The spirochete Borrelia burgdorferi sensu lato is the causative agent of Lyme disease (LD). The spirochetes produce the CspZ protein that binds to a complement regulator, factor H (FH). Such binding downregulates activation of host complement to facilitate spirochete evasion of complement killing. However, vaccination with CspZ does not protect against LD infection. In this study, we demonstrated that immunization with CspZ-YA, a CspZ mutant protein with no FH-binding activity, protected mice from infection by several spirochete genotypes introduced via tick feeding.
KEYWORDS: Borrelia, CspZ, Lyme disease, vaccine
ABSTRACT
The spirochete Borrelia burgdorferi sensu lato is the causative agent of Lyme disease (LD). The spirochetes produce the CspZ protein that binds to a complement regulator, factor H (FH). Such binding downregulates activation of host complement to facilitate spirochete evasion of complement killing. However, vaccination with CspZ does not protect against LD infection. In this study, we demonstrated that immunization with CspZ-YA, a CspZ mutant protein with no FH-binding activity, protected mice from infection by several spirochete genotypes introduced via tick feeding. We found that the sera from CspZ-YA-vaccinated mice more efficiently eliminated spirochetes and blocked CspZ FH-binding activity than sera from CspZ-immunized mice. We also found that vaccination with CspZ, but not CspZ-YA, triggered the production of anti-FH antibodies, justifying CspZ-YA as an LD vaccine candidate. The mechanistic and efficacy information derived from this study provides insights into the development of a CspZ-based LD vaccine.
INTRODUCTION
Lyme disease (LD) is the most common vector-borne disease in both North America and Europe. In the United States, an estimated 300,000 individuals develop new cases each year, and in the past 20 years, the number of reported cases has drastically increased by over 200% (1). The Centers for Disease Control and Prevention (CDC) recently included LD in the top zoonotic disease of national concern, with an annual economic burden over $700,000,000 in the United States (2). The only commercially available human LD vaccine was withdrawn from the market nearly 20 years ago due to limited efficacy and perceived safety concerns, and there is still no human vaccine against the pathogenic spirochete that causes this disease, Borrelia burgdorferi sensu lato (3–5). Of all the Lyme borreliae, B. burgdorferi sensu stricto (here B. burgdorferi), B. garinii, and B. afzelii are the three species that cause the majority of infections in North America, Europe, and Asia (1, 6). Lyme borreliae are transmitted to humans during the feeding of the hard tick vector Ixodes, after which the spirochetes colonize the feeding site of the skin and disseminate through the bloodstream to distal tissues and organs, causing symptoms, including erythema migrans, acrodermatitis chronica atrophicans, arthritis, carditis, and neuroborreliosis (7, 8).
In order to traverse the bloodstream, Lyme borreliae must survive the vertebrate host innate immune defenses located in the blood, including complement (9–11) (for reviews, see references 12, 13, and 14). This system is composed of three distinct pathways, the classical, the lectin, and the alternative pathway. The classical pathway is initiated by antigen-antibody interactions, the lectin pathway is activated after recognition of microbial carbohydrates, and the alternative pathway is triggered by the binding of complement protein C3b to the pathogen surface. All three pathways ultimately result in pathogen lysis or phagocytosis. To prevent self-damage of human cells and tissues in the absence of microbial pathogens, complement is kept in check by a number of cell-associated and fluid phase complement regulatory proteins (CRP), including factor H (FH), which modulates the alternative pathway by inactivating C3b (12–14). Lyme borreliae encode several CRP-binding proteins (9–11), one of which is the outer surface FH-binding protein CspZ (15, 16). CspZ is not produced when spirochetes are in the tick vector but rather is upregulated when spirochetes reside in mammalian hosts (17). When bound by CspZ, the FH regulatory activity is maintained, and complement activation is inhibited on the spirochete surface to promote infectivity (15, 18, 19).
While a minority of individual Lyme borrelia strains do not harbor cspZ, patients across North America and Europe with confirmed LD develop antibodies against CspZ, suggesting the strains that cause human infections produce this antigen (20–22). These results support previous findings that CspZ is present in multiple Lyme borrelia strains that cause human infection (20–24). Further, CspZ is highly conserved among different variants, even among those that do not bind human FH, emphasizing this protein as an attractive vaccine target (20, 21, 25). Surprisingly, vaccination with the wild-type (WT) CspZ from B. burgdorferi strain B31 does not protect mice from spirochete colonization (22, 26, 27). We recently generated a CspZ mutant protein, CspZ-Y207A/Y211A (CspZ-YA, derived from that WT variant), and conjugated this protein to a virus-like particle (VLP). We found that this conjugated CspZ protein prevents infection in mice after needle inoculation of B. burgdorferi B31-A3 (27). These findings elicit several intriguing questions: does this regimen also protect against infections caused by tick-transmitted Lyme borreliae, and what is the protective mechanism that differentiates the efficacy between CspZ and CspZ-YA? In this study, we examined this CspZ-based vaccine in protecting against multiple strains of tick-transmitted Lyme borreliae and elucidated the potential mechanisms that allow this vaccine to be efficacious against LD.
RESULTS
Vaccination with CspZ-YA but not CspZ prevented spirochete colonization and arthritis after tick transmission of B. burgdorferi strain B31-A3.
We first examined the ability of the CspZ-YA vaccine to protect mice from tissue colonization of B. burgdorferi strain B31-A3 after tick feeding, the natural infection route of LD. Mice were inoculated and subsequently given two boosters of CspZ-YA or WT CspZ or the same proteins conjugated to VLP (VLP-CspZ-YA or VLP-CspZ) (see Fig. S1 in the supplemental material). Mice inoculated with VLP or phosphate-buffered saline (PBS) were included as controls, as these offer no protection against B. burgdorferi infection (Fig. S1). Ixodes scapularis nymphal ticks carrying B. burgdorferi B31-A3 were allowed to feed on the immunized mice, and the levels of spirochete colonization were determined at 21 days postfeeding (dpf) (Fig. S1).
As expected, PBS- or VLP-inoculated mice fed on by nymphs carrying B. burgdorferi strain B31-A3 had at least 30-fold-higher spirochete burdens at the tick feeding sites of the skin, bladder, knees, and ears than the negative-control, PBS-inoculated mice fed on by naive nymphs (Fig. 1). After being fed on by nymphs carrying strain B31-A3, mice vaccinated with VLP-CspZ developed bacterial loads indistinguishable from those mice inoculated with CspZ in all tested tissues, and all tissues tested in both groups had bacterial loads that were at least 30-fold greater than those of the negative control (Fig. 1). These results suggest that CspZ conjugation to VLP is dispensable for preventing spirochete colonization caused by tickborne transmission and also suggest the inability of CspZ as a vaccine to prevent spirochete colonization regardless of VLP conjugation. In contrast, after nymphs carrying strain B31-A3 fed on mice immunized with CspZ-YA or VLP-CspZ-YA, spirochete levels were indistinguishable from those in negative-control mice for all tissues tested (Fig. 1). These results clearly indicate the efficacy of CspZ-YA as a vaccine to prevent spirochete colonization, as well as the unessential role of VLP conjugation of CspZ-YA to prevent spirochete colonization via tick transmission.
FIG 1.
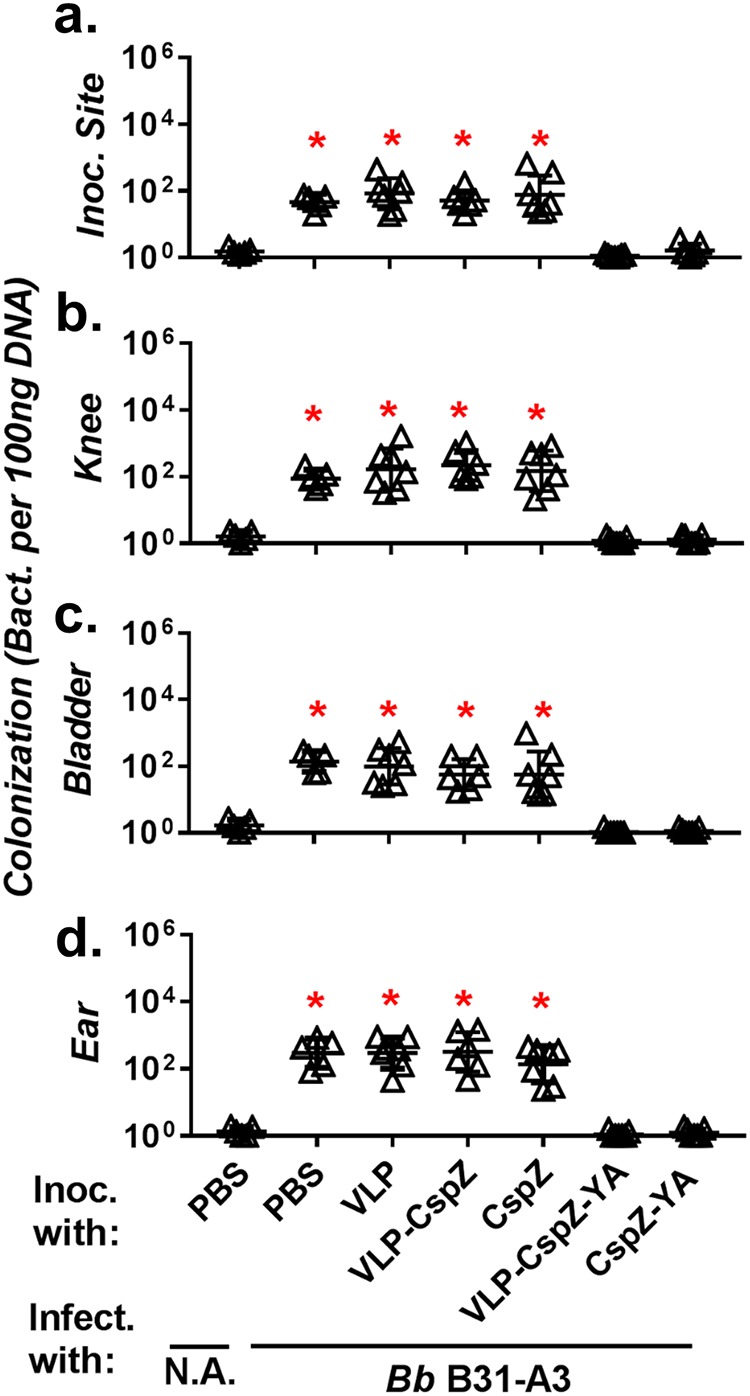
Vaccination with VLP-CspZ-YA or CspZ-YA protected mice from borrelial tissue colonization caused by tick feeding. Five PBS-inoculated C3H/HeN mice, six VLP-CspZ-, VLP-CspZ-YA-, or CspZ-YA-inoculated C3H/HeN mice, or seven VLP- or CspZ-inoculated C3H/HeN mice were fed on by nymphs carrying B. burgdorferi strain B31-A3. Uninfected nymphs were placed on an additional five mice inoculated with PBS as a negative control (N.A.). Spirochete burdens at the tick feeding site (Inoc. Site) (a), knees (b), bladder (c), and ears (d) were quantitatively measured at 21 dpf and are shown as the number of spirochetes per 100 ng total DNA. Data shown are the geometric mean ± geometric standard deviation of the spirochete burdens from each group of mice. Asterisks indicate the statistical significance (P < 0.05, Kruskal-Wallis test with Dunn’s multiple comparisons) of differences in bacterial burden relative to that of uninfected mice.
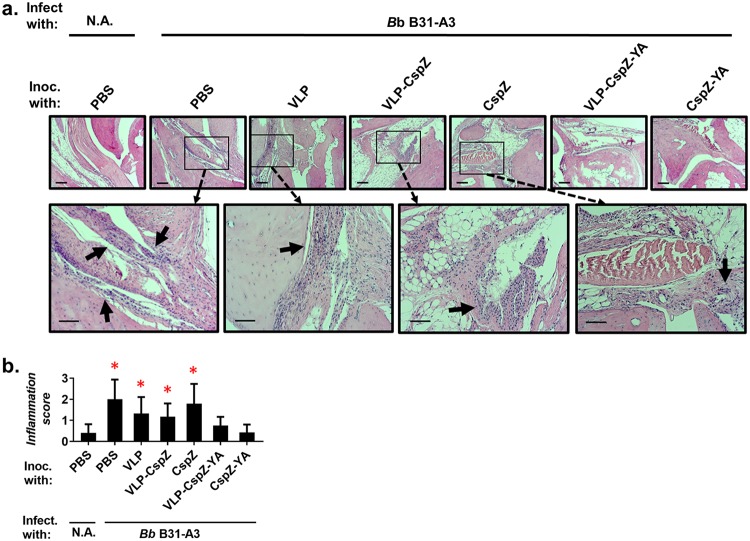
We also determined if vaccination with VLP-CspZ-YA or CspZ-YA prevents arthritis caused by tick-transmitted B. burgdorferi B31-A3. After immunization and infection of mice (Fig. S1), the tibiotarsus joints were histologically accessed for arthritic severity. The PBS- or VLP-inoculated mice reflected severe infiltration of inflammatory cells (Fig. 2a, arrows), whereas the negative-control mice did not (Fig. 2a). Similarly, the joints from the mice immunized with VLP-CspZ or CspZ also showed substantial cellular infiltration both between and within the connective tissues and muscle (Fig. 2a, arrows). The arthritic severity of the two mouse groups, as judged by the infiltration of inflammatory cells, was scored similarly (Fig. 2b). Conversely, the joints of the mice vaccinated with VLP-CspZ-YA or CspZ-YA displayed no detectable signs of inflammation, with the score indistinguishable from that of the negative-control mice (Fig. 2b). Taken together, these results indicate that CspZ-YA vaccination protects mice from LD-associated arthritis after tickborne transmission of spirochetes, and VLP conjugation of this antigen is irrelevant for this protection.
FIG 2.
Vaccination of VLP-CspZ-YA- or CspZ-YA-protected mice from LD-associated arthritis caused by tick feeding. Five PBS-inoculated C3H/HeN mice, six VLP-CspZ-, VLP-CspZ-YA-, or CspZ-YA-inoculated C3H/HeN mice, or seven VLP- or CspZ-inoculated C3H/HeN mice were fed on by nymphs carrying B. burgdorferi strain B31-A3. Uninfected nymphs were placed on an additional five mice inoculated with PBS as a negative control (N.A.). Tibiotarsus joints were collected from these mice at 21 dpf. To assess inflammation, tissues were fixed and stained with hematoxylin and eosin. (a) Representative images from one mouse per group are shown. Top panels are lower-resolution images (joint, ×10 magnification [bar, 160 μm]); bottom panels are higher-resolution images (joint, ×20 magnification [bar, 80 μm]) of selected areas (highlighted in top panels). Arrows indicate infiltration of immune cells. (b) To quantitate inflammation of joint tissues, at least 10 random sections of tibiotarsus joints from each mouse were scored on a scale of 0 to 3 for the severity of arthritis. Data shown are the mean inflammation score ± standard deviation of the arthritis scores from each group of mice. Asterisks indicate the statistical significance (P < 0.05, Kruskal-Wallis test with Dunn’s multiple comparisons) of differences in inflammation relative to that of uninfected mice.
CspZ-YA vaccination protected mice from tissue colonization and arthritis caused by tickborne transmission of multiple Lyme borrelia strains.
As VLP conjugation is dispensable for CspZ-YA in protecting against tickborne spirochete infection, we assessed whether unconjugated CspZ-YA protects against infection caused by additional Lyme borrelia strains. Mice were immunized with CspZ-YA or PBS (control) in the same fashion as described above, followed by challenge with nymphal ticks carrying B. burgdorferi strain 297 or B. afzelii strain VS461-JL (Fig. S1). These strains encode CspZ with 96 or 74% amino acid identity, respectively, to the variant from B. burgdorferi strain B31-A3 (Fig. S2). Whereas CspZ from strain VS461-JL displayed human and mouse FH-binding activity (Fig. S3), CspZ from strain 297 appears to not bind human or mouse FH (21, 22). Therefore, these strains were used, as they represent pathogenic genotypes of Lyme borreliae harboring CspZ variants with or without FH-binding activity.
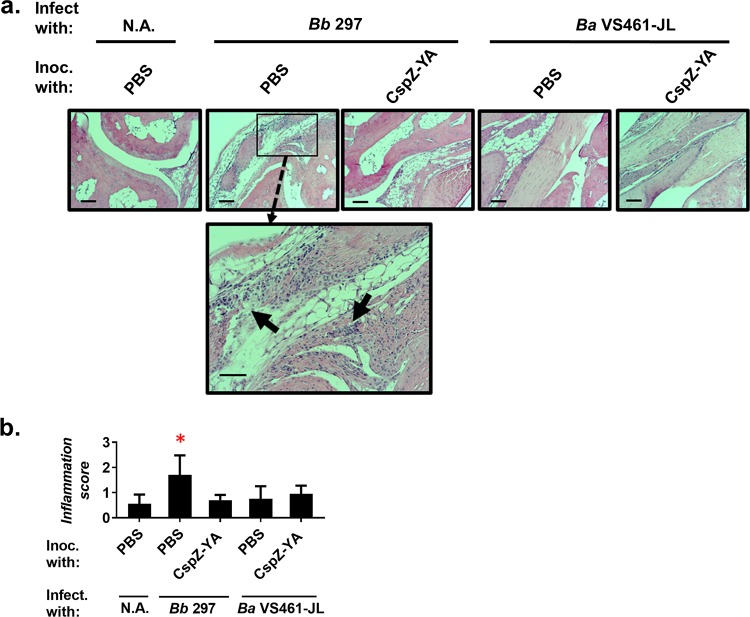
At 21 days post-initial immunization (dpii), the PBS-inoculated mice fed on by ticks carrying B. burgdorferi strain 297 had at least 172-fold-higher spirochete burdens than the PBS-inoculated mice fed on by uninfected nymphs (negative-control mice) at the tick feeding sites of the skin, bladder, knees, and ears (Fig. 3). These PBS-inoculated, strain 297-challenged mice displayed apparent cellular infiltrations between the connective tissues and muscle of the tibiotarsus joints, with arthritic inflammation scored as high as 2 (Fig. 4). In contrast, after exposure to ticks carrying strain 297, the spirochete burdens of CspZ-YA-immunized mice were no greater than those of the negative-control mice for all tested tissues (Fig. 3). Moreover, no visual signs of arthritis were identified in the CspZ-YA-immunized mice (Fig. 4). Similarly, the PBS-inoculated mice fed on by nymphs carrying strain VS461-JL had at least 261-fold-greater bacterial loads in all tested tissues than the negative-control mice (Fig. 3). After exposure to nymphs harboring strain VS461-JL, the spirochete burdens in CspZ-YA-immunized mice were no different from those in the negative-control mice (Fig. 3). Surprisingly, the PBS-inoculated mice fed on by ticks carrying strain VS461-JL did not show any signs of arthritis, with scores similar to those of both negative-control and CspZ-YA-immunized mice (Fig. 4). These results reflect the fact that not every Lyme borrelia strain causes arthritis (28). Taken together, vaccination with CspZ-YA is capable of preventing tissue colonization by multiple spirochete strains, as well as alleviating arthritis caused by the strains that trigger such a manifestation.
FIG 3.
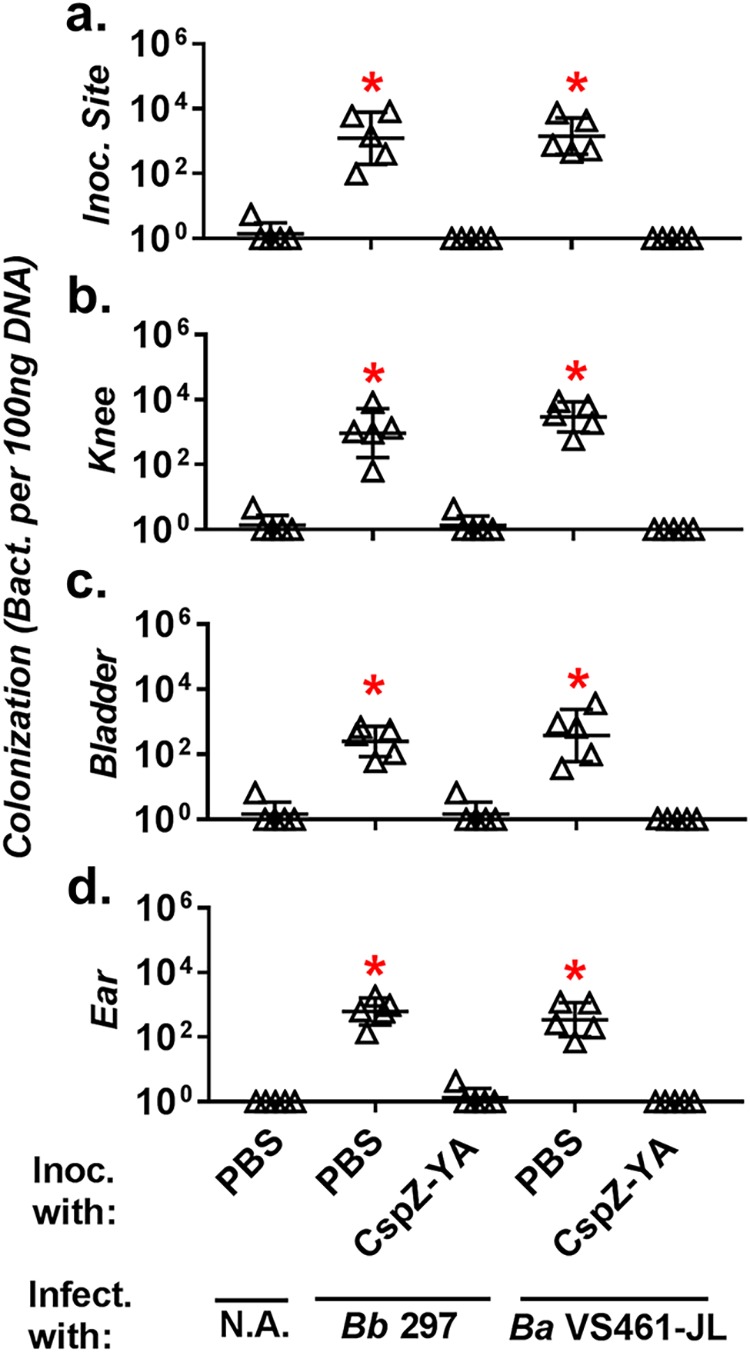
Immunization with CspZ-YA prevented tissue colonization after exposure to ticks carrying multiple Lyme borrelia strains. Five C3H/HeN mice were vaccinated with CspZ-YA or inoculated with PBS and subsequently infected by nymphal ticks carrying B. burgdorferi strain 297 or B. afzelii strain VS461-JL. Uninfected nymphs were placed on an additional five mice inoculated with PBS as a negative control (N.A.). Spirochete burdens at the tick feeding site (Inoc. Site) (a), knees (b), bladder (c), and ears (d) were quantitatively measured at 21 dpf and are indicated as the number of spirochetes per 100 ng total DNA. Data shown are the geometric mean ± geometric standard deviation of the spirochete burdens from each group of the mice. Asterisks indicate the statistical significance (P < 0.05, Kruskal-Wallis test with Dunn’s multiple comparisons) of differences in bacterial burden relative to that of uninfected mice.
FIG 4.
Immunization with CspZ-YA prevented LD-associated arthritis after exposure to ticks carrying multiple Lyme borrelia strains. Five C3H/HeN mice were vaccinated with CspZ-YA or inoculated with PBS and subsequently infected by nymphal ticks carrying B. burgdorferi strain 297 or B. afzelii strain VS461-JL. Uninfected nymphs were placed on an additional five mice inoculated with PBS as a negative control (N.A.). Tibiotarsus joints were collected from these mice at 21 dpf. To assess inflammation, tissues were fixed and stained with hematoxylin and eosin. (a) Representative images from one mouse per group are shown. Top panels are lower-resolution images (joint, ×10 magnification [bar, 160 μm]); bottom panels are higher-resolution images (joint, ×20 magnification [bar, 80 μm]) of selected areas (highlighted in top panels). Arrows indicate infiltration of immune cells. (b) To quantitate inflammation of joint tissues, at least 10 random sections of tibiotarsus joints from each mouse were scored on a scale of 0 to 3 for the severity of arthritis. Data shown are the mean inflammation score ± standard deviation of the arthritis scores from each group of mice. Asterisks indicate the statistical significance (P < 0.05, Kruskal-Wallis test with Dunn’s multiple comparisons) of differences in inflammation relative to that of uninfected mice.
Sera from CspZ-YA-immunized mice more efficiently eradicated multiple Lyme borrelia strains than sera from CspZ-vaccinated mice.
To identify the protective mechanism of the CspZ-YA vaccine, we sought to determine the burdens of B. burgdorferi strain B31-A3 or 297 or B. afzelii strain VS461-JL in the fully engorged nymphs that fed on PBS- or CspZ-YA-inoculated mice. Compared to flat nymphs, spirochete burdens increased in ticks after feeding regardless of spirochete strain or mouse vaccination, as expected (Fig. S4) (29). There was no difference in spirochete burden in any replete nymphs regardless of vaccination or spirochete strain (Fig. S4). In support of the fact that cspZ is not expressed when spirochetes reside in ticks (17), this result suggests that CspZ-YA vaccination does not target spirochetes in nymphs as a protective mechanism.
We next compared the titers of anti-CspZ IgG and IgM (prior to tick feeding) (Fig. S1) in mice vaccinated with CspZ-YA or CspZ (which did not protect mice from infection). We found that the anti-CspZ IgM and IgG titers were indistinguishable between these two groups, and these titers were 5- to 60-fold greater, respectively, than those of the negative-control mice (Fig. S5). These results are consistent with the previous observation that these proteins, when conjugated to VLP, induce similar levels of IgM and IgG against CspZ (27). We also determined the titers of different IgG subclasses in sera from CspZ- or CspZ-YA-immunized mice and detected 10- to 45-fold-greater titers than in the negative-control mouse sera (Fig. S5). There was no difference between the levels of IgG1, IgG2a, and IgG2b in the two vaccination groups (Fig. S5). These results suggest that the quantity of antibodies against CspZ may not be the determining factor to differentiate the efficacies of CspZ and CspZ-YA immunization.
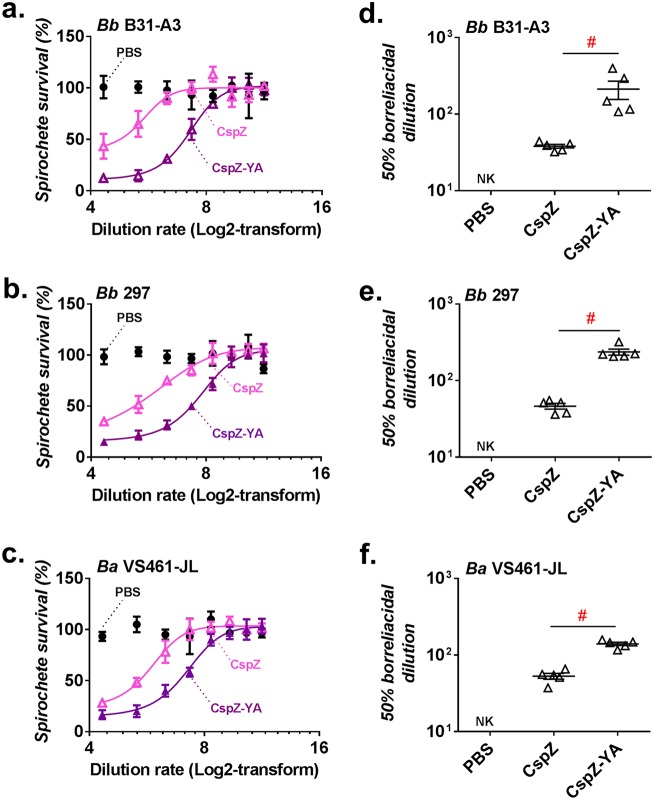
We further assessed the ability of sera from CspZ- or CspZ-YA-immunized mice to kill spirochetes in vitro. Both sera were capable of eradicating B. burgdorferi strain B31-A3; however, the CspZ-YA sera displayed 5.5-fold-more efficient killing than the CspZ sera (Fig. 5a and d; Table S1). These results agree with our previous findings with mice immunized with these proteins conjugated to VLP (27). We further incubated those sera with B. burgdorferi strain 297 or B. afzelii strain VS461-JL. The sera from the CspZ-YA-vaccinated mice killed these strains 5- and 2.6-fold more efficiently than the sera from CspZ-vaccinated mice, respectively (Fig. 5b, c, e, and f; Table S1). These results indicate that sera from CspZ-YA-vaccinated mice display spirochete killing that is superior to that from CspZ-immunized mice.
FIG 5.
Sera from mice immunized with CspZ-YA had more robust levels of borreliacidal activity than those from CspZ-vaccinated mice. Sera from five C3H/HeN mice inoculated with CspZ, CspZ-YA, or PBS (negative control) were obtained at 42 dpii. Sera were serially diluted as indicated and mixed with guinea pig complement and B. burgdorferi strain B31-A3 (a and d) or 297 (b and e) or with B. afzelii strain VS461-JL (c and f) (5 × 105 cells ml−1). After incubation for 24 h, surviving spirochetes were quantified from three fields of view for each sample by using dark-field microscopy. The work was performed in three independent experiments. (a to c) The survival percentage was derived from the proportion of serum-treated to untreated spirochetes. Data shown are the mean ± standard error of the mean (SEM) of the survival percentage from three replicates in one representative experiment. (d to f) The 50% borreliacidal dilution of each serum sample, representing the dilution rate that effectively killed 50% of spirochetes, was obtained from curve-fitting and extrapolation of data from panels A to C. Data shown are the mean ± SEM of the borreliacidal titers from three experiments. The exact values are shown in Table S1 in the supplemental material. PBS-vaccinated mouse sera displayed no bactericidal activity (no killing [NK]). Statistical significance (P < 0.05, Mann-Whitney test) of differences in borreliacidal titers between groups are indicated (#).
Sera from CspZ-YA- but not CspZ-vaccinated mice blocked the CspZ FH-binding activity.
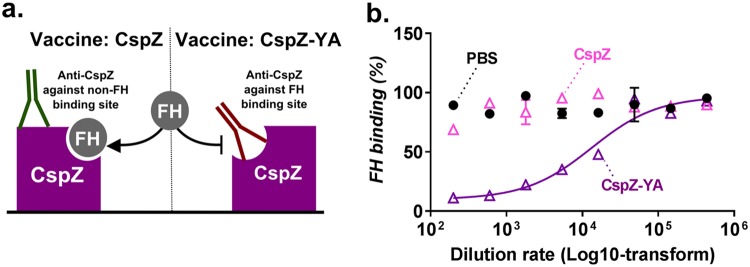
We next examined whether there are differences in epitope recognition between the sera from mice immunized with CspZ-YA or CspZ. The fact that CspZ but not CspZ-YA binds to FH raises the possibility that the sera from mice immunized with the latter protein recognize epitopes within the CspZ FH-binding site and block the FH-binding activity. To test this possibility, we coated microtiter wells with CspZ and then incubated these wells with serially diluted sera from CspZ-YA-, CspZ-, or PBS-inoculated mice. CspZ-coated wells incubated with PBS were included as a control. Following the addition of human FH to the wells, the levels of FH immobilized by CspZ in each reaction were normalized to the control wells to determine the percent FH binding (Fig. 6a). As expected, the levels of FH bound by CspZ were not impacted in the presence of sera from the PBS-inoculated mouse serum (Fig. 6b). Whereas the addition of sera from CspZ-vaccinated mice did not reduce the levels of FH bound by CspZ, our results clearly indicate that sera from CspZ-YA-vaccinated mice decreased the levels of FH bound to CspZ (50% inhibitory dilution rate, 1:16,072 ± 1,595) (Fig. 6b). These results suggest that the sera from CspZ-YA- but not CspZ-immunized mice contain antibodies that recognize the epitopes within the CspZ FH-binding site.
FIG 6.
Sera from CspZ-YA- but not CspZ-vaccinated mice blocked FH binding to CspZ. Sera from five C3H/HeN mice inoculated with CspZ, CspZ-YA, or PBS (negative control) were obtained at 42 dpii. (a) Schematic diagram showing the experimental setup. GST-CspZ was added to a microtiter plate coated with anti-GST, in order to orient CspZ with the FH-binding site exposed. Wells were treated with PBS or the indicated dilution rates of mouse sera and then incubated with human FH. The levels of bound FH were quantified using sheep anti-human FH and goat anti-sheep HRP IgG as primary and secondary antibodies, respectively. The work was performed in three independent experiments; within each experiment, samples were run in triplicate. (b) Data are expressed as the percent FH binding, derived by normalizing the levels of bound FH from mouse serum-treated wells to that in PBS-treated wells. Data shown are the mean ± SEM of the percent FH binding from three replicates in one representative experiment. The dilution rate of the sera to inhibit 50% of FH bound by CspZ was obtained from curve-fitting and extrapolation of data from panel A (50% inhibitory dilution rate, 1:16,072 ± 1,595).
Immunization with CspZ and but not CspZ-YA generated antibodies against mammalian FH.
A meningococcal vaccine based on a Neisseria meningitidis FH-binding protein, FHbp, triggers antibodies against FH from vaccinated individuals (30–32). To determine if a CspZ-based vaccine also induces the production of anti-FH antibodies, we quantified the titers of IgG that recognize human or mouse FH in sera collected from mice at 42 dpii (prior to the infection). As a negative-control group, PBS-inoculated mice developed IgG against either FH variant at titers close to the detection limits (limit of detection = 1 arbitrary unit [AU]) (Fig. 7). The sera from CspZ-vaccinated mice had approximately 10-fold-greater titers of anti-human or anti-mouse FH IgG than the negative-control mice. However, the sera from CspZ-YA-immunized mice had titers comparable to those from negative-control mice (Fig. 7). These results indicate that immunization with CspZ, but not CspZ-YA, triggers antibodies that recognize mammalian FH.
FIG 7.
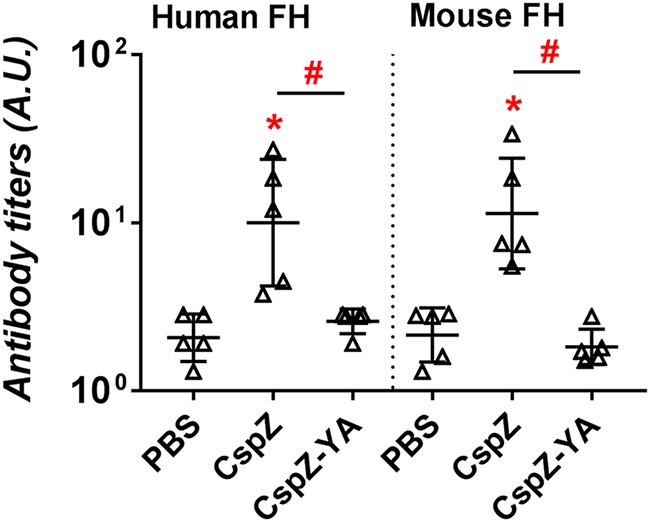
Vaccination with CspZ triggered antibodies against FH. Sera from five C3H/HeN mice inoculated with CspZ, CspZ-YA, or PBS (negative control) at 42 dpii were incubated with microtiter plate wells coated with human or mouse FH. Mouse IgG antibodies bound to FH were detected using goat anti-mouse IgG. Data shown are the geometric mean ± geometric standard deviation of antibody titers from each group of mice. Significant differences (P < 0.05, Mann-Whitney test) in levels of antibody titers relative to that in sera from PBS-inoculated mice (*) or between two groups relative to each other (#) are indicated.
DISCUSSION
The route to introduce arthropod-borne pathogens into hosts often determines the infectivity of these pathogens (33–35). Thus, an antigen that prevents infection via needle inoculation may not necessarily protect against infections transmitted via vectors such as ticks (36, 37). In fact, we previously reported that VLP conjugation enhances the efficacy of CspZ-based antigens in protecting mice after needle inoculation of B. burgdorferi (27). In contrast, we showed in the current study that such a regimen is dispensable for those CspZ proteins in preventing LD-associated spirochete colonization through tick transmission. Such disagreement in vaccine efficacy could be due to the fundamental differences between infection by different routes (e.g., the dosage of spirochetes, the presence of tick-specific proteins, and differential surface protein expression of spirochetes cultivated in vitro and derived from ticks).
In this report, we observed that CspZ-YA vaccination of mice prevents infection caused by ticks carrying different Lyme borrelia strains. This cross-protection via such a physiologically relevant infection route highlights the feasibility of using CspZ-YA as an LD vaccine. It should be noted that we used I. scapularis as a model to examine the efficacy of the CspZ-YA vaccine, allowing us to attribute any differences of efficacy to particular spirochete strains or species. However, the caveat is that some Lyme borreliae (e.g., B. afzelii) are often isolated from Ixodes ricinus but not I. scapularis ticks (1), and vector adaption appears to contribute to the transmission efficiency of spirochetes (38–40). Further investigation of the impact of such a difference for the efficacy of CspZ-YA vaccine is warranted.
Our previous findings that passive immunization with sera from CspZ-YA- but not CspZ-vaccinated mice prevents B. burgdorferi colonization indicate an antibody-dependent protective mechanism for this antigen (27). Consistent with our previous finding using needle infection of Lyme borreliae, CspZ-YA but not CspZ vaccination protects mice from colonization and LD-associated arthritis via tick feeding. These results suggest that the efficacy of a CspZ-based vaccine is dependent on mutating the FH-binding site. FH is present in human blood at concentrations as high as 600 μg ml−1 (41). A physiological range of CspZ’s affinity in binding to mammalian FH (dissociation constant [Kd], ∼10−7 M) suggests a tight association of the two proteins upon vaccination. Such interactions would then prevent the exposure of epitopes within the CspZ FH-binding site. Thus, one model to address the finding of CspZ-YA as a more efficacious vaccine than CspZ is that the antibodies induced by the former but not the latter antigen compete with FH to bind to such epitopes, resulting in the blocking of the spirochetes’ FH-binding-mediated complement evasion. In fact, our finding that sera from CspZ-YA-immunized mice block the binding of FH to CspZ in a dose-dependent manner clearly supports this possibility. It is noteworthy that polyclonal sera from CspZ-YA-immunized mice are used in this study, and a 1:100 dilution of those sera nearly completely inhibited the binding of FH to CspZ. This result suggests that the undiluted sera with a physiological relevant amount/concentration of anti-CspZ antibodies is also capable of inhibiting FH binding to CspZ. Additionally, CspZ-mediated FH-binding activity is allelically variable, despite 98% identity among variants (22). One variant used in this study (CspZ from B. burgdorferi strain 297) appears to not bind to mammalian FH (21, 22), but CspZ-YA-immunized mice were protected from the infection by this spirochete strain. Sera from CspZ-YA-vaccinated mice eliminated B. burgdorferi strain 297 more efficiently than sera from CspZ-immunized mice. Taken together, these results thus suggest an additional protective mechanism of CspZ-YA: the resulting antibodies that recognize the epitopes within the FH-binding site trigger bacterial killing through the classical pathway.
We found that sera from CspZ- but not CspZ-YA-vaccinated mice recognize human and mouse FH, suggesting that CspZ but not CspZ-YA triggers the production of anti-FH antibodies. A previous study indicates that FH’s conformation alters upon its binding by a Streptococcus pneumoniae antigen, PspC (42). These observations lead to a possibility that the formation of an FH neoepitope in the binding interface with CspZ contributes to the induction of anti-FH antibodies. Such antibodies in CspZ-vaccinated mice may prevent FH from downregulating complement, which may exhaust C3 and reduce complement-mediated bactericidal activity, resulting in inefficacious protection of the CspZ vaccination (43, 44, 45). Thus, the lack of anti-FH antibodies in CspZ-YA-vaccinated mice may prevent the exhaustion of complement components, allowing this vaccine to be efficacious. This notion warrants future studies. Further, our finding of anti-FH antibodies induced after CspZ vaccination also leads to another intriguing but unanswered question: do those antibodies promote manifestations mediated by autoimmune responses? In fact, a Neisseria meningitidis FH-binding protein, FHbp, has been used clinically as a human meningococcal vaccine, and humans develop anti-FH antibodies after vaccination. To date, no autoimmune manifestations have been attributed to such antibodies, possibly due to transient and low levels of these antibodies (30). In spite of that, the possibility of autoimmunity triggered by anti-FH antibodies from CspZ vaccination still cannot be excluded, emphasizing the benefit in opting for CspZ-YA as human vaccines from a safety perspective.
In summary, we used a tick infection model to demonstrate the efficacy of vaccination with CspZ-YA, an antigen previously shown to prevent B. burgdorferi colonization and arthritis after needle inoculation. This antigen-mediated prevention can also protect against infections caused by tickborne transmission of other spirochete strains or species that can cause human LD. In addition, we elucidated the potential protective mechanisms of this antigen as well as tested its safety by detecting the levels of anti-FH antibodies. The mechanistic and efficacy information derived from this study will provide insights into antigen engineering to develop a vaccine for LD and can be extended to other pathogens.
MATERIALS AND METHODS
Ethics statement.
All mouse experiments were performed in strict accordance with all provisions of the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals, and the PHS Policy on Humane Care and Use of Laboratory Animals. The protocol (docket no. 16-451 and 19-451) was approved by the Institutional Animal Care and Use Agency of Wadsworth Center, New York State Department of Health. All efforts were made to minimize animal suffering.
Mouse, ticks, and bacterial strains.
Three-week-old, female C3H/HeN mice were purchased from Charles River (Wilmington, MA, USA). This mouse strain was utilized because it develops LD manifestations (e.g., arthritis) during B. burgdorferi infection and thus is commonly used to test the efficacy of LD vaccines (46, 47). BALB/c C3-deficient mice from in-house breeding colonies were used for generating infected ticks (19, 29). Ixodes scapularis tick larvae were obtained from BEI Resources (Manassas, VA).
Escherichia coli strain BL21(DE3) and derivatives were grown at 37°C in Luria-Bertani (BD Bioscience, Franklin Lakes, NJ) broth or agar, supplemented with kanamycin (25 μg/ml), ampicillin (100 μg/ml), or no antibiotics when appropriate. Borrelia strains were grown at 33°C in BSK II complete medium (48) (see Table S2 in the supplemental material). Cultures of B. burgdorferi strain B31-A3 were tested with PCR to ensure a full plasmid profile prior to use (49, 50), whereas B. burgdorferi strain 297 and B. afzelii strain VS461-JL were maintained as fewer than 10 passages.
Generation of recombinant proteins and vaccines.
The genomic DNA of B. afzelii strain VS461 provided by John Leong (designated VS461-JL) (Fig. S2) and Peter Kraiczy (designated VS461-PK) (Fig. S2) was used to amplify cspZ. The DNA sequences containing cspZ were amplified and sequenced by PCR using primers BspA14s-CspZ-Fwd and BspA14s-CspZ-Rev (Table S3) (NCBI accession no. MN809989 and MN809990). The sequences of the two alleles were identical (Fig. S2). The open reading frames of cspZ from strain VS461-JL lacking the putative signal sequences were amplified (residues 24 to 280) (Table S3) and cloned into pGEX-4T-2 at the BamHI and SalI sites (GE Healthcare, Piscataway, NJ) (19, 27). This plasmid was transformed into E. coli strain BL21(DE3), and the plasmid insert was sequenced (Wadsworth ATGC Core Facility, NYS Department of Health, Albany, NY, USA). The glutathione S-transferase (GST)-tagged CspZ (GST-CspZ) variants from strains VS461-JL and B31-A3 were produced and purified by glutathione chromatography according to the manufacturer’s instructions (BD Bioscience, Franklin Lakes, NJ) (27).
Recombinant CspZ and CspZ-YA from strain B31-A3 were purified for mouse vaccination with a HisTrap FF column (GE Healthcare, Chicago, IL, USA) from E. coli strain BL21(DE3) containing pETm_11 encoding CspZ from strain B31-A3 (residues 21 to 236) or an altered open reading frame encoding CspZ-Y207A/Y211A (residues 21 to 236 with tyrosine-207 and -211 replaced by alanine), followed by a TEV protease cleavage site (Table S3). These proteins were conjugated to purified VLP Qβ with SMPH {succinimidyl-6-[(β-maleimidopropionamido) hexanoate]} at the N terminus of the protein as described previously (27).
Generation of infected ticks.
Generating infected I. scapularis ticks has been described previously (46). Basically, BALB/c C3-deficient mice were infected subcutaneously with 105 bacteria of strain B31-A3, 297, or VS461-JL (19, 29). Ear tissues were collected via ear punch, and bacterial genomic DNA (gDNA) was purified for detection with quantitative PCR (qPCR) to confirm infection (see “Quantification of spirochete burden”). Approximately 100 to 200 uninfected larvae were then allowed to feed to repletion on the infected mice as described previously (46). The engorged larvae were collected and allowed to molt into nymphs in a desiccator at room temperature with 95% relative humidity and light-to-dark control (16 h light:8 h dark).
Mouse immunization and infection.
Mice were vaccinated as described previously, with slight modifications (27). Fifty microliters of PBS (control) or 25 μg of VLP, CspZ, VLP-CspZ, CspZ-YA, or VLP-CspZ-YA in 50 μl of PBS was thoroughly mixed with 50 μl TiterMax Gold adjuvant (Norcross, GA, USA). C3H/HeN mice were immunized subcutaneously with 100 μl of the vaccine. Mice received boosters of the same composition at 14 and 28 dpii for a total of three immunizations over 6 weeks. At 42 dpii, blood was collected via submandibular bleeding to isolate serum (Fig. S1). At 49 dpii, uninfected (control) or infected flat nymphs were placed in a chamber on the immunized or PBS-inoculated C3H/HeN mice as described previously (Fig. S1) (26). Five nymphs were allowed to feed to repletion on each mouse, and a subset of nymphs was collected pre- and postfeeding. DNA was purified from these nymphs, and spirochetes were quantified by following the parameters described in “Quantification of spirochete burdens.”
Quantification of spirochete burdens.
To quantify spirochete burdens, mice were sacrificed at 21 dpf and the tick feeding sites of the skin, knees, bladder, and ears were collected (Fig. S1). DNA was purified using an EZ-10 spin column animal genomic DNA mini-prep kit (Bio Basic, Inc., Markham, Ontario, CA). Spirochete burdens were quantified based on the amplification of the 16S rRNA gene (Table S3) by qPCR using an Applied Biosystems 7500 real-time PCR system (ThermoFisher) in conjunction with PowerUp SYBR green master mix (ThermoFisher) as described previously (27, 51). The number of 16S rRNA gene copies was calculated by establishing a quantification cycle (Cq) standard curve of a known number of 16S rRNA genes extracted from strain B31-A3, and burdens were normalized to 100 ng of total DNA.
Histological analysis of LD-associated arthritis.
Mice were sacrificed at 21 dpf, and tibiotarsus joints were collected to assess arthritis via tissue histopathology. Tissues were fixed, decalcified, and prepared as slides stained with hematoxylin and eosin as described previously (Wadsworth Histopathology Core Facility, NYS Department of Health, Albany, NY, USA) (27). At least 10 sections per mouse were blindly evaluated for signs of arthritis by using histological parameters for Borrelia-induced inflammation and scored as described previously (51). The image was scored based on the severity of the inflammation as 0 (no inflammation), 1 (mild inflammation with less than two small foci of infiltration), 2 (moderate inflammation with two or more foci of infiltration), or 3 (severe inflammation with focal and diffuse infiltration covering a large area).
ELISAs.
An ELISA to determine the human or mouse FH-binding ability of CspZ was performed as described previously (19, 52). In brief, microtiter plate wells were coated with human (ComTech, Tyler, TX) or mouse (MyBiosource, San Diego, CA) FH. Serially diluted GST (negative control) or GST‐tagged CspZ from strain B31-A3 or VS461-JL was added to the wells. The binding of GST‐tagged proteins was detected using mouse anti-GST tag (ThermoFisher, Waltham, MA; 1:200) and horseradish peroxidase‐conjugated goat anti-mouse IgG (ThermoFisher; 1:1,000). Tetramethylbenzidine solution (ThermoFisher) was added to each well and incubated for 5 min, and then the reaction was stopped with hydrosulfuric acid. The plates were read at an optical density of 405 nm (OD405) using a Tecan Sunrise microplate reader (Tecan, Morrisville, NC). To determine the dissociation constant (Kd), the data were fitted to the following equation using GraphPad Prism software (GraphPad, La Jolla, CA): OD405 = (OD405, max × CspZ protein concentration)/(Kd + CspZ protein concentration).
To determine the titers of anti-CspZ antibodies, 50 μl of serially diluted mouse serum (1:100, 1:300, 1:900) from 42 dpii was added to microtiter wells coated with recombinant CspZ. Total IgG and IgM were detected using horseradish peroxidase (HRP)-conjugated goat anti-mouse IgM and IgG, respectively (1:20,000; Bethyl, Montgomery, TX, USA). The IgG subclasses were detected using HRP-conjugated goat anti-mouse IgG1, IgG2a, and IgG2b (1:8,000; SouthernBiotech, Birmingham, AL). To measure the levels of anti-FH antibodies in the same vaccinated mouse sera, microtiter wells were coated with 1 μg of human or mouse FH and then incubated with sera, followed by the addition of conjugated goat anti-mouse IgG (1:20,000; Bethyl). After the addition of antibodies, tetramethyl benzidine solution (ThermoFisher) was added, and the absorbance was detected at 620 nm for 10 cycles of 60-s kinetic intervals with a 10-s shaking duration in a Sunrise absorbance ELISA plate reader (Tecan, Männedorf, Switzerland). For each serum sample, the maximum slope of optical density/minute of all the dilutions was multiplied by the respective dilution factor, and the greatest value was used as representative of antibody titers (arbitrary units [A.U.]).
To examine the ability of antibodies to block the FH-binding activity of CspZ, ELISA plate wells were coated with 1 μg of mouse anti-GST IgG (ThermoFisher), followed by incubation with 1 μM recombinant GST-CspZ. After blocking with 5% bovine serum albumin (BSA) in PBS buffer, the wells were incubated with PBS (control) or serially diluted mouse sera collected at 42 dpii (1:200, 1:600, 1:1,80, 1:5,400, 1:16,200, 1:48,600, 1:145,800, and 1:437,400), followed by mixing with 1 μM human FH. Sheep anti-human FH (1:200; ThermoFisher) and then goat anti-sheep HRP (1:2,000; ThermoFisher) were added, and the levels of FH binding were detected as mentioned above. Data were expressed as the proportion of FH binding from serum-treated to PBS-treated wells. The 50% inhibitory dilution, representing the serum dilution rate that blocks 50% of FH binding, was calculated using dose-response stimulation fitting in GraphPad Prism 5.04.
Borreliacidal assays.
Mouse sera collected at 42 dpii were used to determine the bactericidal activity against B. burgdorferi as described previously (27). Briefly, these mouse sera were heat treated to inactivate complement, serially diluted, and mixed with complement-preserved guinea pig serum (Sigma-Aldrich, St. Louis, MO) or heat-inactivated guinea pig serum (negative control). After the addition of strain B31-A3, 297, or VS461-JL, the mixture was incubated at 33°C for 24 h. Surviving spirochetes were quantified by directly counting the motile spirochetes using dark-field microscopy and expressed as the proportion of serum-treated to untreated Lyme borreliae. The 50% borreliacidal titer, representing the serum dilution rate that kills 50% of spirochetes, was calculated using dose-response stimulation fitting in GraphPad Prism 5.04.
Statistical analyses.
Significant differences were determined with a Mann-Whitney test (between two groups) or a Kruskal-Wallis test with Dunn’s multiple comparisons (more than two groups) using GraphPad Prism 5.04. A P value of <0.05 was used to determine significance.
Supplementary Material
ACKNOWLEDGMENTS
We thank John Leong for providing B. burgdorferi strains B31-A3 and 297 and B. afzelii strain VS461-JL and Sanjay Ram for valuable advice. We also thank the Wadsworth Animal Core for assistance with animal care, the ATGC Core for sequencing plasmids, and Abigail Snyder-Keller and Helen Johnson of the Wadsworth Histopathology Core for generating the histopathology slides.
This work was supported by NSF grant IOS1755286 (Y.-P.L., A.L.M., P.L.L, T.M.H.), DoD grant TB170111 (Y.-P.L., A.L.M., P.L.L., T.M.H.), NIAID contract 75N93019C00040 (Y.-P.L., A.L.M., P.L.L., T.M.H., J. Y., N.J.M.), NIH grant R21AI144891 (Y.-P.L., A.L.M., P.L.L., T.M.H., M.E.B., W.-H.C.), NIH grant R01AI080615, NIH grant R01AI116620 (U.P., X.Y.), and NIH grant R01AI121401 and by the LOEWE Center DRUID (Novel Drug Targets against Poverty-Related and Neglected Tropical Infectious Diseases), project C3 (P.K.). The work was also supported by a New York State Department of Health Wadsworth Center start-up grant (Y.-P.L., A.L.M., P.L.L., T.M.H.) and by ERDF grant 2014/0014/2DP/2.1.1.1.0/14/APIA/VIAA/013 (K.T. and I.L.).
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We have no conflicts of interest to declare.
Ashley L. Marcinkiewicz and Yi-Pin Lin designed the study, acquired, analyzed, and interpreted the data, and drafted the article.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Steere AC, Strle F, Wormser GP, Hu LT, Branda JA, Hovius JW, Li X, Mead PS. 2016. Lyme borreliosis. Nat Rev Dis Primers 2:16090. doi: 10.1038/nrdp.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mac S, da Silva SR, Sander B. 2019. The economic burden of Lyme disease and the cost-effectiveness of Lyme disease interventions: a scoping review. PLoS One 14:e0210280. doi: 10.1371/journal.pone.0210280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nigrovic LE, Thompson KM. 2007. The Lyme vaccine: a cautionary tale. Epidemiol Infect 135:1–8. doi: 10.1017/S0950268806007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Federizon J, Lin YP, Lovell JF. 2019. Antigen engineering approaches for Lyme disease vaccines. Bioconjug Chem 30:1259–1272. doi: 10.1021/acs.bioconjchem.9b00167. [DOI] [PubMed] [Google Scholar]
- 5.Gomes-Solecki M, Arnaboldi PM, Backenson PB, Benach JL, Cooper CL, Dattwyler RJ, Diuk-Wasser M, Fikrig E, Hovius JW, Laegreid W, Lundberg U, Marconi RT, Marques AR, Molloy P, Narasimhan S, Pal U, Pedra JHF, Plotkin S, Rock DL, Rosa P, Telford SR, Tsao J, Yang XF, Schutzer SE. 2019. Protective immunity and new vaccines for Lyme disease. Clin Infect Dis doi: 10.1093/cid/ciz872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radolf JD, Caimano MJ, Stevenson B, Hu LT. 2012. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol 10:87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosa PA, Tilly K, Stewart PE. 2005. The burgeoning molecular genetics of the Lyme disease spirochaete. Nat Rev Microbiol 3:129–143. doi: 10.1038/nrmicro1086. [DOI] [PubMed] [Google Scholar]
- 8.Brisson D, Drecktrah D, Eggers CH, Samuels DS. 2012. Genetics of Borrelia burgdorferi. Annu Rev Genet 46:515–536. doi: 10.1146/annurev-genet-011112-112140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kraiczy P. 2016. Hide and seek: how Lyme disease spirochetes overcome complement attack. Front Immunol 7:385. doi: 10.3389/fimmu.2016.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcinkiewicz A, Kraiczy P, Lin Y-P. 2017. There is a method to the madness: strategies to study host complement evasion by Lyme disease and relapsing fever spirochetes. Front Microbiol 8:328. doi: 10.3389/fmicb.2017.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caine JA, Coburn J. 2016. Multifunctional and redundant roles of Borrelia burgdorferi outer surface proteins in tissue adhesion, colonization, and complement evasion. Front Immunol 7:442. doi: 10.3389/fimmu.2016.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zipfel PF, Skerka C. 2009. Complement regulators and inhibitory proteins. Nat Rev Immunol 9:729–740. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- 13.Sjoberg AP, Trouw LA, Blom AM. 2009. Complement activation and inhibition: a delicate balance. Trends Immunol 30:83–90. doi: 10.1016/j.it.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Meri S. 2016. Self-nonself discrimination by the complement system. FEBS Lett 590:2418–2434. doi: 10.1002/1873-3468.12284. [DOI] [PubMed] [Google Scholar]
- 15.Hartmann K, Corvey C, Skerka C, Kirschfink M, Karas M, Brade V, Miller JC, Stevenson B, Wallich R, Zipfel PF, Kraiczy P. 2006. Functional characterization of BbCRASP-2, a distinct outer membrane protein of Borrelia burgdorferi that binds host complement regulators factor H and FHL-1. Mol Microbiol 61:1220–1236. doi: 10.1111/j.1365-2958.2006.05318.x. [DOI] [PubMed] [Google Scholar]
- 16.Kraiczy P, Stevenson B. 2013. Complement regulator-acquiring surface proteins of Borrelia burgdorferi: structure, function and regulation of gene expression. Ticks Tick Borne Dis 4:26–34. doi: 10.1016/j.ttbdis.2012.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bykowski T, Woodman ME, Cooley AE, Brissette CA, Brade V, Wallich R, Kraiczy P, Stevenson B. 2007. Coordinated expression of Borrelia burgdorferi complement regulator-acquiring surface proteins during the Lyme disease spirochete’s mammal-tick infection cycle. Infect Immun 75:4227–4236. doi: 10.1128/IAI.00604-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siegel C, Schreiber J, Haupt K, Skerka C, Brade V, Simon MM, Stevenson B, Wallich R, Zipfel PF, Kraiczy P. 2008. Deciphering the ligand-binding sites in the Borrelia burgdorferi complement regulator-acquiring surface protein 2 required for interactions with the human immune regulators factor H and factor H-like protein 1. J Biol Chem 283:34855–34863. doi: 10.1074/jbc.M805844200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcinkiewicz AL, Dupuis AP II, Zamba-Campero M, Nowak N, Kraiczy P, Ram S, Kramer LD, Lin YP. 2019. Blood treatment of Lyme borreliae demonstrates the mechanism of CspZ-mediated complement evasion to promote systemic infection in vertebrate hosts. Cell Microbiol 21:e12998. doi: 10.1111/cmi.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogers EA, Marconi RT. 2007. Delineation of species-specific binding properties of the CspZ protein (BBH06) of Lyme disease spirochetes: evidence for new contributions to the pathogenesis of Borrelia spp. Infect Immun 75:5272–5281. doi: 10.1128/IAI.00850-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kraiczy P, Seling A, Brissette CA, Rossmann E, Hunfeld KP, Bykowski T, Burns LH, Troese MJ, Cooley AE, Miller JC, Brade V, Wallich R, Casjens S, Stevenson B. 2008. Borrelia burgdorferi complement regulator-acquiring surface protein 2 (CspZ) as a serological marker of human Lyme disease. Clin Vaccine Immunol 15:484–491. doi: 10.1128/CVI.00415-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogers EA, Abdunnur SV, McDowell JV, Marconi RT. 2009. Comparative analysis of the properties and ligand binding characteristics of CspZ, a factor H binding protein, derived from Borrelia burgdorferi isolates of human origin. Infect Immun 77:4396–4405. doi: 10.1128/IAI.00393-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kraiczy P, Skerka C, Brade V, Zipfel PF. 2001. Further characterization of complement regulator-acquiring surface proteins of Borrelia burgdorferi. Infect Immun 69:7800–7809. doi: 10.1128/IAI.69.12.7800-7809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kraiczy P, Skerka C, Kirschfink M, Brade V, Zipfel PF. 2001. Immune evasion of Borrelia burgdorferi by acquisition of human complement regulators FHL-1/reconectin and Factor H. Eur J Immunol 31:1674–1684. doi:. [DOI] [PubMed] [Google Scholar]
- 25.Brangulis K, Petrovskis I, Kazaks A, Bogans J, Otikovs M, Jaudzems K, Ranka R, Tars K. 2014. Structural characterization of CspZ, a complement regulator factor H and FHL-1 binding protein from Borrelia burgdorferi. FEBS J 281:2613–2622. doi: 10.1111/febs.12808. [DOI] [PubMed] [Google Scholar]
- 26.Coleman AS, Yang X, Kumar M, Zhang X, Promnares K, Shroder D, Kenedy MR, Anderson JF, Akins DR, Pal U. 2008. Borrelia burgdorferi complement regulator-acquiring surface protein 2 does not contribute to complement resistance or host infectivity. PLoS One 3:3010e. doi: 10.1371/journal.pone.0003010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcinkiewicz AL, Lieknina I, Kotelovica S, Yang X, Kraiczy P, Pal U, Lin YP, Tars K. 2018. Eliminating factor H-binding activity of Borrelia burgdorferi CspZ combined with virus-like particle conjugation enhances its efficacy as a Lyme disease vaccine. Front Immunol 9:181. doi: 10.3389/fimmu.2018.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones KL, McHugh GA, Glickstein LJ, Steere AC. 2009. Analysis of Borrelia burgdorferi genotypes in patients with Lyme arthritis: high frequency of ribosomal RNA intergenic spacer type 1 strains in antibiotic-refractory arthritis. Arthritis Rheum 60:2174–2182. doi: 10.1002/art.24812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hart T, Nguyen NTT, Nowak NA, Zhang F, Linhardt RJ, Diuk-Wasser M, Ram S, Kraiczy P, Lin YP. 2018. Polymorphic factor H-binding activity of CspA protects Lyme borreliae from the host complement in feeding ticks to facilitate tick-to-host transmission. PLoS Pathog 14:e1007106. doi: 10.1371/journal.ppat.1007106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharkey K, Beernink PT, Langley JM, Gantt S, Quach C, Dold C, Liu Q, Galvan M, Granoff DM. 2019. Anti-factor H antibody reactivity in young adults vaccinated with a meningococcal serogroup B vaccine containing factor H binding protein. mSphere 4:e00393-19. doi: 10.1128/mSphere.00393-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costa I, Pajon R, Granoff DM. 2014. Human factor H (FH) impairs protective meningococcal anti-FHbp antibody responses and the antibodies enhance FH binding. mBio 5:e01625-14. doi: 10.1128/mBio.01625-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giuntini S, Beernink PT, Granoff DM. 2015. Effect of complement factor H on anti-FHbp serum bactericidal antibody responses of infant rhesus macaques boosted with a licensed meningococcal serogroup B vaccine. Vaccine 33:7168–7175. doi: 10.1016/j.vaccine.2015.10.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edwards JF, Higgs S, Beaty BJ. 1998. Mosquito feeding-induced enhancement of Cache Valley virus (Bunyaviridae) infection in mice. J Med Entomol 35:261–265. doi: 10.1093/jmedent/35.3.261. [DOI] [PubMed] [Google Scholar]
- 34.Styer LM, Lim PY, Louie KL, Albright RG, Kramer LD, Bernard KA. 2011. Mosquito saliva causes enhancement of West Nile virus infection in mice. J Virol 85:1517–1527. doi: 10.1128/JVI.01112-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dudley DM, Newman CM, Lalli J, Stewart LM, Koenig MR, Weiler AM, Semler MR, Barry GL, Zarbock KR, Mohns MS, Breitbach ME, Schultz-Darken N, Peterson E, Newton W, Mohr EL, Capuano Iii S, Osorio JE, O'Connor SL, O'Connor DH, Friedrich TC, Aliota MT. 2017. Infection via mosquito bite alters Zika virus tissue tropism and replication kinetics in rhesus macaques. Nat Commun 8:2096. doi: 10.1038/s41467-017-02222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanson MS, Cassatt DR, Guo BP, Patel NK, McCarthy MP, Dorward DW, Hook M. 1998. Active and passive immunity against Borrelia burgdorferi decorin binding protein A (DbpA) protects against infection. Infect Immun 66:2143–2153. doi: 10.1128/IAI.66.5.2143-2153.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hagman KE, Yang X, Wikel SK, Schoeler GB, Caimano MJ, Radolf JD, Norgard MV. 2000. Decorin-binding protein A (DbpA) of Borrelia burgdorferi is not protective when immunized mice are challenged via tick infestation and correlates with the lack of DbpA expression by B. burgdorferi in ticks. Infect Immun 68:4759–4764. doi: 10.1128/iai.68.8.4759-4764.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeidner NS, Schneider BS, Nuncio MS, Gern L, Piesman J. 2002. Coinoculation of Borrelia spp. with tick salivary gland lysate enhances spirochete load in mice and is tick species-specific. J Parasitol 88:1276–1278. doi: 10.1645/0022-3395(2002)088[1276:COBSWT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 39.Pospisilova T, Urbanova V, Hes O, Kopacek P, Hajdusek O, Sima R. 2019. Tracking of Borrelia afzelii transmission from infected Ixodes ricinus nymphs to mice. Infect Immun 9:1464–1467. doi: 10.1128/IAI.00896-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sertour N, Cotte V, Garnier M, Malandrin L, Ferquel E, Choumet V. 2018. Infection kinetics and tropism of Borrelia burgdorferi sensu lato in mouse after natural (via ticks) or artificial (needle) infection depends on the bacterial strain. Front Microbiol 9:1722. doi: 10.3389/fmicb.2018.01722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiler JM, Daha MR, Austen KF, Fearon DT. 1976. Control of the amplification convertase of complement by the plasma protein beta1H. Proc Natl Acad Sci U S A 73:3268–3272. doi: 10.1073/pnas.73.9.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herbert AP, Makou E, Chen ZA, Kerr H, Richards A, Rappsilber J, Barlow PN. 2015. Complement evasion mediated by enhancement of captured factor H: implications for protection of self-surfaces from complement. J Immunol 195:4986–4998. doi: 10.4049/jimmunol.1501388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pickering MC, Cook HT, Warren J, Bygrave AE, Moss J, Walport MJ, Botto M. 2002. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat Genet 31:424–428. doi: 10.1038/ng912. [DOI] [PubMed] [Google Scholar]
- 44.Meri S, Koistinen V, Miettinen A, Tornroth T, Seppala IJ. 1992. Activation of the alternative pathway of complement by monoclonal lambda light chains in membranoproliferative glomerulonephritis. J Exp Med 175:939–950. doi: 10.1084/jem.175.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jokiranta TS, Solomon A, Pangburn MK, Zipfel PF, Meri S. 1999. Nephritogenic lambda light chain dimer: a unique human miniautoantibody against complement factor H. J Immunol 163:4590–4596. [PubMed] [Google Scholar]
- 46.Kern A, Zhou CW, Jia F, Xu Q, Hu LT. 2016. Live-vaccinia virus encapsulation in pH-sensitive polymer increases safety of a reservoir-targeted Lyme disease vaccine by targeting gastrointestinal release. Vaccine 34:4507–4513. doi: 10.1016/j.vaccine.2016.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hahn BL, Padmore LJ, Ristow LC, Curtis MW, Coburn J. 2016. Live attenuated Borrelia burgdorferi targeted mutants in an infectious strain background protect mice from challenge infection. Clin Vaccine Immunol 23:725–731. doi: 10.1128/CVI.00302-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barbour AG, Burgdorfer W, Grunwaldt E, Steere AC. 1983. Antibodies of patients with Lyme disease to components of the Ixodes dammini spirochete. J Clin Invest 72:504–515. doi: 10.1172/jci110998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Purser JE, Norris SJ. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc Natl Acad Sci U S A 97:13865–13870. doi: 10.1073/pnas.97.25.13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bunikis I, Kutschan-Bunikis S, Bonde M, Bergström S. 2011. Multiplex PCR as a tool for validating plasmid content of Borrelia burgdorferi. J Microbiol Methods 86:243–247. doi: 10.1016/j.mimet.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 51.Lin YP, Benoit V, Yang X, Martinez-Herranz R, Pal U, Leong JM. 2014. Strain-specific variation of the decorin-binding adhesin DbpA influences the tissue tropism of the Lyme disease spirochete. PLoS Pathog 10:e1004238. doi: 10.1371/journal.ppat.1004238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin YP, Lee DW, McDonough SP, Nicholson LK, Sharma Y, Chang YF. 2009. Repeated domains of leptospira immunoglobulin-like proteins interact with elastin and tropoelastin. J Biol Chem 284:19380–19391. doi: 10.1074/jbc.M109.004531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.