Abstract
Keratin constitutes the major component of the feather, hair, hooves, horns, and wool represents a group of biological material having high cysteine content (7–13%) as compared to other structural proteins. Keratin -based biomaterials have been investigated extensively over the past few decades due to their intrinsic biological properties and excellent biocompatibility. Unlike other natural polymers such as starch, collagen, chitosan, the complex three-dimensional structure of keratin requires the use of harsh chemical conditions for their dissolution and extraction. The most commonly used methods for keratin extraction are oxidation, reduction, steam explosion, microbial method, microwave irradiation and use of ionic liquids. Keratin -based materials have been used extensively for various biomedical applications such as drug delivery, wound healing, tissue engineering. This review covers the structure, properties, history of keratin research, methods of extraction and some recent advancements related to the use of keratin derived biomaterials in the form of a 3-D scaffold, films, fibers, and hydrogels.
Keywords: Natural polymer, Keratin, Wool, Biomedical applications
Graphical abstract
Highlights
-
•
Keratin a versatile material having unique structural moiety.
-
•
Keratin sources and methods of extraction.
-
•
Keratin-based materials for biomedical applications.
-
•
3-D scaffold, films, fibers, and hydrogels.
1. Introduction
There has been growing interest by both the scientific and industrial communities to utilize the biomass and natural raw materials as a source of different specialty products mainly due to the issues related to the environment with an accumulation of waste [1,2]. Raw materials derived from livestock and agriculture like proteins, sugar, cellulose, oils can play a vital role as sustainable and cheap source of materials for various biomedical applications [3]. Some polymers from natural, renewable sources (such as starch, proteins, cellulose, etc) have been investigated widely as they exhibit intrinsic properties that offer new perspectives for designing novel biomaterials [[4], [5], [6]].
Among all these biodegradable natural polymers “Keratin-Based materials “revolutionized the field of modern biomaterials due to their distinct properties like biodegradability, biocompatibility and mechanical durability [7]. Moreover, they can be cast as sponges, films, and hydrogels for various biomedical applications [7]. Keratin constitutes the major components of hair, horns, wool, feathers, and nails. These biopolymers offer wide variations in their structure and properties. Major sources of keratin extraction are wool, hair, nails, feathers, and horns as they served as the most under-exploited sources of protein [8]. Wool fibers which are mainly high in sulfur content are multi-cell structures [8]. New Zealand, China, Australia, Iran, and Argentina are the top five countries in wool production worldwide [9,10]. There are over 2.5 million tonnes of wool and more than 65 million tonnes of feathers production annually [11,12].
This review will mainly focus on the sources, structure, properties, history of the development of keratin-based biomaterials, methods of extraction and biomedical applications.
2. History of keratin research from 16th century till present
According to literature, the earliest use of keratin was reported in the 16th century for medicinal applications by a Chinese herbalist, Li Shi –Zhen [13]. Shi-Zhen also wrote 800 books and described thousands of therapeutic prescriptions. To accelerate the process of wound healing he suggested a substance called Xue Yu Tan which was mainly derived from pyrolyzed human hair. During 1850, the word keratin first appeared in the literature to mainly describe the material derived from animal hooves, horns and other hard tissues [14].
During the early twentieth century, research mainly focused on the methods to extract keratin from different sources and to develop a method to solubilize it like other proteins. In 1905, a patent [15] described a process for keratin extraction from horns using a lime solution. The use of keratin for various medical applications became topic of interest including keratin powders for drug coatings and keratin-based gels [16,17]. During the 1920s, the focus of research was not to just extract keratin proteins but also to understand its structure, function, and properties [14].
During the years of World War and after that the most research projects were to utilize keratin for textile production. In 1940, to expand the potential applications of wool and keratin, the Council for Scientific and Industrial Research established the Division of Protein Chemistry in Australia. The main role of this division was to better understand the properties of keratin fiber. By using electron microscopy, X-Ray diffraction and some other methods CSIRO produced the complete diagram of a hair fiber for the first time [18]. Other investigations conducted by the University of Leeds and the Wool Industries Research Association in the UK, revealed that the structure of keratin fiber consists of a central cortex and an outer cuticle. Researchers in Netherlands patented a method to fabricate keratin-based films and textile fiber derived from hooves [14]. In the next thirty years Japanese patent office received more than 700 applications of keratin-based inventions.
In short, the keratin research conducted between the years of 1940 and 1970, laid a new foundation towards the fundamentals of biomaterials. Many classes and subclasses of keratins along with their properties were studied by scientists [[19], [20], [21]].
Since the 1970s, there were exponential advancements in the techniques used to extract and characterize keratins and their derivatives. As a result, several research societies worked to utilize extracted keratin in the form of fibers, films, foam, gels, and coatings [[22], [23], [24]]. In the past decades, the use of keratin-based materials for various biomedical applications such as drug delivery wound healing, tissue engineering continued to be the topic of interest.
In the 1980s, collagen, alginate, chitosan and hyaluronic acid became the most widely used biomolecules for various medical applications. Japanese researchers published their study in 1982 explaining the role of keratin coatings on the surface of vascular grafts to prevent blood clotting. They also performed experiments to test the biocompatibility of keratins [25,26].
Thus, all these investigations led a solid foundation for further research and opened new perspectives in the field of keratin-based biomaterials for various applications.
3. Keratin structure and properties
Keratin is the cysteine-rich fibrous protein that associate with intermediate filaments (IFs) forming the bulk of cytoskeleton and epidermal appendageal structures such as hair, horns, feathers, wool and nails [7]. These biopolymers present wide variations in their properties and structure and can be classified on the basis of sulfur amount as hard and soft keratins. Hard keratins with a higher amount of sulfur cross-links mainly contribute to the tough epidermal structure and consist of Intermediate filaments arranged in ordered arrays embedded in a cross-linked matrix [27,28]. For more than three decades these hard keratins have been subjected to various in vitro and in vivo investigations to utilize them for the fabrication of biomaterials [[29], [30], [31]]. Soft keratins, having a low amount of sulfur, formed by bundles of cytoplasmic filaments which are loosely packed and provide resilience to the epithelial tissues (epidermis) [27].
Structure of keratins are often discussed in the literature as alpha-keratins and beta-keratins [32]. As far as the distribution of alpha and beta keratin is concerned alpha-keratin is the primary component of wool, hairs, hooves, nails, horns, and the stratum corneum. Whereas, beta-keratin is the primary constituent of feathers, avian claws and beaks, reptilian claws and scales [27,28]. The structure of alpha-keratin can be illustrated diagrammatically as shown in Fig. 1. In alpha-keratin, the polypeptide chains are arranged as alpha-helices whereas, in beta-keratin, they are in the form of pleated beta-sheets. The two twisted polypeptide chains in alpha-keratin formed coiled-coil structure [33].
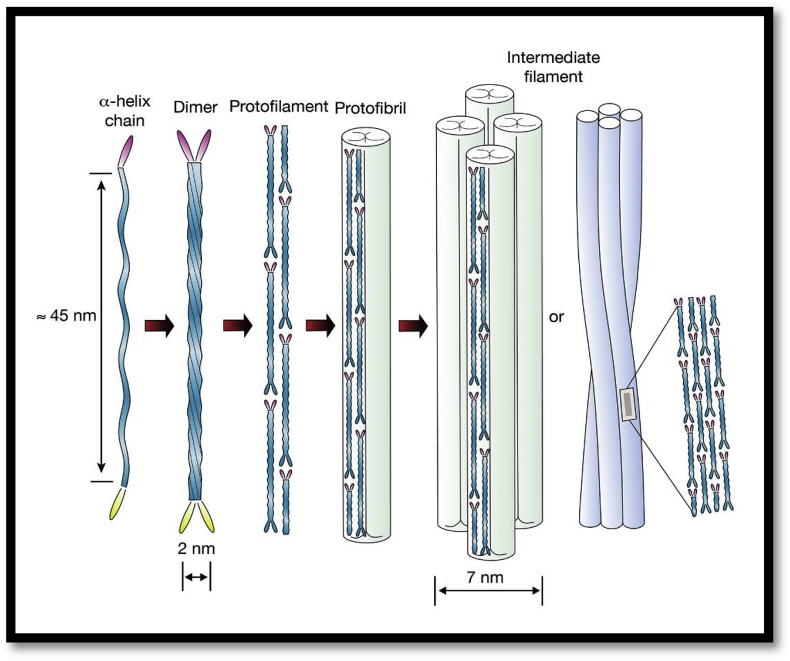
Fig. 1.
(a) Alpha-helices are right-handed individually (b) Two individual right-handed alpha-helix polypeptide further crosslinked by disulfide bonds to form left-handed coiled-coil dimers. (c) side-by-side or end-to-end aggregation of dimers by sulfide crosslinking forms protofilaments (d) two protofilaments when laterally associated forms protofibrils. (e) Intermediate filaments (7 mm diameter) consist of four protofibrils associated with a helical or circular manner. These intermediate filaments are surrounded by amorphous keratin matrix and constitute the basic structural units of keratin [34].
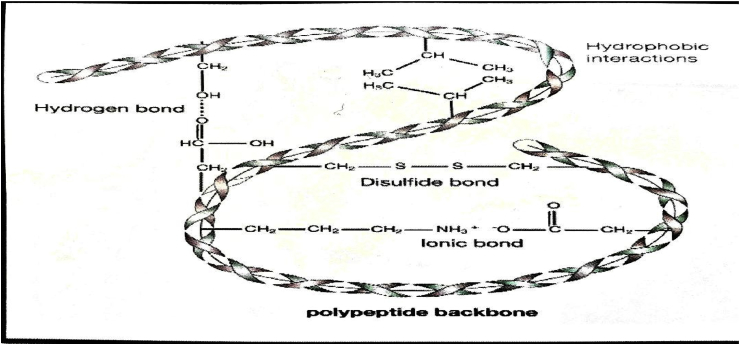
In the last few decades, wool has been investigated extensively as a representative of alpha-keratin material [[35], [36], [37]]. At nanoscale level there is a fine characteristic presentation of filament-matrix structure for both alpha and beta-keratins. The alpha-keratin filament has a diameter of 7–10 nm while, 3–4 nm for beta-keratin filament [38,39]. Wool keratin has a distinct three-dimensional structure and it contains about 95% of proteins, 0.5% minerals and trace amount of lipids (0.1%) [40,41]. The structure of keratin mainly consists of polypeptide chain of different amino acids which served as a backbone having inter and intramolecular bonding as shown in Fig. 2. All these disulfide, hydrogen and ionic bonds increased the stability and strength of keratin structure [42,43]. These proteins are insoluble in majority of solvents like organic solvents, water, weak acids, alkali solutions and protein-digesting enzymes such as trypsin or pepsin. These biopolymers contain glycine, proline, serine, and cysteine in high concentrations whereas low contents of lysine, methionine and histidine (44). Physiochemical properties of wool keratin are different as compared to other proteins such as higher stability and lower solubility. This is mainly due to the cysteine disulphide bonds. However, the presence of other ionic, hydrogen and hydrophobic bonds influenced the properties and stability of wool keratin. The ionic bonds between carboxylic anions and ammonium cations are mainly dependent on pH and at the isoelectric point of pH 4.9 it is highest. These ionic bonds are at its lowest level in extreme conditions of acidity or basicity. At low pH levels these bonds can be reduced by protonation of the carboxylic group and amine group deprotonation at high pH levels [45]. The high stability, resistance to dissolution in various solvents and three-dimensional mesh structure of keratin is due to the presence of disulphide bonds. Solubilization of wool structure mainly occurs by disruption of complex structure of keratin [45].
Fig. 2.
Diagrammatic illustration of keratin structure. Various inter and intramolecular bonding (hydrogen, ionic, disulfide bonds) increased the stability/strength of the keratin materials [46].
4. Methods for keratin extraction
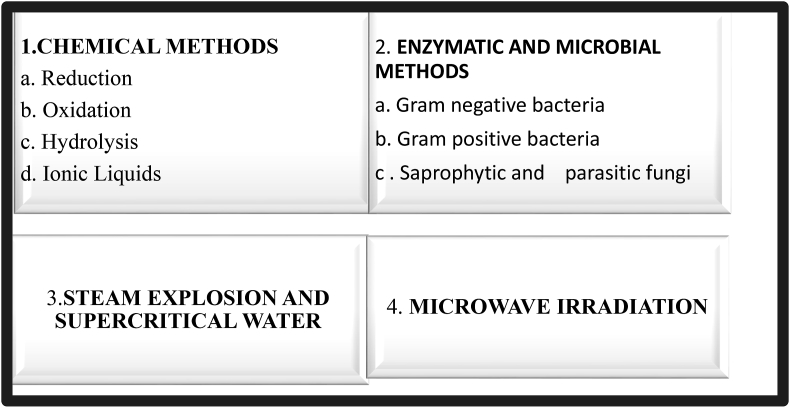
The commonly used methods for keratin extraction from keratin-rich materials are a reduction [47], oxidation [48], alkali extraction [49], microwave irradiation (50), steam explosion [51] and by means of ionic liquids [52]. Fig. 3 summarizes the major methods used to extract and solubilize keratin.
Fig. 3.
Classification of methods for keratin extraction from different sources such as feathers, wool, and hooves.
4.1. Reduction method for keratin extraction
The disulfide linkage in stable keratin structure can be reduced by using chemicals containing thiol. Several reducing agents have been used under varying processing conditions [[53], [54], [55]]. Various chemicals used in this process of reduction will be discussed in the following section.
4.1.1. Effect of different reducing, denaturing and surfactant agents
The use of thiols as a reducing agent is reported back to 1930–1940. Different concentrations of sodium thioglycolate and thioglycolic acid were used for wool keratin reduction in early studies [53,54]. Several investigations observed the effect of pH variation on the degree of keratin reduction [53,54]. According to Goddard at al reduction of wool can only be observed at a pH of 10.5 or higher (53). Patterson et al. also reported increased dissolution at pH values > 11 [54]. Later considerable extraction of protein was observed by Savige et al. at pH 2 by using thiols at a processing temperature of 50–60 °C [55]. In 1962, thioglycolate and mercaptoethanol (MEC) were compared to reducing agents at pH 5 by Thompson and O'Donnell [56]. It was observed that the extent of reduction both chemicals were similar when the concentration of thiol was low. The maximum extractability of 75% was reported by authors and it was suggested that by using 4 M MEC 96% of the wool cystine can be reduced.
Urea has been used commonly as a denaturing agent to increase the solubility of keratin in water [57]. At higher concentration Urea weakens the hydrophobic bondings within the polypeptide chain structure [58]. Several investigations have been done to obtain a high yield of undegraded protein [59,60]. If the extraction of keratin involved high temperature or pH conditions it resulted in severe degradation of keratin structure along with lanthionine formation [61]. Therefore, there was dearth of information on the biochemical and physical properties of extracted keratin under harsh chemical or temperature conditions [47].
According to Yamauchi et al., the use of sodium dodecyl sulfate (SDS) as surfactant improved the stability of extracted keratin and increased the rate of extraction. In 1996 he successfully extracted the keratin with 2-mercaptoethanol urea and SDS with extraction yield of 45–50%. The final extracted keratin contains a small amount of SDS as there was complex formation between the keratin and surfactant [47]. Schrooyen et al. also supported these findings and observed that the final extracted keratin contained 20% of the added SDS [62]. It was also reported that anionic surfactants such as SDS are more effective as compared to cationic and neutral surfactants. Later, Nakamura et al. modified Yamauchi's method of keratin extraction by combining urea and thiourea with MEC. Nakamura et al. also observed that by this modified method protein can be removed from cortex more effectively as compared to conventional method by Yamauchi et al. [47]. This method was developed at Shinshu University, so it was named as “Shindai method “. The extraction yield of keratin by this modified method was >65% [63]. Researchers also extracted keratin from different sources, such as wool and feather by the Shindai method and a yield of >75% was visible which was higher than 5–12% yield obtained from Yamauchi's method of keratin extraction [63]. In Shindai method the addition of thiourea not only improved the keratin dissociation but also significantly increased the extraction yield. Barrows patented a method to overcome the instability of protein extracted by Shindai method [64]. Later the reducing agent MEC was replaced with cysteine by Xu et al. as it is environment friendly in nature [58]. It was also observed that using cysteine controlled breakdown of disulfide bonds occurred and the final product had improved mechanical properties.
4.2. Alkaline method for keratin extraction
The high concentration of hot alkali solutions has the potential to solubilize wool as upon exposure there is cystine residue degradation along with splitting of a sulfur nucleus [65]. A high amount of alkali reagents used during the process and damage to the polypeptide chain structure of keratin are two main drawbacks associated with this method due to which it cannot be used for commercial purposes on large scale [66,67].
For keratin extraction, the required amount of NaOH can be reduced by using strong alkali solutions. Harris et al. observed more rapid degradation of wool in about half an hour when 1% of sodium sulfide was added to NaOH solution (0.065 N) as compared to NaOH solution [68]. However, this combination of NaOH and sodium sulfide resulted in residual wool with high sulfur content. The extracted keratin from wool and feather has 11–17% and 7% of cysteine [49,69].
During the whole extraction process, it is very important to preserve the major amino acids such as cystine as they can be readily decomposed in the presence of strong alkali solutions [70]. Oxalic and pyruvic acids are the major by-products of cystine decomposition. Nagai et al. observed the yield of protein recovery from alkali treatment [71]. He reported that half of the starting material was lost during the process. He further exposed the feathers with 0.1 N NaOH solution for 15 min at 90 °C and found different amino acids composition in the final product than the standard composition of the untreated feather samples. The final extracted keratin from this method had low contents of serine, arginine, cystine while, high amount of lysine, methionine and glutamic acid.
A two-step alkali-reduction method was recently proposed by Jiang-tao et al. to extract keratin from hair samples [72]. He first treated the selected samples with 0.1 mol/L of Na2SO3, SDS, and urea for 5 h. The obtained keratin presented preserved alpha-helix and beta sheet structure. In short, harsh chemical conditions during alkaline treatment process limits the use of keratin on a large commercial scale [71].
4.3. Oxidation method for keratin extraction
The early work on keratin extraction by means of oxidation was reported by Earland et al. [73]. He used 2% peracetic acid, mild ammonia, and HCl for keratin extraction. The oxidation method was mainly used for keratin extraction from hair and wool. During the extraction process, the wool was not completely solubilized and insoluble beta-keratin component was observed in all studies. The completely solubilize component of keratin mainly consist of alpha-keratin. It has also been observed that extended beta-disulfide form of keratin is less soluble than alpha-form [73]. In another study by Weston, it was observed that disulfide bonds were oxidised to sulfonate groups when wool was exposed to 2% peracetic acid for 30 h [74]. Later, Strasheim and Buijs performed infrared analysis to confirmed these results and found cystine monoxide, dioxide and sulfonate groups [75]. Thus, all these oxidation reactions mainly involved the disulfite transformation to sulfonate [76].
The keratin samples after peracetic acid treatment can be divided into alpha, beta, gamma keratose depending on their solubility level at different values of pH [73,77]. Alexander and Earland introduced a process of “keratose” in which they separated keratins depending on their solubility [73,78]. Alpha-keratose was precipitated by adjusting the pH value of the oxidised sample to 4, while beta-keratose was separated after adjusting the pH of oxidised mixture to alkaline [78]. When compared with other methods, the keratin obtained from oxidation (keratosis) exhibited different physicochemical properties due to the conversion of bisulfite bonds to sulfonic acids [79,80].
The major drawbacks associated with the oxidation method for keratin extraction as compared to other methods are the partial oxidation of cystine to cysteic acid by peracetic acid and the loss of some aminoacyls [81]. Simmonds et al. observed that threonine, serine, tyrosine, histidine, and phenylalanine loss from wool after exposure to performic acid, while the concentration of nitrogen increased [82]. Smith and Stockell slowly recovered tyrosine and phenylalanine after oxidation reaction, by using hydrogen peroxide (9:1 v/v) and performic acid (87%) [83].
4.4. Ionic liquids for keratin extraction
Ionic liquids are molten salts mainly composed of organic/inorganic anions and bulky cations. They possessed some unique physiochemical properties like high melting points, thermal stability, high boiling points, chemical stability, high ion conductivity, non-flammability, recyclability, low vapor pressure and high salvation for certain specific solutes (84). In addition to all these properties, ionic liquids also served as a green solvent due to which it has been used widely for various applications such as catalysts, solvent for different polymers and ion conductive media [[85], [86], [87]]. It is often difficult to dissolve wool in a single solvent as many covalent and noncovalent interactions are present [52].
The relationship between different ionic liquids, temperature and solubility were evaluated by Xie et al. in 2005 as shown in Table 1 [52]. He observed that as compared to other tested anions such as BF4, Br, PF6 chloride-containing ionic liquid served as the best solvent [52,84,88]. After dissolution, the keratin was then separated by precipitation with ethanol or water. The regenerated keratin mainly consists of beta-sheet structure as the alpha-helix structure was abolished during the extraction process. The thermal stability of the extracted keratin was higher than the natural wool.
Table 1.
Some of the wool keratin extraction conditions by using ionic liquid.
|
IONIC LIQUIDS |
MATERIAL | PROCESSING CONDITIONS |
% YIELD OF KERATIN | REF | |||
|---|---|---|---|---|---|---|---|
| Temperature (°C) | Time | Solubility | Solid: liquid ratio | ||||
| [Bmim]Br | Wool | 130 | 10 h | 2% | – | – | [52] |
| [Bmim]Cl | Wool | 100 | 10 h | 4% | – | – | [52] |
| [Bmim]Cl | Wool | 130 | 10 h | 11% | – | – | [52] |
| [Amim]Cl | Wool | 130 | 10 h | 8% | – | – | [52] |
| [Bmim]BF4 | Wool | 130 | 24 h | insoluble | – | – | [52] |
| [Bmim]PF6 | Wool | 130 | 24 h | insoluble | – | – | [52] |
| [Amim]Cl | Wool | 130 | 640min | 21% | – | – | [88] |
| [Bmim]Cl | Wool | 130 | 535min | 15% | – | – | [88] |
| [Bmim]Cl | Wool | 120 | 30min | – | 1:6 | 57% | [90] |
| [Bmim]Cl | Wool | 150 | 30min | – | 1:6 | 35% | [90] |
| [Bmim]Cl | Wool | 180 | 30min | – | 1:6 | 18% | [90] |
| [Amim][dca] | Wool | 130 | – | 23% | – | – | [99] |
| [Bmim]Cl | Wool | 130 | – | 12% | – | – | [99] |
| [Amim]Cl | Wool | 130 | – | 10% | – | – | [99] |
| Choline thioglycolate | Wool | 130 | – | 11% | – | – | [99] |
Three major ionic liquids [Amim]Cl, [Bmim]Cl and [Bmim]Br were investigated by Ji et al., for keratin dissolution from feathers [89]. To further facilitate the breakdown of the disulfide bonds Na₂SO₃ was added. It was again observed that chloride-containing ionic liquid was the best solvent for keratin dissolution as it has high Cl‾ concentration and nucleophilic activity which break hydrogen bonds (89, 90). The [Bmim]Cl exhibited slightly lower dissolution as compared to [Amim]Cl but it was recommended due to its cheaper price [52,89].
Ji et al. obtained a keratin yield of 75% with 10% Na₂SO₃ and liquid/feather, a weight ratio of 20 [89]. The whole processing time comprised of 1 h and the extraction was carried out at 90 °C. The processing time and temperature reported by Ji et al. was much lower than the parameters recommended by Xie et al. [52]. Xie et al. observed only 4% wool solubilization after 10 h at 130 °C in [Bmim]Cl. This may be due to the structural differences between wool and feather along with the addition of Na₂SO₃. In both the studies the details regarding amino acid composition were not reported.
Ghosh et al. investigated the effect of temperature on keratin dissolution and the final obtained yield of keratin [90]. The keratin dissolution was observed at different temperatures such as 120 °C, 150 °C, and 180 °C in [Bmim]Cl. At 120 °C, the maximum yield of 57% was reported whereas, 35% and 18% yield were obtained at 150 °C and 180 °C respectively. The researchers concluded that this low yield can be due to the water-soluble amino acids that remained in the ionic liquid and was not precipitated. The hygroscopic nature of ionic liquid required the extraction process to be done under an inert atmosphere [90].
4.4.1. Mechanism of keratin Dissolution and role of temperature
There is a layer of lipid covering the wool fiber surface mainly consist of 18-methyleicosanoic acids [90]. This layer of methyleicosanoic acids is bounded to the proteins in the inner layers by thioester bonds. The ionic liquid reaches the cortex layer after breaking thioesters by Cl‾ ions. After dissociation BMIM ions form complexes with hydroxyl protons which cause hydrogen bonds disruption while Cl‾ anions bonds with hydroxyl protons and as a result keratin dissolution starts [91,92].
The process of keratin dissolution improves by increasing the temperature as more ions become mobile at low viscosity [90]. However, this higher extraction temperature results in a more disrupted structure of the final product. Thus, these two parameters of temperature and final keratin yield should be selected carefully depending on the required properties of the final product. Ghosh et al. also reported that the average cysteine content declined from 8.91 to 0.99 mol% by raising the temperature from 120 to 180 °C [90]. This high-temperature range should be avoided if these amino acids are needed in the final product. The dissolution of wool in ionic liquid is a time - consuming process as very small amount of wool usually added to the solution, e. g. only 1% until it completely dissolved [52,88]. Up to, 11% of wool was dissolved in [Bmim]Cl at 130 °C by Xi et al. during a 10-h long process [52]. Feathers showed higher dissolution rates as compared to wool [93].
A series of thioglycolate ionic liquids were synthesized by Idris et al. to determine their wool solubilization potential [93]. Choline thioglycolate reduced the disulfide bonds which resulted in wool fibers dissolution [94]. According to thermogravimetric analysis (TGA), the regenerated keratin exhibited lower thermal stability as compared to natural wool [88]. However, the findings by Xi et al. showed higher thermal stability of regenerated keratins [52].
The use of ionic liquids for wool dissolution usually required temperature not lower than 90 °C. All physical and chemical changes accelerate at high temperatures along with the unfolding of protein structure which allows their reaction with ionic liquids [95].
Wang and Cao used 1-hydroxyethyl-3-methylimidazoliumbis (trifluoromethanesulfonyl) amide to extract keratin from chicken feathers [95]. They also added NaHSO₃ in different ratios to reduce the disulfide bonds. The extraction process took 4 h to obtain a maximum yield of 21.5%. It was also reported that the ionic liquid can be reused for five cycles. This potential of ionic liquid to be recycled could be a major advantage of this method as compared to other reported methods for keratin dissolution.
4.4.2. The use of ionic liquid as co-solvent
Ionic liquids can be used as a co-solvent in a biphasic system or in aqueous systems [87]. Several investigations have been done to determine the potential of ionic liquids for wool and other polymers dissolution such as cellulose dissolution in co-synthesize techniques (96, 97). Hameed and Guo extracted a blend of wool and cellulose by using [BMIM]Cl at room temperature to obtain blended biopolymer materials [96]. A film was fabricated by dissolving 1g of wool and cellulose in ionic liquid at a ratio of 1:20. The obtained film showed improved mechanical and thermal properties as compared to individual components. Another research was conducted by Tran and Mututuvari in which chitosan, cellulose, and keratin were blended by using [BMIM]Cl to fabricate a film for the release of drug [97]. According to the results keratin incorporation slowed down the process of drug release regardless of the cellulose and chitosan concentration in the film. This factor played a very important role in controlling the drug release amount by incorporating different concentrations of keratin in the film.
According to the published studies, the structure of the ionic liquid extracted keratin showed a weak XRD band of alpha structure [88,98,99]. Similarly, methanol treatment instead of ethanol or water induced the beta-sheet structure regeneration of polypeptide chain similar to the original keratin [88,100,101].
4.5. Steam explosion and supercritical water technique for keratin extraction
This method of the steam explosion was first invented by Manson in 1928 [59]. Many associated factors related to temperature, resistance time, particle size and moisture greatly affect the final results [102,103]. Steam flash explosion (SFE) which is a green hydrolysis process has been used widely for bio-based materials production such as the delignification of wood, bioconversion of barley [104], pulping of lignocellulosic biomasses [105], extraction of sugar from corn starch [106]. However, this steam explosion technique has been mainly used for bio-conversion of the cellulosic materials.
Miyamoto et al. in 1982 used this technique for the first time to extract keratin from wool [107]. The researchers were able to convert about 80% of the wool to pepsin digestible materials by using saturated steam (6 kg/cm2) at 164.2 °C [107]. It was also observed that cysteine content in the final product was 50% less than the original wool. Xu et al. observed decreased crystallinity of the wool by increasing pressure [108]. He exposed wool samples to a pressure range of 0.2–0.8 MPa which resulted in damaging the disulfide bridges. This treatment only damaged the wool surface and was not sufficient to break the disulfide linkages and hydrogen bonds [108].
Tonin et al. treated the wool with saturated steam (220 °C) for 10 min and reported process yield of 62.4% of a solid product, 1.1% of sediment and 18.7% for a water-soluble fraction [51]. About 17.9% of the initial wool mass was lost but surface cuticle and wool fragments can be seen in Scanning electron micrographs. It was again reported that cysteine content was reduced in the final product as strong heating involved in the process destroyed this amino acid. Protein profile by using SDS-PAGE gel also confirmed that the chemical structure of keratin was disintegrated due to the exposure to the high temperature and pressure conditions.
Zhao et al. supported the use of the Steam Flash Explosion (SFE) technique to extract wool keratin instead of conventional steam explosion (CSE) [109]. The high temperature and pressure conditions during CSE resulted in a loss of cysteine and reduced the quality of the final product. SFE technique offers fast processing time, higher digestibility, dissolubility and use of low-temperature range as compared to other conventional steam explosion methods. However, it was observed that the cystine content of the wool extracted from SFE was also low.
4.6. Microwave irradiation for keratin extraction
In this technology, the main role of microwave irradiation is to lower the processing time by heating up the solution. Zoccola et al. used microwave irradiation with power in the range of 150–570 W at a temperature of 180 °C for 7 min and obtained an extraction yield of about 60% [50]. When compared with the conventional steam processing method, the microwave method appeared to be faster.
The major drawback associated with this technique is the significant loss of amino acids such as cysteine from 9.4 mol% to about 0.5mol% in the final keratin sample obtained from wool after about 90 min of treatment [50]. According to Chen et al., the use of microwave heating technique resulted in significant reduction of activation energy required for keratin extraction as compared to the irregular, non-uniform heating found in conventional heating [110]. There is still dearth of information regarding the interaction of electromagnetic radiation with wool matrix due to complex structure of keratin. However, it was suggested that due to ester groups hydrolysis lower energy is required for activation.
4.7. Microbial and enzymatic methods for keratin extraction
These methods used for the microbial and enzymatic degradation of keratin-rich materials such as feathers and wool, for their application in biotechnology and food industries. Enzymes are commonly used on a commercial scale for hydrolysis of keratin as they act as a catalyst and is green, environmentally safe technique. When compared to other commonly used chemical methods this technology consumed lower energy and has less harsh treatment conditions [111].
Keratinases are produced by certain microorganisms and these microbial proteases have the potential to hydrolyze keratin. Keratinases are mainly used in cleaning wool, textile industries, leather industry, to treat obstruction in sewage systems, etc [[112], [113], [114]]. The most commonly investigated bacteria for the degradation of beta-keratin based materials are Gram-positive bacteria [115,116]. Keratinophilic fungi and Prokaryote can degrade the alpha-keratin-based materials. Certain bacteria from Bacillus genus and fungi such as keratinophilic fungi, Actinomycetes can completely disintegrate the keratin [44]. According to several investigation strains of Bacillus licheniformis was able to degrade feather proteins [[117], [118], [119]]. Similarly, species of the genus Chrysosporium and Dermatophytes represented as keratinolytic fungi in the literature [44]. These keratinocytes fungi are environmentally friendly, mesophilic and cost-effective options for keratin degradation (44).
Kornillowicz-Kowalska observed keratin degradation by using a mixture containing 16 strains of different keratinophilic fungi [44]. They reported 50% solubilization of peptide after 21 days of culture. It was also observed that when Chrysosporium was used on the feather-based substrate for 21 days about 75% of nitrogen was converted into ammonium form. Similarly, when the wool-based substrate was observed by using bacteria (Streptomyces fradiae) as a source of degradation again about 75% of nitrogen was converted to ammonia [120]. When feathers were used as substrate about 20% of nitrogen was converted to peptide and amino acids. 10–20% of the lysate protein consist of cystine/cysteine and serine constituted about 20–30% [44].
Some sulfur containing products were also produced during keratin hydrolysis by keratinolytic microorganisms [44]. About 50% of the sulfur content can be converted to sulfite products mainly depending upon the genus of fungi [44]. It was also observed that sulfur compounds were produced differently from the keratin substrate by aerobic or anaerobic strains. For example, higher sulfhydryl compounds were produced by Bacillus licheniformis when cultured in aerobic environment as compared to anaerobic culture [121]. To understand the exact mechanism involved in keratin degradation dermatophytes were used by Kunert et al. [122]. The authors reported that keratin degradation by proteolysis and sulfitolysis resulted in cleaving of disulfide bonds of protein to cysteine and S-sulfocysteine by means of sulfite material released by microorganisms. The reaction involved is similar to sulfitolysis and as follows
| cys – SS - cys (cystine)+ HSO₃ ⇔ cySH (cysteine) + cyS.SO₃ (S-sulocysteine) |
A similar mechanism of keratinolysis was proposed by Lechenne et al. (2007) and Monod et al. (2008) for dermatophyte Trichophyton rubrum, Arthroderma benhamiae and Aspergillus fumigatus [123,124]. The air oxidation of sulfur amino acids resulted in the formation of cysteic acid in the reaction products [125].
5. Keratin biomaterials - current and potential biomedical applications
The extensive research on the overall chemical, physical and biological properties of keratin led to the development of these biomaterials (Table 2). Keratin based biomaterials have an intrinsic ability to self -assembly, biocompatibility, biodegradation and support cellular proliferation [7]. This property of self-assembly has been investigated extensively at both the nanoscale and macro scale levels, and it allows them to polymerize into porous scaffolds [8,36]. Wool and hair derived keratin biomaterials have shown the capability to support cellular attachment as they possess cell-binding motifs, such as glutamic acid-aspartic acid-serine (EDS) and leucine-aspartic acid-valine (LDV) binding residues (36). All these properties favor the cellular attachment, proliferation in a three-dimensional matrix structure. Over the past three decades, many investigations have been conducted to fabricate new keratin-based biomaterials in the form of films, sponges, fibers, gels and scaffolds (Table 2).
Table 2.
Keratin based biomaterials for various biomedical applications.
| COMPOSITION | PROCESSING CONDITIONS | APPLICATIONS | REF | ||
|---|---|---|---|---|---|
| 1. | Keratin dialysate (aq) with alkaline keratin dialysate | Glycerol (1%) was used as a softening agent. Curing of aqueous/alkaline keratin dialysate for 2 h at 100 °C. | Wound healing of corneal epithelial was observed in vitro | [177] | |
| 2. | Photo active keratin derived films | Films were doped with varying concentrations of methylene blue | Photodynamic therapy treatment, wound healing, tissue engineering | [178] | |
| 3. | Keratin film crosslinked by transglutaminase (TG) | 18 h treatment with TGase (30 U/g keratin) at 40 °C | Drug delivery, improve stability in artificial gastric juice environment | [179] | |
| 4. | Photo cross-linkable keratin-polyethylene glycol (PEG) hydrogels via the thiol-norborene “click” reaction | Thiol–norborene click reaction to fabricate keratin-polyethylene glycol (PEG) hydrogels. Eosin Y used as a photoinitiator |
2-D & 3-D cell culture substrates, microfabrication techniques such as photopatterning, wet spinning. | [180] | |
| 5. | Keratin film | Mixing ratios of keratin dialysate and alkaline keratin dialysate were 100, 90/10, 80/20, 70/30 and 50/50. | Reconstruction of ocular surface | [126] | |
| 6. | Keratin film | Glycerol and shindai keratin dried for about 24 h in a 50 °C ventilated oven. | These films continuously released loaded Rhodamine B for 12 h. | [181] | |
| 7. | Keratin, chitosan/gelatine 1:1:2 (w/w) | Gelatine concentration in solution was 5 mg/ml. Keratin & chitosan was 2.5 mg/ml each. Frozen at −40 °C |
soft tissue engineering | [182] | |
| 8. | Keratin -chitosan | CH solutions 2% (w/v), ethylene glycol (1.5 ml). | Wound dressing material | [183] | |
| 9. | PLA/chitosan/keratin composites | A 111: PLA (70%), chitosan (30%). A121: PLA (68%), chitosan (30%), keratin (2%). A131: PLA (66%), chitosan (30%), keratin (4%) |
Facilitates attachment and proliferation of osteoblast | [184] | |
| 10. | Keratin/poly (vinyl alcohol) composite | 10% keratin poly (vinyl alcohol) cross linked with 10% glyoxal | Nano fibres with high optical transmittance | [185] | |
| 11. | Keratin wound dressing | Porcine lethal extremity hemorrhage model | As hemostatic material | [186] | |
| 12. | Keratin gel | Feather-keratin derived hydrogel | The drug release rate was 97% at pH 8.4 for 24 h. | [187] | |
| 13. | Keratin gel | Human hair keratin alkylation | Act as a substrate for cellular attachment and proliferation, delivery of therapeutic agents | [188] | |
| 14. | Hydrogels in injectable forms | - | For repairing cardiac tissue after myocardial infarction. | [189] | |
| 15. | Keratin hydrogel | Glycerol (3%) to formulate 20% (w/v) solution. | Pupal tissue regeneration | [190] | |
| 16. | Keratin hydrogel | - | Fibroblasts culturing | [191] | |
| 17. | Recombinant keratin proteins | Two recombinant trichocyte keratins expressed by using a bacterial expression system. Recombinant keratin nanoparticles prepared by ultrasonic dispersion technique. |
Dermal wound healing | [192] | |
| 18. | Keratin hydrogel | Konjac glucomannan (KGM), human hair proteins (KER), ethanolic extract of Avena sativa (OAT) | Dressing material for diabetic wounds. | [193] | |
| 19. | Keratin based therapeutic dermal patches | Mixing keratin and exopolysaccharide solutions in defined concentrations. Blended solution exposed to −20 °C for 3 days followed by placing in 90% ethanol containing 1% CaCl₂ for 15 min. Incubation of frozen dermal patches at 37 °C. |
Wound healing | [194] | |
| 20. | Keratin/poly (vinyl alcohol) nanofibers | Keratose/PVA mass ratios (1:1, 1:3, 1:5 and 1:7). Electrospinning parameters (voltage, flow rate, tip, and receptor distance) were optimized. Collection of keratose/PVA blended nanofibers on nylon gauze followed by drying at room temperature. |
Tissue engineering. | [195] | |
| 21. | Keratin derived eco-friendly bioplastic film | Bioplastic film fabrication using glycerol (3.5%), microcrystalline cellulose (0.2%) in NaOH at 60 °C for 48 h. | Biopolymer, biomedical and pharmaceutical industries. | [196] | |
5.1. Keratin films application as a bone morphogenic protein (BMP2) carrier and for ocular surface reconstruction
The most commonly used methods for keratin film production are solvent casting technique, compress moulding, thermal pressing, electrospinning and layer by layer (LbL) deposition [[126], [127], [128], [129], [130]]. Over the past few decades, several investigations have been conducted to determine the biological and physicochemical properties of keratin films [7,131,132]. The initial investigations related to the keratin extracted from wool were conducted by Yamauchi at al. and reported the biodegradation, physical and chemical properties of the solvent-cast keratin films [47]. He also investigated the cellular attachment and proliferation of these keratin films and observed that these keratin films served as a more adhesive substrate for cells as compared to collagen [133].
Research conducted by Fujii et al. demonstrated a rapid casting method and proved that hair-based keratin films can incorporate bioactive molecules such as alkaline phosphatase and serve as a suitable substrate for controlled release of these molecules [134]. However, these films had poor mechanical strength. All these early investigations showed the potential applications of keratin films for various medical applications.
5.1.1. Different approaches for the incorporation of various natural and synthetic polymers
Later on, several investigations have been done to improve the physical properties of these films while preserving their excellent biological properties [59,135,136]. Different approaches have been used for the incorporation of various synthetic and natural polymers to pure keratin preparations. Yamauchi's group conducted another study in 2002, they further improved the mechanical strength of their keratin films by incorporation of chitosan [137]. This new chitosan-keratin films not only exhibited improved mechanical properties but also showed antibacterial properties. The incorporation of gelatine into the mixture containing keratin and chitosan was also observed in another study. It was reported that both the oxygen permeability and hydrophilicity of the film were improved by increasing gelatine concentration [138].
The combination of silk fibroin (SF) and keratin film has been investigated extensively to determine the interactions between these two polymers [135,139]. Lee et al. reported a change from random coil to beta-sheet structure for fibroin as there is an interaction with polar amino acids of the keratin [140,141]. Improved antithrombogenic properties were observed for these blended films as compared to pure keratin or SF films [141]. Vasconcelos et al. studied the degradation and physical properties of keratin- SF films and observed unique intermolecular interactions between the two proteins [135].
5.1.2. Application as a bone morphogenic protein (BMP) carrier
In another, in vivo investigation keratose (water-soluble fraction of the keratin) was studied as a BMP2 carrier for bony regeneration of rat femoral bone defect. There was enhanced regeneration of bone along with reduced adipose tissues [142].
5.1.3. Keratin Film for ocular surface reconstruction
The application of keratin film for ocular surface reconstruction was proposed by Reichl et al. [143]. As compared to the human amniotic membrane (AM), keratin film was more transparent and cytocompatibility. The same group of researchers also compared epithelial wound healing potential of keratin film and compared the results to that of polystyrene plates and AM. The keratin film (KF) also supported the migration and proliferation of epithelial HCE-T cell line. In another, in vivo investigation the biocompatibility of keratin-based film for ocular regeneration was observed and it was found that these keratin films exhibited good corneal biocompatibility with minor host reaction and there was preservation of corneal transparency [126].
Similarly, to avoid the use of human skin for various in vitro researches the combination of ceramide and keratin was used for the development of human epidermis [144]. These keratin films can be used as a substrate for drug release in a controlled manner. The incorporation of alkaline phosphatase was observed in keratin film and it was reported that during 14 days of controlled drug release period it remained biologically active [134]. Vasconcelos et al. used the combination of wool derived keratin and silk fibroin for the fabrication of matrix to investigate the release of elastase inhibiting agents to the wound site [145]. It was observed that the concentration of keratin in matrix play an important role in the rate of degradation and the release of elastase inhibiting agent.
5.2. Keratin based scaffolds and their application in urinary tract tissue engineering
Keratin based scaffolds have emerged as an interesting material for various biomedical applications mainly due to their ability to polymerize and self-assembly into 3-dimensional porous structure [146]. Tachibana et al. first fabricated the keratin scaffolds derived from wool for long term cell cultivation in 2001 [147]. These scaffolds were created by freeze-drying in a controlled manner which resulted in a homogenous porous, heat-stable structure. The technique to hybridized keratin sponges with calcium phosphate was demonstrated by Tachibana et al. in 2005 [148]. These calcium and phosphate ions were either bound chemically within the sponges or by incorporating the hydroxyapatite particles within the keratin carboxy-sponges structure. Osteoblastic cultivation was supported by both the hybridized sponges positively. In order to allow the cellular infiltration and adequate delivery of nutrient, the pore diameter of the porous scaffold needed to be regulated. However, it is difficult to control the pore size and porosity of scaffolds using lyophilisation technique. Katoh at al. used compression moulding technique to regulate pore size of keratin scaffolds [57].
In order to determine the relationship between physical strength and mass, Peplow et al. investigated the in vivo degradation of rectangular bars of reconstituted keratin [149]. These keratin bars were then implanted subcutaneously into rats. Both elastic modulus and dry weight were observed over a time period of 18 weeks. It was reported there was about 22% weight degradation at 18 weeks and an abrupt reduction of elastic modulus between 3 and 6 weeks. Thus, it was concluded that keratin in this form can be used as an implant material in non-load bearing areas as there was abrupt declination of mechanical integrity and gradual rate of degradation (149).
Pure keratin composites have poor mechanical properties and brittle structure. In order to improve mechanical properties, some researchers incorporate some synthetic polymers such as PVA, PCL, PLLA, and PEO as shown in Table 2 [[150], [151], [152], [153]]. As compared to other techniques, electrospinning is an efficient technique to generate fibers with high porosity and surface area. At the same time, the structure produced has destabilized the beta-sheet structure [153]. Aluigi et al. electrospun the mixture containing keratin/PEO with a ratio of 50:50 [154]. PCL/keratin fiber was electrospun by Edwards et al. at different ratios [155]. Zhao et al. improved the biocompatibility and mechanical properties of PCL/keratin by the incorporation of hydroxyapatite particles (151).
5.2.1. In vivo evaluation of oxygenated keratin/Silk fibroin Scaffold for their application in urinary tract tissue engineering
In recent years, tissue engineering approaches have shown promising results to restore urinary tract strictures or defects such as malignancy, congenital diseases, trauma and inflammation [156]. Biomaterials played an important in this regard as they served as a three-dimensional structure for seeded cells. Various matrices such as synthetic polyesters, protein, and collagen-based matrices have been investigated in this regard [[157], [158], [159]]. Several limitations regarding the survival of seeded cells, the anti-bacterial property of the implanted biomaterial requires the establishment of an ideal strategy to overcome these problems. In order to improve all these limitations, oxygen-generating biomaterials have been developed to ensure sufficient diffusion of oxygen and to prevent necrosis in cell-seeded implants [160]. It has been observed that these biomaterials not only maintain cell viability under hypoxic conditions but also prevent necrosis [161].
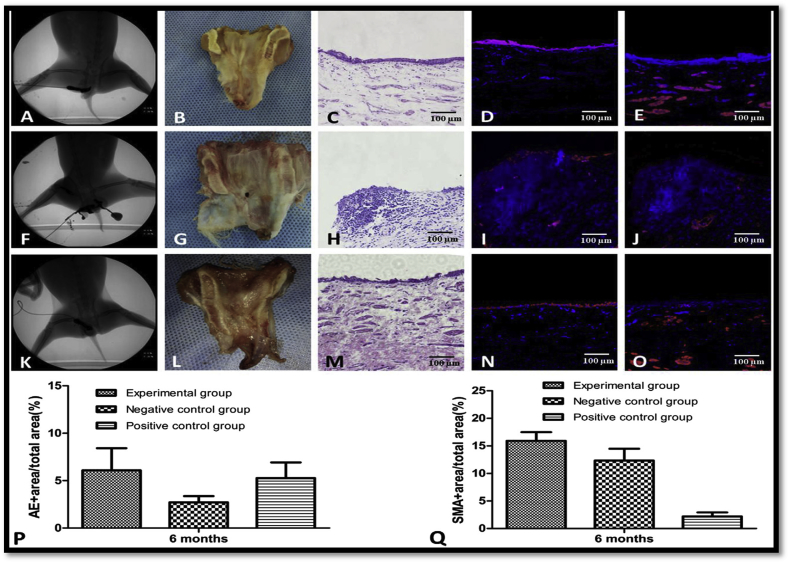
Recently, a novel biomaterial has been fabricated incorporating human-hair derived keratin, gelatin, silk and calcium peroxide (CPO) [161]. This film mainly composed of 2% gelatin, silk/keratin (60:40) and calcium peroxide about 20%. This biomaterial showed in vitro the potential to release oxygen steadily over a period of two weeks. The final film was tested for tensile measurement, Fourier Transform Infrared (FTIR) spectroscopy, scanning electron microscopy and confocal laser scanning microscopy. Other aspects like cellular proliferation, antibacterial activity, cytotoxicity analysis, degradation, and oxygen -release behavior were also assessed. CPO incorporated films showed enhanced repair of dog urethral defects. Muscle bundles and epithelial layer showed an organized pattern in animals treated with CPO incorporated films as compared to non-CPO treated films as shown in Fig. 4.
Fig. 4.
A. The urethrography of Group A (experimental group) showed patent urethra. B. Group A reconstructed urethra showed minor scar in the reconstructed site. C. Intact epithelial layer observed by H & E staining in group A. D, E. confirmation of regenerated epithelial layer in group A on the repaired site by immunohistochemical examination of AE1/AE3 and - smooth actin antibody. F. Group B (negative control) urethrography showed urethral fistula. G. Gross view of fistula in a reconstructed site in Group B. H. inflammatory response observed by H&E staining in group B. I, J. Epithelial layer and smooth muscle cells cannot be viewed in group B by immunohistochemical examination of AE1/AE3 and - smooth actin antibody. K. The urethrography of group C (positive control group) showed patent urethra. L. Group C reconstructed urethra showed minor scar in reconstructed site. M. Intact epithelial layers observed by H & E staining in group C. N, O. Confirmation of regenerated epithelial layer on the repaired site by immunohistochemical examination of group C (161).
5.3. Keratin hydrogels for drug delivery and as a dynamic matrices for wound healing
The use of various natural and synthetic materials in the form of film, gel, scaffold or nano-particles is very common to carry therapeutic agents to the targeted site to prevent infection and enhance the healing process [[162], [163], [164], [165], [166]]. Various natural and synthetic polymers such as polyphosphazene, polyanhydrides, polyorthoesters, alginates, chitosan, collagen, and keratin have been investigated for drug delivery (Table 2). Protein derived biomaterials are gaining interest in drug delivery due to their high abundance, low toxicity, emulsifying and water holding ability [166].
For the preparation of keratin hydrogel, keratose (water-soluble fraction of keratin) has been used commonly [167,168]. The incorporation of Ciprofloxacin in a keratin hydrogel resulted in 60% of the drug release during 10 days [169]. In order to prevent postoperative adhesion after abdominal or peritoneal surgeries, keratin hydrogel played an important role [170]. Peyton et al. used keratose hydrogel as the physical barrier along with Halofuginone (HF) as drug and observed that HF-keratin hydrogel reduced the density of adhesion in rodent cecal abrasion model (170). It was also reported that this hydrogel has the potential to function as a multi-purpose gel and to deliver drug to the targeted site. A high rate of degradation and stability of drug in the keratin matrix are certain limitations associated with the use of these hydrogels for its versatile drug release application.
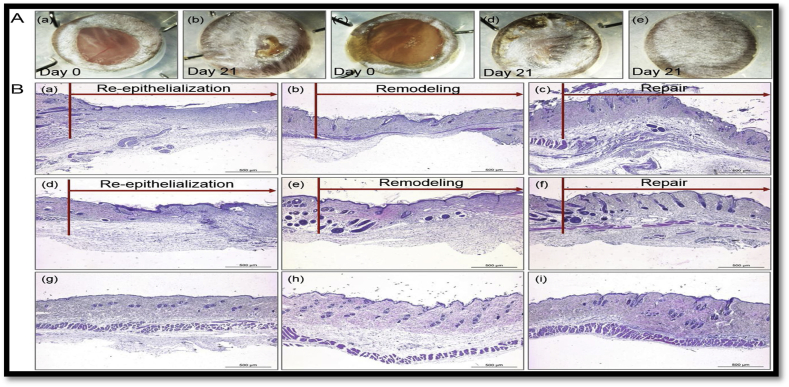
Wound healing involves various matrix and cellular components to restore the integrity of injured or lost tissue by a complex biological process [171]. Human hair derived keratin hydrogel has been investigated extensively as a matrix for enhanced wound healing due to its excellent biocompatibility and abundant availability [36,172,173]. Recently human hair derived keratin-based in situ cross-linkable hydrogels has been designed to serve as a matrix for better wound healing [172]. It was observed that these hydrogels enhanced the process of re-epithelization in the full-thickness animal model. Various cellular interactions between human skin keratinocyte and hair-derived keratin were observed such as proliferation, change in the morphology, gene and molecular expression profiles. Additionally, hydrogel was assessed for their wound healing potential by using an animal model with full-thickness skin defect as shown in Fig. 5.
Fig. 5.
A: Stereo microscopic images of negative control (a & b) of non-treated mouse sites, (c & d) keratin-hydrogel treated mouse, (e) post wounding natural mouse skin at day 0 and 21. B: Histological images of eosin (H&E) and hematoxylin staining of non-treated (a–c), keratin -hydrogels treated (d–f), and natural skin (g–i) [172].
5.3.1. Role of keratin hydrogel for nerve regeneration
A major challenge clinically is to treat peripheral nerve defects and to restore the autonomic and sensory functions. Once the degeneration of nerve fiber starts the condition becomes more serious. There are only few treatment options for such conditions such as tubular conduits, end to end repair and autologous grafts. While using conduit as a treatment option, the defect mostly filled with polysaccharide or protein biomaterial such as chitosan, fibrin and hyaluronic acid [174,175]. According to Sierpinski and Apel keratin hydrogel has the potential to enhance the activity and proliferation of nerve cells through a chemotactic mechanism [174,176]. Later, axon regeneration was confirmed by in vivo investigation. In order to determine the exact time course of nerve regeneration the same group conducted a histological study by using the keratin hydrogel filled conduit [174,176].
6. Concluding remarks
Various keratin-based biomaterials have been fabricated and investigated extensively over the past few decades for their application in the field of biomedical sciences in the form of hydrogels, films, fibers, hydrogels, sponges, scaffolds and wound patches. Keratin biomaterials demonstrated excellent biocompatibility, unique chemical structure, and biodegradability. Additionally, cheap raw materials such as wool and hair are the rich sources of this biopolymer. Despite all these properties only a few of these biomaterials progressed to clinical trials and hold a small share in the commercial market as compared to other biomaterials. There are certain aspects related to these biomaterials that need to be addressed in future to make keratin as the mainstream material for various biomedical application such as.
-
I.
Further detail studies to better understand the cellular interactions with keratin and its role to support cell attachment and proliferation
-
II.
There is still a dearth of information regarding the improvement of mechanical-physical properties of keratin-based sponges, films, composites, and molecular level investigations to determine the keratin interaction with other natural or synthetic polymers.
-
III.
Further investigations are needed to find a green, environmentally friendly, easy, cost-effective and less time-consuming methods to extract keratin such as ionic liquid-based green solvents or supercritical fluids technology for efficient keratin extraction from hair and wool
Declaration of competing interest
There is no conflict of interest. We would like to submit a review article entitled Keratin - Based Materials for Biomedical Applications in your esteemed journal solely with mutual understanding of all co-authors.
Acknowledgment
This work has been supported by Department of Anatomy, University of Otago, Otago, 9016, New Zealand.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Nawshad Muhammad, Email: nawshadmuhammad@cuilahore.edu.pk.
George Dias, Email: george.dias@otago.ac.nz.
References
- 1.Chang S., Wasser S. Current and future research trends in agricultural and biomedical applications of medicinal mushrooms and mushroom products. Int. J. Med. Mushrooms. 2018 doi: 10.1615/IntJMedMushrooms.2018029378. [DOI] [PubMed] [Google Scholar]
- 2.Feroz S., Moeen F., Haq S.N. Protective effect of chicken egg shell powder solution (CESP) on artificially induced dental erosion: an in vitro atomic force microscope study. Int. J. Dental Sci. Res. 2017;5(3):49–55. [Google Scholar]
- 3.Felician F.F., Xia C., Qi W., Xu H. Collagen from marine biological sources and medical applications. Chem. Biodivers. 2018;15(5) doi: 10.1002/cbdv.201700557. [DOI] [PubMed] [Google Scholar]
- 4.Vaz J.M., Pezzoli D., Chevallier P., Campelo C.S., Candiani G., Mantovani D. Antibacterial coatings based on chitosan for pharmaceutical and biomedical applications. Curr. Pharmaceut. Des. 2018;24(8):866–885. doi: 10.2174/1381612824666180219143900. [DOI] [PubMed] [Google Scholar]
- 5.Aigner T.B., DeSimone E., Scheibel T. Biomedical applications of recombinant silk‐based materials. Adv. Mater. 2018;30(19):1704636. doi: 10.1002/adma.201704636. [DOI] [PubMed] [Google Scholar]
- 6.Du H., Liu W., Zhang M., Si C., Zhang X., Li B. Cellulose nanocrystals and cellulose nanofibrils based hydrogels for biomedical applications. Carbohydrate Polym. 2019 doi: 10.1016/j.carbpol.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 7.McLellan J., Thornhill S.G., Shelton S., Kumar M. Springer; 2019. Keratin-based Biofilms, Hydrogels, and Biofibers. Keratin as a Protein Biopolymer; pp. 187–200. [Google Scholar]
- 8.Costa F., Silva R., Boccaccini A. Elsevier; 2018. Fibrous Protein-Based Biomaterials (Silk, Keratin, Elastin, and Resilin Proteins) for Tissue Regeneration and Repair. Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; pp. 175–204. [Google Scholar]
- 9.Zhang Y., Yang R., Zhao W. Improving digestibility of feather meal by steam flash explosion. J. Agric. Food Chem. 2014;62(13):2745–2751. doi: 10.1021/jf405498k. [DOI] [PubMed] [Google Scholar]
- 10.Tubiello F.N., Salvatore M., Rossi S., Ferrara A., Fitton N., Smith P. The FAOSTAT database of greenhouse gas emissions from agriculture. Environ. Res. Lett. 2013;8(1) [Google Scholar]
- 11.Jin E., Reddy N., Zhu Z., Yang Y. Graft polymerization of native chicken feathers for thermoplastic applications. J. Agric. Food Chem. 2011;59(5):1729–1738. doi: 10.1021/jf1039519. [DOI] [PubMed] [Google Scholar]
- 12.Dou Y., Huang X., Zhang B., He M., Yin G., Cui Y. Preparation and characterization of a dialdehyde starch crosslinked feather keratin film for food packaging application. RSC Adv. 2015;5(34):27168–27174. [Google Scholar]
- 13.Zhen L., Mu B.C.G. Jilin; China: 2005. The Time Literature & Art Press: Changchun. [Google Scholar]
- 14.Rouse J.G., Van Dyke M.E. A review of keratin-based biomaterials for biomedical applications. Materials. 2010;3(2):999–1014. [Google Scholar]
- 15.Hofmeier J. 1905. Horn-lime Plastic Masses from Keratin Substances; p. 18. German Pat DE184915. [Google Scholar]
- 16.Dale H. Keratin and other coatings for pills. Pharmacol. J. 1932;129:494–495. [Google Scholar]
- 17.Beyer C. The keratin or horny substance of the hair. Ger pat. 1907:22643. [Google Scholar]
- 18.Rivett D., Ward S., Belkin L., Ramshaw J., Wilshire J. The Lennox Legacy; 1996. Keratin and Wool Research. [Google Scholar]
- 19.Rogers G. Electron microscope studies of hair and wool. Ann. N. Y. Acad. Sci. 1959;83(3):378–399. doi: 10.1111/j.1749-6632.1960.tb40914.x. [DOI] [PubMed] [Google Scholar]
- 20.Baumann H., Parry D., Creamer L., editors. Proc Znt Conf. 1979. Fibrous proteins: scientific, industrial and medical aspects. [Google Scholar]
- 21.Earland C., Knight C. Studies on the structure of keratin II. The amino acid content of fractions isolated from oxidized wool. Biochim. Biophys. Acta. 1956;22(3):405–411. doi: 10.1016/0006-3002(56)90048-8. [DOI] [PubMed] [Google Scholar]
- 22.Anker C.A. Google Patents; 1972. Method of Preparing Keratin-Containing Films and Coatings. [Google Scholar]
- 23.Kawano Y., Okamoto S. Film and gel of keratins. Kagaku to Seibutsu. 1975;13(5):291–292. [Google Scholar]
- 24.Okamoto S. On the formation of films from some proteins. Nippon. Shokuhin Kogyo Gakkaishi. 1977;24(1):40–50. [Google Scholar]
- 25.Noishiki Y., Ito H., Miyamoto T., Inagaki H. Application of denatured wool keratin derivatives to an antithrombogenic biomaterial-vascular graft coated with a heparinized keratin derivative. Kobunshi Ronbunshu. 1982;39(4):221–227. [Google Scholar]
- 26.Miyamoto T., Takahashi Si, Ito H., Inagaki H., Noishiki Y. Tissue biocompatibility of cellulose and its derivatives. J. Biomed. Mater. Res. 1989;23(1):125–133. doi: 10.1002/jbm.820230110. [DOI] [PubMed] [Google Scholar]
- 27.Fraser R., MacRae T., Rogers G.E. 1972. Keratins: Their Composition, Structure and Biosynthesis. [Google Scholar]
- 28.Schweizer J., Bowden P.E., Coulombe P.A., Langbein L., Lane E.B., Magin T.M. New consensus nomenclature for mammalian keratins. J. Cell Biol. 2006;174(2):169–174. doi: 10.1083/jcb.200603161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Potter N.A., Van Dyke M. Effects of differing purification methods on properties of keratose biomaterials. ACS Biomater. Sci. Eng. 2018;4(4):1316–1323. doi: 10.1021/acsbiomaterials.7b00964. [DOI] [PubMed] [Google Scholar]
- 30.Li Y., Wang Y., Ye J., Yuan J., Xiao Y. Fabrication of poly (ε-caprolactone)/keratin nanofibrous mats as a potential scaffold for vascular tissue engineering. Mater. Sci. Eng. C. 2016;68:177–183. doi: 10.1016/j.msec.2016.05.117. [DOI] [PubMed] [Google Scholar]
- 31.Navarro J., Swayambunathan J., Lerman M., Santoro M., Fisher J.P. Development of keratin-based membranes for potential use in skin repair. Acta Biomater. 2019;83:177–188. doi: 10.1016/j.actbio.2018.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spearman R. On the nature of the horny scales of the pangolin. Zool. J. Linn. Soc. 1967;46(310):267–273. [Google Scholar]
- 33.Jones L., Simon M., Watts N., Booy F., Steven A., Parry D. Intermediate filament structure: hard α-keratin. Biophys. Chem. 1997;68(1–3):83–93. doi: 10.1016/s0301-4622(97)00013-6. [DOI] [PubMed] [Google Scholar]
- 34.McKittrick J., Chen P.-Y., Bodde S., Yang W., Novitskaya E., Meyers M. The structure, functions, and mechanical properties of keratin. JOM (J. Occup. Med.) 2012;64(4):449–468. [Google Scholar]
- 35.Kumawat T.K., Sharma A., Sharma V., Chandra S. Keratin; 2018. Keratin Waste: the Biodegradable Polymers. IntechOpen. [Google Scholar]
- 36.Patrucco A., Visai L., Fassina L., Magenes G., Tonin C. Elsevier; 2019. Keratin-based Matrices from Wool Fibers and Human Hair. Materials for Biomedical Engineering; pp. 375–403. [Google Scholar]
- 37.Rajabinejad H., Bucişcanu I.-I., Maier S.S. Current approaches for raw wool waste management and unconventional valorization: a review. Environ. Eng. Manag. J. (EEMJ). 2019;18(7) [Google Scholar]
- 38.Squire J., Vibert P.J. Academic Press; 1987. Fibrous Protein Structure. [Google Scholar]
- 39.Fraser R.B., Parry D.A. The structural basis of the filament-matrix texture in the avian/reptilian group of hard β-keratins. J. Struct. Biol. 2011;173(2):391–405. doi: 10.1016/j.jsb.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 40.Ayutthaya S.I.N., Tanpichai S., Wootthikanokkhan J. Keratin extracted from chicken feather waste: extraction, preparation, and structural characterization of the keratin and keratin/biopolymer films and electrospuns. J. Polym. Environ. 2015;23(4):506–516. [Google Scholar]
- 41.Aluigi A., Tonetti C., Rombaldoni F., Puglia D., Fortunati E., Armentano I. Keratins extracted from Merino wool and Brown Alpaca fibres as potential fillers for PLLA-based biocomposites. J. Mater. Sci. 2014;49(18):6257–6269. [Google Scholar]
- 42.Park M., Kim B.-S., Shin H.K., Park S.-J., Kim H.-Y. Preparation and characterization of keratin-based biocomposite hydrogels prepared by electron beam irradiation. Mater. Sci. Eng. C. 2013;33(8):5051–5057. doi: 10.1016/j.msec.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 43.Poole A.J., Lyons R.E., Church J.S. Dissolving feather keratin using sodium sulfide for bio-polymer applications. J. Polym. Environ. 2011;19(4):995–1004. [Google Scholar]
- 44.Korniłłowicz-Kowalska T., Bohacz J. Biodegradation of keratin waste: theory and practical aspects. Waste Manag. 2011;31(8):1689–1701. doi: 10.1016/j.wasman.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 45.Tonin C., Aluigi A., Varesano A., Vineis C. Keratin-based nanofibres. nanofibers InTech. 2010:139–158. [Google Scholar]
- 46.Wang B., Yang W., McKittrick J., Meyers M.A. Keratin: structure, mechanical properties, occurrence in biological organisms, and efforts at bioinspiration. Prog. Mater. Sci. 2016;76:229–318. [Google Scholar]
- 47.Yamauchi K., Yamauchi A., Kusunoki T., Kohda A., Konishi Y. Preparation of stable aqueous solution of keratins, and physiochemical and biodegradational properties of films. J. Biomed. Mater. Res.: Off. J. Soc. Biomater. Jpn. Soc. Biomater. 1996;31(4):439–444. doi: 10.1002/(SICI)1097-4636(199608)31:4<439::AID-JBM1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 48.Buchanan J.H. A cystine-rich protein fraction from oxidized α-keratin. Biochem. J. 1977;167(2):489–491. doi: 10.1042/bj1670489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsuda Y., Nomura Y. Properties of alkaline‐hydrolyzed waterfowl feather keratin. Anim. Sci. J. 2014;85(2):180–185. doi: 10.1111/asj.12093. [DOI] [PubMed] [Google Scholar]
- 50.Zoccola M., Aluigi A., Patrucco A., Vineis C., Forlini F., Locatelli P. Microwave-assisted chemical-free hydrolysis of wool keratin. Textil. Res. J. 2012;82(19):2006–2018. [Google Scholar]
- 51.Tonin C., Zoccola M., Aluigi A., Varesano A., Montarsolo A., Vineis C. Study on the conversion of wool keratin by steam explosion. Biomacromolecules. 2006;7(12):3499–3504. doi: 10.1021/bm060597w. [DOI] [PubMed] [Google Scholar]
- 52.Xie H., Li S., Zhang S. Ionic liquids as novel solvents for the dissolution and blending of wool keratin fibers. Green Chem. 2005;7(8):606–608. [Google Scholar]
- 53.Goddard D.R., Michaelis L. Derivatives of keratin. J. Biol. Chem. 1935;112(1):361–371. [Google Scholar]
- 54.Patterson W.I., Geiger W., Mizell L., Harris M. The rôle of cystine in the structure of the fibrous protein, wool. Text. Res. 1941;11(9):379–393. [Google Scholar]
- 55.Savige W. The dispersion of wool protein by thiols in acid solution. Textil. Res. J. 1960;30(1):1–10. [Google Scholar]
- 56.Thompson E., O'donnell I. Studies on reduced wool I. The extent of reduotion of wool with inoreasing conoentrations of thiol, and the extraotion of proteins from reduoed and alkylated wool. Aust. J. Biol. Sci. 1962;15(4):757–768. [Google Scholar]
- 57.Katoh K., Tanabe T., Yamauchi K. Novel approach to fabricate keratin sponge scaffolds with controlled pore size and porosity. Biomaterials. 2004;25(18):4255–4262. doi: 10.1016/j.biomaterials.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 58.Xu H., Ma Z., Yang Y. Dissolution and regeneration of wool via controlled disintegration and disentanglement of highly crosslinked keratin. J. Mater. Sci. 2014;49(21):7513–7521. [Google Scholar]
- 59.Shavandi A., Silva T.H., Bekhit A.A., Bekhit A.E.-D.A. Keratin: dissolution, extraction and biomedical application. Biomater. Sci. 2017;5(9):1699–1735. doi: 10.1039/c7bm00411g. [DOI] [PubMed] [Google Scholar]
- 60.Patinvoh R.J., Feuk-Lagerstedt E., Lundin M., Horváth I.S., Taherzadeh M.J. Biological pretreatment of chicken feather and biogas production from total broth. Appl. Biochem. Biotechnol. 2016;180(7):1401–1415. doi: 10.1007/s12010-016-2175-8. [DOI] [PubMed] [Google Scholar]
- 61.Maclaren J.A. Maximum extraction of wool proteins by thiol-urea solutions. Textil. Res. J. 1987;57(2):87–92. [Google Scholar]
- 62.Schrooyen P.M., Dijkstra P.J., Oberthür R.C., Bantjes A., Feijen J. Stabilization of solutions of feather keratins by sodium dodecyl sulfate. J. Colloid Interface Sci. 2001;240(1):30–39. doi: 10.1006/jcis.2001.7673. [DOI] [PubMed] [Google Scholar]
- 63.Nakamura A., Arimoto M., Takeuchi K., Fujii T. A rapid extraction procedure of human hair proteins and identification of phosphorylated species. Biol. Pharm. Bull. 2002;25(5):569–572. doi: 10.1248/bpb.25.569. [DOI] [PubMed] [Google Scholar]
- 64.Barrows T.H. Google Patents; 2015. Biomaterials Made from Human Hair. [Google Scholar]
- 65.Blackburn S., Lee G. The reaction of wool keratin with alkali. Biochim. Biophys. Acta. 1956;19:505–512. doi: 10.1016/0006-3002(56)90474-7. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Y., Zhao W., Yang R. Steam flash explosion assisted dissolution of keratin from feathers. ACS Sustain. Chem. Eng. 2015;3(9):2036–2042. [Google Scholar]
- 67.Evans R.L. Google Patents; 1948. Regenerated Keratin. [Google Scholar]
- 68.Smith A.L., Harris M. Oxidation of wool: photochemical oxidation. J. Res. Natl. Bur. Stand. (U.S.) 1936;17:97–100. [Google Scholar]
- 69.Arai K.M., Takahashi R., Yokote Y., Akahane K. Amino‐acid sequence of feather keratin from fowl. Eur. J. Biochem. 1983;132(3):501–507. doi: 10.1111/j.1432-1033.1983.tb07389.x. [DOI] [PubMed] [Google Scholar]
- 70.Thor C.J., Gortner R.A. Sulfur IN proteins V. The effect OF alkalies UPON cystine, with special reference to the action OF sodium hydroxide. J. Biol. Chem. 1933;99(2):383–403. [Google Scholar]
- 71.Nagai Y., Nishikawa T. Alkali solubilization of chicken feather keratin. Agric. Biol. Chem. 1970;34(1):16–22. [Google Scholar]
- 72.Jiang-Tao X., Ping Z., Lin Z., Shu-ying S., Zhao-hong D., Yi Z. Study of keratin extraction from human hair. Wool Text. J. 2015;43(5) [Google Scholar]
- 73.Earland C., Knight C. Studies on the structure of keratin: I. The analysis of fractions isolated from wool oxidized with peracetic acid. Biochim. Biophys. Acta. 1955;17:457–461. doi: 10.1016/0006-3002(55)90406-6. [DOI] [PubMed] [Google Scholar]
- 74.Weston G. The infra-red spectrum of peracetic acid-treated wool. Biochim. Biophys. Acta. 1955;17:462–464. doi: 10.1016/0006-3002(55)90407-8. [DOI] [PubMed] [Google Scholar]
- 75.Strasheim A., Buijs K. An infra-red study of the oxidation of the disulphide bond in wool. Biochim. Biophys. Acta. 1961;47(3):538–541. [Google Scholar]
- 76.Robbins C. Infrared analysis of oxidized keratins. Textil. Res. J. 1967;37(9):811–813. [Google Scholar]
- 77.Corfield M., Robson A., Skinner B. The amino acid compositions of three fractions from oxidized wool. Biochem. J. 1958;68(2):348. doi: 10.1042/bj0680348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Robbins C.R. Springer; 2012. Chemical Composition of Different Hair Types. Chemical and Physical Behavior of Human Hair; pp. 105–176. [Google Scholar]
- 79.de Guzman R.C., Merrill M.R., Richter J.R., Hamzi R.I., Greengauz-Roberts O.K., Van Dyke M.E. Mechanical and biological properties of keratose biomaterials. Biomaterials. 2011;32(32):8205–8217. doi: 10.1016/j.biomaterials.2011.07.054. [DOI] [PubMed] [Google Scholar]
- 80.Buchanan J.H. A cystine-rich protein fraction from oxidized alpha-keratin. Biochem. J. 1977;167(2):489. doi: 10.1042/bj1670489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Toennies G., Homiller R.P. The oxidation of amino acids by hydrogen peroxide in formic acid. J. Am. Chem. Soc. 1942;64(12):3054–3056. [Google Scholar]
- 82.Simmonds D. The amino acid composition of keratins II. The amino acid composition of a keratin derivative extracted from wool with alkaline thioglycollate solution. Aust. J. Biol. Sci. 1955;8(1):114–121. [Google Scholar]
- 83.Hill R.L., Kimmel J., Smith E.L. The structure of proteins. Annu. Rev. Biochem. 1959;28(1):97–144. doi: 10.1146/annurev.bi.28.070159.000525. [DOI] [PubMed] [Google Scholar]
- 84.Wang H., Gurau G., Rogers R.D. Springer; 2014. Dissolution of Biomass Using Ionic Liquids. Structures and Interactions of Ionic Liquids; pp. 79–105. [Google Scholar]
- 85.Yoo C.G., Pu Y., Ragauskas A.J. Ionic liquids: promising green solvents for lignocellulosic biomass utilization. Curr. Opinion Green Sustain. Chem. 2017;5:5–11. [Google Scholar]
- 86.Elgharbawy A.A., Riyadi F.A., Alam M.Z., Moniruzzaman M. Ionic liquids as a potential solvent for lipase-catalysed reactions: a review. J. Mol. Liq. 2018;251:150–166. [Google Scholar]
- 87.Fernández J.F., Waterkamp D., Thöming J. Recovery of ionic liquids from wastewater: aggregation control for intensified membrane filtration. Desalination. 2008;224(1–3):52–56. [Google Scholar]
- 88.Li R., Wang D. Preparation of regenerated wool keratin films from wool keratin–ionic liquid solutions. J. Appl. Polym. Sci. 2013;127(4):2648–2653. [Google Scholar]
- 89.Ji Y., Chen J., Lv J., Li Z., Xing L., Ding S. Extraction of keratin with ionic liquids from poultry feather. Separ. Purif. Technol. 2014;132:577–583. [Google Scholar]
- 90.Ghosh A., Clerens S., Deb-Choudhury S., Dyer J.M. Thermal effects of ionic liquid dissolution on the structures and properties of regenerated wool keratin. Polym. Degrad. Stabil. 2014;108:108–115. [Google Scholar]
- 91.Zhang H., Wu J., Zhang J., He J. 1-Allyl-3-methylimidazolium chloride room temperature ionic liquid: a new and powerful nonderivatizing solvent for cellulose. Macromolecules. 2005;38(20):8272–8277. [Google Scholar]
- 92.Feng L., Chen Z-l. Research progress on dissolution and functional modification of cellulose in ionic liquids. J. Mol. Liq. 2008;142(1–3):1–5. [Google Scholar]
- 93.Idris A., Vijayaraghavan R., Rana U.A., Fredericks D., Patti A.F., Macfarlane D.R. Dissolution of feather keratin in ionic liquids. Green Chem. 2013;15(2):525–534. [Google Scholar]
- 94.Gillespie J., Lennox F. Preparation of an electrophoretically homogenous keratin derivative from wool. Biochim. Biophys. Acta. 1953;12(3):481–482. doi: 10.1016/0006-3002(53)90169-3. [DOI] [PubMed] [Google Scholar]
- 95.Wang Y.-X., Cao X.-J. Extracting keratin from chicken feathers by using a hydrophobic ionic liquid. Process Biochem. 2012;47(5):896–899. [Google Scholar]
- 96.Hameed N., Guo Q. Blend films of natural wool and cellulose prepared from an ionic liquid. Cellulose. 2010;17(4):803–813. [Google Scholar]
- 97.Tran C.D., Mututuvari T.M. Cellulose, chitosan, and keratin composite materials. Controlled drug release. Langmuir. 2015;31(4):1516–1526. doi: 10.1021/la5034367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sun P., Liu Z.-T., Liu Z.-W. Particles from bird feather: a novel application of an ionic liquid and waste resource. J. Hazard Mater. 2009;170(2–3):786–790. doi: 10.1016/j.jhazmat.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 99.Idris A., Vijayaraghavan R., Rana U.A., Patti A.F., Macfarlane D.R. Dissolution and regeneration of wool keratin in ionic liquids. Green Chem. 2014;16(5):2857–2864. [Google Scholar]
- 100.Phillips D.M., Drummy L.F., Conrady D.G., Fox D.M., Naik R.R., Stone M.O. Dissolution and regeneration of Bombyx mori silk fibroin using ionic liquids. J. Am. Chem. Soc. 2004;126(44):14350–14351. doi: 10.1021/ja046079f. [DOI] [PubMed] [Google Scholar]
- 101.Jin H.-J., Fridrikh S.V., Rutledge G.C., Kaplan D.L. Electrospinning Bombyx mori silk with poly (ethylene oxide) Biomacromolecules. 2002;3(6):1233–1239. doi: 10.1021/bm025581u. [DOI] [PubMed] [Google Scholar]
- 102.Sanchez O.J., Cardona C.A. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour. Technol. 2008;99(13):5270–5295. doi: 10.1016/j.biortech.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 103.Sun X., Xu F., Sun R., Geng Z., Fowler P., Baird M. Characteristics of degraded hemicellulosic polymers obtained from steam exploded wheat straw. Carbohydr. Polym. 2005;60(1):15–26. doi: 10.1016/j.carres.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 104.Zhang L., Wang T., Jiao S., Hao C., Mao Z., editors. Effect of Steam-Explosion on Biodegradation of Lignin in Wheat Straw. American Society of Agricultural and Biological Engineers; 2007. (ASAE Annual Meeting). 2007. [Google Scholar]
- 105.Young R.A., Akhtar M. John Wiley & Sons; 1997. Environmentally Friendly Technologies for the Pulp and Paper Industry. [Google Scholar]
- 106.McKendry P. Energy production from biomass (part 2): conversion technologies. Bioresour. Technol. 2002;83(1):47–54. doi: 10.1016/s0960-8524(01)00119-5. [DOI] [PubMed] [Google Scholar]
- 107.Miyamoto T., Amiya T., Inagaki H. Preparation of wool powder by explosive-puffing treatment. Kobunshi Ronbunshu. 1982;39(11):679–685. [Google Scholar]
- 108.Xu W., Ke G., Wu J., Wang X. Modification of wool fiber using steam explosion. Eur. Polym. J. 2006;42(9):2168–2173. [Google Scholar]
- 109.Zhao W., Yang R., Zhang Y., Wu L. Sustainable and practical utilization of feather keratin by an innovative physicochemical pretreatment: high density steam flash-explosion. Green Chem. 2012;14(12):3352–3360. [Google Scholar]
- 110.Chen J., Ding S., Ji Y., Ding J., Yang X., Zou M. Microwave-enhanced hydrolysis of poultry feather to produce amino acid. Chem. Eng. Process: Process Intensif. 2015;87:104–109. [Google Scholar]
- 111.Brandelli A. Bacterial keratinases: useful enzymes for bioprocessing agroindustrial wastes and beyond. Food Bioprocess Technol. 2008;1(2):105–116. [Google Scholar]
- 112.Saleem M., Rehman A., Yasmin R., Munir B. Biochemical analysis and investigation on the prospective applications of alkaline protease from a Bacillus cereus strain. Mol. Biol. Rep. 2012;39(6):6399–6408. doi: 10.1007/s11033-011-1033-6. [DOI] [PubMed] [Google Scholar]
- 113.Brandelli A., Sala L., Kalil S.J. Microbial enzymes for bioconversion of poultry waste into added-value products. Food Res. Int. 2015;73:3–12. [Google Scholar]
- 114.Chaturvedi V., Bhange K., Bhatt R., Verma P. Production of kertinases using chicken feathers as substrate by a novel multifunctional strain of Pseudomonas stutzeri and its dehairing application. Biocatal. Agric. Biotechnol. 2014;3(2):167–174. [Google Scholar]
- 115.Tork S.E., Shahein Y.E., El-Hakim A.E., Abdel-Aty A.M., Aly M.M. Production and characterization of thermostable metallo-keratinase from newly isolated Bacillus subtilis NRC 3. Int. J. Biol. Macromol. 2013;55:169–175. doi: 10.1016/j.ijbiomac.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 116.Daroit D.J., Corrêa A.P.F., Brandelli A. Keratinolytic potential of a novel Bacillus sp. P45 isolated from the Amazon basin fish Piaractus mesopotamicus. Int. Biodeterior. Biodegrad. 2009;63(3):358–363. [Google Scholar]
- 117.Burtt E.H., Jr., Ichida J.M. Occurrence of feather-degrading bacilli in the plumage of birds. Auk. 1999;116(2):364–372. [Google Scholar]
- 118.Goldstein G., Flory K.R., Browne B.A., Majid S., Ichida J.M., Burtt E.H., Jr. Bacterial degradation of black and white feathers. Auk. 2004;121(3):656–659. [Google Scholar]
- 119.Gunderson A.R., Forsyth M.H., Swaddle J.P. Evidence that plumage bacteria influence feather coloration and body condition of eastern bluebirds Sialia sialis. J. Avian Biol. 2009;40(4):440–447. [Google Scholar]
- 120.Noval J.J., Nickerson W.J. Decomposition of native keratin by Streptomyces fradiae. J. Bacteriol. 1959;77(3):251. doi: 10.1128/jb.77.3.251-263.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lin X., Lee C.-G., Casale E.S., Shih J.C. Purification and characterization of a keratinase from a feather-degrading Bacillus licheniformis strain. Appl. Environ. Microbiol. 1992;58(10):3271–3275. doi: 10.1128/aem.58.10.3271-3275.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kunert J. Keratin decomposition by dermatophytes: evidence of the sulphitolysis of the protein. Cell. Mol. Life Sci. 1972;28(9):1025–1026. doi: 10.1007/BF01918649. [DOI] [PubMed] [Google Scholar]
- 123.Lechenne B., Reichard U., Zaugg C., Fratti M., Kunert J., Boulat O. Sulphite efflux pumps in Aspergillus fumigatus and dermatophytes. Microbiology. 2007;153(3):905–913. doi: 10.1099/mic.0.2006/003335-0. [DOI] [PubMed] [Google Scholar]
- 124.Monod M. Secreted proteases from dermatophytes. Mycopathologia. 2008;166(5–6):285. doi: 10.1007/s11046-008-9105-4. [DOI] [PubMed] [Google Scholar]
- 125.Kunert J. Keratin decomposition by dermatophytes II. Presence of S‐sulfocysteine and cysteic acid in soluble decomposition products. Z. Allg. Mikrobiol. 1976;16(2):97–105. doi: 10.1002/jobm.3630160203. [DOI] [PubMed] [Google Scholar]
- 126.Borrelli M., Joepen N., Reichl S., Finis D., Schoppe M., Geerling G. Keratin films for ocular surface reconstruction: evaluation of biocompatibility in an in-vivo model. Biomaterials. 2015;42:112–120. doi: 10.1016/j.biomaterials.2014.11.038. [DOI] [PubMed] [Google Scholar]
- 127.Ramirez D.O.S., Carletto R.A., Giachet F.T. Springer; 2019. Keratin Processing. Keratin as a Protein Biopolymer; pp. 77–121. [Google Scholar]
- 128.Barone J.R., Schmidt W.F., Liebner C.F. Thermally processed keratin films. J. Appl. Polym. Sci. 2005;97(4):1644–1651. [Google Scholar]
- 129.Fortunato G.M., Da Ros F., Bisconti S., De Acutis A., Biagini F., Lapomarda A. Electrospun structures made of a hydrolyzed keratin-based biomaterial for development of in vitro tissue models. Front. Bioeng. Biotechnol. 2019;7:174. doi: 10.3389/fbioe.2019.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yang X., Zhang H., Yuan X., Cui S. Wool keratin: a novel building block for layer-by-layer self-assembly. J. Colloid Interface Sci. 2009;336(2):756–760. doi: 10.1016/j.jcis.2009.04.050. [DOI] [PubMed] [Google Scholar]
- 131.Rasheed T., Bilal M., Zhao Y., Raza A., Shah S.Z.H., Iqbal H.M. Physiochemical characteristics and bone/cartilage tissue engineering potentialities of protein-based macromolecules—a review. Int. J. Biol. Macromol. 2019;121:13–22. doi: 10.1016/j.ijbiomac.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 132.Thonpho A., Songeon W., Srihanam P. Effect of cross-linked agents on keratin films property. Int. J. Geomate. 2016;11:2866–2869. [Google Scholar]
- 133.Yamauchi K., Maniwa M., Mori T. Cultivation of fibroblast cells on keratin-coated substrata. J. Biomater. Sci. Polym. Ed. 1998;9(3):259–270. doi: 10.1163/156856298x00640. [DOI] [PubMed] [Google Scholar]
- 134.Fujii T., Ogiwara D., Arimoto M. Convenient procedures for human hair protein films and properties of alkaline phosphatase incorporated in the film. Biol. Pharm. Bull. 2004;27(1):89–93. doi: 10.1248/bpb.27.89. [DOI] [PubMed] [Google Scholar]
- 135.Vasconcelos A., Freddi G., Cavaco-Paulo A. Biodegradable materials based on silk fibroin and keratin. Biomacromolecules. 2008;9(4):1299–1305. doi: 10.1021/bm7012789. [DOI] [PubMed] [Google Scholar]
- 136.Fujii T., Ide Y. Preparation of translucent and flexible human hair protein films and their properties. Biol. Pharm. Bull. 2004;27(9):1433–1436. doi: 10.1248/bpb.27.1433. [DOI] [PubMed] [Google Scholar]
- 137.Tanabe T., Okitsu N., Tachibana A., Yamauchi K. Preparation and characterization of keratin–chitosan composite film. Biomaterials. 2002;23(3):817–825. doi: 10.1016/s0142-9612(01)00187-9. [DOI] [PubMed] [Google Scholar]
- 138.Bazargan-Lari R., Bahrololoom M., Nemati A. Preparation and mechanical evaluations of a novel keratin-chitosan-gelatin composite film. World Appl. Sci. J. 2009;7(6):763–768. [Google Scholar]
- 139.Srisuwan Y., Thonpho A., Srihanam P. Effect of starch on property of silk fibroin/keratin blend films. Int. J. 2016;11(28):2870–2873. [Google Scholar]
- 140.Lee K.Y., Ha W. DSC studies on bound water in silk fibroin/S-carboxymethyl kerateine blend films. Polymer. 1999;40(14):4131–4134. [Google Scholar]
- 141.Lee K.Y., Kong S., Park W., Ha W., Kwon I.C. Effect of surface properties on the antithrombogenicity of silk fibroin/S-carboxymethyl kerateine blend films. J. Biomater. Sci. Polym. Ed. 1998;9(9):905–914. doi: 10.1163/156856298x00235. [DOI] [PubMed] [Google Scholar]
- 142.de Guzman R.C., Saul J.M., Ellenburg M.D., Merrill M.R., Coan H.B., Smith T.L. Bone regeneration with BMP-2 delivered from keratose scaffolds. Biomaterials. 2013;34(6):1644–1656. doi: 10.1016/j.biomaterials.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 143.Reichl S., Borrelli M., Geerling G. Keratin films for ocular surface reconstruction. Biomaterials. 2011;32(13):3375–3386. doi: 10.1016/j.biomaterials.2011.01.052. [DOI] [PubMed] [Google Scholar]
- 144.Selmin F., Cilurzo F., Aluigi A., Franze S., Minghetti P. Regenerated keratin membrane to match the in vitro drug diffusion through human epidermis. Results in Pharma Sci. 2012;2:72–78. doi: 10.1016/j.rinphs.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vasconcelos A., Pêgo A.P., Henriques L., Lamghari M., Cavaco-Paulo A. Protein matrices for improved wound healing: elastase inhibition by a synthetic peptide model. Biomacromolecules. 2010;11(9):2213–2220. doi: 10.1021/bm100537b. [DOI] [PubMed] [Google Scholar]
- 146.Rahmani Del Bakhshayesh A., Annabi N., Khalilov R., Akbarzadeh A., Samiei M., Alizadeh E. Recent advances on biomedical applications of scaffolds in wound healing and dermal tissue engineering. Artif. Cells, Nanomed. Biotechnol. 2018;46(4):691–705. doi: 10.1080/21691401.2017.1349778. [DOI] [PubMed] [Google Scholar]
- 147.Tachibana A., Furuta Y., Takeshima H., Tanabe T., Yamauchi K. Fabrication of wool keratin sponge scaffolds for long-term cell cultivation. J. Biotechnol. 2002;93(2):165–170. doi: 10.1016/s0168-1656(01)00395-9. [DOI] [PubMed] [Google Scholar]
- 148.Tachibana A., Kaneko S., Tanabe T., Yamauchi K. Rapid fabrication of keratin–hydroxyapatite hybrid sponges toward osteoblast cultivation and differentiation. Biomaterials. 2005;26(3):297–302. doi: 10.1016/j.biomaterials.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 149.Peplow P., Dias G. A study of the relationship between mass and physical strength of keratin bars in vivo. J. Mater. Sci. Mater. Med. 2004;15(11):1217–1220. doi: 10.1007/s10856-004-5803-8. [DOI] [PubMed] [Google Scholar]
- 150.El-Kheir A.A., Popescu C., Mowafi S., Salama M., El-Sayed H. Physico-chemical properties of keratin-polyvinyl alcohol composite. Fibers Polym. 2015;16(3):537–542. [Google Scholar]
- 151.Zhao X., Lui Y.S., Choo C.K.C., Sow W.T., Huang C.L., Ng K.W. Calcium phosphate coated Keratin–PCL scaffolds for potential bone tissue regeneration. Mater. Sci. Eng. C. 2015;49:746–753. doi: 10.1016/j.msec.2015.01.084. [DOI] [PubMed] [Google Scholar]
- 152.Li J.-S., Li Y., Liu X., Zhang J., Zhang Y. Strategy to introduce an hydroxyapatite–keratin nanocomposite into a fibrous membrane for bone tissue engineering. J. Mater. Chem. B. 2013;1(4):432–437. doi: 10.1039/c2tb00460g. [DOI] [PubMed] [Google Scholar]
- 153.Aluigi A., Vineis C., Varesano A., Mazzuchetti G., Ferrero F., Tonin C. Structure and properties of keratin/PEO blend nanofibres. Eur. Polym. J. 2008;44(8):2465–2475. [Google Scholar]
- 154.Aluigi A., Varesano A., Montarsolo A., Vineis C., Ferrero F., Mazzuchetti G. Electrospinning of keratin/poly (ethylene oxide) blend nanofibers. J. Appl. Polym. Sci. 2007;104(2):863–870. [Google Scholar]
- 155.Edwards A., Jarvis D., Hopkins T., Pixley S., Bhattarai N. Poly (ε‐caprolactone)/keratin‐based composite nanofibers for biomedical applications. J. Biomed. Mater. Res. B Appl. Biomater. 2015;103(1):21–30. doi: 10.1002/jbm.b.33172. [DOI] [PubMed] [Google Scholar]
- 156.Horst M., Madduri S., Milleret V., Sulser T., Gobet R., Eberli D. A bilayered hybrid microfibrous PLGA–acellular matrix scaffold for hollow organ tissue engineering. Biomaterials. 2013;34(5):1537–1545. doi: 10.1016/j.biomaterials.2012.10.075. [DOI] [PubMed] [Google Scholar]
- 157.Zou Q., Fu Q. Tissue engineering for urinary tract reconstruction and repair: progress and prospect in China. Asian J. Urol. 2018;5(2):57–68. doi: 10.1016/j.ajur.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sievert K.-D., Daum L., Maurer S., Toomey P., Vaegler M., Aufderklamm S. Urethroplasty performed with an autologous urothelium-vegetated collagen fleece to treat urethral stricture in the minipig model. World J. Urol. 2019:1–9. doi: 10.1007/s00345-019-02888-3. [DOI] [PubMed] [Google Scholar]
- 159.Xie M., Song L., Wang J., Fan S., Zhang Y., Xu Y. Evaluation of stretched electrospun silk fibroin matrices seeded with urothelial cells for urethra reconstruction. J. Surg. Res. 2013;184(2):774–781. doi: 10.1016/j.jss.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 160.Gholipourmalekabadi M., Zhao S., Harrison B.S., Mozafari M., Seifalian A.M. Oxygen-generating biomaterials: a new, viable paradigm for tissue engineering? Trends Biotechnol. 2016;34(12):1010–1021. doi: 10.1016/j.tibtech.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 161.Lv X., Li Z., Chen S., Xie M., Huang J., Peng X. Structural and functional evaluation of oxygenating keratin/silk fibroin scaffold and initial assessment of their potential for urethral tissue engineering. Biomaterials. 2016;84:99–110. doi: 10.1016/j.biomaterials.2016.01.032. [DOI] [PubMed] [Google Scholar]
- 162.Kumar D., Babu G., Krishnan S. Study on mechanical & thermal properties of PCL blended graphene biocomposites. Polímeros. 2019;29(2) [Google Scholar]
- 163.Lee K., Kaplan D. Springer; 2006. Tissue Engineering I: Scaffold Systems for Tissue Engineering. [Google Scholar]
- 164.Mano J., Silva G., Azevedo H.S., Malafaya P., Sousa R., Silva S.S. Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends. J. R. Soc. Interface. 2007;4(17):999–1030. doi: 10.1098/rsif.2007.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Malafaya P.B., Silva G.A., Reis R.L. Natural–origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv. Drug Deliv. Rev. 2007;59(4–5):207–233. doi: 10.1016/j.addr.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 166.Curcio M., Blanco-Fernandez B., Diaz-Gomez L., Concheiro A., Alvarez-Lorenzo C. Hydrophobically modified keratin vesicles for GSH-responsive intracellular drug release. Bioconjugate Chem. 2015;26(9):1900–1907. doi: 10.1021/acs.bioconjchem.5b00289. [DOI] [PubMed] [Google Scholar]
- 167.Tomblyn S., Pettit Kneller E.L., Walker S.J., Ellenburg M.D., Kowalczewski C.J., Van Dyke M. Keratin hydrogel carrier system for simultaneous delivery of exogenous growth factors and muscle progenitor cells. J. Biomed. Mater. Res. B Appl. Biomater. 2016;104(5):864–879. doi: 10.1002/jbm.b.33438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ham T.R., Lee R.T., Han S., Haque S., Vodovotz Y., Gu J. Tunable keratin hydrogels for controlled erosion and growth factor delivery. Biomacromolecules. 2015;17(1):225–236. doi: 10.1021/acs.biomac.5b01328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Saul J.M., Ellenburg M.D., de Guzman R.C., Dyke M.V. Keratin hydrogels support the sustained release of bioactive ciprofloxacin. J. Biomed. Mater. Res. 2011;98(4):544–553. doi: 10.1002/jbm.a.33147. [DOI] [PubMed] [Google Scholar]
- 170.Peyton C.C., Keys T., Tomblyn S., Burmeister D., Beumer J.H., Holleran J.L. Halofuginone infused keratin hydrogel attenuates adhesions in a rodent cecal abrasion model. J. Surg. Res. 2012;178(2):545–552. doi: 10.1016/j.jss.2012.07.053. [DOI] [PubMed] [Google Scholar]
- 171.Pastar I., Stojadinovic O., Yin N.C., Ramirez H., Nusbaum A.G., Sawaya A. Epithelialization in wound healing: a comprehensive review. Adv. Wound Care. 2014;3(7):445–464. doi: 10.1089/wound.2013.0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kim S.Y., Park B.J., Lee Y., Park N.J., Park K.M., Hwang Y.-S. Human hair keratin-based hydrogels as dynamic matrices for facilitating wound healing. J. Ind. Eng. Chem. 2019;73:142–151. [Google Scholar]
- 173.Gao F., Li W., Deng J., Kan J., Guo T., Wang B. Recombinant human hair keratin nanoparticles accelerate dermal wound healing. ACS Appl. Mater. Interfaces. 2019 doi: 10.1021/acsami.9b01725. [DOI] [PubMed] [Google Scholar]
- 174.Sierpinski P., Garrett J., Ma J., Apel P., Klorig D., Smith T. The use of keratin biomaterials derived from human hair for the promotion of rapid regeneration of peripheral nerves. Biomaterials. 2008;29(1):118–128. doi: 10.1016/j.biomaterials.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 175.Lin Y.-C., Ramadan M., Van Dyke M., Kokai L.E., Philips B.J., Rubin J.P. Keratin gel filler for peripheral nerve repair in a rodent sciatic nerve injury model. Plast. Reconstr. Surg. 2012;129(1):67–78. doi: 10.1097/PRS.0b013e3182268ae0. [DOI] [PubMed] [Google Scholar]
- 176.Apel P.J., Garrett J.P., Sierpinski P., Ma J., Atala A., Smith T.L. Peripheral nerve regeneration using a keratin-based scaffold: long-term functional and histological outcomes in a mouse model. J. Hand Surg. 2008;33(9):1541–1547. doi: 10.1016/j.jhsa.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 177.Feng Y., Borrelli M., Meyer-ter-Vehn T., Reichl S., Schrader S., Geerling G. Epithelial wound healing on keratin film, amniotic membrane and polystyrene in vitro. Curr. Eye Res. 2014;39(6):561–570. doi: 10.3109/02713683.2013.853804. [DOI] [PubMed] [Google Scholar]
- 178.Aluigi A., Sotgiu G., Torreggiani A., Guerrini A., Orlandi V.T., Corticelli F. Methylene blue doped films of wool keratin with antimicrobial photodynamic activity. ACS Appl. Mater. Interfaces. 2015;7(31):17416–17424. doi: 10.1021/acsami.5b04699. [DOI] [PubMed] [Google Scholar]
- 179.Cui L., Gong J., Fan X., Wang P., Wang Q., Qiu Y. Transglutaminase‐modified wool keratin film and its potential application in tissue engineering. Eng. Life Sci. 2013;13(2):149–155. [Google Scholar]
- 180.Yue K., Liu Y., Byambaa B., Singh V., Liu W., Li X. Visible light crosslinkable human hair keratin hydrogels. Bioeng. Transl. Med. 2018;3(1):37–48. doi: 10.1002/btm2.10077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yin X.-C., Li F.-Y., He Y.-F., Wang Y., Wang R.-M. Study on effective extraction of chicken feather keratins and their films for controlling drug release. Biomater. Sci. 2013;1(5):528–536. doi: 10.1039/c3bm00158j. [DOI] [PubMed] [Google Scholar]
- 182.Kakkar P., Verma S., Manjubala I., Madhan B. Development of keratin–chitosan–gelatin composite scaffold for soft tissue engineering. Mater. Sci. Eng. C. 2014;45:343–347. doi: 10.1016/j.msec.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 183.Singaravelu S., Ramanathan G., Raja M., Barge S., Sivagnanam U.T. Preparation and characterization of keratin-based biosheet from bovine horn waste as wound dressing material. Mater. Lett. 2015;152:90–93. [Google Scholar]
- 184.Tanase C.E., Spiridon I. PLA/chitosan/keratin composites for biomedical applications. Mater. Sci. Eng. C. 2014;40:242–247. doi: 10.1016/j.msec.2014.03.054. [DOI] [PubMed] [Google Scholar]
- 185.Choi J., Panthi G., Liu Y., Kim J., Chae S.-H., Lee C. Keratin/poly (vinyl alcohol) blended nanofibers with high optical transmittance. Polymer. 2015;58:146–152. [Google Scholar]
- 186.Burnett L.R., Richter J.G., Rahmany M.B., Soler R., Steen J.A., Orlando G. Novel keratin (KeraStat™) and polyurethane (Nanosan®-Sorb) biomaterials are hemostatic in a porcine lethal extremity hemorrhage model. J. Biomater. Appl. 2014;28(6):869–879. doi: 10.1177/0885328213484975. [DOI] [PubMed] [Google Scholar]
- 187.Guo J., Pan S., Yin X., He Y.F., Li T., Wang R.M. p H‐sensitive keratin‐based polymer hydrogel and its controllable drug‐release behavior. J. Appl. Polym. Sci. 2015;132(9) [Google Scholar]
- 188.Han S., Ham T.R., Haque S., Sparks J.L., Saul J.M. Alkylation of human hair keratin for tunable hydrogel erosion and drug delivery in tissue engineering applications. Acta Biomater. 2015;23:201–213. doi: 10.1016/j.actbio.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hasan A., Khattab A., Islam M.A., Hweij K.A., Zeitouny J., Waters R. Injectable hydrogels for cardiac tissue repair after myocardial infarction. Adv. Sci. 2015;2(11):1500122. doi: 10.1002/advs.201500122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ajay Sharma L., Ali M., Love R., Wilson M., Dias G. Novel keratin preparation supports growth and differentiation of odontoblast‐like cells. Int. Endod. J. 2016;49(5):471–482. doi: 10.1111/iej.12476. [DOI] [PubMed] [Google Scholar]
- 191.Wang S., Wang Z., Foo S.E.M., Tan N.S., Yuan Y., Lin W. Culturing fibroblasts in 3D human hair keratin hydrogels. ACS Appl. Mater. Interfaces. 2015;7(9):5187–5198. doi: 10.1021/acsami.5b00854. [DOI] [PubMed] [Google Scholar]
- 192.Gao F., Li W., Deng J., Kan J., Guo T., Wang B. Recombinant human hair keratin nanoparticles accelerate dermal wound healing. ACS Appl. Mater. Interfaces. 2019;11(20):18681–18690. doi: 10.1021/acsami.9b01725. 2019/05/22. [DOI] [PubMed] [Google Scholar]
- 193.Veerasubramanian P.K., Thangavel P., Kannan R., Chakraborty S., Ramachandran B., Suguna L. An investigation of konjac glucomannan-keratin hydrogel scaffold loaded with Avena sativa extracts for diabetic wound healing. Colloids Surf. B Biointerfaces. 2018;165:92–102. doi: 10.1016/j.colsurfb.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 194.Nayak K.K., Gupta P. Study of the keratin-based therapeutic dermal patches for the delivery of bioactive molecules for wound treatment. Mater. Sci. Eng. C. 2017;77:1088–1097. doi: 10.1016/j.msec.2017.04.042. [DOI] [PubMed] [Google Scholar]
- 195.Wang J., Hao S., Luo T., Zhou T., Yang X., Wang B. Keratose/poly (vinyl alcohol) blended nanofibers: fabrication and biocompatibility assessment. Mater. Sci. Eng. C. 2017;72:212–219. doi: 10.1016/j.msec.2016.11.071. [DOI] [PubMed] [Google Scholar]
- 196.Sharma S., Gupta A., Kumar A., Kee C.G., Kamyab H., Saufi S.M. An efficient conversion of waste feather keratin into ecofriendly bioplastic film. Clean Technol. Environ. Policy. 2018;20(10):2157–2167. [Google Scholar]