Abstract
Purpose
To report a case of unilateral idiopathic elevated episcleral venous pressure (IEEVP) in a 15-year-old patient. We reviewed and summarized published case reports of IEEVP to determine how to manage this challenging and rare condition.
Observations
A 15-year-old Caucasian male presented with elevated intraocular pressures (IOP), blood in Schlemm canal in the left eye, and asymmetric cupping with corresponding glaucomatous findings on testing. We diagnosed the patient with IEEVP and describe successful surgical intervention with deep sclerectomy and viscocanalostomy.
Conclusions and Importance
IEEVP is a diagnosis of exclusion and based on clinical findings of dilated episcleral veins, blood in Schlemm canal and glaucomatous changes. If glaucomatous progression occurs with medication, filtration surgery is usually required, and most patients have good results in the literature. Care should be taken to prevent post-operative hypotony and serous choroidal detachment.
Keywords: Idiopathic elevated episcleral venous pressure, Radius-Maumenee syndrome, Idiopathic dilated episcleral veins, Deep sclerectomy, Viscocanalostomy, Schlemm canal
1. Introduction
Elevated episcleral venous pressure (EVP) is a rare condition that can present with elevated intraocular pressure (IOP) and blood in Schlemm canal. The causes of increased EVP include arteriovenous malformations, such as carotid-cavernous sinus fistula, orbital varix and Sturge-Weber syndrome, and conditions that lead to venous obstruction such as retrobulbar tumor, thyroid eye disease and superior vena cava syndrome.1 It can also be idiopathic, referred to as idiopathic elevated EVP (IEEVP), idiopathic dilated episcleral veins or Radius-Maumenee syndrome,2 and considered a diagnosis of exclusion. Here, we present a case of IEEVP in a teenager.
1.1. Case report
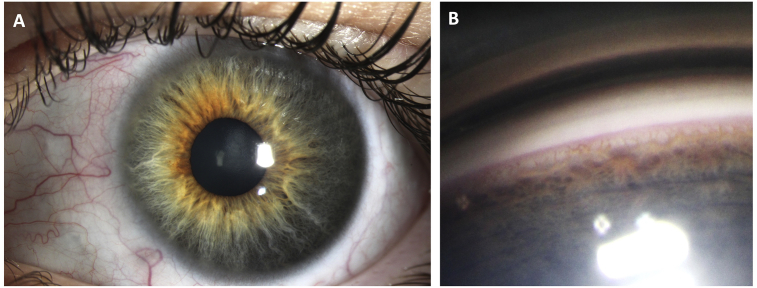
A 15 year-old healthy male was referred to our glaucoma service for elevated IOP in both eyes. He was born full-term and met all developmental milestones. His past medical history was only notable for childhood asthma. He had no prior ocular surgery, laser or trauma, and no family history of glaucoma. Prior to presentation, he was seen by an outside ophthalmologist with IOP of 30 mmHg in the right eye (OD) and 32 mmHg in the left eye (OS) on no medications, and subsequently started on brinzolamide and brimonidine two times a day in both eyes (OU). At his presenting visit, his vision was 20/20 OU with IOP of 15 mmHg OD and 18 mmHg OS on brinzolamide and brimonidine OU, with no afferent pupillary defect. His central corneal thicknesses were 588 μm OD and 570 μm OS. Slit lamp exam revealed normal anterior segments OU except for a few dilated episcleral vessels OS (Fig. 1A). On gonioscopy, his angles were open to ciliary body band 360°, and there was blood in Schlemm canal 270° in the left eye only (Fig. 1B). In the right eye, he had a cup-to-disc ratio of 0.3 with no disc hemorrhage. In the left eye, his cup-to-disc ratio was 0.6 with no disc hemorrhage or focal notching (Fig. 2). His dilated fundus exam was normal OU.
Fig. 1.
A) Slit lamp photo of left eye (OS) showing several dilated, tortuous episcleral vessels.
B) Gonioscopy photo OS showing blood in Schlemm canal.
Fig. 2.
Fundus photos of right eye (OD) and left eye (OS) showing asymmetric cup-to-disc ratio.
At presentation, his Humphrey visual field (HVF) was full in the right eye and showed early superior and inferior arcuate changes in the left eye (Fig. 3A). Optical coherence tomography (OCT) of the circumpapillary retinal nerve fiber layer (RNFL) showed normal thickness in the right optic nerve, and superior and inferior thinning in the left optic nerve (Fig. 3B). Magnetic resonance imaging (MRI) of the orbit and magnetic resonance angiogram (MRA) of the head were unremarkable with no evidence of intracranial aneurysm, vascular stenosis, venous sinus thrombosis, or cavernous carotid fistula.
Fig. 3.
A) Humphrey Visual Field (HVF) of left eye (OS) with early superior and inferior arcuate scotomas at presentation.
B) Optical coherence tomography (OCT) of the retinal nerve fiber layer (RNFL) showing superior and inferior thinning OS at presentation.
C) HVF OS with progressive inferior arcuate scotoma after 1.5 years.
D) OCT RNFL showing progressive thinning OS after 1.5 years.
The patient was diagnosed with Radius-Maumenee syndrome OS based on the elevated IOP, optic nerve cupping, blood in Schlemm canal, and unremarkable head and orbit imaging. He required escalation of medical therapy in his left eye. At his 1.5 year follow-up from presentation, his IOP was 28 mmHg OS on maximum topical medical therapy including brimonidine, brinzolamide, latanoprost, betaxolol and netarsudil. His OCT demonstrated progressive RNFL thinning from an average RNFL thickness of 76 μm to 63 μm over 1.5 years, which corresponded to a progressive inferior arcuate defect on HVF OS (Fig. 3C and D). The patient underwent a deep sclerectomy and viscocanalostomy OS. His IOP was 9 mmHg on post-operative day 1 on no drops and remained at 16 mmHg on no drops at post-operative month 3.
2. Discussion
IEEVP was first described by Minas and Podos in 1968 when they described two cases of unilateral dilated episcleral veins, elevated EVP and blood in Schlemm canal in a mother and daughter.3 In 1978, Radius and Maumenee reported 4 additional cases of idiopathic dilated episcleral vessels and open-angle glaucoma.2 Approximately 55 cases of IEEVP have been reported in the literature in all languages.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 We conducted a PubMed search using the following search terms: idiopathic elevated EVP, idiopathic dilated episcleral veins or Radius-Maumenee syndrome (date 1/29/20). In Table 1, we present the cases reported in the literature in English that required surgical intervention for glaucoma control, grouped by surgery type.
Table 1.
Surgical interventions for IEEVP reported in the English literature.
| Paper | Age (years) | Sex | Affected eye(s) with IEEVP | Surgical Intervention | Last reported IOP after surgery (mmHg) | Presence of choroidal effusion after intervention |
|---|---|---|---|---|---|---|
| Radius and Maumenee, 19782 | 42 | M | OD > OS | Filtration surgery OD | OD 15 | No |
| Radius and Maumenee, 19782 | 48 | F | OD > OS | Filtration surgery OD | OD 19 | No |
| Radius and Maumenee, 19782 | 63 | F | OD > OS | Penetrating cyclodiathermy ×2 OD | OU < 20 | No |
| Minas and Podos, 19683 | 60 | F | OU | Sclerectomy OD | OD 4 | No |
| Bellows et al., 197922 | 27 | F | OU | Sclerectomy + posterior sclerostomy OD | OD 7 | Yes OD |
| Bellows et al., 197922 | 25 | F | OU | Sclerectomy + posterior sclerostomy ×2 OD Sclerectomy + posterior sclerostomy OS |
OD 10, OS 11 | Yes OU |
| Guven et al., 20029 | 32 | M | OD | Non-penetrating deep sclerectomy OD | OD 16-19 | No |
| Parikh et al., 201115 | 65 | F | OD > OS | Sclerectomy with sclerostomy inferotemporally + trabeculectomy superiorly OD | OD 22 | Yes OD |
| Talusan et al., 198320 | 68 | F | OD | Trabecular trephine OD | OD 8 | No |
| Bigger, 197523 | 50 | F | OD | Trabeculectomy OD | OD 12 | No |
| Lanzl et al., 199613 | 34 | M | OS | Trabeculectomy OS | OS < 20 | No |
| Grieshaber et al., 20077 | 39 | M | OS | Trabeculectomy + MMC OS | OS 9 | No |
| Rhee et al., 200917 | 50 | M | OD | Trabeculectomy with MMC OD | OD 11 | No |
| Rhee et al., 200917 | 40 | F | OS > OD | Trabeculectomy with MMC OS | OS 16 | Yes OS |
| Rhee et al., 200917 | 60 | M | OS > OD | Trabeculectomy with MMC OS | OD 10 | No |
| Cymbor et al., 20135 | 15 | M | OD | Trabeculectomy OD | OD 5 | No |
| Stock et al., 201319 | 69 | M | OS | Trabeculectomy OS | OS 12 | Yes OS |
| Pradhan et al., 201516 | 40 | F | OU | Trabeculectomy with MMC OD, followed by sclerostomy for choroidal effusion on POD4 Trabeculectomy with MMC OS |
OD 13, OS 8 | Yes OU |
| Rong and Li, 201818 | 50 | F | OU | Trabeculectomy with 5FU OU | OU 8-11 | No |
Abbreviations: IEEVP, Idiopathic elevated episcleral venous pressure; IOP, Intraocular pressure; M, Male; F, Female; OD, Right eye; OS, Left eye; OU, Both eyes; MMC, Mitomycin C; 5FU, Fluorouracil; POD4, Post-operative day 4.
Our patient presented at age 15, matching the youngest age of presentation reported in the literature.5 The reported age of onset ranges from mid-teens to 70s, and most cases appear to be unilateral or bilateral with asymmetric involvement.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 The pathogenesis is currently unknown, though congenital vascular abnormalities and genetic predisposition have been proposed.2,6 Mechanistically, increased EVP can prevent normal aqueous outflow and lead to elevated IOP. The normal range for EVP is 8–10 mmHg, and based on the Goldmann equation, there should theoretically be a linear relationship between EVP and IOP.1
Since the diagnosis of IEEVP is one of exclusion, it is necessary to rule out other causes of increased EVP, namely pathologic conditions such as carotid-cavernous sinus fistulas that lead to arteriovenous communication and venous obstructive disorders such as thyroid eye disease. Computed tomography (CT) or MRI can help identify any intraorbital or intracranial pathology, and CT or MR angiography is necessary to exclude intracranial vascular abnormalities. If identified, management of the primary neurovascular disorders usually results in the normalization of IOP.1 Our patient had a negative MRI/MRA ruling out a neurovascular abnormality.
The diagnosis of IEEVP is clinical based on findings of dilated episcleral veins, open angle and elevated IOP with corresponding optic nerve and visual field findings consistent with glaucoma. Gonioscopy typically shows an open angle with blood in Schlemm canal. Our patient presented with unilateral dilated episcleral veins and blood in Schlemm canal suggesting elevated episcleral venous pressure. The patient's episcleral veins were noted to be more prominently dilated during surgery due to masking by thick Tenon's capsule. The measurement of EVP can be performed through direct and indirect methods, but no commercially available device is available, making it difficult to perform in the routine clinical setting.17,24,25 Glaucoma status should be assessed with automated perimetry, and OCT of the RNFL and ganglion cell complex. Optic nerve photos can be taken at the time of presentation to allow for future comparison.
Treatment can be challenging in these cases. Typically, topical medications are started, but most patients in the literature required maximum medical therapy, and few were stable only on medications.4,14,17 The efficacy of laser trabeculoplasty in this patient population has not been well studied. There are reports of argon laser trabeculoplasty and micropulse diode laser trabeculoplasty having minimal effect on IOP in IEEVP.9,18 If there is continued progression on maximum-tolerated medical therapy, glaucoma surgery should be performed. Most cases of IEEVP were managed with filtration procedures, including trabeculectomy, penetrating cyclodiathermy, sinusotomy, deep sclerectomy and viscocanalostomy, with favorable outcomes reported in the English literature (Table 1).2,3,5,7,9,12,13,15, 16, 17, 18, 19,23 There is a risk of choroidal effusion or suprachoroidal hemorrhage with rapid reduction of IOP after filtration surgery.15, 16, 17,19,22 Most of these cases resolved spontaneously with conservative management.15, 16, 17,19,22 One case required choroidal drainage on post-operative day 4 after trabeculectomy with mitomycin C.15 Prophylactic sclerectomies could be considered at the time of filtration surgery to prevent intraoperative and post-operative serous choroidal detachments.
Alternative therapies include glaucoma drainage device or bleb-forming minimally invasive glaucoma surgery (MIGS), such as the XEN45 gel stent, but use of these devices for IEEVP has not been reported in the literature. Cyclodestructive procedures may require high amounts of energy to achieve near-complete ciliary body shut-down to reach target IOP and cause post-operative complications such as inflammation and macular edema in a well-seeing eye. In patients with limited visual potential, it may be a reasonable first-line option given the serious complications that can occur with filtration surgery. Trabecular meshwork-bypassing MIGS (e.g. iStent, Kahook dual blade, gonioscopy-assisted transluminal trabeculotomy) likely will not lower the IOP enough or resolve the issue of elevated EVP if the obstruction is distal to the trabecular meshwork.
In our patient, we elected to perform a non-penetrating deep sclerectomy and viscocanalostomy, which theoretically reduces the risk of hypotony and choroidal detachment compared to a trabeculectomy. Mechanistically, the viscocanalostomy dilated the Schlemm canal, which may improve outflow by recruiting collector channels without elevated venous pressure. The deep sclerectomy left a patent space behind the anterior trabeculum and Descemet membrane, which allows aqueous to efflux into a proposed intrascleral lake. The surgery and post-operative course were uncomplicated and he has maintained IOP of 16 mmHg on no drops at post-operative month 3.
3. Conclusions
In young patients presenting with elevated IOP and blood in Schlemm canal, it is important to rule out vision and life-threatening causes of increased EVP, prior to arriving at the diagnosis of IEEVP. Dilated episcleral vessels may not be obvious in young patients due to a thick Tenon's capsule. In these patients, glaucoma evaluation should be performed and patients should be managed similarly to primary open-angle glaucoma with medications as first line therapy. If surgery is needed, nonpenetrating procedures such as deep sclerectomy with viscocanalostomy may be a safe and efficacious alternative to bleb-forming or cyclodestructive procedures. We may be able to have better diagnostic criteria for IEEVP if more objective techniques for EVP measurement are developed for routine use. Lastly, the creation of an international registry-style database may be useful in gathering long-term outcomes data on this uncommon disease.
Patient consent
The patient's legal guardian consented to publication of the case in writing.
Funding
Supported by NIH Center Core Grant P30EY014801, Research to Prevent BlindnessUnrestricted Grant, The University of Miami Institute for Advanced Study of the Americas 2019 Pilot Grant
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Funding
supported by NIH Center Core Grant P30EY014801, Research to Prevent Blindness Unrestricted Grant, The University of Miami Institute for Advanced Study of the Americas 2019 Pilot Grant.
Declaration of competing interest
The following authors have no financial disclosures: CQS, CMM, TCC.
Acknowledgements
The authors thank Mr. Frank J. Semcer Sr. for his generous philanthropic support of The Samuel & Ethel Balkan International Pediatric Glaucoma Center’s research efforts.
References
- 1.Cioffi G.A., editor. Basic and Clinical Science Course. American Academy of Ophthalmology; Section 10: Glaucoma San Francisco, CA: 2015-2016. [Google Scholar]
- 2.Radius R.L., Maumenee A.E. Dilated episcleral vessels and open-angle glaucoma. Am J Ophthalmol. 1978;86:31–35. doi: 10.1016/0002-9394(78)90010-7. [DOI] [PubMed] [Google Scholar]
- 3.Minas T.F., Podos S.M. Familial glaucoma associated with elevated episcleral venous pressure. Arch Ophthalmol. 1968;80:202–208. doi: 10.1001/archopht.1968.00980050204010. [DOI] [PubMed] [Google Scholar]
- 4.Breazzano M.P., Mawn L.A., Kuchtey R.W. Spontaneous resolution of presumed idiopathic elevated episcleral venous pressure. J Glaucoma. 2016;25:e751–e752. doi: 10.1097/IJG.0000000000000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cymbor M., Knapp E., Carlin F. Idiopathic elevated episcleral venous pressure with secondary glaucoma. Optom Vis Sci : official publication of the American Academy of Optometry. 2013;90:e213–e217. doi: 10.1097/OPX.0b013e31829689a6. [DOI] [PubMed] [Google Scholar]
- 6.Foroozan R., Buono L.M., Savino P.J., Sergott R.C. Idiopathic dilated episcleral veins and increased intraocular pressure. Br J Ophthalmol. 2003;87:652–654. doi: 10.1136/bjo.87.5.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grieshaber M.C., Dubler B., Knodel C., Killer H.E., Flammer J., Orgul S. Retrobulbar blood flow in idiopathic dilated episcleral veins and glaucoma. Klinische Monatsblatter fur Augenheilkunde. 2007;224:320–323. doi: 10.1055/s-2007-962946. [DOI] [PubMed] [Google Scholar]
- 8.Groh M.J., Kuchle M. [Idiopathic episcleral venous stasis with secondary open-angle glaucoma (Radius-Maumenee syndrome)] Klinische Monatsblatter fur Augenheilkunde. 1997;211:131–132. doi: 10.1055/s-2008-1035110. [DOI] [PubMed] [Google Scholar]
- 9.Guven D., Karakurt A., Ziraman I., Hasiripi H. Non-penetrating deep sclerectomy in unilateral open-angle glaucoma secondary to idiopathic dilated episcleral veins. Eur J Ophthalmol. 2002;12:66–68. doi: 10.1177/112067210201200113. [DOI] [PubMed] [Google Scholar]
- 10.Jorgensen J.S., Guthoff R. [Pathogenesis of unilateral dilated episcleral vessels and increase in intraocular pressure] Klinische Monatsblatter fur Augenheilkunde. 1987;190:428–430. doi: 10.1055/s-2008-1050426. [DOI] [PubMed] [Google Scholar]
- 11.Jorgensen J.S., Guthoff R. [The role of episcleral venous pressure in the development of secondary glaucomas] Klinische Monatsblatter fur Augenheilkunde. 1988;193:471–475. doi: 10.1055/s-2008-1050284. [DOI] [PubMed] [Google Scholar]
- 12.Kazerounian S., Rickmann A., Helaiwa K., Waizel M. [Management of Radius-Maumenee syndrome : treatment with deep sclerectomy, viscocanalostomy and collagen matrix implantation] Ophthalmologe : Zeitschrift der Deutschen Ophthalmologischen Gesellschaft. 2016;113:775–778. doi: 10.1007/s00347-015-0213-5. [DOI] [PubMed] [Google Scholar]
- 13.Lanzl I.M., Welge-Luessen U., Spaeth G.L. Unilateral open-angle glaucoma secondary to idiopathic dilated episcleral veins. Am J Ophthalmol. 1996;121:587–589. doi: 10.1016/s0002-9394(14)75444-3. [DOI] [PubMed] [Google Scholar]
- 14.Marques S.H.M., Farinha C., Martins A., Faria P. Radius-Maumenee syndrome: a rare cause of glaucoma. BMJ Case Rep. 2018:2018. doi: 10.1136/bcr-2017-223255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parikh R.S., Desai S., Kothari K. Dilated episcleral veins with secondary open angle glaucoma. Indian J Ophthalmol. 2011;59:153–155. doi: 10.4103/0301-4738.77045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pradhan Z.S., Kuruvilla A., Jacob P. Surgical management of glaucoma secondary to idiopathic elevated episcleral venous pressure. Oman J Ophthalmol. 2015;8:120–121. doi: 10.4103/0974-620X.159266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhee D.J., Gupta M., Moncavage M.B., Moster M.L., Moster M.R. Idiopathic elevated episcleral venous pressure and open-angle glaucoma. Br J Ophthalmol. 2009;93:231–234. doi: 10.1136/bjo.2007.126557. [DOI] [PubMed] [Google Scholar]
- 18.Rong X., Li M. Advanced glaucoma secondary to bilateral idiopathic dilated episcleral veins - a case report. BMC Ophthalmol. 2018;18:207. doi: 10.1186/s12886-018-0892-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stock R.A., Fernandes N.L., Pastro N.L., Oliveira R.S., Bonamigo E.L. Idiopathic dilated episcleral vessels (Radius-Maumenee syndrome): case report. Arq Bras Oftalmol. 2013;76:45–47. doi: 10.1590/s0004-27492013000100013. [DOI] [PubMed] [Google Scholar]
- 20.Talusan E.D., Fishbein S.L., Schwartz B. Increased pressure of dilated episcleral veins with open-angle glaucoma without exophthalmos. Ophthalmology. 1983;90:257–265. doi: 10.1016/s0161-6420(83)34565-6. [DOI] [PubMed] [Google Scholar]
- 21.Ruprecht K.W., Naumann G.O. [Unilateral secondary open-angle glaucoma with idiopathically dilated episcleral vessels] Klinische Monatsblatter fur Augenheilkunde. 1984;184:23–27. doi: 10.1055/s-2008-1054402. [DOI] [PubMed] [Google Scholar]
- 22.Bellows A.R., Chylack L.T., Jr., Epstein D.L., Hutchinson B.T. Choroidal effusion during glaucoma surgery in patients with prominent episcleral vessels. Arch Ophthalmol. 1979;97:493–497. doi: 10.1001/archopht.1979.01020010243011. [DOI] [PubMed] [Google Scholar]
- 23.Bigger J.F. Glaucoma with elevated episcleral venous pressure. South Med J. 1975;68:1444–1448. doi: 10.1097/00007611-197511000-00026. [DOI] [PubMed] [Google Scholar]
- 24.Brubaker R.F. Determination of episcleral venous pressure in the eye. A comparison of three methods. Arch Ophthalmol. 1967;77:110–114. doi: 10.1001/archopht.1967.00980020112024. [DOI] [PubMed] [Google Scholar]
- 25.Sit A.J., Ekdawi N.S., Malihi M., McLaren J.W. A novel method for computerized measurement of episcleral venous pressure in humans. Exp Eye Res. 2011;92:537–544. doi: 10.1016/j.exer.2011.03.018. [DOI] [PubMed] [Google Scholar]