Abstract
Introduction
Electronic nicotine delivery systems (ENDS) vary on a wide range of characteristics that may affect reinforcement value and use. One characteristic is the ratio of two solvents commonly used in most e-liquids: propylene glycol (PG) and vegetable glycerin (VG). The goal of this study was to understand how PG/VG ratio affects subjective effects, reinforcement value, and tobacco use patterns among current smokers who try using ENDS.
Aims and Methods
Current smokers with minimal ENDS use history (n = 30) sampled, in a double-blind fashion, three different e-liquids that varied in PG/VG ratio (70/30, 50/50, 0/100) while holding constant other aspects of the e-liquid and ENDS. Participants tried each e-liquid before rating the subjective effects on a modified version of the Cigarette Evaluation Questionnaire. Reinforcement value was assessed using a preference task where participants chose between the three e-liquids. The impact of each e-liquid on cigarette reinforcement was assessed using a modified version of the Cigarette Purchase Task. Participants were randomly assigned to receive one e-liquid to take home for 1 week.
Results
PG/VG ratio had minimal impact on most of the tested outcomes. Participants rated the highest PG concentration as having a stronger “throat hit” than the other two. There was no significant difference between the number of participants who preferred each of the PG/VG ratios in the preference assessment. PG/VG ratio did not affect cigarette or ENDS use during the sampling week.
Conclusions
These data suggest that PG/VG ratio has minimal impact on subjective effects and reinforcement value in ENDS naive current smokers.
Implications
These data suggest that PG/VG ratio, within the range that is commonly used, has minimal impact on subjective effects, reinforcement value, or uptake in current smokers with minimal ENDS experience.
Introduction
Electronic nicotine delivery system (ENDS) use now surpasses the use of any other noncigarette tobacco product in the United States.1 Most ENDS users are current or former cigarette smokers, and the most common reason among smokers for trying ENDS is to quit or reduce their smoking.2 However, although many smokers have successfully adopted ENDS, a significant proportion have also tried and abandoned them.2,3 Additionally, a large proportion of those using ENDS are still unable to stop smoking completely (i.e., dual use).2,3 ENDS vary widely on a number of characteristics including type of device (disposable vs. tank vs. pod-mod), battery power, wattage, resistance, nicotine content, flavoring, and other constituents. Although the impact of these characteristics is complicated, we know that they can impact nicotine delivery and subjective effects4,5 (i.e., ratings of product liking, satisfaction). Nicotine delivery and subjective effects are likely to influence the subjective value of an ENDS product (i.e., reinforcement value), and thus by extension whether smokers are able to successfully transition from cigarettes to ENDS. Indeed, users of ENDS cite these device characteristics as contributing to their choice to use ENDS and to their choice of product.6,7 Because ENDS probably carry a reduced health burden in comparison to traditional cigarettes,8,9 it is important that we understand how various ENDS characteristics contribute to reinforcement value and use among current smokers who try using them. Furthermore, ENDS are regulated by the U.S. Food and Drug Administration (FDA), and the FDA could require changes to ENDS product characteristics if doing so would be likely to improve public health.
At a minimum, ENDS e-liquid is comprised of propylene glycol (PG), vegetable glycerin (VG), nicotine, and flavoring constituents.4,10 One characteristic that varies across e-liquids is the ratio of PG and VG that comprise the e-liquid.11 The ratio of these two constituents (PG/VG) is considered by both ENDS manufacturers and users to be an important determinant of the sensory characteristics of using ENDS. Vaping blogs often describe PG as contributing to the “throat hit” associated with vaping, whereas VG contributes to a smoother flavor and cloud production.12 Despite the perceived importance of this ratio among the vaping community, there is little existing research on how the ratio of PG to VG affects subjective effects, reinforcement value, or use. To date, we are aware of only one published report that investigated the impact of PG/VG ratio on nicotine delivery and subjective effects in 30 experienced ENDS users, holding all other device features constant.13 This study found that at high PG concentrations (PG/VG ratio: 100/0, 55/45), nicotine delivery was increased, and at the highest PG concentration, participants found the e-liquid to be less pleasant and less satisfying. However, to our knowledge, no study has assessed the impact of PG/VG ratio in current smokers who are naive to ENDS, nor has any study assessed the impact of PG/VG ratio on reinforcement value or use.
This study investigates how different PG/VG ratios affect the sensory characteristics and reinforcement value of e-liquid in current smokers who are naive to ENDS. Adult smokers with minimal ENDS experience sampled three tobacco-flavored e-liquids with different PG/VG ratios (70/30, 50/50, 0/100) and evaluated their subjective effects. To understand the impact of PG/VG ratio on reinforcement value, participants completed a preference assessment where they chose between the three e-liquids across a series of trials. To test the impact of PG/VG ratio on uptake and downstream changes in smoking, participants were then randomly assigned to take home one of the e-liquids to use for a 1-week sampling period ad libitum and asked to complete daily electronic diaries to assess their cigarette and ENDS use throughout the sampling period. This study was approved by the Medical University of South Carolina Institutional Review Board.
Methods
Participants
Non-menthol daily cigarette smokers (N = 30), naive to ENDS, were recruited from the local area via advertising on craigslist and on social media. Inclusion and exclusion criteria were designed to enroll smokers with limited ENDS experience. To reduce variability given the small sample size, we opted to only offer one e-liquid flavor (classic tobacco), and only non-menthol cigarette smokers were recruited to increase the likelihood of uptake. Inclusion criteria included (1) adults age ≥18 who have been smoking at least five cigarettes daily for the past year (expired carbon monoxide (CO) > 8), (2) usual brand is non-menthol, (3) use of ENDS on five or fewer lifetime occasions, and (4) regular use of e-mail or smartphone ownership with capacity to receive SMS text and internet access (necessary for electronic diaries). Exclusionary criteria included (1) unwilling to use ENDS as part of the trial, (2) use of smokeless, hookah, or tobacco products other than cigarettes ≥ 10 days in the past 30 days, (3) pregnant, trying to become pregnant, or breastfeeding, (4) recent history of cardiovascular distress in the last 3 months (arrhythmia, heart attack, stroke, uncontrolled hypertension), (5) current use of cessation medications, and (6) another household member currently enrolled in the study (to prevent contamination of e-liquid assignment during sampling).
Procedures
Study participation consisted of three visits to the lab and electronic daily diaries assessing tobacco use completed at home throughout the study. Potential participants were screened on the phone for initial eligibility. Those meeting criteria were invited for in-person screening and consented. Eligible participants completed baseline questionnaires, and daily electronic diaries were initiated to assess daily cigarette and ENDS use during the study. Participants were instructed to smoke their own cigarettes as normal for a 1-week baseline period and complete the diary entry each day.
One week after the screening session, participants attended a lab session in which they sampled a usual brand cigarette (provided by the participant) and the three e-liquids (four puffs each, 30-second interpuff interval, 15-minute interproduct interval) and completed questionnaires about each one. Only four puffs were required to prevent satiation. Usual brand was sampled at the outset of this lab session to standardize time since last cigarette and provides a within-subject comparison to traditional cigarettes on questionnaires. Participants were shown how to operate the ENDS, and the order of e-liquid sampling was randomized. After sampling all e-liquids, participants completed a lab-based preference assessment to assess reinforcement value (described below). At the conclusion of the lab visit, participants were randomized and assigned to take home one of the three e-liquids to use at home for a 1-week sampling period (10 participants/ratio). We provided 10 cartomizers for the 1-week sampling period to ensure that participants did not run out if they chose to exclusively use the ENDS during that period. Participants were instructed to use the assigned ENDS as much as they would like over the week and to continue completing the daily diaries. There were no instructions to reduce or alter combustible smoking.
Following the end of the sampling week, participants returned to the lab to return any unused product. Participants could earn up to $150 for completing all visits and up to an additional $50 for completing all diary entries.
ENDS and E-liquid
All aspects of the ENDS device and e-liquid were held constant with the exception of PG/VG ratio. We utilized an ego-T 1100 mAh battery and disposable cartomizers (510 Smoketech, 1.5-Ω dual coil), a product that has been well characterized.14 The device has an LCD screen with a puff counter which resets when the device is charged. During the take-home week, participants were told (1) to use this puff counter to aid in reporting the number of ENDS puffs per day on the daily diaries and (2) to charge their device at night to reset the puff counter each day.
E-liquid was tobacco flavored (Classic Tobacco, American E-liquid) and contained 18 mg nicotine/ml, a moderate level of nicotine, which delivers nicotine levels comparable to combustible cigarettes in ENDS naive smokers using this same device.15 Three PG/VG ratios were used: 70/30, 50/50, and 0/100. These ratios were chosen to span the range of PG/VG ratios that are common among commercially available e-liquids. These ratios refer to the commercially advertised ratio of the base e-liquid, prior to adding flavoring and nicotine. The final ratios reported as net weight percentage are as follows: 70.8/22.8, 56.8/36.9, and 24.8/69.2, respectively (private communication, American E-liquids). Percentages do not add to 100 because remainder consists of nicotine and flavoring constituents. To confirm the nicotine concentration and PG/VG ratios of the e-liquids, samples were independently analyzed using gas chromatography with mass spectrometry by NicoTar lab (Roswell Park Cancer Institute, PI: Goniewicz). Results of these analyses were within 10% of expected values. PG/VG ratio was blinded from participant and staff members who conducted experimental sessions. Cartomizers were loaded with 1 ml of e-liquid by staff with no participant contact prior to being distributed to participants.
Measures
Subjective Effects
After sampling each product, participants completed the Product Evaluation Scale,16 a modified version of the Cigarette Evaluation Questionnaire,17 which asks participants to rate each product’s subjective effects on a 1–7 Likert scale from “not at all” to “extremely.” Questions were modified for ENDS. Five subscales were created: Satisfaction, Psychological Reward, Craving Relief, Enjoyment of Respiratory Tract Sensations, and Aversion.17 Two additional questions were added that asked participants how strong the throat hit was and how much vapor (smoke) the e-cigarette (cigarette) produced, based on the hypothesis that PG/VG ratio would affect these sensory effects specifically.
Reinforcement Value
To assess the impact of PG/VG ratio on reinforcement value, participants completed a preference task at Visit 2 in which they chose between the three e-liquids across a series of trials. Discrete-choice trials and preference tasks are common assessments of reinforcement value in drug self-administration laboratory research.18,19 During each trial, participants could choose between taking two puffs of any one e-liquid or abstaining from all e-liquids. Each trial lasted two minutes, and participants completed 15 trials. The primary outcome was the e-liquid preferred most often, both ignoring and including trials in which participants chose to abstain.
Any benefits to public health from ENDS use are likely to come from a reduction in the rate and prevalence of cigarette smoking.9 Thus, it is important to understand the impact of ENDS characteristics on the relative reinforcement of cigarettes. To assess the impact of PG/VG ratio on the relative reinforcement value of cigarettes, participants also completed a modified version of the Cigarette Purchase Task20 after sampling each e-liquid. In this version, participants were asked to estimate how many cigarettes they would smoke at a variety of prices (FREE, $0.02, $0.05, $0.10, $0.20, $0.30, $0.40, $0.50, $0.60, $0.70, $0.80, $0.90, $1.00, $2.00, $3.00, $4.00, $5.00). After sampling their usual brand cigarette, participants were told to complete the task after imagining that their usual brand was the only nicotine or tobacco product available. After sampling each e-liquid, participants were told to complete the task after imagining that they also had access to the e-liquid they just sampled. This modification assessed the impact of each e-liquid on demand for usual brand cigarette smoking. Five demand parameters were of interest: (1) intensity, the number of cigarettes participants said they would smoke if cigarettes were free, (2) breakpoint, the lowest price to suppress cigarette consumption to zero, (3) Omax, the maximum amount of money participants report they would spend on cigarettes in a single day, (4) Pmax, the price that produces Omax, and (5) α, elasticity of demand for cigarettes. All outcomes except α were obtained empirically from the data. To calculate α, each person’s responses were fit to an exponential function: Q = Q0 × 10k(e−αQ0C−1),21 in which Q was cigarettes smoked per day at each cost (C), Q0 was the estimated number of cigarettes smoked per day when cigarettes were free, k is a constant set to the logarithmic range of the dependent variable, and α is estimated elasticity of demand.
Changes in Cigarette Smoking and Uptake of ENDS
Each day, participants completed an electronic diary about their tobacco use the previous day. Outcomes included the number of cigarettes smoked each day during the baseline week and during the ENDS sampling week, and the average number of ENDS puffs taken during the sampling week. The number of ENDS puffs was chosen as the outcome of interest rather than puffing episodes because the ENDS puff counter could be used by participants to increase accuracy. Participants provided a CO sample at each visit.
Statistical Analysis
For questionnaires completed after sampling each product (the five Product Evaluation Scale subscales, two items assessing throat hit and vapor production, demand parameters), a repeated-measures analysis of variance (ANOVA) was used. First, to test the impact of e-liquids compared with usual brand, a repeated-measures ANOVA was conducted with all four products (e-liquids and usual brand), and in the case of significant omnibus tests, follow-up t-tests compared each PG/VG ratio with usual brand. No adjustment was made for multiple comparisons because they were only conducted in the case of a significant omnibus test. Second, to test the impact of PG/VG ratio between e-liquids, a repeated-measures ANOVA was conducted with only the three e-liquids, and in the case of a significant omnibus test, follow-up t-test compared each of the three PG/VG ratios to each other. Thus, the PG/VG ratios were only compared with each other using pairwise tests when there was a significant omnibus test that included only the three e-liquids.
For the preference test, a chi-square goodness-of-fit test was used to assess whether there were differences between the proportion of participants that preferred each of the three e-liquids, conducted twice: both ignoring and including the option to abstain. For both tests, participants who preferred more than one option (i.e., ties) were excluded, but sensitivity tests were conducted in which those participants were divided equally between their highest chosen categories, and results were consistent with those reported here.
To test the impact of ENDS sampling and PG/VG ratio on cigarette smoking, a 2 × 3 mixed ANOVA model was conducted testing the impact of sampling (average cigarettes per day [CPD] during baseline week, average CPD during sampling week) and PG/VG ratio (70/30, 50/50, 0/100). A one-way between-subject ANOVA also tested whether there were differences in average number of ENDS puffs per day.
Results
Participant Demographics
Thirty-one participants were eligible and consented. One participant withdrew for personal reasons prior to the sampling visit and randomization, and thus the final analyzed sample consists of 30 participants. Participants were mostly white (80% white, 10% black, 10% another race; 13% identify as Hispanic), 70% male, with an average age of 43.7 (SD = 12.4). Participants self-reported an average of 18.5 CPD at baseline (SD = 7.3), and the average FTND score was 5.4 (SD = 1.7). Participants had used an e-cigarette an average of 1.6 times in their life, and no one reported use in the last 30 days.
Subjective Effects
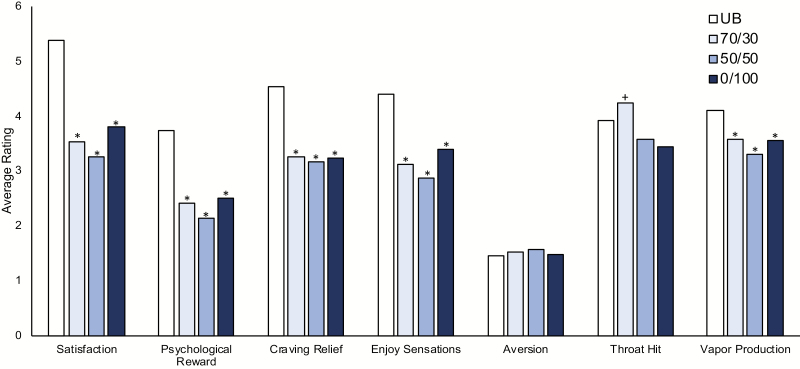
In general, e-liquids were rated as having less satisfying subjective effects than participants’ usual brand of cigarettes. As shown in Figure 1, PG/VG ratio had very little impact on subjective ratings. In the omnibus test that included usual brand and all three e-liquids, there was a significant effect of product on the Satisfaction, Psychological Reward, Craving Relief, and Enjoyment of Respiratory Tract Sensations subscales (Fs(3,87) = 16.8; 25.2; 8.3; 7.6, ps < .01). In follow-up tests for these scales, all e-liquids were rated lower than participants’ usual brand cigarette (ps < .01). There was no significant effect on the aversion subscale (p > .05). In the omnibus test that only included the three e-liquids, there was no significant effect of product on any of the five subscales (Satisfaction, Psychological Reward, Craving Relief, Enjoyment of Respiratory Tract Sensations, Aversion: Fs(2,58) = 1.5; 2.6; 0.1, 1.2; 0.6; ps > .05).
Figure 1.
The impact of product and propylene glycol (PG)/vegetable glycerin (VG) ratio on subjective effects. Average product ratings on five subscales, throat hit, and vapor production after sampling each product. Significant pairwise difference from usual brand indicated by “*”. Significant difference from two other PG/VG ratios indicated by “+”.
When usual brand and all three e-liquids were included in the omnibus test, there was a significant effect of product on the single item assessing “throat hit” (F(3,84) = 2.7, p < .05). However, this effect was not driven by differences between usual brand and the e-liquids (pairwise t-test, ps > .05). When only the three e-liquids were included, the significant effect of product on “throat hit” persisted (F(2,56) = 3.5, p < .05). In follow-up tests comparing the PG/VG ratios to each other, the 70/30 PG/VG ratio was rated as having significantly better “throat hit” than both the 50/50 and the 0/100 PG/VG ratios (ps < .05), but the 50/50 and the 0/100 were not rated as significantly different from each other (p > .05). When usual brand and all three e-liquids were included in the omnibus test, there was a significant main effect of product type on vapor/smoke production (F(3,84) = 6.0, p < .01), and all three e-liquids were rated as providing less pleasing vapor/smoke production than the usual brand cigarette (ps < .01). When only the three e-liquids were included, there was no significant main effect of product type on vapor production (p > .05).
Cigarette and ENDS Reinforcement Value
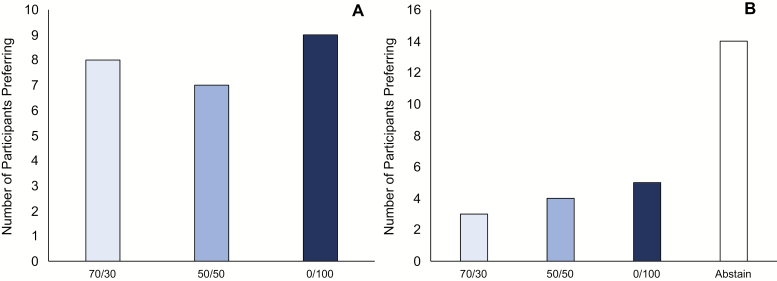
As shown in Figures 2, A and B, e-liquid reinforcement value as measured using a preference task was unaffected by PG/VG ratio. Average number of choices for the 70/30, 50/50, 0/100, and abstaining options was 2.9, 2.7, 4.2, and 5.3 choices. When participants were categorized into their most preferred e-liquid (ignoring the option to abstain), there was no significant difference between the distribution of participants’ preferred options (p > .05). When the choice to abstain was included as an option, there were significant differences between the distribution of participants’ preferred options (p < .05), indicating that the most popular option was the choice to abstain.
Figure 2.
Impact of propylene glycol (PG)/vegetable glycerin (VG) ratio on e-liquid preference. Number of participants preferring each of the available options during preference task when excluding (A) and including (B) the option to abstain. Ties are excluded (A = 4; B = 6).
Demand for usual brand cigarettes, as measured using a modified version of the Cigarette Purchase Task, was unaffected by having access to e-liquid or by PG/VG ratio. There were no significant differences between demand parameters (intensity, Omax, Pmax, breakpoint, α; ps > .05) for either the omnibus test that included all four product conditions (F(3,87) = intensity: 2.7; Omax: 1.0; Pmax: 0.8; breakpoint: 0.5; α: 2.4; ps > .05) or the omnibus test that only included the three e-liquids (F(2,58) = intensity: 0.2; Omax: 0.45; Pmax: 1.1; breakpoint: 0.1; α: 1.7; ps > .05).
Cigarette Smoking and ENDS Uptake
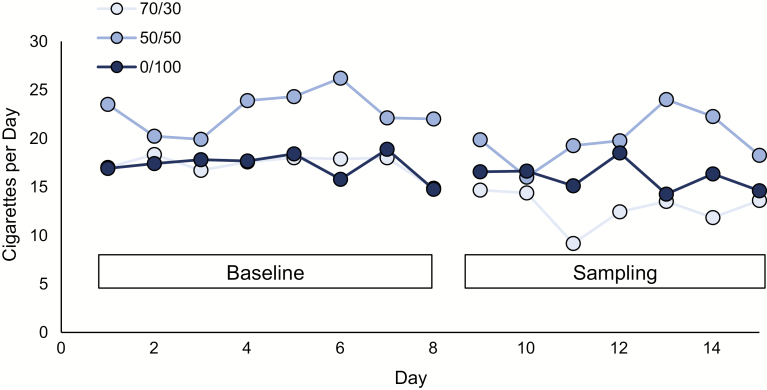
There was a significant reduction in average CPD between weeks for all groups (F(1, 25) = 12.2, p < .01), but there was no main effect of PG/VG ratio nor interaction between e-liquid assignment and week (ps > .05) (Figure 3). CPD reduced 23%, 17%, and 12% in the 70/30, 50/50, and 0/100 groups, respectively. Similarly, there was a significant reduction in average CO between Visit 2 (end of baseline period) and Visit 3 (end of sampling) (F(1, 25) = 8.5, p < .01), but there was no main effect of PG/VG ratio nor interaction between PG/VG ratio and sampling week (ps > .05). CO decreased 20%, 18%, and 22% in the 70/30, 50/50, and 0/100 groups, respectively. The average number of ENDS puffs taken per day during the sampling week was not affected by PG/VG ratio: 67.0 in the 70/30 group (SD = 55.6), 68.8 in the 50/50 group (SD = 25.5), and 63.1 in the 0/100 group (SD = 60.9) (ns, p > .05).
Figure 3.
The impact of e-liquid sampling and propylene glycol (PG)/vegetable glycerin (VG) ratio on cigarette smoking behavior. Average cigarettes smoked per day in each of the PG/VG ratio e-liquid groups during baseline naturalistic week (Days 1–8) and electronic nicotine delivery system (ENDS) sampling (Days 9–15). For participants who had more than 8 days included in baseline or 7 days included in the sampling week, additional days are not shown. Participants received the ENDS during their sampling visit on Day 9.
Discussion
This study investigated the impact of ENDS e-liquid PG/VG ratio on subjective effects, reinforcement value, and tobacco use patterns in current smokers without a significant history of prior ENDS use. PG/VG ratio had minimal impact on any of the tested outcomes. Participants rated all e-liquids as less satisfying than their usual brand of cigarettes, but there were no differences between PG/VG ratios on any of the product evaluation subscales. The only exception was that participants rated the highest PG concentration (70/30) as having a stronger “throat hit” compared with the other PG/VG ratios we tested. This is consistent with online materials citing PG as contributing to a satisfying throat hit22 as well as the one prior report published on PG/VG ratio.13 A relatively equal number of participants preferred each of the e-liquids during the preference assessment, and approximately 20% of participants preferred at least two of the options equally. Use of an e-liquid at home reduced self-reported CPD and CO across groups consistent with other studies, but these results were consistent across the assigned PG/VG ratios. Taken together, these data suggest that PG/VG ratios in this range have minimal impact on ratings of subjective effects and reinforcement value of e-liquids and little effect on cigarette use patterns, although we would acknowledge that a larger study is probably needed to reliably assess the impact on smoking behaviors.
Our findings are in agreement with the one published report investigating the impact of PG/VG ratio in experienced ENDS users, in which increased PG concentrations increased perceived “throat hit,” and the highest PG concentration (100/0) was rated as more aversive than other ratios, but within the range investigated in this study, satisfaction was generally similar across ratios.13 This prior study also found that increasing the PG concentration of e-liquid increased nicotine delivery, which contribute to the stronger throat hit delivered at these concentrations. Very high PG concentrations are not commonly available commercially, which may be reflective of aversive subjective effects produced by strong throat hits for high PG concentrations.
Our findings also raise questions about the validity of information provided by commercial e-liquid vendors and by many vaping blogs, which seem to suggest that PG/VG ratios are an important determinant of subjective effects and user preference.22 For example, there is a common perception that increased VG ratios produce a larger vape cloud, but in the present study, participants did not rate the cloud production of the PG/VG ratios differently. Taken together, the published research suggests PG/VG ratio has relatively little impact on subjective ratings of e-liquids, despite what vendors and individuals in the vape community have asserted.
This study has several limitations. First, although these data suggest that PG/VG ratio has little impact with this device and e-liquid, other characteristics may moderate the impact of PG/VG ratio. The device utilized here was a pen-style ego device, highly popular at one time, but now relatively obscure. PG/VG ratio may be a more important characteristic in more modern devices (e.g., pod-mods) or for other flavors, other nicotine concentrations, other device wattages, etc. Second, the study utilized current smokers with minimal ENDS experience, and there was no requirement that participants be interested in switching to e-cigarettes or quitting smoking. PG/VG ratio may be a more important characteristic after extended use, or when using e-cigarettes exclusively. For example, extended experience with vaping may produce a different vaping topography that increases the impact of PG/VG ratio on cloud production. The impact of PG/VG ratio on tobacco use may have also been exaggerated if participants had been encouraged or required to abstain from smoking. However, the prior lab study investigating the impact of PG/VG ratio utilized a population of experienced ENDS users and also found that within the range tested here, PG/VG ratio did not affect subjective effects.13 Third, the most popular choice during the preference assessment was abstaining from using, which may be a reflection of task parameters (e.g., too many trials, no overnight abstinence required, short intertrial interval, limited sampling experience with each product). However, when all e-liquid options are combined, participants chose to use an e-liquid on more trials than they chose to abstain (9.8 trials vs. 5.2 trials). Future studies may consider adjusting the task parameters to encourage use during the preference assessment. Fourth, the results from the at-home sampling period must be thought of as exploratory. This portion of the experiment utilized a between-subject design and a small sample size per group (n = 10 per group). The sampling week was also only 1 week long, which may not be long enough to allow differences between PG/VG ratios to emerge, especially in naive users. Finally, the present study did not measure nicotine delivery or blood nicotine levels. Given the importance of nicotine delivery on reinforcement and tobacco use, a direct measure of nicotine delivery would have strengthened the design of the study.
The FDA has the authority to set product standards for tobacco products, including ENDS, if appropriate for the protection of public health. The FDA could consider regulating ENDS product characteristics in a way that would encourage switching from cigarettes to ENDS among adult current smokers. However, the data from this trial suggest that regulation surrounding PG/VG ratio is unlikely to be necessary, given that this characteristic does not affect reinforcement value, at least under these conditions in this population. Future studies may investigate the impact of PG/VG ratio in a larger population with more varied ENDS experience and with a variety of ENDS and e-liquid characteristics.
Funding
Funding for this project was provided by pilot funding from the National Cancer Institute (P01CA200512 to K.M.C.). Salary support provided by the National Institute on Drug Abuse (K12DA031794 to T.T.S., K23DA041616 to B.W.H.).
Declaration of Interests
M.J.C. has received consulting honoraria from Pfizer. K.M.C. has received payment as a consultant to Pfizer, Inc., for service on an external advisory panel to assess ways to improve smoking cessation delivery in health care settings. He also has served as paid expert witness in litigation filed against the tobacco industry.
Acknowledgments
The authors Dr. Maciej Goniewicz and his team at the NicoTar lab for analysis of PG/VG and nicotine content. Thank you to the staff who conducted the experimental sessions including Noelle Natale and Lisa Coles.
References
- 1. Hu SS, Neff L, Agaku IT, et al. Tobacco product use among adults – United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2016;65(27):685–691. [DOI] [PubMed] [Google Scholar]
- 2. Action on Smoking or Health (ASH). Use of e-cigarettes (vapourisers) among adults in Great Britain, 2018; https://ash.org.uk/download/ash-use-of-e-cigarettes-by-adults-in-great-britain-2018-pdf/. Accessed August 21, 2019. [Google Scholar]
- 3. Coleman B, Rostron B, Johnson SE, et al. Transitions in electronic cigarette use among adults in the Population Assessment of Tobacco and Health (PATH) Study, Waves 1 and 2 (2013–2015). Tob Control. 2018;(1):50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. DeVito EE, Krishnan-Sarin S. E-cigarettes: impact of e-liquid components and device characteristics on nicotine exposure. Curr Neuropharmacol. 2018;16(4):438–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wagener TL, Floyd EL, Stepanov I, et al. Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tob Control. 2017;26(e1):e23–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simmons VN, Quinn GP, Harrell PT, et al. E-cigarette use in adults: a qualitative study of users’ perceptions and future use intentions. Addict Res Theory. 2016;24(4):313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bono RS, Barnes AJ, Lester RC, Cobb CO. Effects of electronic cigarette liquid flavors and modified risk messages on perceptions and subjective effects of e-cigarettes. Health Educ Behav. 2019;46(2):197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nutt DJ, Phillips LD, Balfour D, et al. E-cigarettes are less harmful than smoking. Lancet. 2016;387(10024):1160–1162. [DOI] [PubMed] [Google Scholar]
- 9. National Academy of Sciences E, and Medicine. Public Health Consequences of E-cigarettes. Washington, DC: The National Academies Press; 2018. [PubMed] [Google Scholar]
- 10. Beauval N, Antherieu S, Soyez M, et al. Chemical evaluation of electronic cigarettes: multicomponent analysis of liquid refills and their corresponding aerosols. J Anal Toxicol. 2017;41(8):670–678. [DOI] [PubMed] [Google Scholar]
- 11. Peace MR, Baird TR, Smith N, Wolf CE, Poklis JL, Poklis A. Concentration of nicotine and glycols in 27 electronic cigarette formulations. J Anal Toxicol. 2016;40(6):403–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jones I. PG vs VG 2016; http://vaping360.com/pg-vs-vg-what-is-the-difference-and-what-should-i-use/. Accessed May 22, 2017.
- 13. Spindle TR, Talih S, Hiler MM, et al. Effects of electronic cigarette liquid solvents propylene glycol and vegetable glycerin on user nicotine delivery, heart rate, subjective effects, and puff topography. Drug Alcohol Depend. 2018;188:193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hiler M, Breland A, Spindle T, et al. Electronic cigarette user plasma nicotine concentration, puff topography, heart rate, and subjective effects: influence of liquid nicotine concentration and user experience. Exp Clin Psychopharmacol. 2017;25(5):380–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lopez AA, Hiler MM, Soule EK, et al. Effects of electronic cigarette liquid nicotine concentration on plasma nicotine and puff topography in tobacco cigarette smokers: a preliminary report. Nicotine Tob Res. 2016;18(5):720–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hatsukami DK, Zhang Y, O’Connor RJ, Severson HH. Subjective responses to oral tobacco products: scale validation. Nicotine Tob Res. 2013;15(7):1259–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG. Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire. Addict Behav. 2007;32(5):912–923. [DOI] [PubMed] [Google Scholar]
- 18. Cassidy RN, Tidey JW, Kahler CW, Wray TB, Colby SM. Increasing the value of an alternative monetary reinforcer reduces cigarette choice in adolescents. Nicotine Tob Res. 2015;17(12):1449–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stoops WW, Poole MM, Vansickel AR, Rush CR. Influence of escalating alternative reinforcer values on cigarette choice. Behav Processes. 2011;87(3):302–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jacobs EA, Bickel WK. Modeling drug consumption in the clinic using simulation procedures: demand for heroin and cigarettes in opioid-dependent outpatients. Exp Clin Psychopharmacol. 1999;7(4):412–426. [DOI] [PubMed] [Google Scholar]
- 21. Koffarnus MN, Franck CT, Stein JS, Bickel WK. A modified exponential behavioral economic demand model to better describe consumption data. Exp Clin Psychopharmacol. 2015;23(6):504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kershaw O. All about Throat Hit! Vaping Guides; 2016. http://vaping.com/blog/guides/throat-hit. Accessed December 19, 2017.