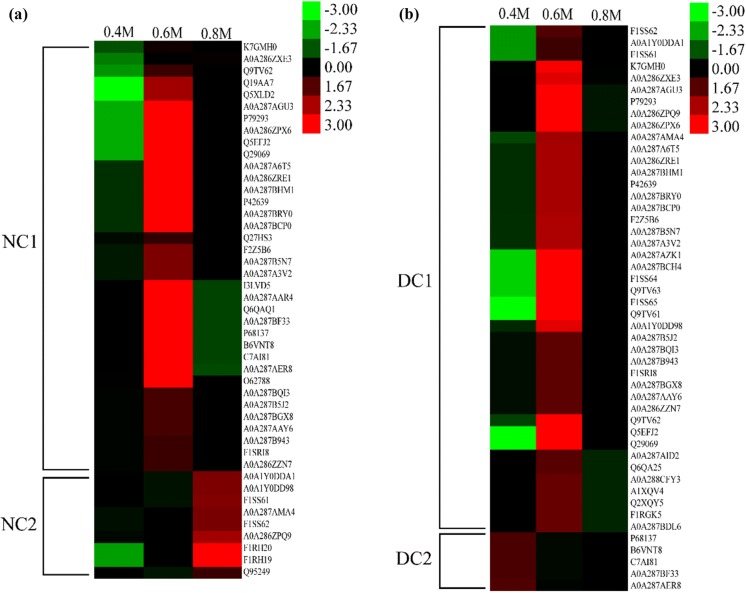
Quantitative analysis of peptide composition in protein digests: To quantify the digested products from actomyosin, the intensities of matched proteins were calculated as the sum of peaks area of corresponding peptides using Proteome Discoverer software. The results were shown by the heatmap in Figs. 5 and 6. Before heat treatment, peptides in digests mainly matched actin, myosin, tropomyosin and myosin binding protein C, which were clustered into two groups in Fig. 5. Cluster NC1 includes myosin, actin, tropomyosin and myosin binding protein C, from which the most abundant peptides were found in sample treated in 0.6 M NaCl solution and the least peptides were found in actomyosin treated in 0.4 M NaCl solution (Fig. 5a). Cluster NC2 mainly includes myosin heavy chain, from which the most abundant peptides were identified in actomyosin in 0.8 M NaCl solution and the least peptides were identified in actomyosin in 0.4 M NaCl solution (Fig. 5a). When treated with DTT, two clusters were obtained in Fig. 6b. In cluster DC1, matched proteins were myosin, tropomyosin and myosin binding protein C that were well digested in 0.6 M NaCl solution but less digested in 0.4 M NaCl solution. In cluster DC2, actin was well digested in 0.4 M NaCl solution but less digested in 0.8 M NaCl solution.
Fig. 5.
Heat maps of the peptides information in the gastrointestinal digests of actomyosin with different salting process before heat treatment: a refer to samples that were not treated with DTT, and b refer to samples that were treated by DTT. Abundance of matched proteins were calculated as the sum of corresponding peptides using Proteome Discoverer software
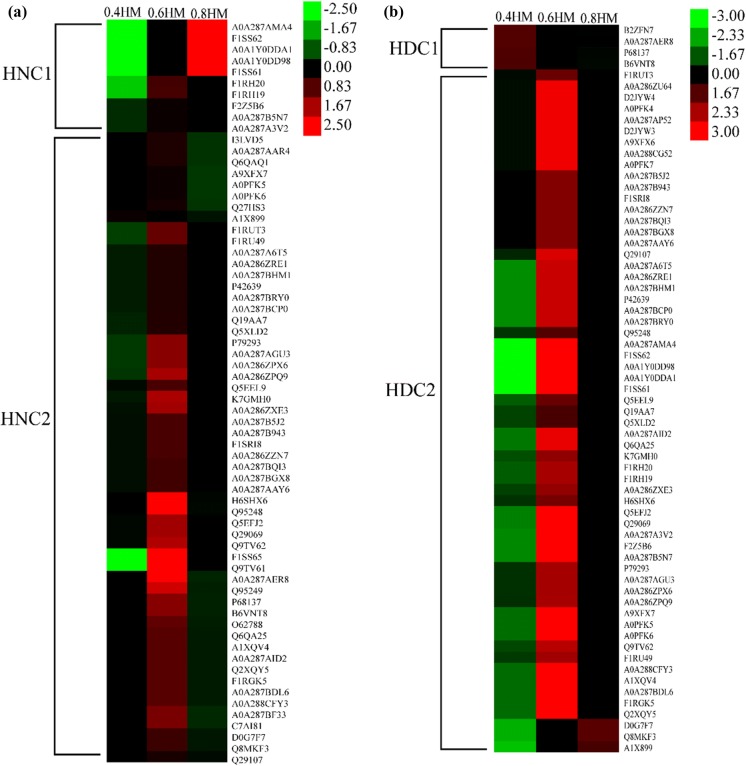
Fig. 6.
Heat maps of the peptides information in the gastrointestinal digests of actomyosin with different salting process after heat treatment: a refer to samples that were not treated by DTT, and b refer to samples that were treated by DTT. Abundance of matched proteins were calculated as the sum of corresponding peptides using Proteome Discoverer software
For the heated samples, the matched proteins were clustered into two groups. In cluster HNC1, the matched proteins were mainly myosin heavy chain, and the most abundant digests was found in sample treated with 0.8 M NaCl solution (Fig. 6a). In cluster HNC2, the matched proteins were mainly myosin, actin, tropomyosin and myosin binding protein C, and the most abundant digests of these proteins were found in sample treated with 0.6 M NaCl solution. After DTT treatment, digests of heated samples were two clusters. In cluster HDC1, the matched protein was actin. In cluster HDC2, the matched proteins were myosin, tropomyosin and myosin binding protein C (Fig. 6b), and the most abundant peptides were found in sample treated with 0.6 M of NaCl.
These qualitative and quantitative analysis results generally indicated that actomyosins in 0.6 M NaCl were more prone to be digested by proteases during in vitro system, which is followed by sample in 0.8 M and 0.4 M NaCl in descending order. The formation of disulfide bond was proved to be a crucial factor that hindered the digestibility of actomyosin, since addition of DTT largely increased the peptide of each sample qualitatively and quantitatively. The relationship between decrease in disulfide bond and elevation in protein digestibility was established in the study of sorghum protein (Hamaker et al. 1987). The higher content of disulfide bonds in sample treated in 0.4 M and 0.8 M NaCl (Fig. 1) could be an important reason that was responsible for the lower digestibility of these two samples. In addition, the severer aggregation of actomyosin in 0.4 M NaCl (Table 1) could have buried more residues inside the aggregates and decreased the accessibility of these residues to pepsin and trypsin during simulated digestion. This could account for the lowest number and abundant of peptides in samples treated in 0.4 M NaCl. What’s more, increased concentration of NaCl have been revealed to decrease the activity of pepsin to some extent, possible due to the denaturation of pepsin (Fox and Walley 1971). This could be a reason for the lower digestibility of sample in 0.8 M of NaCl than the sample in 0.6 M NaCl. Additionally, the higher extent of surface oxidation (Fig. 3) could be another reason for the lower digestibility of actomyosin in 0.8 M after heat treatment. Accordingly, the concentration of NaCl is a crucial factor that can be regulated to change the structure including oxidation, disulfide bonds formation, unfolding and aggregation processes of actomyosin, which largely changed the subsequent digestibility of proteins before and after heat treatment. Similar results were reported in several studies that established the relationship between structural and digestive changes of proteins in Nanjing dry-cured duck during processing. Oxidation, degradation and unfolding of proteins were found to be the key factors in these studies (Du et al. 2018a, b).
Conclusion
Salt concentration had a significant effect on change in structure, oxidation and digests of actomyosin. Actomyosin in 0.6 M was found to be more easily digested than that in 0.4 M and 0.8 M NaCl before and after heat treatment. Correlation between the structural and digestibility changes was found. The higher content of disulfide bonds could account for the reduced digestibility of protein. The severer aggregation behavior of actomyosin may buried partial residues and hinder the action of proteases to some extent. These results indicated the huge influence of salting in the digestibility of meat protein which is closely related with the nutrition of meat.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31530054), the modern agricultural industry technology system (CARS35), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PADP) and Overseas Expertise Introduction Center for Discipline Innovation (“111 Center”) On Quality & Safety Control and Nutrition of Muscle Food.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aliño M, Grau R, Fernández-Sánchez A, Arnold A, Barat JM. Influence of brine concentration on swelling pressure of pork meat throughout salting. Meat Sci. 2010;86:600–606. doi: 10.1016/j.meatsci.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Bax ML, Aubry L, Ferreira C, Daudin JD, Gatellier P, Rémond D, Santé-Lhoutellier V. Cooking temperature is a key determinant of in vitro meat protein digestion rate: investigation of underlying mechanisms. J Agric Food Chem. 2012;60:2569–2576. doi: 10.1021/jf205280y. [DOI] [PubMed] [Google Scholar]
- Berhe DT, Engelsen SB, Hviid MS, Lametsch R. Raman spectroscopic study of effect of the cooking temperature and time on meat proteins. Food Res Int. 2014;66:123–131. doi: 10.1016/j.foodres.2014.09.010. [DOI] [Google Scholar]
- Beveridge T, Toma SJ, Nakai S. Determination of SH-and SS-groups in some food proteins using Ellman’s reagent. J Food Sci. 1974;39:49–51. doi: 10.1111/j.1365-2621.1974.tb00984.x. [DOI] [Google Scholar]
- Brisson G, Britten M, Pouliot Y. Heat-induced aggregation of bovine lactoferrin at neutral pH: effect of iron saturation. Int Dairy J. 2007;17:617–624. doi: 10.1016/j.idairyj.2006.09.002. [DOI] [Google Scholar]
- Cao Y, Xia T, Zhou G, Xu X. The mechanism of high pressure-induced gels of rabbit myosin. Innov Food Sci Emerg. 2012;16:41–46. doi: 10.1016/j.ifset.2012.04.005. [DOI] [Google Scholar]
- Chen X, Tume RK, Xu XL, Zhou GH. Solubilization of myofibrillar proteins in water or low ionic strength media: classical techniques, basic principles and novel functionalities. Crit Rev Food Sci. 2015;57:3260–3280. doi: 10.1080/10408398.2015.1110111. [DOI] [PubMed] [Google Scholar]
- Debiemme-Chouvy C, Haskouri S, Cachet H. Study by XPS of the chlorination of proteins aggregated onto tin dioxide during electrochemical production of hypochlorous acid. Appl Surf Sci. 2007;253:5506–5510. doi: 10.1016/j.apsusc.2006.12.077. [DOI] [Google Scholar]
- Du XJ, Sun YY, Pan DD, Wang Y, Ou CR, Cao JX. The effect of structural change on the digestibility of sarcoplasmic proteins in Nanjing dry-cured duck during processing. Poultry Sci. 2018;97:4450–4457. doi: 10.3382/ps/pey316. [DOI] [PubMed] [Google Scholar]
- Du XJ, Sun YY, Pan DD, Wang Y, Ou CR, Cao JX. Change of the structure and the digestibility of myofibrillar proteins in Nanjing dry-cured duck during processing. J Sci Food Agric. 2018;98:3140–3147. doi: 10.1002/jsfa.8815. [DOI] [PubMed] [Google Scholar]
- Dunn BM. Overview of pepsin-like aspartic peptidases. Curr Protoc Protein Sci. 2001;25:1–21. doi: 10.1002/0471140864.ps2103s25. [DOI] [PubMed] [Google Scholar]
- Fox PF, Walley BF. Influence of sodium chloride on the proteolysis of casein by rennet and by pepsin. J Dairy Res. 1971;38:165–170. doi: 10.1017/S0022029900019282. [DOI] [Google Scholar]
- Gilani GS, Xiao CW, Cockell KA. Impact of antinutritional factors in food proteins on the digestibility of protein and the bioavailability of amino acids and on protein quality. Brit J Nutr. 2012;108:S315–S332. doi: 10.1017/S0007114512002371. [DOI] [PubMed] [Google Scholar]
- Guo X, Yao H, Chen Z. Effect of heat, rutin and disulfide bond reduction on in vitro pepsin digestibility of Chinese tartary buckwheat protein fractions. Food Chem. 2007;102:118–122. doi: 10.1016/j.foodchem.2006.04.039. [DOI] [Google Scholar]
- Hamaker BR, Kirleis AW, Butler LG, Axtell JD, Mertz ET. Improving the in vitro protein digestibility of sorghum with reducing agents. Proc Natl Acad Sci USA. 1987;84:626–628. doi: 10.1073/pnas.84.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Ren S, Shen Q, Ye X, Chen J, Ling J. Protein oxidation and proteolysis during roasting and in vitro digestion of fish (Acipenser gueldenstaedtii) J Sci Food Agric. 2018;98:5344–5351. doi: 10.1002/jsfa.9075. [DOI] [PubMed] [Google Scholar]
- Kumar P, Chatli MK, Verma AK, Mehta N, Malav OP, Kumar D. Sharma N Quality, functionality and shelf life of fermented meat and meat products. A review. Crit Rev Food. 2015;57:2844–2856. doi: 10.1080/10408398.2015.1074533. [DOI] [PubMed] [Google Scholar]
- Li L, Liu Y, Zou X, He J, Xu X, Zhou G, Li C. In vitro protein digestibility of pork products is affected by the method of processing. Food Res Int. 2017;92:88–94. doi: 10.1016/j.foodres.2016.12.024. [DOI] [PubMed] [Google Scholar]
- Li-Chan ECY. The applications of Raman spectroscopy in food science. Trends Food Sci Technol. 1996;11:361–370. doi: 10.1016/S0924-2244(96)10037-6. [DOI] [Google Scholar]
- Liu R, Zhao SM, Xiong SB, Qiu CG, Xie BJ. Rheological properties of fish actomyosin and pork actomyosin solutions. J Food Eng. 2008;85:173–179. doi: 10.1016/j.jfoodeng.2007.06.031. [DOI] [Google Scholar]
- Montel MC, Reitz J, Talon R, Berdague JL, Roussetakrim S. Biochemical activities of micrococcaceae and their effects on the aromatic profiles and odours of a dry sausage model. Food Microbiol. 1996;13:489–499. doi: 10.1006/fmic.1996.0056. [DOI] [Google Scholar]
- Nayak R, Kenney PB, Slider S. Protein extractability of turkey breast and thigh muscle with varying sodium chloride solutions as affected by calcium, magnesium and zinc chloride. J Food Sci. 1996;61:1149–1154. doi: 10.1111/j.1365-2621.1996.tb10950.x. [DOI] [Google Scholar]
- Nguyen MV, Thorarinsdottir KA, Gudmundsdottir A, Thorkelsson G, Arason S. The effects of salt concentration on conformational changes in cod (Gadus morhua) proteins during brine salting. Food Chem. 2011;125:1013–1019. doi: 10.1016/j.foodchem.2010.09.109. [DOI] [Google Scholar]
- Offer G, Trinick J. On the mechanism of water holding in meat: the swelling and shrinking of myofibrils. Meat Sci. 1983;8:245–281. doi: 10.1016/0309-1740(83)90013-X. [DOI] [PubMed] [Google Scholar]
- Promeyrat A, Daudin JD, Gatellier P. Kinetics of protein physicochemical changes induced by heating in meat using mimetic models: (1) Relative effects of heat and oxidants. Food Chem. 2013;138:581–589. doi: 10.1016/j.foodchem.2012.10.084. [DOI] [PubMed] [Google Scholar]
- Sharedeh D, Gatellier P, Astruc T, Daudin JD. Effects of pH and NaCl levels in a beef marinade on physicochemical states of lipids and proteins and on tissue microstructure. Meat Sci. 2015;110:24–31. doi: 10.1016/j.meatsci.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Si JL, Zheng JQ, Li H, Zhang YL. Effect of salt content on the denaturation of pike eel (Muraenesox cinereus Forsskål, 1775) actomyosin. J Appl Ichthyol. 2015;31:767–770. doi: 10.1111/jai.12681. [DOI] [Google Scholar]
- Soladoye OP, Juárez ML, Aalhus JL, Shand P, Estévez M. Protein oxidation in processed meat: mechanisms and potential implications on human health. Compr Rev Food Sci Food Saf. 2015;14:106–122. doi: 10.1111/1541-4337.12127. [DOI] [PubMed] [Google Scholar]
- Sun WZ, Li QY, Zhou FB, Zhao HF, Zhao MM. Surface characterization of oxidized myofibrils using X-ray photoelectron spectroscopy and scanning electron microscopy. J Agric Food Chem. 2014;62:7507–7514. doi: 10.1021/jf501272p. [DOI] [PubMed] [Google Scholar]
- Thorarinsdottir KA, Arason S, Geirsdottir M, Bogason SG, Kristbergsson K. Changes in myofibrillar proteins during processing of salted cod (Gadus morhua) as determined by electrophoresis and differential scanning calorimetry. Food Chem. 2002;77:377–385. doi: 10.1016/S0308-8146(01)00349-1. [DOI] [Google Scholar]
- Thorarinsdottir KA, Arason S, Sigurgisladottir S, Valsdottir T, Tornberg E. Effects of different pre-salting methods on protein aggregation during heavy salting of cod fillets. Food Chem. 2011;124:7–14. doi: 10.1016/j.foodchem.2010.05.095. [DOI] [Google Scholar]
- Zhao D, Li L, Le TT, Larsen LB, Su G, Liang Y, Li B. Digestibility of glyoxal-glycated β-casein and β-lactoglobulin and distribution of peptide-bound advanced glycation end products in gastrointestinal digests. J Agric Food Chem. 2017;65:5778–5788. doi: 10.1021/acs.jafc.7b01951. [DOI] [PubMed] [Google Scholar]
- Zhao D, Li L, Xu D, Sheng B, Qin D, Chen J, Li B, Zhang X. Application of ultrasound pretreatment and glycation in regulating the heat-induced amyloid-like aggregation of β-lactoglobulin. Food Hydrocoll. 2018;80:122–129. doi: 10.1016/j.foodhyd.2018.02.001. [DOI] [Google Scholar]
- Zhao D, He J, Zou X, Xie Y, Xu X, Zhou G, Li C. Influence of hydrothermal treatment on the structural and digestive changes of actomyosin. J Sci Food Agric. 2019;99:6209–6218. doi: 10.1002/jsfa.9893. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.