Abstract
Background
Lung toxicity in patients undergoing cetuximab and radiotherapy (Cetux-RT) for head and neck squamous cell carcinoma (HNSCC) has been reported in literature and represents a serious side effect of concurrent therapies.
Methods
We report a case of a HNSCC patient that developed neck emphysema during the course of Cetux-RT. The patient was an old male (80 years old) in a good performance status, with an oropharyngeal cancer (T4aN3a).
Results
During RT, cone-beam computed tomography (CBCT) showed bilateral neck emphysema that was confirmed at restaging CT. We decided to stop the treatment and to treat the neck emphysema with conservative strategies. After one week CT was repeated and the neck emphysema had improved, so we decided to complete the RT treatment.
Conclusions
Patients undergoing Cetux-RT must be properly selected, whereas IGRT imaging must be viewed carefully in order to permit an early diagnosis and careful management of the patients.
Keywords: Neck emphysema, HNSCC, Cetuximab, Radiation therapy
1. Introduction
Head and neck squamous cell carcinoma (HNSCC) represents a heterogeneous group of diseases, so it is of great interest to define subgroups of patients that might benefit from different treatment strategies.1, 2, 3
Cisplatin and radiotherapy (chemoradiotherapy, CRT) is considered to be the standard treatment of locally advanced HNSCC, although it is difficult to use in elderly patients or in patients with impaired renal function. Cetuximab is a monoclonal antibody that targets the epidermal growth factor receptor (EGFR) and has become one of the treatment options for advanced HNSCC, either in combination with RT in locally advanced disease (bioradiotherapy, BRT) or in combination with chemotherapy in recurrent or metastastic disease.
The common side effects of cetuximab include skin reactions, fatigue, weight loss, diarrhea, nausea and stomatitis, whereas rare complications include an increased risk of heart attack and severe pulmonary toxicity, including interstitial lung disease (ILD).4
Aim of this report is the description of a challenging side effect in a HNSCC patient undergoing concurrent Cetux-RT.
1.1. Case report
The reported patient was a 80 year-old male that was diagnosed in July 2019 with a locally advanced oropharyngeal cancer (tongue base).
The PET/CT and the MRI showed a bulky lesion (>6 cm) of the base of the tongue, on the left, reaching the piriform sinus and showing invasion of the parapharyngeal space. There was also a nodal involvement on left level III, with a big necrotic left mass (>6 cm) showing signs of muscle invasion. The clinical staging was scored as cT4aN3a.
The PET/CT examination also showed a small but widespread uptake of the lungs (SUV max 2), that was interpreted as flogistic. The patient was a non-smoker, with no associate co-phatologies, in a good performance status (G8 score: 15, KPS 90%).
The case was discussed in a multidisciplinary tumor board (MTB) and we decided to start Cetuximab and Radiotherapy, with a curative intent.
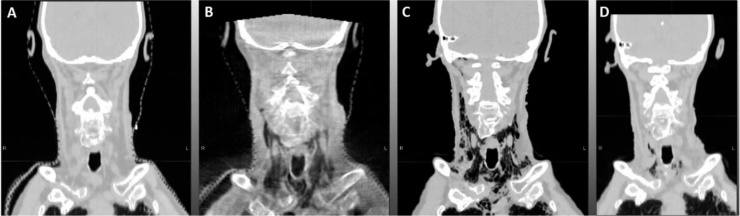
The patient began cetuximab one week before the initiation of the course of RT, with the initial dose of 400 mg/m2 followed by weekly dose of 250 mg/m2 concurrently with RT. The RT course was planned to a total dose of 6996 cGy to the planned target volume 1 (PTV1), including gross tumor volume and positive neck node, with a fractionation of 212 cGy per fraction, 5940 cGy to PTV2, including left omolateral neck nodes levels Ib-II-III-IV, with a fractionation of 180 cGy per fraction, and 5445 cGy to PTV3, including neck node levels V left, IB-II-III-IV right and retropharyngeal nodes, with a fractionation of 165 cGy per fraction (Fig. 1).
Fig. 1.
The figure shows:
A) the simulation CT at baseline before RT;
B) the CBCT, showing suspicious neck emphysema;
C) the diagnostic CT, confirming upper mediastinum and bilateral neck emphysema;
D) the simulation CT one week after the conservative compressive bandage, showing good response of the neck emphysema.
The RT was planned as volumetric modulated arc therapy (VMAT) using simultaneous integrated boost (SIB) and image guided RT (IGRT), as each day the patient underwent cone-beam computer tomography (CB-CT) for pretreatment position verification and online correction of set-up errors with a 6D couch. At this regard, it is noteworthy to underline that pretreatment position verification with CB-CT is usually made by a clinician or a trained radiotherapist that is used to look at target volumes and critical organs at risks only (Fig. 1).
The patient was visited weekly and showed only limited toxicity (G1 skin toxicity and G1 dysphagia according NCI-CTC scoring criteria).
After three weeks, during the pretreatment position verification, the clinician noticed, however, suspicious air in the neck; therefore, the patient underwent diagnostic CT that confirmed the presence of upper mediastinum and bilateral neck emphysema (Fig. 1). The patient was referred to a thoracic surgeon and otorhinolaryngologist and the case was again discussed in a MTB. There was no pneumothorax and a laryngoscopy examination, as well as CT, showed a good initial response of the tumour, so we decided to stop the treatment for one week and to treat the emphysema with a conservative strategy applying a compression bandage.
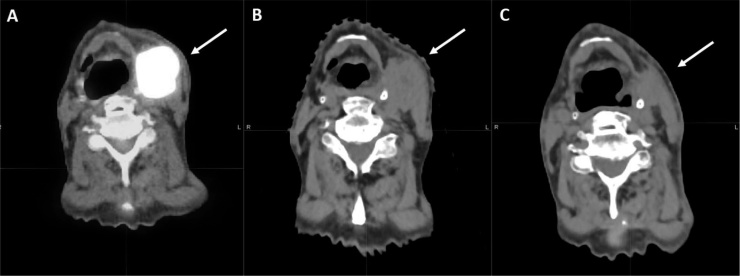
The subsequent follow up simulation CT showed a good response of the neck emphysema and the patient restarted RT treatment after adaptive replanning procedure due to the good response and shrinking of the gross tumor volumes (Fig. 2).
Fig. 2.
The figure shows:
A) the diagnostic PET/CT at baseline before RT;
B) the simulation CT, showing pathologic neck node before RT;
D) the simulation CT before replanning, showing good response of the irradiated lesion.
At the end of the RT course the patient showed mild toxicity (G2 skin toxicity and G2 mucositis, G1 dysphagia and xerostomia according to the NCI-CTC scoring criteria) and was referred to usual follow up examinations.
2. Discussion
The correlation of pulmonary toxicity with epidermal growth factor receptor inhibitors is known in literature,5 although cetuximab related pulmonary toxicity is less frequently described.6
Hoag et al.7 found that the overall rates of pulmonary complications were similar between cetuximab and standard therapy groups, for HNSCC (respectively, 17.9% versus 20.1%, p: NS). Dyspnea was more common in the cetuximab group (8.7% versus 5%, p < 0,02), whereas sputum production and cough were lower (respectively, 3% versus 6.6%, p < 0,01, and 4.5% versus 7.8%, p < 0,01).
Komada et al.8 have investigated the time-to-onset and onset-pattern of drug-induced ILD after the administration of monoclonal antibodies. The authors found that cetuximab showed a median time-to-onset of 1–2 months and was estimated to fit the random failure type profile.8
Incidence of ILD in HNSCC undergoing cetuximab was 4.5% in the work of Nakano et al.9 The same authors reported that high Krebs von den Lungen-L (KL-6) and >50 pack-years of smoking were significantly associated with this side effect.
Okamoto et al.,10 on the other hand, reported an overall incidence of ILD of 9.5% and correlated this toxicity to pulmonary emphysema. The authors analyzed the degree of pulmonary emphysema with scoring according to the Goddard classification guidelines and concluded that even mild pulmonary emphysema would represent a risk factor for ILD in patients undergoing cetuximab.
Satoh et al.11 described a small series of 24 patients undergoing cetuximab for colorectal cancer who developed acute lung injury. Cetuximab-related lung toxicity occurred in 1.2% and was significantly higher in elderly patients, and in patients with prior interstitial lung disease.
The mechanism of pulmonary toxicity in patients undergoing cetuximab for HNSCC is poorly understood. Cetuximab may damage respiratory epithelium, and this could exacerbate the underlying pulmonary inflammatory process.6
Despite the differences of incidence among different studies, the development of pulmonary toxicity in this fragile population of patients is always considered a severe adverse event. Thus, the identification of risk factors is of critical importance in clinical practice.
KL-6 level, high smoking pack-years and mild pulmonary emphysema were identified in previous studies, but in our patient we only noticed a small but widespread uptake of the lungs at PET/CT examination that was interpreted as flogistic.
3. Conclusions
Our reported patient developed neck emphysema, a rare side effect during concurrent cetuximab and RT. Patients with HNSCC undergoing radiation therapy are exposed to many severe and potentially fatal side effects and, accordingly, their early diagnosis and management is extremely important for the dedicated clinicians.
It is noteworthy to underline that the early diagnosis in our case was due to daily IGRT that must be viewed with a critical approach with different window levels.
In the concurrent use of biological therapies and RT (BRT), finally, the pulmonary toxicity must be taken always in consideration also for the selection of the proper patients.
Conflict of interest
None.
Financial disclosure
None.
References
- 1.Lombardi M., Cascone T., Guenzi E. Predictive value of pre-treatment apparent diffusion coefficient (ADC) in radio-chemiotherapy treated head and neck squamous cell carcinoma. Radiol Med. 2017;122(5):345–352. doi: 10.1007/s11547-017-0733-y. [DOI] [PubMed] [Google Scholar]
- 2.Nardone V., Tini P., Nioche C. Texture analysis as a predictor of radiation-induced xerostomia in head and neck patients undergoing IMRT. Radiol Med. 2018;123(6):415–423. doi: 10.1007/s11547-017-0850-7. [DOI] [PubMed] [Google Scholar]
- 3.Deantonio L., Masini L., Brambilla M., Pia F., Krengli M. Dysphagia after definitive radiotherapy for head and neck cancer. Correlation of dose-volume parameters of the pharyngeal constrictor muscles. Strahlenther Onkol. 2013;189(3):230–236. doi: 10.1007/s00066-012-0288-8. [DOI] [PubMed] [Google Scholar]
- 4.Hirasawa K., Okamoto I., Motohashi R. The efficiency and adverse events of radiotherapy with cetuximab for Japanese head and neck cancer patients. Auris Nasus Larynx. 2017;44(6):724–728. doi: 10.1016/j.anl.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Barber N.A., Ganti A.K. Pulmonary toxicities from targeted therapies: A review. Target Oncol. 2011;6(4):235–243. doi: 10.1007/s11523-011-0199-0. [DOI] [PubMed] [Google Scholar]
- 6.Mayfield J.D., Mercado C.E., Kaye F.J., Mendenhall W.M. Cetuximab-associated pulmonary toxicity in concurrent chemoradiation for the treatment of a squamous cell carcinoma of the head and neck. Yakugaku Zasshi. 2019;41(4) doi: 10.1002/hed.25528. E55-e8. [DOI] [PubMed] [Google Scholar]
- 7.Hoag J.B., Azizi A., Doherty T.J., Lu J., Willis R.E., Lund M.E. Association of cetuximab with adverse pulmonary events in cancer patients: A comprehensive review. J Exp Clin Cancer Res. 2009;28:113. doi: 10.1186/1756-9966-28-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Komada F., Nakayama Y., Takara K. [Analysis of time-to-onset and onset-pattern of interstitial lung disease after the administration of monoclonal antibody agents] Yakugaku Zasshi. 2018;138(12):1587–1594. doi: 10.1248/yakushi.18-00094. [DOI] [PubMed] [Google Scholar]
- 9.Nakano K., Seto A., Sasaki T. Incidence and risk factors of interstitial lung disease of patients with head and neck cancer treated with cetuximab. Head Neck. 2019;41(8):2574–2580. doi: 10.1002/hed.25727. [DOI] [PubMed] [Google Scholar]
- 10.Okamoto I., Tsukahara K., Sato H., Motohashi R., Yunaiyama D., Shimizu A. Mild pulmonary emphysema a risk factor for interstitial lung disease when using cetuximab for squamous cell carcinoma of the head and neck. Acta Otolaryngol. 2017;137(12):1288–1291. doi: 10.1080/00016489.2017.1355566. [DOI] [PubMed] [Google Scholar]
- 11.Satoh T., Gemma A., Kudoh S. Incidence and clinical features of drug-induced lung injury in patients with advanced colorectal cancer receiving cetuximab: Results of a prospective multicenter registry. Jpn J Clin Oncol. 2014;44(11):1032–1039. doi: 10.1093/jjco/hyu128. [DOI] [PMC free article] [PubMed] [Google Scholar]