Abstract
Introduction
To examine the interaction between an added flavoring (cherry) and nicotine on the perception of electronic cigarette (e-cigarette) aerosol and how this impacts the appeal of flavored liquids for e-cigarette (e-liquids).
Methods
A total of 19 subjects (13 male, 6 female) vaped six commercially available e-liquids with varying contents of nicotine (0, 6, 12 mg/mL) and cherry flavor (4.7% or 9.3% vol/vol). For each e-liquid, subjects first rated overall liking/disliking of the aerosol using the Labeled Hedonic Scale, followed by perceived intensities of sweetness, bitterness, harshness (irritation), and cherry flavor of the aerosol using the general version of Labeled Magnitude Scale.
Results
The main findings were that (1) added nicotine increased perceived irritation and bitterness, and decreased the perceived sweetness of the e-cigarette aerosol; (2) cherry flavoring added a characteristic “cherry flavor” and an increase in the flavoring concentration from 4.7% to 9.3% tended to increase perceived intensities of sweetness, harshness, and bitterness; and (3) hedonic ratings of the e-cigarette aerosol decreased as nicotine level increased, but were not affected by flavor level.
Conclusions
Our findings indicate that the appeal of the e-cigarette aerosol decreases as nicotine concentration increases. Conversely, perceived sweetness improved liking. An increase in the concentration of cherry flavoring did not appear to impact any of the measured attributes to a significant degree.
Implications
This work demonstrates that the perception of specific sensory attributes of e-cigarettes and their overall appeal are affected by the e-liquid constituents. Most significantly, the results suggest that nicotine decreases the sensory appeal of e-cigarettes by contributing to the perceived irritation and bitterness of the aerosol. These data have implications for the role that nicotine plays in the sensory perception and appeal of e-cigarettes aerosol and further how these sensory factors can be modulated by sweet flavoring.
Introduction
The popularity of electronic cigarettes (e-cigarettes) has risen substantially in recent years among adults and adolescents.1,2 Perhaps the main driver of the product’s success is the perception that it is a safer alternative to combustible tobacco cigarettes, with adverse health effects being generally mild and short-term in comparison,3 though this is still debated.4 In fact, numerous studies have reported that e-cigarette users are most often either current and/or former tobacco cigarette smokers.1,5 However, it is unclear whether the rise in e-cigarette popularity serves to encourage a substantial number of nonsmokers to initiate use.5–7 Nonetheless, the increase in use of e-cigarettes among any population warrants concern about the perpetuation of nicotine addiction, which remains a critical issue in the United States.8
At least 30% of adolescent e-cigarette users have reported using electronic liquids (e-liquids) that contain nicotine.9 For adults, the prevalence of using nicotine-containing e-liquids may be closer to 99%.10 Nicotine is a psychoactive substance,11 which also elicits irritation such as burning, stinging, and painful sensations.12,13 Irritation is generally an undesirable sensory quality that reduces appeal and may deter the initiation and use of nicotine-containing products. To reduce irritation from nicotine, manufacturers use flavor additives, which increase product appeal by adding a desirable sensory dimension. Such an approach had previously been used in conventional tobacco cigarettes through addition of “characterizing” flavors (eg, vanilla, cloves). Recognizing the role of these flavor additives in improving the appeal of cigarettes, in 2009, the US Food and Drug Administration (FDA) banned the addition of “characterizing” flavors to cigarettes (with the exception of menthol).14 Most recently, the FDA announced new restrictions that include a ban on the sale of flavored e-cigarettes, with the exception of menthol and mint flavors, in retail outlets that do not have areas restricted from children under 18 years. Other flavored varieties will now only be sold at age-restricted stores or through online merchants that use age-verification checks.15 Although menthol was excluded from this new e-cigarette restriction, the FDA announced its intent to seek a ban on menthol cigarettes and cigars, which is considered to pose a greater public health risk.15 Further policy actions are expected to be announced based on more data regarding the impact of flavors on use of products across youth and adult populations.
The number of e-liquid flavors available on the market has been increasing exponentially, with more than 7700 unique flavors reported to be available in 2012–2014 online market, increasing from the prior year at a rate of nearly 250 new flavors a month.2 Little is known about how specific classes of flavor impact adoption, preference, and use of e-cigarettes. Studies have shown that flavor additives play an important role in e-cigarette use behaviors.16 In particular, “sweet” flavors (commonly fruit, but also candy and dessert-like flavors), are highly popular among users,17 and there is a significant positive association between sweet flavors and liking.18 Notably, adolescents show greatest preference for sweet-flavored e-liquids.9
There is little empirical evidence regarding how flavor additives perceptually interact with nicotine. Existing literature suggests that other irritants (eg, capsaicin) often interact with flavor compounds (eg, aroma compounds and tastants) resulting in either inhibition or enhancement of perceived flavor intensities, and that the interactions are specific to the chemicals involved.19 In particular, evidence regarding how specific flavor additives may modify or mediate nicotine irritation in the aerosol of e-cigarettes is limited.
This study examined the impacts of nicotine and flavoring on the sensory perception of e-cigarette aerosol by investigating the occurrence and degree of perceptual interaction between fruit flavoring and nicotine, and further how such perception modulates the appeal of e-liquids. Established psychophysical methods, typically used in studies of food and consumer products, were adopted to measure aspects of e-cigarette aerosol perception. Such methods have successfully been used to evaluate e-cigarette perception in two recent studies,18,20 and offer a more controlled approach over survey methods.21
Methods
Subjects
Nineteen current e-cigarette users (13 male, 6 female) between 21 and 35 years of age (mean = 24.5) were recruited from the Oregon State University campus and surrounding areas. All subjects were required to be (1) healthy per self-report, (2) 21–35 years of age, inclusive, (3) established e-cigarette users, defined as having vaped a nicotine-containing e-cigarette for at least 1 month prior to the study, and (4) a user (currently or in the past) of e-liquids with nicotine levels at or greater than 9 mg/mL. Study exclusion criteria included (1) mouth or throat problems that would prevent vaping comfortably; (2) health problems that would prevent tasting or smelling normally; (3) allergies to propylene glycol, vegetable glycerin, natural or artificial cherry flavors or fragrances; (4) respiratory allergies (ie, frequent sneezing, nasal congestion, nasal discharge) or a history of pulmonary disease or asthma; (5) a desire to quit vaping; and (6) pregnancy, breast feeding, or trying to become pregnant. A negative pregnancy result was confirmed for all female subjects prior to the experiment session using the BFP Midstream Early Pregnancy test (Fairhaven Health, Bellingham, WA). Subjects were not further screened for olfactory and gustatory deficits to capture how typical e-cigarette users would perceive test stimuli. Subjects were asked to not eat or drink except water, and not use any tobacco products at least 2 hours prior to their scheduled session. The experimental protocol was approved by the Oregon State University Institutional Review Board and was registered under the Clinical Trial registry (NCT03332953) at www.clinicaltrials.gov. Subjects gave written informed consent and were compensated at the end of the session.
Questionnaires
Subjects were asked to fill out two questionnaires before attending the experimental session. The first questionnaire served as a screener for study participation and included questions regarding the eligibility and exclusion criteria listed earlier. The second questionnaire collected information on the participant’s e-cigarette and conventional tobacco cigarette use, including frequency of use and flavor preferences. E-cigarette dependence was calculated from the Penn State Electronic Cigarette Dependence Index22 (see Table 1).
Table 1.
Characteristics of Study Participants (N = 19)
| Characteristic | N (%) |
|---|---|
| Sex | |
| Male | 13 (68.4) |
| Female | 6 (31.6) |
| Age (y) | 24.5 ± 3.6 |
| Ethnicities | |
| Caucasian | 11 (57.9) |
| Asian, Middle Eastern or Indian | 8 (42.1) |
| Pacific Islander/Native Hawaiian | 1 (5.3) |
| Length of e-cigarette use | |
| 1 mo to 6 mo | 7 (36.8) |
| 6 mo to 1 y | 6 (31.6) |
| Greater than 1 y | 6 (31.6) |
| Frequency of e-cigarette use | |
| Every day | 14 (73.7) |
| 3–6 d per wk | 3 (15.8) |
| 1–2 d per wk | 2 (10.5) |
| Current e-liquid nicotine strength | |
| Low (3–6 mg/mL nicotine) | 7 (36.8) |
| Medium (9–12 mg/mL nicotine) | 8 (42.1) |
| High (18–36 mg/mL nicotine) | 4 (21.1) |
| Past highest used e-liquid nicotine strength | |
| Medium (9–12 mg/mL nicotine) | 11 (57.9) |
| High (18–36 mg/mL nicotine) | 8 (42.1) |
| Current other tobacco products use | |
| No | 11 (57.9) |
| Yes | 8 (42.1) |
| Past other tobacco products use | |
| No | 1 (5.3) |
| Yes | 18 (94.7) |
| Categories of e-liquid flavors used | |
| Fruit flavors (eg, cherry, pineapple, berry) | 19 (100) |
| Dessert flavors (eg, marshmallow, cinnamon, cotton candy) | 8 (42.1) |
| Menthol flavored | 7 (36.8) |
| Tobacco flavored | 2 (10.5) |
| Beverage flavors (eg, chai, coffee, mojito, cola) | 1 (5.3) |
| Other | 1 (5.3) |
| Penn State Electronic Cigarette Dependence Index | |
| Not dependent | 4 (21.1) |
| Low dependence | 6 (31.6) |
| Medium dependence | 5 (26.3) |
| High dependence | 4 (21.1) |
Materials
E-cigarettes were composed of a V2 Ex Blank Cartridge and a V2 Standard 79-mm E-cig Battery (4.2 volts) (VMR Products LLC, Miami, FL). The blank cartridge component consisted of a mouthpiece (drip tip), and a “clearomizer,” composed of a graduated e-liquid tank, silica wick, and heating coil. A new blank cartridge was used for each sample for each subject and was attached to a reusable battery. All batteries were fully charged immediately before use. Six e-liquid formulations (3 nicotine levels × 2 flavor levels) were purchased premade from VaporFi (Miami Lakes, FL). According to the manufacturer, the formulations had 0, 6, or 12 mg/mL of nicotine and contained either “1 shot” (4.66% vol/vol) or “2 shots” (9.33% vol/vol) of cherry flavor. All e-liquid formulations had a 70:30 propylene glycol:vegetable glycerin ratio and were registered with the FDA prior to August of 2016, and thus were not defined as investigational tobacco products. Stimuli were prepared by adding 0.5 mL of e-liquid to the blank cartridge and were vaped within 1 minute of preparation.
Test Environment
The experiment took place in an enclosed lab with a testing space sectioned off by portable dividers. The testing room was ventilated using a negative air machine (OmniAire Nitro 600, 600 ft3/minute) that was exhausted outdoors. The room air was filtered for odors and particles using an ionizer (Honeywell Air Genius) that was run for the full day when testing sessions occurred. All data were collected on a laptop computer connected to a display monitor.
Test Procedure
Each participant was tested in a single 45-minute session, which consisted of two parts: training on hedonic and intensity scales, and data collection.
Training on Scales
Prior to vaping the e-cigarettes, subjects were trained on use of the Labeled Hedonic Scale (LHS)23,24 and the general version of the Labeled Magnitude Scale (gLMS)25,26 to measure liking/disliking and the perceived intensity of specific characteristics about the e-cigarette aerosol, respectively. The LHS is a bipolar categorical ratio scale bounded by the descriptors “most disliked sensation imaginable” on the bottom and “most liked sensation imaginable” at the top, with intermediate hedonic labels (like or dislike: slightly, moderately, very much, extremely) spaced at empirically derived intervals and “neutral” at the midpoint. Importantly, the capacity of this scale to measure the entire range of hedonic sensations one can experience allows ratio-level data to be obtained and hedonic implications to be made across individuals. After a brief explanation of the scale and instruction on its usage, subjects practiced making ratings on the scale using a list of 15 remembered or imagined items representing a broad frame of reference for possible hedonic sensations (eg, the taste of plain bread, the smell of bad body odor).
The gLMS is also a categorical ratio scale used to quantify the intensity of any sensation imaginable and shares similar data analysis implications (eg, ratio-level data) to the LHS discussed earlier. The gLMS is bounded by “no sensation” at the bottom and “strongest imaginable sensation of any kind” at the top, with intermediate labels (from bottom to top: barely detectable, weak, moderate, strong, and very strong) spaced quasi-logarithmically based on empirically derived magnitudes. Subjects were similarly trained on this scale using a list of 15 remembered or imagined items representing a broad context of sensations with varying intensity (eg, the bitterness of fresh spring water, the heat from sipping boiling hot tea). Subjects’ responses from the training session were monitored and further instruction was given if there were inconsistencies with scale usage (eg, ratings abnormally high or low). Training was completed when responses fell within a normal range.
Data Collection
Prior to receiving the first sample, subjects rinsed their mouth three times with room temperature water and expectorated. Next, subjects were instructed on the vaping procedure, which involved inhaling the e-cigarette for 2 seconds, then exhaling, and repeating twice more (total of three puffs, 2 seconds each). Subjects were told that they could use their normal depth of inhalation for each e-cigarette sampled, but to stay consistent between successive samples. Further sampling of the e-cigarette was not allowed. Note the e-cigarette power was simply activated by inhaling; pilot testing did not reveal the necessity to take priming puffs to “warm up” the device. After the vaping procedure was finished, subjects were instructed to begin making ratings immediately. The overall liking/disliking of the aerosol was rated first using the LHS, then subsequent ratings were made using the gLMS on perceived sweetness, bitterness, harshness (irritation), and cherry flavor in that order. The cartridge containing the mouthpiece and e-liquid was disposed of after sampling. A 1-minute break was given between stimuli presentations; during this time, subjects were instructed to rinse their mouth and spit at least three times with room-temperature water. Additional time was offered to subjects between stimuli if they experienced irritation or negative effects (eg, dizziness from nicotine) or flavor carry-over; however, this was not reported by anyone tested. Presentation order of the six stimuli were randomized between subjects using a Williams design, and subjects were blind to the conditions.
Data Preparation and Analysis
All rating data were collected using Compusense V.8.8 (Compusense). Hedonic ratings from the LHS were translated into a range of −100 to +100 for analysis.24 The gLMS ratings were log-transformed in Compusense, as ratings from this scale are typically log-normally distributed.26 Repeated measures analysis of variance was performed on all specific ratings (ie, overall liking/disliking, sweetness, bitterness, harshness, and cherry flavor) using flavor level and nicotine level as factors, followed by Tukey’s honest significant difference test. Statistical significance was set at p = .05. All statistical analyses were performed with Statistica 8 (StatSoft).
Results
Subject Characteristics
Characteristics of the subjects tested are reported in Table 1.
Perception of E-cigarette Aerosol
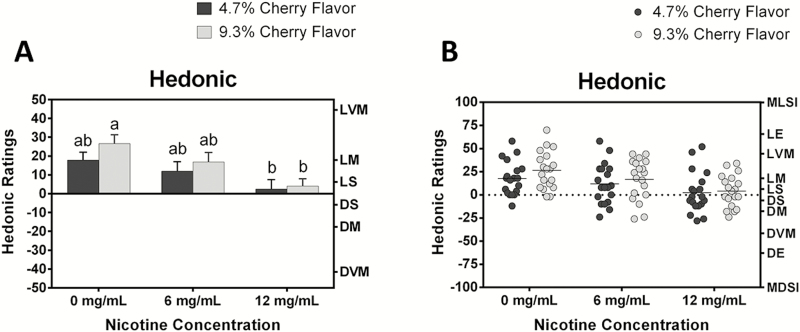
For hedonic ratings, there was a significant effect of nicotine level [F(2,36) = 11.00, p <.0005], but not flavor level [F(1,18) = 2.72, p > .05] on liking. As shown in Figure 1A, hedonic ratings among the six samples (3 nicotine levels × 2 flavor levels) decreased systematically as the nicotine content increased. In contrast, hedonic ratings tended to increase as the concentration of cherry flavor increased from 4.7% to 9.3%, although this tendency did not reach statistical significance. The sample containing higher cherry flavor (9.3%) without nicotine was liked significantly more than two samples that contained 12 mg/mL nicotine at both flavor levels. Nonetheless, mean hedonic ratings for all six samples were above neutral (“0” on left y-axis). Hedonic responses were symmetrically distributed (Figure 1B), suggesting that there were no individuals with a strong disliking of the e-liquid aerosols.
Figure 1.
(A) Mean hedonic ratings ± standard error and (B) individual hedonic ratings of the six e-cigarettes sampled (2 flavor levels × 3 nicotine levels) by 19 subjects. E-cigarettes contained either 4.7% or 9.3% cherry flavoring and 0, 6, or 12 mg/mL of nicotine. Different letters above the bars in (A) indicate significant differences in hedonic ratings per Tukey’s honest significant difference test, p < .05. Note the difference in y-axis range between (A) and (B) for clarity. Abbreviations on the right y-axes represent semantic labels of the labeled hedonic scale. MLSI = most liked sensation imaginable; LE = like extremely; LVM = like very much; LM = like moderately; LS = like slightly; DS = dislike slightly; DM = dislike moderately; DVM = dislike very much; DE = dislike extremely; MDSI = most disliked sensation imaginable.
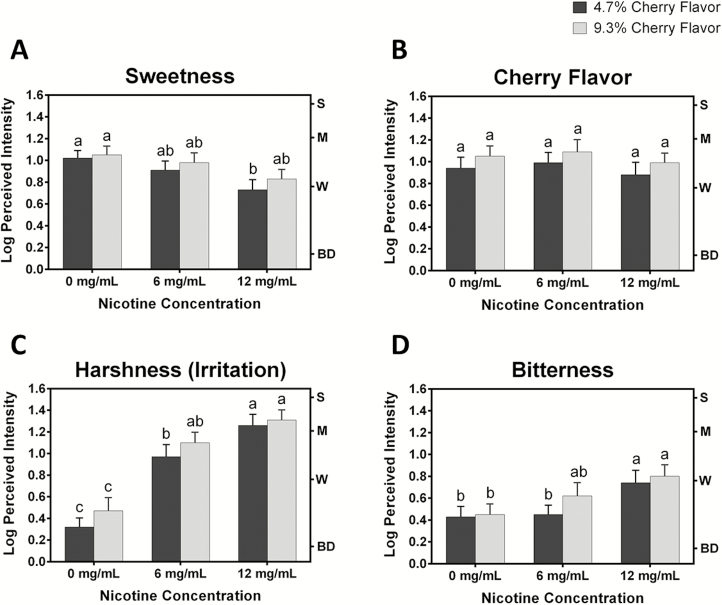
Figure 2 displays log mean intensity ratings for four attributes—sweetness, cherry flavor, harshness, and bitterness—at two flavor levels (black bars, 4.7% cherry flavor; gray bars, 9.3% cherry flavor) and three nicotine concentrations (0, 6, and 12 mg/mL). Repeated measures analysis of variance for sweetness ratings (Figure 2A) revealed a significant effect of nicotine level [F(2,36) = 5.73, p < .01], but not flavor level [F(1,18) = 1.27, p > .05]. As expected, the two e-cigarette samples that did not contain nicotine were rated as sweetest and the sweetness ratings decreased as nicotine concentration increased. The e-cigarette containing the highest nicotine concentration (12 mg/mL) and the lower flavor (4.7%) level was significantly least sweet in rating. For cherry flavor ratings (Figure 2B), flavor level had a significant effect [F(1,18) = 4.43, p = .05]; the ratings increased as the flavor concentration increased from 4.7% to 9.3%, although Tukey’s test did not identify significant groupings. Importantly, nicotine level did not have a significant effect on cherry flavor ratings [F(2,36) = 1.38, p > .05].
Figure 2.
Mean log perceived intensity ratings ± standard error of the six e-cigarettes sampled by 19 subjects (A) sweetness, (B) cherry flavor, (C) harshness (irritation), and (D) bitterness. E-cigarettes contained either 4.7% or 9.3% cherry flavoring and 0, 6, or 12 mg/mL of nicotine. Different letters above the bars indicate significant differences in intensity ratings per Tukey’s honest significant difference test, p < .05. Abbreviations on the right y-axes represent semantic labels of a selected portion of the general version of the labeled magnitude scale. S = strong, M = moderate, W = weak, BD = barely detectable.
A systematic increase in harshness (irritation) was observed as nicotine concentration increased [F(2,36) = 38.35, p < .0001] (Figure 2C). Although there exists a visible trend of increased harshness at increasing flavor concentrations, the effect of flavor level was not significant [F(1,18) = 3.55, p > .05]. The two e-cigarette samples that contained no nicotine were rated as having significantly lower harshness; the two e-cigarette samples that contained the highest concentration of nicotine were rated as significantly harsher. For bitterness (Figure 2D), nicotine level had a significant effect [F(2,36) = 7.44, p < .005], but flavor level did not [F(1,18) = 2.81, p > .05]. The two e-cigarettes that contained no nicotine were rated as significantly less bitter than the two samples containing 12 mg/mL of nicotine. Interestingly, the differences in perceived bitterness were greater between 6 and 12 mg/mL nicotine versus no nicotine and 6 mg/mL nicotine.
Discussion
Sensory Perception of Nicotine in E-cigarette Aerosol
E-cigarettes administer nicotine to the user without combustion and tobacco smoke. Although e-cigarettes lack the added irritation attributed to the chemical and particulate irritants in combusted cigarette smoke,27 nicotine itself is capable of eliciting irritation in the oral cavity12 and respiratory airways.13,28 The sensory irritation from nicotine is mediated through the activation of nicotinic acetylcholine receptors within nerve fibers28 and potentially other channels,29 which are distributed in the mouth and airways. The current study clearly demonstrates that nicotine elicits irritation (harshness) when inhaled, and that this sensation increased in a monotonic manner as nicotine concentration increased from 0 to 12 mg/mL. Similar results have previously been reported in these devices.20
Although some degree of irritation can be desirable for some individuals in certain contexts (eg, spicy food),30 it is typically considered a negative attribute in consumer products. Our data support this notion, as evidenced by reduced e-cigarette liking with presence and increasing concentrations of nicotine. It is worth noting, however, that some perceptible level of irritation may be desirable to some e-cigarette users. Accordingly, it has been postulated as a reason for the greater use of these devices as compared to FDA-approved nicotine cessation methods (patches, lozenges, inhaler).31 This sensation, referred to as “throat hit,” is defined as desirable airway irritation caused by nicotine during smoking.32 One study surveyed more than 1600 e-cigarette users and found that the strength of the throat hit was associated with higher nicotine and higher satisfaction levels, though these results were not determined experimentally and may only be generalizable to some users.33 Whereas other focus group-based studies support these claims,31,32 the actual impact of this sensation on e-cigarette appeal in a controlled study of e-cigarette users was negative.34 It is likely that the appeal of this sensation is related to the users’ smoking history.35 Indeed, our data showed that dual users (ie, e-cigarette users that concurrently use combustible tobacco products) produced higher hedonic ratings for aerosols that they also rated as more harsh, based on a positive coefficient of interaction term between dual-use status and harshness rating on predicting liking score.
In addition to irritation, nicotine is also known to evoke bitterness,36 which is elicited by T2R taste receptors on the tongue and other oral surfaces.37 Our study confirmed that nicotine evokes bitter taste in a dose-response manner in e-cigarette aerosol. Similar to harshness, bitterness is commonly considered a negative attribute in consumer products, although learning can reverse the innate aversive responses.38 Our data demonstrate that there is a negative relationship between e-cigarette liking and bitterness perception, most likely caused by the inhaled nicotine. A similar negative correlation between perceived bitterness and liking of e-cigarette aerosol was reported previously when subjects vaped different flavored e-cigarettes containing a constant level of nicotine.18 Recognizing such effects in nicotine-containing products,39 the industry has put significant effort into creating strategies to improve their sensory appeal, including the use of flavor additives.
Impacts of an Added Flavor on the Sensory Perception of E-cig Aerosol
Flavorants are common additives in tobacco products, including electronic nicotine delivery systems. Flavor additives include aroma compounds (eg, vanillin for vanilla), chemesthetic agents (eg, l-menthol for menthol), and sweet tastants (eg, sucralose), which are sensed by different sensory systems: aroma compounds by olfactory receptors,40 chemesthetic agents by cold-sensitive nerve fibers (TRPM8),41 and sweeteners by the sweet taste receptor (hT1R2-hT1R3).42 These compounds are added to tobacco products, including e-liquids, for the purpose of modulating hedonic responses.18,20,34 Specifically, flavor additives can (1) add a desirable, defining characteristic,18,20,34 (2) suppress irritation and bitterness produced by nicotine and other irritants,18,20 and (3) enhance a positive attribute such as sweetness produced by other ingredients (eg, sweeteners).18,34
Flavorants add a desirable sensory dimension and thereby enhance the rewarding and reinforcing value of e-cigarettes.43 In the current study, we specifically investigated the role of a cherry flavoring on the sensory perception of e-cigarettes. Fruit flavors are reported to be the most commonly used flavor category among established e-cigarette users,17 and are thus particularly relevant when examining the sensory interactions between e-liquid constituents. As expected, the addition of two different concentrations of cherry flavoring elicited the characterizing percept (ie, “cherry flavor”), and the perception of cherry flavor tended to increase slightly as the concentration of flavor increased from 4.7% to 9.3% (see Figure 2B). There also appeared to be a slight trend for increased liking as cherry flavor concentration increased, which could have been attributed to the increased perception of cherry flavoring.
Flavor components have been reported to interact with irritants to inhibit or enhance their perceived intensities.19 Sweeteners, for example, are added both to increase sweetness as well as to suppress bitter taste and to inhibit irritation.44 Aroma compounds have been shown to decrease irritation in some circumstances.45 Contrary to expectations, our results showed that cherry flavoring, which contained sweeteners and aroma compounds, did not lower harshness ratings when its concentration was increased from 4.7% to 9.3%. In fact, there was a trend of increased harshness between 4.7% and 9.3% flavor, though this trend was not significant. It has been previously reported that some aroma compounds are capable of producing irritating sensations at higher concentrations.19 In the case of e-liquids, certain volatile additives may be capable of producing irritation when vaporized, condensed into an aerosol, and inhaled.35 In particular, many cherry-flavored e-liquids contain the aroma compound benzaldehyde. Although generally regarded as safe for ingestion, benzaldehyde is an airway irritant when inhaled,46 and therefore could explain why higher concentrations of cherry flavor did not lower harshness ratings. We speculate that the harshness ratings of these samples are lower than what may be observed in the absence of added flavor (ie, 0% cherry flavor), however a 0% flavor condition was not included in our study. Whereas the increase of cherry flavoring did not significantly impact hedonic responses, the e-cigarette samples that produced a higher perception of sweetness tended to have an increased overall appeal. It is likely that at least some of the sweetness observed was due to the cherry flavoring.
Impacts of Other E-liquid Constituents on the Perception of E-cigarettes
In addition to nicotine and cherry flavoring, e-liquids typically contain a base of propylene glycol, glycerin (ie, glycerol), or a mixture of the two.3 During vaping, these chemicals vaporize and condense to form an aerosol that looks similar to combustible cigarette smoke.47 Propylene glycol is faintly sweet tasting,48 and glycerin tastes about half as sweet as table sugar, that is, granulated sucrose, on a weight basis.49 Given the high concentrations of these two substances in e-liquids, unflavored nicotine-free e-liquid aerosol can possess a low, but detectable sweet quality.34 Our results indicate that the aerosol possessed considerable sweetness that was likely independent of the cherry flavoring, as sweetness did not significantly increase between the two flavor concentrations (4.7% vs. 9.3%). Therefore, we hypothesize that the e-liquid base components (ie, propylene glycol, glycerin) contributed to the sweet quality of the aerosol. Notably, the ratio of propylene glycol to glycerin in an e-liquid is important, because the two constituents differ in terms of aerosol quality and flavor delivery; particularly, high-propylene glycol liquids produce a thinner aerosol, but deliver more flavor and throat hit.35 When aerosolized, both propylene glycol and glycerin have been reported to produce mouth and throat irritation.50,51 It is therefore possible that the base components of the e-liquid, in addition to aroma compounds associated with the cherry flavoring (eg, benzaldehyde), contributed to the baseline harshness ratings observed for e-cigarette samples void of nicotine.
Study Limitations
The study was conducted using one manufacturer’s set of e-liquids and one e-cigarette device, and therefore the results may not be generalizable across all e-liquids and e-cigarette devices. In addition, no attempt was made to verify or measure the variability of the nicotine and cherry flavoring content of the e-liquids as declared by the manufacturer. More importantly, this study did not include an unflavored sample as it was not offered commercially by the e-liquid supplier. The lack of baseline measurement does not allow us to directly test the impact of cherry flavor; nonetheless, this study offers important information regarding how nicotine may impact sensory and hedonic responses to e-liquids in the presence of added flavor.
Conclusion
The results suggest that the perception of specific sensory attributes of e-cigarettes, as well as their overall appeal, are differentially affected by the e-liquid constituents (ie, flavor additives, nicotine). Our results show that nicotine contributes to the irritation and bitter quality of e-cigarette aerosol, with the observed effect of nicotine level on harshness being more dramatic. In contrast, the perceived sweetness of the e-cigarette aerosol decreased in the presence of nicotine. We found that an increase of cherry flavoring concentration resulted in increased ratings for all attributes tested (ie, sweetness, cherry flavor, harshness, and bitterness), although the differences did not reach statistical significance. Hedonic ratings of the e-cigarette aerosol decreased as nicotine level increased, with no effect between the two flavor levels tested. The impact of flavors on e-cigarette use has been shown to be important. More scientific evidence is needed to inform the role of flavors in e-cigarette appeal, uptake and continued use. This study was conducted with a small number of participants to generate comprehensive hypotheses as to how the interaction between flavors and nicotine impact the sensory perception and appeal of e-liquid; the findings should be interpreted as preliminary.
Funding
Research reported in this manuscript was supported by grant number P50CA180523 from the National Cancer Institute (NIH) and US Food and Drug Administration Center for Tobacco Products awarded to the University of Maryland Tobacco Center of Regulatory Sciences (TCORS). The content is solely the responsibility of the authors and does not represent the official views of the NIH or the US Food and Drug Administration.
Declaration of Interests
None declared.
References
- 1. McMillen R, Maduka J, Winickoff J. Use of emerging tobacco products in the United States. J Environ Public Health. 2012;2012:989474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: Implications for product regulation. Tob Control. 2014;23(suppl 3):iii3–iii9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Glasser AM, Collins L, Pearson JL, et al. Overview of electronic nicotine delivery systems: a systematic review. Am J Prev Med. 2017;52(2):e33–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cai H, Wang C. Graphical review: the redox dark side of e-cigarettes; exposure to oxidants and public health concerns. Redox Biol. 2017;13:402–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Delnevo CD, Giovenco DP, Steinberg MB, et al. Patterns of electronic cigarette use among adults in the United States. Nicotine Tob Res. 2016;18(5):715–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Filippidis FT, Laverty AA, Fernandez E, Mons U, Tigova O, Vardavas CI. Correlates of self-reported exposure to advertising of tobacco products and electronic cigarettes across 28 European Union member states. Tob Control. 2017;26(e2):e130–e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klein JD. Electronic cigarettes are another route to nicotine addiction for youth. JAMA Pediatr. 2015;169(11):993–994. [DOI] [PubMed] [Google Scholar]
- 8. Kutlu MG, Parikh V, Gould TJ. Chapter seven—nicotine addiction and psychiatric disorders. In: De Biasi M, ed. International Review of Neurobiology. Vol 124 Cambridge, MA: Academic Press; 2015:171–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Krishnan-Sarin S, Morean ME, Camenga DR, Cavallo DA, Kong G. E-cigarette use among high school and middle school adolescents in connecticut. Nicotine Tob Res. 2015;17(7):810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marynak KL, Gammon DG, Rogers T, Coats EM, Singh T, King BA. Sales of nicotine-containing electronic cigarette products: United States, 2015. Am J Public Health. 2017;107(5):702–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jaffe AJ, Glaros AG. Taste dimensions in cigarette discrimination: a multidimensional scaling approach. Addict Behav. 1986;11(4):407–413. [DOI] [PubMed] [Google Scholar]
- 12. Dessirier JM, Nguyen N, Sieffermann JM, Carstens E, O’Mahony M. Oral irritant properties of piperine and nicotine: psychophysical evidence for asymmetrical desensitization effects. Chem Senses. 1999;24(4):405–413. [DOI] [PubMed] [Google Scholar]
- 13. Lee LY, Gerhardstein DC, Wang AL, Burki NK. Nicotine is responsible for airway irritation evoked by cigarette smoke inhalation in men. J Appl Physiol (1985). 1993;75(5):1955–1961. [DOI] [PubMed] [Google Scholar]
- 14. Enforcement of General Tobacco Standard Special Rule for Cigarettes. Federal register https://www.federalregister.gov/documents/2009/09/25/E9-23144/enforcement-of-general-tobacco-standard-special-rule-for-cigarettes. Published September 25, 2009. Accessed December 27, 2018.
- 15. Office of the Commissioner C for TP. Press Announcements—Statement from FDA Commissioner Scott Gottlieb, M.D., on proposed new steps to protect youth by preventing access to flavored tobacco products and banning menthol in cigarettes https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm625884.htm. Accessed December 27, 2018.
- 16. Feirman SP, Lock D, Cohen JE, Holtgrave DR, Li T. Flavored tobacco products in the United States: a systematic review assessing use and attitudes. Nicotine Tob Res. 2016;18(5):739–749. [DOI] [PubMed] [Google Scholar]
- 17. Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Spyrou A, Voudris V. Impact of flavour variability on electronic cigarette use experience: an internet survey. Int J Environ Res Public Health. 2013;10(12):7272–7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim H, Lim J, Buehler SS, et al. Role of sweet and other flavours in liking and disliking of electronic cigarettes. Tob Control. 2016;25(suppl 2):ii55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Delwiche J. The impact of perceptual interactions on perceived flavor. Food Quality and Preference. 2004;15(2):137–146. [Google Scholar]
- 20. Rosbrook K, Green BG. Sensory effects of menthol and nicotine in an E-Cigarette. Nicotine Tob Res. 2016;18(7):1588–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Czoli CD, Goniewicz M, Islam T, Kotnowski K, Hammond D. Consumer preferences for electronic cigarettes: results from a discrete choice experiment. Tob Control. 2016;25(e1):e30. [DOI] [PubMed] [Google Scholar]
- 22. Foulds J, Veldheer S, Yingst J, et al. Development of a questionnaire for assessing dependence on electronic cigarettes among a large sample of ex-smoking E-cigarette users. Nicotine Tob Res. 2015;17(2):186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lim JI, Lee YK, Shin JS, Lim KJ. Preparation of interconnected porous chitosan scaffolds by sodium acetate particulate leaching. J Biomater Sci Polym Ed. 2011;22(10):1319–1329. [DOI] [PubMed] [Google Scholar]
- 24. Lim J, Wood A, Green BG. Derivation and evaluation of a labeled hedonic scale. Chem Senses. 2009;34(9):739–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bartoshuk LM, Duffy VB, Green BG, et al. Valid across-group comparisons with labeled scales: the gLMS versus magnitude matching. Physiol Behav. 2004;82(1):109–114. [DOI] [PubMed] [Google Scholar]
- 26. Green BG, Shaffer GS, Gilmore MM. Derivation and evaluation of a semantic scale of oral sensation magnitude with apparent ratio properties. Chemical Senses. 1993;18(6):683–702. [Google Scholar]
- 27. McAuley TR, Hopke PK, Zhao J, Babaian S. Comparison of the effects of e-cigarette vapor and cigarette smoke on indoor air quality. Inhal Toxicol. 2012;24(12):850–857. [DOI] [PubMed] [Google Scholar]
- 28. Lee LY, Burki NK, Gerhardstein DC, Gu Q, Kou YR, Xu J. Airway irritation and cough evoked by inhaled cigarette smoke: role of neuronal nicotinic acetylcholine receptors. Pulm Pharmacol Ther. 2007;20(4):355–364. [DOI] [PubMed] [Google Scholar]
- 29. Talavera K, Gees M, Karashima Y, et al. Nicotine activates the chemosensory cation channel TRPA1. Nat Neurosci. 2009;12(10):1293–1299. [DOI] [PubMed] [Google Scholar]
- 30. Green BG. Chemesthesis: pungency as a component of flavor. Trends in Food Science and Technology. 1996;7(12):415–420. [Google Scholar]
- 31. Barbeau AM, Burda J, Siegel M. Perceived efficacy of e-cigarettes versus nicotine replacement therapy among successful e-cigarette users: a qualitative approach. Addict Sci Clin Pract. 2013;8(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pokhrel P, Herzog TA, Muranaka N, Fagan P. Young adult e-cigarette users’ reasons for liking and not liking e-cigarettes: a qualitative study. Psychol Health. 2015;30(12):1450–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Etter JF. Throat hit in users of the electronic cigarette: an exploratory study. Psychol Addict Behav. 2016;30(1):93–100. [DOI] [PubMed] [Google Scholar]
- 34. Goldenson NI, Kirkpatrick MG, Barrington-Trimis JL, et al. Effects of sweet flavorings and nicotine on the appeal and sensory properties of e-cigarettes among young adult vapers: application of a novel methodology. Drug Alcohol Depend. 2016;168:176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li Q, Zhan Y, Wang L, Leischow SJ, Zeng DD. Analysis of symptoms and their potential associations with e-liquids’ components: a social media study. BMC Public Health. 2016;16:674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oliveira-Maia AJ, Stapleton-Kotloski JR, Lyall V, et al. Nicotine activates TRPM5-dependent and independent taste pathways. Proc Natl Acad Sci U S A. 2009;106(5):1596–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Behrens M, Meyerhof W. Bitter taste receptor research comes of age: from characterization to modulation of TAS2Rs. Semin Cell Dev Biol. 2013;24(3):215–221. [DOI] [PubMed] [Google Scholar]
- 38. Breslin PAS. An evolutionary perspective on food and human taste. Curr Biol. 2013;23(9):R409–R418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wayne GF, Connolly GN. How cigarette design can affect youth initiation into smoking: camel cigarettes 1983–93. Tob Control. 2002;11(suppl 1):i32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Buck LB. Olfactory receptors and odor coding in mammals. Nutr Rev. 2004;62(suppl 3):S184–S188. [DOI] [PubMed] [Google Scholar]
- 41. Bautista DM, Siemens J, Glazer JM, et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448(7150):204–208. [DOI] [PubMed] [Google Scholar]
- 42. Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci USA. 2002;99(7):4692–4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Audrain-McGovern J, Strasser AA, Wileyto EP. The impact of flavoring on the rewarding and reinforcing value of e-cigarettes with nicotine among young adult smokers. Drug Alcohol Depend. 2016;166:263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Miao S, Beach ES, Sommer TJ, Zimmerman JB, Jordt SE. High-Intensity sweeteners in alternative tobacco products. Nicotine Tob Res. 2016;18(11):2169–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cain WS, Murphy CL. Interaction between chemoreceptive modalities of odour and irritation. Nature. 1980;284(5753):255–257. [DOI] [PubMed] [Google Scholar]
- 46. Kosmider L, Sobczak A, Prokopowicz A, et al. Cherry-flavoured electronic cigarettes expose users to the inhalation irritant, benzaldehyde. Thorax. 2016;71(4):376–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Goniewicz ML, Kuma T, Gawron M, Knysak J, Kosmider L. Nicotine levels in electronic cigarettes. Nicotine Tob Res. 2013;15(1):158–166. [DOI] [PubMed] [Google Scholar]
- 48. Woelfel K, Hartman TG. Mass spectrometry of the acetal derivatives of selected generally recognized as safe listed aldehydes with ethanol, 1,2-propylene glycol and glycerol. In: Flavor Analysis. Vol 705 ACS Symposium Series. Washington, DC: American Chemical Society; 1998:193–210. [Google Scholar]
- 49. Moskowitz HR. Ratio scales of sugar sweetness. Perception and Psychophysics. 1970;7(5):315–320. [Google Scholar]
- 50. Callahan-Lyon P. Electronic cigarettes: human health effects. Tob Control. 2014;23(suppl 2):ii36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kienhuis AS, Soeteman-Hernandez LG, Bos PM, Cremers HW, Klerx WN, Talhout R. Potential harmful health effects of inhaling nicotine-free shisha-pen vapor: a chemical risk assessment of the main components propylene glycol and glycerol. Tob Induc Dis. 2015;13(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]