Gamma interferon (IFN-γ)-induced innate immune responses play important roles in the inhibition of Toxoplasma gondii infection. It has been reported that IFN-γ stimulates non-acidification-dependent growth restriction of T. gondii in HeLa cells, but the mechanism remains unclear. Here, we found that γ-aminobutyric acid (GABA) receptor-associated protein-like 2 (GABARAPL2) plays a critical role in parasite restriction in IFN-γ-treated HeLa cells.
KEYWORDS: Toxoplasma gondii, GABARAPL2, IFN-γ, autophagy adaptors
ABSTRACT
Gamma interferon (IFN-γ)-induced innate immune responses play important roles in the inhibition of Toxoplasma gondii infection. It has been reported that IFN-γ stimulates non-acidification-dependent growth restriction of T. gondii in HeLa cells, but the mechanism remains unclear. Here, we found that γ-aminobutyric acid (GABA) receptor-associated protein-like 2 (GABARAPL2) plays a critical role in parasite restriction in IFN-γ-treated HeLa cells. GABARAPL2 is recruited to membrane structures surrounding parasitophorous vacuoles (PV). Autophagy adaptors are required for the proper localization and function of GABARAPL2 in the IFN-γ -induced immune response. These findings provide further understanding of a noncanonical autophagy pathway responsible for IFN-γ-dependent inhibition of T. gondii growth in human HeLa cells and demonstrate the critical role of GABARAPL2 in this response.
INTRODUCTION
Infections by the protozoan parasite Toxoplasma gondii are widely prevalent worldwide in animals and humans. All warm-blooded animals can be infected (1). T. gondii infection can lead to opportunistic and latent infections in healthy animals, as well as reactivation and fatal encephalitis in immunocompromised individuals suffering from autoimmune deficiency syndrome (AIDS) or receiving chemotherapy (2). T. gondii invades any nucleated cells and forms parasitophorous vacuoles (PV), which protect the parasite from detection by host innate immune mechanisms and allow for efficient proliferation (3).
Gamma interferon (IFN-γ) is the major mediator of innate immunity against T. gondii in both mouse and human cells (4–6). In IFN-γ-stimulated mouse cells, immunity-related GTPases (IRGs) and guanylate binding proteins (GBPs) are recruited (4–6) to the PV membrane (PVM), and LC3 homologs are conjugated to the PVM by autophagy-related protein 3 (ATG3), ATG7, and a complex of ATG12, ATG5, and ATG16L1 (4–7). The T. gondii PVM is ruptured, which causes the death of the parasite, further promoting inflammation and inducing apoptosis (8, 9). Ubiquitin and p62 are also recruited and contribute to the inhibition of T. gondii growth (8).
Although the T. gondii PVs in both mouse and human cells are decorated by ubiquitin, autophagy adaptors, ATG8, etc. (10), human cells lack IRG proteins, which are critical for inhibiting T. gondii growth in mice (11). Human GBP1 was recently reported to inhibit the growth of T. gondii by inducing apoptosis (12). The ubiquitinated vacuoles are targeted for destruction in acidified lysosomal-associated membrane protein 1 (LAMP1)-positive endo-lysosomal compartments in HUVEC cells (10). In HeLa cells, autophagy proteins are required for the recruitment of LC3 to PVs and the restriction of parasite growth without fusing with the lysosome. These processes are quite different from those in mouse cells.
There are three distinct clusters of ATG8 orthologs: (i) LC3a and LC3b; (ii) γ-aminobutyric acid (GABA) receptor-associated protein (GABARAP) and GABARAP-like 1 (GABARAPL1); and (iii) GABARAPL2 (11). In a recent study, GABARAPL2, but not LC3a or LC3b, was found to be critical for IFN-γ-induced LC3-associated phagocytosis (LAP) associated with T. gondii infection in mouse cells (13). The authors suggest a pathway in which GABARAPL2 recruits GBPs via interaction with ADP ribosylation factor 1 (Arf1) to destroy parasite-containing PVs (14).
Here, we report that in HeLa cells, GABARAPL2 plays an essential role in inhibiting the growth of T. gondii. Since GBPs are dispensable for the control of T. gondii growth in human cells stimulated with IFN-γ (5), these data may suggest a novel role for GABARAPL2 in the human immune response against T. gondii.
RESULTS
GABARAPL2 is critical for the growth restriction of T. gondii in IFN-γ-stimulated HeLa cells.
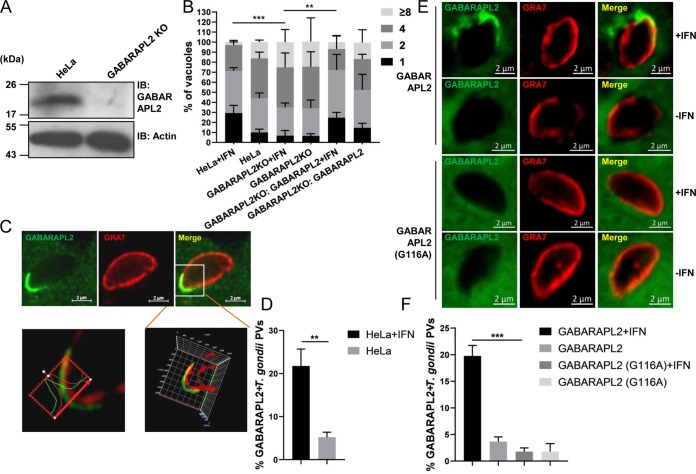
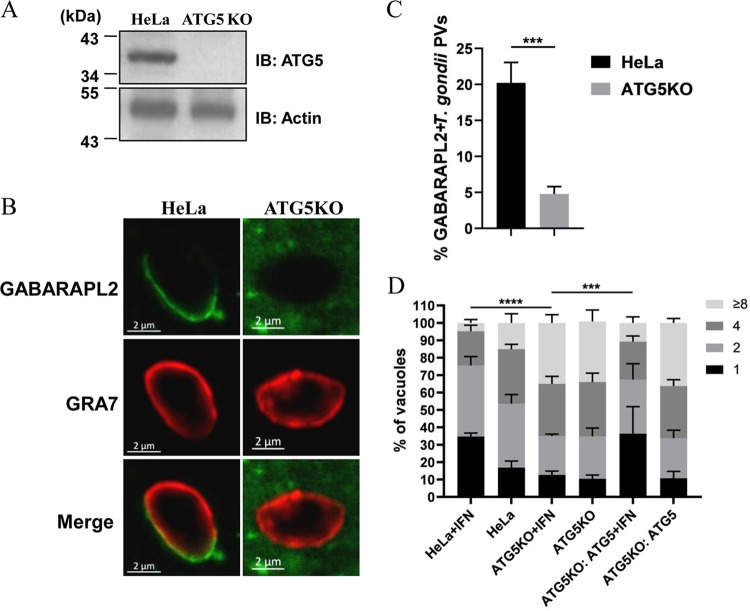
To determine the role of GABARAPL2 for the growth restriction of T. gondii, GABARAPL2 knockout (KO) cells were first generated using the clustered regularly interspersed short palindromic repeats (CRISPR)/Cas9 method. Immunoblot analysis confirmed GABARAPL2 deficiency in the knockout cells (Fig. 1A). After stimulation with IFN-γ, replication of the parasite was notably higher in the GABARAPL2KO cells than in the wild-type cells, with 40% and 25% of the vacuoles containing four and eight parasites, respectively (Fig. 1B). Complementation of GABARAPL2 expression in the KO cell line restored the growth restriction, with 20% and 7% of the vacuoles containing four and eight parasites (Fig. 1B).
FIG 1.
IFN-γ mediates GABARAPL2 recruitment to PVs and restricts T. gondii replication. (A) Wild-type and GABARAPL2KO HeLa cells were lysed and the lysates were analyzed by Western blotting with the indicated antibodies. (B) HeLa and GABARAPL2KO HeLa cells were transfected with control (pCAGGS) or complemented plasmids (pCAGGS-GFP-GABARAPL2) for 24 h and either left unactivated or activated with IFN-γ for 24 h. Cells were infected with T. gondii PLK strain (MOI = 10) and harvested at 30 h postinfection. The infected cells were assessed by indirect immunofluorescence for GFP and the parasite surface marker SAG2. T. gondii replication in vacuoles was counted by laser confocal microscopy. The average number of parasites per vacuole was determined from at least 100 vacuoles on three individual coverslips. Values are the mean ± the standard deviation (SD) of three independent experiments and were compared by two-way ANOVA. ** P < 0.01, *** P < 0.001. (C and D) HeLa cells expressing GFP-GABARAPL2 were left untreated or treated with IFN-γ for 24 h, infected with parasite (MOI = 10), and harvested at 4 h postinfection. Cells were stained for GRA7 and GFP by indirect immunofluorescence, followed by detection with secondary antibodies conjugated to either Alexa Fluor 594 (red) or 488 (green). Laser confocal microscopy shows the three-dimensional localization of GABARAPL2 around the PVM. The average number of vacuoles was determined from at least 100 vacuoles on three individual coverslips. Values are the mean ± the standard deviation (SD) of three independent experiments and were compared by t tests; ** P < 0.01. (E and F) GABARAPL2KO cells transfected with GFP-GABARAPL2 or GFP-GABARAPL2 (G116A) were left unactivated or activated with IFN-γ for 24 h, infected with the parasite (MOI = 10), and harvested at 4 h postinfection. Cells were treated as above. The average number of vacuoles was determined from at least 100 vacuoles on three individual coverslips. Values are the mean ± SD of three independent experiments; *** P < 0.001.
To observe the localization of GABARAPL2, a HeLa cell line stably expressing a green fluorescent protein (GFP)-tagged GABARAPL2 fusion protein (GFP-GABARAPL2) was generated. The recruitment of GFP-GABARAPL2 to PVs in IFN-γ-stimulated and unstimulated HeLa cells was observed by indirect immunofluorescence. In IFN-γ-stimulated HeLa cells, GFP-GABARAPL2 significantly accumulated in PVs, which were visualized by staining with an anti-GRA7 antibody (Ab). Laser confocal microscopy was used to analyze the images three-dimensionally. We found there was some distance between GABARAPL2 and GRA7 staining, which indicated that GABARAPL2 might be located on membrane structures around the PV rather than directly on the PVM (Fig. 1C). We analyzed the percentages of GABARAPL2 associated with PVs and found that about 5% of GABARAPL2 accumulated in PVs in unstimulated cells, while about 22% of GABARAPL2 accumulated in PVs in IFN-γ-treated cells (Fig. 1D).
GABARAPL2 is a ubiquitin-like protein that can be conjugated to the membrane lipid phosphatidylethanolamine (PE) in a conjugation reaction known as lipidation. Lipidation of GABARAPL2 at the C-terminal glycine residue Gly116 is essential for the induction of autophagy (14, 15). We next wanted to determine whether lipidation of GABARAPL2 was involved in this IFN-γ-induced, cell-autonomous immune response. In GABARAPL2KO cells, reintroduced GFP-GABARAPL2, but not the GFP-GABARAPL2 (G116A) mutant protein, was recruited to T. gondii PVs (Fig. 1E and F). Thus, IFN-γ-dependent GABARAPL2 accumulation in T. gondii PVs plays an important role in anti-T. gondii cellular host defenses.
GABARAPL2 accumulates to the T. gondii PVs by an IFN-γ- and ubiquitin-dependent mechanism.
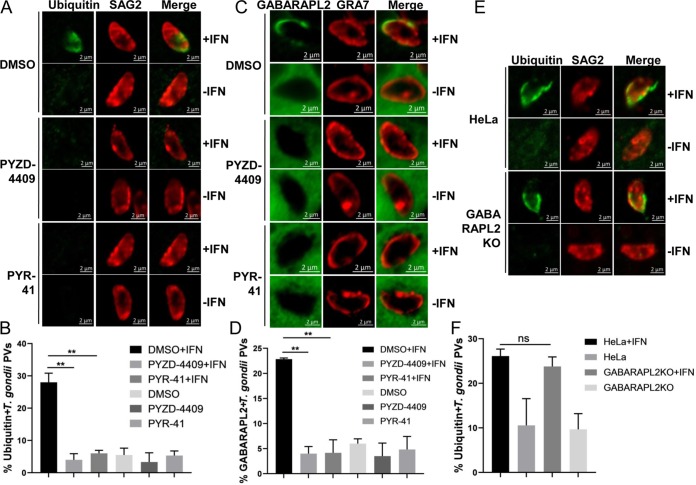
It was reported that in IFN-γ-treated human HeLa cells, there are significantly more ubiquitin-tagged PVs than LC3-tagged PVs, suggesting that ubiquitin is recruited to the PVM before LC3 (13). The non-acidification-dependent growth restriction of T. gondii is dependent on PV ubiquitination induced by IFN-γ in HeLa cells (12). To verify the contribution of the ubiquitin system to GABARAPL2 recruitment to PVs in IFN-γ-treated HeLa cells, E1 inhibitors were used to inhibit ubiquitination in these cells. Recruitment of ubiquitin to PVs was abolished by inhibiting the E1 with PYR-41 or PYZD-4409 (Fig. 2A and B). Importantly, the accumulation of GABARAPL2 to PVs was also reduced in response to E1 inhibition in IFN-γ-stimulated HeLa cells, suggesting that GABARAPL2 accumulation to PVs requires the ubiquitin system (Fig. 2C and D).
FIG 2.
GABARAPL2 accumulated on T. gondii PVs by an IFN-γ and ubiquitin-dependent mechanism. (A to D) HeLa cells were left unactivated or activated with IFN-γ for 24 h and treated with the E1 inhibitor PYR-41 or PYZD-4409 for 6 h. Cells were infected with parasites (MOI = 10) and harvested at 4 h postinfection. Cells were stained with antibodies against GRA7 and ubiquitin or GFP, followed by secondary antibodies conjugated to either Alexa Fluor 594 (red) or 488 (green). The average number of vacuoles was determined from at least 100 vacuoles on three individual coverslips. Values are the mean ± SD of two independent experiments, and statistical analysis was done by t tests. ** P < 0.01. (E and F) GABARAPL2KO cells were left unactivated or were activated with IFN-γ for 24 h, infected with the parasite (MOI = 10), and harvested at 4 h postinfection. Cells were treated as above. The average number of vacuoles was determined from at least 100 vacuoles on three individual coverslips. Values are the mean ± SD of three independent experiments, and statistical analysis was done by t tests; ns, not significant.
In IFN-γ-treated mouse embryonic fibroblasts (MEFs), T. gondii PVs were ubiquitinated through both p62-dependent and -independent mechanisms (8). We thus asked whether the ubiquitination of PVs in IFN-γ-stimulated HeLa cells is regulated by GABARAPL2. The localization of ubiquitin to PVs in IFN-γ-treated GABARAPL2KO cells was similar to that in wild-type HeLa cells. Consistent with previous results, the deletion of GABARAPL2 did not affect the recruitment of ubiquitin to T. gondii PVs (Fig. 2E and F).
Multiple autophagy adaptors are recruited to PVs in IFN-γ-stimulated HeLa cells.
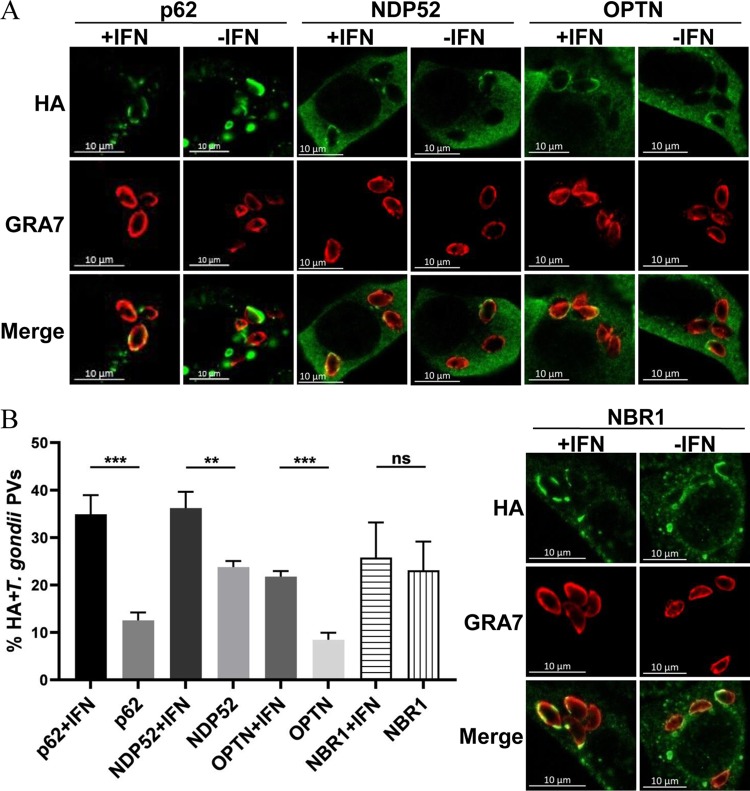
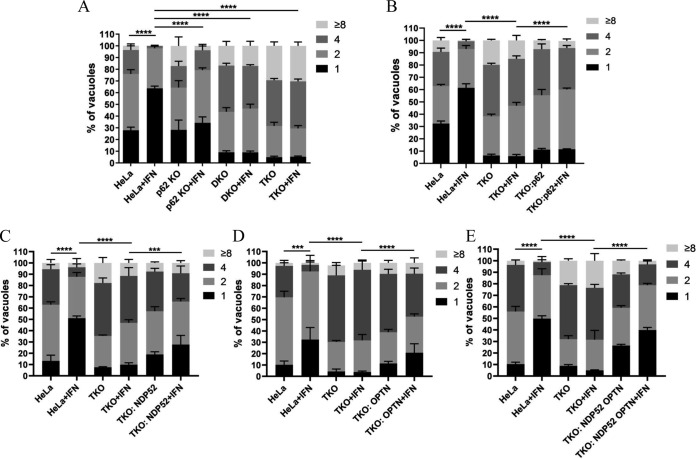
The results above demonstrated that GABARAPL2 was recruited to T. gondii PVs. The selectivity of the autophagic processes is mediated by cargo adaptors that link the cargo to isolation membranes by simultaneously binding to the cargo and ATG8 family proteins on the isolation membrane (16–18). Prior studies have shown that ubiquitin, the autophagy adaptors p62 and NDP52, and LC3 are highly recruited to PVs containing susceptible strains of T. gondii in HeLa cells (12–13). In ubiquitin-positive PVs, the majority of the PVs (72.7%) were positive for both ubiquitin and p62/NDP52, while 23.7% were positive for ubiquitin alone, and no PVs were positive for p62/NDP52 alone (13). It seems that the ubiquitination of PVs drives the recruitment of autophagy adaptors to PVs. However, these PVs were not delivered to lysosomes for degradation. This led to the question of whether autophagy adaptors were still recruited to these membranes. To answer this, we screened for autophagy adaptors that efficiently associated with PVs upon IFN-γ stimulation. The autophagy adaptors p62, NDP52, and OPTN were found to co-localize with PVs in IFN-γ-treated HeLa cells but not untreated cells (Fig. 3). By contrast, there was no significant difference in the co-localization of NBR1 with PVs between IFN-γ-treated and untreated HeLa cells (Fig. 3).
FIG 3.
Multiple autophagy adaptors were recruited to PVs in IFN-γ-stimulated HeLa cells. (A and B) HeLa cells were transfected with HA-p62, HA-NDP52, HA-OPTN, or HA-NBR1. Cells were left unactivated or were activated with IFN-γ for 24 h, infected with parasites (MOI = 10), and harvested at 6 h postinfection. Infected cells were stained with primary antibodies against GRA7 and the hemagglutinin (HA) tag, followed by secondary antibodies conjugated to either Alexa Fluor 594 (red) or 488 (green). The average number of vacuoles was determined from at least 100 vacuoles on three individual coverslips. Values are the mean ± SD of two independent experiments, and statistical analysis was done by t tests; ns, not significant; ** P < 0.01, *** P < 0.001.
The loss of autophagy adaptors affects the recruitment of GABARAPL2 to PVs.
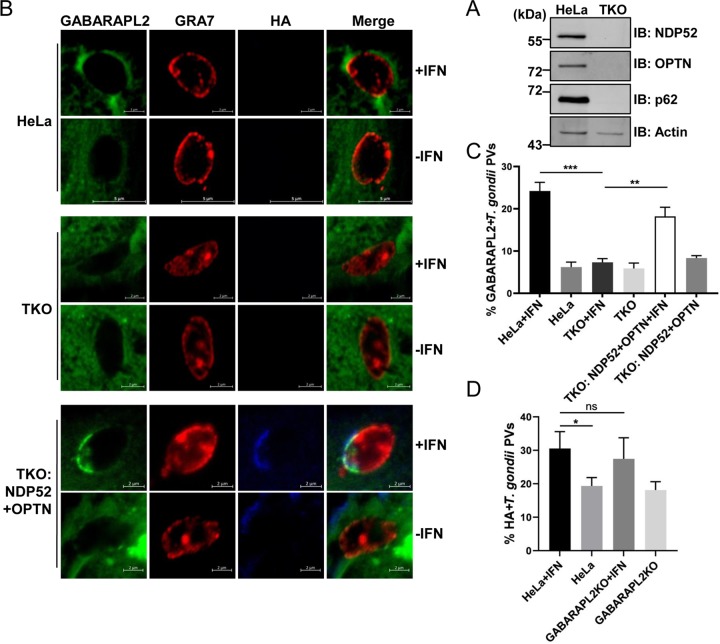
To verify that autophagy adaptors bridge GABARAPL2 and PVs, p62, NDP52, and OPTN were depleted in one triple knockout cell line (TKO) (Fig. 4A). Hemagglutinin (HA)-NDP52 and HA-OPTN were then transfected into TKO cells expressing GFP-GABARAPL2, and GABARAPL2 recruitment to PVs was examined. Transfected cells were left untreated or treated with IFN-γ, infected with the PLK strain of T. gondii, and subsequently stained for GRA7 and GABARAPL2. Representative confocal images of GABARAPL2 recruited to PVs are shown in Fig. 4B. In parental HeLa cells, about 24% of vacuoles were decorated with GABARAPL2 after IFN-γ stimulation. However, in IFN-γ-treated TKO cells, targeting of GABARAPL2 to PVs occurred in less than 10% of vacuoles (Fig. 4C). The IFN-γ-dependent recruitment of GABARAPL2 to PVs in TKO cells may suggest that other autophagy adaptors have similar functions. Complementary expression of NDP52 and OPTN rescued the localization of GABARAPL2 to PVs in TKO cells (Fig. 4C). By contrast, the localization of the autophagy adaptor NDP52 was not affected by the deletion of GABARAPL2 (Fig. 4D). These results indicate that the autophagy adaptors NDP52 and OPTN recruit GABARAPL2 to PVs in an IFN-γ-dependent manner.
FIG 4.
Loss of autophagy adaptors affects the recruitment of GABARAPL2 to PVs. (A) HeLa and TKO HeLa cells were lysed and the lysates were analyzed by Western blotting with the indicated antibodies. (B and C) Wild-type and autophagy receptor TKO HeLa cells were transfected with the empty pCAGGS vector or constructs expressing HA-NDP52 and HA-OPTN. Cells were left unactivated or were activated with IFN-γ for 24 h, infected with parasites (MOI = 10), and harvested at 4 h postinfection. Infected cells were stained with antibodies against GRA7 and the HA tag, followed by secondary antibodies conjugated to either Alexa Fluor 594 (red) or 405 (blue). The average number of vacuoles was determined from at least 100 vacuoles on three individual coverslips. Values are the mean ± SD of two independent experiments, and statistical analysis was done by t tests; ** P < 0.01, *** P < 0.001. (D) Wild-type and GABARAPL2KO HeLa cells were transfected with a plasmid expressing HA-NDP52, and experiments were performed as described above. The average number of vacuoles was determined from at least 100 vacuoles on three individual coverslips. Values are the mean ± SD of three independent experiments, and statistical analysis was done by t tests; ns, not significant; * P < 0.05.
ATG5 is required for the localization of GABARAPL2 to the PVM.
It is well known that an ATG16L1–ATG5–ATG12 complex determines ATG8 lipidation. Consistent with this, only about 5% of GABARAPL2 accumulated around the PVs of ATG5KO cells activated with IFN-γ (Fig. 5A), while about 20% of GABARAPL2 accumulated in parental HeLa cells activated with IFN-γ (Fig. 5B and C). ATG5 deletion also abolished the inhibition of T. gondii growth by IFN-γ (Fig. 5D). Growth restriction of T. gondii was restored by trans‐complementation of ATG5 in the KO cell line (Fig. 5D). Therefore, the role of GABARAPL2 in the inhibition of T. gondii growth depends on its lipidation, which is mediated by the ATG16L1–ATG5–ATG12 complex. It has been reported that autophagy adaptors directly interact with ATG5 and recruit the ATG16L1–ATG12–ATG5 complex to lipidize ATG8 on the isolation membrane (19–21). It is reasonable to speculate that a similar pathway contributes to the formation of multiple membranes around PVs. On the other hand, some previous studies have indicated that the cargo receptor NDP52 binds FIP200 via its N-terminal SKICH domain and recruits the upstream autophagy machinery to cytosol-invading bacteria (22–24). p62 was also found to directly interact with the C-terminal region of FIP200 and to promote autophagosome formation in the vicinity of ubiquitin-positive condensates (25, 26). We evaluated the localization of FIP200 in IFN-γ-activated and unactivated HeLa cells. FIP200 was more highly associated with PVs in the presence of IFN-γ than in the absence of IFN-γ (Fig. S1A and B in the supplemental material). About 15% of NDP52-positive PVs were also positive for FIP200 in IFN-γ-activated HeLa cells, compared with 5% in untreated cells (Fig. S1C). Therefore, autophagy adaptors may also recruit FIP200 to form the multiple membranes around T. gondii PVs in HeLa cells.
FIG 5.
ATG5 mediates GABARAPL2 recruitment to PVs. (A) HeLa and ATG5KO HeLa cells were lysed and the lysates were analyzed by Western blotting with the indicated antibodies. (B and C) Wild-type HeLa and ATG5KO cells expressing GFP-GABARAPL2 were left unactivated or activated with IFN-γ for 24 h, infected with the parasite (MOI = 10), and harvested at 4 h postinfection. Infected cells were stained with antibodies against GRA7 and GFP, followed by secondary antibodies conjugated to either Alexa Fluor 594 (red) or 488 (green). The average number of vacuoles was determined from at least 100 vacuoles on three individual coverslips. Values are the mean ± SD of three independent experiments, and statistical analysis was done by t tests; *** P < 0.001. (D) Wild-type and ATG5KO HeLa cells were transfected with control (pCAGGS) or complemented plasmids (pCAGGS-Flag-ATG5) for 24 h, left unactivated or activated with IFN-γ for 24 h, infected with the parasite (MOI = 10), and harvested at 30 h postinfection. Infected cells were harvested and stained with anti-Flag and anti-SAG2 antibodies. T. gondii replication in vacuoles was counted by laser confocal microscopy. The average number of parasites per vacuole was determined from at least 100 vacuoles on three individual coverslips. Values are the mean ± SD of three independent experiments, and statistical analysis was conducted by two-way ANOVA; *** P < 0.001, **** P < 0.0001.
Autophagy adaptors are required for the restriction of T. gondii growth in IFN-γ-activated HeLa cells.
A previous study found that the replication of T. gondii was significantly slower in p62-positive PVs than in p62-negative PVs in IFN-γ-activated HeLa cells (13). We also found that autophagy adaptors were required for the recruitment of GABARAPL2 to PVs. These data indicate that autophagy adaptors are still necessary for the inhibition of T. gondii growth in the IFN-γ-induced noncanonical pathway (13). To further clarify the functional consequence of the targeting of PVs by p62, NDP52, and OPTN, we tested the replication of the parasite in the TKO cells. T. gondii replication was significantly greater in autophagy adaptor-deficient cells than in wild-type cells activated with IFN-γ. In contrast, complementary re-expression of the adaptors rescued the inhibition of T. gondii growth (Fig. 6).
FIG 6.
Replication of T. gondii is restricted by autophagy adaptors. (A) Wild-type HeLa cells and the indicated knockout cells were left unactivated or activated with IFN-γ for 24 h, infected with the parasite (MOI = 10), and harvested at 30 h postinfection. Infected cells were harvested and stained with anti-GRA7 and anti-SAG2 antibodies. T. gondii replication in vacuoles was counted by fluorescence microscopy. The average number of parasites per vacuole was determined from at least 100 vacuoles on three individual coverslips. Values are the mean ± SD of three independent experiments, and statistical analysis was conducted by two-way ANOVA; **** P < 0.0001. (B to E) HeLa and TKO cells were transfected with either empty pCAGGS vectors or plasmids expressing autophagy adaptors, left unactivated or activated with IFN-γ for 24 h, infected with the parasite (MOI = 10), and harvested at 30 h postinfection. Cells were then treated as above. The average number of parasites per vacuole was determined from at least 100 vacuoles on three individual coverslips. Values are the mean ± SD of three independent experiments, and statistical analysis was done by two-way ANOVA; *** P < 0.001, **** P < 0.0001.
DISCUSSION
When T. gondii invades host cells, it establishes a replication-permissive niche within infected host cells. This niche, the parasitophorous vacuole (PV), is separated from the host cell cytoplasm by the PV membrane (PVM). This structure provides a protective environment for T. gondii replication and prevents the parasite from being targeted by cytosolic host defense systems (27). To control parasite replication, host cells must first destroy the PVM to expose the parasite to host defense systems. Studies have shown that diverse mechanisms are involved in parasite growth restriction in different cell types. Here, we show that a noncanonical autophagy process results in the formation of multiple membranes that engulf PVs. Furthermore, we show for the first time that GABARAPL2 around the PVM plays a vital role in inhibiting parasite growth.
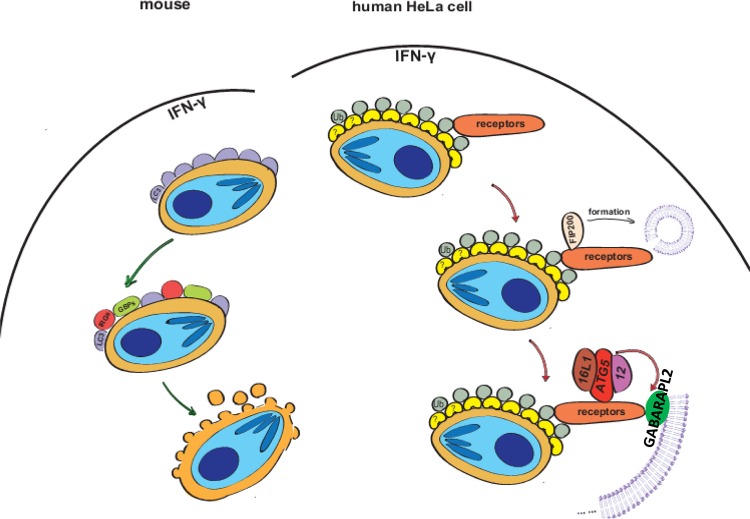
GABARAPL2 was recruited to PVs in HeLa cells in an IFN-γ-dependent manner. GABARAPL2 did not completely colocalize with the PVM marker GRA7, but it could be visualized near the PVM, indicating that GABARAPL2 is bound to a specific structure around the PVM (Fig. 7). This result is similar to the results of a previous study in which LC3 was localized to multiple membranes engulfing the T. gondii PVM (13). Although we did not exclude the possibility that other LC3 family proteins also contribute to this growth restriction pathway, we did find that the deletion of GABARAPL2 almost completely abolished the restriction of PLK-strain parasite growth in IFN-γ-stimulated cells (Fig. 1). Therefore, we conclude that GABARAPL2 is a dominant effector induced by IFN-γ for controlling parasite growth in HeLa cells. Previous studies showed that GABARAPLs and LC3s play opposite roles in regulating ULK1 activity (28). Furthermore, in addition to noncanonical autophagy, GABARAPL2 and LC3 may function in other ways.
FIG 7.
Model for IFN-γ-mediated control of T. gondii infection. In mouse cells, upon infection by T. gondii, GMS IRGs and GBPs are recruited to the T. gondii PVM in response to IFN-γ and disrupt it by LC3-associated phagocytosis (LAP). The T. gondii is then exposed to cytosolic host defense systems and killed. In human cells, which are deficient in IRGs, the T. gondii PVM is ubiquitinated, and autophagy adaptors (p62, NDP52, OPTN, etc.) are recruited to PVs by interaction with ubiquitin. The FIP200 complex, ATG5 complex, and GABARAPL2 are recruited to the PV by autophagy adaptors to form multiple membranes that envelope the PVs and subsequently inhibit the growth of T. gondii.
Autophagy adaptor-deficient cells also showed a significant defect in IFN-γ-induced inhibition of T. gondii growth, and, in a reconstitution experiment, re-expression of NDP52 and OPTN rescued the inhibition of T. gondii growth. Therefore, these multiple adaptors have complementary roles in this growth restriction pathway. In addition, the deletion of these three autophagy adaptors did not reduce IFN-γ-induced growth inhibition to the same extent as GABARAPL2 deletion. We did not compare the growth inhibition of the PLK strain in TKO and GABARAPL2 cells. It is, therefore, possible that other autophagy adaptors also contribute to growth restriction. In bacterum-induced xenophagy, V-ATPase recruits LC3 to the bacterum-containing vacuole by interacting with ATG16L1 to inhibit bacterial growth (29). Thus, GABARAPL2 could be recruited through autophagy adaptor-independent pathways.
In our results, both autophagy adaptors and GABARAPL2 encompassed only a portion of the PV, which might suggest they conjugated to nonclosed membrane structures. Our data suggested that these membranes were segregated from the PV membranes. Results of previous reports that LC3-positive vacuoles do not fuse with lysosomal-associated membrane glycoprotein 1 (LAMP1) suggest that these structures are noncanonical autophagy structures (12, 30). We showed that FIP200 accumulation at the PVM significantly increased after IFN-γ stimulation and that the ATG16L1–ATG5–ATG12 complex was also necessary for the localization of GABARAPL2 to T. gondii PVs. Therefore, both the initial complex and the extension complex of autophagy machinery are involved in the formation of the multiple membranes (Fig. 7). However, our data do not indicate a clear link between the isolation membrane formed by the autophagy machinery and the multiple membranes surrounding PVs. This will require further investigation.
MATERIALS AND METHODS
Cells and parasites.
The T. gondii strain PLK was maintained in human foreskin fibroblast (HFF) cells grown in Dulbecco’s modified Eagle medium (DMEM) (Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS) (HyClone), 100 U/ml penicillin, and 0.1 mg/ml streptomycin (Gibco/Thermo Fisher Scientific) at 37°C in 5% CO2. HeLa cells were maintained in DMEM supplemented with 10% FBS, 100 U/ml penicillin, and 0.1 mg/ml streptomycin at 37°C in 5% CO2.
Reagents.
Recombinant human IFN-γ was obtained from PrimeGene. The ubiquitin E1 inhibitors PYR-41 (11117) and PYZD-4409 (15183) were purchased from MedChem Express. Antibodies (Abs) against p62 (55274), NDP52 (12229), OPTN (10837), and NBR1 (16004) were purchased from Proteintech. Polyethylenimine (PEI) and Abs against ATG5 (A0856), green fluorescent protein (GFP) (SAB4301138), and β-actin (A8481) were purchased from Sigma-Aldrich. Abs against GABARAPL2 (D1W97) and HA (C29F4) were purchased from Cell Signaling. The Flag tag Ab (GTX77457) was purchased from Gene Tex. The Ab against ubiquitin (2739407) was purchased from EMD Millipore. Secondary antibodies labeled with Alexa Fluor 594 and Alexa Fluor 488 (A11032 and A11001) were purchased from Invitrogen/Thermo Fisher Scientific. The pGEM-T easy vector (A1360) was purchased from Promega. SuperSignal West Pico Plus (34578) was purchased from Thermo Fisher Scientific. Abs against T. gondii GRA7 and SAG2 were prepared in our laboratory.
Generation of knockout HeLa cells.
The plasmid expressing mammalian Cas9 was obtained from Addgene (plasmid pMJ920; 42234). Fragments encoding guide RNAs (gRNAs) for ATG5, GABARAPL2, p62, NDP52, and OPTN were inserted into the pGEM-T easy vector to generate the gRNA-expressing plasmids pgRNA-ATG5, pgRNA-GABARAPL2, pgRNA-p62, pgRNA-NDP52, and pgRNA-OPTN, respectively. HeLa cells were cotransfected with the pMJ920 vector and individual pgRNAs using PEI, and cultured in DMEM containing 10% FBS for 2 days. Cells expressing green fluorescence were sorted by flow cytometry. Limiting dilution of the sorted cells was performed, and single colonies were expanded for analysis of protein expression by Western blotting. Clones showing downregulated protein expression by Western blotting were subsequently sequenced to confirm the genetic deletion.
Western blotting.
Cells (5 × 105) were washed with phosphate-buffered saline (PBS) and lysed in lysis buffer containing 1.0% NP-40, 150 mM NaCl, 20 mM Tris-Cl (pH 7.5), and 5 mM EDTA. The cell lysates were separated by SDS-PAGE, transferred to polyvinylidene fluoride (PVDF) membranes, blocked for 1 h in 5% skim milk, and incubated with primary antibody diluted in 5% milk in TBST (20 mM Tris-Cl, 150 mM NaCl, 0.05% Tween 20) for 1 h at room temperature (RT). After several washes in TBST, blots were incubated for 1 h at RT with secondary antibody. The blots were washed again in TBST and then developed using SuperSignal West Pico Plus (34578; Thermo Fisher Scientific).
Indirect immunofluorescence assays.
HeLa and gene-deficient HeLa cells were cultured on coverslips, transfected with plasmids for 24 h, and left untreated or treated with 500 ng/ml IFN-γ for 24 h. The cells were then infected with T. gondii (at a multiplicity of infection [MOI] of 10) for 2 h, washed three times with PBS, and cultured in fresh medium for varying lengths of time. Cells were washed with PBS three times, fixed in 4% paraformaldehyde for 30 min, permeabilized with 0.3% Triton X-100 for 15 min, blocked with 3% bovine serum albumin (BSA) in PBS for 30 min, and stained with primary antibodies for 1 h at RT, washed with PBS, and then stained with Alexa Fluor 488- or Alexa Fluor 594-conjugated secondary antibodies for 1 h at RT. Coverslips were then mounted onto glass slides and analyzed by confocal laser microscopy (Zeiss). Images are shown at 63× magnification.
Intracellular replication assays.
Cells (1 × 105) were left untransfected or were transfected with specific plasmids for 24 h and then left untreated or treated with 500 ng/ml IFN-γ for 24 h. The cells were next infected with T. gondii (MOI = 10) for 2 h, washed three times with PBS, and fed fresh medium. To measure T. gondii titers at 30 h postinfection, the cells were fixed in 4% paraformaldehyde for 30 min, permeabilized with 0.3% Triton X-100 for 15 min, stained with primary antibodies, washed with PBS, and then stained with secondary antibodies. The number of parasites per vacuole was determined by manual counting on a confocal laser microscope. The average number of parasites per vacuole was determined from at least 100 vacuoles on three individual coverslips.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Key Research and Development Program of China (grant no. 2017YFD0501304 to H.J.) and the National Natural Science Foundation of China (grant no. 31702231 to Z.Z.).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Montoya JG, Liesenfeld O. 2004. Toxoplasmosis. Lancet 363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 2.Israelski DM, Remington JS. 1993. Toxoplasmosis in patients with cancer. Clin Infect Dis 17:S423–S435. doi: 10.1093/clinids/17.Supplement_2.S423. [DOI] [PubMed] [Google Scholar]
- 3.Blader IJ, Koshy AA. 2014. Toxoplasma gondii development of its replicative niche: in its host cell and beyond. Eukaryot Cell 13:965–976. doi: 10.1128/EC.00081-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao Z, Fux B, Goodwin M, Dunay IR, Strong D, Miller BC, Cadwell K, Delgado MA, Ponpuak M, Green KG, Schmidt RE, Mizushima N, Deretic V, Sibley LD, Virgin HW. 2008. Autophagosome-independent essential function for the autophagy protein Atg5 in cellular immunity to intracellular pathogens. Cell Host Microbe 4:458–469. doi: 10.1016/j.chom.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohshima J, Youngae L, Sasai M, Saitoh T, Ma JS, Kamiyama N, Matsuura Y, Pann-Ghill S, Hayashi M, Ebisu S, Takeda K, Akira S, Yamamoto M. 2014. Role of mouse and human autophagy proteins in IFN-γ- induced cell-autonomous responses against Toxoplasma gondii. J Immunol 192:3328–3335. doi: 10.4049/jimmunol.1302822. [DOI] [PubMed] [Google Scholar]
- 6.Park S, Choi J, Biering SB, Dominici E, Williams LE, Hwang S. 2016. Targeting by AutophaGy proteins (TAG): targeting of IFNG-inducible GTPases to membranes by the LC3 conjugation system of autophagy. Autophagy 12:1153–1167. doi: 10.1080/15548627.2016.1178447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hurley JH, Young LN. 2017. Mechanisms of autophagy initiation. Annu Rev Biochem 86:225–244. doi: 10.1146/annurev-biochem-061516-044820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haldar AK, Foltz C, Finethy R, Piro AS, Feeley EM, Pilla-Moffett DM, Komatsu M, Frickel E, Coers J. 2015. Ubiquitin systems mark pathogen-containing vacuoles as targets for host defense by guanylate binding proteins. Proc Natl Acad Sci U S A 112:E5628–E5637. doi: 10.1073/pnas.1515966112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martens S, Parvanova I, Zerrahn J, Griffiths G, Schell G, Reichmann G, Howard JC. 2005. Disruption of Toxoplasma gondii parasitophorous vacuoles by the mouse p47-resistance GTPases. PLoS Pathog 1:e24. doi: 10.1371/journal.ppat.0010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clough B, Wright JD, Pereira PM, Hirst EM, Johnston AC, Henriques R, Frickel E. 2016. K63-linked ubiquitination targets Toxoplasma gondii for endo-lysosomal destruction in IFNγ-stimulated human cells. PLoS Pathog 12:e1006027. doi: 10.1371/journal.ppat.1006027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bekpen C, Xavier RJ, Eichler EE. 2010. Human IRGM gene “to be or not to be”. Semin Immunopathol 32:437–444. doi: 10.1007/s00281-010-0224-x. [DOI] [PubMed] [Google Scholar]
- 12.Fisch D, Bando H, Clough B, Hornung V, Yamamoto M, Shenoy AR, Frickel E. 2019. Human GBP1 is a microbe-specific gatekeeper of macrophage apoptosis and pyroptosis. EMBO J 38:e100926. doi: 10.15252/embj.2018100926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selleck EM, Orchard RC, Lassen KG, Beatty WL, Xavier RJ, Levine B, Virgin HW, Sibley LD. 2015. A noncanonical autophagy pathway restricts Toxoplasma gondii growth in a strain-specific manner in IFN-γ-activated human cells. mBio 6:e01157-15. doi: 10.1128/mBio.01157-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sasai M, Sakaguchi N, Ma JS, Nakamura S, Kawabata T, Bando H, Lee Y, Saitoh T, Akira S, Iwasaki A, Standley DM, Yoshimori T, Yamamoto M. 2017. Essential role for GABARAP autophagy proteins in interferon-inducible GTPase-mediated host defense. Nat Immunol 18:899–910. doi: 10.1038/ni.3767. [DOI] [PubMed] [Google Scholar]
- 15.Scherz-Shouval R, Sagiv Y, Shorer H, Elazar Z. 2003. The COOH terminus of GATE-16, an intra-golgi transport modulator, is cleaved by the human cysteine protease HsApg4A. J Biol Chem 278:14053–14058. doi: 10.1074/jbc.M212108200. [DOI] [PubMed] [Google Scholar]
- 16.Lee Y, Sasai M, Ma JS, Sakaguchi N, Ohshima J, Bando H, Saitoh T, Akira S, Yamamoto M. 2015. p62 plays a specific role in interferon-γ-induced presentation of a Toxoplasma vacuolar antigen. Cell Rep 13:223–233. doi: 10.1016/j.celrep.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Rogov V, Dötsch V, Johansen T, Kirkin V. 2014. Interactions between autophagy receptors and ubiquitin-like proteins form the molecular basis for selective autophagy. Mol Cell 53:167–178. doi: 10.1016/j.molcel.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Stolz A, Ernst A, Dikic I. 2014. Cargo recognition and trafficking in selective autophagy. Nat Cell Biol 16:495–501. doi: 10.1038/ncb2979. [DOI] [PubMed] [Google Scholar]
- 19.Fujita N, Itoh T, Omori H, Fukuda M, Noda T, Yoshimori T. 2008. The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Mol Biol Cell 19:2092–2100. doi: 10.1091/mbc.e07-12-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fracchiolla D, Sawa-Makarska J, Zens B, Ruiter AD, Zaffagnini G, Brezovich A, Romanov J, Runggatscher K, Kraft C, Zagrovic B, Martens S. 2016. Mechanism of cargo-directed Atg8 conjugation during selective autophagy. Elife 5:e18544. doi: 10.7554/eLife.18544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abert C, Kontaxis G, Martens S. 2016. Accessory interaction motifs in the Atg19 cargo receptor enable strong binding to the clustered ubiquitin-related Atg8 protein. J Biol Chem 291:18799–18808. doi: 10.1074/jbc.M116.736892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravenhill BJ, Boyle KB, von Muhlinen N, Ellison CJ, Masson GR, Otten EG, Foeglein A, Williams R, Randow F. 2019. The cargo receptor NDP52 initiates selective autophagy by recruiting the ULK complex to cytosol-invading bacteria. Mol Cell 74:320–329. doi: 10.1016/j.molcel.2019.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turco E, Witt M, Abert C, Bock-Bierbaum T, Su MY, Trapannone R, Sztacho M, Danieli A, Shi X, Zaffagnini G, Gamper A, Schuschnig M, Fracchiolla D, Bernklau D, Romanov J, Hartl M, Hurley JH, Daumke O, Martens S. 2019. How RB1CC1/FIP200 claws its way to autophagic engulfment of SQSTM1/p62-ubiquitin condensates. Autophagy 15:1475–1477. doi: 10.1080/15548627.2019.1615306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turco E, Witt M, Abert C, Bock-Bierbaum T, Su M, Trapannone R, Sztacho M, Danieli A, Shi X, Zaffagnini G, Gamper A, Schuschnig M, Fracchiolla D, Bernklau D, Romanov J, Hartl M, Hurley JH, Daumke O, Martens S. 2019. FIP200 claw domain binding to p62 promotes autophagosome formation at ubiquitin condensates. Mol Cell 74:330–346. doi: 10.1016/j.molcel.2019.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hara T, Takamura A, Kishi C, Iemura S, Natsume T, Guan J, Mizushima N. 2008. FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cells. J Cell Biol 181:497–510. doi: 10.1083/jcb.200712064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vargas JNS, Wang C, Bunker E, Hao L, Maric D, Schiavo G, Randow F, Youle RJ. 2019. Spatiotemporal control of ULK1 activation by NDP52 and TBK1 during selective autophagy. Mol Cell 74:347–362. doi: 10.1016/j.molcel.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Besteiro S. 2019. The role of host autophagy machinery in controlling Toxoplasma infection. Virulence 10:438–447. doi: 10.1080/21505594.2018.1518102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grunwald DS, Otto NM, Park JM, Song D, Kim DH. 2020. GABARAPs and LC3s have opposite roles in regulating ULK1 for autophagy induction. Autophagy 16:600–614. doi: 10.1080/15548627.2019.1632620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yue X, Ping Z, Sen C, Qiuhe L, Kathrin N, Ann-Katrin H, Lin L, Xuyan S, Zhiwei Z, Wenqing G, Da L, Huabin H, Xiaoyun L, Jingjin D, Michael OH, Feng S. 2019. A bacterial effector reveals the V-ATPase-ATG16L1 axis that initiates xenophagy. Cell 178:552–566. doi: 10.1016/j.cell.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 30.Coers J, Haldar AK. 2015. Ubiquitination of pathogen-containing vacuoles promotes host defense to Chlamydia trachomatis and Toxoplasma gondii. Commun Integr Biol 8:e1115163. doi: 10.1080/19420889.2015.1115163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.