Abstract
Background
Although various methods have been developed to directly identify bacteria from positive blood cultures by matrix‐assisted laser desorption ionization time‐of‐flight mass spectrometry (MALDI‐TOF MS), the necessity of using commercial kits still leads to a high cost and long assay time. Moreover, few evaluations of these methods have been conducted. This study aimed to evaluate the feasibility of an optimized MALDI‐TOF MS method for direct identification of bacteria in positive blood cultures.
Methods
A total of 829 non‐repeated positive cultures were collected from July 2018 to August 2019, and direct identification was performed by an optimized MALDI‐TOF MS method. The same positive blood cultures were sub‐cultivated to obtain a single bacterial colony and identified by classical biochemical BD testing, which is the gold standard to compare the accuracy of direct identification of positive blood cultures by MALDI‐TOF MS.
Results
After excluding 7 false‐positive samples from the 829 positive blood cultures, the most accurate rate of direct identification by this optimized MALDI‐TOF MS method was for gram‐negative bacteria (91.5%), followed by gram‐positive bacteria (88.3%), fungi (84.8%), anaerobic bacteria (80%), and other rare bacteria (66.67%).
Conclusion
Common bacteria in positive blood cultures can be identified directly within 1 hour by MALDI‐TOF MS, and thus, this optimized method can be used as a primary identification method by clinicians. Routine implementation of this method may significantly increase the optimal utilization rate of antibiotics and decrease mortality in bacteremia patients.
Keywords: bacteria, blood culture, identification, MALDI‐TOF MS
1. INTRODUCTION
Bloodstream infection (BSI), one of the most common infections in hospitals, has attracted attention due to its high mortality and rapid change.1 Blood culture is one of the most important diagnostic methods for BSI,2 in which Gram staining serves as the first step in the identification of positive blood culture samples according to the traditional report flowchart, followed by isolation, phenotypic and biochemical identification, and drug susceptibility testing, taking a total of 24 or 48 hours to report results to clinicians. This traditional detection method for BSI is thus slow and increases patient mortality.3 In particular, in instances where pathogens are rare or difficult to grow, reporting results regarding these pathogens may take a long time. Therefore, it is critically important to develop methods to rapidly identify pathogens from positive blood culture.4
In 2009, matrix‐assisted laser desorption ionization time‐of‐flight mass spectrometry (MALDI‐TOF MS) was first introduced for clinical microbial identification in Europe5 and in the United States.6 The principle is to identify bacteria according to their protein profiles, which are compared with standard profiles of known bacteria in a database.7 Because this method does not require conventional Gram staining and biochemical reactions, bacterial identification with pure colonies can be completed within a few minutes;8 thus, it is considered to be simple, fast, high‐throughput, and cost‐effective.9 In almost all studies, the accuracy of MS‐based identification was better or equivalent to that of the commonly used biochemical identification methods, but with a short inspection cycle and a reduced cost.10
In recent years, the rapid development of MS technology has shortened the identification time of bacteria from positive blood culture to less than 2 hours when pure colonies are not obtained; thus, MS provides a timely and reliable basis for treating patients with BSI.11 Because patient conditions are relatively critical and complicated, clinicians urgently need to know the identity of causal pathogens. However, the current MS identification from positive blood cultures still requires more time than desired, given the urgency of cases with BSI. Considering these limitations, it is necessary to develop a more rapid, direct identification method for bacterial identification based on MS from positive blood cultures in order to improve treatment strategies. Although various efforts have been made to directly identify bacteria from positive blood cultures by MS, the necessity of using commercial kits still leads to a high cost and long assay time.12 Thus, the aim of this study was to overcome these shortcomings by exploring a simple, rapid, and inexpensive method for direct identification of bacteria from positive blood cultures. Moreover, the efficiency of this optimized method was evaluated by comparing its consistency rate with that of the routine biochemical method used for bacterial identification.
2. MATERIALS AND METHODS
2.1. Sample collection
A retrospective study was conducted to collect 829 bottles of positive blood culture that were sent for clinical examination by the Henan Provincial People's Hospital (Zhengzhou, China) from July 2018 to August 2019. The inclusion criterion for the blood samples was as follows: if the BD automatic blood culture system issued a positive alarm, the positive bottle was removed from the BD automatic blood culture system, confirmed by microscopy after routine smear Gram staining, and included in the study. The exclusion criterion was as follows: if the positive culture was found to be bacteria‐free by microscopy after routine Gram staining, the sample was excluded from the study. For patients from whom multiple blood cultures were obtained simultaneously, only the blood culture that was first reported to be positive was used for further experiments to avoid repetition.
2.2. Routine biochemical identification
Positive cultures were simultaneously transferred onto a blood agar plate, chocolate agar plate, and MacConkey agar plate (Autobio Diagnostics Ltd), and pure colonies were obtained for routine biochemical identification. The Phoenix 100 automatic biochemical identification system (BD Diagnostics) was used for routine biochemical identification.
2.3. Statistical software
FlexControl 3.0, Biotype 3.0, and ChinProTool 3.0 (Bruker Company) were used to output credit scores of isolates for statistical analyses. Prism 7.0 (GraphPad) software was used to plot the results. Statistical analyses were performed with SPSS 20.0 software (IBM Corporation). Two‐tailed P values <.05 were considered statistically significant.
2.4. Microbe identification by MALDI‐TOF MS
2.4.1. MS pre‐treatment
Through a preliminary experiment, the experimental group was compared by several different specimen‐processing methods, and the standard experimental procedure was established after optimization and improvement: 3.0 mL of blood was taken from the positive culture flask and transferred to a 3.5‐mL tube containing plasma separation gel, which was centrifuged at 3000g for 10 minutes. After discarding the supernatant, 1.0 mL deionized water was added to resuspend the precipitate and then transferred into a new 1.5‐mL Eppendorf tube to repeat the centrifugation. The supernatant was discarded, and the upper liquid was carefully removed to retain the white bacterial membrane. Next, 1 µL of the white bacterial membrane was subjected to MS.
2.4.2. MALDI‐TOF MS analysis
After drying the bacterial pellet on a MALDI‐TOF MS target plate (Bruker Daltonics), 1 μL of alpha‐cyano‐4‐hydroxycinnamic acid (HCCA) matrix solution was placed onto each spot and air‐dried. Triplicate spots were generated for every sample. MALDI‐TOF MS analysis was performed by the Microflex LT system (Bruker Daltonics) with MALDI BIOTYPER 3.3 (Bruker Daltonics) software. Analysis results were presented as an average score of three repeated values for every sample. According to the manufacturer's instructions, a score <1.7 indicates no reliable identification, a score between 1.7 and 1.999 indicates identification to the genus level, and a score ≥2 indicates identification to the species level.
3. RESULTS
3.1. Distribution of pathogens
Seven false‐positive blood bottles were excluded, and from the positive cultures, 822 strains of bacteria and fungi were isolated, including 325 strains of gram‐positive bacteria (39.5%), 402 strains of gram‐negative bacteria (48.9%), 33 strains of fungi (4.01%), 20 strains of anaerobic bacteria (2.4%), and 42 strains of rare bacteria (5.1%). All the samples were identified using Microflex LT/SH MS with a total consistency rate of 88.7%; the highest identification consistency rate was of gram‐negative bacteria, followed by that of gram‐positive bacteria, yeast, and rare bacteria, in that order. There was a significant difference between the identification consistency rates of the different species of bacteria and fungi (P = .002; Figure 1). Additionally, among the 822 strains of bacteria, the MS score for 713 strains (86.7%) was higher than 1.7, and 485 strains (59%) had a score of more than 2.0 (Table S1).
Figure 1.
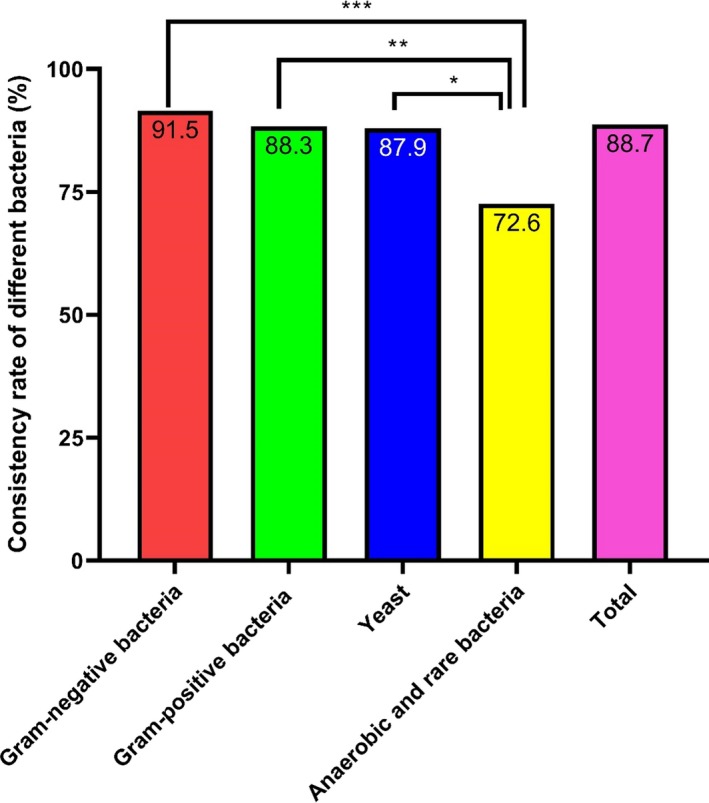
Consistency rates of the pathogens identified by MALDI‐TOF MS. Gram‐negative bacteria had the highest identification consistency rates. *P < .05, **P < .01, and ***P < .001
3.2. Gram‐negative bacteria
Overall, 402 isolates were identified as gram‐negative bacteria. Among them, 91.5% (368/402) were identified consistently. There was a significant difference in consistency rates among different species of gram‐negative bacteria (P < .01). For example, the identification consistency rate was 100% for Pseudomonas aeruginosa, Aeromonas hydrophaga, Klebsiella onionensis, and Baumannii/acinetobacter acetate complex, followed by that for Klebsiella pneumoniae and Escherichia coli (96%; Figure 2). Additionally, among the 402 strains of gram‐negative bacteria, the MS score for 371 strains (92.3%) was identified as at the genus level with a score >1.7; 267 strains (66.4%) were identified as species with a score >2.0 (Table S2).
Figure 2.
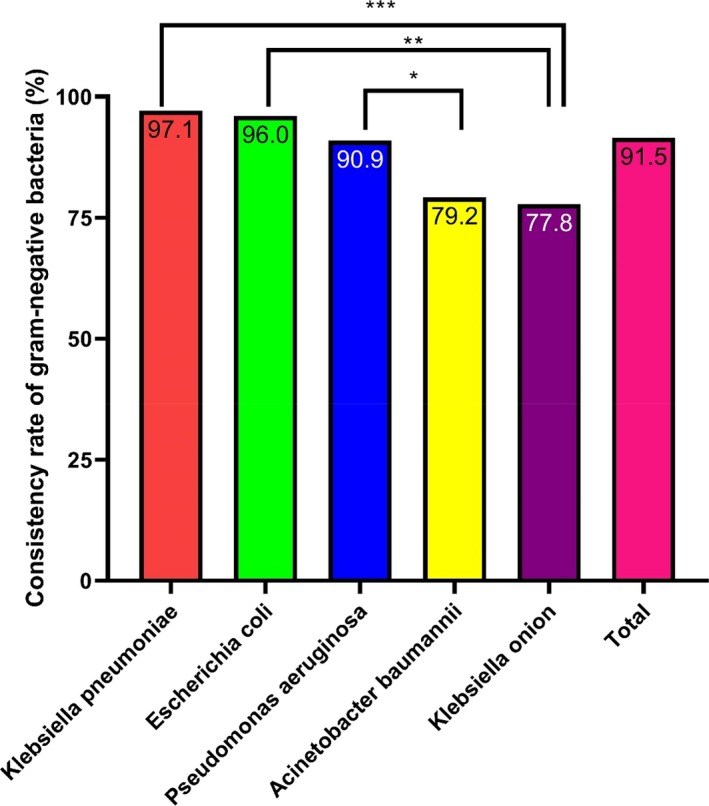
Consistency rates of the gram‐negative bacteria identified by MALDI‐TOF MS Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa had high identification consistency rates. *P < .05, **P < .01, and ***P < .001
3.3. Gram‐positive bacteria
Three hundred and twenty‐five isolates were identified as gram‐positive bacteria. A total of 88.3% (287/325) of the isolates were consistently identified using this optimized method. Among them, the highest consistency rate of identification was of Staphylococcus haemolyticus, Staphylococcus cephalococcus, Staphylococcus goat, and Staphylococcus intermediate, which was 100%, followed by that of Staphylococcus epidermidis, Staphylococcus humanus, and Staphylococcus aureus, which was above 90%. Therefore, there was a significant difference in the consistency rates among different species of gram‐positive bacteria (P < .01; Figure 3). Additionally, among the 325 strains of gram‐positive bacteria, the MS score for 279 strains (85.8%) was higher than 1.7, and 189 strains (58.2%) had a score of more than 2.0 (Table S3).
Figure 3.
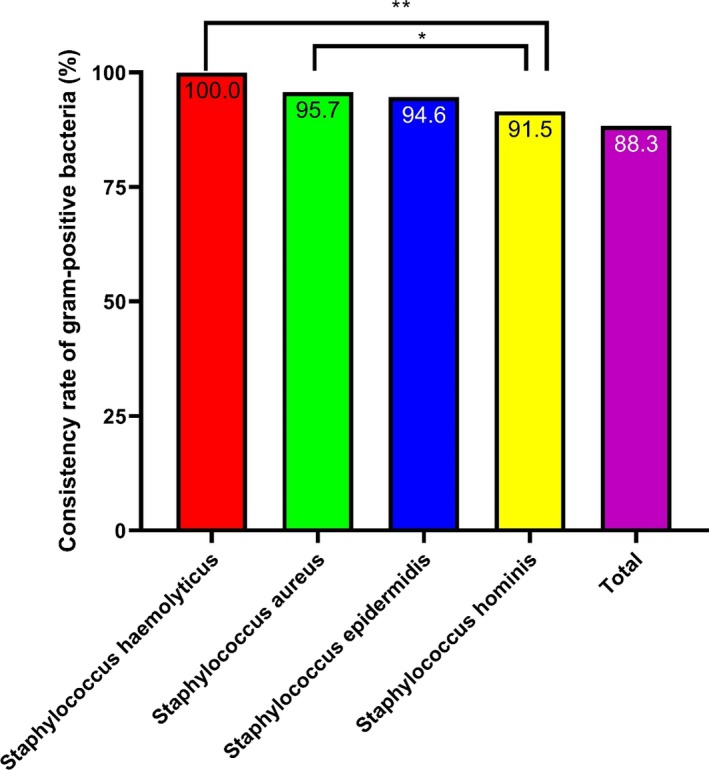
Consistency rates of the gram‐positive bacteria identified by MALDI‐TOF MS Staphylococcus haemolyticus, Staphylococcus epidermidis, and Staphylococcus aureus had high identification consistency rates. *P < .05, **P < .01, and ***P < .001
3.4. Yeasts
Yeasts were isolated from 33 positive blood cultures. Among them, 87.9% (29/33) were identified consistently. Moreover, 25 strains were identified as at the genus level with a score >1.7, and 7 strains were identified as species with a score >2.0. Both Candida parapsilosis and Candida glabrata had an identification consistency rate of 100%, followed by Candida albicans and Candida tropicalis with an identification consistency rate of 80%. There was a significant difference in consistency rates among different species of yeast (P < .001), as shown in Figure 4 and Table S4.
Figure 4.
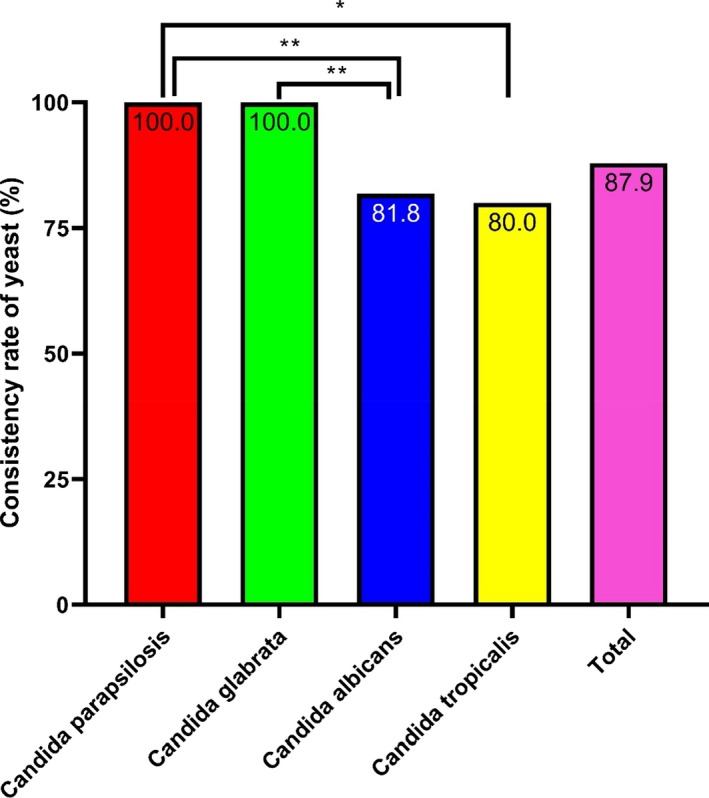
Consistency rates of the yeast identified by MALDI‐TOF MS Candida parapsilosis and Candida glabrata had high identification consistency rates. *P < .05, **P < .01, ***P < .001
3.5. Anaerobic bacteria and rare bacteria
Sixty‐two anaerobic and rare bacteria were isolated in this study; 72.6% (45/62) of the isolates were consistently identified. Moreover, there was a significant difference in the consistency rates among different species of anaerobic bacteria and rare bacteria (P < .001; Figure 5). Further, 39 strains were identified as at the genus level with a score >1.7, and 23 strains were identified as species with a score >2.0, as shown in Table S5.
Figure 5.
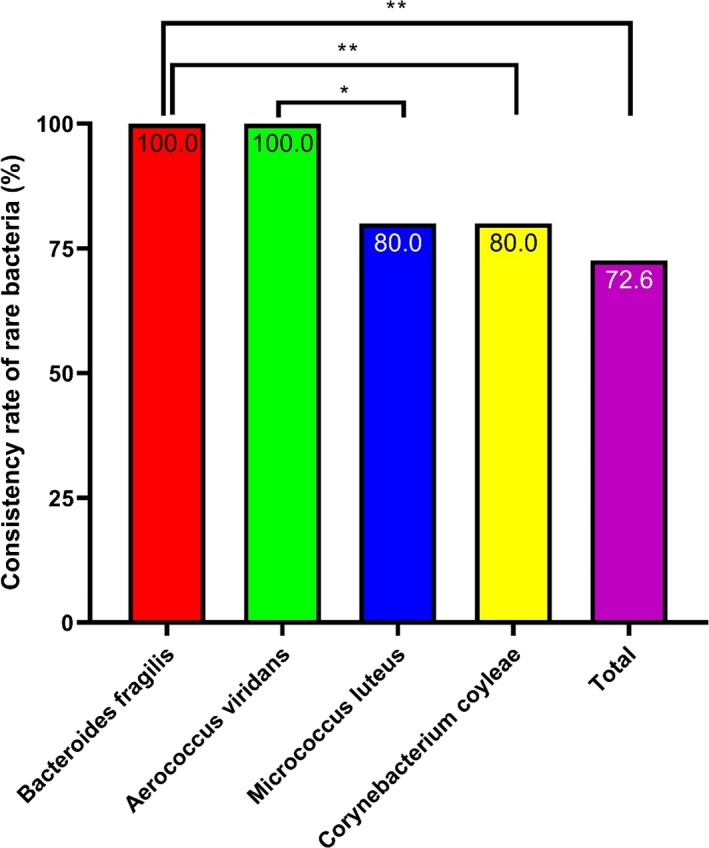
Consistency rates of the rare bacteria identified by MALDI‐TOF MS Bacteroides fragilis and Aerococcus viridans had high identification consistency rates. *P < .05, **P < .01, and ***P < .001
3.6. Aerobic and anaerobic blood culture bottles
After excluding seven false‐positive blood bottles (four aerobic vials and three anaerobic vials), 822 positive vials were obtained from the 829 positive culture vials; these samples included 549 aerobic vials and 273 anaerobic vials. Among these samples, there was a significant difference in the consistency rates among different types of blood bottles (P < .01; Figure 6).
Figure 6.
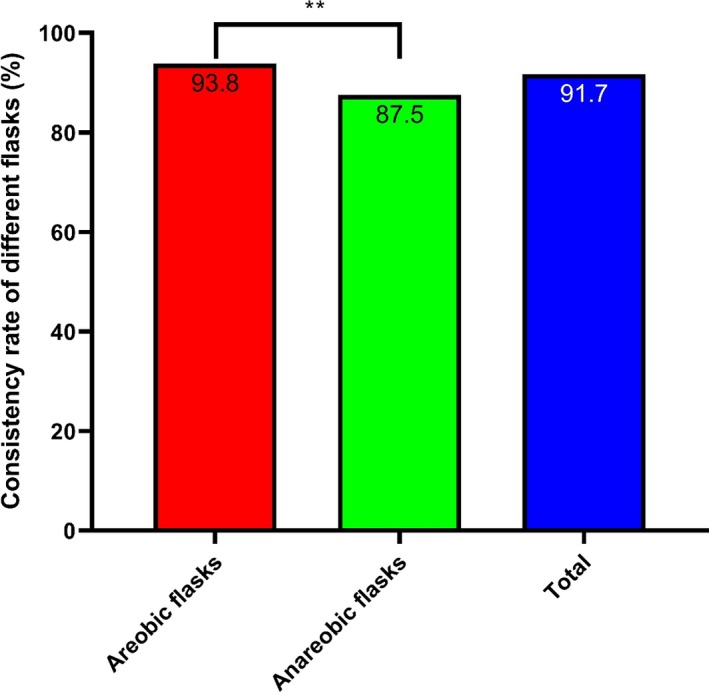
Consistency rates of bacteria from the blood culture flasks identified by MALDI‐TOF MS. Aerobic blood flasks had high identification consistency rates. *P < .05, **P < .01, and ***P < .001
4. DISCUSSION
After the evaluation of identification consistency rates of MALDI‐TOF MS with 822 positive blood samples, we found that by utilizing this optimized identification method, the exact identity of the pathogen responsible for BSI can be obtained within 1 hour, which provides time to administer rapid and accurate treatment to patients with BSI. Compared with traditional phenotypic identification methods, the advantage of this optimized method is that it enables direct and rapid identification of microbes in positive blood culture bottles; thus, it saves about 48 hours, which is typically required for isolation, culturing, and identification.10 Moreover, compared with other MALDI‐TOF MS methods,11, 12 this optimized method requires less time to rapidly and directly identify common bacteria from positive blood culture. Additionally, this method is simple, accurate, and cost‐effective. Further, microbiological laboratory staff can select the appropriate kits for routine drug susceptibility testing based on the identification of bacteria by this method. It is tempting to speculate that routine implementation of this method will significantly increase the optimal utilization rate of antibiotics and further decrease mortality in bacteremia patients.
In this study, we found that gram‐negative bacteria had the highest percentage of credibility scores (greater than 2), followed by gram‐positive bacteria, fungi, anaerobic bacteria, and rare bacteria. These findings were consistent with previous reports, which found that the accuracy of MS for bacterial identification was higher for gram‐negative bacteria than for gram‐positive bacteria.11, 12 Additionally, the accuracy of MS was high in the identification of common bacteria, including common gram‐positive bacteria, such as S aureus, Staphylococcus epidermis, Corynebacterium striatum, Enterococcus faecium, and Enterococcus faecalis, as well as common gram‐negative bacteria, such as E coli, K pneumoniae, P aeruginosa, and Acinetobacter baumannii. We also found that MS showed a high consistency rate in the identification of fungi, such as C parapsilosis and C glabrata; anaerobic bacteria, such as Bacteroides fragilis; and rare bacteria, such as Garcinia micrococcus. The low accuracy of identification of Streptococcus by MS may be related to high similarity between different species in the genus Streptococcus, such as Streptococcus mitis, Streptococcus sanguis, and Streptococcus oralis.13 For Staphylococcus, the main purpose is to distinguish S aureus from coagulase‐negative Staphylococcus.
MS is more accurate in the differential diagnosis of two kinds of similar bacteria. Rapid and accurate identification of bacteria from positive blood culture flasks is the greatest advantage of MS. In our study, the results for identification at the species and genus levels could be obtained from a positive blood culture flask within 1 hour, which is in sharp contrast to the present routine laboratory analysis that can only report the results of Gram staining, and requires an additional 24‐48 hours for species identification.14 It is well known that an identification method that saves time is critical for BSI, especially for patients in need of quick treatment. Therefore, early identification of the causative pathogen may lead to a better, more efficient, and less expensive antibiotic treatment.15 If this technology is adapted and combined with drug resistance detection data in hospitals for the diagnosis and treatment of BSI, anti‐infection treatment plans can be more accurately selected, which can enable curing of pathogenic infections and prevent the overuse of broad‐spectrum antibiotics.16
It should be noted that there are some limitations in this study. For example, due to the number of specimens, the results of the first identification of some bacteria may be a bit far‐fetched. However, the results of this study support the accuracy and rapidity of MS for direct identification of bacteria in positive blood culture flasks. Sample processing procedures, blood culture bottle types, and differences in the distribution of common strains may lead to differences in the results from different studies.17, 18 Despite these limitations, MALDI‐TOF MS has been proven to be a rapid and reliable method for the identification of pathogens directly from blood culture bottles. The technique is inexpensive, the identification can be completed within 1 hour, and specialized personnel is not required.19
In conclusion, this optimized method can be used to rapidly, simply, accurately, and cost‐effectively identify common bacteria in positive blood cultures to meet the clinical demand for rapid diagnosis of common BSI pathogens. To carry out reasonable antibiotic treatment in early clinical stages and improve the survival rate of BSI patients, this method should be promoted.
AUTHORS' CONTRIBUTIONS
YY and JW contributed to the writing of the manuscript; JW, SG, and ZY analyzed and interpreted the clinical data; BM, BW, YL, and NJ analyzed the antimicrobial susceptibility results; YY, SW, QZ, and JZ performed the statistical analyses; and all authors read and approved the final manuscript.
Supporting information
ACKNOWLEDGMENTS
Henan Provincial Key Programs in Science and Technology (182102310576), and Henan Provincial Key Program in Health (201702198) supported this work.
Yuan Y, Wang J, Zhang J, et al. Evaluation of an optimized method to directly identify bacteria from positive blood cultures using MALDI‐TOF mass spectrometry. J Clin Lab Anal. 2020;34:e23119 10.1002/jcla.23119
Youhua Yuan and Junjie Wang contributed equally to this work.
REFERENCES
- 1. Buehler SS, Madison B, Snyder SR, et al. Effectiveness of practices to increase timeliness of providing targeted therapy for inpatients with bloodstream infections: a laboratory medicine best practices systematic review and meta‐analysis. Clin Microbiol Rev. 2016;29:59‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cattoir L, Coorevits L, Leroux‐Roels I, Claeys G, Verhasselt B, Boelens J. Improving timelines in reporting results from positive blood cultures: simulation of impact of rapid identification on therapy on a real‐life cohort. Eur J Clin Microbiol Infect Dis. 2018;37:2253‐2260. [DOI] [PubMed] [Google Scholar]
- 3. Huang CT, Tsai YJ, Tsai PR, Yu CJ, Ko WJ. Severe sepsis and septic shock: timing of septic shock onset matters. Shock. 2016;45:518‐524. [DOI] [PubMed] [Google Scholar]
- 4. Joshi RD, Khadka S, Joshi DM, et al. Antimicrobial sensitivity trend in blood culture positive enteric fever. J Nepal Health Res Counc. 2018;16:228‐232. [PubMed] [Google Scholar]
- 5. Kohlmann R, Hoffmann A, Geis G, Gatermann S. MALDI‐TOF mass spectrometry following short incubation on a solid medium is a valuable tool for rapid pathogen identification from positive blood cultures. Int J Med Microbiol. 2015;305:469‐479. [DOI] [PubMed] [Google Scholar]
- 6. Van Belkum A, Chatellier S, Girard V, Pincus D, Deol P, Dunne WM Jr. Progress in proteomics for clinical microbiology: MALDI‐TOF MS for microbial species identification and more. Expert Rev Proteomics. 2015;12:595‐605. [DOI] [PubMed] [Google Scholar]
- 7. Angeletti S. Matrix assisted laser desorption time of flight mass spectrometry (MALDI‐TOF MS) in clinical microbiology. J Microbiol Methods. 2017;138:20‐29. [DOI] [PubMed] [Google Scholar]
- 8. Van Veen SQ, Claas EC, Kuijper EJ. High‐throughput identification of bacteria and yeast by matrix‐assisted laser desorption ionization‐time of flight mass spectrometry in conventional medical microbiology laboratories. J Clin Microbiol. 2010;48:900‐907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Osthoff M, Gurtler N, Bassetti S, et al. Impact of MALDI‐TOF‐MS‐based identification directly from positive blood cultures on patient management: a controlled clinical trial. Clin Microbiol Infect. 2017;23:78‐85. [DOI] [PubMed] [Google Scholar]
- 10. Sherwin R, Winters ME, Vilke GM, Wardi G. Does early and appropriate antibiotic administration improve mortality in emergency department patients with severe sepsis or septic shock? J Emerg Med. 2017;53:588‐595. [DOI] [PubMed] [Google Scholar]
- 11. Jo SJ, Park KG, Han K, Park DJ, Park YJ. Direct identification and antimicrobial susceptibility testing of bacteria from positive blood culture bottles by matrix‐assisted laser desorption/ionization time‐of‐flight mass spectrometry and the Vitek 2 system. Ann Lab Med. 2016;36:117‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mestas J, Felsenstein S, Bard JD. Direct identification of bacteria from positive BacT/ALERT blood culture bottles using matrix‐assisted laser desorption ionization‐time‐of‐flight mass spectrometry. Diagn Microbiol Infect Dis. 2014;80:193‐196. [DOI] [PubMed] [Google Scholar]
- 13. Ha J, Hong SK, Han GH, Kim M, Yong D, Lee K. Same‐day identification and antimicrobial susceptibility testing of bacteria in positive blood culture broths using short‐term incubation on solid medium with the MicroFlex LT, Vitek‐MS, and Vitek2 systems. Ann Lab Med. 2018;38:235‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin HC, Lu JJ, Lin LC, et al. Identification of a proteomic biomarker associated with invasive ST1, serotype VI Group B Streptococcus by MALDI‐TOF MS. J Microbiol Immunol Infect. 2019;52:81‐89. [DOI] [PubMed] [Google Scholar]
- 15. Van Belkum A, Bachmann TT, Ludke G, et al. Developmental roadmap for antimicrobial susceptibility testing systems. Nat Rev Microbiol. 2019;17:51‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Labriola L. Antibiotic locks for the treatment of catheter‐related blood stream infection: still more hope than data. Semin Dial. 2019;32:402‐405. [DOI] [PubMed] [Google Scholar]
- 17. Rand KH, Delano JP. Direct identification of bacteria in positive blood cultures: comparison of two rapid methods, FilmArray and mass spectrometry. Diagn Microbiol Infect Dis. 2014;79:293‐297. [DOI] [PubMed] [Google Scholar]
- 18. Kato H, Yoshimura Y, Suido Y, et al. Mortality and risk factor analysis for Candida blood stream infection: a multicenter study. J Infect Chemother. 2019;25:341‐345. [DOI] [PubMed] [Google Scholar]
- 19. Becker P, Normand AC, Vanantwerpen G, et al. Identification of fungal isolates by MALDI‐TOF mass spectrometry in veterinary practice: validation of a web application. J Vet Diagn Invest. 2019;31:471‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


