Abstract
Background
The present experiments evaluated the effects of acute high-intensity resistance exercise on episodic memory.
Methods
Two experiments were conducted. For Experiment 1, participants (N = 40; Mage = 21.0 years) were randomized into one of two groups, including an experimental exercise group and a control group (seated for 20 min). The experimental group engaged in an acute bout of resistance exercises (circuit style exercises) for 15 minutes, followed by a 5-min recovery period. Memory function was subsequently assessed using a multiple trial (immediate and delay), word-list episodic memory task (Rey Auditory Verbal Learning Test, RAVLT), and then followed by a comprehensive, computerized assessment of episodic memory (Treasure Hunt task, THT). The THT involved a spatio-temporal assessment of what, where, and when components of episodic memory. Experiment 2 evaluated if altering the recovery period would influence the potential negative effects of high-intensity resistance exercise on episodic memory function. For Experiment 2, participants (N = 51) were randomized into the same acute resistance exercise protocol but either with a 10-min recovery period, 20-min recovery period, or a control group.
Results
For Experiment 1, for RAVLT, the exercise group performed worse (Fgroup × time = 3.7, p = .001, η2p = .09). Across nearly all THT outcomes, the exercise group had worse spatio-temporal memory than the control group. These results suggest that high-intensity resistance exercise (with a 5-min recovery) may have a detrimental effect on episodic memory function. For Experiment 2, for RAVLT, the exercise with 10-min recovery group performed better (Fgroup × time = 3.1, p = .04, η2p = .11). Unlike Experiment 1, exercise did not impair spatio-temporal memory, with the 20-min exercise recovery group having the best “where” component of episodic memory.
Conclusion
Collectively, the results from these two experiments suggest that acute high-intensity resistance exercise may impair episodic memory when a short exercise recovery period (e.g., 5-min) is employed, but with a longer recovery period (10+ min), acute high-intensity resistance exercise may, potentially, enhance episodic memory.
Keywords: Consolidation, Encoding, Memory function, Physical activity
INTRODUCTION
Declarative memory includes the recall of factual-based information (semantic memory) or episodes/events (episodic memory) that occur in a spatial and/or temporal context. Recent work from this [1-6] and other labs [7-10] demonstrate that acute aerobic exercise can enhance short- and long-term episodic memory function, as well as semantic memory [11,12]. These exercise-induced improvements in memory function have important health implications, as memory function is an independent predictor of premature mortality [13].
We have previously discussed the potential mechanisms through which acute aerobic exercise may influence episodic memory function [14-18]. These postulated acute aerobic exercise-related mechanisms include, for example, 1) enhanced neuronal excitability; 2) enhanced attentional resource allocation to facilitate memory encoding; 3) upregulation of AMPA receptor levels, opening NMDA channels, and increasing EPSP (excitatory post-synaptic potentials) in the hippocampus; 4) the priming of neurons to be encoded in the memory trace by increasing CREB transcription; 5) BDNF (brain-derived neurotropic factor) expression; and 6) enhanced dendritic spine growth. Such effects may arise from physiological changes that enhance memory consolidation, as well as psychological effects, such as exercise-induced enhancement in attention, which may facilitate enhanced memory encoding [19,20]. As detailed elsewhere [21], attentional processes may involve involuntary or bottom-up attention, as well as voluntary or top- down attention. Acute aerobic exercise has also been shown to induce neuronal excitation in the mesencephalic reticular formation [22,23], thalamus [24], and limbic structures [25], which are key brain structures involved in bottom-up attentional processes. Similarly, acute aerobic exercise may help to facilitate top-down attention via increases in neuronal activity in both the frontal and parietal structures [26,27]. Notably, however, emerging research demonstrates that both aerobic and resistance exercise may enhance episodic memory function, but they may activate unique intracellular pathways to exert such memory-enhancing effects [28-32].
Emerging work is starting to demonstrate that resistance exercise may confer unique health benefits when compared to aerobic exercise [33,34]. Although recent work suggests that aerobic exercise may confer mnemonic benefits, much less investigated is whether resistance exercise confers similar benefits to episodic memory as does aerobic exercise [35-42]. As we detailed in a recent systematic review [43], of the 7 chronic training studies, 4 [36,37,39,41] did not demonstrate beneficial effects of resistance training on episodic memory function, whereas 3 studies [38,40,42] provided some evidence that chronic resistance training was beneficial in improving episodic memory function. A recent review [44] demonstrates that acute resistance exercise may enhance cognition, particularly inhibitory control.
To our knowledge, only one study has examined the effects of an acute resistance training bout (isokinetic dynamometer knee extension exercises) on memory function [35]. This study demonstrated mostly null effects, potentially because the knee extension exercises were employed only after memory encoding. Recent work from this [4,5] and other labs [8-10] suggest that exercise occurring prior to encoding (vs. other temporal periods) may be more beneficial in enhancing episodic memory.
Given the dearth of research in this area, the purpose of this study was to examine the effects of an acute bout of resistance exercise on episodic memory function. There is plausibility for resistance exercise to confer unique effects on memory, when compared to other modalities of exercise (e.g., aerobic exercise). For example, Ozkaya et al. [45] evaluated neuroelectrical correlates of memory function among those engaging in an aerobic vs. resistance exercise program. Both interventions improved various event-related potential parameters, but the strength training group produced a shorter latency for P2/N2 and a larger amplitude for N1P2, P2N2, and N2P3 (event-related potentials), which may help to facilitate information processing. Further, emerging work in animal models suggest that the mechanisms through which resistance exercise improves memory function may differ from mechanisms modulated by aerobic exercise, with different intracellular pathways being activated [28-32].
We recently demonstrated that the post-exercise recovery period may play an important role in influencing the relationship between acute moderate-intensity aerobic exercise on cognitive function [3]. This is also supported by meta- analytic research [7]. Herein, we evaluate the experimental effects of acute high-intensity resistance exercise on episodic memory function. Specifically, we conducted two experimental studies. Experiment 1 evaluated the effects of acute high-intensity resistance exercise, with a 5-minute recovery period, on episodic memory function. We initially hypothesized that acute resistance exercise would enhance episodic memory function. As will be discussed, these findings provided evidence that acute high-intensity resistance exercise with a relatively short recovery period had a potentially detrimental effect on episodic memory function. This served as the motivation for Experiment 2, in which two additional high-intensity resistance exercise groups were included, involving longer recovery periods (10-min and 20-min). For this second experiment, we hypothesized that extending the post-exercise recovery period would attenuate any potential negative effects on memory, and possibly, enhance memory function. These two experiments were conducted sequentially (Experiment 1 in the Spring of 2018, with Experiment 2 in the Summer of 2018). These findings may have important implications for exercise prescription purposes. For example, these implications include the timing of exercise and the duration of recovery period to try and optimize memory function, and regarding the latter, may provide individuals with evidence that other modalities of exercise, besides aerobic exercise, may improve their health, which may influence their initiation and maintenance of resistance exercise behavior. We study these research questions among a convenience sample of young adults. This study serves as a proof-of-concept study to evaluate whether acute resistance exercise may influence memory function. As discussed previously [46], this is a population where memory may start to decline, and as such, is a useful population to evaluate this question. Of course, for generalizability purposes, future studies on this topic, among other populations, will be needed.
MATERIALS AND METHODS – Experiment 1
1. Study design
A two-arm, parallel-group randomized controlled intervention was employed. Participants were randomized into one of two groups, including an experimental group and a control group. The experimental group engaged in an acute bout of resistance exercises for 15 minutes, while the control group engaged in a seated task that involved playing an on-line game (Sudoku). All memory assessments occurred after the exercise or control periods. This study was approved by the authors’ institutional review board and participants provided written consent prior to study participation.
2. Participants
Each group included 20 participants (N = 40). This is based from a power analysis indicating adequate statistical power for sample sizes ranging from 8-24 (d, 0.84-1.36; two-tailed α error probability, 0.05; 1-β error probability, 0.80; allocation ratio, 1). This also aligns with our other related experiment work on this topic demonstrating adequate statistical power [2-5]. Recruitment occurred via a convenience-based, non-probability sampling approach (classroom announcement and word-of-mouth). Participants included undergraduate and graduate students between the ages of 18 and 35 yrs. Students were sampled from a variety of disciplines, such as exercise science, psychology, and biology. Additionally, participants were excluded if they:
Self-reported as a daily smoker [47,48]; self-reported being pregnant [49]; exercised within 5 hours of testing [8]; consumed caffeine within 3 hours of testing [50]; had a concussion or head trauma within the past 30 days [51]; took marijuana or other illegal drugs within the past 30 days [52] or were considered a daily alcohol user (> 30 drinks/ month for women; > 60 drinks/month for men) [53].
3. Resistance exercise protocol
All resistance exercises were weight-free, i.e., only using the human body and no external loads. This approach was employed to maximize generalizability. Participants performed 5 supervised circuits, with each circuit lasting 3 minutes. Thus, the bout of exercise was 15-minutes in duration. Each circuit, in this order, involved:
• Human squats for 30 seconds
• Push-ups for 30 seconds
• Sit-ups for 30 seconds
• Plank exercise for 30 seconds
• Rest (laying) for 60 seconds*
*For the last circuit, instead of a rest period for 60-seconds, participants completed push-ups (or sit-ups, if they could not maintain the push-ups for the full 60-secs) to failure for one-minute. This was performed to ensure that all participants had a similar level of physical exertion by the end of the bout of exercise. After the 15-minute bout of exercise, participants sat (and played Sudoku) quietly for 5-minutes before commencing the memory task.
4. Control protocol
Similar to other related experiments [54], those randomized to the control group completed a medium-level, on-line administered, Sudoku puzzle. Participants engaged in this task to prevent boredom. Participants completed this time-matched puzzle for 20-minutes prior to completing the memory task (described below). The website for this puzzle is located here: https://www.websudoku.com/. We have experimental evidence that playing Sudoku does not prime or enhance episodic memory function; thus, we have limited concern as to whether this type of control activity would induce any cognitive benefits.
5. Memory assessments
Participants completed two memory tasks in a fixed order. The first memory task was a multiple trial, word-list episodic memory task (Rey Auditory Verbal Learning Test; RAVLT) [55]. This task, described in more detail below, involves an immediate memory assessment, as well as a delayed free-recall test, occurring approximately 10-minutes after memory encoding. Between the immediate and delayed free recall RAVLT assessments, participants completed a comprehensive, computerized ‘what-where-when’ assessment of episodic memory (Treasure Hunt Task; THT).
1) RAVLT
Identical to our other experimental work [4,5], short-term (immediate) and longer-term memory (10-min delay, i.e., 10-minutes after the completion of the final trial of the RAVLT) were assessed using the standardized Rey Auditory Verbal Learning Test (RAVLT) [56], which takes approximately 10-15 minutes to complete. Participants were asked to listen to and immediately recall a recording of a list of 15 words (List A) five times in a row (Trials 1-5). Each word list was played at a rate of approximately 1 word per second. Participants were then asked to listen to and immediately recall a list of 15 new words (List B). Immediately following the recall of List B (Trial 6), participants were required to recall the words from List A (Trial 7). The outcome variables included in the number of words recalled for Trials 1-7, as well as the 20-min delay free recall. The RAVLT has been shown to provide evidence of reliability and validity [57].
2) THT
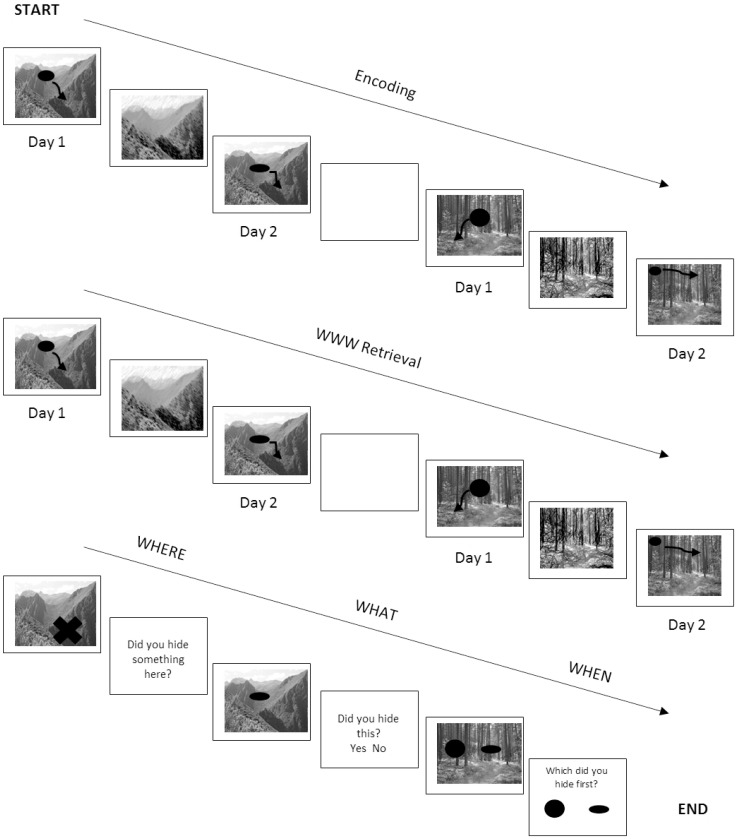
The THT (Treasure Hunt Task) is a computerized task assessing ‘what-where-when’ episodic memory, taking approximately 10-minutes to complete. Details of this THT have been discussed elsewhere [58,59]. In brief, this task involved ‘hiding’ items in various scenes, then later indicating what items were hidden, where and on what occasion. This requires the integration of item, location and temporal memory into a single coherent representation (What- Where-When memory, WWW). Participants are also assessed for their memory for the individual components (what, where and when) without requirement for integration. Fig. 1 displays a schematic of the WWW task. Reliability for these tasks has been previously demonstrated (ICCs > 0.7) [59]. The outcome variables assessed included an absolute WWW score (in which the location of the correct object for the correct time is identified exactly), and the proportion of correct responses for the separate what, where and when sub-tasks. This study used the ‘medium’ difficulty version of the task, assessing 16 unique item-location-time combinations.
Fig. 1.
Schematic of an example version of the Treasure Hunt Task (THT). Participants “hid” items in two different scenes, across days that were labeled as “day 1” and “day 2”. After this encoding task, participants then were prompted to indicate where they hid each of the items on each day (WWW score). Following this, participants completed recognition and discrimination tasks for the calculation of the “where”, “what”, and “when” parameters.
6. Additional assessments
Various behavioral and psychological assessments were completed (at the beginning of the visit) to ensure that the two groups were similar on these parameters. To assess mood status, participants completed the Positive and Negative Affect Schedule (PANAS) [60]. For this mood survey, participants rated 20 items (e.g., excited, upset, irritable, attentive) on a Likert scale (1, very slightly or not at all; to 5, extremely), with half of the items constituting a “positive” mood state, with the other half being a “negative” mood state. As a measure of habitual physical activity behavior, participants also completed a survey (Physical Activity Vital Signs Questionnaire) and reported time spent per week in moderate-to-vigorous physical activity (MVPA) [61]. Further, participants self-reported whether they currently participate in resistance exercise at least 2 days per week (yes/no). Height/weight (BMI) were measured to provide anthropometric characteristics of the sample. Lastly, before, during and after the exercise and control conditions, heart rate (chest-strapped Polar monitor, F1 model) and rating of perceived exertion (RPE, range 6-20) were assessed. For the 6-20 RPE scale, 6 represented no exertion at all, 9 was light exertion, 13 somewhat hard, 15 hard, and 20 being maximal exertion.
7. Statistical analysis
All statistical analyses were computed in SPSS (v. 24). Independent sample t-tests were computed to examine group differences in the THT sub-tasks as well as for manipulation checks (e.g., heart rate differences). A repeated measures ANOVA (RM-ANOVA) was employed to evaluate group differences in the RAVLT outcomes. For the RM-ANOVA for the RAVLT outcomes, group assignment served as the between-subject variable, time-point served as the within-subject variable, and group × time interactions were evaluated for interaction effects. In addition to RM- ANOVA analyses, we computed RM-ANCOVA analyses, controlling for post-exercise heart rate and RPE. Statistical significance was set at an alpha of 0.05. Partial eta-squared (η2p) effect size estimates were calculated.
RESULTS – Experiment 1
Table 1 displays the demographic and behavioral characteristics of the experimental and control groups for Experiment 1. Groups were similar regarding the demographic (e.g., age) and behavioral (e.g., habitual physical activity, engagement in habitual resistance exercise) characteristics (all p’s > .05).
Table 1.
Characteristics of the study variables
| Variable | Experiment 1 | Experiment 2 | |||||
|---|---|---|---|---|---|---|---|
| Exercise w/5 min recovery | Control | p-value | Exercise w/10 min recovery | Exercise w/20 min recovery | Control | p-value | |
| n | 20 | 20 | 17 | 16 | 18 | ||
| Age, mean years | 20.8 (1.2) | 21.2 (1.4) | 0.35 | 22.3 (2.1) | 21.0 (1.8) | 21.9 (2.2) | 0.15 |
| % Female | 55.0 | 40.0 | 0.34 | 47.1 | 73.7 | 84.2 | |
| % White | 90.0 | 80.0 | 0.55 | 41.2 | 57.9 | 36.8 | |
| Body mass index, mean kg/m2 | 25.5 (4.1) | 25.9 (5.4) | 0.76 | 24.0 (3.3) | 25.3 (3.6) | 26.8 (6.5) | 0.20 |
| % Taking medication to regulate mood | 5.0 | 5.0 | 1.00 | 5.9 | 5.3 | 5.3 | |
| Affect, mean (PANAS) | |||||||
| Positive | 27.9 (6.2) | 31.1 (6.5) | 0.12 | 31.1 (6.9) | 29.7 (7.0) | 30.6 (7.6) | 0.84 |
| Negative | 2.2 (3.1) | 12.6 (4.0) | 0.70 | 11.7 (2.5) | 11.5 (1.7) | 11.4 (2.3) | 0.94 |
| MVPA, mean | 257.5 (166.9) | 273.5 (126.2) | 0.73 | 190.0 (113.6) | 205.3 (160.0) | 196.6 (147.0) | 0.95 |
| Resistance Exercise, % | |||||||
| Currently engaging in resistance exercise | 85.0 | 75.0 | 0.43 | 64.7 | 52.6 | 68.4 | |
| Heart Rate, mean | |||||||
| Resting | 75.0 (10.8) | 70.2 (10.6) | 0.16 | 77.0 (11.0) | 74.1 (13.9) | 72.1 (12.7) | 0.52 |
| Midpoint | 144.5 (19.6) | 76.7 (9.5) | < 0.001 | 129.3 (21.4) | 127.5 (21.1) | 73.7 (11.5) | < 0.001 |
| Endpoint | 155.5 (18.9) | 73.7 (10.4) | < 0.001 | 135.7 (24.6) | 137.8 (21.1) | 68.6 (11.8) | < 0.001 |
| Post | 102.7 (12.7) | 71.7 (8.4) | < 0.001 | 91.8 (14.5) | 86.2 (14.5) | - | 0.26 |
| Rating of Perceived Exertion (RPE), mean | |||||||
| Resting | 6.0 (0.2) | 6.0 (0.0) | 0.32 | 6.4 (1.2) | 6.1 (0.3) | 6.2 (1.1) | 0.64 |
| Midpoint | 12.8 (1.6) | 6.0 (0.0) | < 0.001 | 13.1 (1.9) | 12.4 (1.6) | 6.5 (1.6) | < 0.001 |
| Endpoint | 15.9 (1.5) | 6.0 (0.0) | < 0.001 | 15.5 (2.2) | 14.2 (2.3) | 6.3 (1.1) | < 0.001 |
| Post | 7.9 (1.9) | 6.0 (0.0) | < 0.001 | 7.6 (1.7) | 6.2 (0.4) | 6.0 (0.0) | 0.008 |
| Resistance training circuit | |||||||
| # of circuits completed (max = 5) | 5.0 (0.0) | - | N/A | 4.9 (.26) | 5.0 (0.0) | - | 0.15 |
| Duration engaged in circuit (max = 15 min) | 15.0 (0.0) | - | N/A | 13.3 (4.7) | 15.0 (0.0) | - | 0.13 |
Values in parentheses are SD estimates.
MVPA: Moderate to vigorous physical activity.
Table 1 also displays the physiological (heart rate) and psychological (RPE) responses to the exercise bout and seated control condition. There were no significant respective differences in resting (baseline) heart rate (75.0 vs. 70.2 bpm; t(38) = 1.41, p = .16) or resting RPE (6.0 vs. 6.0; t(38) = 1.00, p = .32) between the groups. However, both heart rate (155.5 vs. 73.7 bpm; t(38) = 16.8, p < .001) and RPE (15.9 vs. 6.0; t(38) = 28.5, p < .001) were significantly higher at the end of the exercise bout when compared to the end of the control condition. Similarly, even 5-minutes post-exercise (i.e., immediately prior to the start of the memory tasks), heart rates was significantly higher in the exercise group compared to the control group (102.7 vs. 71.7 bpm, t(38) = 9.06, p < .001).
Table 2 displays the memory (RAVLT and THT) scores between the exercise and control groups. For the RAVLT, the exercise group had slightly higher free recall scores for Trials 1-3. However, these did not reach statistical significance (Trial 1: t(38) = 1.85, p = .07; Trial 2: t(38) = .76, p = .44; Trial 3: t(38) = .09, p = .93). After the third trial, there was an orthogonal shift occurring for Trials 4-7, in that the exercise group performed worse for these respective trials. Indeed, a group × trial interaction effect was statistically significant (Fgroup × time = 3.7, p = .001, η2p = .09). Results were also similar for the THT task. Across nearly all THT outcomes, the exercise group had numerically worse spatio-temporal memory than the control group. Specifically, spatial memory (“where”) was significantly worse in the exercise vs. control group (81.5 vs. 88.2; t(38) = 2.05, p = .04), but results did not reach statistical significance for the item (“what”) (99.0 vs. 97.1; t(38) = .96, p = .33) or temporal (“when”) memory (88.4 vs. 92.5; t(38) = 1.40, p = .15) parameters.
Table 2.
Memory scores across the two experiments
| Variable | Experiment 1 | Test-statistic | |||
| Exercise w/5 min recovery | Control | ||||
| RAVLT, mean recall of words | |||||
| Trial 1 | 7.55 (2.0) | 6.60 (1.0) | Ftime = 97.91, p < .001, η2p = .72 | ||
| Trial 2 | 9.60 (1.9) | 9.15 (1.7) | Fgroup × time = 2.86, p = .007, η2p = .07 | ||
| Trial 3 | 11.75 (1.9) | 11.70 (1.6) | |||
| Trial 4 | 12.25 (1.9) | 12.85 (1.8) | |||
| Trial 5 | 12.75 (2.5) | 13.50 (1.0) | |||
| Trial 6 | 5.70 (1.5) | 6.20 (1.7) | |||
| Trial 7 | 11.00 (3.8) | 12.45 (1.5) | |||
| 20-min delay | 10.70 (3.6) | 12.40 (1.9) | |||
| WWW, mean proportion correct | |||||
| WWW | 47.90 (18.0) | 56.7 (18.0) | t(38) = 1.54, p = .13 | ||
| What | 99.00 (2.4) | 97.10 (8.4) | t(38) = .96, p = .33 | ||
| Where | 81.85 (11.9) | 88.20 (6.9) | t(38) = 2.05, p = .04 | ||
| When | 88.40 (10.1) | 92.85 (9.0) | t(38) = 1.40, p = .15 | ||
| Experiment 2 | Test-statistic | ||||
| Exercise w/10 min recovery | Exercise w/20 min recovery | Control | |||
| RAVLT, mean recall of words | |||||
| Trial 1 | 7.47 (1.9) | 6.94 (1.8) | 7.06 (1.6) | Ftime = 180.03, p < .001, η2p = .79 | |
| Trial 2 | 9.76 (2.3) | 9.63 (2.7) | 9.94 (2.2) | Fgroup × time = 3.08, p = .04, η2p = .11 | |
| Trial 3 | 12.00 (2.1) | 10.50 (2.6) | 11.17 (1.9) | ||
| Trial 4 | 12.65 (2.4) | 11.31 (2.6) | 11.61 (2.6) | ||
| Trial 5 | 13.35 (2.0) | 12.00 (2.2) | 11.94 (2.2) | ||
| Trial 6 | 5.35 (1.1) | 5.00 (1.8) | 5.39 (1.6) | ||
| Trial 7 | 12.00 (2.5) | 10.94 (2.2) | 10.78 (2.7) | ||
| 20-min delay | 11.71 (3.1) | 10.31 (3.1) | 10.20 (2.5) | ||
| WWW, mean proportion correct | |||||
| WWW | 39.06 (24.3) | 37.17 (27.5) | 35.52 (18.8) | F(2) = .10, p = .90 | |
| What | 99.62 (1.5) | 99.17 (2.0) | 97.03 (7.3) | F(2) = 1.70, p = .19 | |
| Where | 80.50 (12.8) | 74.66 (17.6) | 80.57 (21.0) | F(2) = .69, p = .50 | |
| When | 77.20 (17.3) | 88.15 (11.0) | 76.97 (16.6) | F(2) = 3.29, p = .04 | |
DISCUSSION – Experiment 1
The motivation for Experiment 1 was threefold: Emerging work suggests that acute aerobic exercise is favorably associated with episodic memory function, meanwhile, research demonstrates that resistance exercise may confer unique health benefits, however very little research has examined the effects of acute resistance exercise on episodic memory function. The main finding of Experiment 1 was that, a relatively high-intensity acute bout of resistance exercise, compared to a seated control condition, was associated with worse episodic memory function.
Recent meta-analytic research [9] focused on aerobic exercise suggests that exercise intensity may moderate the relationship between acute aerobic exercise and memory function. Specifically, low- to moderate-intensity aerobic exercise (vs. higher intensity aerobic exercise) appears to be optimal for enhancing memory function [62,63]. This intensity-specific effect also aligns with our previous experimental work on aerobic exercise and cognition [2]. Although the collective empirical research suggests that moderate-intensity aerobic exercise may be optimal in enhancing episodic memory function [9], others [64,65] have suggested that, on physiological grounds, high-intensity exercise should be more beneficial in enhancing episodic memory. In theory, high-intensity exercise is hypothesized to be superior in enhancing episodic memory, as higher intensity exercise is more effective in augmenting neurotrophic factors (e.g., BDNF) and catecholamines (e.g., dopamine, epinephrine, and norepinephrine), which are suggested to play an integral role in the exercise-memory link [17]. Indeed, recent work from this [4] and other labs [66,67] demonstrate that acute high-intensity aerobic exercise has been shown to enhance memory function. Of course, high-intensity exercise would also augment cortisol concentrations, which may impair memory function [68]. However, and as suggested elsewhere, acute exercise-induced increases from exercise, as opposed to a psychosocial stressor, may attenuate HPA axis activity via increases in dopamine in the medial prefrontal cortex, as well as favorably influence the cortisol recovery curve [69]. Taken together, the studies examining the effects of high-intensity exercise and memory function are equivocal. From a mechanistic perspective, theoretically, it would seem that high-intensity exercise would be optimal for enhancing episodic memory. However, the dose-response effects of BDNF, for example, may follow an inverted U-shaped relationship, with intermediate concentrations yielding optimal benefits (e.g., sprouting of serotoninergic neurons) [70,71]. An example of this can be seen in evidence that high levels of BDNF may induce TrkB desensitization, impairing memory function [72]. High-intensity may also increase levels of fatigue, and thus, induce an attention deficit effect, ultimately impairing memory encoding or inhibiting resources to facilitate memory retrieval [73].
Couched within the above, it is possible that the mixed findings regarding high-intensity exercise on episodic memory function is a result two primary factors. First, it is possible that the post-exercise recovery period may play an important role in subsequent memory performance [10]. Shorter post-exercise recovery periods, for example, may impair memory via depletion of cognitive resources needed to effectively encode the stimuli. In Experiment 2, we specifically evaluated whether changing the duration of the post-exercise recovery period would have a differential effect on episodic memory. We have provided suggestive evidence of this (implications of recovery period) for acute, moderate-intensity aerobic exercise [3]. It seems conceivable that post-exercise recovery would play an even more important role for higher-intensity exercise. Our recent work [4] suggests that high-intensity aerobic exercise is not associated with immediate, short-term episodic memory function, but is associated with improved episodic memory function 20-minutes (aligning with the temporal period of the present study’s [Experiment 2] THT assessment) and 24-hours post-memory encoding.
In addition to the post-exercise recovery period (Experiment 2), it would be worth investigating whether the familiarity of the modality of exercise plays a moderating role. Although speculative, even at the same relative degree of high-intensity exercise, those with a history of engaging in that high-intensity modality of exercise may have a differential memorial effect from the bout of exercise. Although measured in our study (Table 1), most of the participants reported currently engaging in resistance exercise; thus, this prevented our ability to evaluate this as a potential moderator. Of course, our results somewhat contradict this speculation, as we observed a worse episodic memory performance among the exercise group, which included a high proportion of individuals currently engaging in habitual resistance exercise. A group × time interaction effect continued to remain even when controlling for post-exercise (i.e., immediately prior to the memory task) heart rate (Fgroup × time = 2.00, p = .06, η2p = .05) and RPE (Fgroup × time = 4.1, p = .001, η2p = .10).
Experiment 1 provides some suggestive evidence that an acute bout of high-intensity resistance exercise may have a detrimental effect on episodic memory function. Based on this, we conducted a follow-up experiment to evaluate whether altering the post-exercise recovery period would influence the potential negative effects of high-intensity resistance exercise on episodic memory function. It is conceivable that a short duration recovery period after a high-intensity bout of exercise will induce cognitive fatigue, and thus, impair subsequent memory encoding. Memory encoding may be influenced by the degree of cognitive attention toward the memory stimuli [74].
MATERIALS AND METHODS – Experiment 2
A three-arm, parallel-group randomized controlled intervention was employed. Participants were randomized into one of three groups, including two experimental groups and a control group. The experimental groups included an acute bout of resistance exercises for 15 minutes, with either a 10-min (n = 17) or 20-min (n = 16) seated recovery period, while the control group (n = 18) engaged in a seated task that involved playing an on-line game (Sudoku). This study was approved by the authors’ institutional review board and participants provided written consent prior to study participation.
All other aspects of Experiment 2 were identical to Experiment 1.
RESULTS – Experiment 2
Table 1 displays the study variable characteristics for the 3 groups for Experiment 2. Groups were similar regarding the demographic (e.g., age) and behavioral (e.g., habitual physical activity, engagement in habitual resistance exercise) characteristics (all p’s > .05). Table 1 also displays the physiological (heart rate) and psychological (RPE) responses to the 3 conditions. There were no significant respective differences in resting (baseline) heart rate (F(2) = .66, p = .52) or resting RPE (F(2) = .44, p = .64) between the groups. However, both heart rate (F(2) = 70.9, p < .001) and RPE (F(2) = 108.3, p < .001) were significantly higher at the end of the exercise conditions when compared to the end of the control condition.
Table 2 displays the memory (RAVLT and THT) scores for Experiment 2. Similar to Experiment 1, results for Experiment 2 showed a statistically significant main effect for time (F = 180.03, p < .001, η2p = .79) and a group by time interaction (F = 3.08, p = .04, η2p = .11). For the RAVLT, the exercise group with a 10-min recovery period had higher free recall scores across nearly all trials. Results were in partial agreement for the THT task (Table 2). For the THT outcomes, there were no group differences for absolute WWW (F = .10, p = .90), “what” (F = 1.70, p = .19), or “where” (F = .69, p = .50), but the exercise group with a 20-min recovery period had the highest “when” episodic memory (F = 3.29, p = .04).
DISCUSSION – Experiment 2
The results from Experiment 1 provide evidence that acute high-intensity resistance exercise with a short (5-min) recovery period was associated with worse episodic memory performance. The results from Experiment 2 extend these findings by providing suggestive evidence that extending this post-exercise recovery period may have enhancing effects on memory. Specifically, for Experiment 2, we observed that acute high-intensity exercise with a 10-min recovery period resulted in higher memory scores on the word-list task, whereas the exercise group with a 20-min recovery period had the highest “when” performance on the computerized episodic memory task. These collective findings are in alignment with assertions mentioned elsewhere, noting that “...the exercise stimulus can mask gains in memory performance easily due to disproportionate exercise-induced fatigue and/or arousal, especially when exercise is performed at higher intensity” [10]. Further, our findings also are in alignment with meta-analytic research among predominately aerobic exercise studies, which showed that the greatest beneficial effects on cognition occurred approximately 11-20 minutes post-acute exercise [7].
Memory encoding, in part, is influenced by the perceived nature of the event. The degree of mental fatigue may influence attentional resources needed to effectively encode the memory stimuli [75]. This suggests that fatigue may induce depletion of cognitive resources needed to effectively encode (and/or retrieve) memories. Relatedly, research demonstrates that cognitive fatigue may reduce error-related negativity (ERN), and in turn, impair cognitive control [76]. Additionally, cognitive fatigue may reduce hippocampal activation, leading to reduced memory encoding [77]. Further, and as noted previously, high-intensity exercise may sub-optimally increase neurotrophic and catecholamine levels, which may have an unfavorable effect of memory [70-73]. This explanation, however, is an unlikely candidate to explain our findings, as it is unlikely that levels of these parameters would be drastically different across the recovery periods employed herein.
From Experiment 2, we observed that exercise with a 10-min recovery period appeared to favor the word-list memory task, whereas exercise with a 20-min recovery period was more advantageous for improving the “when” component of episodic memory function. This potential exercise recovery-dependent difference is difficult to explain, and thus, should not be overemphasized. Although we observed exercise-induced differences for select components of episodic memory, we notably did not observe any differences for this bound what-where-when-memory. This, however, does not discount the possibility that exercise may have a differential effect on the individual components (what, where, when) that comprise episodic memory. In fact, recent meta-analytic research demonstrates that exercise appears to have a greater enhancement effect for spatial-based episodic memories [9]. Critical thought and experimentation will be needed to determine if exercise does indeed have a unique role in the constituents of episodic memory, and if so, what underlying mechanisms are responsible for this potential effect.
GENERAL DISCUSSION
As stated in the introduction, the majority of work examining the effects of acute exercise on episodic memory function have focused on aerobic exercise. Among the studies examining the effects of resistance exercise on memory function, findings are equivocal, with nearly all of these studies employing a chronic resistance training program [43]. The one study employing an acute resistance exercise protocol used a knee extension task, which did not show convincing evidence of memory benefits [35]. Thus, the effects of acute resistance exercise on memory function is an under-investigated area of research. The present study builds upon this gap in the literature by evaluating the effects of acute resistance exercise on episodic memory function. We employed a circuit-style resistance exercise program (no weights) to maximize the potential for greater application into society. Our main findings are as follows. High-intensity acute resistance exercise may have an unfavorable effect of memory if the recovery period is of an insufficient duration (e.g., 5-minutes) (Experiment 1). However, we also provide some suggestive evidence that if this recovery period is lengthened (e.g., 10-20 minutes), then acute high-intensity exercise may have a beneficial effect on episodic memory (Experiment 2).
In addition to future research evaluating the effects of exercise recovery on episodic memory (including its constituents), we are in need of future modality-specific work employing a side-to-side comparison of aerobic and resistance exercise (matched for intensity and duration) on episodic memory function. Other work suggests that aerobic walking may enhance memory function over aerobic cycling exercise [9]. However, it is uncertain as to whether there is an exercise modality effect for aerobic exercise vs. resistance exercise on memory function in humans. Such an effect is plausible, as aerobic exercise may have a differential effect on BDNF when compared to resistance exercise [78]. Similarly, animal work suggests that different intracellular pathways are activated based on the modality of exercise [28-32].
Such work should also consider whether the time course of memory assessment plays a moderating role. It is conceivable that high-intensity resistance exercise may have a detrimental effect on short-term memory but may help to facilitate the consolidation of the memory trace (via increases in plasticity-related proteins), and in turn, positively influence long-term memory function. Whether this potential enhanced consolidation effect occurs when the exercise bout is placed before memory encoding or during memory consolidation, should also be investigated. That is, whether preferentially priming encoding, consolidation, or both has a unique effect on episodic memory.
Limitations of this study include the relatively small homogenous sample, limiting the study’s generalizability and statistical power. Further, we employed a between-subject post-test comparison, as opposed to a between-subject pretest posttest comparison. Our employed design was specifically chosen because, for example, for the RAVLT assessment, which involves encoding a list of words, if this protocol was employed pretest and posttest, then this could have induced a proactive memory interference effect. That is, the pretest word list may interfere with the encoding and retrieval of the different posttest word list. When feasible (based on the cognitive parameter evaluated), future carefully designed work should consider employing a between-subject pretest posttest comparison or a within-subject crossover pretest posttest comparison. Our second experiment would have also benefited by employing a 5-minute recovery period group. Another potential limitation is that our experiments appeared to have elicited a perceived intensity at the lower end of high-intensity exercise. For logistical reasons, we employed a progressive bout of resistance exercise, with the last portion inducing a larger exertion level. Future work is needed to carefully design circuit-style resistance exercise bouts to elicit sustained high- intensity exercise. Strengths of our study include the experimental design, study novelty, and integration of two experimental studies.
In conclusion, we provide some suggestive evidence that high-intensity resistance exercise may have a detrimental effect on memory performance if it is coupled with a short-duration recovery period. Our results did not provide convincing evidence that lengthening the recovery period would reverse this effect. However, our data suggest that the recovery period may, potentially, play a role in the exercise-memory interaction. Future confirmatory work on this topic is needed. Such work should evaluate high-intensity exercise for multiple modalities, should vary the intensity within the higher-intensity range, and should also vary the recovery period beyond our evaluated recovery periods. If confirmed by future work, then these findings may have important implications for exercise prescription purposes, such as the timing of exercise and the duration of recovery period to optimize memory function. Further, if future work demonstrates enhancement effects of acute resistance exercise on memory, then this will provide individuals with evidence that other modalities of exercise, besides aerobic exercise, may improve their memory function, which may influence their initiation and maintenance of resistance exercise behavior. Future research should consider conducting a side-to-side comparison between aerobic and resistance exercise on human memory function.
Footnotes
CONFLICTS OF INTERESTS
No financial support was used to prepare this manuscript.
REFERENCES
- 1.Loprinzi PD, Frith E, Edwards MK, Sng E, Ashpole N. The effects of exercise on memory function among young to middle-aged adults: systematic review and recommendations for future research. Am J Health Promot. 2018;32:691–704. doi: 10.1177/0890117117737409. [DOI] [PubMed] [Google Scholar]
- 2.Loprinzi PD, Kane CJ. Exercise and cognitive function: a randomized controlled trial examining acute exercise and free-living physical activity and sedentary effects. Mayo Clin Proc. 2015;90:450–60. doi: 10.1016/j.mayocp.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 3.Crush EA, Loprinzi PD. Dose-response effects of exercise duration and recovery on cognitive functioning. Percept Mot Skills. 2017;124:1164–93. doi: 10.1177/0031512517726920. [DOI] [PubMed] [Google Scholar]
- 4.Frith E, Sng E, Loprinzi PD. Randomized controlled trial evaluating the temporal effects of high-intensity exercise on learning, short-term and long-term memory, and prospective memory. Eur J Neurosci. 2017;46:2557–64. doi: 10.1111/ejn.13719. [DOI] [PubMed] [Google Scholar]
- 5.Sng E, Frith E, Loprinzi PD. Temporal effects of acute walking exercise on learning and memory function. Am J Health Promot. 2018;32:1518–25. doi: 10.1177/0890117117749476. [DOI] [PubMed] [Google Scholar]
- 6.Haynes JTt, Frith E, Sng E, Loprinzi PD. Experimental effects of acute exercise on episodic memory function: considerations for the timing of exercise. Psychol Rep. 2019;122:1744–54. doi: 10.1177/0033294118786688. [DOI] [PubMed] [Google Scholar]
- 7.Chang YK, Labban JD, Gapin JI, Etnier JL. The effects of acute exercise on cognitive performance: a meta- analysis. Brain Res. 2012;1453:87–101. doi: 10.1016/j.brainres.2012.02.068. [DOI] [PubMed] [Google Scholar]
- 8.Labban JD, Etnier JL. Effects of acute exercise on long-term memory. Res Q Exerc Sport. 2011;82:712–21. doi: 10.1080/02701367.2011.10599808. [DOI] [PubMed] [Google Scholar]
- 9.Roig M, Nordbrandt S, Geertsen SS, Nielsen JB. The effects of cardiovascular exercise on human memory: a review with meta-analysis. Neurosci Biobehav Rev. 2013;37:1645–66. doi: 10.1016/j.neubiorev.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Roig M, Thomas R, Mang CS, Snow NJ, Ostadan F, Boyd LA, Lundbye-Jensen J. Time-dependent effects of cardiovascular exercise on memory. Exerc Sport Sci Rev. 2016;44:81–8. doi: 10.1249/JES.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 11.Smith JC, Nielson KA, Antuono P, Lyons JA, Hanson RJ, Butts AM, Hantke NC, Verber MD. Semantic memory functional MRI and cognitive function after exercise intervention in mild cognitive impairment. J Alzheimers Dis. 2013;37:197–215. doi: 10.3233/JAD-130467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loprinzi PD, Edwards MK. Exercise and cognitive-related semantic memory function. J Cognit Behav Psychotherap Res. 2018;7:51–2. doi: 10.5455/JCBPR.284864. [DOI] [Google Scholar]
- 13.Frith E, Addoh O, Mann JR, Windham BG, Loprinzi PD. Individual and combined associations of cognitive and mobility limitations on mortality risk in older adults. Mayo Clin Proc. 2017;92:1494–501. doi: 10.1016/j.mayocp.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 14.Loprinzi PD, Edwards MK, Frith E. Potential avenues for exercise to activate episodic memory-related pathways: a narrative review. Eur J Neurosci. 2017;46:2067–77. doi: 10.1111/ejn.13644. [DOI] [PubMed] [Google Scholar]
- 15.Loprinzi PD, Ponce P, Frith E. Hypothesized mechanisms through which acute exercise influences episodic memory. Physiol Int. 2018;105:285–97. doi: 10.1556/2060.105.2018.4.28. [DOI] [PubMed] [Google Scholar]
- 16.Loprinzi PD. IGF-1 in exercise-induced enhancement of episodic memory. Acta Physiol (Oxf) 2019;226:e13154. doi: 10.1111/apha.13154. [DOI] [PubMed] [Google Scholar]
- 17.Loprinzi PD, Frith E. A brief primer on the mediational role of BDNF in the exercise-memory link. Clin Physiol Funct Imaging. 2019;39:9–14. doi: 10.1111/cpf.12522. [DOI] [PubMed] [Google Scholar]
- 18.Frith E, Loprinzi PD. Physical activity and individual cognitive funcion parameters: unique exercise-induced mechansims. J Cognit Behav Psychother Res. 2018;7:92–106. doi: 10.5455/JCBPR.284071. [DOI] [Google Scholar]
- 19.Alves CR, Tessaro VH, Teixeira LA, Murakava K, Roschel H, Gualano B, Takito MY. Influence of acute high-intensity aerobic interval exercise bout on selective attention and short-term memory tasks. Percept Mot Skills. 2014;118:63–72. doi: 10.2466/22.06.PMS.118k10w4. [DOI] [PubMed] [Google Scholar]
- 20.McMorris T, Davranche K, Jones G, Hall B, Corbett J, Minter C. Acute incremental exercise, performance of a central executive task, and sympathoadrenal system and hypothalamic-pituitary-adrenal axis activity. Int J Psychophysiol. 2009;73:334–40. doi: 10.1016/j.ijpsycho.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Fingelkurts AA, Fingelkurts AA. Attentional state: From automatic detection to willful focused concentration. In: Marchetti G, Benedetti G, Alharbi A, editors. Attention and Meaning. The Attentional Basis of Meaning. Nova Science Publishers; 2015. pp. 133–50. [Google Scholar]
- 22.Iwamoto GA, Kaufman MP. Caudal ventrolateral medullary cells responsive to muscular contraction. J Appl Physiol (1985) 1987;62:149–57. doi: 10.1152/jappl.1987.62.1.149. [DOI] [PubMed] [Google Scholar]
- 23.Dietrich A, Audiffren M. The reticular-activating hypofrontality (RAH) model of acute exercise. Neurosci Biobehav Rev. 2011;35:1305–25. doi: 10.1016/j.neubiorev.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Rajab AS, Crane DE, Middleton LE, Robertson AD, Hampson M, MacIntosh BJ. A single session of exercise increases connectivity in sensorimotor-related brain networks: a resting-state fMRI study in young healthy adults. Front Hum Neurosci. 2014;8:625. doi: 10.3389/fnhum.2014.00625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z, Myers KG, Guo Y, Ocampo MA, Pang RD, Jakowec MW, Holschneider DP. Functional reorganization of motor and limbic circuits after exercise training in a rat model of bilateral parkinsonism. PLoS One. 2013;8:e80058. doi: 10.1371/journal.pone.0080058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Enders H, Cortese F, Maurer C, Baltich J, Protzner AB, Nigg BM. Changes in cortical activity measured with EEG during a high-intensity cycling exercise. J Neurophysiol. 2016;115:379–88. doi: 10.1152/jn.00497.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsujii T, Komatsu K, Sakatani K. Acute effects of physical exercise on prefrontal cortex activity in older adults: a functional near-infrared spectroscopy study. Adv Exp Med Biol. 2013;765:293–8. doi: 10.1007/978-1-4614-4989-8_41. [DOI] [PubMed] [Google Scholar]
- 28.Cassilhas RC, Lee KS, Fernandes J, Oliveira MGM, Tufik S, Meeusen R, de Mello MT. Spatial memory is improved by aerobic and resistance exercise through divergent molecular mechanisms. Neuroscience. 2012;202:309–17. doi: 10.1016/j.neuroscience.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 29.Cassilhas RC, Tufik S, de Mello MT. Physical exercise, neuroplasticity, spatial learning and memory. Cell Mol Life Sci. 2016;73:975–83. doi: 10.1007/s00018-015-2102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee MC, Okamoto M, Liu YF, Inoue K, Matsui T, Nogami H, Soya H. Voluntary resistance running with short distance enhances spatial memory related to hippocampal BDNF signaling. J Appl Physiol (1985) 2012;113:1260–6. doi: 10.1152/japplphysiol.00869.2012. [DOI] [PubMed] [Google Scholar]
- 31.Vilela TC, Muller AP, Damiani AP, Macan TP, da Silva S, Canteiro PB, de Sena Casagrande A, Pedroso GDS, Nesi RT, de Andrade VM, de Pinho RA. Strength and aerobic exercises improve spatial memory in aging rats through stimulating distinct neuroplasticity mechanisms. Mol Neurobiol. 2017;54:7928–37. doi: 10.1007/s12035-016-0272-x. [DOI] [PubMed] [Google Scholar]
- 32.Tang L, Kang YT, Yin B, Sun LJ, Fan XS. [Effects of weight-bearing ladder and aerobic treadmill exercise on learning and memory ability of diabetic rats and its mechanism] Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2017;33:436–40. doi: 10.12047/j.cjap.5570.2017.105. [DOI] [PubMed] [Google Scholar]
- 33.Stamatakis E, Lee IM, Bennie J, Freeston J, Hamer M, O'Donovan G, Ding D, Bauman A, Mavros Y. Does strength promoting exercise confer unique health benefits? A pooled analysis of eleven population cohorts with all-cause, cancer, and cardiovascular mortality endpoints. Am J Epidemiol. 2018;187:1102–12. doi: 10.1093/aje/kwx345. [DOI] [PubMed] [Google Scholar]
- 34.Chang YK, Pan CY, Chen FT, Tsai CL, Huang CC. Effect of resistance-exercise training on cognitive function in healthy older adults: a review. J Aging Phys Act. 2012;20:497–517. doi: 10.1123/japa.20.4.497. [DOI] [PubMed] [Google Scholar]
- 35.Weinberg L, Hasni A, Shinohara M, Duarte A. A single bout of resistance exercise can enhance episodic memory performance. Acta Psychol (Amst) 2014;153:13–9. doi: 10.1016/j.actpsy.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yerokhin V, Anderson-Hanley C, Hogan MJ, Dunnam M, Huber D, Osborne S, Shulan M. Neuropsychological and neurophysiological effects of strengthening exercise for early dementia: a pilot study. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2012;19:380–401. doi: 10.1080/13825585.2011.628378. [DOI] [PubMed] [Google Scholar]
- 37.Alves CRR, Merege Filho CAA, Benatti FB, Brucki S, Pereira RMR, de Sá Pinto AL, Lima FR, Roschel H, Gualano B. Creatine supplementation associated or not with strength training upon emotional and cognitive measures in older women: a randomized double-blind study. PLoS One. 2013;8:e76301. doi: 10.1371/journal.pone.0076301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perrig-Chiello P, Perrig WJ, Ehrsam R, Staehelin HB, Krings F. The effects of resistance training on well-being and memory in elderly volunteers. Age Ageing. 1998;27:469–75. doi: 10.1093/ageing/27.4.469. [DOI] [PubMed] [Google Scholar]
- 39.Iuliano E, Fiorilli G, Aquino G, Di Costanzo A, Calcagno G, di Cagno A. Twelve-Week Exercise Influences Memory Complaint but not Memory Performance in Older Adults: A Randomized Controlled Study. J Aging Phys Act. 2017;25:612–20. doi: 10.1123/japa.2016-0249. [DOI] [PubMed] [Google Scholar]
- 40.Best JR, Chiu BK, Liang Hsu C, Nagamatsu LS, Liu-Ambrose T. Long-term effects of resistance exercise training on cognition and brain volume in older women: results from a randomized controlled trial. J Int Neuropsychol Soc. 2015;21:745–56. doi: 10.1017/S1355617715000673. [DOI] [PubMed] [Google Scholar]
- 41.van de Rest O, van der Zwaluw NL, Tieland M, Adam JJ, Hiddink GJ, van Loon LJC, de Groot LCPGM. Effect of resistance-type exercise training with or without protein supplementation on cognitive functioning in frail and pre-frail elderly: Secondary analysis of a randomized, double-blind, placebo-controlled trial. Mech Ageing Dev. 2014;136:85–93. doi: 10.1016/j.mad.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 42.Fiatarone Singh MA, Gates N, Saigal N, Wilson GC, Meiklejohn J, Brodaty H, Wen W, Singh N, Baune BT, Suo C, Baker MK, Foroughi N, Wang Y, Sachdev PS, Valenzuela M. The Study of Mental and Resistance Training (SMART) study-resistance training and/or cognitive training in mild cognitive impairment: a randomized, double-blind, double-sham controlled trial. J Am Med Dir Assoc. 2014;15:873–80. doi: 10.1016/j.jamda.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 43.Loprinzi PD, Frith E, Edwards MK. Resistance exercise and episodic memory function: a systematic review. Clin Physiol Funct Imaging. 2018 doi: 10.1111/cpf.12507. doi: 10.1111/cpf.12507. [DOI] [PubMed] [Google Scholar]
- 44.Soga K, Masaki H, Gerber M, Ludyga S. Acute and long-term effects of resistance training on executive function. J Cogn Enhanc. 2018;2:200–7. doi: 10.1007/s41465-018-0079-y. [DOI] [Google Scholar]
- 45.Ozkaya GY, Aydin H, Toraman FN, Kizilay F, Ozdemir O, Cetinkaya V. Effect of strength and endurance training on cognition in older people. J Sports Sci Med. 2005;4:300–13. [PMC free article] [PubMed] [Google Scholar]
- 46.Salthouse TA. When does age-related cognitive decline begin? Neurobiol Aging. 2009;30:507–14. doi: 10.1016/j.neurobiolaging.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jubelt LE, Barr RS, Goff DC, Logvinenko T, Weiss AP, Evins AE. Effects of transdermal nicotine on episodic memory in non-smokers with and without schizophrenia. Psychopharmacology (Berl) 2008;199:89–98. doi: 10.1007/s00213-008-1133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klaming R, Annese J, Veltman DJ, Comijs HC. Episodic memory function is affected by lifestyle factors: a 14-year follow-up study in an elderly population. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2017;24:528–42. doi: 10.1080/13825585.2016.1226746. [DOI] [PubMed] [Google Scholar]
- 49.Henry JD, Rendell PG. A review of the impact of pregnancy on memory function. J Clin Exp Neuropsychol. 2007;29:793–803. doi: 10.1080/13803390701612209. [DOI] [PubMed] [Google Scholar]
- 50.Sherman SM, Buckley TP, Baena E, Ryan L. Caffeine enhances memory performance in young adults during their non-optimal time of day. Front Psychol. 2016;7:1764. doi: 10.3389/fpsyg.2016.01764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wammes JD, Good TJ, Fernandes MA. Autobiographical and episodic memory deficits in mild traumatic brain injury. Brain Cogn. 2017;111:112–26. doi: 10.1016/j.bandc.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 52.Hindocha C, Freeman TP, Xia JX, Shaban NDC, Curran HV. Acute memory and psychotomimetic effects of cannabis and tobacco both 'joint' and individually: a placebo-controlled trial. Psychol Med. 2017;47:2708–19. doi: 10.1017/S0033291717001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Le Berre AP, Fama R, Sullivan EV. Executive functions, memory, and social cognitive deficits and recovery in chronic alcoholism: a critical review to inform future research. Alcohol Clin Exp Res. 2017;41:1432–43. doi: 10.1111/acer.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McNerney MW, Radvansky GA. Mind racing: The influence of exercise on long-term memory consolidation. Memory. 2015;23:1140–51. doi: 10.1080/09658211.2014.962545. [DOI] [PubMed] [Google Scholar]
- 55.Sng E, Frith E, Loprinzi PD. Experimental effects of acute exercise on episodic memory acquisition: Decomposition of multi-trial gains and losses. Physiol Behav. 2018;186:82–4. doi: 10.1016/j.physbeh.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 56.Rey A. The psychological examination in cases of traumatic encepholopathy. Arch Psychol (Geneve) 1941;28:215–85. [Google Scholar]
- 57.Magalhaes S, Malloy-Diniz LF, Hamdan A. Validity convergent and reliability test-retest of the rey auditory verbal learning test. Clin Neuropsychiatry. 2012;9:129–37. [Google Scholar]
- 58.Cheke LG. What-where-when memory and encoding strategies in healthy aging. Learn Mem. 2016;23:121–6. doi: 10.1101/lm.040840.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheke LG, Simons JS, Clayton NS. Higher body mass index is associated with episodic memory deficits in young adults. Q J Exp Psychol (Hove) 2016;69:2305–16. doi: 10.1080/17470218.2015.1099163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–70. doi: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 61.Ball TJ, Joy EA, Gren LH, Shaw JM. Concurrent validity of a self-reported physical activity "Vital Sign" questionnaire with adult primary care patients. Prev Chronic Dis. 2016;13:E16. doi: 10.5888/pcd13.150228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diederich K, Bastl A, Wersching H, Teuber A, Strecker JK, Schmidt A, Minnerup J, Schabitz WR. Effects of different exercise strategies and intensities on memory performance and neurogenesis. Front Behav Neurosci. 2017;11:47. doi: 10.3389/fnbeh.2017.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bosch BM, Bringard A, Logrieco MG, Lauer E, Imobersteg N, Thomas A, Ferretti G, Schwartz S, Igloi K. Acute physical exercise improves memory consolidation in humans via BDNF and endocannabinoid signaling. bioRxiv. 2018 doi: 10.1101/211227:211227.. doi: 10.1101/211227:211227.. [DOI] [Google Scholar]
- 64.McMorris T. Developing the catecholamines hypothesis for the acute exercise-cognition interaction in humans: Lessons from animal studies. Physiol Behav. 2016;165:291–9. doi: 10.1016/j.physbeh.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 65.McMorris T, Turner A, Hale BJ, Sproule J. Beyond the catecholamines hypothesis for an acute exercise-cognition interaction: A neurochemical perspective. In: McMorris T, editor. Exercise-cognition interaction: Neuroscience perspectives. Elsevier Academic Press; San Diego: 2016. pp. 65–103. [DOI] [Google Scholar]
- 66.Griffin EW, Mullally S, Foley C, Warmington SA, O'Mara SM, Kelly AM. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol Behav. 2011;104:934–41. doi: 10.1016/j.physbeh.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 67.Winter B, Breitenstein C, Mooren FC, Voelker K, Fobker M, Lechtermann A, Krueger K, Fromme A, Korsukewitz C, Floel A, Knecht S. High impact running improves learning. Neurobiol Learn Mem. 2007;87:597–609. doi: 10.1016/j.nlm.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 68.Salehi B, Cordero MI, Sandi C. Learning under stress: the inverted-U-shape function revisited. Learn Mem. 2010;17:522–30. doi: 10.1101/lm.1914110. [DOI] [PubMed] [Google Scholar]
- 69.Chen C, Nakagawa S, An Y, Ito K, Kitaichi Y, Kusumi I. The exercise-glucocorticoid paradox: How exercise is beneficial to cognition, mood, and the brain while increasing glucocorticoid levels. Front Neuroendocrinol. 2017;44:83–102. doi: 10.1016/j.yfrne.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 70.Mamounas LA, Altar CA, Blue ME, Kaplan DR, Tessarollo L, Lyons WE. BDNF promotes the regenerative sprouting, but not survival, of injured serotonergic axons in the adult rat brain. J Neurosci. 2000;20:771–82. doi: 10.1523/JNEUROSCI.20-02-00771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moreira FA, Aguiar DC, Guimaraes FS. Anxiolytic-like effect of cannabinoids injected into the rat dorsolateral periaqueductal gray. Neuropharmacology. 2007;52:958–65. doi: 10.1016/j.neuropharm.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 72.Cunha C, Brambilla R, Thomas KL. A simple role for BDNF in learning and memory? Front Mol Neurosci. 2010;3:1. doi: 10.3389/neuro.02.001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hartley JT. Aging and prose memory: tests of the resource-deficit hypothesis. Psychol Aging. 1993;8:538–51. doi: 10.1037/0882-7974.8.4.538. [DOI] [PubMed] [Google Scholar]
- 74.Muzzio IA, Kentros C, Kandel E. What is remembered? Role of attention on the encoding and retrieval of hippocampal representations. J Physiol. 2009;587:2837–54. doi: 10.1113/jphysiol.2009.172445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klaassen EB, Plukaard S, Evers EA, de Groot RH, Backes WH, Veltman DJ, Jolles J. Young and middle-aged schoolteachers differ in the neural correlates of memory encoding and cognitive fatigue: A functional MRI study. Front Hum Neurosci. 2016;10:148. doi: 10.3389/fnhum.2016.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lorist MM, Boksem MA, Ridderinkhof KR. Impaired cognitive control and reduced cingulate activity during mental fatigue. Brain Res Cogn Brain Res. 2005;24:199–205. doi: 10.1016/j.cogbrainres.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 77.Klaassen EB, de Groot RH, Evers EA, Nicolson NA, Veltman DJ, Jolles J. Cortisol and induced cognitive fatigue: effects on memory activation in healthy males. Biol Psychol. 2013;94:167–74. doi: 10.1016/j.biopsycho.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 78.Hamedinia M, Sharifi M, Hosseini-Kakhak A. The effect of eight weeks of aerobic, anaerobic and resistance training on some factor of endocannabinoid system, serotonin, beta-endorphin and BDNF in young men. Biosci Biotechnol Res Asia. 2017;14:1201–10. doi: 10.13005/bbra/2562. [DOI] [Google Scholar]