Implanted medical device-associated infections pose significant health risks, as they are often the result of bacterial biofilm formation. Staphylococcus aureus is a leading cause of biofilm-associated infections which persist due to mechanisms of device surface adhesion, biofilm accumulation, and reprogramming of host innate immune responses. We found that the S. aureus fibronectin binding protein A (FnBPA) is required for normal biofilm development in mammalian serum and that the SaeRS two-component system is required for functional FnBPA activity in serum.
KEYWORDS: S. aureus, fibronectin binding protein, biofilm, infection
ABSTRACT
Implanted medical device-associated infections pose significant health risks, as they are often the result of bacterial biofilm formation. Staphylococcus aureus is a leading cause of biofilm-associated infections which persist due to mechanisms of device surface adhesion, biofilm accumulation, and reprogramming of host innate immune responses. We found that the S. aureus fibronectin binding protein A (FnBPA) is required for normal biofilm development in mammalian serum and that the SaeRS two-component system is required for functional FnBPA activity in serum. Furthermore, serum-developed biofilms deficient in FnBPA were more susceptible to macrophage invasion, and in a model of biofilm-associated implant infection, we found that FnBPA is crucial for the establishment of infection. Together, these findings show that S. aureus FnBPA plays an important role in physical biofilm development and represents a potential therapeutic target for the prevention and treatment of device-associated infections.
INTRODUCTION
Staphylococcus aureus is a leading cause of both health care- and community-associated infections (1), often manifesting as skin and soft tissue infections, sepsis, endocarditis, and osteomyelitis. However, infection risk is substantially increased with the presence of an implanted medical device, such as an indwelling catheter, orthopedic prosthesis, or cardiac device. Of all health care-associated infections in the United States, more than 25% are attributed to infection associated with an implanted medical device, and staphylococci are the leading cause of device-associated infections (2–5). With the expansion of antibiotic resistance in S. aureus and increased clinical use of medical implants, novel therapeutic strategies are urgently needed to prevent and treat device-associated infections. Moreover, a detailed understanding of how S. aureus establishes biofilm-mediated infections within the host will help guide the development of effective antibiofilm treatments.
Medical implants are rapidly coated with host plasma and extracellular matrix proteins, such as elastin, fibrinogen, and fibronectin (Fn), that help modulate a foreign body reaction (6) but also represent a target for bacterial attachment and biofilm formation (7). As opposed to a planktonic lifestyle, biofilms are heterogeneous communities of surface-attached bacterial cells encased within a self-produced extracellular matrix of DNA, proteins, and polysaccharides. Three-dimensional biofilm architecture not only poses a physical barrier to immune cell infiltration and phagocytosis, but biofilm products actively skew immune responses toward an alternatively activated status and enable infection persistence (8–10). S. aureus produces several cell wall-anchored adhesins that may contribute to device adherence and biofilm development, but their role and regulation during biofilm-mediated infection have yet to be fully elucidated.
The S. aureus biofilm mode of growth is regulated by a complex network of genetic factors responding to various environmental cues, including available metabolites, host components, and quorum sensing molecules. The SaeRS two-component system, composed of a surface sensor kinase (SaeS) and response regulator (SaeR), responds to several known signals, including activation by human neutrophil peptide 1 (HNP1) and inhibition by some divalent metal ions, such as zinc (11). Upon stimulation, SaeS autophosphorylates and transfers the phosphoryl group to SaeR, which then binds to a direct repeat sequence in the promoters of target genes, either enhancing or inhibiting their transcription (11). SaeR modulates the expression of more than 20 known adhesins, toxins, and immune modulators, thereby playing a major role in S. aureus pathogenesis. One of the known SaeRS-regulated genes, fibronectin binding protein A (fnbA; FnBPA), has been found to be responsible for binding host matrix molecules and mediating biofilm accumulation via intercellular homophilic bonds (12–17). Sae inactivation has been shown to significantly reduce the abundance of FnBPA in vitro (18), and an Sae mutation has been show to eliminate FnBPA-specific IgG1 antibody production in a mouse model of S. aureus subcutaneous infection (19).
Most S. aureus isolates express two cell wall-anchored fibronectin binding proteins, FnBPA and FnBPB, with the known exception of two strains, UAMS-1 and Mu50, which harbor only FnBPA (20). FnBPs are adhesins in the family of microbial surface components recognizing adhesive matrix molecules (MSCRAMMs) (21) and have been shown to play crucial roles in surface attachment and biofilm formation in vitro (12, 22). It was previously found that 99% of 191 S. aureus orthopedic device infection isolates carry fnbA (23), and fnbA is one of seven virulence factors significantly more common in invasive S. aureus isolates (24). The FnBPs are complex proteins, containing two multifunctional domains: an N-terminal A domain and a fibronectin-binding repeat (FnBR) domain. The A domain is responsible for forming intercellular homophilic bonds (12) and binding of elastin and fibrinogen (13–15). The FnBR domain is composed of 10 (FnBPB) or 11 (FnBPA) tandem repeat motifs, and each motif can bind the N terminus of a single type I Fn module using a tandem β-zipper mechanism (16, 17, 25). Additionally, only one Fn-binding motif is necessary and sufficient for S. aureus to bind Fn-coated surfaces in vitro (22, 26).
Despite previous work examining the role of FnBPA in adherence and cellular invasion in vitro, remarkably few studies have investigated a role and regulation for FnBPA in preclinical models of S. aureus infection. These include a pair of intravenous sepsis infection models (26, 27), the expression of S. aureus FnBPA conferring virulence to Lactococcus lactis in a rat model of valve endocarditis (21), and a competitive index model of device-associate infection (28); the latter, however, is limited to assessing a single coinfection experiment measuring bacterial burdens within the lumen of an implanted catheter. Thus, it remains unclear how FnBPA contributes to S. aureus biofilm infection development, and its role in mediating recalcitrance to immune cell biofilm invasion is unknown. Here, we show that SaeS-mediated FnBPA activity is required for S. aureus biofilm architecture development in serum and thereby contributes to the resistance of macrophage invasion. In a mouse model of device-associated infection, we found that both FnBPA and SaeS contribute significantly to device colonization and spread into soft tissue surrounding the infection. The results of these studies demonstrate an essential role for Sae-regulated FnBPA activity and further signify FnBPA as a target for preventing biofilm formation and facilitating biofilm eradication in device-associated infections.
RESULTS
Sae-mediated biofilm development in fetal bovine serum.
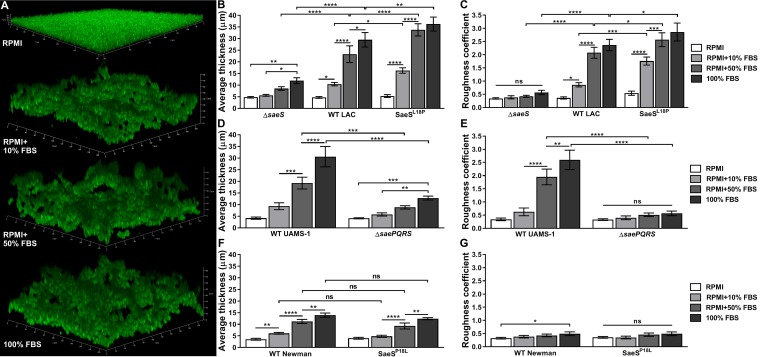
To examine the impact of animal serum on S. aureus biofilm development, static biofilms were cultured in RPMI medium alone or supplemented with fetal bovine serum (FBS). As shown in Fig. 1A to C, FBS induced a complex dose-dependent change in biofilm architecture in wild-type (WT) S. aureus strain LAC, characterized by increasing average biofilm thickness (Fig. 1B) and roughness (Fig. 1C). We hypothesized that this FBS-induced alteration in biofilm architecture was a result of surface sensing of serum molecules, and so we examined the effects of a mutation in the surface sensor kinase SaeS. Compared to that for WT LAC, an ΔsaeS mutant exhibited significantly reduced biofilm thickness and roughness in serum (Fig. 1B and C). Importantly, while the ΔsaeS mutant biofilm thickness was significantly increased in 100% FBS compared to that in RPMI with or without 10% FBS, the ΔsaeS mutant biofilm roughness remained unchanged, indicating that FBS merely provided additional nutrients for growth, as shown in Fig. S1 in the supplemental material, and did not influence biofilm architecture. Next, to determine if a role in biofilm development in serum exists for the Sae two-component system in unique S. aureus strains, we also examined the methicillin-susceptible S. aureus (MSSA) osteomyelitis isolate UAMS-1 (29). As shown in Fig. 1D and E, WT UAMS-1 exhibited an FBS dose-dependent biofilm thickness and roughness phenotype analogous to that of WT LAC. Moreover, these effects were dependent on Sae signaling, as shown with a complete ΔsaePQRS operon mutant, establishing a conserved role for this system in serum-induced S. aureus biofilm development.
FIG 1.
Sae-mediated biofilm development in fetal bovine serum. GFP+ S. aureus strains LAC, UAMS-1, and Newman, including their respective isogenic mutants, were cultured statically in chambered cover glass at 37°C for 24 h in RPMI with/without 10% or 50% fetal bovine serum (FBS) or in 100% FBS. (A) Representative three-dimensional (3D) Z-stack confocal microscopy images of WT LAC biofilms developed in RPMI alone or with FBS as indicated. COMSTAT2 quantification of average biofilm thickness (in microns) and dimensionless roughness coefficients in LAC (B and C), UAMS-1 (D and E), and Newman (F and G) strains. Data are from three independent experiments and presented as means ± standard deviations (SDs). Statistical analyses were performed using a two-way analysis of variance (ANOVA) with Tukey’s post hoc multiple-comparison test. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; ns, no statistically significant differences across the groups.
To further assess the role of SaeS activity on biofilm development, we tested an LAC SaeSL18P mutant, which results in constitutive SaeS kinase activity (30). As shown in Fig. 1B and C, compared to WT LAC, the SaeSL18P mutant exhibited increased biofilm height and roughness under all FBS-containing conditions tested. Interestingly, while roughness was moderately increased, there were no significant differences between WT LAC and the SaeSL18P mutant in RPMI, suggesting that SaeS activity alone is insufficient for the development of a complex biofilm architecture and that serum components themselves also contribute to biofilm architecture. Finally, we examined if the naturally occurring SaeSL18P mutation in S. aureus strain Newman (31) also increased biofilm architecture in FBS compared to that of a reverted nonconstitutive SaeSP18L mutant. As shown in Fig. 1F and G, neither WT Newman nor the SaeSP18L mutant formed a complex biofilm architecture (as defined by increased thickness and roughness) as observed in WT LAC and UAMS-1. These data indicate that an inherent property of S. aureus strain Newman, independent of SaeS signaling, prevented normal biofilm accumulation under serum-containing conditions.
S. aureus biofilm development in serum requires FnBPA.
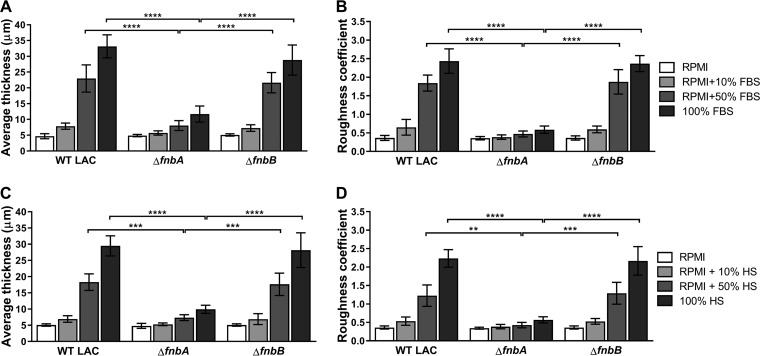
We hypothesized that the observed FBS-induced biofilm development phenotype resulted from altered expression and/or activity of surface adhesins responsible for intercellular interactions. Importantly, strain Newman and its SaeSP18L mutant harbor truncated fibronectin binding proteins A and B (FnBPA and FnBPB) (32). Thus, we examined the role of these proteins in S. aureus strain LAC biofilm formation and found that FnBPA, but not FnBPB, was required for biofilm architecture under conditions containing FBS (Fig. 2A and B). Expression of fnbA from a plasmid significantly increased biofilm thickness and roughness of the ΔfnbA mutant in RPMI plus 50% FBS compared to that from a vector control (see Fig. S2); however, in trans expression did not completely restore ΔfnbA biofilms to WT levels. In addition, as shown in Fig. 2C and D, human serum (HS) elicited a similar biofilm formation response and requirement for FnBPA in S. aureus strain LAC, indicating that conserved mammalian serum elements elicit FnBPA-dependent biofilm development.
FIG 2.
FnBPA-dependent biofilm development in FBS and human serum (HS). GFP+ S. aureus strain LAC and its isogenic ΔfnbA and ΔfnbB mutants were cultured statically in chambered cover glass at 37°C for 24 h in RPMI with/without 10% or 50% FBS or in 100% FBS (A and B) or HS (C and D) followed by COMSTAT2 quantification of average biofilm thickness (in microns) and dimensionless roughness coefficients. Data are from three independent experiments and presented as means ±SDs. Statistical analyses were performed using a two-way ANOVA with Tukey’s post hoc multiple-comparison test. **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Sae signaling is known to regulate extracellular protease production in RPMI (18), and FnBPA extracellular stability has been shown to be increased in an extracellular protease-deficient strain (33). To assess the contribution of the major extracellular proteases in the serum-dependent biofilm phenotype, we generated a ΔsaePQRS mutation in the protease-deficient strain AH1919 (34). AH1919 biofilm development appeared analogous to that of WT S. aureus in RPMI plus 50% FBS, while the AH1919 ΔsaePQRS mutant lacked biofilm architecture (see Fig. S3). In addition, serum-induced biofilm development was not affected in single Δaur and ΔsspAB mutants (data not shown). Together, these data demonstrate that the observed biofilm phenotype is independent of Sae regulation of extracellular proteases.
Genetic regulation of fnbA during biofilm development in serum.
We hypothesized that SaeRS signaling directly regulates FnBPA production and/or function during S. aureus biofilm development in serum. To determine the role of SaeS in regulating fnbA expression during biofilm development in FBS and HS, fnbA and fnbB RNA levels were quantified in WT LAC, ΔsaeS, and SaeSL18P mutant biofilms using reverse transcription-quantitative PCR (qRT-PCR). Samples were taken after 5 h to assess transcriptional regulation during the initial phases of biofilm formation. As shown in Fig. 3A, compared to that in WT LAC, expression of fnbA was not significantly reduced in the ΔsaeS mutant under the conditions tested. However, fnbA expression in the SaeSL18P mutant was significantly increased in 50% FBS compared to that in WT LAC and the ΔsaeS mutant and significantly upregulated in 50% FBS compared to that of the SaeSL18P mutant in RPMI alone (Fig. 3A). Interestingly, the additive effect of serum on SaeSL18P fnbA expression was diminished in 50% HS. Furthermore, after 24 h, the SaeSL18P mutant maintained significantly increased fnbA expression in RPMI with/without 10% HS (see Fig. S4). These results are in contrast to fnbB expression, which showed no significant differences in any strain under any condition tested (Fig. 3B). Examination of fnbA expression in WT UAMS-1 and the ΔsaePQRS mutant also showed no significant differences under these conditions (data not shown). These data indicate that while strong, constitutive SaeS activity promotes fnbA expression, and that mammalian serum has no direct effect on SaeS-mediated fnbA transcription in WT S. aureus under the conditions examined here.
FIG 3.
Fibronectin binding protein gene expression in serum. Analysis of fnbA (A) and fnbB (B) expression in S. aureus LAC WT and isogenic ΔsaeS and SaeSL18P mutant biofilms developed in RPMI with/without 10% or 50% FBS or 50% HS after 5 h. Data are from at least two independent experiments and presented as mean fold changes from LAC in RPMI ± SDs. Statistical analysis was performed using a two-way ANOVA with Tukey’s post hoc multiple-comparison test. *, P < 0.05; **, P < 0.01; ns, no statistically significant difference across the groups.
Immobilized human fibronectin binding.
As a direct measure of cell-bound FnBPA function and activity, we next examined the capacity of S. aureus cells harvested from biofilms to bind immobilized human Fn. As shown in Fig. 4A, WT LAC exhibited significantly increased Fn binding following biofilm development in 10% and 50% FBS compared to that in RPMI alone. Consistent with the biofilm architecture phenotypes, both ΔfnbA and ΔsaeS mutants had significantly reduced Fn binding that approached baseline levels, and binding in the ΔfnbB mutant was unaltered from that of the WT under all conditions tested. Surprisingly, despite the aforementioned increase in fnbA expression, the SaeSL18P mutant was significantly less capable of binding immobilized Fn under all FBS-containing conditions than the WT.
FIG 4.
Immobilized human Fn binding. Biofilm-associated cells from S. aureus strains LAC (A), UAMS-1 (B), and Newman (C), including their respective isogenic mutants, cultured in RPMI with/without 10% or 50% FBS or in 100% FBS were collected and assessed for their binding capacity to immobilized human Fn as measured by crystal violet staining. Data are from at least two independent experiments and presented as mean absorbances at 595 nm (OD595) ± SDs. Statistical analyses were performed using a two-way ANOVA with Tukey’s post hoc multiple-comparison test. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; ns, no statistically significant difference across the groups.
We further examined the contribution of the Sae two-component system to human Fn binding in S. aureus strain UAMS-1. As shown in Fig. 4B, UAMS-1 biofilm-associated cells showed significantly increased Fn binding in 50% and 100% FBS compared to that in RPMI alone, and consistent with LAC, the ΔsaePQRS mutation significantly reduced Fn binding. We also examined strain Newman and its SaeSP18P mutant and found that neither strain was capable of binding immobilized human Fn (Fig. 4C), likely attributable to the truncated FnBPA. Together, these data demonstrate a clear role for the Sae two-component system in regulating the activity and function of FnBPA in S. aureus biofilms cultured in FBS.
FnBPA-mediated inhibition of macrophage biofilm invasion.
S. aureus biofilms are known to inhibit macrophage invasion and phagocytosis, thereby contributing to the establishment and chronicity of biofilm-mediated infections (8, 10, 35). To assess the contribution of FnBPA to the inhibition of macrophage biofilm invasion, murine bone marrow-derived macrophages (BMDMs) were added to WT LAC and ΔfnbA mutant biofilms, and invading BMDMs were quantified. As shown in Fig. 5, ΔfnbA mutant biofilms developed in RPMI plus 50% FBS were significantly more susceptible to macrophage invasion than WT LAC biofilms. Moreover, BMDM invasion in ΔfnbA mutant biofilms developed in RPMI plus 50% FBS was not significantly different from invasion in ΔfnbA biofilms developed in RPMI alone (Fig. 5B), suggesting an important role for FnBPA in preventing immune-mediated clearance of S. aureus biofilms.
FIG 5.
FnBPA-mediated inhibition of macrophage biofilm invasion. CellTracker violet-labeled bone marrow-derived macrophage (MΦ) were added to WT LAC and ΔfnbA biofilms cultured in RPMI plus 50% FBS and assessed for MΦ invasion after 4 h. (A) Representative 3D Z-stack confocal images of BMDM (blue) invasion into LAC (top) and ΔfnbA (bottom) biofilms developed in RPMI plus 50% FBS. White arrows indicate BMDMs not clearly visible. (B) Quantified BMDM invasion as percentage of visible BMDMs in a sterile sample within the same x/y/z dimensions. Data are from three independent experiments and presented as means ± SDs. Statistical analyses were performed using a two-way ANOVA with Tukey’s post hoc multiple-comparison test. ****, P < 0.0001.
FnBPA and SaeS are required in a biofilm infection model.
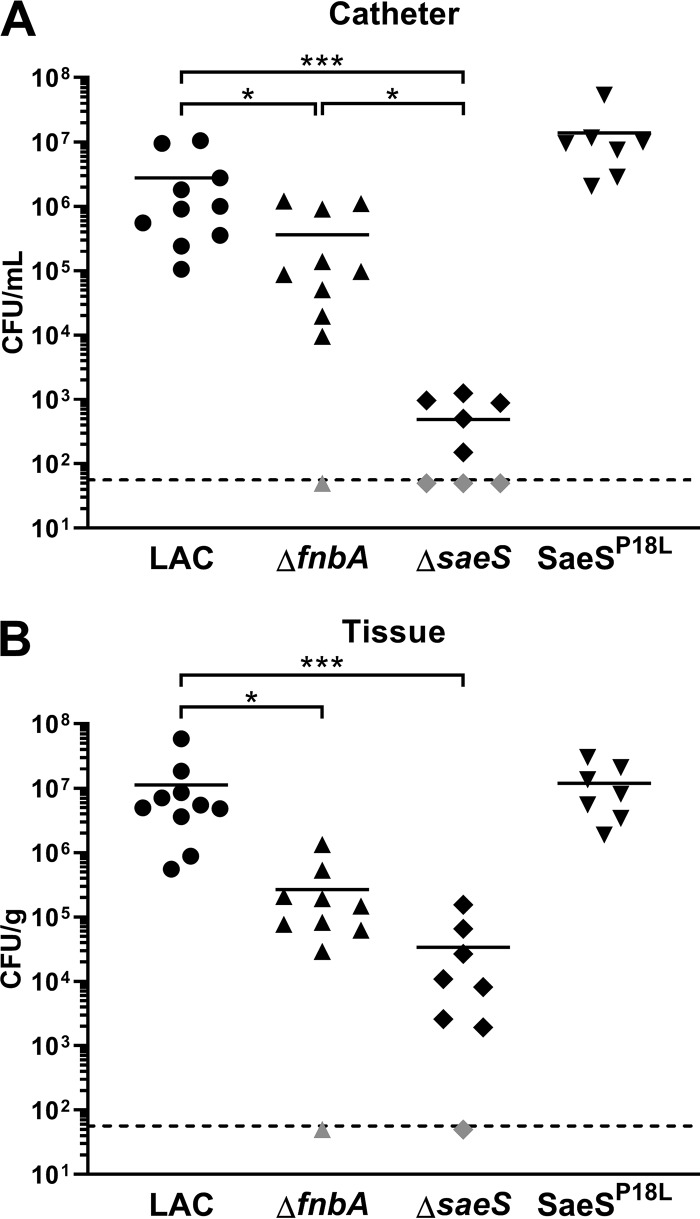
The establishment of device-associated infections requires initial surface adhesion and subsequent biofilm accumulation to prevent immune-mediated clearance (36–38). To assess the contribution of both SaeS and FnBPA to biofilm-mediated infection, we utilized a mouse flank catheter infection model. As shown in Fig. 6, both ΔsaeS and ΔfnbA mutations resulted in significantly reduced bacterial titers associated with the catheter and surrounding soft tissue. The significant decrease in catheter-associated bacteria observed with the ΔsaeS mutant appeared in part due to FnBPA, as the associated titers were statistically significantly different. However, statistical significance was not reached between ΔsaeS and ΔfnbA mutants in the tissue, indicating their role in bacterial spread from the biofilm to surrounding soft tissues. Interestingly, the SaeSL18P mutation resulted in markedly increased bacterial burdens associated with the catheter (P = 0.096), but not the surrounding tissue, suggesting that constitutive SaeS kinase activity enhanced primary biofilm formation, as we observed in vitro. Together, these findings demonstrate a clear role for FnBPA activity in biofilm development and immune evasion.
FIG 6.
FnBPA is required for S. aureus catheter-associated infection. Implanted subcutaneous catheters were infected with 103 WT LAC, ΔfnbA, ΔsaeS, or SaeSL18P mutant strains. Mice were sacrificed 7 days postinfection, whereupon bacterial burdens associated with the catheter (A) and surrounding soft tissue (B) were determined. Data are from two independent experiments, and the horizonal lines represent the mean CFUs. Each symbol denotes an individual mouse, and gray symbols indicate bacterial titers falling below the limit of detection (dashed lines). Statistical analyses were performed using a Kruskal-Wallis one-way ANOVA with an uncorrected Dunn’s test for multiple comparisons. *, P < 0.05; ***, P < 0.001.
DISCUSSION
Biofilm-mediated infections of implanted medical devices are particularly difficult to treat and often require a two-stage removal and replacement of the infected device. This is a long and debilitating process associated with significant morbidity and financial burden for patients. Biofilm-associated cells are heterogenous in activity; therefore, antibiotic therapy often fails due to incomplete microbicidal action. In addition, S. aureus biofilm products actively inhibit productive innate immune responses, including polarizing infiltrating macrophages toward an anti-inflammatory state and recruitment of myeloid-derived suppressor cells (8–10, 35, 39), thereby leading to infection establishment and chronicity. In this report, we demonstrate that mammalian serum promotes a robust FnBPA-dependent biofilm architecture that contributes to the inhibition of macrophage invasion. These findings are consistent with previous findings of device-associated biofilm architecture, which revealed irregular biofilm surface structures and tower formation with a predominantly hollow interior (8), thereby establishing physical biofilm architecture as a mechanism of immune deviation during device-associated infection.
To assess the underlying mechanism of S. aureus biofilm architecture development when exposed to mammalian serum, we investigated known regulators of various secreted proteins and surface adhesins. These studies revealed that biofilm architecture formation in serum was dependent on the S. aureus SaeRS two-component system in two diverse clinical isolates, the community-associated methicillin-resistant S. aureus (CA-MRSA) strain LAC and the MSSA strain UAMS-1. While it is unknown how the serum concentrations used in our present study relate to what S. aureus would be exposed to during infection, they provide evidence that as little as 10% serum can elicit marked biofilm structural arrangement. Interestingly, we found that the accessory gene regulatory (Agr) system, known to play important roles in S. aureus biofilm formation (40, 41), was not required for the observed biofilm architecture in the LAC strain (data not shown). The SaeRS two-component system responds to environmental stimuli and regulates the production and activity of numerous proteins involved in host cell adhesion, invasion, and toxicity (30, 31, 42, 43, and reviewed in reference 11). We initially screened several known SaeRS-regulated genes for their contribution to the biofilm architecture phenotype in serum and found that fibronectin binding protein A (FnBPA), but not FnBPB, was necessary and sufficient for the development of this antiphagocyte biofilm structure, closely resembling the structure of the SaeS mutant strain. A previous study showed that SaeS-mediated coagulase production facilitates attachment and biofilm accumulation on plasma-coated surfaces under flow conditions in RPMI medium (44). While no phenotype was observed for coagulase in our initial screen with static growth conditions in serum, it would not be surprising to find that SaeRS signaling effects multiple proteins to promote complex biofilm formation under shear conditions. In addition, serum lacks plasma clotting proteins such as fibrinogen, which indicates that the static biofilm architecture phenotype is independent of plasma components. Together, these data suggest that S. aureus senses serum components via SaeS and responds by promoting FnBPA activity, leading to complex biofilm accumulation.
We hypothesized that host serum induces SaeRS signaling pathways, leading to upregulated fnbA transcription. However, our qRT-PCR results demonstrated that neither human nor fetal bovine serum (FBS) had a significant effect on fnbA expression in WT S. aureus. Rather, only the constitutively active SaeSL18P mutant resulted in upregulated fnbA expression. Expression of fnbA is modulated by a low-affinity class I SaeR promoter, requiring high levels of phosphorylated SaeR for regulation. Our results are consistent with findings in S. aureus strain Newman (31), which harbors a naturally occurring SaeSL18P, demonstrating that SaeRS-mediated transcriptional regulation of fnbA in USA300 requires additional SaeS stimulation. Our data do demonstrate the feasibility of serum in regulating SaeRS signaling activity, as shown by the dose-dependent increase of SaeSL18P mutant fnbA expression in FBS. Moreover, previous studies have shown that that fnbA is upregulated 17-fold in a human S. aureus prosthetic join infection (45) and, importantly, that SaeS signaling effects FnBPA production (18). Together, while these findings suggest a role for SaeRS in regulating fnbA expression during biofilm-mediation infection, they indicate that the primary effect of SaeRS signaling on FnBPA during in vitro biofilm formation occurs posttranscriptionally.
From these data, we hypothesized that SaeRS signaling regulates the posttranslational modification of the surface-bound FnBPA protein. Indeed, we found that a SaeS mutant harvested from biofilms cultured in FBS was defective in binding immobilized human Fn, comparable to that of an FnBPA mutant. These findings indicate that SaeRS signaling regulated FnBPA function and corroborate our biofilm developmental phenotype showing similar architecture development in SaeS- and FnBPA-deficient S. aureus. Moreover, Fn binding was significantly increased in biofilms cultured in FBS compared to those in RPMI alone, indicating that, rather than modulating fnbA expression, serum promotes Sae-dependent FnBPA activity. It remains unclear how FnBPA activity, but not expression, is regulated by SaeRS signaling activity, which is the subject of future investigation. A clue to this may lie in the upregulation of fnbA in the SaeSL18P mutant, yet its Fn-binding capacity is decreased. It is possible that SaeRS-regulated SspA (V8) protease activity (46), known to cleave FnBPA (47), contributes to enhanced proteolysis of FnBPA in this mutant. However, because this phenotype was found in an artificially induced SaeSL18P mutant strain, the significance of this finding requires validation in a real model. In addition, we speculated whether dysregulation of extracellular proteases as a result of SaeS inactivation contributed to the observed biofilm development phenotype. However, no effect was observed in a mutant lacking 10 major secreted proteases or in individual aureolysin or SspAB mutants, indicating that Sae likely regulates FnBPA activity independently of secreted proteases. Finally, while it was previously shown that expression of either fnbA or fnbB was able restore immobilized Fn binding of a ΔfnbA ΔfnbB double mutant (48), our data indicate that FnBPA contributes the dominant role in Fn binding despite the high homology between FnBPA and FnBPB Fn-binding domains. A possible explanation for this is the effect of serum components on the function and/or stability of FnBPA versus that of FnBPB, as illustrated by dose-dependent results shown in Fig. 4. In addition, the contribution for these proteins to Fn binding may vary depending on modes of growth (biofilm versus planktonic). Further examination of the individual function of these proteins during biofilm formation in serum will be required to distinguish their contributions under these conditions.
We further assessed the impact of FnBPA-promoted biofilm architecture on immune cell infiltration and found that macrophages were significantly more capable of invading the FnBPA-deficient biofilms than the complex WT biofilms developed in serum. These data demonstrate that the physical structure of S. aureus biofilms directly influences macrophage invasion and suggest that FnBPA is employed during biofilm-mediated infection to resist immune cell invasion. In support of this, we found that both SaeS and FnBPA mutant strains were significantly attenuated in a mouse catheter-associated biofilm infection model. Interestingly, the SaeS mutant was significantly less capable of adhering to and/or colonizing the implanted catheter than the FnBPA mutant, indicating that other SaeS-regulated adherence mechanisms are altered. However, bacterial spread into the surrounding soft tissue was comparable in the SaeS and FnBPA mutants, suggesting that FnBPA is required to promote tissue invasion from established S. aureus device-associated biofilms.
In summary, S. aureus biofilms attenuate immune antibacterial activity by inhibiting effector phagocyte invasion, phagocytosis, and proinflammatory activity (8–10), due to a combination of both physical and biochemical inhibition. Our results implicate FnBPA as an essential component to normal S. aureus biofilm development, inhibition of macrophage invasion, and device-associated infection. We hypothesize that the FnBRs are required for initial seeding of Fn-coated devices and that A-domain-mediated intercellular bonds contribute significantly to biofilm accumulation and architecture.
MATERIALS AND METHODS
Bacterial strains.
The S. aureus strains used in these studies are described in Table 1, including a USA300 MRSA skin and soft tissue infection isolate cured of plasmid p03 (49), referred to as LAC in this work, USA200 methicillin-sensitive osteomyelitis isolate UAMS-1 (29), and the methicillin-sensitive osteomyelitis isolate strain Newman (50). Transposon insertion mutants were acquired from the Nebraska Transposon Mutant Library (49), and the transposons were transduced into WT LAC using bacteriophage ϕ11, as previously described (51). Colonies were selected by plating on tryptic soy agar (TSA) containing erythromycin (5 μg ml−1), and correct transposon insertion was confirmed by PCR.
TABLE 1.
Bacterial strains, qRT-PCR oligonucleotides, and plasmids
| Strain, oligonucleotide, or plasmid | Properties or sequence (5′→3′) | Reference |
|---|---|---|
| Strains | ||
| LAC | Wild-type S. aureus CA-MRSA (USA300) isolate cured of plasmid p03 | 49 |
| ΔsaeS | Transduction of saeS::ΦNΣ into LAC | 49, this work |
| ΔfnbA | Transduction of fnbA::ΦNΣ into LAC | 49, this work |
| ΔfnbB | Transduction of fnbB::ΦNΣ into LAC | 49, this work |
| JLB29 (SaeSL18P) | SaeSL18P chromosomal mutant in LAC lacking p03 | 58 |
| UAMS-1 | Wild-type S. aureus MSSA isolate | 29 |
| ΔsaePQRS | AH2333; UAMS-1 ΔsaePQRS::spec | 59 |
| Newman | Wild-type S. aureus human infection isolate | 50 |
| NewmanP18L | SaeSP18L chromosomal mutant in WT Newman | 30 |
| RN4220 | Highly transformable restriction-deficient strain | 60 |
| AH1919 | LAC Δaur ΔsspAB ΔscpA ΔsplA-F | 34 |
| CG100 | AH1919 ΔsaePQRS | This work |
| Oligonucleotides | ||
| fnbA up/fwd | CGCGGATCCTCTTCATTTTATTCATTAGCTTATTTG | This work |
| fnbA down/rev | TAAGTCGACAGCTCTTGAACACCTTGATA | This work |
| rpoD Fwd qRT-PCR | CGTGCAATTGCTGACCAAGCACG | This work |
| rpoD Rev qRT-PCR | TCTTCTGGTGCTGGATCTCGACCT | This work |
| fnbA Fwd qRT-PCR | TGGCACAGCCAAGAACGGCA | This work |
| fnbA Rev qRT-PCR | TGTACCCGTTTCCACTTTCGCGT | This work |
| fnbB Fwd qRT-PCR | AACTTGGAAAAATGGCGTTG | This work |
| fnbB Rev qRT-PCR | CATGACCTTCTGCACCTTCA | This work |
| JBKU130 | CAGGTACCGACATTACGTCATAATCCGATTTATTTAT | This work |
| JBKU131 | CAGTCGACTATGATCAAGTGATTGCAAAGCGTG | This work |
| CNK29 | CCGGAATTCCGATGTTTATCATTTGGACGATACTACAG | This work |
| CNK30 | CGGGGTACCGCTAACTCCTCATTTCTTCAATTTGATAAG | This work |
| Plasmids | ||
| pCM28 | E. coli-S. aureus shuttle vector | 61 |
| pCM29 | sarAP1::sGFP in pCM28 | 61 |
| pFnbA | fnbA in pCM28 | This work |
| pJB1047 | saePQRS allelic exchange plasmid in pJB38 | 52, this work |
The fnbA complementation vector was generated by amplifying fnbA from LAC (see Table 1), including 500 bp upstream and downstream to include native promoter and terminator elements. The PCR product was digested with BamHI and SalI and then ligated into likewise-digested pCM28, generating pFnbA. For the saePQRS allelic exchange vector, saeS downstream was amplified with primers JBKU130 and JBKU131, digested with KpnI and SalI, and ligated into the same sites of pJB38 to create pJB1046. Next, saeP upstream was amplified with CNK29 and CNK30, digested with EcoRI and KpnI, and ligated into the same sites of pJB1046 to create pJB1047. pJB1047 was phage transduced into S. aureus AH1919, and allelic exchange was performed as previously described (52), using primers JBKU131 and CNK29 to confirm saePQRS deletion. Transduction of pJB1047, pFnbA, and the constitutive superfolder green fluorescent protein (GFP) plasmid was performed using ϕ11 propagated on S. aureus RN4220.
Static biofilm development and microscopy.
Isolated colonies on TSA were grown overnight in RPMI 1640 (Becton, Dickinson, USA) at 37°C with orbital shaking at 250 rpm. Chloramphenicol (10 μg ml−1) was used when plasmid maintenance was required. Cultures were diluted to an optical density at 600 nm (OD600) of 0.05 in fresh RPMI 1640 supplemented with or without fetal bovine serum (Gibco Life Technologies, USA) or normal human serum (EMD Millipore, USA). Bacterial suspensions were added to wells of an 8-well chambered cover glass (Cellvis, USA) or 24- or 96-well plate at a surface-to-volume ratio of 0.19 and incubated statically at 37°C for the designated time.
Confocal analysis of constitutive GFP or nucleic acid dye fluorescence during static biofilm growth was performed using a CSU-X-1 spinning-disk confocal imager (Yokogawa, Japan) attached to a Zeiss Axio Observer inverted microscope. Hardware, including the Prim 95B digital camera (Photometrics, USA), was controlled by Micro-Manager imaging software. Images were analyzed using ZEN software (Zeiss, Germany). For strains lacking GFP fluorescence, Hoechst 33342 nucleic acid dye (Invitrogen, Molecular Probes, USA) was used at a 1:500 dilution and added to wells 30 min prior to imaging. Hoechst was visualized with a 405-nm excitation laser and DAPI (4′,6-diamidino-2-phenylindole) filter set. GFP fluorescence was acquired with 488-nm excitation laser and fluorescein isothiocyanate (FITC) filter set. Biofilm thickness and roughness coefficient were quantified by COMSTAT2 measurement (53).
RNA isolation and analysis.
Analysis of fnbA and fnbB transcription was performed by isolating RNA from static biofilms cultured in 24-well plates. Cells were suspended in 1 ml of TRIzol (Life Technologies, USA), transferred to screw cap tubes containing 0.1-mm-diameter glass beads (Bio Spec Products, USA), and lysed in BioSpec 1001 Mini-Beadbeater twice for 2 min at 4°C. Subsequent RNA purification was performed using a Direct-zol RNA isolation kit (Zymo Research, USA) according to the manufacturer’s recommendations. cDNA was generated using the Verso cDNA Synthesis kit (Thermo Scientific, USA) using random hexamer primers, and qRT-PCR was performed using iTaq Universal SYBR green supermix (Bio-Rad, USA) with primers specific to rpoD, fnbA, and fnbB (Table 1) designed using Primer3Plus (54). Data were analyzed using CFX Manager software (Bio-Rad) with gene expression levels normalized to rpoD and presented as the fold-induction threshold cycle (2−ΔΔCT) value relative to that in LAC in RPMI alone.
Immobilized human Fn binding.
Experiments were performed similarly to previously described methods (26, 48, 55–57). High-protein-binding 96-well plates (Costar, USA) were coated with 0.1 ml 10 μg/ml human Fn (Becton Dickenson, USA) or 1% bovine serum albumin (BSA; for control wells) overnight at 4°C. Biofilms were developed in 96-well plates for 17 h at 37°C as described above. The BSA and Fn solutions were removed, and the remaining binding sites were blocked with 0.1 ml 2% BSA for 1 h at room temperature. Biofilm cells were washed with phosphate-buffered saline (PBS) and suspended to an OD600 of 1.0. Blocked wells were washed three times with PBS, and then 0.1 ml of the biofilm suspensions was added and incubated for 1 h at 37°C. Nonadherent bacteria were removed by washing wells three times with PBS, and adherent bacteria were fixed with 0.1 ml 2% glutaraldehyde for 15 min. Wells were washed twice with PBS, and fixed adherent bacteria were stained with 0.1 ml 0.5% crystal violet (wt/vol in 12% ethanol [EtOH]) for 3 min. Unbound dye was removed with four washes with PBS, and bound dye was released with 0.1 ml 7% acetic acid and gentle shaking. The OD595 was measured and normalized against bacterial binding to wells coated only with BSA.
Bone marrow-derived macrophage isolation and biofilm invasion assay.
To prepare bone marrow-derived macrophages (BMDMs), bone marrow was isolated from the long bones (femurs and tibias) of C57BL/6 mice (Jackson Laboratories, USA) as previously described (9). These studies were conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (62). Fully differentiated BMDMs (2 × 105) were stained with 5 μM CellTracker Violet (Invitrogen, USA) for 1 h and cocultured for 4 h with 1-day-old biofilms in an 8-well chamber slide (Thermo Scientific Nunc, USA). Quantified BMDM invasion was analyzed using ZEN (Carl Zeiss) software and calculated as percentage of visible BMDMs in a sterile control well within the same x/y/z dimensions.
Mouse flank catheter model of infection.
S. aureus catheter-associated biofilm infections were performed as previously described (39). Briefly, isolated colonies on TSA were cultured in 25 ml of brain heart infusion (BHI) broth for 16 h and washed with PBS, and the OD600 was measured to estimate appropriate bacterial dilution prior to infection. C57BL/6 mice were anesthetized with ketamine-xylazine (100 mg/kg-5 mg/kg of body weight), and the surgical site was disinfected with povidone-iodine or 70% ethanol. A small subcutaneous incision was made in the mouse flank, and a blunt instrument was used to create a pocket for insertion of a 1-cm sterile 16-gauge Teflon-coated intravenous catheter (Excel International, USA). The incision was then sealed using Vetbond tissue adhesive (3M, USA), and 103 CFU of S. aureus in 20 μl PBS was injected directly into the catheter lumen using a 29-gauge insulin syringe (BD, USA). For postinfection bacterial enumeration, catheters were removed and placed in 100 μl of PBS for sonication to dissociate bacteria from the catheter surface. The soft tissue surrounding the catheter was collected, weighed, and dissociated using the blunt end of a 3-ml syringe in 500 μl of PBS. Bacterial titers were quantified on TSA and expressed as CFU per milliliter for catheters or CFU per gram of tissue.
Statistical analyses.
Significant differences between experimental groups were determined as described in the respective figure legends and/or Materials and Methods. GraphPad Prism 8 (GraphPad, La Jolla, CA) was used for all statistical analysis calculations, and a P value of <0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health NIAID under award numbers R01AI063426 to D.D.L., 3P01AI083211 project 4 to T.K., and R01AI121073 to J.L.B.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Grundmann H, Aires-de-Sousa M, Boyce J, Tiemersma E. 2006. Emergence and resurgence of methicillin-resistant Staphylococcus aureus as a public-health threat. Lancet 368:874–885. doi: 10.1016/S0140-6736(06)68853-3. [DOI] [PubMed] [Google Scholar]
- 2.Arciola CR, Campoccia D, Montanaro L. 2018. Implant infections: adhesion, biofilm formation and immune evasion. Nat Rev Microbiol 16:397–409. doi: 10.1038/s41579-018-0019-y. [DOI] [PubMed] [Google Scholar]
- 3.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, Ray SM, Thompson DL, Wilson LE, Fridkin SK, Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. 2014. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walls RJ, Roche SJ, O'Rourke A, McCabe JP. 2008. Surgical site infection with methicillin-resistant Staphylococcus aureus after primary total hip replacement. J Bone Joint Surg Br 90:292–298. doi: 10.1302/0301-620X.90B3.20155. [DOI] [PubMed] [Google Scholar]
- 5.Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. 2008. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res 466:1710–1715. doi: 10.1007/s11999-008-0209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson JM, Rodriguez A, Chang DT. 2008. Foreign body reaction to biomaterials. Semin Immunol 20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Francois P, Vaudaux P, Lew PD. 1998. Role of plasma and extracellular matrix proteins in the physiopathology of foreign body infections. Ann Vasc Surg 12:34–40. doi: 10.1007/s100169900112. [DOI] [PubMed] [Google Scholar]
- 8.Thurlow LR, Hanke ML, Fritz T, Angle A, Aldrich A, Williams SH, Engebretsen IL, Bayles KW, Horswill AR, Kielian T. 2011. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J Immunol 186:6585–6596. doi: 10.4049/jimmunol.1002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gries CM, Bruger EL, Moormeier DE, Scherr TD, Waters CM, Kielian T. 2016. Cyclic di-AMP released from Staphylococcus aureus biofilm induces a macrophage type I interferon response. Infect Immun 84:3564–3574. doi: 10.1128/IAI.00447-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gries CM, Kielian T. 2017. Staphylococcal biofilms and immune polarization during prosthetic joint infection. J Am Acad Orthop Surg 25(Suppl 1):S20–S24. doi: 10.5435/JAAOS-D-16-00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Q, Yeo WS, Bae T. 2016. The SaeRS two-component system of Staphylococcus aureus. Genes (Basel) 7:E81. doi: 10.3390/genes7100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herman-Bausier P, El-Kirat-Chatel S, Foster TJ, Geoghegan JA, Dufrêne YF. 2015. Staphylococcus aureus fibronectin-binding protein a mediates cell-cell adhesion through low-affinity homophilic bonds. mBio 6:e00413-15. doi: 10.1128/mBio.00413-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ponnuraj K, Bowden MG, Davis S, Gurusiddappa S, Moore D, Choe D, Xu Y, Hook M, Narayana SV. 2003. A “dock, lock, and latch” structural model for a staphylococcal adhesin binding to fibrinogen. Cell 115:217–228. doi: 10.1016/s0092-8674(03)00809-2. [DOI] [PubMed] [Google Scholar]
- 14.Keane FM, Loughman A, Valtulina V, Brennan M, Speziale P, Foster TJ. 2007. Fibrinogen and elastin bind to the same region within the A domain of fibronectin binding protein A, an MSCRAMM of Staphylococcus aureus. Mol Microbiol 63:711–723. doi: 10.1111/j.1365-2958.2006.05552.x. [DOI] [PubMed] [Google Scholar]
- 15.Stemberk V, Jones RP, Moroz O, Atkin KE, Edwards AM, Turkenburg JP, Leech AP, Massey RC, Potts JR. 2014. Evidence for steric regulation of fibrinogen binding to Staphylococcus aureus fibronectin-binding protein A (FnBPA). J Biol Chem 289:12842–12851. doi: 10.1074/jbc.M113.543546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bingham RJ, Rudiño-Piñera E, Meenan NAG, Schwarz-Linek U, Turkenburg JP, Höök M, Garman EF, Potts JR. 2008. Crystal structures of fibronectin-binding sites from Staphylococcus aureus FnBPA in complex with fibronectin domains. Proc Natl Acad Sci U S A 105:12254–12258. doi: 10.1073/pnas.0803556105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meenan NA, Visai L, Valtulina V, Schwarz-Linek U, Norris NC, Gurusiddappa S, Hook M, Speziale P, Potts JR. 2007. The tandem beta-zipper model defines high affinity fibronectin-binding repeats within Staphylococcus aureus FnBPA. J Biol Chem 282:25893–25902. doi: 10.1074/jbc.M703063200. [DOI] [PubMed] [Google Scholar]
- 18.Cassat JE, Hammer ND, Campbell JP, Benson MA, Perrien DS, Mrak LN, Smeltzer MS, Torres VJ, Skaar EP. 2013. A secreted bacterial protease tailors the Staphylococcus aureus virulence repertoire to modulate bone remodeling during osteomyelitis. Cell Host Microbe 13:759–772. doi: 10.1016/j.chom.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao F, Cheng BL, Boyle-Vavra S, Alegre ML, Daum RS, Chong AS, Montgomery CP. 2015. Proteomic identification of saeRS-dependent targets critical for protective humoral immunity against Staphylococcus aureus skin infection. Infect Immun 83:3712–3721. doi: 10.1128/IAI.00667-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cassat JE, Dunman PM, McAleese F, Murphy E, Projan SJ, Smeltzer MS. 2005. Comparative genomics of Staphylococcus aureus musculoskeletal isolates. J Bacteriol 187:576–592. doi: 10.1128/JB.187.2.576-592.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Que YA, Haefliger JA, Piroth L, Francois P, Widmer E, Entenza JM, Sinha B, Herrmann M, Francioli P, Vaudaux P, Moreillon P. 2005. Fibrinogen and fibronectin binding cooperate for valve infection and invasion in Staphylococcus aureus experimental endocarditis. J Exp Med 201:1627–1635. doi: 10.1084/jem.20050125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Neill E, Pozzi C, Houston P, Humphreys H, Robinson DA, Loughman A, Foster TJ, O'Gara JP. 2008. A novel Staphylococcus aureus biofilm phenotype mediated by the fibronectin-binding proteins, FnBPA and FnBPB. J Bacteriol 190:3835–3850. doi: 10.1128/JB.00167-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arciola CR, Campoccia D, Gamberini S, Baldassarri L, Montanaro L. 2005. Prevalence of cna, fnbA and fnbB adhesin genes among Staphylococcus aureus isolates from orthopedic infections associated to different types of implant. FEMS Microbiol Lett 246:81–86. doi: 10.1016/j.femsle.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 24.Peacock SJ, Moore CE, Justice A, Kantzanou M, Story L, Mackie K, O'Neill G, Day NPJ. 2002. Virulent combinations of adhesin and toxin genes in natural populations of Staphylococcus aureus. Infect Immun 70:4987–4996. doi: 10.1128/iai.70.9.4987-4996.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwarz-Linek U, Werner JM, Pickford AR, Gurusiddappa S, Kim JH, Pilka ES, Briggs JA, Gough TS, Hook M, Campbell ID, Potts JR. 2003. Pathogenic bacteria attach to human fibronectin through a tandem beta-zipper. Nature 423:177–181. doi: 10.1038/nature01589. [DOI] [PubMed] [Google Scholar]
- 26.Edwards AM, Potts JR, Josefsson E, Massey RC. 2010. Staphylococcus aureus host cell invasion and virulence in sepsis is facilitated by the multiple repeats within FnBPA. PLoS Pathog 6:e1000964. doi: 10.1371/journal.ppat.1000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shinji H, Yosizawa Y, Tajima A, Iwase T, Sugimoto S, Seki K, Mizunoe Y. 2011. Role of fibronectin-binding proteins A and B in in vitro cellular infections and in vivo septic infections by Staphylococcus aureus. Infect Immun 79:2215–2223. doi: 10.1128/IAI.00133-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vergara-Irigaray M, Valle J, Merino N, Latasa C, García B, Ruiz de Los Mozos I, Solano C, Toledo-Arana A, Penadés JR, Lasa I. 2009. Relevant role of fibronectin-binding proteins in Staphylococcus aureus biofilm-associated foreign-body infections. Infect Immun 77:3978–3991. doi: 10.1128/IAI.00616-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillaspy AF, Hickmon SG, Skinner RA, Thomas JR, Nelson CL, Smeltzer MS. 1995. Role of the accessory gene regulator (agr) in pathogenesis of staphylococcal osteomyelitis. Infect Immun 63:3373–3380. doi: 10.1128/IAI.63.9.3373-3380.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krute CN, Rice KC, Bose JL. 2017. VfrB Is a key activator of the Staphylococcus aureus SaeRS two-component system. J Bacteriol 199:e00828-16. doi: 10.1128/JB.00828-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mainiero M, Goerke C, Geiger T, Gonser C, Herbert S, Wolz C. 2010. Differential target gene activation by the Staphylococcus aureus two-component system saeRS. J Bacteriol 192:613–623. doi: 10.1128/JB.01242-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grundmeier M, Hussain M, Becker P, Heilmann C, Peters G, Sinha B. 2004. Truncation of fibronectin-binding proteins in Staphylococcus aureus strain Newman leads to deficient adherence and host cell invasion due to loss of the cell wall anchor function. Infect Immun 72:7155–7163. doi: 10.1128/IAI.72.12.7155-7163.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolar SL, Ibarra JA, Rivera FE, Mootz JM, Davenport JE, Stevens SM, Horswill AR, Shaw LN. 2013. Extracellular proteases are key mediators of Staphylococcus aureus virulence via the global modulation of virulence-determinant stability. Microbiologyopen 2:18–34. doi: 10.1002/mbo3.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wormann ME, Reichmann NT, Malone CL, Horswill AR, Grundling A. 2011. Proteolytic cleavage inactivates the Staphylococcus aureus lipoteichoic acid synthase. J Bacteriol 193:5279–5291. doi: 10.1128/JB.00369-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanke ML, Heim CE, Angle A, Sanderson SD, Kielian T. 2013. Targeting macrophage activation for the prevention and treatment of Staphylococcus aureus biofilm infections. J Immunol 190:2159–2168. doi: 10.4049/jimmunol.1202348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paharik AE, Horswill AR. 2016. The staphylococcal biofilm: adhesins, regulation, and host response. Microbiol Spectr 4:VMBF-0022-2015. doi: 10.1128/microbiolspec.VMBF-0022-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moormeier DE, Bayles KW. 2017. Staphylococcus aureus biofilm: a complex developmental organism. Mol Microbiol 104:365–376. doi: 10.1111/mmi.13634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamada KJ, Kielian T. 2019. Biofilm-leukocyte cross-talk: impact on immune polarization and immunometabolism. J Innate Immun 11:280–288. doi: 10.1159/000492680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamada KJ, Heim CE, Aldrich AL, Gries CM, Staudacher AG, Kielian T. 2018. Arginase-1 expression in myeloid cells regulates Staphylococcus aureus planktonic but not biofilm infection. Infect Immun 86:e00206-18. doi: 10.1128/IAI.00206-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Neill E, Pozzi C, Houston P, Smyth D, Humphreys H, Robinson DA, O'Gara JP. 2007. Association between methicillin susceptibility and biofilm regulation in Staphylococcus aureus isolates from device-related infections. J Clin Microbiol 45:1379–1388. doi: 10.1128/JCM.02280-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boles BR, Horswill AR. 2008. Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog 4:e1000052. doi: 10.1371/journal.ppat.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Voyich JM, Vuong C, DeWald M, Nygaard TK, Kocianova S, Griffith S, Jones J, Iverson C, Sturdevant DE, Braughton KR, Whitney AR, Otto M, DeLeo FR. 2009. The SaeR/S gene regulatory system is essential for innate immune evasion by Staphylococcus aureus. J Infect Dis 199:1698–1706. doi: 10.1086/598967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mashruwala AA, Gries CM, Scherr TD, Kielian T, Boyd JM. 2017. SaeRS is responsive to cellular respiratory status and regulates fermentative biofilm formation in Staphylococcus aureus. Infect Immun 85:e00157-17. doi: 10.1128/IAI.00157-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zapotoczna M, McCarthy H, Rudkin JK, O'Gara JP, O'Neill E. 2015. An essential role for coagulase in Staphylococcus aureus biofilm development reveals new therapeutic possibilities for device-related infections. J Infect Dis 212:1883–1893. doi: 10.1093/infdis/jiv319. [DOI] [PubMed] [Google Scholar]
- 45.Xu Y, Maltesen RG, Larsen LH, Schonheyder HC, Le VQ, Nielsen JL, Nielsen PH, Thomsen TR, Nielsen KL. 2016. In vivo gene expression in a Staphylococcus aureus prosthetic joint infection characterized by RNA sequencing and metabolomics: a pilot study. BMC Microbiol 16:80. doi: 10.1186/s12866-016-0695-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McGavin MJ, Zahradka C, Rice K, Scott JE. 1997. Modification of the Staphylococcus aureus fibronectin binding phenotype by V8 protease. Infect Immun 65:2621–2628. doi: 10.1128/IAI.65.7.2621-2628.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karlsson A, Saravia-Otten P, Tegmark K, Morfeldt E, Arvidson S. 2001. Decreased amounts of cell wall-associated protein A and fibronectin-binding proteins in Staphylococcus aureus sarA mutants due to up-regulation of extracellular proteases. Infect Immun 69:4742–4748. doi: 10.1128/IAI.69.8.4742-4748.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCourt J, O'Halloran DP, McCarthy H, O'Gara JP, Geoghegan JA. 2014. Fibronectin-binding proteins are required for biofilm formation by community-associated methicillin-resistant Staphylococcus aureus strain LAC. FEMS Microbiol Lett 353:157–164. doi: 10.1111/1574-6968.12424. [DOI] [PubMed] [Google Scholar]
- 49.Fey PD, Endres JL, Yajjala VK, Widhelm TJ, Boissy RJ, Bose JL, Bayles KW. 2013. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. mBio 4:e00537-12. doi: 10.1128/mBio.00537-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duthie ES, Lorenz LL. 1952. Staphylococcal coagulase; mode of action and antigenicity. J Gen Microbiol 6:95–107. doi: 10.1099/00221287-6-1-2-95. [DOI] [PubMed] [Google Scholar]
- 51.Gries CM, Bose JL, Nuxoll AS, Fey PD, Bayles KW. 2013. The Ktr potassium transport system in Staphylococcus aureus and its role in cell physiology, antimicrobial resistance and pathogenesis. Mol Microbiol 89:760–773. doi: 10.1111/mmi.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bose JL, Fey PD, Bayles KW. 2013. Genetic tools to enhance the study of gene function and regulation in Staphylococcus aureus. Appl Environ Microbiol 79:2218–2224. doi: 10.1128/AEM.00136-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heydorn A, Nielsen AT, Hentzer M, Sternberg C, Givskov M, Ersboll BK, Molin S. 2000. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146:2395–2407. doi: 10.1099/00221287-146-10-2395. [DOI] [PubMed] [Google Scholar]
- 54.Untergasser A, Nijveen H, Rao X, Bisseling T, Geurts R, Leunissen JA. 2007. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res 35:W71–W74. doi: 10.1093/nar/gkm306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y, Ren B, Zhou X, Liu S, Zhou Y, Li B, Jiang Y, Li M, Feng M, Cheng L. 2017. Growth and adherence of Staphylococcus aureus were enhanced through the PGE2 produced by the activated COX-2/PGE2 pathway of infected oral epithelial cells. PLoS One 12:e0177166. doi: 10.1371/journal.pone.0177166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peacock SJ, Foster TJ, Cameron BJ, Berendt AR. 1999. Bacterial fibronectin-binding proteins and endothelial cell surface fibronectin mediate adherence of Staphylococcus aureus to resting human endothelial cells. Microbiology 145:3477–3486. doi: 10.1099/00221287-145-12-3477. [DOI] [PubMed] [Google Scholar]
- 57.Ahmed S, Meghji S, Williams RJ, Henderson B, Brock JH, Nair SP. 2001. Staphylococcus aureus fibronectin binding proteins are essential for internalization by osteoblasts but do not account for differences in intracellular levels of bacteria. Infect Immun 69:2872–2877. doi: 10.1128/IAI.69.5.2872-2877.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bose JL, Lehman MK, Fey PD, Bayles KW. 2012. Contribution of the Staphylococcus aureus Atl AM and GL murein hydrolase activities in cell division, autolysis, and biofilm formation. PLoS One 7:e42244. doi: 10.1371/journal.pone.0042244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olson ME, Nygaard TK, Ackermann L, Watkins RL, Zurek OW, Pallister KB, Griffith S, Kiedrowski MR, Flack CE, Kavanaugh JS, Kreiswirth BN, Horswill AR, Voyich JM. 2013. Staphylococcus aureus nuclease is an SaeRS-dependent virulence factor. Infect Immun 81:1316–1324. doi: 10.1128/IAI.01242-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kreiswirth BN, Löfdahl S, Betley MJ, O'Reilly M, Schlievert PM, Bergdoll MS, Novick RP. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305:709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- 61.Pang YY, Schwartz J, Thoendel M, Ackermann LW, Horswill AR, Nauseef WM. 2010. agr-dependent interactions of Staphylococcus aureus USA300 with human polymorphonuclear neutrophils. J Innate Immun 2:546–559. doi: 10.1159/000319855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.