Peptidoglycan, the sugar-amino acid polymer that composes the bacterial cell wall, requires a significant expenditure of energy to synthesize and is highly immunogenic. To minimize the loss of an energetically expensive metabolite and avoid host detection, bacteria often recycle their peptidoglycan, transporting its components back into the cytoplasm, where they can be used for subsequent rounds of new synthesis. The peptidoglycan-recycling substrate binding protein (SBP) MppA, which is responsible for recycling peptidoglycan fragments in Escherichia coli, has not been annotated for most intracellular pathogens.
KEYWORDS: Chlamydia trachomatis, oligopeptide transport (Opp), nutrient acquisition, metabolism, peptidoglycan recycling, triornithine, tri-DAP, pathoadaptation, immune dampening
ABSTRACT
Peptidoglycan, the sugar-amino acid polymer that composes the bacterial cell wall, requires a significant expenditure of energy to synthesize and is highly immunogenic. To minimize the loss of an energetically expensive metabolite and avoid host detection, bacteria often recycle their peptidoglycan, transporting its components back into the cytoplasm, where they can be used for subsequent rounds of new synthesis. The peptidoglycan-recycling substrate binding protein (SBP) MppA, which is responsible for recycling peptidoglycan fragments in Escherichia coli, has not been annotated for most intracellular pathogens. One such pathogen, Chlamydia trachomatis, has a limited capacity to synthesize amino acids de novo and therefore must obtain oligopeptides from its host cell for growth. Bioinformatics analysis suggests that the putative C. trachomatis oligopeptide transporter OppABCDF (OppABCDFCt) encodes multiple SBPs (OppA1Ct, OppA2Ct, and OppA3Ct). Intracellular pathogens often encode multiple SBPs, while only one, OppA, is encoded in the E. coli opp operon. We hypothesized that the putative OppABCDF transporter of C. trachomatis functions in both oligopeptide transport and peptidoglycan recycling. We coexpressed the putative SBP genes (oppA1Ct, oppA2Ct, oppA3Ct) along with oppBCDFCt in an E. coli mutant lacking the Opp transporter and determined that all three chlamydial OppA subunits supported oligopeptide transport. We also demonstrated the in vivo functionality of the chlamydial Opp transporter in C. trachomatis. Importantly, we found that one chlamydial SBP, OppA3Ct, possessed dual substrate recognition properties and is capable of transporting peptidoglycan fragments (tri-diaminopimelic acid) in E. coli and in C. trachomatis. These findings suggest that Chlamydia evolved an oligopeptide transporter to facilitate the acquisition of oligopeptides for growth while simultaneously reducing the accumulation of immunostimulatory peptidoglycan fragments in the host cell cytosol. The latter property reflects bacterial pathoadaptation that dampens the host innate immune response to Chlamydia infection.
INTRODUCTION
Chlamydia trachomatis belongs to the family Chlamydiaceae and causes both trachoma, an ocular infection that leads to blindness, and sexually transmitted infections. Genital C. trachomatis infections can result in serious health consequences, such as pelvic inflammatory disease, ectopic pregnancy, chronic pain, and epididymitis (1). C. trachomatis possesses a biphasic developmental cycle. The rigid and infectious form, known as the elementary body (EB), initiates infection by adhering to susceptible host cells. After internalization, EBs differentiate over the next 6 to 10 h into a larger, metabolically active, and osmotically fragile vegetative form known as the reticulate body (RB). RBs grow and divide inside a protective host cell phagosome, termed an inclusion. Dividing RBs secrete effector proteins via a type III secretion system, which results in the redirection of host nutrients to the growing inclusion. In the late phase of the developmental cycle, RBs reduce their volume, condense their chromosome, and transition back into infectious EBs. After 48 to 72 h of infection, EBs are predominant in the mature inclusion and subsequent host cell lysis releases EBs to initiate a new infection cycle (2, 3).
The intracellular nature of Chlamydia and its small genome, which lacks many biosynthetic genes, makes Chlamydia dependent on the host for nutrients (4–6). According to the Kyoto Encyclopedia of Genes and Genomes (KEGG) (7), C. trachomatis cannot perform de novo synthesis for 14 amino acids, including alanine, valine, leucine, isoleucine, phenylalanine, methionine, proline, cysteine, threonine, asparagine, glutamine, histidine, arginine, and lysine. Importantly, valine, leucine, isoleucine, phenyl alanine, cysteine, histidine, tyrosine, and the tripeptide γ-l-glutamyl-l-cysteinylglycine are essential because their absence from the growth medium prevents inclusion formation (8).
Chlamydia must meet its nutritional requirements by obtaining either individual essential amino acids or oligopeptides. Strategically, oligopeptide transport is more advantageous because the same amount of energy (ATP) is required to transport either individual amino acids or amino acid-rich oligopeptides through an ATP binding cassette (ABC)-type transporter (9). In this context, oligopeptide transporters are crucial for growth because these ABC-type transporters transport oligopeptides more efficiently than non-ATP-utilizing transporters (10, 11). A typical oligopeptide permease transporter (Opp) is composed of a substrate binding protein (SBP; OppA), two transmembrane proteins (OppB and OppC), and two ATPase subunits (OppD and OppF), encoded by an oppABCDF operon (12). The putative transporter in C. trachomatis is more complex. given that, in addition to a complete C. trachomatis oppA3BCDF (oppA3BCDFCt) operon, two other oppA-encoding genes (oppA1Ct and oppA2Ct) are present elsewhere in the genome. The presence of multiple oppACt paralogs suggests either that they are pseudogenes or that the chlamydial Opp transporter has a broad capacity for transporting various substrates.
Apart from oligopeptide transport, the Opp transporter in many bacterial species, including Listeria monocytogenes, Escherichia coli, and Neisseria gonorrhoeae, also recycles periplasmic amidase-generated small fragments of peptidoglycan (e.g., l-Ala-γ-d-Glu-mDAP). A specialized murein binding subunit (MppA) forms a complex with the core (OppBCDF) of the Opp transporter to mediate this transport (13–16). In contrast, larger and intact muropeptides, disaccharide-anhydro tri- and tetrapeptides (e.g., GlcNAc-anhMurNAc-l-Ala-d-Glu-meso-diaminopimelic acid [mDAP]) (17), are transported by AmpG, a permease-type transporter belonging to the major facilitator family.
Nonpathogenic bacteria recycle 40 to 50% of their peptidoglycan to conserve the energy required for its de novo synthesis (15, 18). However, for intracellular pathogens like C. trachomatis, peptidoglycan recycling can be a two-part strategy for survival in the host. First, peptidoglycan recycling can conserve a significant amount of energy since the host cannot provide certain peptidoglycan precursors, such as mDAP. The de novo synthesis of mDAP to construct mDAP-containing peptidoglycan stem peptides requires six independent, energy-intensive biochemical reactions in C. trachomatis (19). Second, recycling of peptidoglycan fragments in C. trachomatis could be a result of pathoadaptation because it limits the release of immunogenic fragments that can stimulate the innate host immune response. No studies have been conducted to date in C. trachomatis to identify an Opp transporter or its role(s) in metabolite acquisition and peptidoglycan recycling.
Peptidoglycan recycling in the intracellular pathogen Neisseria meningitidis (20) minimizes its detection by host cytosolic NOD-based pathogen-associated molecular pattern (PAMP) receptors, which could otherwise lead to immune clearance (21). A similar mechanism could be operative in C. trachomatis because the peptidoglycan degradation products of C. trachomatis are immunostimulatory ligands for both NOD1 and NOD2 (22). Recycling would prevent immune recognition as well as conserve peptidoglycan precursors for assembly of nascent peptidoglycan in new RBs. Interestingly, neither an MppA nor an AmpG homolog exists in the C. trachomatis genome. Instead, genes encoding three putative substrate binding proteins (OppA1Ct, OppA2Ct, OppA3Ct) for the oligopeptide transporter are present. In E. coli, the murein binding MppA (MppAEc) shares significant sequence homology (46%) with the OppA of E. coli, involved in oligopeptide transport, which suggests its evolution from a common ancestor following gene duplication (14). In most free-living pathogens and nonpathogens, an MppA subunit evolved following the duplication of an opp subunit gene. However, it is not known whether the same evolutionary process took place in intracellular pathogens, such as C. trachomatis, which contain multiple oppA genes.
The Opp transporter plays diverse roles in fitness, virulence, the modulation of membrane protein stability, and regulation of the function of two-component systems (23). Due to the obligate intracellular nature of Chlamydia and the limited tools available to manipulate the chlamydial genome, we sought to test the function of chlamydial Opp proteins in an E. coli surrogate system (24). In the present study, the putative chlamydial Opp transporter system was reconstructed in a ΔoppABCDF mutant of E. coli and oligopeptide transport and peptidoglycan uptake were tested. After determining the functions of chlamydial Opp components in E. coli mutants, we demonstrated the oligopeptide transport activity of all three OppAs and the additional role of OppA3 in peptidoglycan recycling in vivo in C. trachomatis. Thus, we characterized an Opp transporter in which a bifunctional OppA3Ct module recovers mDAP-containing peptidoglycan fragments that would otherwise be released into the host to initiate an immune response. Because genome annotation cannot always predict the existence of a peptidoglycan-recycling protein, the experimental approach presented in the current study could be helpful for characterizing the role of Opp in other intracellular pathogens that lack an annotated MppA.
RESULTS
Opp paralogs in pathogens and their phylogenetic relationships to MppA.
E. coli synthesizes one OppA protein, which is encoded within the oppABCDF operon, to form the oligopeptide transporter. In addition, E. coli synthesizes a specialized muropeptide (tri-diaminopimelic acid [tri-DAP]) substrate binding subunit, MppA, which complexes with OppBCDF to transport peptidoglycan fragments. mppA is not part of the oppABCDF operon but instead is encoded elsewhere in the E. coli genome (see Fig. S1 in the supplemental material). Thus, it is likely regulated independently of the OppABCDFEc operon. In contrast, the C. trachomatis genome contains an operon encoding oppA3BCDF along with two OppA paralogs (OppA1 and OppA2) that are encoded in the genome as monocistronic genes. Interestingly, the C. trachomatis genome has no annotated MppA protein. Like C. trachomatis, many intracellular pathogens (including other human- and animal-pathogenic chlamydiae), with a few exceptions, possess multiple OppAs. Remarkably, the majority of intracellular pathogens also lack an authentic MppA protein (Table 1). While all species of intracellular Chlamydiaceae possess more than one OppA protein, environmental Chlamydia strains do not encode multiple OppA proteins. The significance of multiple OppA subunits and the absence of MppA in these pathogens is unknown. In E. coli, mppA diverged from oppA to gain the specialized function of recycling tri-DAP (14). To test whether any of the oppA genes of C. trachomatis followed a similar evolution, a phylogenetic tree analysis was performed. Consistent with a previous report (14), the phylogenetic tree concluded that MppAEc evolved from OppAEc by accumulating specific mutations. OppA3Ct has a similar evolutionary pattern as MppAEc which makes it an outgroup to OppA1Ct and OppA2Ct (Fig. 1). The diversification of OppA3Ct from OppA1Ct and OppA2Ct and its evolutionary distance suggest that these two periplasmic components of the Chlamydia Opp transporter have divergent substrates. While experimental evidence demonstrated a function for MppAEc (i.e., the transport of tri-DAP) distinct from that of its own homolog, OppAEc (14), despite similar sequence divergence, a peptidoglycan transport function for OppA3Ct has not been tested.
TABLE 1.
Distribution of oligopeptide and tri-DAP binding protein subunits in intracellular pathogens and the numbers of their paralogs
| Organism name | No. of OppA proteinsa | Type of lifestyle | No. of annotated MppA proteins |
|---|---|---|---|
| Chlamydia trachomatis L2/434/Bu | 3 (CTL0450, CTL0394, and CTL0427) | Obligate intracellular | None predicted |
| Chlamydia muridarum Nigg | 3 (TC_0471, TC_416, and TC_446) | Obligate intracellular | None predicted |
| Chlamydia pneumoniae | 4 (CPn0195, CPn0196, CPn0197, and CPn0198) | Obligate intracellular | None predicted |
| Chlamydia pecorum | 4 (G5S_0973, G5S_0968, G5S_0970, and G5S_0972) | Obligate intracellular | None predicted |
| Chlamydia psittaci | 4 (CPSIT_0640, CPSIT_0638, CPSIT_0639, and CPSIT_0585) | Obligate intracellular | None predicted |
| Chlamydia avium | 3 (M832_03820, M832_03530, and M832_03810) | Obligate intracellular | None predicted |
| Chlamydia abortus | 4 (CAB574, CAB527, CAB572, and CAB573) | Obligate intracellular | None predicted |
| “Candidatus Protochlamydia amoebophila” | 1 (pc1502) | Obligate intracellular | None predicted |
| Parachlamydia acanthamoebae | 1 (PUV_22640) | Obligate intracellular | None predicted |
| Simkania negevensis | 7 (SNE_A05670, SNE_A10450, SNE_A10490, SNE_A10700, SNE_A10710, SNE_A23170, and SNE_A10480) | Obligate intracellular | None predicted |
| Waddlia chondrophila | 1 (wcw_0284) | Obligate intracellular | None predicted |
| Coxiella burnetii | 1 (CBU_1860) | Obligate intracellular | None predicted |
| Brucella abortus | 1 (BAbS19_I00090) | Facultative intracellular | None predicted |
| Mycobacterium tuberculosis | 1 (Rv1280c) | Facultative intracellular | None predicted |
| Listeria monocytogenes | 5 (lmo0135, lmo0152, lmo2044, lmo2196, and lmo2569) | Facultative intracellular | None predicted |
| Shigella dysenteriae | 4 (SDY_1409, SDY_1301, SDY_3217, and SDY_3631) | Facultative intracellular | None predicted |
| Yersinia pseudotuberculosis | 1 (BN7064_RS10080) | Facultative intracellular | None predicted |
| Yersinia enterocolitica | 2 (BFS78_RS12050 and BFS78_RS12045) | Facultative intracellular | None predicted |
| Yersinia pestis biovar Orientalis CO92 | 4 (YPO2182, YPO4003, YPO2355, and YPO2660) | Facultative intracellular | 1 |
| Borrelia burgdorferi | 3 chromosome encoded (BB_0328, BB_0329, and BB_0330) plus 2 plasmid encoded (BB_B16 and BB_A34) | Facultative intracellular | None predicted |
| Treponema pallidum subsp. pallidum Nichols | 1 (TPANIC_0585) | Obligate intracellular | None predicted |
The locus tags of each organism are listed in parentheses.
FIG 1.

Phylogenetic relationship of C. trachomatis OppAs to MppA and OppA of E. coli.
The predicted 3-D structure of OppA3Ct suggests functional similarity to MppAEc.
In order to determine if the substrate binding sites between the Chlamydia paralogs are significantly different, the predicted crystal structures of all three chlamydial OppAs were superimposed, separately, on the structure of E. coli MppA cocrystalized with the tri-DAP substrate (PDB accession number 3O9P). Based on the three-dimensional (3-D) structural alignment, OppA3Ct has a higher percent identity (29%) to MppA than OppA1Ct (14%) and OppA2Ct (10%) do (Fig. S2A). The crucial part of MppA is the binding pocket where tri-DAP binds to the nearby residues through hydrogen bonds. After separately superimposing the putative crystal structures of OppA1Ct, OppA2Ct, and OppA3Ct on the MppA–tri-DAP crystal structure, the binding pocket of MppA of E. coli was analyzed to identify amino acids in other chlamydial OppAs that could potentially interact with tri-DAP (Fig. S2C). Using the MppA–tri-DAP crystal structure, it was determined that Lys20, Leu34, Gln267, Arg402, Arg411, Ser413, Val415, and Asp417 are the important residues (9, 13) in MppA that interact with tri-DAP through hydrogen bonds (Fig. S2B). Interestingly, Lys41, Leu55, Gln272, and Asp424 of OppA3Ct, which correspond to Lys20, Leu34, Gln267, and Asp417 of E. coli MppA, were conserved in the 3-D space of the binding pocket (Fig. S2C and D). Neither of the other two chlamydial OppAs (OppA1Ct and OppA2Ct) contained any of these residues (Table S1). This conservation predicts an ability of OppA3Ct to interact with tri-DAP. To determine the amino acid conservation among OppA proteins of the other Chlamydia species, we aligned all putative OppA amino acid sequences of various chlamydial species with OppA3 of C. trachomatis and compared eight residues in the predicted substrate binding site of OppA3Ct (Table 2). Of the three C. muridarum OppAs, TC_0471 has eight residues identical to those in OppA3Ct, suggesting that it is a potential tri-DAP-transporting protein. Similarly, from each chlamydial species, one of their Opp proteins had a significantly high match to the predicted substrate binding amino acids of OppA3Ct. For example, CPSIT_0585 of C. psittaci, CAB527 of C. abortus, Cpn0195 of C. pneumoniae, and G5S_0968 of C. pecorum have six, six, five, and four matches, respectively, to the predicted substrate binding amino acids of OppA3Ct. Interestingly, the environmental Chlamydia species (Parachlamydia acanthamoebae, Waddlia chondrophila, and Simkania negevensis) had two to four conserved residues, despite the sequence divergence in their OppAs. The conserved amino acid residues in the binding pocket of OppA3Ct and their comparisons to other OppAs of various chlamydial species could be a feature that identifies a tri-DAP binding protein. This model is further supported by the phylogenetic relatedness that clustered CPSIT_0585 of C. psittaci, CAB527 of C. abortus, M832_03530 of C. avium, Cpn0195 of C. pneumoniae, G5S_0968 of C. pecorum, and TC0407 of C. muridarum Nigg with CTL0450 (OppA3) of C. trachomatis (see Fig. 7).
TABLE 2.
Amino acid conservation in the predicted substrate binding pocket of C. trachomatis OppA3 with OppA residues of various Chlamydia species
| Organism name | OppA allelea | Conserved amino acid residues | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Chlamydia trachomatis L2/434/Bu | CTL0450 | K41 | L55 | Q272 | D424 | L409 | I418 | G420 | F422 |
| Chlamydia muridarum Nigg | TC_0416 | ||||||||
| TC_0471 | K43 | L57 | Q274 | D426 | L411 | I420 | G0422 | F424 | |
| TC_0446 | D432 | ||||||||
| Chlamydia pneumoniae | Cpn0195 | L55 | D437 | L422 | G433 | F435 | |||
| Cpn0196 | K36 | ||||||||
| Cpn0197 | |||||||||
| Cpn0198 | G431 | F426 | |||||||
| Chlamydia pecorum | G5S_0973 | G460 | |||||||
| G5S_0968 | K51 | L65 | Q292 | D447 | |||||
| G5S_0970 | K37 | L419 | I428 | ||||||
| G5S_0972 | |||||||||
| Chlamydia psittaci | CPSIT_0640 | D433 | |||||||
| CPSIT_0639 | |||||||||
| CPSIT_0585 | K2 | L16 | D397 | L382 | G393 | F395 | |||
| CPSIT_0638 | |||||||||
| Chlamydia avium | M832_03820 | D458 | |||||||
| M832_03530 | L52 | D435 | L420 | F433 | |||||
| M832_03810 | |||||||||
| Chlamydia abortus | CAB574 | L53 | D433 | ||||||
| CAB573 | G433 | ||||||||
| CAB527 | K38 | L52 | D433 | L418 | G429 | F431 | |||
| “Candidatus Protochlamydia amoebophila” | pc1502 | L53 | D428 | L413 | I422 | ||||
| Parachlamydia acanthamoebae | PUV_22640 | L101 | G591 | ||||||
| Waddlia chondrophila | wcw_0284 | L89 | G574 | ||||||
| Simkania negevensis | SNE_A05670 | K266 | L1026 | ||||||
| SNE_A10450 | L680 | ||||||||
| SNE_A10490 | Q284 | L421 | |||||||
| SNE_A10700 | L417 | ||||||||
| SNE_A10710 | Q286 | L424 | |||||||
| SNE_A23170 | L58 | Q287 | D439 | F437 | |||||
| SNE_A10480 | K38 | ||||||||
Bold alleles have high matches to OppA3Ct.
FIG 7.
Phylogenetic relationship of C. trachomatis OppA3 (CTL0450) to the OppA proteins of various species of Chlamydia. Protein sequences were aligned to construct a phylogenetic tree using the neighbor-joining (N-J) method considering 500 replicates (repl.). The alleles clustering with oppA3Ct are in the square.
OppAs of C. trachomatis restore oligopeptide transport function in an E. coli OppA mutant.
While the amino acid ornithine is nontoxic to bacteria, its oligopeptide form, triornithine (TO), is toxic. TO is taken up by bacteria solely via the Opp transporter and subsequently blocks protein synthesis (25). The functionality of OppABCDFCt was demonstrated in the ΔoppABCDF mutant of E. coli. ΔoppABCDF mutants of E. coli are resistant to TO because of their inability to transport TO. The TO-resistant phenotype was confirmed in our ΔoppABCDF mutant (strain ATM1387). Sensitivity (i.e., the absence of growth for 10 h) to 300 μM TO was restored upon expression of the cloned oppABCDFEc operon (Fig. 2A). Repression of the oppABCDFEc operon by 0.5% glucose resulted in resistance to TO. Similarly, complementation with chlamydial oppA1, oppA2, and oppA3 along with chlamydial oppBCDF resulted in sensitivity to TO upon induction with l-arabinose and resistance to TO upon repression by glucose (Fig. 2B to D). These results confirm that oppA1BCDFCt, oppA2BCDFCt, and oppA3BCDFCt form fully functional oligopeptide transporters in the E. coli ΔoppABCDF mutant and that the chlamydial Opp transporter functions analogously to the OppABCDF transporter of E. coli. The growth kinetics and doubling times in M9 medium supplemented with 0.5% glycerol of the E. coli ΔoppABCDF mutants complemented with either oppABCDF from E. coli or C. trachomatis were comparable (Table 3). These data suggest that the Opp transporter of C. trachomatis is as efficient as the Opp of E. coli for transport of oligopeptides.
FIG 2.
Genetic complementation of an E. coli ΔoppABCDF mutant with the Opp transporter of E. coli (A) and the Opp transporter of C. trachomatis (B, C, and D). l-Arabinose induced gene expression, resulting in sensitivity to 300 μM TO (open symbols), and glucose repression imparted resistance to TO (filled symbols) in the complemented E. coli ΔoppABCDF mutant. The subunits of OppABCDF inserted in the outer membrane are shown in orange (E. coli) and purple (C. trachomatis) in each panel. Growth was measured in M9 minimal medium supplemented with 0.5% glycerol. Model fittings were carried out to determine growth kinetics (Table 3), which were statistically significant (P < 0.05).
TABLE 3.
Growth kinetics in M9 minimal medium supplemented with 0.5% glycerol
| Transporter component and protein | μmax (1/h) | Xmax (OD units/ml) | Doubling time (h) | R2 | P value |
|---|---|---|---|---|---|
| Oligopeptide | |||||
| OppA1Ct | 0.598 ± 0.012 | 0.650 ± 0.016 | 0.83 | 0.99 | <0.05 |
| OppA2Ct | 0.554 ± 0.011 | 0.642 ± 0.019 | 0.78 | 0.99 | <0.05 |
| OppA3Ct | 0.524 ± 0.013 | 0.631 ± 0.026 | 0.82 | 0.99 | <0.05 |
| OppAEc | 0.578 ± 0.020 | 0.668 ± 0.031 | 0.83 | 0.99 | <0.05 |
| Peptidoglycan | |||||
| MppA | 1.45 ± 0.07 | 0.40 ± 0.04 | 0.83 | 0.98 | <0.05 |
| OppA3Ct | 0.1326 ± 0.01 | 0.599 ± 0.03 | 3.87 | 0.99 | <0.05 |
OppA3 of C. trachomatis transports tripeptide/tri-DAP (l-Ala-γ-d-Glu-mDAP).
MppA, an OppA homolog in E. coli, binds to tri-DAP (l-Ala-γ-d-Glu-mDAP), a peptidoglycan fragment. Tri-DAP bound to MppA interacts with OppBCDFEc, leading to the transport of the tri-DAP inside the bacterium. However, an mppA-like gene has not been annotated in C. trachomatis. Instead, three oppA paralogs are present. We hypothesized that one of these OppA paralogs can serve the tri-DAP transport function of MppA. To test this hypothesis, an E. coli ΔmppA ΔdapD double mutant was constructed. The ΔdapD mutation renders the strain auxotrophic for mDAP, and the defect can be rescued by addition of mDAP or tri-DAP. However, the ΔmppA mutation makes the mutant unable to grow in medium containing tri-DAP. As predicted, this double mutant did not grow in broth containing tri-DAP. Complementation with E. coli mppA and induction with l-arabinose restored growth on tri-DAP, whereas repression by 0.5% glucose prevented its growth on tri-DAP (Fig. 3A). While l-arabinose induction of oppA1Ct and oppA2Ct did not support the growth of the mutant on tri-DAP out to 50 h (Fig. S6), induction of oppA3Ct resulted in growth restoration on tri-DAP, although it did more slowly than the E. coli mppA (Fig. 3B). A strain possessing pBAD33::oppA3BCDFCt (i.e., a complete chlamydial transporter plus substrate binding oppA3) exhibited a growth trend similar to that of a strain possessing oppA3Ct on tri-DAP and showed no growth when the expression was repressed by 0.5% glucose (Fig. 3C).
FIG 3.
Determining the role of chlamydial oppA in peptidoglycan recycling using an E. coli ΔdapD ΔmppA mutant that requires DAP. Growth of the ΔdapD ΔmppA mutant in LB in the presence of tri-DAP when complemented with the E. coli mppA (A), oppA3Ct (B), and oppA3BCDFCt (C) genes induced by l-arabinose was determined. Model fittings were carried out to determine the growth kinetics, which were statistically verified by the sensitivity analysis (P < 0.05).
Growth of C. trachomatis in tissue culture is sensitive to TO.
TO has previously been used to characterize Opp transporters in extracellular bacteria in minimal medium lacking competing oligopeptides. Though we characterized the functionality of the chlamydial opp genes in E. coli mutants in M9 minimal medium, we were interested in demonstrating the in vivo function of the chlamydial Opp transport system by measuring the uptake of the toxic TO by C. trachomatis. The obligate intracellular nature of C. trachomatis presents challenges to testing the in vivo function of its Opp transporter because the fetal calf serum present in complete tissue culture medium contains many unknown oligopeptides that may slow down or outcompete the transport of the TO oligopeptide. Increasing concentrations of TO were added to the tissue culture media, and sensitivity was assessed via an immunofluorescence assay (IFA) and a chlamydial titer assay at 40 h postinfection (hpi) (Fig. 4C) (at all concentrations tested, TO was not cytotoxic to HeLa cells; see Materials and Methods). We observed a 25% reduction in EB production at 3 mM TO (P < 0.05). Unknown tripeptides present in the tissue culture media likely competed with TO, preventing a more severe inhibition (Fig. 4). Together, these results demonstrate that C. trachomatis likely utilizes a functional oligopeptide transport system in vivo.
FIG 4.
In vivo functionality of the OppABCDFCt transporter in C. trachomatis, as measured by growth arrest in the presence of TO. (A and B) Titer of C. trachomatis (IFU, inclusion-forming units) (A) and stained inclusions (B) after treatment with TO. HeLa cells were infected with C. trachomatis, and an anti-Chlamydia LPS antibody was used to visualize chlamydial inclusions. DAPI was used as a counterstain to visualize host cell nuclei. A reduction in titer represents the growth reduction of C. trachomatis. (C) Cytotoxicity assessment of HeLa cells for TO, as measured by the MTT assay. Data were evaluated by Student's t test (P < 0.05).
Overexpression of oppA3Ct significantly decreases the release of NOD1-stimulatory muropeptides by C. trachomatis.
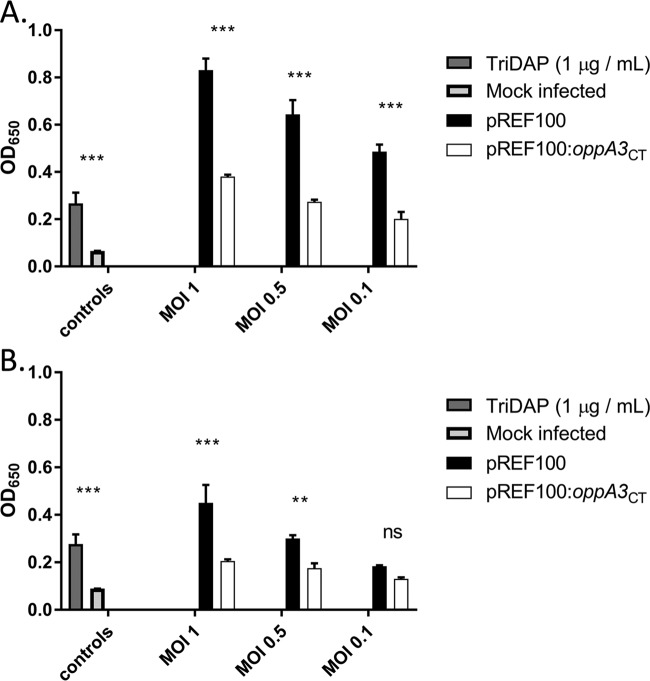
The transport of tri-DAP by OppA3Ct in an E. coli ΔmppA ΔdapD double mutant suggested that peptidoglycan fragments are recycled by OppA3Ct in C. trachomatis. Typically, peptidoglycan fragments, including tri-DAP, are generated during the breakdown of peptidoglycan, and their subsequent extracellular release is sensed by host cytoplasmic pathogen recognition receptors. NOD1 receptors specifically detect l-Ala-d-Glu-mDAP or d-Glu-mDAP. We predicted that if OppA3Ct is responsible for salvaging DAP-containing peptidoglycan fragments in C. trachomatis, its overexpression should reduce NOD1 signaling. We used a human NOD1 (hNOD1) secreted embryonic alkaline phosphatase (SEAP) reporter assay to compare two Chlamydia transformants: one containing an empty shuttle vector (pREF100) (Fig. S3) and one expressing oppA3Ct (pREF100::oppA3). The NOD1-dependent immunostimulatory potential of these strains was determined by infecting HEK-Blue hNOD1 and HEK-Blue Null1 cells with either transformed strain at three different multiplicities of infection (MOIs; 1, 0.5, and 0.1). At 18 (Fig. 5A) and 24 (Fig. 5B) hpi, we observed that at each MOI, the strain expressing additional oppA3 exhibited significantly reduced levels of production of SEAP over time. No significant activity (optical density [OD], <0.05) was measurable for the mock-infected controls or any of the infections carried out in the Null1 cell line (Fig. S4A). Additionally, no change in the infectivity (Fig. S4B) and titer (Fig. S4C) for C. trachomatis overexpressing oppA3 in comparison with those for the empty vector-transfected control established that there was no negative effect of oppA3 overexpression. We conclude that the overexpression of oppA3Ct reduces NOD1 signaling in Chlamydia-infected cells, suggesting that OppA3 could be involved in peptidoglycan recycling in C. trachomatis.
FIG 5.
C. trachomatis oppA3Ct expression decreases the release of NOD1-stimulatory muropeptides. The ability of oppA3Ct to reduce NF-κB activity was tested in HEK-Blue cell lines expressing or lacking hNOD1 immune receptors and carrying the NF-κB-inducible, secreted embryonic alkaline phosphatase (SEAP) reporter gene. The HEK-Blue hNOD1 and HEK-Blue Null1 cell lines were infected with C. trachomatis strains transformed with either an empty vector (pREF100) or an expression vector for oppA3Ct (pREF100::oppA3). The NOD1-stimulatory ligand tri-DAP was used as a positive control. Cell supernatants were tested at 18 (A) and 24 (B) h postinfection for alkaline phosphatase activity, and the OD was measured at 650 nm. The data presented represent those from three independent biological replicates, and error bars represent the standard deviation of the mean. ***, P < 0.001; **, P < 0.005; ns; not significant.
DISCUSSION
In the current study, we characterized an Opp transporter in the obligate intracellular pathogen C. trachomatis. Our findings suggest that while the interactions of any of the three different OppACt subunits with OppBCDFCt allow oligopeptide transport, only OppA3Ct allows for the transport of tri-DAP. For the first time, these findings assign a role to each predicted SBP in C. trachomatis but, more importantly, unveil the existence of a bifunctional Opp transporter that can reduce the release of immunostimulatory peptidoglycan fragments.
Opp transporters have been characterized in E. coli (26) and Borrelia burgdorferi (24) using a synthetic oligopeptide, triornithine (TO), whose transport is Opp transporter dependent. We used a similar strategy and measured the TO sensitivity and growth kinetics of an E. coli ΔoppABCDF mutant complemented with chlamydial oppA modules. oppA1Ct, oppA2Ct, and oppA3Ct were all as capable of oligopeptide transport as the oppAEc-complemented E. coli ΔoppABCDF strain. Sensitivity to TO also provided evidence of the in vivo functionality of the Opp transporter in C. trachomatis. TO sensitivity studies are typically performed in defined minimal medium to eliminate competition between TO and the undefined oligopeptides present in complex medium (24). However due to a lack of minimal medium (axenic medium) to culture C. trachomatis outside a mammalian cell host, we used higher concentrations of TO to outcompete unknown oligopeptides present in the 10% fetal bovine serum (FBS). The selection of the highest concentration of TO in the current study is based on a previous study in which the transport of a dipeptide (alafosfalin) at 3 mM overcame competition for other dipeptides in tissue culture medium containing 10% FBS (25). For C. trachomatis, while smaller amounts were ineffective, 3 mM TO outcompeted the oligopeptides present in FBS in the culture medium and resulted in 25% growth inhibition without causing cytotoxicity to the host (HeLa) cells. Moderate levels of growth inhibition by TO can also be due to the presence of more unknown oligopeptides than dipeptides in FBS that cannot be outcompeted. While the TO-based growth-inhibitory effect in model organisms, including E. coli, is transient due to the breakdown of TO into its monomeric units by bacterial peptidases (27, 28), growth inhibition of C. trachomatis was evident even after 40 hpi. This suggests that C. trachomatis may lack the enzymatic machinery to cleave TO to its individual ornithine units. Alternatively, the lower growth rate of C. trachomatis compared to that of E. coli could be responsible for the extended toxicity of TO. Though we could not attain 100% growth inhibition in C. trachomatis, the data nevertheless demonstrate an active oligopeptide transport mechanism in C. trachomatis in vivo. Since TO was efficiently transported in the oppA1Ct-, oppA2Ct-, and oppA3Ct-complemented E. coli ΔoppABCDF strains, it is plausible that there is no preference for any given OppACt in C. trachomatis. Thus, transport occurs when any OppA protein bound to a substrate interacts with OppBCDFCt (Fig. 6). The use of multiple OppA subunits might be advantageous to intracellular bacteria, including C. trachomatis, by increasing the chances of oligopeptide transport within host cells.
FIG 6.
Model of the C. trachomatis OppA transporter for oligopeptide and tripeptide transport across the inner membrane. The basic components of an Opp transporter of E. coli are shown (orange and red). Subunit mixing allows the transport of either oligopeptides or tripeptides. A predicted Opp transporter of C. trachomatis (purple) and interactions of the three different paralogs with the core OppBCDF transporter allowing oligopeptide transport and tri-DAP uptake are shown.
In recent years, the Opp transporters have gained attention because they not only play a pivotal role in nutrient transport (26) but also are involved in recapturing fragments generated during peptidoglycan degradation for new peptidoglycan strand synthesis (14). Peptidoglycan-derived fragments are not only immunogenic, but their constituent parts are energetically costly to synthesize. Without a means of recovering them for use in subsequent rounds of cell wall synthesis, these valuable components would simply be released out into the environment and wasted. Both Gram-positive and Gram-negative bacteria recycle ∼50% or more of their cell wall components per generation using an MppA/OppBCDF transporter, representing a significant conservation of metabolic energy (29). However, the exact role of Opp transporters for intracellular pathogens cannot be reliably predicted, as they encode multiple SBP subunits in their genomes (Table 1) but no dedicated peptidoglycan fragment (tri-DAP)-transporting subunits (MppA). Characterization of these transporters in intracellular pathogens is further complicated by the fact that there are limited genetic tools available to manipulate genomes and the unavailability of axenic medium to support host-free culturing of these intracellular pathogens. Despite these limitations, Opp transporters in the intracellular bacteria Listeria monocytogenes (30) and Treponema denticola (31) have been shown to be required for pathogenesis. However, the role of the Opp transporter in peptidoglycan transport remains uncharacterized for the majority of intracellular pathogens.
Here, we describe a plausible peptidoglycan-recycling mechanism in C. trachomatis in the absence of an annotated MppA protein. This system can enable C. trachomatis to reutilize mDAP-containing peptidoglycan fragments and conserve its metabolic energy expenditure while also lowering the bacterium’s immunostimulatory profile. We identified a chlamydial protein, OppA3, that can substitute for MppA and transport peptidoglycan fragments (tri-DAP) in an E. coli ΔmppA ΔdapD mutant. Thus, OppA3Ct is a bifunctional protein capable of transporting both oligopeptides and peptidoglycan fragments. However, the tri-DAP transport function was slower than that of MppAEc, suggesting that chlamydial OppA3Ct functions poorly in E. coli. This could be due to the presence of only four amino acid residues out of eight conserved residues in the binding pocket of OppA3Ct which otherwise might completely convert OppA3Ct into an authentic MppA-like subunit. These residues are so important to MppA and OppA3Ct for the tri-DAP transport function that overexpression of OppAEc, OppA1Ct, and OppA2Ct, proteins lacking binding pocket homology to MppA, in a peptidoglycan-recycling-defective E. coli mutant (the ΔmppA ΔdapD mutant) did not support the growth of this mutant in the presence of tri-DAP (see Fig. S5 and S6 in the supplemental material). The presence of these residues has important implications for the identification of the tri-DAP-transporting subunits in other species of Chlamydia. Based on the number of conserved amino acids of various OppAs of different Chlamydia species matching the putative tri-DAP binding pocket of C. trachomatis OppA3, at least one distinct OppA from each species of Chlamydia that potentially serves to transport peptidoglycan fragments in C. psittaci, C. abortus, C. avium, C. pneumoniae, and C. pecorum can be identified (Table 2; Fig. 7).
Peptidoglycan is a common pathogen-associated molecular pattern molecule recognized by host pathogen recognition receptors (PRRs). Peptidoglycan fragments are primarily recognized by the intracellular NOD-like receptors, NOD1 and NOD2 (32, 33). The minimal peptidoglycan ligand for NOD1 is d-Glu-mDAP, whereas NOD2 requires MurNAc covalently linked to the first two amino acids (l-alanine-d-glutamate) in the stem peptide (34). Detection by NOD1 and NOD2 cytoplasmic receptors requires bacterial entry into the host cell cytosol and the degradation of peptidoglycan into detectable fragments (34). The peptidoglycan degradation products of C. trachomatis are immunostimulatory ligands for both NOD1 and NOD2 (22). Therefore, it has been postulated that the limited amount of peptidoglycan produced by C. trachomatis (35) might also limit PRR stimulation.
Based on the results of the complementation studies in E. coli, we hypothesized that C. trachomatis limits PRR stimulation by managing the release of peptidoglycan fragments, likely through OppA3 binding and transport. Overexpression of oppA3Ct in C. trachomatis significantly reduced the release of tri-DAP, as measured by infection of hNOD1 reporter cells. This result supports our model that OppA3 recycles peptidoglycan fragments back into the bacterial cell to minimize the release of PRR-stimulating molecules. However, it remains to be determined if the transported peptidoglycan fragments are further hydrolyzed into their individual components for use in peptidoglycan.
MppA likely arose from OppA in a gene duplication event in E. coli’s evolutionary history, and over time, it evolved to specifically recognize peptidoglycan-derived substrates only. The driving force behind this change could be the need to synthesize peptidoglycan fragments faster without spending energy in rapidly growing bacteria, such as E. coli. The appearance of MppA is a rather recent evolutionary feature because MppA is absent in most intracellular bacteria (Table 1). In contrast, intracellular pathogens, including C. trachomatis and other species of Chlamydia (C. psittaci, C. pneumoniae, C. muridarum, and C. avium), which had very limited options during evolution due to their intracellular lifestyle, might have retained bifunctional activity in their OppAs and retained multiple OppAs.
In conclusion, this study demonstrates not only that the chlamydial Opp transporter is important for meeting the nutritional requirements of the bacterium but also that it may be important to keep Chlamydia in the stealth mode by lowering the bacterium’s immunologic profile. It also identifies OppA3 as a new bifunctional protein in C. trachomatis that can be added to the list of the previously reported bifunctional proteins DapF (3), TrpF (36), Alr (37), AmiA (38), and SpoIID (in Chlamydia-related Waddlia [39]). Because genome annotation does not assign additional functions of multiple paralogs, this important pathoadaptation strategy employed by C. trachomatis remained unnoticed. A similar situation exists in the majority of intracellular pathogens that lack an MppA-like (tri-DAP binding) protein but that encode multiple OppAs. The methods described in the current study might help to reveal variants of OppA that are involved in turning over mDAP-containing peptidoglycan fragments in other intracellular pathogens.
MATERIALS AND METHODS
Bacterial strains, medium, and culture conditions.
C. trachomatis L2 434/Bu was provided by Harlan Caldwell (Rocky Mountain Laboratories, Hamilton, MT). All E. coli K-12 strains are derivatives of strain MG1655 (40), and bacteriophage lambda red recombineering (41) was used to generate E. coli mutants (Table 4). E. coli ΔdapD ΔmppA (ATM1377) and ΔdapD ΔmppA ΔoppABCDF (ATM1478) were grown in LB supplemented with 100 μg/ml meso-DAP (mDAP), and the ΔoppABCDF mutant (ATM1387) was grown in LB without any supplementation. Strains containing pBAD24- and pBAD33-based plasmids were grown in the presence of 100 μg/ml ampicillin and 10 μg/ml chloramphenicol, respectively. Tripeptide/tri-DAP (l-Ala-γ-d-Glu-mDAP) uptake studies were performed in LB. Uptake studies with triornithine (TO) were done in M9 minimal medium supplemented with 0.5% glycerol as a sole carbon source. All studies were performed on a shaker incubator at 37°C overnight unless otherwise stated. l-Arabinose was used as an inducer, and glucose was used as a repressor. Tri-DAP was purchased from InvivoGen, and TO was purchased from Bachem.
TABLE 4.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotypea | Source |
|---|---|---|
| Strains | ||
| ATM759 | MG1655 ΔdapD::Tet | McCoy et al., 2006 (19) |
| ATM1377 | MG1655 ΔdapD::tet ΔmppA::Kan | This study |
| ATM1381 | MG1655 ΔoppABCDF::Cat | This study |
| ATM1387 | ATM1381 ΔoppABCDF | This study |
| ATM1478 | MG1655 ΔdapD::Tet ΔmppA ΔoppABCDF | This study |
| ATM1479 | MG1655 ΔdapD::Tet ΔmppA ΔoppABCDF/pRS6/pRS7 | This study |
| ATM1480 | MG1655 ΔoppABCDF/pRS5 | This study |
| ATM1481 | MG1655 ΔoppABCDF/pRS5/pRS1 | This study |
| ATM1482 | MG1655 ΔoppABCDF/pRS5/pRS2 | This study |
| ATM1483 | MG1655 ΔoppABCDF/pRS5/pRS3 | This study |
| ATM1484 | MG1655 ΔoppABCDF/pRS6/pRS7 | This study |
| ATM1485 | MG1655 ΔdapD::Tet ΔmppA::Kan/pRS1 | This study |
| ATM1486 | MG1655 ΔdapD::Tet ΔmppA::Kan/pRS2 | This study |
| ATM1487 | MG1655 ΔdapD::Tet ΔmppA::Kan/pRS3 | This study |
| ATM1488 | MG1655 ΔdapD::Tet ΔmppA::Kan/pRS4 | This study |
| ATM1489 | MG1655 ΔdapD::Tet ΔmppA::Kan/pRS8 | This study |
| ACE147 | C. trachomatis L2 434/Bu/pREF100 | This study |
| ACE151 | C. trachomatis L2 434/Bu/pREF100::oppA3Ct | This study |
| Plasmids | ||
| pREF100 | Tetracycline-inducible vector, spectinomycin resistant, high copy number | This study |
| pREF100::oppA3Ct | Contains coding sequence of C. trachomatis oppA3 under the control of the Tet promoter | This study |
| pBAD24 | Arabinose-inducible expression vector, Ampr, moderate copy number | Guzman et al., 1995 (51) |
| pBAD33 | Arabinose-inducible expression vector, Catr, moderate copy number | Guzman et al., 1995 (51) |
| pRS1 | pBAD24::oppA1Ct; contains the dsbAEc signal sequence fused at the 5′ end of the coding sequence of C. trachomatis oppA1 under the control of the ara promoter | This study |
| pRS2 | pBAD24::oppA2Ct; contains the dsbAEc signal sequence fused at the 5′ end of the coding sequence of C. trachomatis oppA2 under the control of the ara promoter | This study |
| pRS3 | pBAD24::oppA3Ct; contains the dsbAEc signal sequence fused at the 5′ end of the coding sequence of C. trachomatis oppA3 under the control of the ara promoter | This study |
| pRS4 | pBAD24::oppA3BCDFCt; contains the dsbAEc signal sequence fused at the 5′ end of the coding sequence of C. trachomatis oppA3BCDF under the control of the ara promoter | This study |
| pRS5 | pBAD33::oppBCDFCt; contains the coding sequence of C. trachomatis oppBCDF under the control of the ara promoter | This study |
| pRS6 | pBAD33::oppAEc; contains the opp regulatory region, the oppA gene, and part of E. coli oppB under the control of the ara promoter | This study |
| pRS7 | pBAD24::oppBCDFEc; contains the end region of E. coli oppA beginning from the EcoRV site and extending through the end of the operon under the control of the ara promoter | This study |
| pRS8 | pBAD33::mppAEc; contains the coding region of mppA under the control of the ara promoter | This study |
Tet, tetracycline resistance gene; Kan, kanamycin resistance gene; Cat, chloramphenicol resistance gene; Catr, chloramphenicol resistance; Ampr, ampicillin resistance.
Cell culture conditions.
HeLa cells were routinely cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% heat-inactivated fetal bovine serum (FBS) in appropriate tissue culture flasks. HEK-Blue hNOD1 and Null1 cells were cultured in T-25 flasks (BD Falcon) at 37°C with 5% CO2. HEK-Blue hNOD1 and Null1 cells were grown in high-glucose Dulbecco’s modified Eagle medium plus GlutaMAX supplement (DMEM; Gibco) and supplemented with 10% heat-inactivated FBS, 100 U per ml penicillin-streptomycin, 100 μg/ml Normocin, 100 μg/ml Zeocin. HEK-Blue hNOD1 cells were also supplemented with 30 μg/ml of blasticidin in order to maintain the plasmid coding for the human NOD1 gene.
Development of a new shuttle vector for C. trachomatis.
We modified the pBOMB-Tet-mCherry plasmid (42) to develop a new shuttle vector to express the genes of interest in C. trachomatis with a new selectable marker, aadA, which encodes spectinomycin resistance. The newly constructed plasmid was named pREF100. Spectinomycin resistance is a useful selectable marker for C. trachomatis, as reported previously by Lowden et al. (43). The rationale for removing the bla gene from pBOMB-Tet-mCherry was to prevent ampicillin-induced persistence in C. trachomatis. We removed the bla and mCherry genes from pBOMB-Tet-mCherry by digesting it with NotI and AhdI and inserted aadA at the same sites (NotI and AhdI) (see Fig. S3 and S7 in the supplemental material). Spectinomycin was used at 100 μg/ml to select E. coli bacteria harboring this plasmid, and 500 μg/ml was used for the isolation of C. trachomatis transformants. Using primers P13/P14, oppA3 from the genome of C. trachomatis was PCR amplified and cloned into pREF100 at NotI/KpnI site, which places this gene under the control of an anhydrotetracycline (aTet)-inducible promoter. This plasmid was called pREF100::oppA3. All plasmids used in the current study were transformed into suitable E. coli strains, as mentioned in Table 4.
Transformation of C. trachomatis.
The transformation of C. trachomatis L2 was performed as previously described (42), with the following modifications. Briefly, each transformation mixture contained 7 μg of plasmid DNA (either pREF100 or pREF100::oppA3), a crude preparation of 1 × 107 C. trachomatis elementary bodies (EBs), and 40 μl 5× CaCl2 buffer (50 mM Tris, 250 mM CaCl2, pH 7.4). Sterile water was added for a total volume of 200 μl per mixture. The transformation mixtures were incubated at room temperature (RT) for 30 to 45 min, at which time 200 μl of 1× CaCl2 buffer was added. The 400-μl mixture was then added to 6 × 106 freshly trypsinized L2 mouse fibroblast cells (L929 variant obtained from K. Holmes [44]) and incubated at RT for 20 min with occasional mixing. The transformation/L2 cell mixtures were distributed equally among the wells of a 6-well plate containing 1.5 ml of 1× DMEM plus 10% FBS, and the plates were incubated at 37°C with 5% CO2. After 12 to 18 h, the medium was replaced with fresh medium containing 1-μg/ml cycloheximide and 500-μg/ml spectinomycin and harvested for the next passage after 40 h. The transformed Chlamydia bacteria were passaged up to 4 times with selection and then plaque purified twice, before being expanded to generate a crude stock.
Construction of complementation and overexpression plasmids.
The complete open reading frame of E. coli mppA was amplified by PCR using the P1 and P2 primers to add a Shine-Dalgarno (S/D) sequence at the 5′ end of this gene. This was subsequently cloned in pBAD33 at the SalI/SphI site. The oppA1Ct, oppA2Ct, and oppA3Ct genes were PCR amplified from the chromosome of C. trachomatis L2, followed by fusion of the periplasmic signal sequence of E. coli dsbA to the 5′ end of each gene (Table 4). These PCR products were further cloned into the multiple-cloning site of pBAD24 using the P3, P4, P5, P6, P7, and P8 primers (Table 5). The oppBCDFCt of C. trachomatis was cloned into pBAD33 at the KpnI/PstI site using the P9/P10 primers (ATM1480). As a positive control, the oppA and oppBCDF genes from E. coli were PCR amplified using P11/P12 (along with an S/D sequence) and P13/P14, followed by cloning these PCR products into pBAD33 and pBAD24, respectively, as described previously (14). For C. trachomatis overexpression studies, pREF100 was used.
TABLE 5.
Primers used in strain construction
| Primer | Sequence (5′ to 3′)a | Restriction site |
|---|---|---|
| P1 | TCAATGCTTCACAATATACATAGTCCGACTG | SphI |
| P2 | TGATTAACTTTATAAGGAGGAAAAACATATGTCGGTTAGAGGGAAACTTATGAAG | SalI |
| P3 | ATGAAAAAGATTTGGCTGGCGCTGGCTGGTTTAGTTTTAGCGTTTAGCGCATCGGCGGCGCAGTATGAAGATTATGTTCGCTCTATCTTTT | KpnI |
| P4 | CTAGTGAAAGTGTGCATAGGTCAAATCAAC | PstI |
| P5 | ATGAAAAAGATTTGGCTGGCGCTGGCTGGTTTAGTTTTAGCGTTTAGCGCATCGGCGGCGCAGTATGAAGATCATCACAGGAAGTTTTTA | KpnI |
| P6 | CTATCCTTCAGCTAATGAAACTCTTTTTAAA | PstI |
| P7 | ATGAAAAAGATTTGGCTGGCGCTGGCTGGTTTAGTTTTAGCGTTTAGCGCATCGGCGGCGCAGTATGAAGATCGCAAGATATCAGTGGGA | KpnI |
| P8 | TTAAGAGTTTTTAGCATACCGCATATCA | SphI |
| P9 | TGATTAACTTTATAAGGAGGAAAAACATATGCTCAGCTACATAAAGAGGCGGCTGTT | KpnI |
| P10 | TCAAAAAGAGAAAGTAAAAGCTGGAATA | PstI |
| P11 | TGATTAACTTTATAAGGAGGAAAAACATATGACCAACATCACCAAGAGAAGTT | SalI |
| P12 | CTGCAGATAGTAATAAGAATAAATAGCGTCGGAATCG | PstR |
| P13 | ATTGTTAATAAAGTGAAAGCGC | EcoRV |
| P14 | TTAAAGCGGATCGACTTTCAGG | PstI |
Underlining indicates Shine-Dalgarno (S/D) sequences.
Bioinformatic analysis of putative OppACt paralogs.
The web-based server Phyre2 was used to predict the three-dimensional (3-D) model for OppA1, OppA2, and OppA3 of C. trachomatis L2. Phyre2 employs homology recognition methods to predict a 3-D structure, ligand-binding site, and the effect of amino acid variants for the submitted query sequence (45). A predicted structure generated by Phyre2 can be used for structural comparisons with a known crystal structure present in the Protein Data Bank (PDB). To determine the important residues that may participate in binding to tri-DAP, all three predicted OppACt crystal structures were superimposed individually onto an E. coli MppA bound to tri-DAP, and further structural analysis was carried out using the UCSF Chimera package (46).
To determine the presence or absence of MppA and the number of paralogs for OppA in the genomes of various intracellular pathogens, the Kyoto Encyclopedia of Genes and Genomes (KEGG) (7) was used, and the findings are summarized in Table 1. To determine the phylogenetic relationship of all three OppAs of C. trachomatis to orthologous MppA and its homologous sequences of E. coli, deduced protein sequences were aligned to construct a phylogenetic tree using the neighbor-joining (N-J) method (MEGA software, version 6.0.6) (47). Five hundred replicates were used to produce reliable taxon clustering. Evolutionary distances (number of amino acid substitutions per site) were computed using the Poisson correction method (48).
Oligopeptide transport functionality of C. trachomatis oppABCDF in an E. coli ΔoppABCDF mutant.
The E. coli ΔoppABCDF mutant (ATM1387) complemented separately with oppBCDFCt and oppA1Ct (ATM1481), oppBCDFCt and oppA2Ct (ATM1482), or oppBCDFCt and oppA3Ct (ATM1483) was pregrown in LB overnight at 37°C. As a control, ATM1387 harboring oppBCDFEc along with oppAEc and the parental ATM1387 strain were grown under similar conditions. To remove residual LB medium components, overnight cultures were washed twice with M9 medium, prior to inoculation into fresh M9 medium supplemented with 0.5% glycerol and 300 μM TO. The inducer (0.5% l-arabinose) and the repressor (0.5% glucose) were added at the beginning, and all samples were incubated in a shaker incubator at 37°C. Growth was measured by taking readings of the optical density at 600 nm (OD600) every 2 h for 8 h. To test the statistical significance of the experimental data, a logistic approach was used to determine the maximum specific growth rate (μmax; in hours−1) and the maximum biomass (Xmax) by employing the Mathematica (version 10.0) package (Wolfram Research Inc, Champaign, IL, USA). The entire data set was statistically analyzed by analysis of variance (ANOVA), and the standard error, t statistic, and P values were estimated as described previously (49).
HeLa cell cytotoxicity assay.
The 3-(4,5-dimethyithiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to observe any cytotoxicity of TO for HeLa cervical carcinoma cells (HeLa-USU, a variant of HeLa-CCL2 from the American Type Culture Collection) (50). The MTT assay measures the amount of active NAD(P)H inside HeLa cells as an indication of cytotoxicity. This method relies on the availability of NAD(P)H in viable cells that reduces the yellow MTT to a purple formazan precipitate, which is subsequently dissolved by HCl, followed by measurement of the optical density at 570 nm. HeLa cells were plated in Dulbecco’s modified Eagle’s medium (DMEM) plus 10% fetal bovine serum (FBS) at a density of 5 × 104 cells/0.2 ml per well in 96-well microtiter plates. After 24 h, the medium was removed and replaced with complete medium containing 0, 0.25, 0.50, or 3 mM TO. The plates were incubated for 40 h at 37°C with 5.0% CO2. At 40 h, the medium containing TO was replaced with phenol red-free DMEM and the MTT assay was performed as described by the manufacturer (Cayman, Ann Arbor, MI, USA). The viability of HeLa cells treated with TO was calculated in terms of the OD570, and the data were plotted as a bar chart. Student’s t-test statistic was applied to validate the experimental data.
Chlamydial infection and TO sensitivity.
HeLa cells were plated at 1.3 × 105 cells/ml in triplicate wells of 24-well plates either with or without glass coverslips for use in immunofluorescence assays (IFAs) and titer assays, respectively. After 24 h, the monolayers were infected with C. trachomatis L2 at a multiplicity of infection of 0.5 in 100 μl sucrose-phosphate-glutamic acid buffer per well. The cultures were incubated at 37°C for 2 h with agitation every 30 min to allow for adsorption. At time zero, the inocula were removed and the cultures were refed with DMEM plus 10% FBS and 1 μg/ml cycloheximide. To test the sensitivity of C. trachomatis to TO, 0, 0.5, or 3 mM TO was added at time zero in the refeeding medium. At 40 h postinfection, cultures seeded on coverslips were fixed with 0.5 ml cold methanol for 20 to 30 min and stored in wash buffer for staining. After 40 h, infected monolayers lacking coverslips were scraped using sterile 200-μl large-orifice pipette tips, harvested, and immediately frozen at −80°C for later use in Chlamydia titer assays. Student’s t-test statistic was applied to validate the experimental data.
Chlamydia IFAs and titer assays.
For immunofluorescence assays (IFAs), fixed coverslips were stained with Pathfinder Chlamydia lipopolysaccharide (LPS) stain (Bio-Rad, Hercules, CA) to visualize chlamydial inclusions and were counterstained with DAPI (4′,6-diamidino-2-phenylindole) to visualize host cell nuclei. Each coverslip was photographed under a ×400 magnification using a Z1 Axiovert Observer epifluorescence microscope and the accompanying Zen Blue software (Zeiss, Oberkochen, Germany). Representative images from three coverslips per sample are shown.
To determine the production of infectious progeny, samples were thawed, vortexed, and sonicated and then serially diluted for infection of a new monolayer of HeLa cells. After 40 h, the coverslips were fixed with methanol and stained as described above to visualize chlamydial inclusions. Inclusions were counted on two coverslips per sample using a Zeiss Z1 Axio Observer inverted light and epifluorescence microscope at a ×200 magnification. Data are expressed as the average number of inclusion-forming units per milliliter ± standard deviation.
Testing chlamydial OppA paralogs for tripeptide/tri-DAP (l-Ala-γ-d-Glu-mDAP) uptake.
For tri-DAP uptake, the E. coli ΔmppA ΔdapD mutant (ATM1377) was used. It is auxotrophic for mDAP (ΔdapD), but it can use tri-DAP to satisfy its mDAP requirement if MppA or an equivalent protein is expressed to transport tri-DAP. To test for the transport of tri-DAP in the E. coli ΔmppA ΔdapD mutant, oppA1Ct, oppA2Ct, oppA3Ct, and mppAEc were expressed individually (Table 4). All complemented strains were grown in LB containing 100 μg/ml mDAP with appropriate antibiotics to maintain the respective plasmids. After overnight growth, the strains were washed with LB to remove residual mDAP. The strains were diluted to an initial OD of 0.01 in LB supplemented with 50 μg/ml tri-DAP (l-Ala-γ-d-Glu-mDAP). l-Arabinose (1%) was added to induce the expression and 0.5% glucose was added to repress the expression of the cloned genes. Statistical significance was determined as described previously (49).
Infection of HEK-Blue hNOD1 cells with oppA3Ct-overexpressing C. trachomatis and NF-κB reporter assay.
HEK-Blue cells expressing the hNOD1 receptor and carrying the NF-κB-inducible, secreted embryonic alkaline phosphatase (SEAP) reporter gene (InvivoGen) were used according to the manufacturer’s instructions and adapted to assess the NOD1-specific NF-κB activity during infection with C. trachomatis. Briefly, 3 × 105 cells/ml of HEK-Blue hNOD1 or HEK-Blue Null1 cells were plated in 96-well plates (total reaction volume, 200 μl per well, or ∼6.0 × 104 cells per well) and allowed to settle/adhere for 6 h at 37°C. These cells were infected with C. trachomatis strains harboring either pREF100::oppA3Ct or pREF100 (empty vector control) at an MOI of 1, 0.5, and 0.1. To ensure synchronous and uniform infections, the plates were centrifuged for 1 h at 2,000 × g. To induce the expression of the gene cloned under the anhydrotetracycline (aTet) promoter, 10-ng/ml anhydrotetracycline was added at 0 hpi. The plates were incubated in a CO2 incubator at 37°C for 18 h and 24 h. To perform the NF-κB reporter assay (NOD1) for estimating the amount of residual MurNAc-l-Ala-d-Glu-mDAP in the experimental samples and controls, 20 μl of supernatants collected at specified times (18 h and 24 h) were added to 180 μl of the SEAP detection solution (InvivoGen), followed by incubation at 37°C for ∼8 h. SEAP enzymatic activity was then quantified using a plate reader set to 650 nm. The results for the two strains were compared to each other, to those for the mock-infected controls, and to those for cells treated with the known NOD1 signaling ligand tri-DAP (1 μg/ml). HEK-Blue hNOD1 SEAP reporter assays were carried out in biological triplicate, statistical analysis was conducted by 2-way ANOVA, and significance values were analyzed utilizing Sidak’s multiple-comparison test.
Accession number.
The complete sequence of pREF100 has been deposited in GenBank under accession number MT241513.
Supplementary Material
ACKNOWLEDGMENTS
We thank Rey E. Fernandez for constructing the pREF100 shuttle vector for gene expression studies in C. trachomatis.
Molecular graphics and analyses were performed using the UCSF Chimera package, developed by the resource for Biocomputing, Visualization and Informatics at the University of California, San Francisco.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Schachter J. 1990. Chlamydial infections. West J Med 153:523–534. [PMC free article] [PubMed] [Google Scholar]
- 2.Moulder JW. 1991. Interaction of chlamydiae and host cells in vitro. Microbiol Rev 55:143–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liechti G, Singh R, Rossi PL, Gray MD, Adams NE, Maurelli AT. 2018. Chlamydia trachomatis dapF encodes a bifunctional enzyme capable of both d-glutamate racemase and diaminopimelate epimerase activities. mBio 9:e00204-18. doi: 10.1128/mBio.00204-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grieshaber S, Swanson JA, Hackstadt T. 2002. Determination of the physical environment within the Chlamydia trachomatis inclusion using ion-selective ratiometric probes. Cell Microbiol 4:273–283. doi: 10.1046/j.1462-5822.2002.00191.x. [DOI] [PubMed] [Google Scholar]
- 5.Thomson NR, Holden MTG, Carder C, Lennard N, Lockey SJ, Marsh P, Skipp P, O'Connor CD, Goodhead I, Norbertzcak H, Harris B, Ormond D, Rance R, Quail MA, Parkhill J, Stephens RS, Clarke IN. 2008. Chlamydia trachomatis: genome sequence analysis of lymphogranuloma venereum isolates. Genome Res 18:161–171. doi: 10.1101/gr.7020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Younes HM, Gussmann J, Braun PR, Brinkmann V, Meyer TF. 2006. Naturally occurring amino acids differentially influence the development of Chlamydia trachomatis and Chlamydia (Chlamydophila) pneumoniae. J Med Microbiol 55:879–886. doi: 10.1099/jmm.0.46445-0. [DOI] [PubMed] [Google Scholar]
- 7.Kanehisa M, Goto S. 2000. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res 28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karayiannis P, Hobson D. 1981. Amino acid requirements of a Chlamydia trachomatis genital strain in McCoy cell cultures. J Clin Microbiol 13:427–432. doi: 10.1128/JCM.13.3.427-432.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hosie AHF, Poole PS. 2001. Bacterial ABC transporters of amino acids. Res Microbiol 152:259–270. doi: 10.1016/S0923-2508(01)01197-4. [DOI] [PubMed] [Google Scholar]
- 10.Payne JW. 1968. Oligopeptide transport in Escherichia coli. Specificity with respect to side chain and distinction from dipeptide transport. J Biol Chem 243:3395–3403. [PubMed] [Google Scholar]
- 11.Singh R, White D, Blum P. 2017. Identification of the ATPase subunit of the primary maltose transporter in the hyperthermophilic anaerobe Thermotoga maritima. Appl Environ Microbiol 83:e00930-17. doi: 10.1128/AEM.00930-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ames GF, Mimura CS, Shyamala V. 1990. Bacterial periplasmic permeases belong to a family of transport proteins operating from Escherichia coli to human: traffic ATPases. FEMS Microbiol Rev 6:429–446. doi: 10.1111/j.1574-6968.1990.tb04110.x. [DOI] [PubMed] [Google Scholar]
- 13.Maqbool A, Levdikov VM, Blagova E, Herve M, Horler RSP, Wilkinson AJ, Thomas GH. 2011. Compensating stereochemical changes allow murein tripeptide to be accommodated in a conventional peptide binding protein. J Biol Chem 286:31512–31521. doi: 10.1074/jbc.M111.267179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park JT, Raychaudhuri D, Li H, Normark S, Mengin-Lecreulx D. 1998. MppA, a periplasmic binding protein essential for import of the bacterial cell wall peptide l-alanyl-γ-d-glutamyl-meso-diaminopimelate. J Bacteriol 180:1215–1223. doi: 10.1128/JB.180.5.1215-1223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doyle RJ, Chaloupka J, Vinter V. 1988. Turnover of cell walls in microorganisms. Microbiol Rev 52:554–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodell EW, Higgins CF. 1987. Uptake of cell wall peptides by Salmonella typhimurium and Escherichia coli. J Bacteriol 169:3861–3865. doi: 10.1128/jb.169.8.3861-3865.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobs C, Huang LJ, Bartowsky E, Normark S, Park JT. 1994. Bacterial cell wall recycling provides cytosolic muropeptides as effectors for beta-lactamase induction. EMBO J 13:4684–4694. doi: 10.1002/j.1460-2075.1994.tb06792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park J. 1995. Why does Escherichia coli recycle its cell wall peptides? Mol Microbiol 17:421–426. doi: 10.1111/j.1365-2958.1995.mmi_17030421.x. [DOI] [PubMed] [Google Scholar]
- 19.McCoy AJ, Adams NE, Hudson AO, Gilvarg C, Leustek T, Maurelli AT. 2006. l,l-Diaminopimelate aminotransferase, a trans-kingdom enzyme shared by Chlamydia and plants for synthesis of diaminopimelate/lysine. Proc Natl Acad Sci U S A 103:17909–17914. doi: 10.1073/pnas.0608643103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun Y-H, Bakshi S, Chalmers R, Tang CM. 2000. Functional genomics of Neisseria meningitidis pathogenesis. Nat Med 6:1269–1273. doi: 10.1038/81380. [DOI] [PubMed] [Google Scholar]
- 21.Boneca IG. 2005. The role of peptidoglycan in pathogenesis. Curr Opin Microbiol 8:46–53. doi: 10.1016/j.mib.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Packiam M, Weinrick B, Jacobs WR, Maurelli AT. 2015. Structural characterization of muropeptides from Chlamydia trachomatis peptidoglycan by mass spectrometry resolves “chlamydial anomaly.” Proc Natl Acad Sci U S A 112:11660–11665. doi: 10.1073/pnas.1514026112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alix E, Blanc-Potard AB. 2009. Hydrophobic peptides: novel regulators within bacterial membrane. Mol Microbiol 72:5–11. doi: 10.1111/j.1365-2958.2009.06626.x. [DOI] [PubMed] [Google Scholar]
- 24.Lin B, Short SA, Eskildsen M, Klempner MS, Hu LT. 2001. Functional testing of putative oligopeptide permease (Opp) proteins of Borrelia burgdorferi: a complementation model in opp- Escherichia coli. Biochim Biophys Acta 1499:222–231. doi: 10.1016/s0167-4889(00)00121-x. [DOI] [PubMed] [Google Scholar]
- 25.Neumann J, Bruch M, Gebauer S, Brandsch M. 2004. Transport of the phosphonodipeptide alafosfalin by the H+/peptide cotransporters PEPT1 and PEPT2 in intestinal and renal epithelial cells. Eur J Biochem 271:2012–2017. doi: 10.1111/j.1432-1033.2004.04114.x. [DOI] [PubMed] [Google Scholar]
- 26.Barak Z, Gilbarg C. 1975. Specialized peptide transport system in Escherichia coli. J Bacteriol 122:1200–1207. doi: 10.1128/JB.122.3.1200-1207.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barak Z, Gilvarg C. 1974. Triornithine-resistant strains of Escherichia coli. Isolation, definition, and genetic studies. J Biol Chem 249:143–148. [PubMed] [Google Scholar]
- 28.Gilvarg C, Levin Y. 1972. Response of Escherichia coli to ornithyl peptides. J Biol Chem 247:543–549. [PubMed] [Google Scholar]
- 29.Johnson JW, Fisher JF, Mobashery S. 2013. Bacterial cell-wall recycling. Ann N Y Acad Sci 1277:54–75. doi: 10.1111/j.1749-6632.2012.06813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borezee E, Pellegrini E, Berche P. 2000. OppA of Listeria monocytogenes, an oligopeptide-binding protein required for bacterial growth at low temperature and involved in intracellular survival. Infect Immun 68:7069–7077. doi: 10.1128/iai.68.12.7069-7077.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fenno JC, Tamura M, Hannam PM, Wong GW, Chan RA, McBride BC. 2000. Identification of a Treponema denticola OppA homologue that binds host proteins present in the subgingival environment. Infect Immun 68:1884–1892. doi: 10.1128/iai.68.4.1884-1892.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rietdijk ST, Burwell T, Bertin J, Coyle AJ. 2008. Sensing intracellular pathogens—NOD-like receptors. Curr Opin Pharmacol 8:261–266. doi: 10.1016/j.coph.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Akira S. 2006. TLR signaling. Curr Top Microbiol Immunol 311:1–16. doi: 10.1007/3-540-32636-7_1. [DOI] [PubMed] [Google Scholar]
- 34.Wolf AJ, Underhill DM. 2018. Peptidoglycan recognition by the innate immune system. Nat Rev Immunol 18:243–254. doi: 10.1038/nri.2017.136. [DOI] [PubMed] [Google Scholar]
- 35.Liechti G, Kuru E, Hall E, Kalinda A, Brun YV, VanNieuwenhze M, Maurelli AT. 2014. A new metabolic cell wall labeling method reveals peptidoglycan in Chlamydia trachomatis. Nature 506:507–510. doi: 10.1038/nature12892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adams NE, Thiaville JJ, Proestos J, Juárez-Vázquez AL, McCoy AJ, Barona-Gómez F, Iwata-Reuyl D, de Crécy-Lagard V, Maurelli AT. 2014. Promiscuous and adaptable enzymes fill “holes” in the tetrahydrofolate pathway in Chlamydia species. mBio 5:e01378-14. doi: 10.1128/mBio.01378-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Benedetti S, Bühl H, Gaballah A, Klöckner A, Otten C, Schneider T, Sahl H-G, Henrichfreise B. 2014. Characterization of serine hydroxymethyltransferase GlyA as a potential source of d-alanine in Chlamydia pneumoniae. Front Cell Infect Microbiol 4:19. doi: 10.3389/fcimb.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klöckner A, Otten C, Derouaux A, Vollmer W, Bühl H, De Benedetti S, Münch D, Josten M, Mölleken K, Sahl H-G, Henrichfreise B. 2014. AmiA is a penicillin target enzyme with dual activity in the intracellular pathogen Chlamydia pneumoniae. Nat Commun 5:4201. doi: 10.1038/ncomms5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacquier N, Yadav AK, Pillonel T, Viollier PH, Cava F, Greub G. 2019. A SpoIID homolog cleaves glycan strands at the chlamydial division septum. mBio 10:e01128-19. doi: 10.1128/mBio.01128-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guyer MS, Reed RR, Steitz JA, Low KB. 1981. Identification of a sex-factor-affinity site in E. coli as γδ. Cold Spring Harbor Symp Quant Biol 45:135–140. doi: 10.1101/SQB.1981.045.01.022. [DOI] [PubMed] [Google Scholar]
- 41.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bauler LD, Hackstadt T. 2014. Expression and targeting of secreted proteins from Chlamydia trachomatis. J Bacteriol 196:1325–1334. doi: 10.1128/JB.01290-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lowden NM, Yeruva L, Johnson CM, Bowlin AK, Fisher DJ. 2015. Use of aminoglycoside 3′ adenyltransferase as a selection marker for Chlamydia trachomatis intron-mutagenesis and in vivo intron stability. BMC Res Notes 8:570. doi: 10.1186/s13104-015-1542-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holmes KV. 1975. Scanning electron microscopic studies of virus-infected cells. I. Cytopathic effects and maturation of vesicular stomatitis virus in L2 cells. J Virol 15:355–362. doi: 10.1128/JVI.15.2.355-362.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelley LA, Sternberg MJE. 2009. Protein structure prediction on the web: a case study using the Phyre server. Nat Protoc 4:363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- 46.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. 2004. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 13:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 47.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zuckerkandl E, Pauling L. 1965. Evolutionary divergence and convergence in proteins, p 97–166. In Evolving genes and proteins. Elsevier, Amsterdam, Netherlands. [Google Scholar]
- 49.Singh R, Tevatia R, White D, Demirel Y, Blum P. 2019. Comparative kinetic modeling of growth and molecular hydrogen overproduction by engineered strains of Thermotoga maritima. Int J Hydrogen Energy 44:7125–7136. doi: 10.1016/j.ijhydene.2019.01.124. [DOI] [Google Scholar]
- 50.Bonaparte MI, Dimitrov AS, Bossart KN, Crameri G, Mungall BA, Bishop KA, Choudhry V, Dimitrov DS, Wang L-F, Eaton BT, Broder CC. 2005. Ephrin-B2 ligand is a functional receptor for Hendra virus and Nipah virus. Proc Natl Acad Sci U S A 102:10652–10657. doi: 10.1073/pnas.0504887102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guzman LM, Belin D, Carson MJ, Beckwith J. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol 177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.