Abstract
Although brain metastases from bone and soft tissue sarcoma are uncommon, advances in sarcoma treatment have led to an increasing incidence of them. We present a 23-year-old male with a history of metastatic femoral osteosarcoma, who presented with headache and unsteady gait and was diagnosed with a cerebellar metastasis. CT scan revealed a mass in the left cerebellar parenchyma with large intralesional central calcification and perilesional edema. Corticosteroid treatment led to neurological symptoms resolution, with a rapid tapering. The patient had also lung metastases and we opted to administer systemic treatment with the tyrosine kinase inhibitor cabozantinib. Given the relative radioresistance of osteosarcomas, the patient did not receive radiation therapy.
Keywords: Brain metastasis, Osteosarcoma, Calcification
Introduction
There is an increase in the incidence of brain metastases in soft tissue and bone sarcomas due to new chemotherapeutic and radiotherapeutic treatments that prolong survival through systemic disease control but without effective intracranial control [1], [2], [3]. Mean time of brain metastasis from initial diagnosis is approximately 20-30 months [4].
Brain metastases are rare in osteosarcoma and lung tumor emboli invading the brain is the main cause of metastases [5]. Yonemoto et al recommended performing brain imaging periodically in patients with known active pulmonary metastasis [6]. Similarly to osteosarcoma metastases to the lungs, brain lesions are characterized by large calcifications.
The primary tumors reported to be responsible for calcified brain metastases are squamous cell carcinoma of the lung, adenocarcinoma of the lung, breast adenocarcinoma, mediastinal sarcoma, squamous cell carcinoma of the cervix, adenocarcinoma of the pancreas, non-Hodgkin's lymphoma, osteosarcoma and mucinous colorectal, and ovarian adenocarcinomas [7].
We report the clinical course of a 23-year-old patient with a history of femoral osteosarcoma, who was diagnosed with brain metastasis. CT scan showed a unique calcified lesion inside the left cerebellar parenchyma, mimicking an osseous lesion.
Case presentation
A 20-year-old male was diagnosed with high-grade osteoblastic osteosarcoma of the right femur, in October 2016. He received neoadjuvant chemotherapy according to MAP protocol (high-dose methotrexate, adriamycin, cisplatin) in the first medical oncology clinic of Saint-Savvas Cancer Hospital (Athens, Greece) between August and October 2016. In October 2016 he underwent surgery and histological examination revealed osteosarcoma with <90% necrosis. He received adjuvant treatment per protocol, combined with mifamurtide, which was maintained until November 2017.
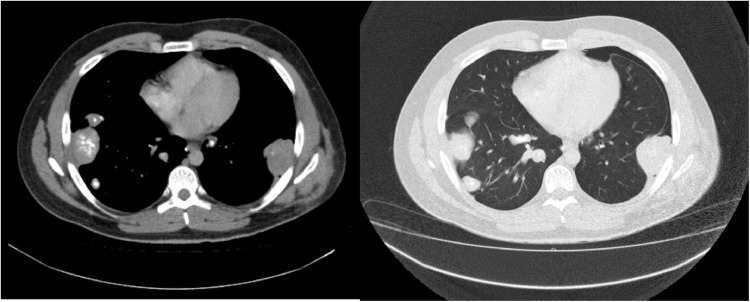
In March 2018 he was diagnosed with solitary lung mass. He underwent lung metastasectomy and histological examination confirmed metastasis from high-grade osteosarcoma. We did not administer to the patient any systemic treatment, in line with the sarcoma tumor board decision for a close follow-up. Six months later imaging revealed a controlateral lung lesion, as well as a left pelvic mass. We opted again for surgical management of the 2 metastases. Therefore the patient underwent lung metastasectomy followed by resection of the pelvic mass. Subsequently, he received 3 cycles of ifosfamide/etoposide chemotherapy. Imaging reevaluation showed unfortunately progression of the disease, with multiple lung nodules. Gemcitabine/docetaxel was then administered as third-line chemotherapy with no efficacy and finally topotecan/cyclophosphamide. CT scan of thorax and abdomen after the third cycle revealed progressive disease in the lungs (Fig. 1), as well as new renal metastases.
Fig. 1.
Thoracic CT scan (axial view) showing multiple partially calcified lung metastases from osteosarcoma.
One month later he visited the oncology clinic with headache and a slight unsteady gait. A brain CT scan was performed showing a left cerebellar metastasis of 2.3 cm in size with perilesional edema (Fig. 2). The patient had a good performance status and neurological symptoms resolved with dexamethasone treatment. We opted to administer off-label cabozantinib, a tyrosine kinase inhibitor (TKI) which was recently reported to have some activity in advanced bone sarcomas. Radiation therapy to the cerebral tumor was not proposed, due to the relative radioresistance of osteosarcoma. Therefore he started cabozantinib 60 mg per day at the end of July 2019, without any complications except for mild transaminitis. Symptoms associated with cerebral metastasis did not recur, despite a rapid cortisone tapering. However, the patient died 2 months later due to respiratory failure in the context of progressive disease in the lungs.
Fig. 2.
A brain CT scan (axial and coronal view) was performed and revealed a solitary space occupying lesion, 2.3 cm in size, in the left cerebellar region with large intralesional central calcification and perilesional edema that was suggestive of metastatic deposit of osteosarcoma.
Discussion
Cerebral metastases from soft tissue or bone sarcomas are not common and therefore brain imaging is not systematically performed. Their incidence is approximately 1%-8% [3,8,9]. In the largest adult series (246 patients), published by the French Sarcoma Group, osteosarcoma represented 6% of cases, whereas more common soft tissue histotypes like leiomyosarcoma represented the majority of cases [10]. Pediatric studies have reported a higher incidence of brain metastases from osteosarcoma, compared to other histologies [1].
Radiological findings of osteosarcoma brain metastases are unique and characterized by large calcifications. Both supratentorial and infratentorial localizations have been reported [11,12]. Our patient exhibited a typical image of a left cerebellar mass with large intralesional central calcification in brain CT scan. He developed also metastases to both kidneys, another rare location for osteosarcoma.
Very sparse data exist regarding the clinical course and management of brain metastases from osteosarcoma and come mainly from case-reports or small retrospective series [8], [9], [10], [11], [12]. Most patients are already diagnosed with metastases in other sites, more commonly the lungs. Despite the poor prognosis of brain metastases, aggressive multimodality treatment has been reported to prolong survival of these patients [4].
Surgical management has traditionally been reported as the main treatment modality for sarcoma brain metastasis [6,[13], [14], [15]]. This is based to the evidence originating from some studies of osteosarcoma lung metastases, in which a survival benefit is reported [16]. Surgical resection of osteosarcoma brain metastases has led in some cases to long-term disease control and survival [6,11].
Evidence for the use of radiation therapy is inconclusive. It has been used in some cases of soft tissue and bone sarcoma metastases [5,17]. According to the French retrospective series, local treatments including surgery and radiation therapy seem beneficial in terms of survival [10]. This approach needs evaluation in the context of prospective trials. We did not deliver radiation therapy to our patient, because osteosarcoma is not considered a radiosensitive sarcoma histotype. In addition he experienced disease progression in the lungs, not amenable to local treatment.
Systemic therapy is the mainstay of treatment for metastatic sarcoma. As for other primary tumors, the question of intracranial efficacy of the different drugs is posed. TKIs represent a relatively new therapeutic class in the armamentarium of bone sarcomas. Sorafenib, regorafenib, lenvatinib, and cabozantininb were tested in phase 2 trials of advanced osteosarcoma and clinically relevant efficacy was found [18], [19], [20], [21]. Cabozantinib yielded the most positive result, with a median progression-free survival of 6.2 months. Our patient was heavily pretreated and received off-label cabozantinib. Despite corticoid tapering, he did not experience any recurrence of neurological symptoms, suggesting a possible control of intracranial metastasis. TKIs like cabozantinib could be an effective treatment for cerebral metastases and warrant further evaluation.
In conclusion, we present an interesting radiological image of osteosarcoma metastasis to the left cerebellar parenchyma in a young male. Our case illustrates a clinical scenario of calcified brain metastases. Although sarcoma metastatic to the brain is uncommon, clinicians should be aware of this condition and request appropriate investigations.
Ethics committee approval
We received consent from the patient. The study was approved by the Ethics Committee of the hospital.
Footnotes
Funding: No funding was received for this work.
Competing Interests: The authors declare no conflict of interest.
References
- 1.Kebudi R, Ayan I, Görgün O, Agaoglu FY, Vural S, Darendeliler E. Brain metastasis in pediatric extracranial solid tumors: survey and literature review. J Neurooncol. 2005;71:43–48. doi: 10.1007/s11060-004-4840-y. [DOI] [PubMed] [Google Scholar]
- 2.Chou YS, Liu CY, Chen WM, Chen TH, Chen PC, Wu HT. Brain, the last fortress of sarcoma: similar dismal outcome but discrepancy of timing of brain metastasis in bone and soft tissue sarcoma. J Surg Oncol. 2011;104:765–770. doi: 10.1002/jso.22011. [DOI] [PubMed] [Google Scholar]
- 3.Salvati M, D'Elia A, Frati A, Santoro A. Sarcoma metastatic to the brain: a series of 35 cases and considerations from 27 years of experience. J Neurooncol. 2010;98:373–377. doi: 10.1007/s11060-009-0085-0. [DOI] [PubMed] [Google Scholar]
- 4.Shweikeh F, Bukavina L, Saeed K, Sarkis R, Suneja A, Sweiss F. Brain metastasis in bone and soft tissue cancers: a review of incidence, interventions, and outcomes. Sarcoma. 2014;2014 doi: 10.1155/2014/475175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deutsch M, Orlando S, Wollman M. Radiotherapy for metastases to the brain in children. Med PediatrOncol. 2002;39(1):60–62. doi: 10.1002/mpo.10042. [DOI] [PubMed] [Google Scholar]
- 6.Yonemoto T, Tatezaki S, Ishii T, Osato K, Takenouchi T. Long-term survival after surgical removal of solitary brain metastasis from osteosarcoma. Int J ClinOncol. 2003;8:340–342. doi: 10.1007/s10147-003-0341-9. [DOI] [PubMed] [Google Scholar]
- 7.Graña L, Santamaría N, Yus M, Méndez R. Calcified cerebral metastases. Radiology. 2007;49:335–337. doi: 10.1016/s0033-8338(07)73788-8. [DOI] [PubMed] [Google Scholar]
- 8.Hoiczyk M, Herbrik M, Grabellus F, Podleska L, Pöttgen C, Schwindenhammer B. Brain metastases in sarcoma patients: Incidence and outcome. J Clin Oncol. 2014;32(15_suppl):10591. doi: 10.1200/jco.2014.32.15_suppl.10591. -10591. [DOI] [Google Scholar]
- 9.Espat NJ, Bilsky M, Lewis JJ, Leung D, Brennan MF. Soft tissue sarcoma brain metastases. Prevalence in a cohort of 3829 patients. Cancer. 2002;10:2706–2711. doi: 10.1002/cncr.10554. 94. [DOI] [PubMed] [Google Scholar]
- 10.Chaigneau L, Patrikidou A, Ray-Coquard I, Valentin T, Linassier C, Bay JO. Brain metastases from adult sarcoma: prognostic factors and impact of treatment. A retrospective analysis from the French sarcoma group (GSF/GETO) Oncologist. Aug 2018;23(8):948–955. doi: 10.1634/theoncologist.2017-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baram TZ, van Tassel P, Jaffe NA. Brain metastases in osteosarcoma: incidence, clinical and neuroradiological findings and management options. J Neurooncol. 1988;6(1):47–52. doi: 10.1007/bf00163540. [DOI] [PubMed] [Google Scholar]
- 12.Marina NM, Pratt CB, Shema SJ, Brooks T, Rao B, Meyer WH. Brain metastases in osteosarcoma. Report of a long-term survivor and review of the St. Jude Children's Research Hospital experience. Cancer. 1993;71(11):3656–3660. doi: 10.1002/1097-0142(19930601)71:11<3656::aid-cncr2820711130>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 13.Bindal RK, Sawaya RE, Leavens ME, Taylor SH, Guinee VF. Sarcoma metastatic to the brain: results of surgical treatment. Neurosurgery. 1994;35(2):185–191. doi: 10.1227/00006123-199408000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Fox BD, Patel A, Suki D, Rao G. Surgical management of metastatic sarcoma to the brain: clinical article. J Neurosurg. 2009;110(1):181–186. doi: 10.3171/2008.4.17505. [DOI] [PubMed] [Google Scholar]
- 15.Wronski M, Arbit E, Burt M, Perino G, Galicich JH, Brennan MF. Resection of brain metastases from sarcoma. Ann Surg Oncol. 1995;2(5):392–399. doi: 10.1007/bf02306371. [DOI] [PubMed] [Google Scholar]
- 16.Bielack SS, Kempf-Bielack B, Branscheid D, Carrle D, Friedel G, Helmke K. Second and subsequent recurrences of osteosarcoma: presentation, treatment, and outcomes of 249 consecutive cooperative osteosarcoma study group patients. J Clin Oncol. 2009;27(4):557–565. doi: 10.1200/JCO.2008.16.2305. [DOI] [PubMed] [Google Scholar]
- 17.Nieto-Coronel MT, López-Vásquez AD, Marroquín-Flores D, Ruiz-Cruz S, Martínez-Tláhuel JL, De la Garza-Salazar J. Central nervous system metastasis from osteosarcoma: case report and literature review. Rep Pract Oncol Radiother. 2018;23(4):266–269. doi: 10.1016/j.rpor.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grignani G, Palmerini E, Ferraresi V, D'Ambrosio L, Bertulli R, Asaftei SD. Sorafenib and everolimus for patients with unresectable high-grade osteosarcoma progressing after standard treatment: a non-randomised phase 2 clinical trial. Lancet Oncol. 2015;16(1):98–107. doi: 10.1016/S1470-2045(14)71136-2.012. [DOI] [PubMed] [Google Scholar]
- 19.Duffaud F, Mir O, Boudou-Rouquette P, Piperno-Neumann S, Penel N, Bompas E. Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: a non-comparative, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 2019;20(January (1)):120–133. doi: 10.1016/S1470-2045(18)30742-3. [DOI] [PubMed] [Google Scholar]
- 20.Gaspa N, Casanova M, Sirvent FJB, Venkatramani R, Morland B, Gambart M. Single-agent expansion cohort of lenvatinib (LEN) and combination dose-finding cohort of LEN + etoposide (ETP) + ifosfamide (IFM) in patients (pts) aged 2 to ≤25 years with relapsed/refractory osteosarcoma (OS) J Clin Onco. 2018;36(15_suppl):11527. -11527. [Google Scholar]
- 21.Italiano A, Mir O, Mathoulin-Pelissier S, Penel N, Piperno-Neumann S, Bompas E. Cabozantinib in patients with advanced Ewing sarcoma or osteosarcoma (CABONE): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;(February) doi: 10.1016/S1470-2045(19)30825-3. pii: S1470-2045(19)30825-3. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]