Abstract
Introduction
Documenting factors that influence differential sensitivity to acutely inhaled nicotine products requires carefully controlling the amount of exposure (dose), and thus a procedure by which to control such exposure.
Methods
We evaluated consistency of puff volume from intermittent acute exposures to smoked tobacco cigarettes (study 1, n = 45, plus a comparison study of uninstructed use with n = 59) and to vaped electronic cigarettes (e-cigarettes; study 2, n = 27 naive to e-cigarettes) in adult-dependent smokers. All in primary studies 1 and 2 participated in research administering different nicotine levels in each product under blind conditions, one per session using within-subject designs. In both studies, participants followed an automated instructional procedure on a computer monitor standardizing the timing and amount of exposure to each product during a given trial, with four trials per session, each separated by 20 minutes. Puff volume per trial via Clinical Research Support System (CReSS) was the primary dependent measure to determine consistency across trials via intraclass correlation coefficients (ICCs).
Results
Control over topography with both inhaled products was demonstrated by highly significant ICCs for puff volume across trials. Instructed control with own brand was generally better in study 1 than with uninstructed smoking in the comparison sample, as expected. As intended, reliability of puff volume generally did not differ by menthol preference or sex in either study, but ICCs in study 2 tended to be lower for some men using the placebo e-cigarette.
Conclusions
This instructional procedure may substantially improve control over amounts of acute exposure to tobacco or e-cigarette use.
Implications
Control over topography in studies of acute exposure to these inhaled products can potentially aid validity of research into differential sensitivity to use, so findings can be attributed to factors of interest and not to variable exposure. Our procedure minimized variability in exposure to the same product and between moderate nicotine products, but remaining differences suggest that compensation for very low or no nicotine commercial products may be difficult to totally eliminate with these instructions alone. Further study is needed to determine this procedure’s utility with other inhaled products among experienced users and when comparing different products in between-groups analyses.
Introduction
Some research on acute smoking or vaping behavior focuses on factors that may account for differential amounts of smoking exposure that are self-administered ad libitum by smokers over a brief period of time (eg, single cigarette, or one “bout” of smoking or vaping).1–5 Separately, other acute research evaluates influences on variable responding to fixed amounts of cigarette smoking (or vaping), which requires careful control over that smoke exposure to establish the factors responsible and rule out differential smoking amounts. In other words, rather than being the dependent measure, as in the first area of research, exposure here is an independent measure (ie, dose), with magnitude of responding to that exposure amount, or sensitivity, being the dependent measure. Relevant studies here are far more common for tobacco smoking, such as those examining variable responding to specific levels of smoking exposure as functions of individual differences, concurrent environmental conditions, differences in the cigarette’s constituents, or other manipulations. Examples include research on responding due to static individual difference factors of high or low dependence,6 menthol or nonmenthol preference,7 or subject sex8; contextual factors of testing location,9 concurrently engaging in exercise,10 or pretreatment with medications11; and controlled variations in nicotine or other content of tobacco in test cigarettes.12,13 Similarly observed variations in amount of self-administered exposure and in differential responses to exposure have been found in acute tests with electronic cigarettes (e-cigarettes).14
Some measures of smoking topography are often consistent within smokers smoking under the same conditions.4,15 However, although smokers can accurately report puff number and approximate interpuff interval (IPI), most are not able to accurately gauge puff volume,16 questioning the ability of instructions limited to just puff number and timing to effectively control total exposure from acute smoking.17,18 Consequently, even videotaped observation of smoking may not accurately assess total topography, given variability in duration and volume of each puff.19 Without tightly controlling amounts of smoke or vape exposure, this research may not easily distinguish between factors affecting differential pharmacodynamic responses (sensitivity) to the same specific amount of exposure versus those altering intensity of smoking or vaping behavior, or differential amount of exposure (ie, puff topography).8,20 Therefore, an instructional procedure that can elicit standardized volumes for puffs may be of considerable utility for research seeking to assess responses to fixed amounts of acute exposure to these inhaled products.
In the current research, we evaluated the effect simplified instructions on the precise timing of puff inhalation, breath hold, and exhalation had on the reliability of the resulting volume of each discrete trial of smoking or vaping exposure, involving a set number of puffs. To evaluate this procedure, we conducted secondary analyses of data from recent studies using very similar methods for dependent smokers to self-administer fixed numbers of puffs under various manipulations. These studies also allowed us to explore generalizability of the control resulting from our computerized instructional procedure across different products, tobacco and e-cigarettes varying in nicotine, and different subgroups of smokers. Using within-subject designs, primary comparisons were differences in consistency across trials between sessions because of cigarette nicotine yield or own brand (study 1, plus comparison data of topography with own brand when not using this instructional procedure), or due to e-cigarette nicotine content (study 2). Secondary between-groups comparisons were differences in consistency of topography due to men versus women or to menthol versus nonmenthol preference (both studies).
All puffing in these studies was done via the portable Clinical Research Support System (CReSS Pocket; Borgwaldt KC, Inc, Richmond, VA; https://www.borgwaldt.com/en/products/smoking-vaping-machines/smoking-topography-devices.html), widely used in research over the past decade to assess acute smoking topography.15,19 The CReSS was used here to objectively assess puff volume (in milliliters),4,15 as well as puff duration, puff number, and IPI, and, thus, to evaluate the reliability of intake per trial with the instructions presented in this computerized puffing procedure. (A prior desktop CReSS version allowed real-time feedback on the immediately obtained volume relative to the instructed volume, possibly improving exposure control beyond our instructional procedure. However, this version is apparently no longer available for purchase from Borgwaldt, the manufacturer; see link mentioned earlier). Importantly, though, the CReSS here is not a component of our instructional procedure but a method to validate the procedure, specifically its control over topography. This procedure potentially can be used alone to improve control over exposure during acute assessments of inhaled nicotine products.
If smoke and vape exposure per trial is confirmed as very reliable, this simple instructional procedure may enable researchers to carefully control acute smoking or vaping topography in experimental studies, with or without a device for objective topography assessment. Such a procedure would allow within-subject examination of responses to a fixed amount of exposure to inhaled products differing in constituents (especially nicotine content) and other influences on pharmacodynamic responses, isolating causal factors in this differential responding. Between-groups differences in responding also may be more valid with better topography control. This procedure may be of particular benefit going forward, as research cigarettes explicitly differing in specific nicotine and other contents recently became available for study of nicotine dosing via smoking (Spectrum),7,12 along with nonsmoked products varying in nicotine content,21–23 all of which require good control over topography to isolate acute effects of nicotine dosing per se.
Study 1
These instructions were presented with an automated computerized procedure displayed in slides on a monitor that advance automatically based on the timing specified (see Supplementary Figure), as later described in more detail (Topography Control section). Study 1 examined the consistency of control over topography with our procedure in dependent smokers instructed to take six puffs from one cigarette at four separate times (trials). Three different types of cigarettes were tested, just one per session. Two sessions involved Quest brand cigarettes differing widely in nicotine yield and administered under blind conditions after brief abstinence. To begin evaluating generalizability of our instructional procedure between different study conditions, a third session entailed administering the participant’s own brand of cigarettes in unblinded manner after no abstinence. Finally, for comparison, topography data from a separate sample smoking own brand in uninstructed (ie, ad libitum) manner were related to study 1 results from the own brand condition to examine differences in control with versus without our procedure. Both of these samples smoked their own brand unblinded and after no abstinence, but with (study 1) or without (comparison sample) this instructional procedure.
Methods
Participants
Data for study 1 were from dependent smokers completing two very similar prior studies examining acute reinforcement-enhancing effects of nicotine via smoking.24,25 These studies compared sessions with virtually identical procedures and involving administration of moderate and very low nicotine Quest brand cigarettes, as well as a session with their own preferred brand. These participants comprised 45 dependent smokers (20 M, 25 W), those who smoked at least 10 cigarettes/day for at least 1 year and met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria for nicotine dependence. We excluded those currently taking medications to treat serious psychological problems (eg, psychosis, major depression) or those interested in quitting smoking (ie, treatment seekers). Mean (± SD) smoking characteristics were 14.6 ± 3.9 cigarettes/day, 4.5 ± 1.7 on the Fagerström Test of Nicotine Dependence (FTND)26; nicotine yield of their preferred brand was 1.0 ± 0.2 mg; 58% were nonmenthol smokers; and they were 26.4 ± 9.7 years of age. Men and women also did not differ on these smoking characteristics or age. All were recruited from the surrounding community and most self-identified as Caucasian (84.4%), with 11.1% African American, 2.2% Asian, and 2.2% Native Indian/Alaskan Native.
To explore topography control with versus without our procedure, we compared topography between that for the own brand session in study 1 versus a participant sample from another study with the same inclusion or exclusion criteria27 who also smoked their own brand via the CReSS unblinded, but in uninstructed manner. Characteristics of the 59 individuals in this sample (36 M, 23 W) were comparable to those 45 for study 1, with means (SD) of 20.1 (6.8) cigarettes/day, 4.7 (2.0) on FTND; nicotine yield of their preferred brand was 1.0 (0.2) mg; 53% were nonmenthol smokers; and they were 26.1 (8.9) years of age, with no sex differences in these characteristics. Also similar, most identified as Caucasian (86%) with others self-reporting as African American (12%) or Hispanic (2%).
Cigarettes
Subjects smoked a different cigarette in these three sessions, involving a moderate nicotine brand, a denicotinized brand, and, for comparison, their own preferred nicotine brand, with just one cigarette type per session. The moderate (nicotine) brand was Quest 1 (yield of 0.6 mg nicotine), and the very low, denicotinized (denic) brand was Quest 3 (yield <0.05 mg nicotine). Both were formerly sold commercially by Vector Group, Ltd (Miami, FL). Menthol-preferring smokers (n = 19) received menthol Quest; nonmenthol smokers (n = 26) received nonmenthol Quest. All Quest cigarettes had identifiable markings covered using Fisherbrand 13 mm labeling tape,28 and subjects were kept blind to brand. By contrast, markings of their preferred brand (mean yield of 1.0 ± 0.2 mg nicotine) were not covered in the nonabstinent comparison session and so were administered unblind. This own brand or satiation condition was intended here to examine generalizability of smoking topography control with our procedure between an unfamiliar brand (Quest) under blinded and overnight abstinence conditions, and a familiar (own brand) cigarette under unblinded and nonabstinence conditions (typical of most smoking in the natural environment). All in the comparison sample also smoked their own brand unblinded but without access to the instructional procedure to allow us to evaluate whether the procedure improved control over topography.
Topography Control
This instructional procedure was designed to standardize the timing and amount of exposure from a fixed number of puffs inhaled from the cigarette being tested, based on typical means from observations of ad libitum smoking in past research. The exact puff timing and durations were guided by computer-presented puffing instructions via Microsoft Office PowerPoint29 slides to standardize the durations of each step in consuming one puff, comprising 30 seconds per puff exposure. As shown in the Supplementary Figure, these steps proceeded from preparation (two slides) of “Get Ready” and then “Put the Mouthpiece to your lips” (2 seconds each), then “Inhale” (ie, puff duration; 2 seconds), “Breathe in and hold” (2 seconds for tobacco cigarette, 4 seconds for e-cigarette), and “Exhale” (2 seconds), followed by “In a moment you’ll do that again. Wait for instructions” (20 seconds for tobacco, 18 seconds for e-cigarette) for the next scheduled puff. The slides ended after the last scheduled puff of the trial. This timing was intended to fix intake from a tobacco cigarette at about 50 mL per puff, consistent with ad libitum puffing.4,19 Participants engaged in these trials of controlled puffing behavior while alone in the study room but were observed by an experimenter in a different room watching via video monitor.
Procedure
After attending a screening session to confirm eligibility and provide informed consent, all engaged in a “practice” period with the topography procedure (described earlier) using an unlit cigarette, to ensure familiarity with following the timing of puffing instructions shown on the monitor prior to the three 2-hour experimental sessions. No other training or feedback on performance was provided. Two sessions followed overnight abstinence and differed only in the acute smoking condition in effect: “nicotine” (Quest 1) or “denic” (Quest 3) cigarettes presented under blind conditions. To compare these results with topography control under more “typical” smoking conditions, the third session involved no overnight abstinence and smoking of one’s preferred cigarette (own brand) in unblinded manner, identified at the eligibility screening session. A fourth experimental session was identical to the other three but did not involve any smoking and is not discussed further. All cigarettes, including the preferred brand, were provided by the experimenter. The order of these three smoking conditions across sessions was counterbalanced between subjects and separated by at least 1 day.
On arrival to each session, expired-air carbon monoxide (CO) was assessed via BreathCo CO monitor (Vitalograph, Lenexa, KS) to confirm overnight (>12 hours) smoking abstinence (CO ≤ 10 ppm) or nonabstinence (CO > 10 ppm),30 according to smoking restrictions required prior to these three sessions. Prior to each of the four puffing trials per session, participants were presented with the cigarette assigned for that session, told to insert it into the CReSS device prior to the puffing trial, and then instructed to light it and follow the computerized puffing procedure via PowerPoint slides on the monitor (see Topography Control). When lighting the cigarette, participants were instructed to do so without taking a puff from the cigarette or CReSS device, preventing additional smoke exposure separate from the puffing procedure. Participants held the flame from the lighter on the tip of the cigarette until the paper caught and were instructed to blow on the tip of the cigarette to ensure it was fully lit before continuing with the puffing procedure. This same procedure was followed on the three subsequent puffing trials, each involving a new cigarette. As noted, the procedure instructed them on when to take six puffs over 3 minutes, with one puff every 30 seconds, and these six puffs/trial were presented on a total of four trials per session, each followed by 20 minutes of rest without any smoking exposure.
Finally, to compare consistency of topography with versus without our instructional procedure, we examined data from a different sample of smokers comparable to those in study 1 but who smoked their own brand without instructions (ie, ad libitum) once upon arrival on four separate sessions (unblinded and after no abstinence, as in study 1). The purpose for these trials in that comparison study was to ensure satiation at baseline prior to the start of testing of smoking responses to other cigarette brands later in the sessions because of different negative affect induction manipulations, described in the original report27 and not discussed further here. Thus, the only relevant procedures in this comparison sample involved telling participants to smoke as usual prior to each of four sessions and then, after arrival, to smoke one of their preferred brand cigarettes however they wished via the CReSS. Study protocols were approved by the University of Pittsburgh Institutional Review Board.
Data Preparation and Analysis Approach
To assess amount of smoking exposure, the primary dependent measure of interest was total puff volume per trial, which was determined by the volume assessed by the CReSS throughout each trial. Secondary topography measures were puff number per trial and means per trial for the individual puff-level data of IPI and puff inhalation duration. Across all participants and cigarette conditions, there were a total of 3362 individual puffs. Prior to analyses, we examined all trials apparently deviating from the procedure’s instructions. Puffs with IPIs less than 1000 ms (n = 26, 0.77% of all puffs) or a combination of IPIs less than 2000 ms and durations less than 1000 ms (n = 3, 0.09% of all puffs) were identified as “stutter puffs.” Stutter puffs were those that could have occurred due to participants changing their lip position on the mouthpiece mid puff, taking a brief pause mid puff, or a false start in anticipation of the “Inhale” slide. Puff volume and duration from these few identified stutter puffs were combined with the preceding puff. Means for puff duration and IPI, along with the number of puffs, were then calculated for each trial.
Preliminary analyses used separate 3 × 4 repeated measures analysis of variance (RM ANOVA) to assess within-subjects differences in puff volume, puff duration, IPI, and absolute number of puffs as a function of cigarette conditions (3) and trial number (1–4). Partial eta squared (η2p) was calculated as a measure of effect size for each ANOVA. Pairwise comparisons using a Bonferroni correction for multiple comparisons were conducted to follow up any significant main effects. For primary analyses, two-way mixed model intraclass correlation coefficients (ICCs)31 were calculated to assess reliability in total puff volume across trials within each cigarette condition. Type C ICC values were used to estimate the consistency of puff volumes. Differences in ICC values between cigarette conditions, menthol preference, and sex were determined by examining overlap of the 95% CIs.
To compare topography results from smoking own brand with versus without our procedure, we conducted similar analyses as described earlier with the own brand session of study 1 versus the comparison sample smoking their own brand in uninstructed manner. RM ANOVA of Study × Trial evaluated differences in the four topography measures noted earlier. ICC values were also computed to estimate consistency of puff volumes across sessions.
Results
Preliminary Analyses
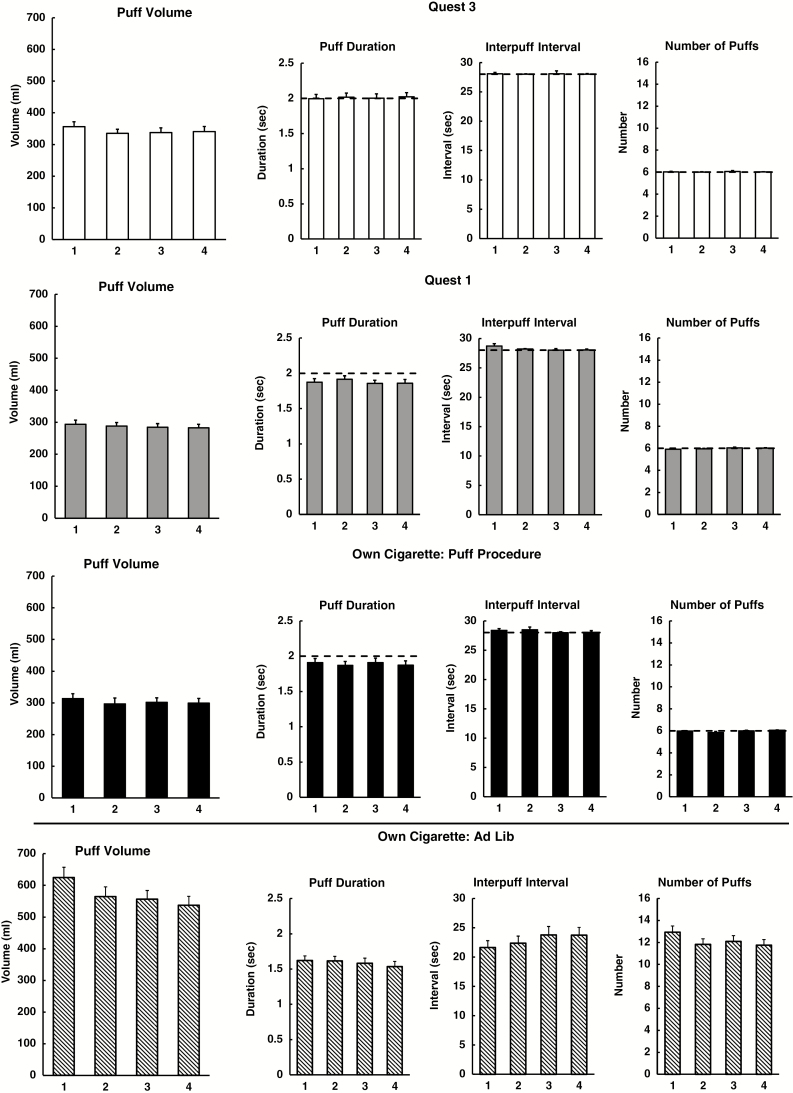
Control of smoking exposure with this procedure is indicated by the means (±SEM) for the topography measures by trial, shown separately for each cigarette condition in Figure 1. Results of the preliminary RM ANOVAs are shown in Table 1. Despite this consistency of puff volume across trials (Figure 1), main effects of cigarette condition and trial were significant. Bonferroni adjusted pairwise comparisons indicated that mean (SEM) volume was higher for the Quest 3 denic, 342.45 (13.53) mL, than for the two nicotine cigarettes, Quest 1, 287.26 (10.75) mL, and own brand, 302.79 (14.54) mL, which did not differ. However, pairwise comparisons found no significant differences in puff volume between trials. Similarly, the main effect of cigarette condition was also significant for puff duration, with longer mean (SEM) durations for Quest 3 denic, 2.01 (0.06) seconds, compared to the nicotine cigarettes of Quest 1, 1.88 (0.04) seconds, and own brand, 1.89 (0.06) seconds. For the other secondary topography measures, IPI and number of puffs, the main effect of trial was significant, but none of the follow-up comparisons were significant.
Figure 1.
Means for the primary outcome of puff volume, and for the secondary topography measures of puff inhalation duration, interpuff interval, and puff number, in each of the four exposure trials with each of the three tobacco cigarettes in study 1. Also shown are means for the uninstructed smoking (“ad lib”) of own brand in the comparison sample (versus instructed smoking of own brand in study 1). Dashed lines indicate expected values due to instructions for fixed timing and puff number of tobacco cigarettes during puffing procedure.
Table 1.
Repeated Measures Analysis of Variance (RM ANOVA) Results for Study 1 (and Comparison Sample) and Study 2
| Instructed puffing (main study) | Uninstructed puffing (comparison sample) | Own cigarette condition comparisons: instructed vs. uninstructed puffing | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cig or e-cig condition | Trial | Cig × Trial | Trial (session) | Study | Trial (session) | Study × Trial | ||||||||
| F | η2p | F | η2p | F | η2p | F | η2p | F | η2p | F | η2p | F | η2p | |
| Study 1 | df = 2, 88 | df = 3, 132 | df = 6, 264 | df = 3, 174 | df = 1, 102 | df = 3, 306 | df = 3, 306 | |||||||
| Puff volume | 11.48*** | 0.21 | 3.74*, a | 0.08 | 0.33 | 0.01 | 5.20*** | 0.08 | 67.43*** | 0.40 | 5.01** | 0.05 | 2.47† | 0.02 |
| Puff duration | 7.04** | 0.14 | 0.27 | 0.01 | 1.18 | 0.03 | 2.91* | 0.05 | 11.16** | 0.10 | 2.44† | 0.02 | 1.35 | 0.01 |
| Interpuff interval | 2.13 | 0.05 | 3.70*, a | 0.08 | 0.80 | 0.02 | 3.05* | 0.05 | 0.22 | 0.00 | 1.54 | 0.02 | 2.83* | 0.03 |
| Number of puffs | 1.19 | 0.03 | 4.29**, a | 0.09 | 0.92 | 0.02 | 2.82* | 0.05 | 143.94*** | 0.59 | 2.24† | 0.02 | 2.09† | 0.02 |
| Study 2 | df = 1, 26 | df = 3, 78 | df = 3, 78 | |||||||||||
| Puff volume | 1.97 | 0.07 | 1.21 | 0.04 | 1.25 | 0.05 | ||||||||
| Puff duration | 0.94 | 0.04 | 2.61 | 0.09 | 0.37 | 0.01 | ||||||||
| Interpuff interval | 0.08 | 0.00 | 2.09 | 0.07 | 1.83 | 0.07 | ||||||||
| Number of puffs | 0.22 | 0.01 | 0.89 | 0.03 | 1.57 | 0.06 | ||||||||
Cig = cigarette, E-cig = electronic cigarette, df = degrees of freedom.
aNo significant differences in follow up pairwise comparisons.
†p ≤ .10; *p < .05; **p < .01, ***p < .001.
Primary Analyses
Table 2 displays ICC values and 95% CIs for each cigarette condition overall, and then separately for the subgroups differing by menthol preference and sex. As suggested by the comparable means per trial shown in Figure 1, total puff volume was consistent across trials for each cigarette condition in all participants, indicated by overlapping 95% confidence interval (CI) bands in the first row of Table 2. When comparing between menthol preference subgroups, ICC values were similar within each cigarette condition. Similarly, puff volume per trial was consistent for each menthol group across cigarette conditions. The same pattern of consistency between groups (within cigarette conditions) and within groups (across cigarette conditions) was found for sex.
Table 2.
ICC Values for Puff Volumes Across Trials by Cigarette for Study 1 (and Comparison Sample) and E-Cigarette Conditions for Study 2
| Instructed puffing | Uninstructed puffing | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quest 3 | Quest 1 | Own cigarette | Own cig comparison sample | |||||||
| Study 1 | n | ICC | (95% CI) | ICC | (95% CI) | ICC | (95% CI) | n | ICC | (95% CI) |
| Overall | 45 | 0.79 | (0.70 to 0.87) | 0.82 | (0.74 to 0.89) | 0.81 | (0.72 to 0.88) | 59 | 0.69 | (0.58 to 0.78) |
| Menthol groups | ||||||||||
| Menthol | 19 | 0.83 | (0.70 to 0.92) | 0.85 | (0.74 to 0.93) | 0.81 | (0.66 to 0.91) | 28 | 0.77 | (0.64 to 0.87) |
| Nonmenthol | 26 | 0.69 | (0.53 to 0.83) | 0.81 | (0.69 to 0.90) | 0.80 | (0.68 to 0.90) | 31 | 0.63 | (0.46 to 0.77) |
| Sex | ||||||||||
| Women | 24 | 0.80 | (0.67 to 0.90) | 0.76 | (0.62 to 0.88) | 0.77 | (0.63 to 0.88) | 23 | 0.77 | (0.62 to 0.88) |
| Men | 21 | 0.72 | (0.55 to 0.86) | 0.82 | (0.70 to 0.92) | 0.85 | (0.74 to 0.93) | 36 | 0.64 | (0.49 to 0.77) |
| Placebo e-cig (0 mg/mL) | Nicotine e-cig (36 mg/mL) | |||||||||
| Study 2 | n | ICC | (95% CI) | ICC | (95% CI) | |||||
| Overall | 27 | 0.59 | (0.41 to 0.76) | 0.90 | (0.83 to 0.95) | |||||
| Menthol groups | ||||||||||
| Menthol | 09 | 0.57 | (0.25 to 0.86) | 0.94 | (0.85 to 0.98) | |||||
| Nonmenthol | 18 | 0.54 | (0.30 to 0.76) | 0.86 | (0.74 to 0.94) | |||||
| Sex | ||||||||||
| Women | 15 | 0.84 | (0.70 to 0.94) | 0.91 | (0.82 to 0.97) | |||||
| Men | 12 | 0.43 | (0.15 to 0.74) | 0.87 | (0.72 to 0.96) | |||||
ICC = intraclass correlation coefficient; CI = confidence interval; cig = cigarette; e-cig = electronic cigarette.
Comparison Analyses
For the noninstructed smoking of own brand in the comparison sample, the RM ANOVA is also shown in Table 1; the ICC values and 95% CIs for topography results are shown in Table 2; and the means per trial for each measure are also displayed at the bottom of Figure 1. As would be expected, total exposure was much higher in the uninstructed sample versus study 1, with 570.39 (21.44) versus 301.79 (24.55) mL for total volume and 12.14 (0.33) versus 5.97 (0.39) for puff number, respectively. Ignoring this expected vast difference in total volume and puff number, comparisons between samples in puff duration and IPI may be more informative, as they assess each puff considered individually. Mean puff duration was shorter in the uninstructed versus study 1, 1.59 (0.06) versus 1.89 (0.07) seconds, respectively, whereas IPI did not differ (perhaps surprisingly; see Figure 1). Better topography control with versus without our instructed puffing procedure also may be shown by the consistency of values across the four exposure trials to own brand within each study sample (comparison versus study 1), as the interaction of Study × Trial was significant for total volume, IPI, and number of puffs, but not puff duration (Table 1). As shown in Figure 1, volume and puff number declined and IPI got longer across trials for the ad libitum smoking sample compared to the consistency across trials observed in study 1, presented earlier. However, although ICC values for the uninstructed sample in Table 2 were somewhat lower than those for the instructed puffing sample in study 1 when using their own brand of cigarette, the differences were not statistically significant.
Study 2
Study 2 gauged generalizability of our computerized puffing procedure for controlling topography to dependent smokers administered e-cigarettes with nicotine versus no nicotine (placebo) content, very similar to the within-subjects design of Quest 1 nicotine versus Quest 3 denic tobacco cigarettes in study 1. In study 2, just as with study 1, each smoker took a fixed number of puffs from the designated e-cigarette four times per occasion, only one e-cigarette per occasion, under blind conditions after abstinence prior to both sessions. An own cigarette session after no overnight abstinence was not assessed here, given stark differences between tobacco and e-cigarette products in how they are used.
Methods
Participants
Participants in study 2 were adult-dependent smokers with little e-cigarette experience (N = 27; 12M, 15 W). All were from a prior study examining reinforcement-enhancing effects of nicotine via e-cigarettes,32 as a direct follow-up to the research comprising study 1 mentioned earlier and using virtually identical procedures. All again met the same dependence eligibility criteria as in study 1, as well as having no current or history of more than once per week e-cigarette use. Respective mean (± SD) tobacco smoking characteristics were 15.5 ± 5.1 cigarettes/day, 4.4 ± 1.9 on the FTND, and nicotine yield of their preferred tobacco cigarette brand of 1.1 ± 0.1 mg (ns of 18 nonmenthol, or 67%, and 9 menthol, or 33%). They were 26.8 ± 6.6 years of age, and men and women did not differ on these smoking characteristics or age. They mostly self-identified as Caucasian (74.1%), with 22.2% African American, and 3.7% more than one ethnicity. No comparison sample of uninstructed e-cigarette use was included in study 2.
Procedures
Participants first attended a screening session to provide informed consent and confirm eligibility, followed by the practice period with the topography procedure using an unlit cigarette (as in study 1) to ensure familiarity with how to follow the timing of puffing instructions shown on the monitor. Then, as in the sessions comparing Quest 1 versus Quest 3 exposures in study 1, subjects abstained overnight from tobacco smoking prior to intermittent e-cigarette use during two sessions on separate days, differing in e-cigarette nicotine content and scheduled in counterbalanced order between days. Participants provided CO upon arrival to each session to confirm compliance with the abstinence instructions (CO ≤ 10 ppm). Before each of four trials, 20 minutes apart, participants self-administered 10 puffs over 5 minutes from the designated e-cigarette, one puff every 30 seconds, similar to exposure procedures from recent studies assessing acute e-cigarette effects.33 Again, the precise timing and durations of each puff were guided by the computer-presented instructions (Supplementary Figure), but with two modifications: (1) increase to 10 for the number of puffs presented, and (2) increase per puff from 2-second to a 4-second “hold” duration for e-cigarettes, as often observed in similar research.14,34 Also as in study 1, all puffing was done via portable CReSS Pocket, this time with an e-cigarette adapter (E-Cig Adaptor 9.00 mm), and both device and adaptor were obtained from Borgwaldt KC, Inc. This protocol was approved by the University of Pittsburgh Institutional Review Board.
The e-cigarettes were obtained from PrimeVapor LLC (Pleasant Prairie, WI), labeled as containing 36 or 0 mg nicotine content per milliliter of liquid in vegetable glycerin, with prefilled cartridges (www.primevapor.com). These different nicotine contents are the same as those tested in many other acute studies of e-cigarettes as a function of nicotine content.21,35 The nicotine and placebo versions were “Rawhide Red (Tobacco)” for nonmenthol and “Freeport (Menthol)” for menthol, all provided with a KR808D-1 type automatic E-cigarette battery.
Data Preparation and Analysis Approach
As with study 1, total puff volume per trial was the main dependent variable in study 2, with puff number, mean IPI, and mean puff inhalation duration (each per trial) as secondary dependent variables. Across all participants and e-cigarette conditions, there were a total of 2224 individual puffs. Examination of trials deviating from 10 puffs identified 45 puffs (2.02% of all puffs) with IPIs less than 1000 ms and another 22 puffs (0.99% of all puffs) with a combination of IPIs less than 2000 ms and durations less than 1000 ms. Consistent with study 1, puff volume and puff duration (inhalation) from these stutter puffs were combined with the preceding puff and means for puff duration and IPI, along with the number of puffs, were then calculated per trial.
Analyses were similar to those used in study 1. Preliminary analyses used separate 2 × 4 RM ANOVA to assess within-subjects differences in puff volume, puff duration, IPI, and absolute number of puffs as a function of e-cigarette conditions (2) and trial number (1–4). Partial eta squared (η2p) was calculated as a measure of effect size for each ANOVA. Pairwise comparisons using a Bonferroni correction for multiple comparisons were conducted to follow up any significant main effects. Again, primary analyses used two-way mixed model Type C ICCs31 to assess reliability in total puff volume across trials within each e-cigarette condition. Differences in ICC values between e-cigarette conditions, menthol preference, and sex were determined by examining overlap of the 95% CIs.
Results
Preliminary Analyses
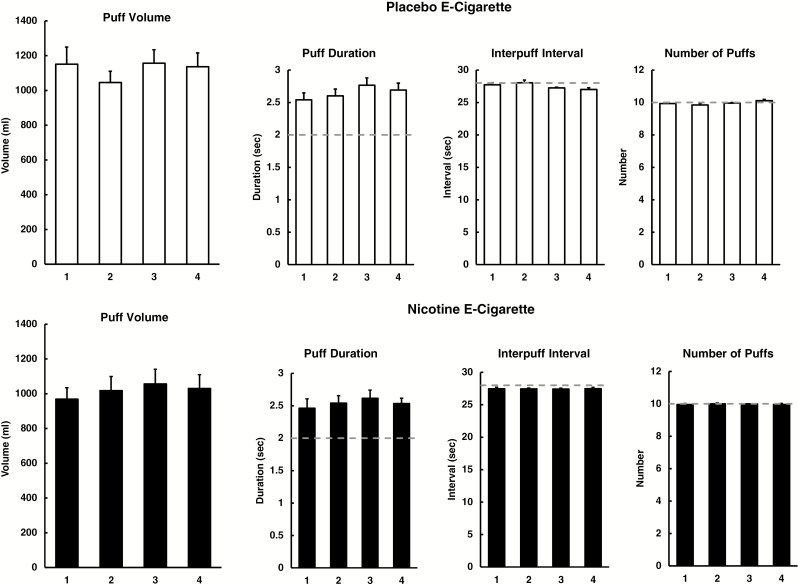
As shown in the bottom half of Table 1, none of the topography measures varied significantly as a function of e-cigarette condition, trial, or the E-cigarette × Trial interaction. Taken together, our puffing procedure minimized within-subject variability in these topography measures, as intended.
Primary Analyses
Means (±SEM) for the topography measures by trial, separately for each e-cigarette condition, are shown in Figure 2. ICC values and 95% CI for study 2 are displayed in the bottom half of Table 2, by e-cigarette condition overall and then separately for the subgroups differing by menthol preference and sex. Consistency in total puff volume across trials was shown in all men and women when vaping on the nicotine e-cigarette, and among women with the placebo e-cigarette, with ICC values at least as strong as those for study 1 (top half of Table 2). However, surprisingly given the RM ANOVA results (Table 1), consistency was lower among men vaping on the placebo e-cigarette, resulting in an overall difference between the nicotine and placebo e-cigarette conditions, indicated by non-overlapping 95% CI bands. Comparing outcomes between menthol preference subgroups, ICC values were similar within each but generally lower for the placebo e-cigarette in both subgroups, due to low ICCs for a few men vaping on the menthol or nonmenthol placebo e-cigarette.
Figure 2.
Means for puff volume, and for measures of puff inhalation duration, interpuff interval, and puff number, in each of the four exposure trials with the nicotine and placebo electronic cigarette (e-cigarette) in study 2. Dashed lines indicate expected values due to instructions for fixed timing and puff number of e-cigarettes during puffing procedure.
Trial level puff volumes were examined for men to follow up the low ICCs for the placebo e-cigarette in this subgroup. Of these 12 men, 3 participants (1 menthol, 2 nonmenthol) had puff volumes per trial during the placebo e-cigarette condition that differed by more than twofold (eg, 2072, 556, 2100, and 1252 mL in trials 1–4 for one of these three men). Exploratory analyses examined the impact of these participants’ erratic puffing behavior by calculating the ICCs without these participants. After excluding these three men, ICCs (95% CI) for the remaining nine indicated much greater consistency in puff volumes across trials between placebo and nicotine e-cigarette conditions, 0.85 (0.75 to 0.93) and 0.83 (0.71 to 0.91), respectively, very similar to the ICCs for the 15 women (Table 2).
General Discussion
Results of this research document strong control over acute puffing topography from these inhaled products with the use of our computerized procedure, instructing smokers or vapers on precisely when and how long to inhale a puff (ie, puff duration), hold it, and then exhale it. Consistency of exposure via puff volume and other topography measures across four trials was shown when participants smoked different tobacco cigarettes in study 1 and puffed on e-cigarettes differing in nicotine content in study 2. No subgroup differences in consistency of topography per trial (ICCs) were observed between men and women smokers, or between smokers differing in menthol preference (although some men in study 2 were less consistent in puffing on the placebo e-cigarette, as discussed in more detail later). In the study 1 comparison analyses, we also found generally better topography control under our instructional procedure in study 1 versus no instructions (ad libitum) in the comparison sample, both involving those smoking own brand of tobacco cigarettes in unblinded manner after no abstinence.
Note that our evaluation was designed to compare consistency of puff topography values within smokers across exposures from the same inhaled product within a session, and from different products between sessions. Such control should aid within-subject analyses of responding to fixed amounts of acute exposure between cigarettes explicitly differing on certain constituents (eg, nicotine content),7 as we intended for this procedure. Although our procedure also minimized variability in exposure between smokers, it did not eliminate it completely, pointing to a need for further refinement of these instructional procedures to ensure identical puff topography when comparing responses to different cigarettes in between-groups analyses.
Moreover, consistency of puff topography was strong with the Quest 1 versus Quest 3 cigarettes in study 1, relative to prior research showing variable topography from these cigarettes under uninstructed conditions.36 Yet, the mean volume was higher for the denic Quest 1 compared to mean volumes for nicotine Quest 1 or the nicotine own brand. This difference perhaps suggests that smoking “compensation”37 cannot be completely overcome with widely varying nicotine commercial cigarettes using these instructions alone. The very similar topography results between the two higher nicotine cigarettes, Quest 1 (after abstinence) and own brand (when not abstinent), indicate the factors differing between those sessions, that is presession abstinence or knowledge of the brand being smoked,28 apparently did not alter topography. Although we saw good control over vape exposure from the nicotine e-cigarette, a bit less control was observed with use of placebo e-cigarettes in some men, perhaps reflecting their attempts at compensation during occasional trials, common with users of e-cigarettes.23,34
A potential limitation with this research was the use in study 2 of smokers without regular experience using e-cigarettes, along with the inadequacy of using an unlit tobacco cigarette to practice our topography procedure during the screening session prior to the sessions with e-cigarette exposure trials. E-cigarette use requires puffing in a manner different from puffing on a tobacco cigarette,38 and those less experienced with e-cigarette use may not take puffs on e-cigarettes as smoothly or consistently as experienced vapers21 (eg, see also the longer mean puff inhalation durations in Figure 2). Yet, wide variability was pronounced in only some men and only when those three were using a placebo e-cigarette, and not in any men when using the nicotine e-cigarette or women under either e-cigarette condition. In sum, replication of study 2 is needed to confirm control over topography by those more familiar with using e-cigarettes, particularly when administering very low or no nicotine products, as well as when manipulating other components of e-cigarette delivery (eg, power settings).2 Individual variability in topography with e-cigarettes may be significant among dual users with differential experience smoking combustible cigarettes.39
Another limitation with all this research is inability of the CReSS to assess the precise duration of the puff hold after the duration of puff inhalation, as that involves removing the cigarette from the mouth (thus, not providing any flow to be assessed). Consequently, compliance with our instruction to exhale cannot be evaluated (see Supplementary Figure). Thus, research relating differential responses to smoking with the assessed puff volumes and timing alone may not include all the relevant topography data needed to fully account for exposure to smoking. Although hold durations have been shown to not alter nicotine retention during controlled smoking, hold duration may affect CO and other smoke constituent exposure.40 Finally, our uninstructed comparison sample involved testing topography across the four trials occurring on four different sessions, rather than consecutively in one session, as in study 1. This difference in timing between smoking trials could have limited the validity of this comparison of topography from own brand after no abstinence between assessments with versus without our instructional procedure. On the other hand, the somewhat similar means between study samples in puff duration and IPI may confirm that the timing of these specific puff behaviors in our procedure’s instructions closely conforms to naturalistic ad libitum smoking puff behaviors, as also shown in prior research on ad libitum smoking.41
Future research may need to compare topography control with our procedure from smoking in a more “conventional” manner, that is, not through the CReSS device, as the device may alter smoking enjoyability42 but does not appear to systematically alter topography.15,19 As noted in Introduction, this procedure does not necessarily require use of a topography assessment device such as the CReSS. It likely can be used to improve control over exposure from conventional use of inhaled nicotine products during acute assessments, but that outcome remains to be confirmed in future research. Moreover, the minimal training required of participants (see practice periods at start of Procedures) further indicates the feasibility of using this procedure in controlling acute smoking exposure, although more extensive training may be required for inexperienced e-cigarette users. We previously used this same automated procedure to carefully control puff topography while smoking commercial cigarettes widely differing in nicotine yield but not via the CReSS, showing no differences in plasma nicotine rise due to each cigarette between men and women, but other measures of exposure were not assessed.43,44 We have also used this procedure to control smoke exposure via CReSS in research using two types of menthol and nonmenthol Spectrum research cigarettes differing in nicotine content, showing virtually identical topography regardless of their nicotine or menthol content.7 However, we could not include those data as part of this evaluation because of the random order of administering both types of cigarettes within the same session (and a smaller number of puffs per trial).
Additional future research should examine the procedure’s control of topography when smoking or vaping under different environmental manipulations,9,10 such as those causing acute negative affect.45 Toward that end, and to conduct such assessments under more naturalistic conditions, our PowerPoint-driven topography instructions could be modified and administered by smart phones for use during mobile assessment of inhaled product use while controlling topography46,47 with or without including the CReSS Pocket device. Our procedure may also be effective in controlling total smoke exposure from a larger bout of smoking, and not just individual puffs. Finally, additional study is needed to verify this procedure is also effective in controlling topography when used with other inhaled products, such as other smoked tobacco (eg, cigars, hookah, pipe), other nonsmoked tobacco (heat-not-burned cigarettes), or newer versions of e-cigarettes or other emerging nontobacco nicotine products,22,48–50 pending their ability to allow careful control over exposure during a defined bout of use.
Funding
Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under grant award DA035774.
Declaration of Interests
None declared.
Supplementary Material
Acknowledgments
The authors thank Valerie Michael for helping with data collection in study 2 of this report.
References
- 1. Bridges RB, Combs JG, Humble JW, Turbek JA, Rehm SR, Haley NJ. Puffing topography as a determinant of smoke exposure. Pharmacol Biochem Behav. 1990;37(1):29–39. [DOI] [PubMed] [Google Scholar]
- 2. Farsalinos KE, Poulas K, Voudris V. Changes in puffing topography and nicotine consumption depending on the power setting of electronic cigarettes. Nicotine Tob Res. 2018;20(8):993–997. [DOI] [PubMed] [Google Scholar]
- 3. McClure EA, Saladin ME, Baker NL, Carpenter MJ, Gray KM. Smoking topography and abstinence in adult female smokers. Addict Behav. 2013;38(12):2833–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA. The reliability of puff topography and subjective responses during ad lib smoking of a single cigarette. Nicotine Tob Res. 2012;14(4):490–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tidey JW, Cassidy RN, Miller ME. Smoking topography characteristics of very low nicotine content cigarettes, with and without nicotine replacement, in smokers with schizophrenia and controls. Nicotine Tob Res. 2016;18(9):1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brauer LH, Hatsukami D, Hanson K, Shiffman S. Smoking topography in tobacco chippers and dependent smokers. Addict Behav. 1996;21(2):233–238. [DOI] [PubMed] [Google Scholar]
- 7. Perkins KA, Karelitz JL, Kunkle N. Evaluation of menthol per se on acute perceptions and behavioral choice of cigarettes differing in nicotine content. J Psychopharmacol. 2018;32(3):324–331. [DOI] [PubMed] [Google Scholar]
- 8. Perkins KA, Karelitz JL, Kunkle N. Sex differences in subjective responses to moderate versus very low nicotine content cigarettes. Nicotine Tob Res. 2018;20(10):1258–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. June KM, Norton KJ, Rees VW, O’Connor RJ. Influence of measurement setting and home smoking policy on smoking topography. Addict Behav. 2012;37(1):42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schneider T, De Jesus S, Prapavessis H. The effect of acute exercise on smoking topography: no evidence for cutting down one puff at a time. J Smoking Cessation. 2014;10(2):146–153. [Google Scholar]
- 11. Hussain S, Zawertailo L, Busto U, Zack M, Farvolden P, Selby P. The impact of chronic bupropion on plasma cotinine and on the subjective effects of ad lib smoking: a randomized controlled trial in unmotivated smokers. Addict Behav. 2010;35(2):164–167. [DOI] [PubMed] [Google Scholar]
- 12. Hatsukami DK, Heishman SJ, Vogel RI, et al. Dose–response effects of Spectrum research cigarettes. Nicotine Tob Res. 2013;15(6):1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoffman AC, Evans SE. Abuse potential of non-nicotine tobacco smoke components: acetaldehyde, nornicotine, cotinine, and anabasine. Nicotine Tob Res. 2013;15(3):622–632. [DOI] [PubMed] [Google Scholar]
- 14. St Helen G, Shahid M, Chu S, Benowitz NL. Impact of e-liquid flavors on e-cigarette vaping behavior. Drug Alcohol Depend. 2018;189(1):42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee EM, Malson JL, Waters AJ, Moolchan ET, Pickworth WB. Smoking topography: reliability and validity in dependent smokers. Nicotine Tob Res. 2003;5(5):673–679. [DOI] [PubMed] [Google Scholar]
- 16. Shahab L, Hammond D, O’Connor RJ, et al. The reliability and validity of self-reported puffing behavior: evidence from a cross-national study. Nicotine Tob Res. 2008;10(5):867–874. [DOI] [PubMed] [Google Scholar]
- 17. Pomerleau OF, Pomerleau CS, Rose JE. Controlled dosing of nicotine: a review of problems and progress. Annals Behav Med. 1989;11(4):158–163. [Google Scholar]
- 18. Weinhold LL, Stitzer ML. Effects of puff number and puff spacing on carbon monoxide exposure from commercial brand cigarettes. Pharmacol Biochem Behav. 1989;33(4):853–858. [DOI] [PubMed] [Google Scholar]
- 19. Blank MD, Disharoon S, Eissenberg T. Comparison of methods for measurement of smoking behavior: mouthpiece-based computerized devices versus direct observation. Nicotine Tob Res. 2009;11(7):896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Purkis SW, Troude V, Hill CA. Effect of puffing intensity on cigarette smoke yields. Regul Toxicol Pharmacol. 2013;66(1):72–82. [DOI] [PubMed] [Google Scholar]
- 21. Hiler M, Breland A, Spindle T, et al. Electronic cigarette user plasma nicotine concentration, puff topography, heart rate, and subjective effects: influence of liquid nicotine concentration and user experience. Exp Clin Psychopharmacol. 2017;25(5):380–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Farsalinos KE, Yannovits N, Sarri T, Voudris V, Poulas K. Nicotine delivery to the aerosol of a heat-not-burn tobacco product: comparison with a tobacco cigarette and e-cigarettes. Nicotine Tob Res. 2018;20(8):1004–1009. [DOI] [PubMed] [Google Scholar]
- 23. Kosmider L, Kimber CF, Kurek J, Corcoran O, Dawkins LE. Compensatory puffing with lower nicotine concentration e-liquids increases carbonyl exposure in e-cigarette aerosols. Nicotine Tob Res. 2018;20(8):998–1003. [DOI] [PubMed] [Google Scholar]
- 24. Perkins KA, Karelitz JL. Reinforcement enhancing effects of nicotine via smoking. Psychopharmacology 2013;228(3):479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perkins KA, Karelitz JL. Sensory reinforcement-enhancing effects of nicotine via smoking. Exp Clin Psychopharmacol. 2014;22(6):511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 27. Perkins KA, Karelitz JL, Conklin CA, Sayette MA, Giedgowd GE. Acute negative affect relief from smoking depends on the affect situation and measure but not on nicotine. Biol Psychiatry. 2010;67(8):707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Perkins KA, Karelitz JL. Acute perceptions of preferred cigarettes when blinded to brand. Tob Control. In press. [DOI] [PubMed] [Google Scholar]
- 29. Microsoft PowerPoint Video Training https://support.office.com/en-us/article/powerpoint-video-training-40e8c930-cb0b-40d8-82c4-bd53d3398787. Accessed June 27, 2018.
- 30. Benowitz NL, Jacob P, Ahijevych K, et al. (SRNT subcommittee). Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. [DOI] [PubMed] [Google Scholar]
- 31. McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychol Meth. 1996;1(1):30–46. Retrieved from http://ft.csa.com/ids70 [Google Scholar]
- 32. Perkins KA, Karelitz JL, Michael VC. Reinforcement enhancing effects of acute nicotine via electronic cigarettes. Drug Alcohol Depend. 2015;153(1):104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vansickel AR, Eissenberg T. Electronic cigarettes: effective nicotine delivery after acute administration. Nicotine Tob Res. 2013;15(1):267–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Spindle TR, Hiler MM, Breland AB, Karaoghlanian NV, Shihadeh AL, Eissenberg T. The influence of a mouthpiece-based topography measurement device on electronic cigarette user’s plasma nicotine concentration, heart rate, and subjective effects under directed and ad libitum use conditions. Nicotine Tob Res. 2017;19(4):469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lopez AA, Hiler MM, Soule EK, et al. Effects of electronic cigarette liquid nicotine concentration on plasma nicotine and puff topography in tobacco cigarette smokers: a preliminary report. Nicotine Tob Res. 2016;18(5):720–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Strasser AA, Lerman C, Sanborn PM, Pickworth WB, Feldman EA. New lower nicotine cigarettes can produce compensatory smoking and increased carbon monoxide exposure. Drug Alcohol Depend. 2007;86(2-3):294–300. [DOI] [PubMed] [Google Scholar]
- 37. Marian C, O’Connor RJ, Djordjevic MV, Rees VW, Hatsukami DK, Shields PG. Reconciling human smoking behavior and machine smoking patterns: implications for understanding smoking behavior and the impact on laboratory studies. Cancer Epidemiol Biomarkers Prev. 2009;18(12):3305–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Simonavicius E, McNeill A, Arnott D, Brose LS. What factors are associated with current smokers using or stopping e-cigarette use?Drug Alcohol Depend. 2017;173(1):139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee YO, Nonnemaker JM, Bradfield B, Hensel EC, Robinson RJ. Examining daily electronic cigarette puff topography among established and nonestablished cigarette smokers in their natural environment. Nicotine Tob Res. 2018;20(10):1283–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zacny JP, Stitzer ML, Brown FJ, Yingling JE, Griffiths RR. Human cigarette smoking: effects of puff and inhalation parameters on smoke exposure. J Pharmacol Exp Ther. 1987;240(2):554–564. [PubMed] [Google Scholar]
- 41. Woodman G, Newman SP, Pavia D, Clarke SW. Inhaled smoke volume and puff indices with cigarettes of different tar and nicotine levels. Eur J Respir Dis. 1987;70(3):187–192. [PubMed] [Google Scholar]
- 42. Ross KC, Juliano LM. Smoking through a topography device diminishes some of the acute rewarding effects of smoking. Nicotine Tob Res. 2016;18(5):564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Perkins KA, Jacobs L, Sanders M, Caggiula AR. Sex differences in the subjective and reinforcing effects of cigarette nicotine dose. Psychopharmacology (Berl). 2002;163(2):194–201. [DOI] [PubMed] [Google Scholar]
- 44. Perkins KA, Sexton JE, Reynolds WA, Grobe JE, Fonte C, Stiller RL. Comparison of acute subjective and heart rate effects of nicotine intake via tobacco smoking versus nasal spray. Pharmacol Biochem Behav. 1994;47(2):295–299. [DOI] [PubMed] [Google Scholar]
- 45. Parkerson HA, Asmundson GJG. The role of pain intensity and smoking expectancies on smoking urge and behavior following experimental pain induction. Drug Alcohol Depend. 2016;164(1):166–171. [DOI] [PubMed] [Google Scholar]
- 46. Karelitz JL, Michael VC, Boldry M, Perkins KA. Validating use of internet-submitted carbon monoxide values by video to determine quit status. Nicotine Tob Res. 2017;19(8):990–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mikheev VB, Buehler SS, Brinkman MC, et al. The application of commercially available mobile cigarette topography devices for e-cigarette vaping behavior measurements. Nicotine Tob Res. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cobb CO, Blank MD, Morlett A, et al. Comparison of puff topography, toxicant exposure, and subjective effects in low- and high-frequency waterpipe users: a double-blind, placebo-control study. Nicotine Tob Res. 2015;17(6):667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Huang J, Duan Z, Kwok J, et al. Vaping versus JUULing: how the extraordinary growth and marketing of JUUL transformed the US retail e-cigarette market. Tob Control. In press. doi: 10.1136/tobaccocontrol-2018-054382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rosenberry ZR, Pickworth WB, Koszowski B. Large cigars: smoking topography and toxicant exposure. Nicotine Tob Res. 2018;20(2):183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.