Endothelial activation and microvascular dysfunction are key pathogenic processes in severe malaria. We evaluated the early role of these processes in experimentally induced Plasmodium falciparum and P. vivax infection. Participants were enrolled in induced blood-stage malaria clinical trials. Plasma osteoprotegerin, angiopoietin-2, and von Willebrand Factor (vWF) levels were measured as biomarkers of endothelial activation. Microvascular function was assessed using peripheral arterial tonometry and near-infrared spectroscopy, and the endothelial glycocalyx was assessed by sublingual videomicroscopy and measurement of biomarkers of degradation.
KEYWORDS: malaria, falciparum, vivax, microvascular, OPG, osteoprotegerin, vWF, von Willebrand factor, Ang-2, angiopoietin 2, glycocalyx, IBSM
ABSTRACT
Endothelial activation and microvascular dysfunction are key pathogenic processes in severe malaria. We evaluated the early role of these processes in experimentally induced Plasmodium falciparum and P. vivax infection. Participants were enrolled in induced blood-stage malaria clinical trials. Plasma osteoprotegerin, angiopoietin-2, and von Willebrand Factor (vWF) levels were measured as biomarkers of endothelial activation. Microvascular function was assessed using peripheral arterial tonometry and near-infrared spectroscopy, and the endothelial glycocalyx was assessed by sublingual videomicroscopy and measurement of biomarkers of degradation. Forty-five healthy, malaria-naive participants were recruited from 5 studies. Osteoprotegerin and vWF levels increased in participants following inoculation with P. vivax (n = 16) or P. falciparum (n = 15), with the angiopoietin-2 level also increasing in participants following inoculation with P. falciparum. For both species, the most pronounced increase was seen in osteoprotegerin. This was particularly marked in participants inoculated with P. vivax, where the osteoprotegerin level correlated with the levels of parasitemia and the malaria clinical score. There were no changes in measures of endothelial glycocalyx or microvascular function. Plasma biomarkers of endothelial activation increased in early P. falciparum and P. vivax infection and preceded changes in the endothelial glycocalyx or microvascular function. The more pronounced increase in osteoprotegerin suggests that this biomarker may play a role in disease pathogenesis.
INTRODUCTION
Microvascular dysfunction is central to the pathogenesis of the organ dysfunction characteristic of severe malaria caused by Plasmodium falciparum (1–3), P. vivax (4–6), and P. knowlesi (7). Despite this, the underlying mechanisms are incompletely understood. Key factors contributing to microvascular dysfunction in falciparum and vivax malaria include endothelial activation (2, 5), the reduced bioavailability of endothelial nitric oxide (NO) (1, 6), and, in falciparum malaria, alterations in the endothelial glycocalyx (8, 9). Microvascular dysfunction contributes to impaired tissue perfusion and oxygenation and is associated with mortality in falciparum malaria (3).
Endothelial activation leads to exocytosis of Weibel Palade bodies (WPBs), which are endothelial cell storage organelles (10). The constituents of WPBs include angiopoeitin-2 (Ang-2), von Willebrand factor (vWF), and osteoprotegerin (OPG); these act as mediators of hemostasis, inflammation, and vascular tone (10). Ang-2 is a key biomarker of disease severity in falciparum malaria and is significantly associated with parasite biomass, microvascular dysfunction, and mortality (2, 11). In vivax malaria, Ang-2 is also associated with parasitemia and clinical severity (5, 12). Similarly, vWF is associated with parasitemia and disease severity in falciparum malaria, even in early infection (13, 14), and with parasitemia in vivax malaria (5). OPG has also recently been shown to be associated with mortality in children with falciparum malaria (15) and with endothelial dysfunction and disease severity in adults with knowlesi malaria (16). As a soluble decoy receptor, OPG reduces the effect of RANKL (receptor activator of NF-κB ligand), which regulates endothelial cell survival in vitro (17). In human endothelial cells, OPG enhances the effects of Ang-2 and mediates the upregulation of cell adhesion molecules in the presence of tumor necrosis factor alpha (TNF-α) (18). OPG has not been evaluated in adults with falciparum or vivax malaria.
The endothelial glycocalyx is a gel-like brush border consisting of transmembrane anchoring proteins, including syndecans and glypicans, covalently linked to glycosaminoglycan side chains (19). It modulates interactions between vascular contents and endothelial cells, mediates flow-dependent NO production, and protects against the cytoadhesion of parasitized red cells, leukocytes, and platelets (19). The glycocalyx is degraded in falciparum malaria and is significantly associated with endothelial activation (9, 20) and with disease severity and mortality (8). How early this glycocalyx degradation occurs in infection has not been studied.
Understanding the cascade of events leading to microvascular dysfunction in malaria may allow for better determination of causal pathways, prognostication, and aid in the development of adjunctive therapies. Volunteer infection studies allow for the prospective evaluation of multiple aspects of early malaria infection, in many cases, prior to the onset of disease, which is not possible in clinical studies (21). In this study, we evaluated markers of endothelial activation, glycocalyx degradation, microvascular reactivity, and tissue perfusion in the early stages of P. falciparum and P. vivax infection in subjects who participated in clinical trials using the induced blood-stage malaria (IBSM) model.
(Preliminary results of this study were presented at the Australian Society for Infectious Diseases Annual Scientific Meeting, Darwin, Australia, 2019.)
RESULTS
Study participants and IBSM studies.
A total of 45 healthy, malaria-naive participants were recruited from 5 IBSM studies between 2014 and 2016. Participants had a median age of 24 years (range, 18 to 48 years), and 36 (80%) were male. Twenty-nine were inoculated with P. falciparum, and 16 were inoculated with P. vivax. The day of treatment differed between studies, ranging from day 7 to day 10. All participants developed a measurable parasitemia (Fig. 1). The median parasitemia on the day of treatment was 7,479/ml (range, 271 to 95,851/ml) for the P. falciparum group and 19,770/ml (range, 1,288 to 175,522/ml) for the P. vivax group. Study population information is described in Table 1, and individual-level information is described in Table S1 in the supplemental material.
FIG 1.
Parasitemia curves. (A) P. falciparum-inoculated group (n = 29). (B) P. vivax-inoculated group (n = 16). Day represents the study day, where day 0 is the day of inoculation. Gray lines, individual participant results; black lines, median results.
TABLE 1.
Study population and study proceduresa
| Study | Cohort | Yr | No. of participants | Inoculum species | Inoculum size (approx no. of viable parasites) | Day of treatment (days after inoculation) | Median (range) age (yr) | No. of males/total no. | Median (range) parasitemia at treatment as: |
Median (range) clinical score | Endothelial activation markersb | GC degradation markersc | Noninvasive measurements |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of parasites/ml | Log10 no. of parasites/ml | PAT | NIRS | Videomicroscopy | ||||||||||||
| NCT02783820 | 5 | 2016 | 8 | P. falciparum | 2,800 | 8 | 29 (20–48) | 8/8 | 11,535 (1,508–95,851) | 4.1 (3.2–5.0) | 1.5 (0–5) | Y | Y | − | − | Y |
| NCT02389348 | 1 | 2015 | 8 | P. falciparum | 1,800 | 7 | 23 (19–55) | 5/8 | 1,556 (271–7,495) | 3.2 (2.5–3.9) | 0 (0–9) | Y | Y | − | Y | − |
| NCT02389348 | 2 | 2015 | 3 | P. falciparum | 1,800 | 7 | 24 (24–27) | 1/3 | 20,281 (12,111–27,284) | 4.3 (4.1–4.4) | 0 (0–0) | − | − | Y | Y | − |
| Collins et al. (31) | 1 | 2015 | 4 | P. falciparum | 2,800 | 7 | 25.5 (23–32) | 3/4 | 12,440 (3,819–61,357) | 4.2 (3.6–4.8) | 1 (0–3) | − | − | Y | Y | − |
| NCT02281344 | 1 | 2014 | 6 | P. falciparum | 1,800 | 7 | 26 (19–29) | 6/6 | 6,411 (3,121–17,890) | 3.8 (3.5–4.3) | NA | − | Y | − | Y | − |
| ACTRN12616000174482 | 1 | 2016 | 8 | P. vivax | 1,100 | 8 | 19.5 (18–30) | 5/8 | 5,737 (1,288–9,209) | 4.0 (3.3–4.2) | 3 (0–11) | Y | Y | Y | Y | Y |
| NCT02573857 | 2 | 2016 | 8 | P. vivax | 1,100 | 10 | 23 (21–31) | 8/8 | 80,149 (30,331–175,522) | 5.1 (4.7–5.5) | 16 (1–35) | Y | Y | − | − | Y |
Abbreviations: GC, glycocalyx; PAT, peripheral arterial tonometry; NIRS, near-infrared spectroscopy; NA, clinical score data not available for this study; Y, marker found or measurement performed; −, marker not found or measurement not performed.
Endothelial activation markers are plasma osteoprotegerin, angiopoietin 2, and von Willebrand factor.
Glycocalyx degradation markers are urinary glycosaminoglycans, plasma syndecan-1, and plasma sphingosine-1-phosphate.
Biomarkers of endothelial activation.
Markers of endothelial activation were measured in participants who had plasma available, which included 15 participants inoculated with P. falciparum and 16 inoculated with P. vivax. The study population with biomarker measurements was predominately male (13/15 for the P. falciparum-inoculated group, 13/16 for the P. vivax-inoculated group).
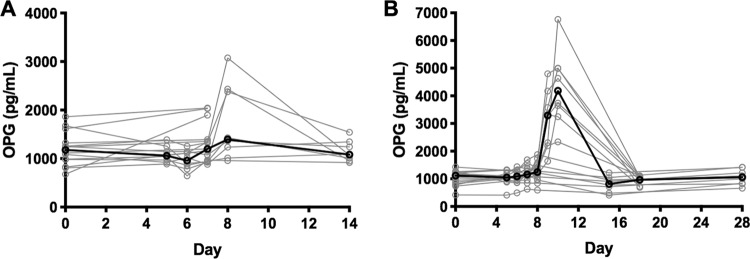
In the P. falciparum-inoculated group, OPG, Ang-2, and vWF levels all increased from baseline to the day of treatment (Fig. 2A to C; Fig. S1A to C). The greatest increase from baseline occurred in OPG, which increased from a median concentration of 1,179 pg/ml (interquartile range [IQR], 678 to 1,863 pg/ml) at baseline to 1,393 pg/ml (IQR, 958 to 3,074 pg/ml) at the day of treatment (P = 0.015; effect size [ES], 0.952). OPG levels returned to normal at follow-up (day 14). A modest increase from baseline to the day of treatment also occurred for Ang-2 (1,712 pg/ml [IQR, 1,009 to 2,682 pg/ml] to 1,874 pg/ml [IQR, 1,205 to 3,039 pg/ml]; P = 0.020; ES, 0.294) and vWF (660 mU/ml [IQR, 392 to 789 mU/ml] to 684 mU/ml [IQR, 485 to 1,193 mU/ml]; P = 0.016; ES, 0.522). At an individual level, a subset of participants experienced more pronounced changes in biomarker levels during the course of parasitemia (Fig. 2A to C). The platelet count decreased from a median of 222 × 109/liter (IQR, 203 × 109 to 306 × 109/liter) to 200 × 109/liter (IQR, 162 × 109 to 259 × 109/liter) (P < 0.001; ES, 0.664) on the day of treatment but returned to baseline values at the follow-up visit (days 23 to 26; median, 233 × 109/liter [IQR, 172 × 109 to 295 × 109/liter]) (Fig. 2D).
FIG 2.

Plasma biomarkers of endothelial activation over time for the P. falciparum-inoculated group (n = 15). (A) OPG concentration (P = 0.015); (B) Ang-2 concentration (P = 0.020); (C) vWF concentration (P = 0.016); (D) platelet count (P < 0.001). Gray lines, individual participant results; black lines, median results; filled data points, value for the individual participant on the day of treatment; *, P < 0.05 for the paired comparison of the participant results at baseline and on the day of treatment. Abbreviations: OPG, osteoprotegerin; Ang-2, angiopoietin 2; vWF, von Willebrand factor; DOT, day of treatment.
In participants inoculated with P. falciparum, none of the biomarkers of endothelial activation correlated highly with parasitemia or the cumulative clinical score. A modest negative correlation between the vWF level and the platelet count was observed (r = −0.326, P = 0.036) (Table 2). At an individual level, two participants (participants 3 and 5 in the trial with ClinicalTrials.gov registration number NCT02783820) demonstrated an increase in the OPG level on the day of treatment without any recorded signs or reported symptoms of malaria infection (cumulative clinical symptoms scores, 0).
TABLE 2.
Spearman’s correlation coefficients for biochemical markers of endothelial activation, parasitemia, clinical score, and platelet countd
| Marker | Spearman’s correlation coefficient |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parasitemia |
Clinical score |
OPG |
Angiopoetin-2 |
vWF |
Platelet count |
|||||||
| P. falciparum | P. vivax | P. falciparum | P. vivax | P. falciparum | P. vivax | P. falciparum | P. vivax | P. falciparum | P. vivax | P. falciparum | P. vivax | |
| Parasitemia | −0.057 | 0.603a | 0.229 | 0.562c | 0.051 | 0.146 | −0.038 | 0.295b | −0.326 | −0.574b | ||
| Clinical score | −0.057 | 0.603a | −0.119 | 0.818c | −0.588a | 0.425 | −0.109 | 0.564a | −0.156 | −0.257 | ||
| OPG | 0.229 | 0.562c | −0.119 | 0.818c | 0.220 | 0.384c | 0.256 | 0.463c | 0.024 | −0.472b | ||
| Angiopoetin-2 | 0.051 | 0.146 | −0.588 | 0.425 | 0.220 | 0.384c | 0.206 | 0.324c | 0.145 | −0.040 | ||
| vWF | −0.038 | 0.295b | −0.109 | 0.564a | 0.256 | 0.463c | 0.206 | 0.324c | −0.384a | −0.057 | ||
| Platelet count | −0.326 | −0.574b | −0.156 | −0.257 | 0.024 | −0.472b | 0.145 | −0.040 | −0.384 | −0.057 | ||
P < 0.05.
P < 0.01.
P < 0.001.
Parasitemia is in log10 number of parasites per milliliter, the clinical score is the cumulative clinical score, OPG is the osteoprotegerin concentration (in picograms per milliliter), angiopoetin-2 is the angiopoetin-2 concentration (in picograms per milliliter), vWF is the von Willebrand factor concentration (in milliunits per milliliter), the platelet count is in 109 cells per liter, P. falciparum represents the P. falciparum-inoculated group, and P. vivax represents the P. vivax-inoculated group. P values represent the P values of the correlation.
In the P. vivax-inoculated group, OPG and vWF levels increased from day 8 (Fig. 3A to C; Fig. S1D to F). As with those inoculated with P. falciparum, the greatest increase occurred in OPG levels, which increased from a median of 1,115 pg/ml (IQR, 420 to 1,425 pg/ml) at baseline to 2,106 pg/ml (IQR, 593 to 6,761 pg/ml) on the day of treatment (P = 0.002; ES, 1.261). Similar to the findings for the P. falciparum-inoculated group, OPG returned to normal levels at the follow-up visits (days 15 to 18) (Fig. 4). The vWF level increased from 579 mU/ml (IQR, 411 to 1,042 mU/ml) to 616 mU/ml (IQR, 396 to 1,265 mU/ml) (P = 0.015; ES, 0.603). The small increase in Ang-2 levels observed was not statistically significant. At an individual level, most participants experienced changes in biomarker levels during the later stages of parasitemia (Fig. 3A to C). The platelet count decreased from a median of 226 × 109/liter (IQR, 211 × 109 to 272 × 109/liter) at baseline to 189 × 109/liter (IQR, 168 × 109 to 218 × 109/liter) on the day of treatment (P < 0.001; ES, 1.217). The platelet count was increased compared to that at baseline at the follow-up visit (days 22 to 25; median, 260 × 109/liter [IQR, 234 × 109 to 300 × 109/liter]; P = 0.001; ES, 0.699) (Fig. 3D).
FIG 3.

Plasma biomarkers of endothelial activation over time for the P. vivax-inoculated group (n = 16). (A) OPG concentration (P = 0.002); (B) Ang-2 concentration (P = 0.669); (C) vWF concentration (P = 0.015); (D) platelet count (day of treatment, P < 0.001; follow up, P = 0.001). Gray lines, individual participant results; black lines, median results; filled data points, value for the individual participant on the day of treatment; *, P < 0.05 for the paired comparison of the participant results at baseline and on the day of treatment; **, P < 0.05 by comparison with the baseline value.
FIG 4.
Osteoprotegerin (OPG) levels at posttreatment follow-up for the P. falciparum-inoculated group (n = 15) (A) and the P. vivax-inoculated group (n = 16) (B). Gray lines, individual participant results; black lines, median results.
In participants inoculated with P. vivax, the OPG level correlated positively with parasitemia (r = 0.562, P < 0.001) and with the cumulative clinical score (r = 0.818, P < 0.001) and negatively with the platelet count (r = −0.472, P = 0.006). vWF and Ang-2 levels were also correlated with the clinical score (r = 0.564 and P = 0.025 for vWF levels and r = 0.425 and P = 0.001 for Ang-2 levels) (Table 2). The platelet count was negatively correlated with parasitemia (r = −0.574, P = 0.001). At an individual level, one participant (participant 6 in the trial with Australian New Zealand Clinical Trials Registry registration number ACTRN12616000174482) demonstrated an increase in the OPG level on the day of treatment without any recorded signs or reported symptoms of malaria infection (cumulative clinical symptoms score, 0).
In both groups, the majority of hematology parameters remained within normal laboratory limits for age and gender at baseline and on the day of treatment.
Urinary and plasma markers of glycocalyx degradation.
Urinary glycosaminoglycan levels were measured in 15 participants inoculated with P. falciparum and 16 inoculated with P. vivax. Plasma syndecan-1 levels were measured in 10 participants inoculated with P. falciparum and 12 inoculated with P. vivax. The plasma sphingosine-1-phosphate level was measured as an upstream marker of glycocalyx integrity in 15 participants inoculated with P. falciparum and 16 inoculated with P. vivax. There was no change in any of the markers of glycocalyx degradation between baseline and the day of treatment for either inoculum group (Fig. S2). The measurements obtained at baseline and on the day of treatment did not differ between the P. falciparum- and P. vivax-inoculated groups.
Noninvasive bedside assessment of microvascular function.
Peripheral arterial tonometry was performed in 16 participants (8 inoculated with P. falciparum, 8 inoculated with P. vivax). Near-infrared spectroscopy was performed in 29 participants (21 inoculated with P. falciparum, 8 inoculated with P. vivax). Sublingual videomicroscopy assessment for glycocalyx thickness, capillary filling, and vessel size distribution was performed in 24 participants (8 inoculated with P. falciparum, 16 inoculated with P. vivax) (Table S1). No differences between the values obtained at baseline and on the day of treatment were observed for any of the noninvasive imaging modalities (Fig. S3).
DISCUSSION
Plasma biomarkers of WPB exocytosis and endothelial activation are increased early in infection with both P. falciparum and P. vivax in the IBSM model. These increases preceded detectable glycocalyx breakdown and microvascular dysfunction and occurred even though the parasitemia values were well below those generally observed in the clinical setting. A subset of participants experienced more pronounced elevations in biomarkers of endothelial activation, and in some participants, they preceded symptom onset. Taken together, these findings suggest that IBSM studies may be used to study the subtle changes that occur early in the disease process and that endothelial activation may be a key upstream factor mediating early disease pathogenesis in both falciparum and vivax malaria.
A notable finding in this study was the early and pronounced increase in OPG levels following inoculation with P. falciparum and P. vivax, with the effect size of this change being greater than the effect sizes of changes in the levels of the other WPB constituents, vWF and Ang-2. OPG is a cytokine of the TNF receptor superfamily that has been well described to be a mediator of bone remodeling by acting as a RANKL decoy receptor to modulate RANK signaling (22). More recently, OPG has been described to be a constituent of WPBs and a marker of endothelial activation and is associated with adhesion molecule upregulation and vascular remodeling (22) and with a reduction of endothelial NO production by inhibiting the RANKL-induced activation of endothelial NO synthase (23). The OPG level increases early in P. berghei-infected mice (15) and is correlated with disease severity and mortality in children with P. falciparum infection (15). In adults with P. knowlesi infection, the OPG level is also increased in proportion to disease severity and is associated with endothelial dysfunction (16). In this study, we demonstrate that the OPG level increases early following infection with P. falciparum and P. vivax, with a return to baseline levels following treatment for both species. The finding that the OPG level correlated with the clinical score and parasitemia in the P. vivax-inoculated group suggests that this biomarker may be involved in the progression of disease. The lack of such correlations in the P. falciparum-inoculated group may relate to the lower level of parasitemia and the lower clinical score in this group at the time of treatment or, potentially, an earlier or greater role for OPG in P. vivax pathogenesis. Furthermore, it is possible that the sequestration of P. falciparum-infected erythrocytes may affect the correlation of biomarkers with the measured peripheral parasitemia.
Increases in vWF and Ang-2 levels in the P. falciparum-inoculated group and the vWF level in the P. vivax-inoculated group provide further evidence of early WPB exocytosis and endothelial activation in primary infection in malaria-naive adults. Whether endothelial activation occurs at such low parasitemias in other settings, such as in tolerant individuals with asymptomatic parasitemia in settings where malaria is endemic, requires further evaluation. This will be an important population to study, as chronic endothelial activation from persistent or frequently recurring P. falciparum or P. vivax infection could have the potential to increase cardiovascular risk (24–26).
In this study, we observed a correlation between the vWF level and the platelet decrement in the P. falciparum-inoculated group and between the OPG level and the platelet decrement in the P. vivax-inoculated group. This is consistent with the findings of previous studies also demonstrating an association between platelet reduction and the vWF level in patients with P. falciparum (13, 35) and P. vivax (35) infection and suggests a link between endothelial activation and thrombocytopenia. The relative increase in the platelet count to suprabaseline levels in the follow-up period observed in the P. vivax-inoculated group has not been previously described but may result from an increase in thrombopoietin associated with hematological recovery from malaria.
Interestingly, a subset of individuals in both the P. falciparum- and P. vivax-inoculated groups appeared to experience more pronounced elevations in the levels of biomarkers of endothelial activation. Although the numbers were small, in this study the variation was not explained by participant age or sex and was not fully accounted for by a variation in parasitemia. Larger studies would be required to evaluate factors which may affect the predisposition of an individual to greater endothelial activation.
We did not observe any change from baseline in any of the specific measures of glycocalyx degradation in this study. While glycocalyx degradation has been observed in proportion to disease severity in adult (8) and pediatric (20) patients with falciparum malaria and in P. berghei-infected mice with experimental cerebral malaria (19), no change from baseline in plasma syndecan-1 or urinary glycosaminoglycan levels or videomicroscopy quantitation of glycocalyx thickness was observed in the study participants. Sphingosine-1-phosphate has a hypothesized upstream role modulating glycocalyx integrity, and reduced levels have been observed in both P. falciparum and P. vivax infection, with the levels being associated with disease severity in patients with P. falciparum malaria (8, 28, 29). Nonetheless, no change to suggest glycocalyx degradation or dysfunction in early experimental infection was observed in our study. Whether such measures are insufficiently sensitive for detecting an early loss of integrity or whether glycocalyx degradation occurs later in disease progression requires further investigation.
No change in peripheral arterial tonometry in our study was observed. While reduced NO-mediated endothelial function has been demonstrated in patients with moderate and severe P. falciparum and P. vivax infection (1, 6, 30), we did not demonstrate this in early-stage disease. Similarly, while reduced tissue perfusion has been shown using near-infrared spectroscopy in clinical P. falciparum (3) and P. vivax (5) infections, no change was observed for participants inoculated with either species in this study. This may suggest that endothelial activation precedes glycocalyx degradation and microvascular dysfunction or that these measures lack the sensitivity required to monitor physiological changes in early infection.
There were some limitations to this study. Due to differences in the day of antimalarial treatment, there were variations in peak parasitemia and clinical scores, with particular differences being seen between the P. falciparum- and P. vivax-inoculated groups. As a result, it is difficult to directly compare biomarkers between individuals inoculated with the different species, and therefore statistical comparisons have not been performed. However, the lack of a significant increase in the Ang-2 level in the P. vivax-inoculated group, despite a higher parasitemia, may suggest that Ang-2 contributes less to the early pathogenesis of P. vivax infection than to that of P. falciparum infection. Although multivariate analysis could be used to control for these factors, a larger study population would be required. Furthermore, the study population was predominately male, which may limit the generalizability of the results to a female population. While this study has allowed for the evaluation of multiple components of microvascular homeostasis using multiple measurement techniques, some of our negative results may be due to a lack of test sensitivity to detect changes early in the course of infection. While we hypothesize that the notable increase in OPG levels in this study reflects endothelial activation and associated WBP release, OPG has a widespread tissue distribution (22), and further studies are required to determine the source of OPG in malaria infection. Finally, we did not measure the levels of all known WPB constituents, which may be also relevant to the pathogenesis of disease (10).
Understanding the pathophysiology of early malaria infection may help us understand the mechanisms of upstream processes contributing to later disease and lead to the development of prognostic markers and adjunctive therapies. Using the IBSM model, we have demonstrated that endothelial WPB exocytosis and activation occur early in both P. falciparum and P. vivax infection and prior to detectable microvascular dysfunction. Our finding that the OPG level demonstrated the earliest and most pronounced change suggests that this biomarker may play a key role in early disease pathogenesis.
MATERIALS AND METHODS
Study participants and procedures.
This was a prospective, single-center exploratory study performed between 2014 and 2016 at Q-Pharm Pty. Ltd., Brisbane, Australia. The participants were recruited to the exploratory study after enrolling in IBSM clinical trials (ClinicalTrials.gov registration numbers NCT02783820 and NCT02389348 [31], ClinicalTrials.gov registration number NCT02281344. Australian New Zealand Clinical Trials Registry registration number ACTRN12616000174482, and ClinicalTrials.gov registration number NCT02573857). In brief, healthy malaria-naive adults aged 18 to 55 years were inoculated with ∼1,800 to 2,800 viable P. falciparum-infected erythrocytes or ∼1,100 to 1,800 viable P. vivax-infected erythrocytes. Peripheral blood parasitemia was monitored at least daily by quantitative PCR as described previously (27). The participants were administered antimalarial treatment following the development of clinical signs of malaria or after reaching a prespecified threshold parasitemia. Clinical signs of malaria were quantified by study staff using a clinical score template described previously (32). Standard safety assessments, including assessments of hematology and biochemistry parameters, were performed at specified times during the studies. Follow-up safety assessments were performed approximately 2 weeks following treatment. The timing of follow-up varied between IBSM trials.
Baseline measurements of endothelial activation, glycocalyx degradation, and microvascular function were conducted prior to malaria parasite inoculation. Longitudinal measurements were collected during routine study visits from the day of inoculation until the day of treatment and on the day of treatment prior to the administration of antimalarials. Some participants underwent further measurements in the posttreatment period. For logistical reasons, not all participants underwent evaluations for all measures of endothelial activation, glycocalyx degradation, and microvascular function.
Ethics statement.
The IBSM studies were approved by the QIMR-Berghofer Medical Research Institute Ethics Committee. The exploratory study was approved by the Menzies School of Health Research and the QIMR-Berghofer Medical Research Institute Ethics Committees. All participants provided informed written consent for the IBSM clinical trials and the exploratory study.
Biomarkers of endothelial activation and glycocalyx degradation.
Plasma OPG, Ang-2, and vWF levels were measured as biomarkers of endothelial activation. Venous blood samples were collected in tubes containing lithium heparin (OPG, Ang-2) or tubes containing sodium citrate (vWF). The blood samples were spun, and plasma was stored at −80°C within 30 min of collection. OPG, Ang-2, and vWF levels were measured by enzyme-linked immunosorbent assays (ELISA) according to the manufacturers’ instructions (DuoSet R&D Systems, Quantikine R&D Systems, and Imubind BioMedica Diagnostics).
Urinary glycosaminoglycan levels were measured as biomarkers of glycocalyx degradation. Urine samples were collected and stored at −80°C within 30 min. Glycosaminoglycan levels were measured by a previously validated dimethylmethylene blue colorimetric assay, and the results were corrected for the urinary creatinine concentration, as previously described (33). Plasma levels of the glycocalyx constituent syndecan-1 and the glycocalyx stabilizer sphingosine-1-phosphate were measured as markers of glycocalyx integrity by ELISA according to the manufacturers’ instructions (Cell Sciences; Echelon Biosciences, Inc.). Blood samples were spun, and heparinized plasma was stored at −80°C within 30 min of collection.
Noninvasive bedside assessment of microvascular function.
Endothelial function was assessed using peripheral arterial tonometry (EndoPAT; Itamar Medical, Israel). The change in the digital pulse wave amplitude in response to reactive hyperemia following the release of a standardized ischemic stress was measured to calculate the reactive hyperemia peripheral arterial tonometry (RH-PAT) index, as previously described (1).
Microcirculatory oxygenation was assessed as a marker of microvascular function using near-infrared spectroscopy (InSpectra 650 instrument; Hutchinson Technology, Hutchinson, MN). A probe applied to the thenar eminence was used to measure the tissue oxygen saturation (StO2) by comparing the relative absorption of near-infrared light by oxy- and deoxyhemoglobin before and after a standardized ischemic stress, as previously described (3). To induce an ischemic stress, a vascular cuff was inflated to 200 mm Hg for 5 min and then rapidly deflated. Tissue reperfusion capacity was defined as the gradient of the StO2 recovery slope following the release of the vascular cuff until StO2 had reached 85% of the baseline value, representing the rate of tissue reoxygenation. Tissue oxygen consumption (VO2) was calculated by measuring the linear average rate of decline in tissue oxygenation during the course of the standardized ischemic stress.
Glycocalyx thickness was assessed as a measure of function by sublingual videomicroscopy using side-stream dark-field imaging per the manufacturer’s instructions (Glycocheck, Netherlands). Oral submucosal capillaries were recorded for 3 min and analyzed for capillary filling, vessel size distribution, and the turbulence of erythrocyte flow using the incorporated software. The variability of erythrocyte blood flow relative to total vessel width was used to calculate the perfused boundary region as a measure of glycocalyx thickness, as previously described (34).
Statistical analysis.
Statistical analysis was performed using Prism (version 7.0) software (GraphPad, San Diego, CA, USA). Baseline participant data prior to inoculation were compared to the results obtained on the day of treatment using paired t tests or Wilcoxon matched-pairs signed-rank tests, depending on the normality of the distribution. For each of the paired tests, effect size (ES) was calculated using Hedges’ g statistic, which expresses the difference in means between groups as the number of pooled standard deviations. Results are presented as the median (interquartile range [IQR]) unless otherwise specified.
Spearman’s correlation was used to explore associations between biomarkers of endothelial activation and parasitemia, the cumulative clinical score, and platelet count. The cumulative clinical score was defined as the sum of all standardized clinical score measurements from the time of inoculation to the day of treatment.
Supplementary Material
ACKNOWLEDGMENTS
We declare that we have no conflict of interest.
This research was supported by the Australian National Health and Medical Research Council (fellowships to B.E.B. [grant 1088738], J.S.M. [grant 1135955], and N.M.A. [grant 1135820] and grants 1037304, 1098334, and 1132975), by the U.S. National Institutes of Health (to J.B.W. and N.M.A. [grant R01-HL130763]), and the U.S. Department of Veterans Affairs Research Service (to J.B.W.).
We thank Peter O’Rourke for assistance with the statistical analysis, Fiona Amante, Suzanne Elliott, Gabriela Minigo, and Tonia Woodberry for logistical assistance, Youwei Chen for laboratory assistance, all of the trial participants for their time, and the Medicines for Malaria Venture for encouraging investigator-driven research.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Yeo TW, Lampah DA, Gitawati R, Tjitra E, Kenangalem E, McNeil YR, Darcy CJ, Granger DL, Weinberg JB, Lopansri BK, Price RN, Duffull SB, Celermajer DS, Anstey NM. 2007. Impaired nitric oxide bioavailability and l-arginine reversible endothelial dysfunction in adults with falciparum malaria. J Exp Med 204:2693–2704. doi: 10.1084/jem.20070819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeo TW, Lampah DA, Gitawati R, Tjitra E, Kenangalem E, Piera K, Price RN, Duffull SB, Celermajer DS, Anstey NM. 2008. Angiopoietin-2 is associated with decreased endothelial nitric oxide and poor clinical outcome in severe falciparum malaria. Proc Natl Acad Sci U S A 105:17097–17102. doi: 10.1073/pnas.0805782105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeo TW, Lampah DA, Kenangalem E, Tjitra E, Price RN, Anstey NM. 2013. Impaired skeletal muscle microvascular function and increased skeletal muscle oxygen consumption in severe falciparum malaria. J Infect Dis 207:528–536. doi: 10.1093/infdis/jis692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeo TW, Lampah DA, Tjitra E, Piera K, Gitawati R, Kenangalem E, Price RN, Anstey NM. 2010. Greater endothelial activation, Weibel-Palade body release and host inflammatory response to Plasmodium vivax, compared with Plasmodium falciparum: a prospective study in Papua, Indonesia. J Infect Dis 202:109–112. doi: 10.1086/653211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barber BE, William T, Grigg MJ, Parameswaran U, Piera KA, Price RN, Yeo TW, Anstey NM. 2015. Parasite biomass-related inflammation, endothelial activation, microvascular dysfunction and disease severity in vivax malaria. PLoS Pathog 11:e1004558. doi: 10.1371/journal.ppat.1004558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barber BE, William T, Grigg MJ, Piera KA, Chen Y, Wang H, Weinberg JB, Yeo TW, Anstey NM. 2016. Nitric oxide-dependent endothelial dysfunction and reduced arginine bioavailability in Plasmodium vivax malaria but no greater increase in intravascular hemolysis in severe disease. J Infect Dis 214:1557–1564. doi: 10.1093/infdis/jiw427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barber BE, Grigg MJ, William T, Piera KA, Boyle MJ, Yeo TW, Anstey NM. 2017. Effects of aging on parasite biomass, inflammation, endothelial activation, microvascular dysfunction and disease severity in Plasmodium knowlesi and Plasmodium falciparum malaria. J Infect Dis 215:1908–1917. doi: 10.1093/infdis/jix193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeo TW, Weinberg JB, Lampah DA, Kenangalem E, Bush P, Chen Y, Price RN, Young S, Zhang HY, Millington D, Granger DL, Anstey NM. 2019. Glycocalyx breakdown is associated with severe disease and fatal outcome in Plasmodium falciparum malaria. Clin Infect Dis 69:1712–1720. doi: 10.1093/cid/ciz038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeo TW, Bush PA, Chen Y, Young SP, Zhang H, Millington DS, Granger DL, Mwaikambo ED, Anstey NM, Weinberg JB. 2019. Glycocalyx breakdown is increased in African children with cerebral and uncomplicated falciparum malaria. FASEB J 33:14185–14193. doi: 10.1096/fj.201901048RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valentijn KM, Sadler JE, Valentijn JA, Voorberg J, Eikenboom J. 2011. Functional architecture of Weibel-Palade bodies. Blood 117:5033–5043. doi: 10.1182/blood-2010-09-267492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanson J, Lee SJ, Hossain MA, Anstey NM, Charunwatthana P, Maude RJ, Kingston HW, Mishra SK, Mohanty S, Plewes K, Piera K, Hassan MU, Ghose A, Faiz MA, White NJ, Day NP, Dondorp AM. 2015. Microvascular obstruction and endothelial activation are independently associated with the clinical manifestations of severe falciparum malaria in adults: an observational study. BMC Med 13:122. doi: 10.1186/s12916-015-0365-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomes LT, Alves-Junior ER, Rodrigues-Jesus C, Nery AF, Gasquez-Martin TO, Fontes CJ. 2014. Angiopoietin-2 and angiopoietin-2/angiopoietin-1 ratio as indicators of potential severity of Plasmodium vivax malaria in patients with thrombocytopenia. PLoS One 9:e109246. doi: 10.1371/journal.pone.0109246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Mast Q, Groot E, Lenting PJ, de Groot PG, McCall M, Sauerwein RW, Fijnheer R, van der Ven A. 2007. Thrombocytopenia and release of activated von Willebrand factor during early Plasmodium falciparum malaria. J Infect Dis 196:622–628. doi: 10.1086/519844. [DOI] [PubMed] [Google Scholar]
- 14.Park GS, Ireland KF, Opoka RO, John CC. 2012. Evidence of endothelial activation in asymptomatic parasitemia and effect of blood group on levels of von Willebrand factor in malaria. J Pediatr Infect Dis Soc 1:16–25. doi: 10.1093/jpids/pis010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Regan N, Moxon C, Gegenbauer K, O'Sullivan JM, Chion A, Smith OP, Preston RJS, Brophy TM, Craig AG, O'Donnell JS. 2016. Marked elevation in plasma osteoprotegerin constitutes an early and consistent feature of cerebral malaria. Thromb Haemost 115:773–780. doi: 10.1160/TH15-10-0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barber BE, Grigg MJ, Piera KA, William T, Cooper DJ, Plewes K, Dondorp AM, Yeo TW, Anstey NM. 2018. Intravascular haemolysis in severe Plasmodium knowlesi malaria: association with endothelial activation, microvascular dysfunction, and acute kidney injury. Emerg Microbes Infect 7:106. doi: 10.1038/s41426-018-0105-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim HH, Shin HS, Kwak HJ, Ahn KY, Kim JH, Lee HJ, Lee MS, Lee ZH, Koh GY. 2003. RANKL regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. FASEB J 17:2163–2165. doi: 10.1096/fj.03-0215fje. [DOI] [PubMed] [Google Scholar]
- 18.Mangan SH, Van Campenhout A, Rush C, Golledge J. 2007. Osteoprotegerin upregulates endothelial cell adhesion molecule response to tumor necrosis factor-alpha associated with induction of angiopoietin-2. Cardiovasc Res 76:494–505. doi: 10.1016/j.cardiores.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hempel C, Pasini EM, Kurtzhals JA. 2016. Endothelial glycocalyx: shedding light on malaria pathogenesis. Trends Mol Med 22:453–457. doi: 10.1016/j.molmed.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Lyimo E, Haslund LE, Ramsing T, Wang CW, Efunshile AM, Manjurano A, Makene V, Lusingu J, Theander TG, Kurtzhals JAL, Paulsen R, Hempel C. 2019. In vivo imaging of the buccal mucosa shows loss of the endothelial glycocalyx and perivascular hemorrhages in pediatric Plasmodium falciparum malaria. Infect Immun 88:e00679-19. doi: 10.1128/IAI.00679-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stanisic DI, McCarthy JS, Good MF. 2018. Controlled human malaria infection: applications, advances, and challenges. Infect Immun 86:e00479-17. doi: 10.1128/IAI.00479-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rochette L, Meloux A, Rigal E, Zeller M, Cottin Y, Vergely C. 2018. The role of osteoprotegerin in the crosstalk between vessels and bone: its potential utility as a marker of cardiometabolic diseases. Pharmacol Ther 182:115–132. doi: 10.1016/j.pharmthera.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 23.Secchiero P, Corallini F, Pandolfi A, Consoli A, Candido R, Fabris B, Celeghini C, Capitani S, Zauli G. 2006. An increased osteoprotegerin serum release characterizes the early onset of diabetes mellitus and may contribute to endothelial cell dysfunction. Am J Pathol 169:2236–2244. doi: 10.2353/ajpath.2006.060398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X, Zhang H, Shi Y, Yu Z, Yan H, Ni Z, Qian J, Fang W. 2018. Association of serum angiopoietin-2 with malnutrition, inflammation, atherosclerosis and valvular calcification syndrome and outcome in peritoneal dialysis patients: a prospective cohort study. J Transl Med 16:312. doi: 10.1186/s12967-018-1687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jian W, Li L, Wei XM, Wu CQ, Gui C. 2019. Prognostic value of angiopoietin-2 for patients with coronary heart disease after elective PCI. Medicine (Baltimore) 98:e14216. doi: 10.1097/MD.0000000000014216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perez de Ciriza C, Lawrie A, Varo N. 2015. Osteoprotegerin in cardiometabolic disorders. Int J Endocrinol 2015:564934. doi: 10.1155/2015/564934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rockett RJ, Tozer SJ, Peatey C, Bialasiewicz S, Whiley DM, Nissen MD, Trenholme K, Mc Carthy JS, Sloots TP. 2011. A real-time, quantitative PCR method using hydrolysis probes for the monitoring of Plasmodium falciparum load in experimentally infected human volunteers. Malar J 10:48. doi: 10.1186/1475-2875-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finney CA, Hawkes CA, Kain DC, Dhabangi A, Musoke C, Cserti-Gazdewich C, Oravecz T, Liles WC, Kain KC. 2011. S1P is associated with protection in human and experimental cerebral malaria. Mol Med 17:717–725. doi: 10.2119/molmed.2010.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Punsawad C, Viriyavejakul P. 2017. Reduction in serum sphingosine 1-phosphate concentration in malaria. PLoS One 12:e0180631. doi: 10.1371/journal.pone.0180631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeo TW, Lampah DA, Kenangalem E, Tjitra E, Weinberg JB, Granger DL, Price RN, Anstey NM. 2014. Decreased endothelial nitric oxide bioavailability, impaired microvascular function, and increased tissue oxygen consumption in children with falciparum malaria. J Infect Dis 210:1627–1632. doi: 10.1093/infdis/jiu308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collins KA, Ruckle T, Elliott S, Marquart L, Ballard E, Chalon S, Griffin P, Mohrle JJ, McCarthy JS. 2019. DSM265 at 400 milligrams clears asexual stage parasites but not mature gametocytes from the blood of healthy subjects experimentally infected with Plasmodium falciparum. Antimicrob Agents Chemother 63:e01837-18. doi: 10.1128/AAC.01837-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Griffin P, Pasay C, Elliott S, Sekuloski S, Sikulu M, Hugo L, Khoury D, Cromer D, Davenport M, Sattabongkot J, Ivinson K, Ockenhouse C, McCarthy J. 2016. Safety and reproducibility of a clinical trial system using induced blood stage Plasmodium vivax infection and its potential as a model to evaluate malaria transmission. PLoS Negl Trop Dis 10:e0005139. doi: 10.1371/journal.pntd.0005139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt EP, Overdier KH, Sun X, Lin L, Liu X, Yang Y, Ammons LA, Hiller TD, Suflita MA, Yu Y, Chen Y, Zhang F, Cothren Burlew C, Edelstein CL, Douglas IS, Linhardt RJ. 2016. Urinary glycosaminoglycans predict outcomes in septic shock and acute respiratory distress syndrome. Am J Respir Crit Care Med 194:439–449. doi: 10.1164/rccm.201511-2281OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dane M, van den Berg B, Rabelink T. 2014. The endothelial glycocalyx: scratching the surface for cardiovascular disease in kidney failure. Atherosclerosis 235:56–57. doi: 10.1016/j.atherosclerosis.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 35.de Mast Q, Groot E, Asih PB, Syafruddin D, Oosting M, Sebastian S, Ferwerda B, Netea MG, de Groot PG, van der Ven AJ, Fijnheer R. 2009. ADAMTS13 deficiency with elevated levels of ultra-large and active von Willebrand factor in P. falciparum and P. vivax malaria. Am J Trop Med Hyg 80:492–498. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.