Abstract
Introduction
The ability to reliably measure real-world vaping behavior is critical to understand exposures to potential toxins. Commercially available mobile topography devices were originally designed to measure cigarette puffing behavior. Information regarding how applicable these devices are to the measurement of electronic cigarette (e-cigarette) vaping topography is needed.
Methods
Clinical Research Support System (CReSS; Pocket) and Smoking Puff Analyzer Mobile (SPA-M) topography devices were tested against the calibrated laboratory-based smoking puff analyzer duplicator (SPA-D) device combined with an analytical smoking machine that generates programmable puffs with high precision. Puff topography of e-cigarettes was measured over a range of puff volumes (10–130 mL) at 2 and 5 s puff durations (using bell- and square-shaped puffs). “Real-world” topography data collected from 10 participants during 1 week of at-home vaping were also analyzed. Recording anomalies and limitations of the devices, such as accuracy of detection of the puff end, flow rate dropouts, unreported puffs, and abandoned vaping sessions for the CReSS, and multi-peak puffs for the SPA-M were defined.
Results
The accuracy of puff volumes and durations was determined for both devices. The error for SPA-M was generally within ±10%, whereas that for the CReSS varied more widely. The CReSS consistently underestimated puff duration at higher flow rates.
Conclusions
CReSS and SPA-M topography devices can be used for real-world e-cigarette topography measurements, but researchers have to be aware of the limitations. Both devices can provide accurate measurements only under certain puff parameter ranges. The SPA-M provided more accurate measurements under a wider range of puffing parameters than the CReSS. Summary data reported by both devices require thorough analysis of the raw data to avoid misleading data interpretation.
Implications
Results of this study provide researchers with valuable information about the capability of commercially available cigarette topography devices to measure real-world vaping behaviors. The differing measurement ranges of the two devices and puff recording limitations and anomalies should be taken into account during analysis and interpretation of real-world data.
Introduction
The use of electronic (e-) cigarettes is on the rise in the United States. More than 10% of all adults and 20% of young adults aged 18–24 years have tried e-cigarettes; approximately 25% of them report consistent use.1 In addition, approximately 5% of middle school and 16% of high school students report vaping e-cigarettes.2
Users of e-cigarettes may be exposed to a variety of toxic and/or carcinogenic compounds during vaping, although the implications and level of exposure to these compounds in e-cigarette aerosol and vapor are not well understood. E-cigarettes deliver nicotine,3 aldehydes,4–10 glycidol,11 enols,12 benzene,13 and metals,14,15 both in vapor and aerosol phases.16–20 Many of these compounds are classified as harmful and potentially harmful constituents of tobacco smoke.21 E-cigarette emissions also contain free radicals, which can adversely affect the respiratory function or cardiac epithelium.22 Although some reports state that levels of the toxins generated by e-cigarettes are below levels generated from combustible cigarettes,23 the limited toxicology data on e-cigarettes are insufficient to allow a comprehensive toxicological evaluation of this relatively new type of tobacco product.24 Nevertheless, the perception of reduced harm persists,25 necessitating a strong science base to better understand the health impact of e-cigarette use.
Understanding vaping behavior (puffing topography) is a critical step in evaluating the potential toxic emissions and human exposures from e-cigarettes. The importance of smoking puffing behavior has been established through several studies of combustible cigarettes dating back to the work of Benowitz et al.26 who first showed that smokers change their behavior in response to changes in product characteristics such as reduced nicotine. Because that time other researchers have established that changes in brand,27 additives such as menthol,28–30 and differing demographics such as gender,29,31 can also alter smoking behavior.
Studies of vaping behavior have relied mostly on laboratory-collected topography data.32–35 However, smoking topography associated with laboratory versus real-world tobacco product use may differ substantially.36 Variations in human smoking behavior can significantly affect exposures and thus accurate topography devices are important tools for informing regulations.37 Limited real-world studies of vaping behavior have been conducted using either devices that were built in-house and not widely available to the research community38 or e-cigarette brands that record puff frequency and puff duration (PD),39 but not puffing flow rate (PFR). Square-shaped vaping puffs were commonly observed as opposed to bell-shaped puffs typical for combustible cigarettes.36,38 Various PDs at different PFRs were seen,32–35,38 confirming that accurate measurement of all the parameters such as PFR, PD, puff volume (PV), inter-puff interval (IPI), and total vaping time are of paramount importance to adequately characterizing exposures from e-cigarette use.
A viable approach to characterizing naturalistic vaping behavior is to use commercially available mobile topography devices, including the CReSS Pocket (Clinical Research Support System, portable version; Borgwaldt-Hauni, herein referred to as CReSS) and SPA-M (Smoking Puff Analyzer, portable version; Sodim, herein referred to as SPA-M). These devices were used to capture combustible cigarette smoking topography in non-laboratory settings; however, limited data are available on the validity of these devices for puffing topography measurements.40 Published data on the CReSS used with cigarettes demonstrate significant limitations of the device such as not identifying puffs below a certain PFR threshold, underreporting PV for lower volumes, and the inaccuracy of PD for the entire tested volume range.40 Although pressure drop, PV and PFR measurements with the CReSS for e-cigarettes were found “acceptable” in one study,34 the range of validation measures evaluated was very limited, and not representative of the range of e-cigarette topography parameters that have been found from vapers.34,41,42 No published data on the accuracy of the SPA-M puff parameters determination, acceptable limits, or level of error are available.
The goal of this study was to comprehensively characterize the utility of the CReSS and SPA-M for capturing e-cigarette puffing topography using a set of laboratory tests to assess the accuracy and limitation range of the main parameters (PV and PD) for both devices. In addition, limited data from an ongoing study of established e-cigarette users were analyzed to provide an understanding of the performance of the CReSS and SPA-M while recording real-world vaping behaviors.
Materials and Methods
Mobile Devices Tested
The study used three CReSS (one purchased in 2013 and two purchased in 2014) and three SPA-M purchased in 2014 (Supplementary Figure 1a and b). Both devices record puffing topography information such as number of puffs, PV, PD, PFR, and IPI. To accommodate the CReSS and SPA-M to test e-cigarettes, special e-cigarette holders were designed for both devices to ensure a leak-free seal to e-cigarettes with varying diameters (Supplementary Figure 2a–f). E-cigarettes were inserted into the Borgwaldt cigarette holders (Supplementary Figure 2a) to make a leak-tight seal with the inner rubber washers. The cigarette holders were connected to the Delrin adaptors (fabricated by Battelle) that fit into the mobile devices. Two types of adaptors were used: one for the CReSS (Supplementary Figure 2b) and one for the SPA-M (Supplementary Figure 2c). The CReSS cigarette inlet has a narrow slit-window for the optical sensor that detects cigarette insertion (Supplementary Figure 2d); therefore, to provide a seal the outer shape of the adaptor has to perfectly match the inner shape of the cigarette inlet. The SPA-M adaptor was easier to manufacture (both type of adaptors could be made in a conventional machine shop).
Before mobile device validation, preliminary limited testing using machine-generated puffs of known PV, PD, and IPI was conducted. Two CReSS devices showed obvious inconsistency with IPI measurements and were sent back to the manufacturer to resolve this issue. SPA-M devices did not show any obvious measurement inconsistencies.
Test Articles, Smoking Machine, and Puff Parameters Used for Laboratory Mobile Devices Testing
Non-refillable fixed-power blu e-cigarette (Classic Tobacco, 0.9%–1.2 % nicotine), refillable power-adjustable Joyetech eVic (4 V, 2.5 Ω), and refillable Smokio (Joyetech eVic and Smokio filled with 50/50 propylene glycol and glycerol mixture) were used to evaluate accuracy of the laboratory puff topography measurements from the CReSS and SPA-M. A standard reference 3R4F cigarette was used to compare mobile device performance between e-cigarettes and combustible tobacco products.
A five-port linear smoking machine (Hawktech FP2000; Tri-City Machine Works, VA) was used to generate the following target conditions: square-shaped puff profile (the flow rate instantaneously grows to a certain value and at the end of the puff rapidly drops down) and bell-shaped puff profile (the flow rate grows and drops following the sine wave) at 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, and 130 mL PV, at 2 and 5 s PD, and 60 s IPI. Cigalike blu e-cigarettes were tested for both bell- and square-shaped profiles whereas Joyetech eVic and Smokio were tested only for square-shaped profiles more relevant to e-cigarette usage. Three replicates were made per each puff topography conditions. The accuracy of the smoking machine was verified using a Soap Bubble Flowmeter (Borgwaldt) and a National Institute of Standards and Technology–calibrated stopwatch (Traceable; Control Company), and was within ±2% for both PV and PD. A third laboratory-based topography device, the SPA-D (Sodim), was calibrated using the smoking machine, and used to determine the error associated with the mobile topography devices. The error in the SPA-D PV and PD, compared to the smoking machine, was within 8% and 5%, respectively. E-cigarettes and 3R4F cigarettes were machine-smoked to collect puff topography data using the CReSS and SPA-M devices. Mobile topography devices were regularly calibrated according to procedures described in the manufacturer’s user manuals.
Participant Processing
To understand the ability of the CReSS and SPA-M to capture human vaping topography outside of a laboratory setting, limited data from an ongoing human subjects study using both devices with a variety of own-brand e-cigarettes were analyzed. Established e-cigarette users (defined as vaping for at least 2 months, every day, eight times or more each day) were recruited to use one of the mobile devices at home each time they vape with their own e-cigarette for 1 week. The topography data from the first 10 participants to use each device were evaluated for operational performance. Devices were randomly assigned to each participant. Five participants overlapped and used both devices. Own-brand e-cigarettes came from multiple manufacturers and ranged from disposable, fixed-power cigalikes, including the blu e-cigarette (27% participants); to refillable, fixed-power devices (33% participants); to refillable and adjustable e-cigarettes (40% participants). The average participant age was 42 ± 16 years; 53% were males and all were white. The analysis was focused on data recording limitations and anomalies pertinent to real-world conditions as described next.
Quantification of CReSS Real-World Recording Data Limitations and Anomalies
A quantitative analysis was performed on the recorded topography from the CReSS to determine the frequency of occurrence for noted recording limitations and anomalies. The following main parameters were under investigation:
1. Incomplete puffs. During the course of the studies, it was confirmed that the CReSS was only able to record a maximum PD of 5 s, as stated in the manufacturer’s user manual. The frequency with which puffs were not completed in the 5 s measurement window was determined for all CReSS-recorded topographies based on the two last recorded flowrates for each puff; if flow rate was increasing at the end of a puff, then puff was considered incomplete.
2. Signal dropouts. The topographies were inspected for the frequency with which the PFR dropped to zero within a puff. Ignoring the first two and last two datapoints (recorded every 20 ms), if the flowrate dropped to zero, the anomaly was counted. Consecutive zeroes were recorded as one instance.
3. Data not recorded. It was observed that after recording approximately 2000 datapoints, CReSS stops recording individual datapoints (this limitation is not described in manual) although still reports a summary of the unrecorded data. The frequency of these occasions was determined.
4. Time limit. The number of abandoned sessions, defined as total session duration exceeds 20 min (default time limit, as specified in the user manual), was assessed.
5. Puff limit. The number of sessions that exceeded 43 puffs, another CReSS limitation (as described in the user manual), was also assessed.
Quantification of SPA-M Real-World Recording Anomalies
Quantitative analysis of puff topography recording anomalies was also performed for the SPA-M. Unlike the CReSS, there are no PD, puff number recording, or total session time limits specified in the user manual. No total number of datapoints recording limit was observed. The following parameters were analyzed:
1) Incomplete puffs. Similar to the CReSS, analysis was conducted if puffs were not completely recorded, as determined by the PFR increasing at the end of a puff.
2) Multiple peaks. Unlike the CReSS where single flowrate dropouts were observed during the puff, the SPA-M topography was found to report multiple peaks being counted as one puff. The frequency with which this occurred in each recorded vaping session was determined for each participant.
Results
Preliminary Laboratory Assessment of the CReSS Devices and IPI Issues
Preliminary laboratory testing of the CReSS devices was conducted using machine sham smoking (3R4F butt was used) with a puffing regimen of 2 s PD, 18 s IPI, and PV of 20, 50, 75, 100, and 150 mL (bell-shaped profile). This initial assessment showed that the two CReSS devices purchased in 2014 had an error associated with IPI data that fell within two ranges: 0%–3% above the true value, and greater than 80% below the true value (the device purchased in 2013 did not show these errors). All three devices were sent to the manufacturer, and after unspecified manufacturer repair, the devices were returned and tested again. The error associated with IPI was greatly reduced and was within 0%–3% above the true value.
Laboratory-Measured PV, PD, and PFR. Accuracy of the Measurements and Validity Limits
The primary goal of this investigation was to assess the accuracy (deviation from the target values) of PV and PD measurements across a PV range (10–130 mL) for puffs with two different PDs (2 and 5 s). Results of the laboratory measurements taken for e-cigarettes and combustible cigarette are summarized in Table 1 and Figure 1 (Supplementary Figures 3–10).
Table 1.
Summary of Puff Volume and Puff Duration Measurements Taken for CReSS and SPA-M
| Cigarette type | Mobile device | Puff shape | Puff duration (s) | Puff volume measurement reliability | Puff duration measurement reliability | ||||
|---|---|---|---|---|---|---|---|---|---|
| Valid puff volume range (mL) | Puff volume accuracy (%)* | Valid puff volume range (mL) | Puff duration accuracy (%)a | ||||||
| Minimum | Maximum | Minimum | Maximum | ||||||
| Blu electronic cigarette | CReSS | Bell | 2 | 20 | 130 | ±15 | 30 | 100 | −8 to −22 |
| 5 | 40 | 130 | ±15 | 60 | 130 | −6 to −21 | |||
| Square | 2 | 20 | 130 | ±10 | 20 | 100 | 0 to −18 | ||
| 5 | 30 | 130 | −16 to +11 | 30 | 130 | 0 to −15 | |||
| SPA-M | Bell | 2 | 10 | 130 | ±10 | 10 | 130 | ±10 | |
| 5 | 20 | 130 | ±10 | 20 | 130 | ±10 | |||
| Square | 2 | 10 | 130 | ±10 | 10 | 130 | ±10 | ||
| 5 | 20 | 130 | ±10 | 10 | 130 | ±10 | |||
| 3R4F cigarette | CReSS | Bell | 2 | 20 | 130 | −10 to +20 | 20 | 90 | −10 to −20 |
| 5 | 40 | 130 | 0 to +20 | 50 | 130 | −5 to −20 | |||
| Square | 2 | 20 | 130 | 0 to +21 | 20 | 100 | 0 to −20 | ||
| 5 | 30 | 130 | −10 to +23 | 30 | 130 | 0 to −20 | |||
| SPA-M | Bell | 2 | 10 | 130 | ±10 | 10 | 130 | ±10 | |
| 5 | 20 | 130 | ±10 | 20 | 130 | ±10 | |||
| Square | 2 | 10 | 130 | ±10 | 10 | 130 | ±10 | ||
| 5 | 20 | 130 | ±10 | 10 | 130 | ±10 | |||
| Joyetech eVic | CReSS | Square | 2 | 20 | 130 | 0 to +23 | 20 | 130 | 0 to −11 |
| 5 | 20 | 130 | −3 to +23 | 20 | 130 | ±10 | |||
| SPA-M | Square | 2 | 20 | 130 | 0 to +15 | 10 | 130 | ±10 | |
| 5 | 20 | 130 | 0 to +22 | 10 | 130 | ±10 | |||
| Smokio | CReSS | Square | 2 | 20 | 130 | 0 to +24 | 20 | 130 | 0 to −12 |
| 5 | 30 | 130 | 0 to +22 | 30 | 130 | ±10 | |||
| SPA-M | Square | 2 | 10 | 130 | −10 to 0 | 10 | 130 | ±10 | |
| 5 | 20 | 130 | ±10 | 10 | 130 | ±10 | |||
aAccuracy = deviation from target. For measures demonstrating consistent variability the error rate is given; for inconsistent measures, the outer ranges of measured values are reported. CReSS = Clinical Research Support System; SPA-M = Smoking Puff Analyzer Mobile.
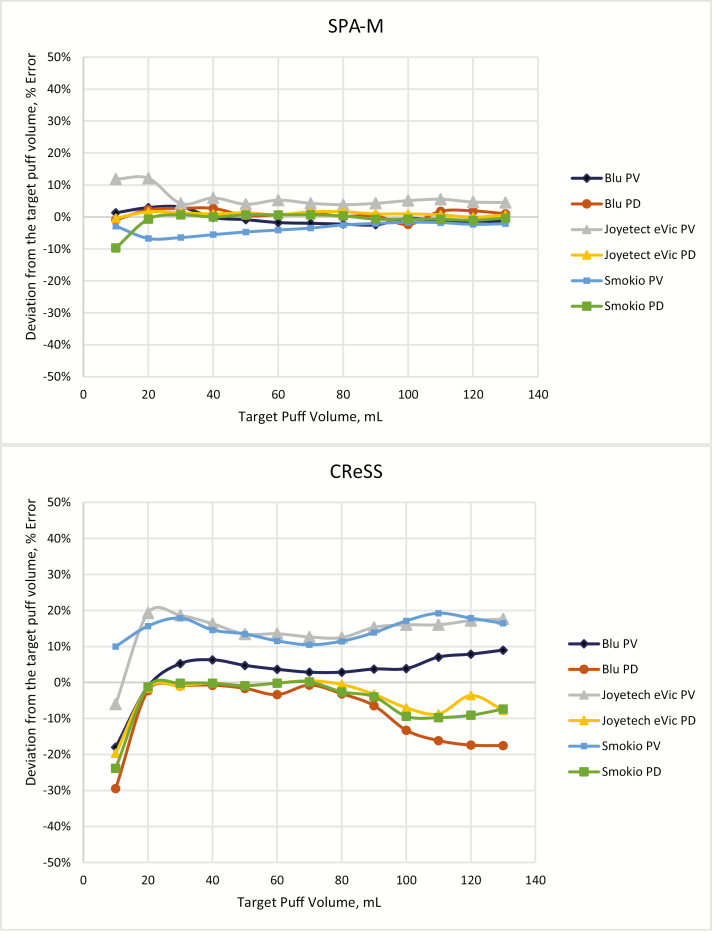
Figure 1.
Average error in puff volume and puff duration measured using Smoking Puff Analyzer Mobile (SPA-M) (top) and Clinical Research Support System (CReSS) (bottom) for square-shaped puff with 2 s duration.
Overall, the SPA-M demonstrated good accuracy of ±10% for both PV and PD either for the entire range of tested PVs (10–130 mL) or for the slightly limited (20–130 mL) range at both 2 and 5 s PDs. Only the Joyetech eVic showed PV outside ±10% accuracy at lower target PV (Table 1, Figure 1, and Supplementary Figure 12).
The CReSS response was more variable. At 5 s PD (square-shaped profile), PV percent error varied from −16% to +24% (for e-cigarettes), and from −10% to 23% (for 3R4F) within a 30–130 mL range of PV. In general, the CReSS consistently underreported PD values and its error increased at low and high PVs (Figure 1 and Supplementary Figures 3–6 and 11). Valid upper PV range (PD error within approximately −20%) could be as small as 90 or 100 mL (at 2 s target PD) and valid lower PV range could be as high as 50 or 60 mL (at 5 s target PD).
Topography Reporting Concerns During Real-World Data Collection
Both the CReSS and SPA-M produced summaries of recorded at-home vaping topography parameters as expected and described in the user manuals. However, during the analysis of the individual puff profiles, topography recording anomalies and limitation issues were observed for both devices.
CReSS
Multiple topography recording anomalies as well as limitations that may impact the reported data were observed and summarized per each participant (Supplementary Table 1) for puff profiles collected using the CReSS.
A 5 s PD limit verified for the CReSS means that puffs lasting longer than 5 s were cut off and the full extent of the puff (i.e., total PV, total PD) may not be properly recorded (Supplementary Figure 13). Nine (of 10) participants were affected by this recording issue, but the fraction of these potentially not-properly-recorded puffs did not exceed 15% per individual participant.
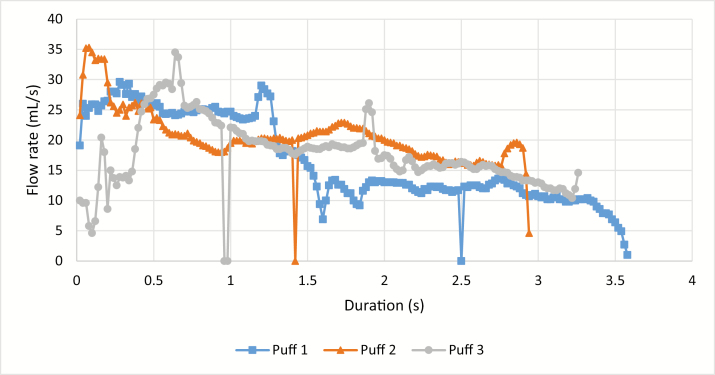
A typical CReSS data recording anomaly was flow rate dropouts, where the recorded signal dropped to 0 mL/s in the middle of a puff, and then went back up to the pre-dropout flow rate (Figure 2 shows an example). Across CReSS vaping topography data collected for 10 participants over a 1-week real-world collection period, these dropouts were found in 13%–49% of the total puffs recorded for a given participant.
Figure 2.
Example data collected with the Clinical Research Support System (CReSS) pocket device showing a signal dropouts in each of the three puffs collected.
A data recording limit (not described in the user manual) was observed for the CReSS. After recording approximately 2010–2020 total datapoints, the CReSS stopped reporting individual datapoints but still reported puff summary data. This issue was observed for three participants at a frequency of 20% and 30%.
The Cigarette Abandon time on the CReSS was left at the recommended default 20 min to conserve battery life over the 1-week of at-home use. In cases where a vaping session lasted more than 20 min, the CReSS stopped recording flow rate information but still reported summary topography information after the Cigarette Abandon time was passed. Data of eight participants showed this issue with a frequency of 3%–80% per individual participant across all recorded vaping sessions. Also, if more than 43 puffs were taken, the data file noted a Puff Overflow, indicating that more puffs were taken but they were not recorded. This was only an issue for two participants, and in one case this affected 78% of the recorded data. In both cases (abandon time or puffs overflow), it is hard to differentiate if these limits were exceeded because of actual long vaping session or the user simply forgot to remove the e-cigarette from the CReSS.
SPA-M
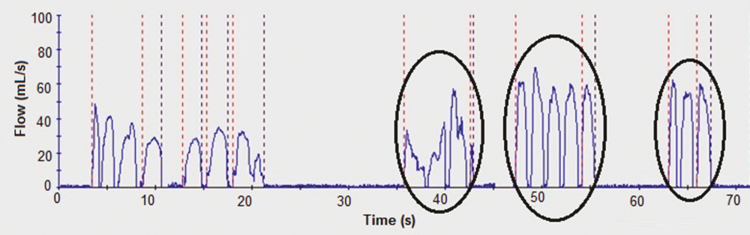
Data recording anomalies were also discovered in the at-home vaping topography data recorded by the SPA-M. When raw flow rate and duration data from the SPA-M were plotted to inspect individual puffs, the data showed multiple peaks being counted as one puff. An example of this is shown in Figure 3. This anomaly might be considered comparable to the signal dropout noted in the CReSS data, as there is no apparent IPI between these multiple peaks to suggest that more than one puff was taken. This anomaly was observed in all but 2 of the 10 participants considered here, affecting 40%–100% of all vaping sessions recorded for a given individual (Supplementary Table 1). Not all puffs were impacted for each vaping session, however.
Figure 3.
Example of the multi-peak puffs recorded by the Smoking Puff Analyzer Mobile (SPA-M) device. The puffs reported by SPA-M software have been circled.
Other topography recording anomalies found in the CReSS were not observed in the SPA-M data. The SPA-M manual did not define a PD limit. The laboratory testing showed that the SPA-M has a PD limit in excess of at least 60 s. No puffs recorded in the participant data exceeded this limit.
Similar to the CReSS, we observed PFR increase at the end of the puffs but analysis of the raw data (PFR vs. elapsed time) showed that SPA-M simply did not include the next few PFR values that in fact dropped down indicating the puff end (Supplementary Figure 14).
Discussion
Recording Accuracy
The SPA-M in comparison with the CReSS demonstrated a noticeable higher accuracy and wider validity range, where the major topography parameters PV and PD can be measured within reasonable accuracy limits. The difference between the two devices is caused by better SPA-M sensitivity that allows for a lower threshold of the minimum PFR detection in comparison with the CReSS. The average PFRs reported by both devices were checked against the calibrated flowmeter and showed that the SPA-M can operate at a minimum PFR of approximately 4 mL/s versus approximately 6 mL/s for the CReSS. As a result, the CReSS shows significant error in PV and PD for a 10 mL square-shaped puff with 2 s duration (that corresponds to ~5 mL/s PFR) as shown in Figure 1. Both power-adjustable and refillable e-cigarettes (Joyetech eVic and Smokio) showed higher PV variability than the blu e-cigarettes (Figure 1). Pressure drop measurements (at 25 mL/s PFR) were approximately 3.3 in of water for both refillable e-cigarettes versus approximately 4.3 in of water for the blu e-cigarette. It could be assumed that lower pressure drop across the e-cigarette may cause additional inaccuracy of the puff topography measurements.
For all types of e-cigarettes, the CReSS showed underestimation of PD at high PVs (Figure 1). The PVs are estimated based on the measured PFRs and the PDs; therefore, underestimated PD may mask imprecise measurements made by the CReSS at high PVs. For example, at 100 mL PV and 2 s PD, which corresponds to approximately 50 mL/s PFR, the CReSS underestimates PD measured for blu e-cigarette by >10% (Figure 1 and Supplementary Figures 3–6) and continues to increase as PV increases. The SPA-M does not have this limitation and records correct PD as well as PV up to 130 mL at 2 s duration (or at ~60 mL/s PFR). It is important to consider how often e-cigarette users vape at a PFR that exceeds 50 mL/s, as this will determine the ability of the CReSS to accurately capture and report real-world topography measurements.
Recording Anomalies
The topography data recording anomalies were found through careful inspection of raw (nonsummarized) data taken from e-cigarette vaping sessions. Some of the CReSS anomalies such as signal dropouts were also observed during laboratory validation of the device using a combustible cigarette. The effect of dropouts on PV determination depends on how long it takes for the signal to return to the normal values. Although normally the duration of dropouts was approximately 20–60 ms, occasionally, for a 0.6 s PD signal, a dropout was observed for 0.2 s (~30% of the entire PD).
The SPA-M multi-peak puff anomaly, which was also seen in the laboratory validation of the SPA-D, was not observed in historic SPA-D topography data collected from humans smoking combustible cigarettes. This implies that some e-cigarette properties might be affecting the topography recordings from the SPA-M. Direct observation of some of the participants whose topography exhibited the multi-peak puff anomaly on the SPA-M while vaping in the laboratory indicated that the participants did not remove the e-cigarette from their mouths. Thus, it was a single puff, as there was no removal of the device or IPI to denote the start of a new puff. This suggests particular vaping behavior may be causing this unusual multi-peak puffing data, and that the data represent the actual puff profile. The multi-peak data pattern was not seen in all participants’ real-world data when using the SPA-M, further indicating participant-specific e-cigarette vaping behavior as the root cause.
Possible influence of e-cigarette generated aerosol on device performance also cannot be excluded. Visible liquid deposits were noticed on the CReSS pressure transducer orifices after prolonged use, and similar deposits were observed inside the SPA-M tubes that connect the e-cigarette holder with the topography device. The deposits accumulated with time and could affect accuracy of the pressure transducers responses. Cleaning the CReSS orifices and drying the SPA-M tubes before giving the mobile devices to participants should be implemented, although this practice is more feasible for laboratory measurements than for the real-world data collection.
Device Limitations and Recommendations
The software of both devices provides not only the summary but also individual PFR per each recorded timepoint. Analysis of the PFR versus elapsed time data allows for detection of the anomalies described in this article and will help researchers discern if data were abandoned, not recorded, or are misinterpreted.
The CReSS 5 s PD limitation should be considered in concert with PFR trend at the end of the puff. If PFR is increasing, then it may indicate that puff was not finished, and therefore, the actual puff topography was not fully captured. The CReSS total number of datapoints recording limitation means that the CReSS still reports a summary of the puffs past the data recording limit, but there is no way to verify summary results as the individual datapoints were not available (as can be seen from the raw data). Abandoned sessions (if the total session duration exceeds 20 min) require analysis to conclude whether it was a very long vaping session, or whether the participant simply forgot to remove the e-cigarette from the CReSS. Both situations may take place, and it is impossible to determine which occurred from the data collected. The CReSS puff number limit of 43 puffs per session caused a significant problem for one of the participants examined for this article (78% of the vaping sessions recorded). The ramifications of this limitation on larger participant populations require further investigation.
It is important to point out that many of the topography recording concerns discussed in this article would not have been detected or even noticed without a visual, detailed inspection of the raw data. CReSS PD limitation may affect both laboratory and at-home measurements whereas total datapoint limit, abandon time, and puff number limit will be of greater concern for real-world data collection. Downloading and using the data as summarized by the device gives no alert regarding these issues and could lead to summarization of incorrect and incomplete topography data. This may result in misrepresentation or misunderstanding of the vaping behavior being studied.
Conclusions
Both mobile puffing topography measuring devices currently available on the market, the CReSS and SPA-M, are capable of recording real-world e-cigarette vaping data, but researchers should be aware of the limitations and errors associated with these two devices. In particular, the CReSS 5 s PD limit requires PFR analysis at the end of a puff to assess if e-cigarette topography was fully recorded. The SPA-M is more accurate over a wider range of PVs than the CReSS. Data collected from both devices during laboratory and real-world vaping demonstrated some anomalies that should be taken into account when analyzing the data. Data summaries provided by the mobile devices’ software have to be verified by examining the raw data to define if the anomalies were present, and if the anomalies were found then an assessment has to be made to evaluate the impact to the study conclusions.
Limitations
Three e-cigarette types were used to assess the performance of the mobile topography devices in the laboratory validation part of this study. It is possible that other types of e-cigarettes could introduce additional error in the topography data recorded. The anomaly percentages described are only for a small number of subjects and may not be representative of a larger number of e-cigarette users; therefore, more real-world data are required to fully validate the use of both devices for human studies.
Supplementary Material
Supplementary data are available at Nicotine and Tobacco Research online.
Funding
This research was supported by grant number P50CA180523 from the National Cancer Institute and Food and Drug Administration (FDA) Center for Tobacco Products awarded to the University of Maryland. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the FDA.
Declaration of Interests
None declared.
References
- 1. Delnevo CD, Giovenco DP, Steinberg MB, et al. Patterns of electronic cigarette use among adults in the United States. Nicotine Tob Res. 2016;18(5):715–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singh T, Arrazola RA, Corey CG, et al. Tobacco use among middle and high school students—United States, 2011–2015. MMWR Morb Mortal Wkly Rep. 2016;65(14):361–367. [DOI] [PubMed] [Google Scholar]
- 3. Laugesen M. Safety Report on the Ruyan E-cigarette Cartridge and Inhaled Aerosol. Health New Zealand Ltd; 2008. [Google Scholar]
- 4. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23(2):133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bekki K, Uchiyama S, Ohta K, Inaba Y, Nakagome H, Kunugita N. Carbonyl compounds generated from electronic cigarettes. Int J Environ Res Public Health. 2014;11(11):11192–11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jensen RP, Luo W, Pankow JF, Strongin RM, Peyton DH. Hidden formaldehyde in e-cigarette aerosols. N Engl J Med. 2015;372(4):392–394. [DOI] [PubMed] [Google Scholar]
- 7. Kosmider L, Sobczak A, Fik M, et al. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res. 2014;16(10):1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Uchiyama S, Inaba Y, Kunugita N. Determination of acrolein and other carbonyls in cigarette smoke using coupled silica cartridges impregnated with hydroquinone and 2,4-dinitrophenylhydrazine. J Chromatogr A. 2010;1217(26):4383–4388. [DOI] [PubMed] [Google Scholar]
- 9. Ogunwale MA, Li M, Ramakrishnam Raju MV, et al. Aldehyde detection in electronic cigarette aerosols. ACS Omega. 2017;2(3):1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khlystov A, Samburova V. Flavoring compounds dominate toxic aldehyde production during e-cigarette vaping. Environ Sci Technol. 2016;50(23):13080–13085. [DOI] [PubMed] [Google Scholar]
- 11. Sleiman M, Logue JM, Montesinos VN, et al. Emissions from electronic cigarettes: key parameters affecting the release of harmful chemicals. Environ Sci Technol. 2016;50(17):9644–9651. [DOI] [PubMed] [Google Scholar]
- 12. Jensen RP, Strongin RM, Peyton DH. Solvent chemistry in the electronic cigarette reaction vessel. Sci Rep. 2017;7:42549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pankow JF, Kim K, McWhirter KJ, et al. Benzene formation in electronic cigarettes. PLoS One. 2017;12(3):e0173055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Williams M, Villarreal A, Bozhilov K, Lin S, Talbot P. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS One. 2013;8(3):e57987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Williams M, To A, Bozhilov K, Talbot P. Strategies to reduce tin and other metals in electronic cigarette aerosol. PLoS One. 2015;10(9):e0138933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mikheev VB, Brinkman MC, Granville CA, Gordon SM, Clark PI. Real-time measurement of electronic cigarette aerosol size distribution and metals content analysis. Nicotine Tob Res. 2016;18(9):1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fuoco FC, Buonanno G, Stabile L, Vigo P. Influential parameters on particle concentration and size distribution in the mainstream of e-cigarettes. Environ Pollut. 2014;184:523–529. [DOI] [PubMed] [Google Scholar]
- 18. Ingebrethsen BJ, Cole SK, Alderman SL. Electronic cigarette aerosol particle size distribution measurements. Inhal Toxicol. 2012;24(14):976–984. [DOI] [PubMed] [Google Scholar]
- 19. Manigrasso M, Buonanno G, Fuoco FC, Stabile L, Avino P. Aerosol deposition doses in the human respiratory tree of electronic cigarette smokers. Environ Pollut. 2015;196:257–267. [DOI] [PubMed] [Google Scholar]
- 20. Pellegrino RM, Tinghino B, Mangiaracina G, et al. Electronic cigarettes: an evaluation of exposure to chemicals and fine particulate matter (PM). Ann Ig. 2012;24(4):279–288. [PubMed] [Google Scholar]
- 21. Harmful and potentially harmful constituents in tobacco products and tobacco smoke; established list. In: Department of Health and Human Services FDA, ed. Federal Register, Vol. 77, No. 64, 2012:20034–20037. [Google Scholar]
- 22. Goel R, Durand E, Trushin N, et al. Highly reactive free radicals in electronic cigarette aerosols. Chem Res Toxicol. 2015;28(9):1675–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Farsalinos KE, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review. Ther Adv Drug Saf. 2014;5(2):67–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Orr MS. Electronic cigarettes in the USA: a summary of available toxicology data and suggestions for the future. Tob Control. 2014;23 (suppl 2):ii18–ii22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Volesky KD, Maki A, Scherf C, Watson LM, Cassol E, Villeneuve PJ. Characteristics of e-cigarette users and their perceptions of the benefits, harms and risks of e-cigarette use: survey results from a convenience sample in Ottawa, Canada. Health Promot Chronic Dis Prev Can. 2016;36(7):130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Benowitz NL, Hall SM, Herning RI, Jacob P 3rd, Jones RT, Osman AL. Smokers of low-yield cigarettes do not consume less nicotine. N Engl J Med. 1983;309(3):139–142. [DOI] [PubMed] [Google Scholar]
- 27. Hammond D, Fong GT, Cummings KM, Hyland A. Smoking topography, brand switching, and nicotine delivery: results from an in vivo study. Cancer Epidemiol Biomarkers Prev. 2005;14(6):1370–1375. [DOI] [PubMed] [Google Scholar]
- 28. MacGregor IC, Stanfill SB, Gordon SM, et al. Custom mentholation of commercial cigarettes for research purposes. Toxicol Rep. 2014;1:1068–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ross KC, Juliano LM. Smoking through a topography device diminishes some of the acute rewarding effects of smoking. Nicotine Tob Res. 2016;18(5):564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buehler SS, Gordon SM, Brinkman MC, Kroeger RR, Clark PI. Effect of menthol cigarette content on mainstream smoke VOC emissions and uptake. Presented at the annual conference of the Society for Research on Nicotine and Tobacco, Philadelphia, PA, February 25–28, 2015.
- 31. Eissenberg T, Adams C, Riggins EC 3rd, Likness M. Smokers’ sex and the effects of tobacco cigarettes: subject-rated and physiological measures. Nicotine Tob Res. 1999;1(4):317–324. [DOI] [PubMed] [Google Scholar]
- 32. Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: implications for research protocol standards definition and for public health authorities’ regulation. Int J Environ Res Public Health. 2013;10(6):2500–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cunningham A, Slayford S, Vas C, Gee J, Costigan S, Prasad K. Development, validation and application of a device to measure e-cigarette users’ puffing topography. Sci Rep. 2016;6:35071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Behar RZ, Hua M, Talbot P. Puffing topography and nicotine intake of electronic cigarette users. PLoS One. 2015;10(2):e0117222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Spindle TR, Breland AB, Karaoghlanian NV, Shihadeh AL, Eissenberg T. Preliminary results of an examination of electronic cigarette user puff topography: the effect of a mouthpiece-based topography measurement device on plasma nicotine and subjective effects. Nicotine Tob Res. 2015;17(2):142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ossip-Klein DJ, Martin JE, Lomax BD, Prue DM, Davis CJ. Assessment of smoking topography generalization across laboratory, clinical, and naturalistic settings. Addict Behav. 1983;8(1):11–17. [DOI] [PubMed] [Google Scholar]
- 37. Marian C, O’Connor RJ, Djordjevic MV, Rees VW, Hatsukami DK, Shields PG. Reconciling human smoking behavior and machine smoking patterns: implications for understanding smoking behavior and the impact on laboratory studies. Cancer Epidemiol Biomarkers Prev. 2009;18(12):3305–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Robinson RJ, Hensel EC, Morabito PN, Roundtree KA. Electronic cigarette topography in the natural environment. PLoS One. 2015;10(6):e0129296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dautzenberg B, Bricard D. Real-time characterization of e-cigarettes use: the 1 million puffs study. J Addict Res Ther. 2015;6(2):1–5. [Google Scholar]
- 40. Oldham MJ, Plunkett SE, Fisher MT, Shafer KH, Morton MJ. Laboratory evaluation of the CReSSmicro™ portable topography device: implications for clinical research. Contributions to Tob Res. 2014;26(1):19–25. [Google Scholar]
- 41. Goniewicz ML, Kuma T, Gawron M, Knysak J, Kosmider L. Nicotine levels in electronic cigarettes. Nicotine Tob Res. 2013;15(1):158–166. [DOI] [PubMed] [Google Scholar]
- 42. Norton KJ, June KM, O’Connor RJ. Initial puffing behaviors and subjective responses differ between an electronic nicotine delivery system and traditional cigarettes. Tob Induc Dis. 2014;12(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.