Abstract
Background
HIV testing rates in many hyper-endemic areas are lower than needed to curtail the HIV epidemic. New HIV testing strategies are needed to overcome barriers to traditional clinic based testing; HIV self-testing is one modality that offers promise in reaching individuals who experience barriers to clinic-based testing.
Methods
We conducted a randomized control trial among young women ages 18-26 living in rural Mpumalanga, South Africa where they were randomized in a 1:1 allocation to either the: (1) HIV Counseling and Testing (HCT) arm: an invitation to test at one of the 9 local government clinics where free HCT is provided and is standard of care (SOC), or (2) choice arm: choice of either a clinic-based HCT invitation or oral HIV Self-Testing (HIVST) kits. Depending on the arm, participants were also provided either: (1) 4 HCT invitations to provide to peers/partners for HIV testing at one of the 9 local clinics, or (2) 4 HIV self-test kits to provide to peers/partners (thus 5 total HIVST kits or HCT invitations). Young women were asked to return 3 months and 9 months after enrollment to assess testing uptake and invitation or kit distribution to peers and partners and experiences with testing. Peers and partners who were reported by index participants to have received kits/invitations during follow-up visits were also invited to attend a study visit to assess their testing experiences. The trial is registered at clinical trials.gov NCT03162965.
Findings
287 young women were enrolled and randomized, with 146 randomized to the HCT arm and 141 to the choice (HCT or HIVST) arm. Of those randomized to the choice arm, over 95% (n=135) chose the HIV self-testing kit and only 6 individuals chose HCT. At the 3-month follow-up visit, 92% of index participants in the choice arm reported having tested for HIV compared to 43% of participants in the HCT arm, resulting in a significant risk difference of 49% (95% CI 40%, 58%). By 9 months, this difference decreased to a risk difference of 25% (95% CI 17%, 33%) between arms (96% in the choice arm and 72% in the HCT arm). Participants in the choice arm were also more likely to invite peers and partners to test compared to the HCT arm (94% vs. 76% or an average of 4.97 vs 2.79 tests). Few male partners were invited to test by index participants; however, index participants in the choice arm were more likely to have their male partners test than index participants in the HCT arm (RR 2.99, 95% CI 1.45, 6.16).
Interpretation
When given a choice between clinic-based HIV testing and HIV oral self-testing, the overwhelming majority of young women chose HIVST. In addition, those offered a choice of HIV testing modality were much more likely to test, distribute test kits to peers and partners, and to have peers and partners who reported testing compared to the HCT arm. Self-testing offers an important opportunity to significantly increase testing rates among young women and their peers and partners compared to clinic-based HCT. Other strategies to reach men with testing are needed.
Funding
US National Institutes of Health
Keywords: HIV testing, Self testing, South Africa, HIV prevention, Young people
Research in context.
Evidence before this study
HIV self-testing (HIVST) offers individuals the opportunity to test on their own terms, reducing many barriers to clinic based HIV testing including wait times, privacy concerns and costs associated with clinic attendance. The evidence on self-testing to date has shown that offering HIVST can increase HIV testing, with the bulk of the data coming from Men who have sex with Men (MSM), sex workers or pregnant women. To date, there is no data from a randomized trial showing that offering HIVST to young women (who are not pregnant, recruited from antenatal care centers, or sex workers) can increase testing in this high priority population and there is also a lack of evidence as to whether young women would distribute test kits to friends and partners.
Added value of this study
This study is the first RCT among young women who are not pregnant or engaged in sex work to examine the impact of offering a choice of HIVST to young women on their testing uptake and on testing uptake among friends and partners as compared to standard, provider delivered HIV Counseling and Testing (HCT). We found that the vast majority (95%) of young women chose HIVST over clinic based HCT and that HIVST resulted in a difference in testing uptake between groups of 49% at the 3 month follow up visit (95% CI 40%, 58%). We also found that young women offered HIVST were more likely than those offered HCT invitations to have friends and partners test. By the 9-month visit, 84% of young women in the choice/HIVST arm had at least one peer or partner who reported testing compared to 44% in the HCT arm for a risk difference of 41% (95% CI 30%, 51%). There were no social harms reported in this study and overall feasibility of conducting HIVST was high.
Implications of all the available evidence
Increasing evidence from different populations and contexts has found that offering HIVST to individuals results in a large and significant increase in HIV testing and that secondary distribution of HIVST to friends and partners can increase testing among an even larger network of individuals. There is minimal evidence that HIVST distribution leads to social harms and in many high prevalence HIV settings, where governments are actively trying to de-congest clinics, HIVST can reduce clinic burden and help reach the first 90 in the UNAIDS testing target; there is a need though for alternative approaches to reach men other than through their female partners.
Alt-text: Unlabelled box
1. Introduction
South Africa has the most Adolescent Girls and Young Women (AGYW) ages 15- 24 years living with HIV in the world. An estimated 6% of South African AGYW ages 15-19 are HIV infected, increasing to over 17% by age 20-24; incidence rates exceed 3% in many sub-groups [1,2]. Despite this, HIV testing and linkage to care remains inadequate in this population; fewer than 50% of all 15-19 year olds reported ever testing in 2016 [3], only 14% of HIV-positive adolescents are on antiretroviral therapy (ART), and just 10% are virally suppressed [1,4]. In addition, young men ages 18-35– the partners of many of these young women– are some of the least likely to test for HIV [3]. Novel strategies to get young people to test for HIV are needed.
A substantial proportion of new infections are spread by persons unaware of their HIV status, making it urgent that we improve testing uptake and reduce the number of undiagnosed cases of HIV [5,6]. Ensuring universal access to HIV prevention, treatment and care in countries with generalized epidemics, such as South Africa, will require near-complete uptake of annual HIV testing by all adults, which could be achieved by expanding delivery options to overcome some of the obstacles to provider-delivered HIV Counseling and Testing (HCT). In light of recent scale-up of HCT in many high prevalence settings not reaching key populations at risk for HIV infection, new strategies are needed to identify hard-to-reach, undiagnosed cases of HIV infection.
HIV self-testing (HIVST) has the potential to increase the proportion of people ever tested, increase testing frequency, and encourage earlier detection of HIV and thus earlier treatment [7,8]. With self-testing, individuals collect their own sample and perform a simple, rapid HIV antibody test in the absence of a provider. As a result, HIVST can address some of the structural barriers to testing, such as privacy concerns, lack of access to care (e.g. lack of transportation), stigma, and clinic wait times [9,10] Self-testing has the potential to reach those who have traditionally been reluctant or unable to attend clinics and has been found to be acceptable and feasible in a number of sub-Saharan African countries among a range of populations [8,11,12,13]. A recent meta-analysis of 5 randomized control trials found HIVST to increase uptake and frequency of HIV testing [7]. Many of the studies among women have focused on pregnant or post-partum women and female sex workers, finding that women have been successful in giving HIV test kits to partners [14], [15], [16], [17]. Secondary distribution of HIV self-test kits is one way to reach populations who test less frequently and hard-to-reach groups like adolescents, men and key populations.
2. Methods
2.1. Study design
This was a 1:1 individually randomized controlled trial aiming to determine whether providing young women with a choice of testing strategies as compared to traditional HCT would result in greater uptake of testing among young women and their peers and partners. Young women ages 18-26 were randomized to either the: 1) HCT arm: an invitation to a local clinic for free HCT (standard of care) or, 2) HCT/HIVST choice arm: choice of either free HCT or oral HIVST kits. Young women choosing HIVST in the choice arm were provided with 5 HIVST kits (OraQuick); young women randomized to or choosing HCT were given 5 invitations to test for free at local government clinics. In both arms, one kit or invitation was intended for the trial (index) participant, while the remaining four were intended for distribution to peers and partners. Young women were asked to return 3 months and 9 months after enrollment to assess testing uptake and kit/invitation distribution to peers and partners and experiences with testing modality. Peers and partners that young women reported giving kits/invitations to at each follow-up visit were also contacted and invited to attend a study visit where they were interviewed about their experiences with the testing modality they were provided with.
The study was conducted in the rural Bushbuckridge sub-district of Mpumalanga Province, South Africa in the Medical Research Council / Wits University Rural Public Health and Health Transitions Research Unit's field center. This area is characterized by high unemployment and temporary migration for work, high uptake of social grants, poor provision of water and sanitation, and high levels of HIV infection in the adult population.
2.2. Ethics
This study was approved by the Institutional Review Boards at the University of North Carolina-Chapel Hill and the University of California-San Francisco, the Human Research Ethics Committee (Medical) at the University of the Witwatersrand in South Africa, and the Mpumalanga Department of Health and Social Development Research Committee. The protocol was registered at clinical trials.gov ID number - NCT03162965.
2.3. Participants
Young women were recruited from the Agincourt Health and socio-Demographic Surveillance System (AHDSS) sampling frame. This is an annual household census of over 120,000 individuals living in 31 villages in the study area (www.agincourt.co.za). A sample was drawn from the AHDSS based on age and not having previously participated in studies with regular HIV testing. Households were visited for each young woman to determine eligibility and willingness to participate in the study. Inclusion criteria included: between the ages of 18-26; not having participated in HPTN 068, an RCT with annual HIV testing; reported having had sex in the past 3 months and planning to have sex again in the next three months; planning to stay in the Agincourt HDSS area for the next nine months; able and willing to provide informed consent; willing to comply with study procedures, and did not self report HIV-positive serostatus.
Peers and partners invited by the young women had to be 18 years old and above to participate in the study. Recruited peers and partners recruited by index participants were not entered into the index participant pool. All primary analyses are based on the original randomized index participants only.
Written informed consent either in English or Shangaan (the local language) was obtained from all participants.
2.4. Randomization
Young women ages 18-26 were randomized using block randomization with a 1:1 allocation to either the HCT arm or the choice arm. Randomization sequences were generated by the data team in the US and sent to South Africa. The random allocation was printed in 440 sealed opaque envelopes ordered 1-440 in South Africa. In every block of 4 there were 2 choice and 2 HCT allocations in random order. Participants randomly selected an envelope from a box with a set number of blocks of envelopes put into the box and opened it to reveal the study arm in the presence of a research team member. The study was not blinded.
2.5. Procedures
Following consent young women were asked to complete a baseline questionnaire and then randomized into the study arms. The questionnaire was interviewer administered and included questions about demographics, history of testing for HIV, and sexual behavior. A finger prick blood sample was collected from all participants onto a dried blood spot (DBS) card for storage, so that at the end of the trial, ie at the 9-month visit, incident cases could be distinguished from those who were already HIV positive at baseline. Young women were followed up 3 and 9 months after baseline to determine their experiences with testing.
Young women who were randomized to the choice arm and chose self-testing were provided with five self-test kits for themselves, their peers and their partner(s). Each test kit was numbered and linked to the index participant using a unique identifier. Participants who chose the HIVST were given the OraQuick InHome Rapid HIV- 1/2 Antibody Test™ (Orasure Technologies) which is FDA approved and WHO pre-qualified and approved for use in South Africa. Young women were provided with a demonstration of how to use the kit, shown an online video created by the manufacturer demonstrating use in the local language (which could be shown to peer/parnters on a smartphone), and given an opportunity to ask any questions about the kit. Test kits included the instructions for use provided by the manufacturer in the local language (Tsonga); these instructions include pre- and post-test counseling information and a “frequently asked questions” document on HIV self-testing. The study team provided a list of local HIV/AIDS and related resources. The study also provided a self-testing log to document use of the self-test to be returned to the study team. The log included date and time of test, people present during testing, and test results (with graphics displaying possible results –participants were to select the image that best represented the test result). It was emphasized that as the test is an antibody-based test, it was important to both test again in 3 months if HIV negative and to get confirmatory testing at the clinic after testing HIV positive.
Young women who were randomized to clinic-based HCT and those who chose clinic-based HCT in the choice arm were provided with an invitation to test at one of the 9 local government primary care clinics in the AHDSS study area where rapid HIV testing is provided free of charge. They were also given linked HCT invitation cards for 4 peers and sex partners to test; in total there were 5 HIVST kits or 5 HCT invitations given to young women. Cards were linked to the index partner using a confidential unique identifying number in order to determine if peers and sex partners of index cases returned to the clinic for testing.
Young women in both arms were advised to provide the test kits/invitations to test to peers and sex partners within 1 month of receiving the kit and advised to tell sex partners and peers to use the self-test kit or go to the clinic within 3 months of receiving it. It was emphasized that participants should think carefully about who it would be appropriate to share the HIV testing kits/invitations with and remind them that they should only provide them to sex partners and peers with whom they felt safe giving HIV test kits/invitations to.
At the 3- and 9-month follow-up visits an assessment for all index participants was undertaken. The assessment included questions about their testing experiences, depression, intimate partner violence, HIV knowledge and any negative or positive experiences as a result of the study. Additionally, they were asked to provide names and contact information for the peers and partners to whom they gave test kits/invitations. All peers and sex partners for whom the participant provided contact information at the 3 or 9 month visit were invited to attend a study visit. Those that were interested were asked to visit the study offices, provide written informed consent and take part in a questionnaire (similar to the survey conducted with the index young women).
At the 3-month follow-up assessment, young women who tested negative were offered the opportunity to receive more HIVST kits or more HCT invitation cards (the same number as at baseline, 5 in total). Those that tested positive were offered more kits to distribute to their peers and partners. Young women who were randomized to the “choice” arm had the option to change their testing choice. That means if they chose HCT the first time, they could chose HIVST kits at month 3 and vice versa.
At the 9-month follow-up visit, young woman who self-reported as HIV negative or status unknown completed an in-office HIV self-test on her own (both study arms), while observed by a study counselor. Participants were given the instruction sheet on how to use the test kit but were not shown how to use it. All index participants also had a dried blood spot (DBS) card collected for confirmatory HIV testing at the 9-month visit. Cards were stored in -80C freezers on site and shipped to the laboratory following cold-chain procedures.
Everyone who tested HIV positive during the study was encouraged to link to care by study interviewers. All individuals who reported testing HIV positive at either the 3-month or 9-month visit were asked whether or not they sought care for their HIV diagnosis, and reminded about the importance of starting treatment. We conducted care calls 4 weeks prior to the 3-month and 9-month visits at which we reminded the participant to invite peers and partners to their testing modality and to use the testing modality if they have not yet used it. If they reported testing HIV-positive at the time of the care call they were encouraged to link to care for confirmatory testing if they have not yet done so.
2.6. Statistical Analysis
The primary outcome of the trial was uptake of HIV testing by study arm at the 3-month visit, measured by the proportion of index participants self-reporting HIV testing in the choice arm (either HCT or HIVST) compared to those in the HCT arm. The secondary outcome of the trial is the proportion of peers and sex partners reporting having tested for HIV in the choice arm compared to those in HCT arm at 3 months. We also assessed the proportion reporting testing by study arm at the 9-month visit for both index participants and their peers and partners.
2.6.1. Sample size
An original sample size of 200 index participants per arm (400 total) was estimated for the main study aims assuming a 20% difference in the take-up of HIV testing between study arms. However, early study results in July 2017 with 272 participants recruited at that time found that the difference in uptake between arms to be greater than four times initial estimates, suggesting that the study would be overpowered to detect differences for the main study aims. The study team simulated the estimated impact of early recruitment cutoff on statistical power using a range of plausible scenarios. The simulation approach is analogous to an empirical power calculation, but better utilizes existing data and expected statistical methods to generate more realistic power estimates [18]. It was determined that detection of the main study aims was more than adequate at current recruitment levels. As a result, the decision was made to cease additional recruitment, resulting in a total enrollment of 287 participants. The study adhered to Consort guideline criteria.
2.6.2. Statistical methods
All main analyses are performed as intent to treat analyses between the two trial arms. Risk and count differences and their respective 95% confidence intervals are estimated by ordinary least squares (OLS) regression – or the untransformed case of generalized linear models – with adjustment to standard errors for heteroskedasticity. For binary outcome variables, risk ratios were also calculated and provided, using the standard 2 × 2 method. For the index participant analyses, HC2 estimators were used for confidence intervals (CIs) for the OLS estimates for heteroskedasticity robustness [19], and the Wald estimator for the risk ratios. Differences between the peers and partners recruited from index participants were also assessed using OLS and risk ratios, with adjusted confidence intervals to account for clustering. CIs were estimated with the CR2 estimator for all OLS estimates [19], and with a cluster-bootstrap estimator for the relative risks. No additional control variables were included for any regression or analysis beyond the binary indicator for the assigned arm of the index participant and an intercept term.
All 9-month statistics are cumulative from baseline, inferring HIV status and testing events from both baseline to 3-month and 3-month to 9-month, to estimate the number of testing events and sero-conversions from baseline. Unless otherwise noted, missing data for any reason, including not attending a visit or refusal to answer a question, are treated as the event or status not detected and count as a 0. This is performed for consistency across 3-month and 9-month measurements.
All calculations were performed in R v3.6.1 [20], with risk ratios calculated with the epitools package v0.5-10 [21] and OLS-based estimators and their confidence intervals in the estimatr package v0.20.0 [22].
2.6.3. Treatment of peers and partners
Peers and partners were each associated with an index participant. In some instances, the same peer/partner could have been invited to test (HCT or Choice arm) by more than one index participant. Each occurrence of a peer or partner invitation is counted separately for each index participant with whom they are associated, and as such the same peer/partner may be counted more than once in the total number of testing invitations or test kits distributed by index cases. However, peers/partners who were invited into the study by more than one index and ultimately entered the study (e.g. signed informed consent and completed a questionnaire) were only allowed to enroll in the study once and complete one questionnaire pertaining to the first index case who invited them, this occurred for 26 peer/partners.
2.7. Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
3.1. Findings with index participants
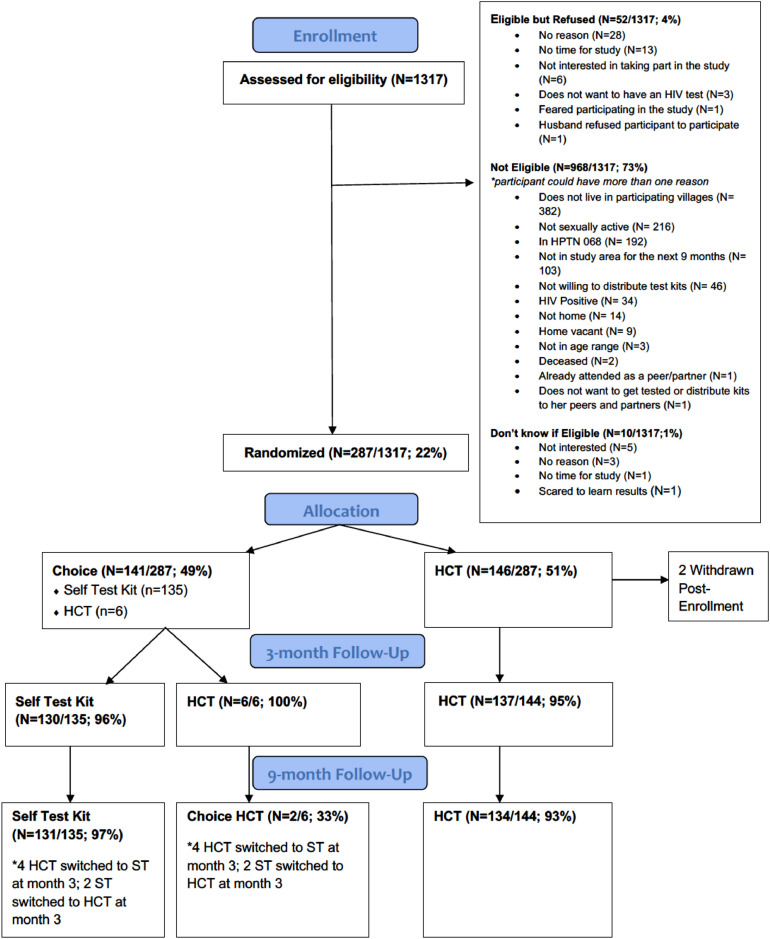
Of 1317 young women contacted and screened for eligibility, 968 (73%) were not eligible: 382 were no longer living in the study area, 216 were not sexually active, 192 had been in a prior trial that offered annual HIV testing, and 103 were not planning to stay in the study area for the study duration (Fig. 1). Other reasons for ineligibility included: not willing to distribute test kits (n=46), self report being HIV positive (n=34), no one home or refused assessment of eligibility (n=37). We could not assess eligibility of 10 participants. The remaining 339 young women were eligible to participate. Of these, 52 (15%) refused to participate (Fig. 1).
Fig. 1.
CONSORT Diagram: Index participants.
Overall, 287 young women were enrolled between December 2016 and July 2017, with 146 randomized to the HCT arm and 141 to the choice arm. Of those randomized to the choice arm, over 95% (n=135) chose to use the HIV self-test kit (HIVST) and only 6 individuals chose HCT.
At baseline, the mean age of index participants in both arms was 21 years, having completed 12 years of schooling on average. The mean number of life time partners was 2.7 vs 2.9 (HCT vs. choice, respectively) and 67% reported having been pregnant before (Table 1). Prior to the study, 94% (265/283) of participants in both arms reported ever having tested for HIV and 76% (n=135, HCT) and 77% (n=130, choice) reported having tested for HIV in the last 12 months. The majority (84%, n=223/265) reported having tested at a government clinic the last time they tested for HIV. At baseline, 80% of participants (n=225/283) also reported feeling very comfortable asking their primary partner to test for HIV, while only 43% (n=122/283) reported being very comfortable asking a non-primary partner to test for HIV.
Table 1.
Baseline descriptive statistics.
| HCT arm (n=146) |
Choice arm (n=141) |
|
|---|---|---|
| Descriptive statistics | mean (SD) | mean (SD) |
| Age | 21.1 (2.2) | 20.7 (2.1) |
| Years of school | 12.2 (0.7) | 12.2 (0.9) |
| Age at first sex | 16.9 (1.6) | 16.7 (1.7) |
| Number of sex partners ever | 2.7 (1.5) | 2.9 (1.8) |
| Number of sex partners in the last 12 months | 1.09 (0.31) | 1.17 (0.41) |
| Received pay for work | 0.15 (0.35) | 0.10 (0.30) |
| Previously used an HIV self-test | 0.02 (0.14) | 0.04 (0.20) |
| Has ever tested for HIV before trial | 0.94 (0.24) | 0.94 (0.25) |
| Has tested for HIV in last 12 months | 0.76 (0.43) | 0.77 (0.42) |
| Ever been pregnant | 0.66 (0.47) | 0.68 (0.47) |
| Arm assignment | n (%) | n (%) |
| Assigned or chose HCT protocol | 146 (100%) | 6 (4.3%) |
| Assigned or chose self-test protocol | 0 (0%) | 135 (96.7%) |
Follow up visits occurred between March 2017 and May 2018. At the 3-month follow-up visit, 92% of index participants in the choice arm reported having tested for HIV compared to 43% of participants in the HCT arm resulting in a risk difference of 49% (95% CI 0.40,0.58) (Table 2). At the 9-month visit, 96% of participants in the choice arm reported having tested for HIV compared to 72% of HCT arm participants, resulting in a 25% difference in risk between arms (95% CI 0.16, 0.33) (Table 2).
Table 2.
Main outcome results for index participants.
| HCT (n=146) | Choice arm (n=141) | |||
|---|---|---|---|---|
| Panel a: Binary outcome variables at Month 3 | Proportion of outcome | Risk difference | Risk ratio | |
| Used at least one test | 0.43 | 0.92 | 0.49 [0.40,0.58] | 2.14 [1.76,2.59] |
| Used test and at least one was HIV positive | 0.02 | 0.04 | 0.01 [-0.02,0.05] | 1.73 [0.42,7.09] |
| Gave at least one test away | 0.57 | 0.87 | 0.30 [0.21,0.40] | 1.53 [1.31,1.79] |
| Gave at least one test to a partner | 0.06 | 0.18 | 0.12 [0.05,0.20] | 2.99 [1.45,6.16] |
| At least one peer/partner linked to trial* | 0.45 | 0.78 | 0.33 [0.23,0.44] | 1.75 [1.43,2.14] |
| At least one peer/partner used at least one test* | 0.31 | 0.76 | 0.45 [0.35,0.55] | 2.46 [1.90,3.19] |
| At least one peer/partner tested positive* | 0.03 | 0.06 | 0.03 [-0.02,0.08] | 1.86 [0.64,5.43] |
| Became pregnant since baseline | 0.07 | 0.02 | -0.05 [-0.09,0.00] | 0.31 [0.09,1.11] |
| HCT (n=146) | Choice arm (n=141) | |||
|---|---|---|---|---|
| Panel b: Count outcome variables at Month 3 | Mean count of outcome | Count difference | ||
| Number of tests used | 0.43 | 0.96 | 0.53 [0.43,0.62] | |
| Number of tests given away | 1.36 | 2.65 | 1.29 [1.00,1.62] | |
| Number of tests given to partner(s) | 0.06 | 0.20 | 0.14 [0.05,0.22] | |
| Number of peers/partners linked to trial* | 0.74 | 1.69 | 0.95 [0.70,1.21] | |
| Number of peer/partners who received at least one test* | 0.74 | 1.65 | 0.91 [0.70,1.17] | |
| Number of peer/partners who used at least one test* | 0.43 | 1.60 | 1.16 [0.90,1.40] | |
| HCT (n=146) | Choice arm (n=141) | |||
|---|---|---|---|---|
| Panel c: Binary outcome variables at Month 9 | Proportion of outcome | Risk difference | Risk ratio | |
| Used at least one test | 0.72 | 0.96 | 0.25 [0.17,0.33] | 1.34 [1.21,1.49] |
| Used test and at least one was HIV positive | 0.03 | 0.05 | 0.02 [-0.03,0.06] | 1.45 [0.47,4.46] |
| Gave at least one test away | 0.76 | 0.94 | 0.18 [0.10,0.26] | 1.23 [1.11,1.36] |
| Gave at least one test to a partner | 0.08 | 0.23 | 0.14 [0.06,0.23] | 2.76 [1.48,5.14] |
| At least one peer/partner linked to trial* | 0.60 | 0.87 | 0.27 [0.17,0.37] | 1.45 [1.25,1.68] |
| At least one peer/partner used at least one test* | 0.44 | 0.84 | 0.41 [0.30,0.51] | 1.93 [1.58,2.34] |
| At least one peer/partner tested positive* | 0.04 | 0.11 | 0.07 [0.00,0.13] | 2.59 [1.03,6.48] |
| Became pregnant since baseline | 0.14 | 0.06 | -0.09 [-0.2,-0.02] | 0.39 [0.20,0.86] |
| HCT (n=146) | Choice arm (n=141) | |||
|---|---|---|---|---|
| Panel d: Count outcome variables at Month 9 | Mean count of outcome | Count difference | ||
| Number of tests used | 1.03 | 1.89 | 0.87 [0.7,1.02] | |
| Number of tests given away | 2.79 | 4.97 | 2.18 [1.6,2.77] | |
| Number of tests given to partner(s) | 0.09 | 0.30 | 0.22 [0.1,0.33] | |
| Number of peers/partners linked to trial* | 1.32 | 2.96 | 1.64 [1.2,2.07] | |
| Number of peer/partners who received at least one test* | 1.32 | 2.91 | 1.59 [1.2,2.02] | |
| Number of peer/partners who used at least one test* | 0.78 | 2.76 | 1.98 [1.6,2.36] | |
Unless otherwise noted, all data are self-reported from the index participant. * Data are from peer/partners linked with index participant.
Index participants reported giving HIVST kits or HCT invitations to 570 peers and partners between baseline and the 3-month visit; 373 in the choice arm and 198 in the HCT arm. 87% of index participants gave at least one test to a peer or partner compared to 60% in the HCT arm, resulting in a 27% risk difference (RR 1.45 95% CI 1.25,1.68) (Table 2). More choice arm participants reported giving a test kit/invitation to at least one male partner than HCT participants at both 3-month (18% in choice vs. 6% in HCT; RR 2.99, 95% CI 1.45, 6.16) and 9-month visits (23% in choice vs. 8% HCT, RR 2.76, 95% CI 1.48, 5.14).
No harms were reported by index participants in the 3 or 9 month survey. One participant in the HCT arm reported as a harm that she was turned away from a clinic when presenting with her study invivation to access HCT, however she did not report this in the survey. One social harm was reported to the IRB for an individual recruited to participate in the study (but did not enroll in the study) because a boyfriend was upset about study staff contacting her to take part.
3.1.1. Findings from peers and partners
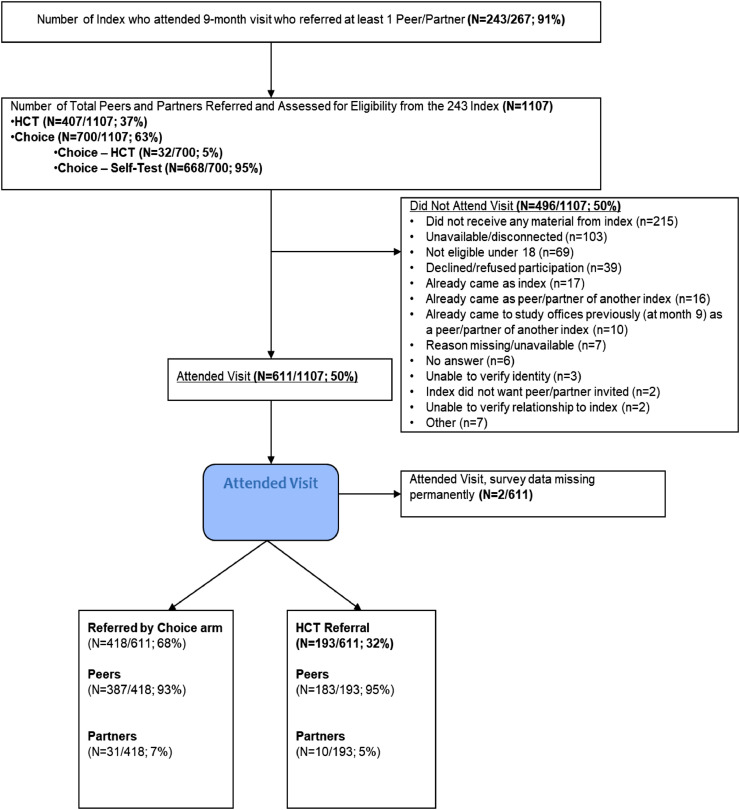
Of the 1107 peers and partners reported by the index cases by the end of the trial, 609 (55%) attended a visit and enrolled in the study, 193 in the HCT arm and 416 in the choice arm (Fig. 2). The majority of peer/partners were female (Table 3) in both arms, although a higher proportion of those invited in the choice arm were male (26%) than those invited from the HCT arm (19%) (RR 1.38, 95% CI: 1.03,1.90).
Fig. 2.
CONSORT Diagram: Peer/partner participants.
Table 3.
Differences in characteristics of peers / partners between invited by index participants from each arm by month 3.
| Panel a: All peers/partners invited by index participant by month 3 | HCT (n=198) | Choice arm (n=373) | ||
|---|---|---|---|---|
| Binary outcome variables | Proportion of outcome | Risk difference | Risk ratio | |
| Male* | 0.19 | 0.26 | 0.08 [0.01,0.16] | 1.43 [1.03,2.10] |
| Sexual partner* | 0.04 | 0.08 | 0.04 [-0.00,0.07] | 1.77 [0.93,4.44] |
| Came in for a study visit | 0.55 | 0.64 | 0.09 [-0.00,0.19] | 1.17 [1.00,1.40] |
| Used at least one kit/card from index | 0.15 | 0.32 | 0.29 [0.19,0.38] | 1.90 [1.52,2.48] |
| Had at least one positive test result | 0.01 | 0.01 | -0.00 [-0.03,0.03] | 0.96 [0.32,4.67] |
| Mean/count outcome variables | Mean of outcome | Mean/count differences | ||
| Age* | 23.9 | 24.0 | 0.35 [-2.00,2.33] | |
| Panel b: Peers/partners who came in for a clinic visit for study | HCT (n=108) | Choice arm (n=238) | ||
|---|---|---|---|---|
| Binary outcome variables | Proportion of outcome | Risk difference | Risk ratio | |
| Male | 0.19 | 0.29 | 0.10 [0.00,0.20] | 1.54 [1.00,2.64] |
| Sexual partner* | 0.06 | 0.08 | 0.02 [-0.03,0.08] | 1.44 [0.66,4.80] |
| Ever previously tested | 0.83 | 0.87 | 0.04 [-0.05,0.12] | 1.04 [0.95,1.16] |
| Used at least one kit/card from index | 0.58 | 0.95 | 0.36 [0.24,0.48] | 1.62 [1.35,2.01] |
| Had at least one positive test result | 0.05 | 0.04 | -0.01 [-0.06,0.04] | 0.82 [0.27,3.58] |
| Mean/count outcome variables | Mean of outcome | Mean/count differences | ||
| Age | 24.9 | 23.9 | -1.08 [-3.56,1.40] | |
| Years of school | 11.9 | 12.1 | 0.24 [-0.07,0.55] | |
| Panel c: All peers/partners invited by index participant by month 9 | HCT (n=406) | Choice arm (n=701) | ||
|---|---|---|---|---|
| Binary outcome variables | Proportion of outcome | Risk difference | Risk ratio | |
| Male* | 0.19 | 0.26 | 0.07 [0.02,0.12] | 1.38 [1.03,1.90] |
| Sexual partner* | 0.04 | 0.08 | 0.04 [0.01,0.07] | 1.93 [1.12,3.79] |
| Came in for a study visit | 0.47 | 0.60 | 0.12 [0.06,0.18] | 1.26 [1.08,1.42] |
| Used at least one kit/card from index | 0.28 | 0.56 | 0.28 [0.22,0.33] | 1.98 [1.62,2.49] |
| Had at least one positive test result | 0.02 | 0.02 | 0.01 [-0.01,0.02] | 1.41 [0.58,5.31] |
| Mean/count outcome variables | Mean of outcome | Mean/count differences | ||
| Age* | 23.9 | 24.0 | 0.12 [-1.00,1.20] | |
| Panel d: Peers/partners who came in for a clinic visit for study | HCT (n=193) | Choice arm (n=417) | ||
|---|---|---|---|---|
| Binary outcome variables | Proportion of outcome | Risk difference | Risk ratio | |
| Male | 0.17 | 0.26 | 0.09 [0.02,0.16] | 1.51 [1.04,2.31] |
| Sexual partner* | 0.05 | 0.07 | 0.02 [-0.02,0.07] | 1.43 [0.72,3.66] |
| Ever previously tested | 0.85 | 0.89 | 0.04 [-0.02,0.10] | 1.05 [0.97,1.13] |
| Used at least one kit/card from index | 0.59 | 0.93 | 0.34 [0.28,0.40] | 1.58 [1.37,1.85] |
| Had at least one positive test result | 0.04 | 0.04 | 0.00 [-0.03,0.04] | 1.12 [0.46,4.22] |
| Mean/count outcome variables | Mean of outcome | Mean/count differences | ||
| Age (indicated by PP) | 25.4 | 24.5 | -0.86 [-2.32,0.60] | |
| Years of school | 11.9 | 12.1 | 0.18 [-0.03,0.40] | |
Unless otherwise noted, all data are self-reported from the peer/partner. * Data are indicated by the index participant.
By the 9-month visit, 84% of index participants in the choice arm had at least one peer/partner report testing for HIV vs. 44% for the HCT arm (RR 1.93, 95% CI 1.58,2.34) (Table 2). This is not only due to simply inviting more peers and partners; peers and partners who were invited by choice arm index cases were individually more likely to enroll in the trial (RR 1.26, 95% CI 1.08,1.48). Overall by 9-months, of those peers and partners who came in for a study visit, 93% of choice arm peer/partners reported having tested compared to 59% peer/partners in the HCT arm (RR 1.58, 95% CI 1.37-1.85) (Table 3).
3.1.2. Experiences with HIV self-testing
Among the index HIVST users, when asked what they did after getting their test result at the 3-month visit, of those that reported testing positive (n=4) most reported calling a friend or relative, talking to a friend or relative, or getting a confirmatory test. Of those who tested negative, most (n=66) reported doing nothing/ not talking to anyone and 23 said they talked to a friend, family member or partner. Thirty-three percent (41/125) reported needing someone to talk to during the HIVST however only 6 reported calling the toll-free study number provided– most (n=63) reported not calling the number because they did not want to talk to the study counselor. Ninety-percent (113/125) reported feeling they received the support they needed to use the HIVST. Of these, friends and family (n=40), the online video (n=28) and partners (n=23) were reported to be the most helpful sources of support. Eighty-one percent of women who received HIV self-test kits reported talking to a partner about HIV testing at the 3 month visit and 36% reported that they offered the kit to someone who refused to use it.
When asked about the circumstances around taking the test, the majority (94% (n=117/125) reported conducting the test at their home or where they live; only 6 reported testing at their sex partner's house. Most reported testing alone (55%, n=69/125), 16 (13%) with a partner, 15 (12%) with a sister, 8 (6%) reported a friend, 6 (5%) their mother, and 5 (5%) another relative. Of those testing with someone present (43%, n=54/125), the majority reported it made testing easier (89%, 48/54). Of these, the main reasons they reported it being easier were because they felt safer and less scared with someone present (n=17) and the person supported them during the test (n=15)
Four percent (n=12/287) of index participants reported testing positive during the trial, 5 in the HCT arm and 7 in the choice arm, yielding little power to detect risk differences of testing positive ever between the two groups (RD=-0.01, 95% CI -0.05,0.04). Of the 7 individuals reporting testing positive in the choice arm, 4 reported going to the clinic for confirmatory testing. At the 9-month visit, all index participants who reported being HIV negative were offered an observed oral self-test, 14 tested positive (12/14 were HIV ELISA positive at 9 months and 10/14 were HIV ELISA positive at baseline –there were two confirmed seroconversions during the study and two false positives). Six refused the observed oral self-test (3 of whom tested HIV ELISA positive at baseline and 9 months). Of the 9 individuals who reported being HIV positive at 3 or 9 months, all 9 tested HIV ELISA positive at baseline. The 14 who tested positive at 9 months on the observed self-test were evenly distributed by study arm. Interestingly, only 2/7 reported testing during the study in the HCT arm (both reported being negative) while 6/7 repored testing using the self-test kit but 5/6 reported being negative.
4. Discussion
In this randomized trial where young women in rural South Africa were randomized to either HCT or to a choice of using an HIV self-test or going for clinic based HCT. We found that providing participants with a choice of testing modality resulted in significantly more testing events at the follow-up visits with the vast majority (96%) of the choice arm choosing self-testing. At 3-months 92% of young women in the choice/self-test arm compared to 43% in the HCT arm tested for HIV resulting in a risk difference of 49%. We also found that participants in the choice arm were significantly more likely to give test kits/invitations to peers and partners compared to the HCT arm and that those peers and partners were more likely to report testing for HIV. The large difference in testing uptake among the choice/self-test group provides more compelling evidence that providing HIVST to individuals could increase testing uptake significantly not only among those receiving self-test kits but also through secondary distribution to friends, family and partners.
HIV self-testing makes HIV testing more accessible to individuals by reducing barriers that are often cited by individuals as reasons for not testing, including access to clinics (distance, opening hours, waiting times) and confidentiality and privacy [9,10]. While recent national surveys suggest that countries in sub-Saharan Africa (SSA) are making great strides towards reaching the UNAIDS goal of 90% of individuals testing and knowing their status, it has also become apparent that younger people are less likely to have tested than other populations [23]. HIVST is becoming more widely available in many countries in SSA with a number of large demonstration studies underway [24], and a growing number of randomized trials and observational studies have shown that offering HIVST can increase the uptake and frequency of testing although these have been conducted to date among pregnant and post-partum women, female sex workers, truck drivers and MSM rather than the general population [7,25].
We found young women strongly prefered self-testing to HCT: in our choice arm 95% of young women chose the HIVST over the option of attending the clinic. We chose this study design as it simulates the choice individuals might have in the real world if HIVST become available through community distribution, clinics, pharmacies or other means (free of charge). In this study HIVST was the clear favorite over clinic-based testing. While the vast majority of participants in this study reported having tested before and tested in the last 12 months, access to HIVST significantly increased testing rates in this group of young women and among their peers and partners. The later findings being particularly significant for men, who were more likely to uptake HIVST as compared to HCT and who have fewer reasons to attend clinics and thus be exposed to HCT. Women who are pregnant, have small children or seeking contraception are likely to attend clinic regularly and thus have greater to access to HIV testing therefore HIVST programs need to focus identifying non-clinic attending young women. It is notable that the difference between the HIVST and HCT arm decreased over time; the largest difference in testing was in the first 3 months highlighting that young people, especially young women, may test at clinics when they access other services over the year. HIVST fills a particular gap for those individuals who do not attend clinics regularly or who are worried about recent exposure to the virus; with the rollout of Pre Exposure Prophylaxis (PrEP) the need for more rapid and on demand testing may increase and HIVST may help fill this important gap.
Distribution of HIVST and HCT invitations to male partners in this population was less than we had hoped but by 9 months 23% of women in the choice/self test arm had given an HIVST kit to a male partner compared to 8% giving an invitation to test in the HCT arm. Other studies of HIVST distribution to partners have found much higher levels of distribution to partners; however, these populations have been among pregnant women (ages 18-39), female sex workers (FSW) and men who have sex with men (MSM) [13,14,15]. In Kenya, 91% of women attending ANC who had a primary partner distributed kits to their partner, and 75% of FSWs distributed to commercial sex clients [15]. In a similar study in Kisumu, the same authors found that 91% of women (again ANC and FSW) reported partner HIV testing in the HIVST group compared to women who invited partners to come to the clinic to test [14]. In a study among MSM in South Africa, 65% gave a test kit to a partner [13]. Differences in distribution rates to partners in our study may be because these are younger women (18-26 years) who were not married or cohabiting with partners and thus may not have been as comfortable providing kits to their partners. Interestingly, 81% of young women who used the HIVST reported talking to their partner about HIV testing after using the HIVST so it is possible testing might encourage male partners to test down the line. Given that this population is one of the highest risk in terms of HIV incidence, it is encouraging that these young women were willing to test and distribute kits to their female friends and family; however given somewhat limited distribution to partners, finding other strategies to reach men with HIV testing, such as direct distribution of self-tests to men in places where they gather and taking testing services to men, is essential.
We did not have any reports of adverse events as a result of secondary distribution of kits to peers and partners. That said, we did advise women not to provide kits to partners where there was a history of violence or if they worried providing a kit might insite violence. Interestingly, 36% of young women reported that they offered the HIVST kit to someone who refused it, so it is possible that male partners were offered kits and refused to use them.
A key rationale for HIVST is to reach those who do not test regularly and do not want or are unable to attend routine testing services. At the 9-month visit, 9 individuals self-reported having tested positive for HIV during the study and another 12 individuals tested positive on the DBS. The majority of those testing positive in the self-test arm did report having tested during the study while few individuals in the HCT arm reported testing. It is most likely those who tested were not comfortable reporting that they were HIV positive to the interviewer given the majority were positive at baseline based on DBS results.
Limitations of the study include the fact that testing uptake are based on self-report. We did try to collect the invitations for HCT from the clinics (we provided boxes for the counselors to put invitations in and all invitations were linked with a unique ID number) but very few individuals returned these invitations to clinics. It is certainly possible that individuals overreported testing, however, there is no reason to believe reporting between arms would be differential. In addition, the self-report of prior HIV testing was very high among index cases and peer/partners thus results may be different in a population with less testing experience. Strengths of this study include that it was randomized, removing potential selection bias from those that might opt to enroll in a self-test study (perhaps they are more likely to test than those not choosing to be in a study). In addition, we followed up with peers and partners who were provided test kits/invitations to determine their experiences with the test.
Given a choice, young women significantly preferred self-testing to clinic testing, and having chosen self-testing, they tested at much higher rates and provided test kits to more of their peers and partners than those being offered standard clinic-based HCT. Future research should pursue the best ways to reach young men and those not in stable partnerships with HIVST and other novel testing approaches that reduce wait times and increase confidentiality. While those that test positive must still attend clinics for confirmatory testing and to access treatment, allowing individuals to test at their convenience and on their own terms can be empowering for young people and will reach more populations in need of routine HIV testing.
Declaration of Competing Interests
Dr. Wagner reports grants from National Institutes of Health during the conduct of the study. There are no other conflicts of interest to declare.
Acknowledgments
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under award number R01HD083033. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Institutes of Health.
We thank all of the study participants who provided their time and commitment to the study.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100279.
Appendix. Supplementary materials
References
- 1.Shisana O., Rehle T., Simbayi L.C., et al. South African National HIV Prevalence, Incidence and Behaviour Survey, 2012. [DOI] [PubMed]
- 2.UNAIDS. Global AIDS Update 2016. 2016. http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf. Accessed December 14, 2017.
- 3.South Africa Demographic and Health Survey 2016 Key Indicators Report. Pretoria; 2016. https://www.dhsprogram.com/pubs/pdf/PR84/PR84.pdf. Accessed November 27, 2017.
- 4.Zanoni B.C., Archary M., Buchan S., Katz I.T., Haberer J.E. Systematic review and meta-analysis of the adolescent HIV continuum of care in South Africa: the Cresting Wave. BMJ Glob Heal. 2016 doi: 10.1136/bmjgh-2015-000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marks G., Crepaz N., Janssen R.S. Estimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS. 2006 doi: 10.1097/01.aids.0000233579.79714.8d. [DOI] [PubMed] [Google Scholar]
- 6.Pinkerton S.D., Holtgrave D.R., Galletly C.L. Infections prevented by increasing HIV serostatus awareness in the United States, 2001 to 2004. J Acquir Immune Defic Syndr. 2008 doi: 10.1097/QAI.0b013e318160d57e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson C.C., Kennedy C., Fonner V. Examining the effects of HIV self-Testing compared to standard HIV testing services: a systematic review and meta-Analysis. J Int AIDS Soc. 2017 doi: 10.7448/IAS.20.1.21594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choko A.T., Desmond N., Webb E.L. The Uptake and Accuracy of Oral Kits for HIV Self-Testing in High HIV Prevalence Setting: A Cross-Sectional Feasibility Study in Blantyre, Malawi. Bangsberg DR, ed. PLoS Med. 2011;8(10) doi: 10.1371/journal.pmed.1001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson C., Baggaley R., Forsythe S. Realizing the potential for HIV self-testing. AIDS Behav. 2014 doi: 10.1007/s10461-014-0832-x. [DOI] [PubMed] [Google Scholar]
- 10.Mavedzenge S.N., Baggaley R., Corbett E.L. A review of self-testing for HIV: Research and policy priorities in a new era of HIV prevention. Clin Infect Dis. 2013 doi: 10.1093/cid/cit156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choko A.T., MacPherson P., Webb E.L. Uptake, Accuracy, Safety, and Linkage into Care over Two Years of Promoting Annual Self-Testing for HIV in Blantyre, Malawi: A Community-Based Prospective Study. PLoS Med. 2015 doi: 10.1371/journal.pmed.1001873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mavedzenge S.N., Sibanda E., Mavengere Y. Supervised HIV self-testing to inform implementation and scale up of self-testing in Zimbabwe. J Int AIDS Soc. 2015;18(Suppl. 4):96. [Google Scholar]
- 13.Lippman S.A., Lane T., Radebe High Acceptability and Increased HIV-Testing Frequency After Introduction of HIV Self-Testing and Network Distribution Among South African MSM. JAIDS. 2018;77(3):279–287. doi: 10.1097/QAI.0000000000001601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masters S.H., Agot K., Obonyo B., Napierala Mavedzenge S., Maman S., Thirumurthy H. Promoting Partner Testing and Couples Testing through Secondary Distribution of HIV Self-Tests: A Randomized Clinical Trial. PLoS Med. 2016 doi: 10.1371/journal.pmed.1002166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thirumurthy H., Masters S.H., Mavedzenge S.N., Maman S., Omanga E., Agot K. Promoting male partner HIV testing and safer sexual decision making through secondary distribution of self-tests by HIV-negative female sex workers and women receiving antenatal and post-partum care in Kenya: a cohort study. Lancet HIV. 2016 doi: 10.1016/S2352-3018(16)00041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ortblad K., Kibuuka Musoke D., Ngabirano T. Direct provision versus facility collection of HIV self-tests among female sex workers in Uganda: A cluster-randomized controlled health systems trial. PLoS Med. 2017;14(11) doi: 10.1371/journal.pmed.1002458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chanda M.M., Ortblad K.F., Mwale M. HIV self-testing among female sex workers in Zambia: A cluster randomized controlled trial. PLoS Med. 2017 21;14(11) doi: 10.1371/journal.pmed.1002442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnold B.F., Hogan D.R., Colford J.M., Hubbard A.E. Simulation methods to estimate design power: An overview for applied research. BMC Med Res Methodol. 2011 doi: 10.1186/1471-2288-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pustejovsky J.E., Tipton E. Small-Sample Methods for Cluster-Robust Variance Estimation and Hypothesis Testing in Fixed Effects Models. J Bus Econ Stat. 2018 doi: 10.1080/07350015.2016.1247004. [DOI] [Google Scholar]
- 20.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2019. R: A language and environment for statistical computing.https://www.R-project.org/ URL. [Google Scholar]
- 21.Tomas J. Aragon. Epitools: Epidemiology Tools. R packageversion 0.5-10. 2017. URLhttps://CRAN.R-project.org/package=epitools
- 22.G. Blair, J. Cooper, A. Coppock, M. Humphreys and Luke Sonnet. estimatr: Fast Estimators for Design-Based Inference. 2019. R Package version 0.20.0. https://CRAN.R-project.org/package=estimatr
- 23.Riggs M.A., Philip N.M., Williams D.B. Status of HIV Epidemic Control Among Adolescent Girls and Young Women Aged 15–24 Years — Seven African Countries, 2015–2017. MMWR Morb Mortal Wkly Rep. 2018 doi: 10.15585/mmwr.mm6701a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neuman M., Indravudh P., Chilongosi R. The effectiveness and cost-effectiveness of community-based lay distribution of HIV self-tests in increasing uptake of HIV testing among adults in rural Malawi and rural and peri-urban Zambia: Protocol for STAR (self-testing for Africa) cluster randomized evaluations. BMC Public Health. 2018 doi: 10.1186/s12889-018-6120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katz D., Golden M., Hughes J.P., Farquhar C., Stekler J. HIV Self-Testing Increases HIV Testing Frequency in High-Risk Men Who Have Sex With Men: A Randomized Controlled Trial. J Acquir Immune Defic Syndr. 2018;78(5):505–512. doi: 10.1097/QAI.0000000000001709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.