Abstract
Our study showed that total urinary arsenic concentrations were positively correlated with renal cell carcinoma (RCC). Chronic inflammation is a key player in the development of RCC. This study explored the association between nucleotide-binding domain-like receptor protein 3 (NLRP3) genotypes and the development of RCC. We also investigated whether any of the NLRP3 genotypes modified the risk between arsenic and RCC. We recruited 350 RCC patients and 700 age-sex matched controls. RCC was confirmed by pathological assessment following surgical resection or image-guided biopsy of a renal tumor. Fifteen sites of NLRP3 gene polymorphisms were identified using the Agena Bioscience MassARRAY platform. The concentrations of the urinary arsenic species were determined by HPLC-HG-AAS. There was a significant dose-dependent association between arsenic and RCC. In addition, six of thirteen NLRP3 alleles, including rs12239046 C, rs10925025 G, rs1539019 C, rs10925026 A, rs10157379 T, and rs12143966 A, had increased odds ratios (ORs) for RCC than other NLRP3 alleles. Among these sites, we found the novel haplotype of five tag-SNPs (C-A-A-A-A) was significantly related to RCC, the OR and 95% confidence interval was 1.44 (1.08–1.92). Furthermore, participants with high total urinary arsenic levels and the NLRP3 rs1539019 C allele had significantly multiplicative and additive interactions for the risk of RCC (p interaction = 0.012). This study is the first to identify the modified effects of NLRP3 risk alleles involved in the association between arsenic and RCC risk in a population with low arsenic exposure.
Subject terms: Environmental impact, Cancer epidemiology
Introduction
Renal cell carcinoma (RCC) represents the most deadly urological malignancy and accounts for 2 to 3% of all adult malignancies. RCC is most commonly diagnosed between the ages of 50 and 75 years old with a ratio of males to females of 1.5:11. The incidence of RCC in most countries has been increasing over the past decade2. In Taiwan, the incidence trend and average annual percentage increase for kidney cancer from 2002 to 2012 was 5.1 and 2.9% for men and women, respectively3. Although cigarette smoking, obesity, and hypertension have been identified as risk factors for RCC4, the etiology of RCC is still unclear.
Chronic inflammation is a key player in the occurrence and development of RCC5. It can be caused by environmental exposure, obesity, tumorigenic pathogens, immune deregulation, and autoimmunity6. Chronic inflammation promotes tumorigenesis by enhancing genomic instability, inducing oncogenic mutations, and altering the immune response6. In addition, arsenic is a Group I carcinogen has been shown to cause lung, skin, liver, kidney and bladder cancer7. Our study showed that subjects with a high total arsenic concentration in their urine had a high odds ratio (OR) for RCC8, even if they are exposed to low arsenic levels. Several studies suggested that arsenic-induced nephrotoxicity may be through activation of inflammasomes and induction of cyclooxygenase-2 (Cox-2) as well as COX-2-derived prostanoids upregulation9–11. Therefore, whether arsenic induces RCC through an inflammatory response is a topic worthy of discussion.
Inflammasomes are newly discovered immune complexes that help a host defend against physiological aberrations and infectious agents12. Absent in melanoma 2 (AIM2)-like receptors (ALRs), leucine-rich repeat-containing receptor (NLR), or the nucleotide-binding domain initiate the inflammasome complex. Nucleotide-binding domain-like receptor protein 3 (NLRP3), caspase-1, and adaptor ASC (apoptosis-associated spot-like protein) constitute the NLRP3 inflammasome, which can be activated through in vivo cell damage and death or invasion of foreign pathogens13. One recent study reported that arsenic could activate the NLRP3 inflammasome and induce pyroptosis14. However, mercury and arsenic can inhibit interleukin (IL)-1β and IL-18 secretion, which is caused by the activation of both the classical and non-classical NLRP3 inflammasomes in macrophages, suggesting that exposure to these heavy metals could destroy the inflammasome-mediated immune responses and cause unexpected side effects15. Because of the inconsistency of these findings, the association between NLRP3 inflammasomes and arsenic exposure remains unclear. In addition, a study has found that NLRP3 is overexpressed in patients with bladder cancer16. However, the association between NLRP3 and RCC needs investigation.
NLRP3 gene variants may influence NLRP3 mRNA stability and expression17. Recent studies have identified an association between NLRP3 gene polymorphisms and both increased blood pressure18 and coronary artery disease19. However, no studies have examined their association with the development of RCC. Therefore, the aims of the study were to investigate the relationship between NLRP3 genotypes and the risk of RCC and explore whether NLRP3 gene polymorphisms could modify the risk between arsenic and RCC.
Materials and Methods
Study subjects
This study was a case-control study. To prevent age and gender from confounding the risk of RCC, we matched the age and gender of the control group with those of the case group. Pathologically-confirmed RCC patients (350) and 700 age- and gender-matched controls (i.e., without RCC or any other malignancy) were recruited from our past study20. Among the RCC patients, about 70% had grade II or III tumors, including 262 clear-cell, 25 papillary, 21 chromophobe, one collecting duct, one sarcomatoid, and five “other” cases. No information was available for 35 of the cases. The Research Ethics Committee of National Taiwan University Hospital approved the study, which complied with the World Medical Association Declaration of Helsinki. All study subjects provided their informed consent before specimen and data collection.
All study subjects were Taipei residents and drank tap water with arsenic levels within the World Health Organization standards21. Although Taipei does not have an arsenic-related factory, the urinary arsenic species present in the study subjects may be due to exposure from seafood22, cereals23, edible oil24, and agricultural rice25.
Biological specimen collection and questionnaire interview
The questionnaire interview, the content of the questionnaires, and the methods used for collecting blood and urine samples were previously described8. Peripheral blood samples (5 to 8 mL) were collected using EDTA-vacuum syringes. The buffy coat was separated for DNA extraction and gene polymorphism determination. Spot urine can reflect arsenic excretion concentrations over 24 h26. Total urinary arsenic levels were adjusted by the urinary creatinine concentration for variation in the hydration states27.
Measurement of arsenic species in urine
The methods for measuring arsenite (AsIII), dimethylarsenic acid (DMAV), monomethylarsonic acid (MMAV), and arsenate (AsV) in urine were previously described28. Urine sample pretreatment, the method of measurement and validity, and the reliability of the arsenic species in the urine are described in Supplemental Table S1. The intake of fish, shellfish, or any other seafood did not affect the method of determining the arsenic species29.
Genetic polymorphisms determination
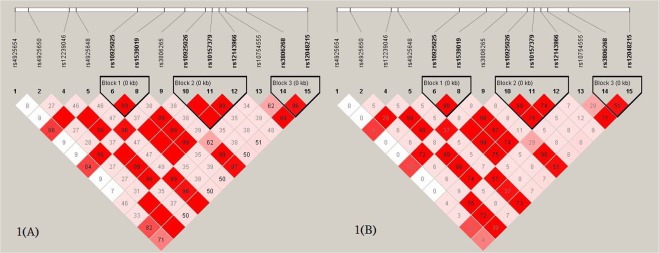
DNA extraction was performed using proteinase K digestion and phenol and chloroform extraction. Seventeen common single nucleotide polymorphisms (SNPs) in the NLRP3 gene region were initially selected from the Han Chinese in Beijing HapMap data with a minor-allele frequency of ≥ 0.2. However, two SNPs failed during the genotyping assay design. Genotyping of 15 SNPs was performed using the Agena Bioscience MassARRAY iPLEX system, according to manufacturer’s instructions (National Genome Medicine Center, Taipei, Taiwan). Two SNPs did not fit the Hardy-Weinberg equilibrium and were removed. Therefore, 13 NLRP3 SNPs were included in the analyses. The NLRP3 gene exhibited three haplotype blocks as shown in Fig. 1.
Figure 1.
(A) Lewontin’s D’ of the NLRP3 block 1 (NLRP3 rs10925025 and NLRP3 rs1539019), NLRP3 block 2 (NLRP3 rs10925026, NLRP3 rs10157379, and NLRP3 rs12143966), and NLRP3 block 3 (NLRP3 rs3806268 and NLRP3 rs12048215) polymorphisms. (B) r2 values for each pair of polymorphisms of NLRP3 block 1, NLRP3 block 2, and NLRP3 block 3.
Statistical analysis
The values for the AsIII and AsV (InAs), MMAV, and DMAV species in the urine were added to determine the total urinary arsenic concentration. The differences in the continuous variables between two groups were compared using the Student’s t-test. Multiple logistic regression models were used to calculate the OR and 95% confidence interval (CI). The continuous variable of the total urinary arsenic levels in the controls was categorized, and the resulting tertile was defined as the cutoff point. The linear trends for the ORs across the strata of independent variables were tested by categorizing the independent variables and treating the score variables as continuous. Haploview 4.1 software was used to calculate D’ and r2 of the Lewontin to determine the strength of the linkage disequilibrium (LD) intensity30. This cutoff value represents the median of the total urinary arsenic concentration of the controls (15.6 μg/g creatinine) and was used for the interaction analysis. We tested the multiplicative interaction of the total urinary arsenic concentration and each NLRP3 genotype using a product term in the logistic regression model. Additive interactions were evaluated using the Synergy (S) Index31. The analysis of all data used SAS 9.4 software (Cary, NC, USA). A two-sided 0.05 < p < 0.1 and p < 0.05 was considered marginally significant and statistically significant, respectively. The statistical power of this study was calculated using Power and Sample Size Calculation online software (http://sampsize.sourceforge.net/iface/s3.html). Based on the number of samples in this study, the odds ratio was about 2, the exposed controls were 5%, the alpha risk was 5%, and the controls/case ratio was 2, resulting in a power of about 80%.
Results
Sociodemographic characteristics of RCC cases and controls
The mean age of 350 RCC patients and 700 healthy controls were 59.29 ± 0.70 and 60.12 ± 0.49 years, respectively in this study. Subjects with higher educational level or who were alcohol drinkers had a lower OR for RCC than those with a lower level of education or who were non-drinkers. Cigarette smoking ≥ 21 pack-years significantly increased the OR 1.60-fold for RCC compared to non-smokers (Table 1). We also analyzed the consumption effect of cumulative cigarette smoking with all 13 NLRP3 SNPs with genotypic form. However, we did not find any difference in the consumption of cumulative cigarette smoking for different genotypes of any NLRP3 SNPs (Supplemental Table S2). Hypertension and diabetes were significantly associated with RCC with ORs (95% CI) of 2.76 (2.07–3.67) and 2.65 (1.80–3.90), respectively (data not shown). In this study, total urinary arsenic levels of RCC cases and controls were 23.72 ± 1.19 and 18.91 ± 0.50 μg/g creatinine, but in the arseniasis endemic area of Taiwan, those of urothelial carcinoma cases and controls were 69.6 ± 11.4 and 63.7 ± 14.2 μg/L respectively32.
Table 1.
Sociodemographic characteristics, lifestyle, disease histories, urinary creatinine, and urinary total arsenic levels of RCC cases and non-RCC controls.
| RCC Cases (n = 350) N (%) | Controls (n = 700) N (%) | |
|---|---|---|
| Age (years) (Mean ± SD) | 59.29 ± 0.70 | 60.12 ± 0.49 |
| Gender | ||
| Male | 231 (66.00) | 462 (66.00) |
| Female | 119 (34.00) | 238 (34.00) |
| Education | ||
| Illiterate/Elementary school | 78 (22.29) | 131 (18.71) |
| Junior/Senior high school | 143 (40.86) | 240 (34.29) |
| College or above | 129 (36.86) | 329 (47.00) |
| Smoking | ||
| No | 221 (63.32) | 472 (67.43) |
| Former or current | 128 (36.68) | 228 (32.57) |
| Cumulative cigarette smoking (pack-years) (Mean ± SD) | 10.73 ± 1.11 | 8.34 ± 0.70 |
| 0 | 221 (65.38) | 472 (69.01) |
| <21 | 44 (13.02) | 106 (15.50) |
| ≥21 | 73 (21.60) | 106 (15.50) |
| Alcohol consumption | ||
| No | 274 (78.29) | 418 (59.71) |
| Occasional or frequent | 76 (21.71) | 282 (40.29) |
| Diabetes | ||
| No | 284 (81.38) | 641 (91.57) |
| Yes | 65 (18.62) | 59 (8.43) |
| Hypertension | ||
| No | 188 (53.71) | 516 (73.71) |
| Yes | 162 (46.29) | 184 (26.29) |
| Urinary creatinine (mg/dL) (Mean ± SD) | 77.21 ± 2.80a | 133.46 ± 3.36a |
| Total urinary arsenic (μg/L) (Mean ± SD) | 18.01 ± 1.11a | 22.79 ± 0.72a |
| <12.2 | 173 (33.43) | 234 (33.43) |
| 12.2–26.2 | 100 (28.57) | 233 (33.29) |
| ≥26.2 | 77 (22.00) | 233 (33.29) |
| Total urinary arsenic (μg/g creatinine) (Mean ± SD) | 23.72 ± 1.19a | 18.91 ± 0.50a |
| <11.5 | 90 (25.71) | 234 (33.43) |
| 11.5–20.4 | 107 (30.57) | 233 (33.29) |
| ≥ 20.4 | 153 (43.71) | 233 (33.29) |
SD: standard deviation. RCC: renal cell carcinoma.
§P < 0.05 for the trend test; ap < 0.05 calculated using the Wilcoxon rank–sum test.
Alleles, genotypes, and haplotype of NLRP3 gene and RCC risk
A marginally significant increased RCC risk was showed among participants with the NLRP3 rs12239046 T allele compared to the NLRP3 rs12239046 C allele, the OR (95% CI) was 1.20 (0.98 to 1.47). Participants with the NLRP3 rs10925025 (G vs. A allele), NLRP3 rs1539019 (C vs. A allele), NLRP3 rs10925026 (A vs. C allele), NLRP3 rs10157379 (T vs. C allele), and NLRP3 rs12143966 (A vs. G allele) genotypes had similar 1.20–1.22-fold risks to that of NLRP3 rs12239046 (C vs. T allele). However, for the NLRP3 rs10925025 GG vs. AA genotype, the OR (95% CI) of RCC was 1.43 (0.95–2.17); for the NLRP3 rs10925026 AA vs. CC genotype, the OR (95% CI) of RCC was 1.42 (0.94–2.15); for the NLRP3 rs1539019 AA vs. CC genotype, the OR (95% CI) of RCC was 0.70 (0.46–1.05). These comparisons were all marginally significant. For the NLRP3 rs12143966 AA genotype compared to the GG genotype, the OR (95% CI) of RCC was 1.50 (1.01–2.22). Other NLRP3 genotypes were not associated with RCC risk (Table 2).
Table 2.
Alleles, genotype and haplotypes of inflammasome gene and the risk of RCC.
| Alleles and haplotypes of NLRP3 | RCC Cases (n = 350) | Controls (n = 700) | Crude ORs (95% CI) | Multivariate adjusted ORs (95% CI)a |
|---|---|---|---|---|
| SNP1: rs4925654 | ||||
| G | 589 (84.63) | 1192 (85.26) | 1.00 | 1.00 |
| A | 107 (15.37) | 206 (14.74) | 1.05 (0.82–1.36) | 1.12 (0.85–1.47) |
| GG | 250 (71.84) | 509 (72.82) | 1.00 | 1.00 |
| GA | 89 (25.57) | 174 (24.89) | 1.04 (0.78–1.40) | 1.09 (0.79–1.51) |
| AA | 9 (2.59) | 16 (2.29) | 1.17 (0.51–2.68) | 1.36 (0.56–3.30) |
| SNP2: rs4925650 | ||||
| G | 357 (51.00) | 761 (54.36) | 1.00 | 1.00 |
| A | 343 (49.00) | 639 (45.64) | 1.14 (0.95–1.37) | 1.12 (0.92–1.37) |
| GG | 97 (27.71) | 203 (29.00) | 1.00 | 1.00 |
| GA | 163 (46.57) | 355 (50.71) | 0.97 (0.71–1.31) | 1.00 (0.71–1.39) |
| AA | 90 (25.71) | 142 (20.29) | 1.34 (0.93–1.91) | 1.27 (0.86–1.87) |
| SNP3: rs12239046 | ||||
| T | 267 (38.25) | 590 (42.14) | 1.00 | 1.00 |
| C | 431 (61.75) | 810 (57.86) | 1.18 (0.98–1.42)+ | 1.20 (0.98–1.47)+ |
| CC | 136 (38.97) | 236 (33.71) | 1.00 | 1.00 |
| CT | 159 (45.56) | 338 (48.29) | 0.82 (0.62–1.09) | 0.84 (0.62–1.14) |
| TT | 54 (15.47) | 126 (18.00) | 0.74 (0.51–1.09) | 0.71 (0.47–1.07) |
| SNP4: rs4925648 | ||||
| T | 169 (24.14) | 353 (25.21) | 1.00 | 1.00 |
| C | 531 (75.86) | 1047 (74.79) | 1.06 (0.86–1.31) | 1.04 (0.83–1.30) |
| CC | 203 (58.00) | 398 (56.86) | 1.00 | 1.00 |
| CT | 125 (35.71) | 251 (35.86) | 0.97 (0.74–1.28) | 1.03 (0.77–1.39) |
| TT | 22 (6.29) | 51 (7.29) | 0.83 (0.49–1.41) | 0.83 (0.46–1.47) |
| SNP5: rs10925025 | ||||
| A | 267 (38.25) | 588 (42.30) | 1.00 | 1.00 |
| G | 431 (61.75) | 802 (57.70) | 1.18 (0.98–1.43)+ | 1.20 (0.98–1.47)+ |
| AA | 53 (15.19) | 125 (17.99) | 1.00 | 1.00 |
| AG | 161 (46.13) | 338 (48.63) | 1.13 (0.78–1.65) | 1.21 (0.81–1.81) |
| GG | 135 (38.68) | 232 (33.38) | 1.37 (0.93–2.02) | 1.43 (0.95–2.17)+ |
| SNP6: rs1539019 | ||||
| A | 265 (37.86) | 585 (41.91) | 1.00 | 1.00 |
| C | 435 (62.14) | 811 (58.09) | 1.18 (0.98–1.43)+ | 1.21 (0.99–1.48)+ |
| CC | 138 (39.43) | 237 (33.95) | 1.00 | 1.00 |
| CA | 159 (45.43) | 337 (48.28) | 0.82 (0.62–1.08) | 0.83 (0.61–1.13) |
| AA | 53 (15.14) | 124 (17.77) | 0.73 (0.50–1.08) | 0.70 (0.46–1.05)+ |
| SNP7: rs3806265 | ||||
| C | 300 (42.86) | 653 (46.64) | 1.00 | 1.00 |
| T | 400 (57.14) | 747 (53.36) | 1.17 (0.97–1.40) | 1.15 (0.94–1.40) |
| TT | 112 (32.00) | 200 (28.57) | 1.00 | 1.00 |
| TC | 176 (50.29) | 347 (49.57) | 0.90 (0.67–1.21) | 0.92 (0.67–1.27) |
| CC | 62 (17.71) | 153 (21.86) | 0.72 (0.49–1.04) + | 0.74 (0.49–1.11) |
| SNP8: rs10925026 | ||||
| C | 267 (38.14) | 587 (42.05) | 1.00 | 1.00 |
| A | 433 (61.86) | 809 (57.95) | 1.18 (0.98–1.42)+ | 1.20 (0.98–1.47)+ |
| CC | 53 (15.14) | 125 (17.91) | 1.00 | 1.00 |
| CA | 161 (46.00) | 337 (48.28) | 1.14 (0.78–1.65) | 1.21 (0.81–1.81) |
| AA | 136 (38.86) | 236 (33.81) | 1.36 (0.93–2.00) | 1.42 (0.94–2.15)+ |
| SNP9: rs10157379 | ||||
| C | 268 (38.29) | 589 (42.19) | 1.00 | 1.00 |
| T | 432 (61.71) | 807 (57.81) | 1.18 (0.98–1.42) | 1.20 (0.98–1.46)+ |
| CC | 53 (15.14) | 124 (17.77) | 1.00 | 1.00 |
| CT | 162 (46.29) | 341 (48.85) | 1.12 (0.77–1.63) | 1.20 (0.80–1.79) |
| TT | 135 (38.57) | 233 (33.38) | 1.36 (0.92–2.00) | 1.42 (0.94–2.14) |
| SNP10: rs12143966 | ||||
| G | 307 (44.62) | 679 (49.20) | 1.00 | 1.00 |
| A | 381 (55.38) | 701 (50.80) | 1.20 (1.00–1.44)+ | 1.22 (1.00–1.49)+ |
| GG | 70 (20.35) | 175 (25.36) | 1.00 | 1.00 |
| GA | 167 (48.55) | 329 (47.68) | 1.27 (0.91–1.77) | 1.39 (0.97–2.00)+ |
| AA | 107 (31.10) | 186 (26.96) | 1.43 (0.99–2.06) + | 1.50 (1.01–2.22) |
| SNP11: rs10754555 | ||||
| G | 259 (37.00) | 552 (39.43) | 1.00 | 1.00 |
| C | 441 (63.00) | 848 (60.57) | 1.11 (0.92–1.34) | 1.10 (0.89–1.34) |
| CC | 147 (42.00) | 261 (37.29) | 1.00 | 1.00 |
| CG | 147 (42.00) | 326 (46.57) | 0.80 (0.61–1.06) | 0.84 (0.62–1.13) |
| GG | 56 (16.00) | 113 (16.14) | 0.87 (0.60–1.27) | 0.89 (0.59–1.34) |
| SNP12: rs3806268 | ||||
| G | 298 (42.57) | 651 (46.50) | 1.00 | 1.00 |
| A | 402 (57.43) | 749 (53.50) | 1.17 (0.98–1.41)+ | 1.15 (0.94–1.40) |
| AA | 113 (32.29) | 203 (29.00) | 1.00 | 1.00 |
| AG | 176 (50.29) | 343 (49.00) | 0.92 (0.68–1.23) | 0.95 (0.69–1.30) |
| GG | 61 (17.43) | 154 (22.00) | 0.70 (0.48–1.03) | 0.74 (0.49–1.11) |
| SNP13: rs12048215 | ||||
| G | 207 (29.57) | 447 (31.93) | 1.00 | 1.00 |
| A | 493 (70.43) | 953 (68.07) | 1.12 (0.92–1.36) | 1.98 (0.88–1.35) |
| AA | 176 (50.29) | 331 (47.29) | 1.00 | 1.00 |
| AG | 141 (40.29) | 291 (41.57) | 0.91 (0.70–1.20) | 0.95 (0.71–1.28) |
| GG | 33 (9.43) | 78 (11.14) | 0.78 (0.50–1.23) | 0.81 (0.50–1.31) |
| NLRP3 block 1: NLRP3 rs10925025 and NLRP3 rs1539019 | ||||
| A-A | 265 (37.86) | 585 (41.79) | 0.85 (0.70–1.02) | 0.83 (0.68–1.02)+ |
| A-C | 2 (0.29) | 4 (0.29) | 0.93 (0.17–5.12) | 1.21 (0.21–7.09) |
| G-A | 0 | 2 (0.14) | ||
| G-C | 433 (61.86) | 809 (57.79) | 1.00 | 1.00 |
| NLRP3 block 2: NLRP3 rs10925026, NLRP3 rs10157379, and NLRP3 rs12143966 | ||||
| A-C-A | 1 (0.14) | 3 (0.21) | 0.61 (0.06–5.87) | 0.76 (0.07–8.30) |
| A-C-G | 0 | 1 (0.07) | ||
| A-T-A | 387 (55.29) | 706 (50.50) | 1.00 | 1.00 |
| A-T-G | 45 (6.43) | 101 (7.22) | 0.81 (0.56–1.18) | 0.80 (0.54–1.20) |
| C-C-G | 267 (38.14) | 587 (41.99) | 0.83 (0.69–1.00)+ | 0.82 (0.66–1.00)+ |
| NLRP3 block 3: NLRP3 rs3806268 and NLRP3 rs12048215 | ||||
| A-A | 397 (56.71) | 743 (53.07) | 1.00 | 1.00 |
| A-G | 5 (0.71) | 6 (0.43) | 1.56 (0.47–5.14) | 2.09 (0.55–7.91) |
| G-A | 96 (13.71) | 210 (15.00) | 0.86 (0.65–1.12) | 0.88 (0.65–1.17) |
| G-G | 202 (28.86) | 441 (31.50) | 0.86 (0.70–1.05) | 0.88 (0.70–1.10) |
| NLRP3 Five Tag-SNPs | ||||
| A-A-A-G-A | 0 | 1 (0.07) | ||
| A-A-G-G-A | 0 | 1 (0.07) | ||
| A-C-G-A-A | 211 (30.14) | 448 (32.00) | 1.00 | 1.00 |
| A-C-G-A-G | 4 (0.57) | 6 (0.43) | 1.42 (0.40–5.07) | 1.74 (0.41–7.38) |
| A-C-G-G-A | 31 (4.43) | 65 (4.64) | 1.01 (0.64–1.60) | 0.96 (0.58–1.57) |
| A-C-G-G-G | 19 (2.71) | 66 (4.71) | 0.61 (0.36–1.05)+ | 0.64 (0.36–1.13) |
| C-A-A-A-A | 149 (21.29) | 220 (15.71) | 1.44 (1.10–1.87) | 1.44 (1.08–1.92)* |
| C-A-A-A-G | 1 (0.14) | 0 | ||
| C-A-A-G-A | 64 (3.05) | 139 (9.93) | 0.98 (0.70–1.37) | 1.04 (0.72–1.50) |
| C-A-A-G-G | 174 (24.86) | 350 (25.00) | 1.06 (0.83–1.35) | 1.07 (0.82–1.40) |
| C-A-G-A-A | 37 (5.29) | 75 (5.36) | 1.05 (0.68–1.60) | 1.05 (0.66–1.65) |
| C-A-G-G-G | 8 (1.14) | 25 (1.79) | 0.68 (0.30–1.53) | 0.71 (0.29–1.76) |
| C-C-G-G-A | 1 (0.14) | 4 (0.29) | 0.53 (0.06–4.78) | 0.64 (0.07–6.25) |
| C-C-G-G-G | 1 (0.14) | 0 | ||
| C-A-A-A-A | 149 (21.29) | 220 (15.71) | 1.45 (1.15–1.83) | 1.43 (1.11–1.84)* |
| others | 551 (78.71) | 1180 (84.29) | 1.00 | 1.00 |
+0.05 ≤ P < 0.1 and *P < 0.05.
aModel was adjusted by age, sex, education, cumulative cigarette smoking, alcohol consumption, diabetes, and hypertension.
NLRP3 Five Tag-SNPs: rs1539019, rs10925026, rs12143966, rs3806268, and rs12048215.
Lewontin’s D’ of polymorphisms NLRP3 block 1 (NLRP3 rs10925025 and NLRP3 rs1539019), NLRP3 block 2 (NLRP3 rs10925026, NLRP3 rs10157379, and NLRP3 rs12143966), and NLRP3 block 3 (NLRP3 rs3806268 and NLRP3 rs12048215) ranged from 0.96 to 0.99 indicating the LD (Fig. 1A), and r2 values (Fig. 1B) for each pair of polymorphisms. The A-A haplotype of NLRP3 block 1 had a marginally significantly inverse OR for RCC compared to the G-C haplotype. Similarly, The OR for RCC was marginally significantly lower in the C-C-G haplotype of NLRP3 block 2 than that of the A-T-A haplotype. Further we found out five Tag-SNPs of NLRP3 gene from above seven sites of SNP, including rs1539019, rs10925026, rs12143966, rs3806268, and rs12048215 (Fig. 2). The results showed the C-A-A-A-A haplotype of the NLRP3 Five Tag-SNPs had a significantly higher risk of RCC than other haplotypes, the OR was 1.43 (95% CI = 1.11 to 1.84).
Figure 2.
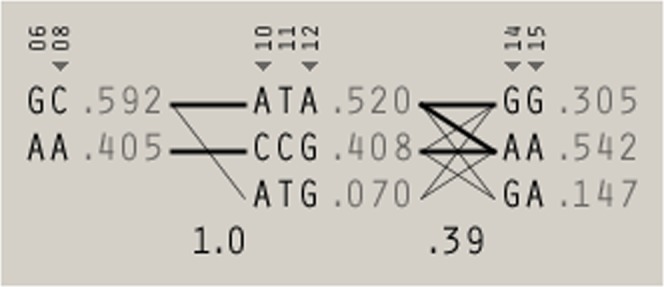
Five Tag-SNPs of NLRP3 gene.
We analyzed whether there were differences in the total urinary arsenic concentrations of the different genotypes of the 13 NLRP3 genes. We found that the total urinary arsenic concentration of the NLRP3 rs4925650 GA genotype was significantly higher than that of the GG genotype in all study subjects. However, the total urinary arsenic concentration of the NLRP3 rs4925650 AA genotype was significantly higher than those of the GA genotype in RCC patients. The total urinary arsenic concentration of the NLRP3 rs3806265 TT genotype was significantly higher than that of the CC genotype in all study subjects. In addition, the total urinary arsenic concentration of the NLRP3 rs3806268 AA genotype was significantly higher than that of the GG genotype in all study subjects (Supplemental Table S3). However, these NLRP3 SNPs were not associated with the risk of RCC.
Joint effects of total urinary arsenic levels and NLRP3 polymorphisms on RCC risk
For the NLRP3 rs1539019 C allele and a total urinary arsenic concentration ≥ 15.6 μg/g creatinine, the risk of developing RCC increased with exposure to an increasing number of risk factors (i.e., none, one, or both risk factors). Subjects with the NLRP3 rs1539019 C allele and total urinary arsenic concentration of ≥ 15.6 μg/g creatinine had a higher OR of RCC (2.33, 1.70–3.19) compared to those with the NLRP3 rs1539019 A allele, whose total urinary arsenic concentration was <15.6 μg/g creatinine after multivariable adjustment (Table 3). The NLRP3 rs1539019 C allele tended to multiplicatively and additively interact significantly with high total urinary arsenic concentrations to change the OR of RCC. In addition, the NLRP3 rs12239046 C, NLRP3 rs10925025 G, NLRP3 rs10925026 A, and NLRP3 rs10157379 T alleles tended to multiplicatively interact with high total urinary arsenic levels to affect RCC risk. However, the NLRP3 rs12143966 A allele and NLRP3 Five Tag-SNPs C-A-A-A-A haplotype did not interact with a high total urinary arsenic concentration in the risk of RCC.
Table 3.
The interaction of total urinary arsenic level and inflammasome gene polymorphisms on RCC risk.
| Total arsenicb | Polymorphisms of NLRP3 | Case/Control Number | Multivariate ORsa (95% CI) | Pinteraction | S index |
|---|---|---|---|---|---|
| Total arsenic | rs12239046 | 0.0219 | 0.66 (0.36–1.23) | ||
| <15.6 | T | 93/303 | 1.00§ | ||
| <15.6 | C | 179/397 | 1.60 (1.17–2.20) | ||
| ≥ 15.6 | T | 174/287 | 2.30 (1.65–3.20) | ||
| ≥ 15.6 | C | 252/413 | 2.26 (1.66–3.09) | ||
| Total arsenic | rs10925025 | 0.0172 | 0.66 (0.43–1.01) | ||
| <15.6 | A | 92/302 | 1.00§ | ||
| <15.6 | G | 180/394 | 1.63 (1.19–2.25) | ||
| ≥ 15.6 | A | 175/286 | 2.35 (1.68–3.28) | ||
| ≥ 15.6 | G | 251/408 | 2.31 (1.69–3.17) | ||
| Total arsenic | rs1539019 | 0.0116 | 0.65 (0.43–0.98) | ||
| <15.6 | A | 91/302 | 1.00§ | ||
| <15.6 | C | 181/396 | 1.67 (1.21–2.29) | ||
| ≥ 15.6 | A | 174/283 | 2.39 (1.71–3.33) | ||
| ≥ 15.6 | C | 254/415 | 2.33 (1.70–3.19) | ||
| Total arsenic | rs10925026 | 0.0187 | 0.66 (0.43–1.01) | ||
| <15.6 | C | 92/301 | 1.00§ | ||
| <15.6 | A | 180/397 | 1.62 (1.18–2.23) | ||
| ≥ 15.6 | C | 175/286 | 2.33 (1.67–3.24) | ||
| ≥ 15.6 | A | 253/412 | 2.29 (1.67–3.13) | ||
| Total arsenic | rs10157379 | 0.0250 | 0.67 (0.44–1.04) | ||
| <15.6 | C | 93/301 | 1.00§ | ||
| <15.6 | T | 179/395 | 1.59 (1.16–2.19) | ||
| ≥ 15.6 | C | 175/288 | 2.28 (1.64–3.17) | ||
| ≥ 15.6 | T | 253/412 | 2.26 (1.65–3.08) | ||
| Total arsenic | rs12143966 | 0.0721 | 0.74 (0.46–1.18) | ||
| <15.6 | G | 109/342 | 1.00§ | ||
| <15.6 | A | 161/344 | 1.54 (1.13–2.10) | ||
| ≥ 15.6 | G | 198/337 | 2.07 (1.52–2.81) | ||
| ≥ 15.6 | A | 220/357 | 2.19 (1.61–2.96) | ||
| Total arsenic | NLRP3 haplotype | 0.5812 | 1.02 (0.50–2.09) | ||
| <15.6 | others | 214/600 | 1.00§ | ||
| <15.6 | C-A-A-A-A | 58/100 | 1.54 (1.04–2.28) | ||
| ≥ 15.6 | others | 337/580 | 1.74 (1.38–2.19) | ||
| ≥ 15.6 | C-A-A-A-A | 91/120 | 2.31 (1.64–3.26) | ||
§tRend p-value <0.05. aModel was adjusted by age, sex, cumulative cigarette smoking, alcohol consumption, diabetes, and hypertension. bThe units of total arsenic in urine were μg/g creatinine.
We also reanalyzed the effect of gene-gene interactions on the risk of developing RCC. The marginally significant genotypes are presented in Table 2. We found that NLRP3 rs10925025 and NLRP3 rs12143966 or NLRP3 rs10925026 and NLRP3 rs12143966 had significantly additive and multiplicative interactions on the risk of RCC (Supplemental Table S4).
Discussion
In this study, we observed a dose-dependent relationship between total urinary arsenic levels and the OR for RCC after multivariate adjustments (i.e., the higher the total urinary arsenic, the higher the OR), which reflects the results of our previous study8. We found that the NLRP3 rs12239046, NLRP3 rs10925025, NLRP3 rs1539019, NLRP3 rs10925026, NLRP3 rs10157379, and NLRP3 rs12143966 genotypes were marginally significantly correlated with the risk of RCC. Additionally, the NLRP3 rs1539019 C, NLRP3 rs12239046 C, NLRP3 rs10925025 G, NLRP3 rs10925026 A, and NLRP3 rs10157379 T alleles tended to multiplicatively interact with high total urinary arsenic concentrations on the risk of RCC. Specifically, the NLRP3 rs1539019 C allele significantly additively interacted with high total arsenic concentration to change the risk of RCC.
Diabetes and hypertension were important risk factors for RCC in this study. Capitanio et al.33 also concluded in a recent review that hypertension is a critical risk factor for RCC. This risk may be caused by chronic renal hypoxia due to oxidative damage and lipid peroxidation caused by hypertension34. A case-control study from Sri Lanka also showed that diabetes was significantly associated with RCC35. This association may be because a high insulin concentration increases the concentration of insulin-like growth factor 1, which, in turn, can upregulate vascular endothelial growth factor secretion and induce tumor angiogenesis, leading to tumorigenesis and metastasis36. These associations need to be explored further.
RCC is a disease that involves complicated interactions between various environmental33 and genetic factors37,38. Recent study pointed out that tumor-associated immune cells play an important role in the initiation and progression of RCC39. The NLRP3 inflammasome is important for innate immune responses. NLRs affect the pathogenesis of many diseases, including neurodegenerative, metabolic, cardiovascular, and kidney diseases40. Cellular stress and tissue damage can activate NLR. One of the models of NLRP3 inflammasome activation is the dependence on reactive oxygen species41. Inflammasome disorders are associated with some inflammatory diseases. NLRP3 interacts with insulin resistance-associated thioredoxin-interacting protein (TXNIP). In response to reactive oxygen species, TXNIP dissociates from thioredoxin and binds to NLRP3 to activate the inflammasome. Lack of TXNIP can impair the activation of the NLRP3 inflammasome and subsequent secretion of IL-1β, which may be related to the pathogenesis of diabetes41. Diabetes is one of the risk factors for RCC42 . The association of NLRP3 with RCC and diabetes needs further investigation.
One study has shown that arsenic trioxide (As2O3) could induce nonalcoholic steatohepatitis, increase autophagy, NLRP3 inflammasome activation, and lipid accumulation, which leads to lipid-related gene dysregulation14. Another study demonstrated that arsenic enhanced the AIM2 inflammasome activation to increase IL-1 β/IL-18 production11. In contrast, Ahn et al.15 demonstrated that arsenic could inhibit the activation of the NLRP3 inflammasome in macrophages in response to lipopolysaccharide treatment, which attenuated the elevation of serum IL-1β in mice. Animal and cell culture studies showed that arsenic trioxide and other arsenic compounds inhibited NLRP3 inflammasome, caspase-1, and IL-1β inflammatory signaling, and played a major role in its anti-cancer effects43. Overall, the correlation between arsenic and the NLRP3 inflammasome remains unclear.
The NLRP3 inflammasome functions in the host immune response but also plays a role in the susceptibility to inflammatory disorders44. The NLRP3 gene is located on the long arm of chromosome 1q4417. It has nine exons within its 32.9 kb sequence17. Paramel et al.45 demonstrated a relationship between NLRP3 SNPs and the susceptibility to some inflammatory diseases. Approximately 60 SNPs have been identified within the NLRP3 gene46. Many studies have examined the association between the NLRP3 genotypes and cardiovascular disease. However, the results are inconsistent. The NLRP3 rs7512998 C allele, but not the TT-genotype, has been significantly associated with higher systolic and diastolic blood pressure18. The NLRP3 rs4612666 gene polymorphism may affect the risk of having a large artery atherosclerosis-induced ischemic stroke47. However, NLRP3 rs12239046 allele was not associated with cardiovascular disease48. Fewer studies have examined the association of the NLRP3 genotypes with cancer. One study found that the NLRP3 rs35829419 C > A (Q705K) genotype was associated with a lower survival rate in patients diagnosed with invasive colorectal cancer49. However, this genotype was not associated with myeloid leukemia50 or pancreatic cancer51. However, the NLRP3 alleles of rs10925025 G, rs1539019 C, rs10925026 A, and rs12143966 A tended to correlate with the risk of RCC in this study.
Studies exploring NLRP3 rs1539019 genotypes showed relationships between the NLRP3 rs1539019 TT genotype and increased risk of pneumoconiosis52 and NLRP3 rs1539019 polymorphisms and heart disease53 but not major blunt trauma17. Our study is the first to demonstrate that the NLRP3 rs1539019 C allele is marginally associated with RCC, but the five NLRP3-tag SNPs were found to be in LD. Haplotype analysis of these five NLRP3 SNPs showed that haplotype C-A-A-A-A (NLRP3 rs1539019, NLRP3 rs10925026, NLRP3 rs12143966, NLRP3 rs3806268, and NLRP3 rs12048215) significantly increased the risk of RCC compared to the other haplotypes after multivariate adjustment. Furthermore, the NLRP3 rs1539019 C allele additively and multiplicatively interacted significantly with the total urinary arsenic concentration in the increased risk of RCC. The relationships identified in this study may result from the effect of arsenic on NLRP3 inflammasome activation, which alters caspase-1 and IL-1β levels11 and the risk of developing RCC. Perhaps, NLRP3 rs1539019 affects the gene expression or linkage disequilibrium of other functional gene polymorphisms, which enhances the risk of RCC. These hypotheses require further investigation. We did find that NLRP3 rs12143966 and NLRP3 rs10925025, or and NLRP3 rs10925026 had a significant additive and multiplicative interactions on the risk of RCC. However, we cannot explain these results at this time. Perhaps in addition to the total urinary arsenic concentration and the NLRP3 rs1539019 genotype interaction, gene-gene interactions may also increase the risk of RCC.
This study had some limitations. This study was a case-control study design, therefore, we cannot rule out that the association between the environmental factors and RCC may be caused by RCC rather than the cause of RCC. A total of 15 SNPs of the NLRP3 gene were examined in this study. However, the correlation between the NLRP3 gene polymorphisms and NLRP3 expression could not be assessed. Therefore, the interpretation of the results should be conservative. Because the sample size was not large; the interpretation of the significance of the findings should be limited. This study showed the joint effect of total urinary arsenic concentration and the NLRP3 genotypes on the risk of developing RCC. However, we did not analyze the interaction between environmental arsenic exposure and the NLRP3 genotype.
Conclusions
This study is the first to identify significant multiplicative and additive interactions between high total urinary arsenic levels, the NLRP3 rs1539019 C allele, and an increased risk of RCC. Furthermore, the NLRP3 Five Tag-SNPs C-A-A-A-A haplotype had a higher OR for RCC than other haplotypes. Our data demonstrate that NLRP3 gene polymorphisms could alter the correlation between total urinary arsenic concentrations and RCC, even in people with low arsenic exposure. In addition, the multivariate analyses showed that high total urinary arsenic concentration and the NLRP3 rs1539019 C allele may indicate a higher OR for RCC. However, these results should be verified using a larger dataset.
Supplementary information
Acknowledgements
This study was supported by grants from the National Science Council of the Republic of China (NSC 100–2314-B-038–026, NSC 101–2314-B-038–051-MY3 (1–3), NSC 101–2314-B-038–051-MY3 (2–3), NSC 101–2314-B-038–051-MY3 (3–3) and Ministry of Science and Technology of the Republic of China (MOST 103–2314-B-038–021-MY2 (1–2), MOST 103–2314-B-038–021-MY2 (2–2), MOST 105–2314-B-038–082, MOST 106–2314-B-038–066, MOST 107–2320-B-039–010, MOST 106–2314-B-002–235-MY3, MOST 107–2314-B-038–073, and MOST 108–2314-B-038–089).
Author contributions
The study was equally conceived and designed by C.-J.C., B.-Y.B., Y.-C.L., Y.-L.H., H.-S.S., Y.-S.P., C.-Y.H. and Y.-M.H.; B.-Y.B., Y.-C.L., H.-S.S.,.-S.P., C.-Y.H. and Y.-M.H. collected the data; C.-J.C., P.-L.A., and Y.-M.H. analyzed the data; C.-J.C., and Y.-M.H. wrote the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chao-Yuan Huang, Email: ymhsueh@tmu.edu.tw.
Yu-Mei Hsueh, Email: cyhuang0909@ntu.edu.tw.
Supplementary information
is available for this paper at 10.1038/s41598-020-63469-8.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J. Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur. Urol. 2015;67:519–530. doi: 10.1016/j.eururo.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Chiang CJ, et al. Incidence and survival of adult cancer patients in Taiwan, 2002-2012. J. Formos. Med. Assoc. 2016;115:1076–1088. doi: 10.1016/j.jfma.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Chow WH, Gridley G, Fraumeni JF, Jr., Jarvholm B. Obesity, hypertension, and the risk of kidney cancer in men. N. Engl. J. Med. 2000;343:1305–1311. doi: 10.1056/NEJM200011023431804. [DOI] [PubMed] [Google Scholar]
- 5.Fox P, et al. Markers of systemic inflammation predict survival in patients with advanced renal cell cancer. Br. J. Cancer. 2013;109:147–153. doi: 10.1038/bjc.2013.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.IARC: Arsenic, metals, fibres, and dusts. In: IARC Monogr Eval Carcinog Risks Hum, 100 ed IARC Press, International Agency for Research on Cancer 11–465 (2012). [PMC free article] [PubMed]
- 8.Huang CY, et al. Effect of urinary total arsenic level and estimated glomerular filtration rate on the risk of renal cell carcinoma in a low arsenic exposure area. J. Urol. 2011;185:2040–2044. doi: 10.1016/j.juro.2011.01.079. [DOI] [PubMed] [Google Scholar]
- 9.Hao CM, Breyer MD. Physiologic and pathophysiologic roles of lipid mediators in the kidney. Kidney Int. 2007;71:1105–1115. doi: 10.1038/sj.ki.5002192. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, et al. Sodium arsenite induces cyclooxygenase-2 expression in human uroepithelial cells through MAPK pathway activation and reactive oxygen species induction. Toxicol. In Vitro. 2013;27:1043–1048. doi: 10.1016/j.tiv.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Zhang M, et al. AIM2 inflammasome mediates Arsenic-induced secretion of IL-1 beta and IL-18. Oncoimmunology. 2016;5:e1160182. doi: 10.1080/2162402X.2016.1160182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Man SM, Kanneganti TD. Regulation of inflammasome activation. Immunol. Rev. 2015;265:6–21. doi: 10.1111/imr.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell. 2002;10:417–426. doi: 10.1016/S1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 14.Qiu T, et al. Taurine attenuates arsenic-induced pyroptosis and nonalcoholic steatohepatitis by inhibiting the autophagic-inflammasomal pathway. Cell Death Dis. 2018;9:946. doi: 10.1038/s41419-018-1004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahn H, et al. Mercury and arsenic attenuate canonical and non-canonical NLRP3 inflammasome activation. Sci. Rep. 2018;8:13659. doi: 10.1038/s41598-018-31717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poli G, et al. Expression of inflammasome-related genes in bladder cancer and their association with cytokeratin 20 messenger RNA. Urol. Oncol. 2015;33:505–507. doi: 10.1016/j.urolonc.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Zhang AQ, et al. Clinical relevance of single nucleotide polymorphisms within the entire NLRP3 gene in patients with major blunt trauma. Crit. Care. 2011;15:R280. doi: 10.1186/cc10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunnas T, Maatta K, Nikkari ST. NLR family pyrin domain containing 3 (NLRP3) inflammasome gene polymorphism rs7512998 (C > T) predicts aging-related increase of blood pressure, the TAMRISK study. Immun. Ageing. 2015;12:19. doi: 10.1186/s12979-015-0047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou D, et al. The NLRP3 rs10754558 Polymorphism Is Associated with the Occurrence and Prognosis of Coronary Artery Disease in the Chinese Han Population. Biomed. Res. Int. 2016;2016:3185397. doi: 10.1155/2016/3185397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsueh YM, et al. Association of Arsenic Methylation Capacity with Developmental Delays and Health Status in Children: A Prospective Case-Control Trial. Sci. Rep. 2016;6:37287. doi: 10.1038/srep37287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO: Guidelines for drinking water quality, fourth edition. (2011).
- 22.Chen BC, Chou WC, Chen WY, Liao CM. Assessing the cancer risk associated with arsenic-contaminated seafood. J. Hazard. Mater. 2010;181:161–169. doi: 10.1016/j.jhazmat.2010.04.112. [DOI] [PubMed] [Google Scholar]
- 23.Tsai CY, Jiang SJ. Microwave-assisted extraction and ion chromatography dynamic reaction cell inductively coupled plasma mass spectrometry for the speciation analysis of arsenic and selenium in cereals. Anal. Sci. 2011;27:271–276. doi: 10.2116/analsci.27.271. [DOI] [PubMed] [Google Scholar]
- 24.Chu YL, Jiang SJ. Speciation analysis of arsenic compounds in edible oil by ion chromatography-inductively coupled plasma mass spectrometry. J. Chromatogr. A. 2011;1218:5175–5179. doi: 10.1016/j.chroma.2011.05.089. [DOI] [PubMed] [Google Scholar]
- 25.Hsu WM, Hsi HC, Huang YT, Liao CS, Hseu ZY. Partitioning of arsenic in soil-crop systems irrigated using groundwater: a case study of rice paddy soils in southwestern Taiwan. Chemosphere. 2012;86:606–613. doi: 10.1016/j.chemosphere.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 26.Calderon RL, Hudgens E, Le XC, Schreinemachers D, Thomas DJ. Excretion of arsenic in urine as a function of exposure to arsenic in drinking water. Environ. Health Perspect. 1999;107:663–667. doi: 10.1289/ehp.99107663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barr DB, et al. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ. Health Perspect. 2005;113:192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsueh YM, et al. Serum beta-carotene level, arsenic methylation capability, and incidence of skin cancer. Cancer Epidemiol. Biomarkers Prev. 1997;6:589–596. [PubMed] [Google Scholar]
- 29.Hsueh YM, et al. Urinary arsenic speciation in subjects with or without restriction from seafood dietary intake. Toxicol. Lett. 2002;133:83–91. doi: 10.1016/S0378-4274(02)00087-5. [DOI] [PubMed] [Google Scholar]
- 30.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 31.Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology. 1992;3:452–456. doi: 10.1097/00001648-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Huang YK, et al. Arsenic exposure, urinary arsenic speciation, and the incidence of urothelial carcinoma: a twelve-year follow-up study. Cancer Causes Control. 2008;19:829–839. doi: 10.1007/s10552-008-9146-5. [DOI] [PubMed] [Google Scholar]
- 33.Capitanio U, et al. Epidemiology of Renal Cell Carcinoma. Eur. Urol. 2019;75:74–84. doi: 10.1016/j.eururo.2018.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gago-Dominguez M, Castelao JE. Lipid peroxidation and renal cell carcinoma: further supportive evidence and new mechanistic insights. Free Radic. Biol. Med. 2006;40:721–733. doi: 10.1016/j.freeradbiomed.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 35.Balagobi B, et al. Risk factors of renal cell carcinoma in a cohort of Sri Lankan patients: A case-control study. J. Cancer Res. Ther. 2019;15:S91–S96. doi: 10.4103/0973-1482.206867. [DOI] [PubMed] [Google Scholar]
- 36.Djiogue S, et al. Insulin resistance and cancer: the role of insulin and IGFs. Endocr. Relat. Cancer. 2013;20:R1–R17. doi: 10.1530/ERC-12-0324. [DOI] [PubMed] [Google Scholar]
- 37.Yang SM, et al. Joint Effect of Urinary Total Arsenic Level and VEGF-A Genetic Polymorphisms on the Recurrence of Renal Cell Carcinoma. PLoS One. 2015;10:e0145410. doi: 10.1371/journal.pone.0145410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang SM, et al. Combined effects of DNA methyltransferase 1 and 3A polymorphisms and urinary total arsenic levels on the risk for clear cell renal cell carcinoma. Toxicol. Appl. Pharmacol. 2016;305:103–110. doi: 10.1016/j.taap.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 39.Geissler K, et al. Immune signature of tumor infiltrating immune cells in renal cancer. Oncoimmunology. 2015;4:e985082. doi: 10.4161/2162402X.2014.985082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mason DR, Beck PL, Muruve DA. Nucleotide-binding oligomerization domain-like receptors and inflammasomes in the pathogenesis of non-microbial inflammation and diseases. J. Innate Immun. 2012;4:16–30. doi: 10.1159/000334247. [DOI] [PubMed] [Google Scholar]
- 41.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 42.Spyridopoulos TN, et al. Insulin resistance and risk of renal cell cancer: a case-control study. Hormones (Athens) 2012;11:308–315. doi: 10.14310/horm.2002.1359. [DOI] [PubMed] [Google Scholar]
- 43.Maier NK, Crown D, Liu J, Leppla SH, Moayeri M. Arsenic trioxide and other arsenical compounds inhibit the NLRP1, NLRP3, and NAIP5/NLRC4 inflammasomes. J. Immunol. 2014;192:763–770. doi: 10.4049/jimmunol.1301434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fritz JH, Ferrero RL, Philpott DJ, Girardin SE. Nod-like proteins in immunity, inflammation and disease. Nat. Immunol. 2006;7:1250–1257. doi: 10.1038/ni1412. [DOI] [PubMed] [Google Scholar]
- 45.Paramel GV, Sirsjo A, Fransen K. Role of genetic alterations in the NLRP3 and CARD8 genes in health and disease. Mediators Inflamm. 2015;2015:846782. doi: 10.1155/2015/846782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.The International HapMap Project The International HapMap Project. Nature. 2003;426:789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 47.Cheng L, Yin R, Yang S, Pan X, Ma A. Rs4612666 Polymorphism of the NLRP3 Gene Is Associated with the Occurrence of Large Artery Atherosclerotic Ischemic Strokes and Microembolic Signals. Biomed Res. Int. 2018;2018:6345805. doi: 10.1155/2018/6345805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lopez-Mejias R, et al. Influence of elevated-CRP level-related polymorphisms in non-rheumatic Caucasians on the risk of subclinical atherosclerosis and cardiovascular disease in rheumatoid arthritis. Sci. Rep. 2016;6:31979. doi: 10.1038/srep31979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ungerback J, et al. Genetic variation and alterations of genes involved in NFkappaB/TNF. Carcinogenesis. 2012;33:2126–2134. doi: 10.1093/carcin/bgs256. [DOI] [PubMed] [Google Scholar]
- 50.Zhang A, et al. The genetic polymorphism and expression profiles of NLRP3 inflammasome in patients with chronic myeloid leukemia. Hum. Immunol. 2018;79:57–62. doi: 10.1016/j.humimm.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 51.Miskiewicz A, et al. The Q705K and F359L Single-Nucleotide Polymorphisms of NOD-Like Receptor Signaling Pathway: Association with Chronic Pancreatitis, Pancreatic Cancer, and Periodontitis. Arch. Immunol. Ther. Exp. (Warsz) 2015;63:485–494. doi: 10.1007/s00005-015-0355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ji X, et al. Polymorphisms in inflammasome genes and risk of coal workers’ pneumoconiosis in a Chinese population. PLoS One. 2012;7:e47949. doi: 10.1371/journal.pone.0047949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dehghan A, et al. Association of novel genetic Loci with circulating fibrinogen levels: a genome-wide association study in 6 population-based cohorts. Circ. Cardiovasc. Genet. 2009;2:125–133. doi: 10.1161/CIRCGENETICS.108.825224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.