Abstract
Background
The aim of this study was to investigate the differences in oncological outcome and inflammatory biomarkers between right‐sided colon cancer (RCC) and left‐sided colorectal cancer (LCRC).
Methods
We retrospectively analyzed 339 patients with stage I‐III colorectal cancer, including 125 RCC patients and 214 LCRC patients, who underwent radical resection from January 2012 to January 2014. Comparison of neutrophil‐to‐lymphocyte ratio (NLR), platelet‐to‐lymphocyte ratio (PLR), and lymphocyte‐to‐monocyte ratio (LMR) between RCC and LCRC was evaluated using the Mann‐Whitney U test. Overall survival (OS) and disease‐free survival (DFS) were analyzed using Kaplan‐Meier analysis and compared using the log‐rank test. Univariate and multivariate Cox regression analyses were used to identify the prognostic value of inflammatory markers.
Results
Patients with RCC had higher NLR (P = .002) and PLR (P < .001) but lower LMR (P = .002) compared to LCRC. In stage I‐III, RCC showed poorer OS and DFS than LCRC (61.6% vs 71.5%, P = .018; 64.8% vs 76.2%, P = .006). Univariate and multivariate analyses indicated that NLR, PLR, and LMR were independent predictors for both OS and DFS in RCC, whereas only PLR was found to be an independent prognostic predictor in LCRC.
Conclusion
The prognosis and prognostic value of inflammatory biomarkers were significantly different between RCC and LCRC. Novel therapeutic strategies are needed, and proper prognostic predictors should be selected according to colorectal tumor location.
Keywords: inflammatory biomarkers, right/left‐sided colorectal adenocarcinoma, lymphocyte‐to‐monocyte ratio, neutrophil‐to‐lymphocyte ratio, oncological outcomes, overall survival, platelet‐to‐lymphocyte ratio
1. INTRODUCTION
Colorectal cancer (CRC) is one of the common cancers around the world, the morbidity and mortality rates of which ranked third in 2018.1 Currently, colorectal cancer is divided into right‐sided colon cancer (RCC) and left‐sided colorectal cancer (LCRC).2 Accumulating studies have demonstrated that RCC and LCRC behave differently in terms of genetic expression, embryologic development, epidemiology, clinicopathological characteristics, and even overall survival.2, 3, 4, 5 Patients with RCC are more likely to be older and female and have larger, more advanced tumors that are poorly differentiated with microsatellite instability‐high (MSI‐high) and 5’‐C‐phosphate‐G‐3’ (CpG) island methylation phenotype‐high (CIMP‐high) phenotypes; these patients typically also have a higher number of BRAF mutations and are more likely to have worse survival outcomes.5, 6, 7 Although differences in oncological outcomes based on tumor sidedness have been reported, it is still controversial. Most studies have reported that RCC patients have worse prognosis compared with LCRC patients.5, 8, 9, 10 However, recent studies have found that there is no difference in 5‐year mortality between RCC and LCRC patients.11
An increasing number of studies have shown that inflammation plays a crucial role in the pathogenesis, development, and progression of various cancers, including colorectal cancer.12, 13, 14 Growing evidence has indicated that a systemic inflammatory response negatively correlates with postoperative survival in CRC patients.15 The systemic inflammatory state can be represented by the level of a variety of biomarkers, such as neutrophil‐to‐lymphocyte ratio (NLR), platelet‐to‐lymphocyte ratio (PLR), lymphocyte‐to‐monocyte ratio (LMR), C‐reactive protein (CRP), procalcitonin (PCT), and erythrocyte sedimentation rate (ESR). However, CRP, PCT, and ESR are not routinely detected in clinical treatment for CRC. In contrast, NLR, PLR, and LMR are simple and easy to measure, so they are widely used in clinical practice. At the same time, recent research has suggested that NLR, PLR, and LMR may be predictors of overall survival in CRC.15, 16 Guo et al17reported that different inflammatory factors exhibit different prognostic roles in unresectable RCC and LCRC. However, the differences in inflammatory biomarkers and their prognostic value between stage I‐III RCC and LCRC are unclear. Therefore, in the present study, we aimed to compare oncological outcomes and inflammatory biomarkers between right‐sided and left‐sided stage I‐III CRC after curative resection.
2. MATERIALS AND METHODS
2.1. Patients
This study included 339 consecutive patients with primary CRC who underwent radical resection in the Department of Gastrointestinal Surgery, the First Affiliated Hospital of Shantou University Medical College between January 2012 and January 2014. The inclusion criteria for patient enrollment were as follows: (a) Patients histologically confirmed to have colorectal adenocarcinoma; (b) patients diagnosed with stage I to III cancer according to the 8th edition of the American Joint Committee on Cancer (AJCC); and (c) patients with complete clinicopathologic and follow‐up data. The exclusion criteria were as follows: (a) a previous history of malignant diseases; (b) preoperative anti‐cancer treatment; (c) evidence of infectious diseases, blood diseases, tissue diseases, immunological diseases, other gastrointestinal diseases, or other cancers; and (d) emergency surgery due to bowel obstruction or perforation. None of the included patients died of surgical complications. Patients with stage III and high‐risk stage II were generally offered 5‐fluorouracil‐based adjuvant chemotherapy. The adjuvant chemotherapy regimen was as follows: oxaliplatin 85 mg/m2 intravenously over 2 hours on day 1, leucovorin 400 mg/m2 intravenously over 2 hours on day 1, 5‐ fluorouracil 400 mg/m2 intravenously bolus on day 1, and then 1200 mg/m2/day for 2 days (total 2400 mg/m2 over 46‐48 hours) continuous infusion, repeated every 2 weeks for a total of 24 weeks.
2.2. Definitions
Currently, there is no consensus on the demarcation between RCC and LCRC. Moreover, the distal third of the transverse colon, which is the embryological boundary line, was difficult to determine in retrospective analyses. Therefore, the splenic flexure was used to distinguish RCC and LCRC in most of the available clinical reports.18, 19, 20 In this study, RCC was defined as a tumor localized from the cecum to transverse colon, and LCRC was defined as a tumor localized from the splenic flexure to rectum. All blood tests were performed within one week before surgery, and the counts of neutrophils (N), platelets (P), lymphocytes (L), and monocytes (M) were measured for calculation of the NLR (N/L), PLR (P/L), and LMR (L/M).
Disease‐free survival (DFS) was defined as the time from the date of surgery to the date of the detection of recurrence, death, or last follow‐up. Overall survival (OS) was defined as the time from the date of operation to the date of death or last follow‐up. The latest follow‐up was conducted on January 31, 2019. The protocol was approved by the Ethics Committee of the First Affiliated Hospital of Shantou University Medical College, China.
2.3. Statistical analysis
All statistical analyses were performed using SPSS software version 24.0 (SPSS). The Kolmogorov‐Smirnov test was selected to assess the normality of continuous variables. Student's t test was used for normally distributed parameters, but otherwise the Mann‐Whitney U test was performed. Categorical variables were analyzed using the chi‐square test or Fisher exact test. The DFS and OS rates were examined using the Kaplan‐Meier method, and the difference was compared using the log‐rank test. The relation between inflammatory biomarkers and prognosis was analyzed using Cox proportional hazards regression. Variables found to be statistically significant in univariate analysis were further assessed with multivariate Cox models using a forward stepwise method. A P‐value <.05 was considered to be significant.
3. RESULTS
3.1. Baseline characteristics
In total, 339 cases were enrolled in the study, including 125 patients with RCC and 214 patients with LCRC. The comparison of baseline characteristics and clinicopathological features according to the tumor location is shown in Table 1.
Table 1.
Clinicopathological characteristics of RCC and LCRC patients
| Characteristics | RCC (%) | LCRC (%) | P‐value |
|---|---|---|---|
| Number of patients | 125 | 214 | |
| Age (years, mean ± SD) | 64.2 ± 11.7 | 61.4 ± 11.5 | .028 |
| Gender | |||
| Male | 67 (53.6%) | 131 (61.2%) | .170 |
| Female | 58 (46.4%) | 83 (38.8%) | |
| T stage | |||
| T1 | 5 (4.0%) | 13 (6.1%) | .650 |
| T2 | 16 (12.8%) | 32 (15.0%) | |
| T3 | 53 (42.4%) | 94 (43.9%) | |
| T4 | 51 (40.8%) | 75 (35.0%) | |
| N stage | |||
| N0 | 65 (52.0%) | 125 (58.4%) | .032 |
| N1 | 32 (25.6%) | 64 (29.9%) | |
| N2 | 28 (22.4%) | 25 (11.7%) | |
| TNM stage | |||
| I | 11 (8.8%) | 34 (15.9%) | .155 |
| II | 54 (43.2%) | 91 (42.5%) | |
| III | 60 (48.0%) | 89 (41.6%) | |
| Differentiation | |||
| Poor | 39 (31.2%) | 43 (20.1%) | .021 |
| Well/moderate | 86 (68.8%) | 171 (79.9%) | |
| Lymphovascular invasion | |||
| + | 65 (52.0%) | 87 (40.7%) | .043 |
| − | 60 (48.0%) | 127 (59.3%) | |
| Perineural invasion | |||
| + | 29 (23.2%) | 41 (19.2%) | .375 |
| − | 96 (73.8%) | 173 (80.8%) | |
| Number of harvested lymph node | |||
| ≥12 | 103 (82.4%) | 172 (80.4%) | .646 |
| <12 | 22 (17.6%) | 42 (19.6%) | |
| Surgical complications | |||
| Yes | 19 (15.2%) | 27 (12.6) | .503 |
| No | 106 (84.8%) | 187 (87.4) | |
| Adjuvant chemotherapy | |||
| Yes | 103 (82.4%) | 165 (77.1%) | .248 |
| No | 22 (17.6%) | 49 (22.9%) | |
| Recurrence | |||
| Local recurrence | 10 (22.7%) | 14 (27.4%) | .649 |
| Metastatic recurrence | 29 (65.9%) | 31 (60.8%) | |
| Both | 5 (11.4%) | 6 (11.8%) | |
| CEA (median, quartile) (ng/mL) | 4.33 (2.28‐9.65) | 4.14 (2.05‐8.76) | .643 |
| NE (median, quartile) (109/L) | 4.68 (3.36‐6.82) | 4.22 (3.17‐5.60) | .105 |
| PLT (median, quartile) (109/L) | 301 (247‐385) | 238 (205‐238) | <.001 |
| LY (median, quartile) (109/L) | 1.72 (1.30‐2.24) | 1.90 (1.48‐2.39) | .016 |
| MO (median, quartile) (109/L) | 0.56 (0.42‐0.68) | 0.52 (0.41‐0.65) | .217 |
| NLR (median, quartile) | 2.6 (1.9‐3.9) | 2.1 (1.5‐3.4) | .002 |
| PLR (median, quartile) | 185.8 (130.6‐274.3) | 127.1 (97.4‐174.4) | <.001 |
| LMR (median, quartile) | 3.2 (2.3‐4.1) | 3.7 (2.7‐4.8) | .002 |
Abbreviations: CEA, carcinoembryonic antigen; LCRC, left‐sided colorectal cancer; LMR, lymphocyte‐monocyte ratio; LY, lymphocytes; MO, monocytes; NE, neutrophils; NLR, neutrophil‐lymphocyte ratio; PLR, platelet‐lymphocyte ratio; PLT, platelets; RCC, right‐sided colon cancer.
Patients with RCC were significantly more likely to be older (mean year, 64.2 vs 61.4, P = .028). In the distribution of gender, there tended to be more females among patients with RCC (46.4% vs 38.8%, P = .170), but this difference was not statistically significant. For TNM stage, N stage was significantly different between the two groups (P = .032), but no significant difference was observed in TNM stage (P = .155) and T stage (P = .650). RCC exhibited more cases of poor differentiation and lymphovascular invasion compared to LCRC (P = .021 and P = .043, respectively).There were no significant differences in perineural invasion, number of harvested lymph nodes, surgical complications, adjuvant chemotherapy, and the pattern of recurrence between RCC and LCRC. Patients with RCC had increased carcinoembryonic antigen, neutrophils, platelets, and monocytes but decreased lymphocytes. However, only platelets (P < .001) and lymphocytes (P = .016) showed significant difference. Compared with LCRC patients, the NLR (P = .002) and PLR (P < .001) were significantly elevated, whereas LMR (P = .002) was obviously lower in RCC patients.
3.2. Oncological outcomes and inflammatory markers
In the survival analyses (Figure 1A,D) with a median follow‐up of 54 months, RCC patients had poorer OS and DFS rates compared with LCRC patients in stage I‐III (61.6% vs 71.5%, P = .018; 64.8% vs 76.2%, P = .006). In the subgroup analyses (Figure 1B,E), no significant differences of OS and DFS were observed according to the tumor location at stage II (68.5% vs 71.4%, P = .589; 70.4% vs 74.7%, P = .464). For Stage III (Figure 1C,F), however, patients with RCC had significantly worse OS and DFS compared to LCRC patients (51.7% vs 64.0%, P = .045; 55.0% vs 68.5%, P = .021).The median patient age, CEA, NLR, PLR, and LMR were 64 years, 4.25 ng/mL, 2.3, 145.2, and 3.5, respectively, and these values were used as cutoffs. Based on these cutoff values, both RCC and LCRC patients were divided into the following two groups for further analysis: high NLR (≥2.3) and low NLR (<2.3); high PLR (≥145.2) and low PLR (<145.2); and high LMR (≥3.5) and low LMR (<3.5).The association between survival and inflammatory markers was analyzed using Kaplan‐Meier analysis. In RCC (Figure 2), patients with high NLR (P = .015; P = .025), high PLR (P = .011; P = .021), and low LMR (P = .018; P = .012) had significantly poorer OS and DFS than those with low NLR, low PLR, and high LMR. For patients with LCRC (Figure 3), high NLR (P = .013) and PLR (P = .004) correlated with significantly shorter OS compared with low NLR and PLR, but no significant correlation was observed between LMR (P = .099) and OS. Similar results were observed between inflammatory markers and DFS (NLR, P = .009; PLR, P = .003; LMR, P = .101).
Figure 1.
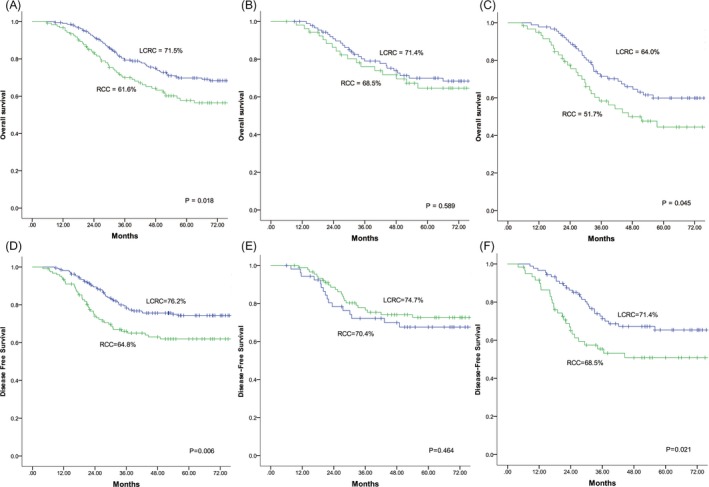
Overall survival and disease‐free survival for patients with RCC vs LCRC after curative surgery. (A, D) Stages I‐III. (B, E) Stage II. (C, F) Stage III. RCC, right‐sided colon cancer; LCRC, left‐sided colorectal cancer
Figure 2.
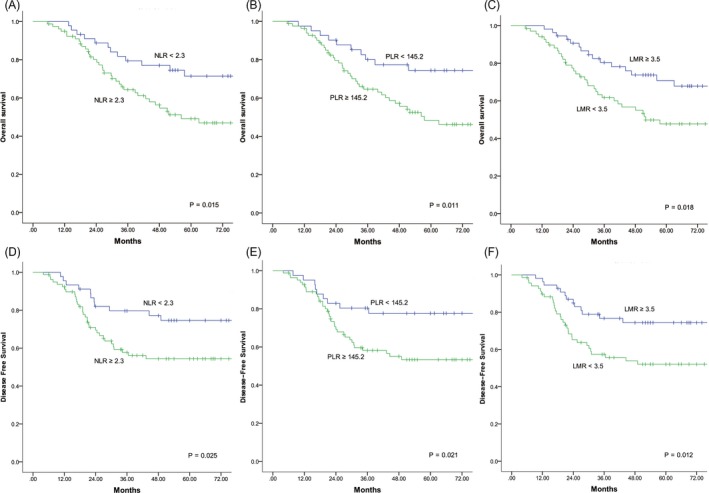
Kaplan‐Meier curves of overall survival (A, B, C) and disease‐free survival (D, E, F) for right‐sided colon cancer patients based on different levels of NLR, PLR, and LMR. NLR, neutrophil‐lymphocyte ratio; PLR, platelet‐lymphocyte ratio; LMR, lymphocyte‐monocyte ratio
Figure 3.
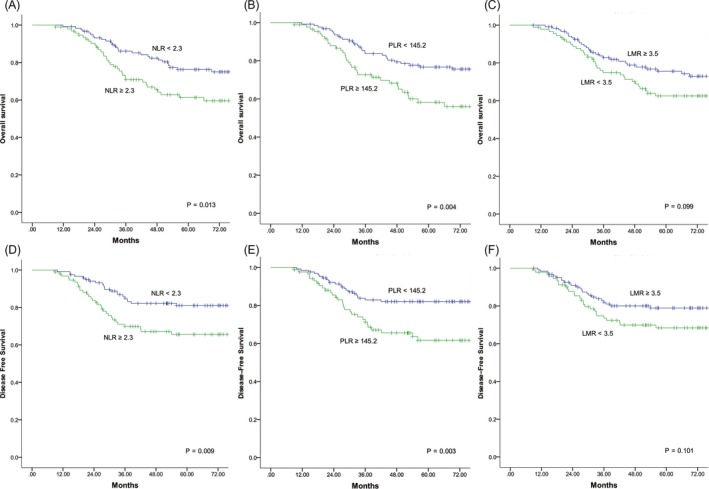
Kaplan‐Meier curves of overall survival (A, B, C) and disease‐free survival (D, E, F) for left‐sided colorectal cancer patients based on different levels of NLR, PLR, and LMR. NLR, neutrophil‐lymphocyte ratio; PLR, platelet‐lymphocyte ratio; LMR, lymphocyte‐monocyte ratio
3.3. Prognostic value of inflammatory biomarkers
In the univariate and multivariate Cox regression analyses, NLR, PLR, LMR, N1‐2 stage, CEA, lymphovascular invasion, and poor differentiation were independent prognostic factors for OS in RCC (Table 2). For LCRC, PLR, T3‐4 stage, N1‐2 stage, CEA, and poor differentiation were significantly associated with worse OS (Table 2).As shown in Table 3, NLR, PLR, LMR, N1‐2 stage, CEA, lymphovascular invasion, and poor differentiation were independent prognostic factors for DFS in RCC, whereas the independent predictors for DFS were T3‐4 stage, N1‐2 stage, poor differentiation, lymphovascular invasion, CEA, and PLR in LCRC.
Table 2.
Univariate and multivariate analyses for prognostic factors of OS after curative resection for stage I‐III RCC and LCRC
| Factors | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |
| RCC | ||||||
| Age (≥64 vs <64 y) | 1.23 | 0.69‐2.17 | .480 | |||
| Gender (male vs female) | 1.46 | 0.82‐2.61 | .197 | |||
| T stage (T3‐4 vs T1‐2) | 2.85 | 1.02‐7.94 | .045 | 1.61 | 0.55‐4.67 | .382 |
| N stage (N1‐2 vs N0) | 2.17 | 1.22‐3.89 | .009 | 1.98 | 1.09‐3.60 | .024 |
| Differentiation (poor vs others) | 2.21 | 1.25‐3.91 | .007 | 1.93 | 1.07‐3.48 | .029 |
| Lymphovascular invasion | 2.53 | 1.39‐4.58 | .002 | 2.13 | 1.13‐4.02 | .020 |
| Perineural invasion | 1.84 | 0.95‐3.55 | .070 | |||
| No. of lymph node (≥12 vs <12) | 1.16 | 0.56‐2.39 | .693 | |||
| CEA(≥4.25 ng/mL vs <4.25 ng/mL) | 1.95 | 1.09‐3.49 | .024 | 1.99 | 1.07‐3.70 | .030 |
| NLR (≥2.3 vs <2.3) | 2.21 | 1.15‐4.25 | .018 | 2.54 | 1.29‐4.98 | .007 |
| PLR (≥145.2 vs < 145.2) | 2.41 | 1.20‐4.85 | .013 | 2.73 | 1.28‐5.83 | .010 |
| LMR (<3.5 vs ≥3.5) | 2.06 | 1.12‐3.79 | .020 | 2.08 | 1.11‐3.89 | .022 |
| LCRC | ||||||
| Age (≥64 vs <64 y) | 1.42 | 0.86‐2.35 | .172 | |||
| Gender (male vs female) | 1.30 | 0.77‐2.20 | .334 | |||
| T stage (T3‐4 vs T1‐2) | 3.30 | 1.42‐7.67 | .006 | 3.39 | 1.43‐8.04 | .005 |
| N stage (N1‐2 vs N0) | 1.98 | 1.20‐3.28 | .008 | 1.94 | 1.17‐3.23 | .011 |
| Differentiation (poor vs others) | 2.48 | 1.45‐4.24 | .001 | 1.66 | 0.94‐2.92 | .082 |
| Lymphovascular invasion | 3.57 | 2.12‐6.03 | <.001 | 4.04 | 2.31‐7.06 | <.001 |
| Perineural invasion | 1.54 | 0.86‐2.76 | .146 | |||
| No. of lymph node (≥12 vs <12) | 1.60 | 0.89‐2.87 | .117 | |||
| CEA(≥4.25 ng/mL vs <4.25 ng/mL) | 2.01 | 1.20‐3.36 | .008 | 2.59 | 1.51‐4.45 | .001 |
| NLR (≥2.3 vs <2.3) | 1.88 | 1.13‐3.11 | .015 | 1.51 | 0.89‐2.55 | .125 |
| PLR (≥145.2 vs <145.2) | 2.05 | 1.24‐3.39 | .005 | 2.69 | 1.57‐4.62 | <.001 |
| LMR (<3.5 vs ≥3.5) | 1.52 | 0.92‐2.52 | .101 | |||
Abbreviations: 95%CI, 95% confidential interval; CEA, carcinoembryonic antigen; HR, hazard ratio; LMR, lymphocyte‐monocyte ratio; NLR, neutrophil‐lymphocyte ratio; OS, overall survival; PLR, platelet‐lymphocyte ratio; RCRC, right‐sided colon cancer.
Table 3.
Univariate and multivariate analyses for prognostic factors of DFS after curative resection for stage I‐III RCC and LCRC
| Factors | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |
| RCC | ||||||
| Age (≥64 vs <64 y) | 1.36 | 0.75‐2.48 | .315 | |||
| Gender (male vs female) | 1.21 | 0.66‐2.19 | .540 | |||
| T stage (T3‐4 vs T1‐2) | 3.48 | 1.08‐11.25 | .037 | 2.07 | 0.62‐6.88 | .235 |
| N stage (N1‐2 vs N0) | 2.16 | 1.17‐3.96 | .013 | 1.98 | 1.09‐3.60 | .036 |
| Differentiation (poor vs others) | 2.31 | 1.27‐4.18 | .006 | 2.02 | 1.10‐3.72 | .024 |
| Lymphovascular invasion | 2.51 | 1.34‐4.69 | .004 | 2.09 | 1.07‐4.10 | .031 |
| Perineural invasion | 1.63 | 0.82‐3.23 | .163 | |||
| No. of lymph node (≥12 vs <12) | 1.33 | 0.59‐2.98 | .495 | |||
| CEA(≥4.25 ng/mL vs <4.25 ng/mL) | 2.07 | 1.12‐3.83 | .021 | 1.95 | 1.02‐3.72 | .044 |
| NLR (≥2.3 vs <2.3) | 2.14 | 1.08‐4.24 | .029 | 2.32 | 1.15‐4.66 | .018 |
| PLR (≥145.2 vs <145.2) | 2.31 | 1.11‐4.81 | .025 | 2.43 | 1.09‐5.42 | .030 |
| LMR (<3.5 vs ≥3.5) | 2.25 | 1.18‐4.31 | .014 | 2.16 | 1.11‐4.20 | .024 |
| LCRC | ||||||
| Age (≥64 vs <64 y) | 1.47 | 0.85‐2.55 | .171 | |||
| Gender (male vs female) | 1.49 | 0.82‐2.69 | .187 | |||
| T stage (T3‐4 vs T1‐2) | 5.42 | 1.69‐17.40 | .005 | 5.16 | 1.58‐16.82 | .007 |
| N stage (N1‐2 vs N0) | 1.96 | 1.13‐3.41 | .017 | 1.85 | 1.06‐3.23 | .031 |
| Differentiation (poor vs others) | 2.94 | 1.67‐5.20 | <.001 | 1.96 | 1.07‐3.60 | .030 |
| Lymphovascular invasion | 3.34 | 1.89‐5.91 | <.001 | 3.54 | 1.92‐6.52 | <.001 |
| Perineural invasion | 1.37 | 0.72‐2.62 | .343 | |||
| No. of lymph node (≥12 vs <12) | 1.40 | 0.73‐2.68 | .310 | |||
| CEA(≥4.25 ng/mL vs <4.25 ng/mL) | 1.90 | 1.08‐3.34 | .025 | 2.45 | 1.35‐4.45 | .003 |
| NLR (≥2.3 vs <2.3) | 2.08 | 1.19‐3.63 | .010 | 1.60 | 0.89‐2.87 | .115 |
| PLR (≥145.2 vs <145.2) | 2.28 | 1.31‐3.97 | .004 | 2.84 | 1.57‐5.15 | .001 |
| LMR (<3.5 vs ≥3.5) | 1.58 | 0.91‐2.74 | .104 | |||
Abbreviations: 95%CI, 95% confidential interval; CEA, carcinoembryonic antigen; DFS, disease‐free survival; HR, hazard ratio; LMR, lymphocyte‐monocyte ratio; NLR, neutrophil‐lymphocyte ratio; PLR, platelet‐lymphocyte ratio; RCRC, right‐sided colon cancer.
4. DISCUSSION
In recent years, more and more studies have been performed to distinguish the differences between right‐sided and left‐sided colorectal cancer. Given the markedly different biological and clinical characteristics, RCC and LCRC are considered as two different disease entities.21, 22 Several publications have reported that older age, females, advanced T stage, node‐positive stage, larger tumor size, and poorer differentiation are more commonly observed for RCC.4, 5, 6
In this study, we found that patients with RCC are older and have tumors with poorer histological grade and more advanced N stage, which was in concordance with the abovementioned studies. For patients with stage I‐III, we found that RCC patients had poorer prognosis compared to LCRC patients. In stage III, survival was significantly worse in RCC compared with that in LCRC, whereas no significant difference of prognosis was found between the two groups in stages I and II. Our results were in agreement with those reported by Meguid et al,23 who identified patients who underwent surgical resection from the SEER database between 1988 and 2003 and found that RCC has poorer prognosis than LCRC. Similarly, Huang et al18 analyzed 1198 consecutive colorectal patients, who received surgical treatment from 2002 to 2008, and they reported significantly poorer OS in stage III patients with right‐sided CRC vs those with left‐sided CRC.
The reason for the difference of oncological outcomes between right‐sided and left‐sided CRC is still unclear. Most studies believe that the differences in embryonic origin, genotypes, and phenotypes play a part.5, 18, 24 The right colon is developed from the midgut, which consists of the cecum, ascending colon, and proximal two‐thirds of the transverse colon. In contrast, the left‐sided colorectum is developed from the hindgut, which is composed of the distal third of the transverse colon, the descending colon, the sigmoid colon, and the upper two‐thirds of the anorectal canal. The right‐sided colon and left‐sided colorectum are mainly supplied by the superior and inferior mesenteric arteries, respectively. Physiologically, the right‐sided colon plays a role of absorbing water, while the left‐sided colorectum assists defecation by peristalsis. Based on the above differences of anatomy and physiology, the range of resection for right hemicolectomy is wider than that for left hemicolectomy or anterior resection, causing more damage to the function of the large intestine, which may lead to worse survival in RCC patients after operation. Narayanan et al25 found that higher rates of MSI and mutated KRAS oncogenes may contribute to poorer OS of RCC compared to LCRC patients. Eklof et al26 also found that RCC with an increase in BRAF mutations exhibits poor prognosis. Moreover, RCC usually presents with advanced stage, increased tumor size, poorer differentiation, and more node positivity than LCRC, which are related to inferior survival.27
Interestingly, we found that the level of inflammatory markers was significantly different between the two groups. Patients with right‐sided cancer had significantly higher NLR and PLR but lower level of LMR compared to patients with left‐sided cancer. Although Yang et al28 investigated different expression levels of NLR and PLR between RCC and LCRC, they did not explore the level of LMR. In addition, these researchers found that more LCRC patients express high levels of NLR and PLR, which was inconsistent with our results. However, these researchers did not report whether the different expression is significant. Therefore, more studies with larger sample size are needed to clarify the contradictory results.
The significant difference in inflammatory markers analyzed as independent prognostic factors was the more interesting finding of our study. In RCC, NLR, PLR, and LMR were associated with significantly poorer overall survival and disease‐free survival in univariate and multivariate analyses. However, only PLR was found to be an independent predictor of worse outcome in LCRC. Although many studies have reported that inflammatory markers, such as NLR, PLR, and LMR predict prognosis of patients with CRC,15, 16 no one has reported the different prognostic values of these parameters according to tumor location in stage I‐III CRC. Corrado et al15 analyzed 603 R0 resected CRC patients and found that both NLR and PLR are independent predictors of 5‐year OS. Joseph et al 16 concluded that LMR is a superior prognostic predictor of OS in patients with CRC undergoing curative resection.
Cancer‐related inflammation is an essential process in malignant tumor growth and can enhance cancer cell invasion, proliferation, metastasis, and immune escape.29 Inflammatory cells may produce growth factors for tumor cells, and the tumor microenvironment, in turn, may provide factors that suppress antitumor immune responses.30 Neutrophils not only promote tumor cell invasion, proliferation, metastasis, and angiogenesis but also help cancer cells escape from immune surveillance by secreting reactive oxygen species, proteases, vascular endothelial growth factors, and hepatocyte growth factors.31, 32 Tumor‐associated neutrophils have been observed to promote lung or liver metastasis in CRC.33, 34 Platelets are remarkably activated in cancer patients and contain a plethora of growth and angiogenic factors that all contribute to tumor growth and angiogenesis.35 Furthermore, platelets protect circulating tumor cells from natural killer cell‐mediated lysis by encasing them in a thrombus.36 Lymphocytes play a key role in cytotoxic cell death and inhibit proliferation and migration of tumor cells.37 A decreased number of lymphocytes are indicative of immune surveillance suppression and ineffectiveness of tumor control, which has been reportedly associated with poor prognosis in CRC patients.38 It has also been reported that tumor‐infiltrating lymphocytes (TILs) correlate with peripheral blood lymphocytes.39 TILs are more often observed in advanced CRC where tumors display higher programmed death‐ligand 1 (PD‐L1) expression and a significant correlation with worse OS.40 Circulating monocytes can improve tumor growth and reduce host immunosurveillance.41 Peripheral monocytes migrate into tumor tissue and differentiate into M2 macrophages, promoting immunosuppression, tumor angiogenesis, and metastasis.42 Moreover, circulating monocytes have been found to upregulate PD‐L1 expression to inhibit antitumor T‐cell responses.43 Considering these reasons, elevated NLR and PLR as well as decreased LMR may contribute to poorer survival in RCC patients.
Although increasing evidence has shown that cancer‐related inflammation has a negative impact on survival,30, 44 the mechanism for the significant difference in inflammatory markers between RCC and LCRC is still unknown. Anatomically, the right‐sided colon is connected to the ileum, which contains many lymphoid follicles, and the right‐sided colon mesentery may contain a more complex lymphatic system, which may more easily cause a cancer‐related inflammatory and immune response in RCC. Epidemiologically, our results demonstrated that RCC more frequently involves older people. Older patients may be more likely to have uncontrolled systemic inflammation as well as have inferior immune function. With respect to intestinal flora, invasive bacterial biofilms have been associated with increased interleukin‐6 (IL‐6), which was found in 89% of RCC but in only 12% of LCRC.45 IL‐6 is a pro‐inflammatory factor and is considered as one of the most important cytokines during tumorigenesis and metastasis.46 With regard to molecular mechanisms, phosphoinositide 3‐kinase pathway mutations, which regulate several key events in the inflammatory and immune response,47 are significantly associated with proximal CRC.48 We propose that greater vascular invasion, more advanced tumor stage, and less differentiation of RCC tumors, such as mucinous and signet‐ring cell adenocarcinoma, result in more intense inflammatory reactions. Neutrophilia, thrombocythemia, lymphopenia, and monocytosis suggest an elevated inflammatory status and decreased immune system response, which contribute to poorer prognosis in RCC patients.
In the present study, we found that the survival and inflammatory markers significantly differed between RCC and LCRC, implying that one of the potential mechanisms for different prognoses is different systemic inflammation. Our study was the first to investigate the different prognostic value of inflammatory biomarkers for RCC and LCRC, suggesting that exploring the difference of inflammatory mechanism may be helpful for tailored treatment for CRC according to tumor location. Therefore, further research on the inflammatory mechanism contributing to the prognosis of CRC is needed for identifying key targets, which will improve the oncological outcomes of RCC and LCRC.
This study had several limitations. First, this was a retrospective and single‐center study with small sample size. Thus, multicenter studies with larger sample size should be performed to validate these results. Second, we did not examine other preoperative inflammatory markers, such as CRP. Hence, the difference in other inflammatory biomarkers between RCC and LCRC is unclear. Third, we did not detect the microsatellite instability status of the included patients, which may result in different expression of inflammatory markers. Fourth, only patients who received curative surgery were enrolled, making the results of this study not applicable to patients with stage IV. Fifth, some baseline variables related to surgical risk, such as physiological and operative scores for the enumeration of mortality and morbidity as well as American Society of Anesthesiologists Physical Status and Charlson Comorbidity Index, were not included.
In conclusion, for stage I‐III CRC, RCC patients had poorer OS and DFS compared with LCRC patients. In stage III, worse OS and DFS were significantly observed in RCC patients. Different inflammatory markers showed different levels and prognostic values in RCC and LCRC. RCC and LCRC should be regarded as two heterogeneous entities. Novel therapeutic strategies are needed, and proper prognostic predictors should be selected according to colorectal tumor location.
ACKNOWLEDGMENTS
We would like to thank the Department of Clinical Medicine Laboratory Center, the First Affiliated Hospital of Shantou University Medical College, PR China.
Guo D, Li X, Xie A, et al. Differences in oncological outcomes and inflammatory biomarkers between right‐sided and left‐sided stage I‐III colorectal adenocarcinoma. J Clin Lab Anal. 2020;34:e23132 10.1002/jcla.23132
Dongming Guo and Xinxin Li contributed equally to this work.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7‐30. [DOI] [PubMed] [Google Scholar]
- 2. Bufill JA. Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med. 1990;113(10):779‐788. [DOI] [PubMed] [Google Scholar]
- 3. Birkenkamp‐Demtroder K. Differential gene expression in colon cancer of the caecum versus the sigmoid and rectosigmoid. Gut. 2005;54(3):374‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Powell AGMT, Wallace R, McKee RF, et al. The relationship between tumour site, clinicopathological characteristics and cancer‐specific survival in patients undergoing surgery for colorectal cancer. Colorectal Dis. 2012;14(12):1493‐1499. [DOI] [PubMed] [Google Scholar]
- 5. Benedix F, Kube R, Meyer F, Schmidt U, Gastinger I, Lippert H. Comparison of 17,641 patients with right‐ and left‐sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum. 2010;53(1):57‐64. [DOI] [PubMed] [Google Scholar]
- 6. Saltzstein SL, Behling CA. Age and time as factors in the left‐to‐right shift of the subsite of colorectal adenocarcinoma: a study of 213,383 cases from the California Cancer Registry. J Clin Gastroenterol. 2007;41(2):173‐177. [DOI] [PubMed] [Google Scholar]
- 7. Shen H. Different treatment strategies and molecular features between right‐sided and left‐sided colon cancers. World J Gastroenterol. 2015;21(21):6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hansen IO, Jess P. Possible better long‐term survival in left versus right‐sided colon cancer ‐ a systematic review. Dan Med J. 2012;59(6):A4444. [PubMed] [Google Scholar]
- 9. Suttie SA, Shaikh I, Mullen R, Amin AI, Daniel T, Yalamarthi S. Outcome of right‐ and left‐sided colonic and rectal cancer following surgical resection. Colorectal Dis. 2011;13(8):884‐889. [DOI] [PubMed] [Google Scholar]
- 10. Yahagi M, Okabayashi K, Hasegawa H, Tsuruta M, Kitagawa Y. The Worse prognosis of right‐sided compared with left‐sided colon cancers: a systematic review and meta‐analysis. J Gastrointest Surg. 2016;20(3):648‐655. [DOI] [PubMed] [Google Scholar]
- 11. Weiss JM, Pfau PR, O'Connor ES, et al. Mortality by stage for right‐ versus left‐sided colon cancer: analysis of surveillance, epidemiology, and end results‐medicare data. J Clin Oncol. 2011;29(33):4401‐4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murata M. Inflammation and cancer. Environ Health Prev Med. 2018;23(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuraishy A, Karin M, Grivennikov SI. Tumor promotion via injury‐ and death‐induced inflammation. Immunity. 2011;35(4):467‐477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cho Y, Lee J, Oh J, et al. Inflammatory dietary pattern, IL‐17F genetic variant, and the risk of colorectal cancer. Nutrients. 2018;10(6):724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pedrazzani C, Mantovani G, Fernandes E, et al. Assessment of neutrophil‐to‐lymphocyte ratio, platelet‐to‐lymphocyte ratio and platelet count as predictors of long‐term outcome after R0 resection for colorectal cancer. Sci Rep. 2017;7(1):1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chan JCY, Chan DL, Diakos CI, et al. The lymphocyte‐to‐monocyte ratio is a superior predictor of overall survival in comparison to established biomarkers of resectable colorectal cancer. Ann Surg. 2017;265(3):539‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guo GF, Chen XX, He WZ, et al. Establishment of inflammation biomarkers‐based nomograms to predict prognosis of advanced colorectal cancer patients based on real world data. PLoS ONE. 2018;13(12):e0208547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang C‐W, Tsai H‐L, Huang M‐Y, et al. Different clinicopathologic features and favorable outcomes of patients with stage III left‐sided colon cancer. World J Surg Oncol. 2015;13(1):257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101(5):403‐408. [DOI] [PubMed] [Google Scholar]
- 20. Yang SY, Cho MS, Kim NK. Difference between right‐sided and left‐sided colorectal cancers: from embryology to molecular subtype. Expert Rev Anticancer Ther. 2018;18(4):351‐358. [DOI] [PubMed] [Google Scholar]
- 21. Hussain M, Waqas O, Hassan U, et al. Right‐sided and left‐sided colon cancers are two distinct disease entities: an analysis of 200 cases in Pakistan. Asian Pac J Cancer Prev. 2016;17(5):2545‐2548. [PubMed] [Google Scholar]
- 22. Baran B, Mert Ozupek N, Yerli Tetik N, Acar E, Bekcioglu O, Baskin Y. Difference between left‐sided and right‐sided colorectal cancer: a focused review of literature. Gastroenterol Res. 2018;11(4):264‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meguid RA, Slidell MB, Wolfgang CL, Chang DC, Ahuja N. Is There a difference in survival between right‐ versus left‐sided colon cancers? Ann Surg Oncol. 2008;15(9):2388‐2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jensen CE, Villanueva JY, Loaiza‐Bonilla A. Differences in overall survival and mutation prevalence between right‐ and left‐sided colorectal adenocarcinoma. J Gastrointest Oncol. 2018;9(5):778‐784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Narayanan S, Gabriel E, Attwood K, Boland P, Nurkin S. Association of clinicopathologic and molecular markers on stage‐specific survival of right versus left colon cancer. Clin Colorectal Cancer. 2018;17(4):e671‐e678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eklof V, Wikberg ML, Edin S, et al. The prognostic role of KRAS, BRAF, PIK3CA and PTEN in colorectal cancer. Br J Cancer. 2013;108(10):2153‐2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lim DR, Kuk JK, Kim T, Shin EJ. Comparison of oncological outcomes of right‐sided colon cancer versus left‐sided colon cancer after curative resection: Which side is better outcome? Medicine (Baltimore). 2017;96(42):e8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang L, He W, Kong P, et al. Clinical baseline and prognostic difference of platelet lymphocyte ratio (PLR) in right‐sided and let‐sided colon cancers. BMC Cancer. 2017;17(1):873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation‐induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759‐771. [DOI] [PubMed] [Google Scholar]
- 30. Candido J, Hagemann T. Cancer‐related inflammation. J Clin Immunol. 2013;33(S1):79‐84. [DOI] [PubMed] [Google Scholar]
- 31. Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11(8):519‐531. [DOI] [PubMed] [Google Scholar]
- 32. Imai Y, Kubota Y, Yamamoto S, et al. Neutrophils enhance invasion activity of human cholangiocellular carcinoma and hepatocellular carcinoma cells: an in vitro study. J Gastroenterol Hepatol. 2005;20(2):287‐293. [DOI] [PubMed] [Google Scholar]
- 33. Yamamoto T, Kawada K, Itatani Y, et al. Loss of SMAD4 promotes lung metastasis of colorectal cancer by accumulation of CCR1+ tumor‐associated neutrophils through CCL15‐CCR1 axis. Clin Cancer Res. 2017;23(3):833‐844. [DOI] [PubMed] [Google Scholar]
- 34. Gordon‐Weeks AN, Lim SY, Yuzhalin AE, et al. Neutrophils promote hepatic metastasis growth through fibroblast growth factor 2‐dependent angiogenesis in mice. Hepatology. 2017;65(6):1920‐1935. [DOI] [PubMed] [Google Scholar]
- 35. Plantureux L, Crescence L, Dignat‐George F, Panicot‐Dubois L, Dubois C. Effects of platelets on cancer progression. Thromb Res. 2018;164:S40‐S47. [DOI] [PubMed] [Google Scholar]
- 36. Nieswandt B, Hafner M, Echtenacher B, Mannel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59(6):1295‐1300. [PubMed] [Google Scholar]
- 37. Ferradini L, Miescher S, Stoeck M, et al. Cytotoxic potential despite impaired activation pathways in T lymphocytes infiltrating nasopharyngeal carcinoma. Int J Cancer. 1991;47(3):362‐370. [DOI] [PubMed] [Google Scholar]
- 38. Tanio A, Saito H, Uejima C, et al. A prognostic index for colorectal cancer based on preoperative absolute lymphocyte, monocyte, and neutrophil counts. Surg Today. 2019;49(3):245‐253. [DOI] [PubMed] [Google Scholar]
- 39. Turner N, Wong HL, Templeton A, et al. Analysis of local chronic inflammatory cell infiltrate combined with systemic inflammation improves prognostication in stage II colon cancer independent of standard clinicopathologic criteria. Int J Cancer. 2016;138(3):671‐678. [DOI] [PubMed] [Google Scholar]
- 40. Ko YS, Pyo JS. Clinicopathological significance and prognostic role of tumor‐infiltrating lymphocytes in colorectal cancer. Int J Biol Markers. 2019;34(2):132‐138. [DOI] [PubMed] [Google Scholar]
- 41. Augier S, Ciucci T, Luci C, Carle GF, Blin‐Wakkach C, Wakkach A. Inflammatory blood monocytes contribute to tumor development and represent a privileged target to improve host immunosurveillance. J Immunol. 2010;185(12):7165‐7173. [DOI] [PubMed] [Google Scholar]
- 42. Galdiero MR, Bonavita E, Barajon I, Garlanda C, Mantovani A, Jaillon S. Tumor associated macrophages and neutrophils in cancer. Immunobiology. 2013;218(11):1402‐1410. [DOI] [PubMed] [Google Scholar]
- 43. Matsunaga T, Saito H, Ikeguchi M. Increased B7–H1 and B7–H4 expressions on circulating monocytes and tumor‐associated macrophages are involved in immune evasion in patients with gastric cancer. Yonago Acta Med. 2011;54(1):1‐10. [PMC free article] [PubMed] [Google Scholar]
- 44. Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5(4):263‐274. [DOI] [PubMed] [Google Scholar]
- 45. Dejea CM, Wick EC, Hechenbleikner EM, et al. Microbiota organization is a distinct feature of proximal colorectal cancers. Proc Natl Acad Sci USA. 2014;111(51):18321‐18326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Taniguchi K, Karin M. IL‐6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol. 2014;26(1):54‐74. [DOI] [PubMed] [Google Scholar]
- 47. Hawkins PT, Stephens LR. PI3K signalling in inflammation. Biochim Biophys Acta. 2015;1851(6):882‐897. [DOI] [PubMed] [Google Scholar]
- 48. Lan YT, Jen‐Kou L, Lin CH, et al. Mutations in the RAS and PI3K pathways are associated with metastatic location in colorectal cancers. J Surg Oncol. 2015;111(7):905‐910. [DOI] [PubMed] [Google Scholar]


