Abstract
Background
The aim of this study was to investigate the infection and antimicrobial resistance of Ureaplasma urealyticum and Mycoplasma hominis in patients with genitourinary symptoms among Hakka population in Meizhou, China.
Methods
A total of 12 633 females and 3315 males who presented urogenital symptoms and were subjected to mycoplasma tests from 2014 to 2018 were enrolled in this study. The mycoplasma detection and antimicrobial susceptibility were tested using the Mycoplasma ID/AST kit.
Results
The total incidence of mycoplasma infection, as well as the incidence of U urealyticum in Hakka population was annually increasing from 2014 to 2018. The total incidences and U urealyticum infection were more prevalent in females than males. Higher positive rate of mycoplasmas infection was observed in women aged 16‐20 (50.9%) and men aged 26‐30 (25.4%). The occurrence of antimicrobial resistance of mycoplasma to antibacterial agents remained relatively similar in the past five years. Ureaplasma urealyticum infection, M hominis infection, and co‐infection of resistance to levofloxacin, erythromycin, ciprofloxacin, ofloxacin, roxithromycin, azithromycin, clarithromycin, and sparfloxacin were dramatically higher in females than in males.
Conclusion
Our findings indicate a high burden of mycoplasmas infection and antimicrobial resistance of mycoplasmas infection among females, and josamycin and minocycline may be recommended as the primary choice in clinical treatment of anti‐mycoplasmas.
Keywords: antimicrobial susceptibility, Hakka, infection, mycoplasma
1. INTRODUCTION
Mycoplasma is known as the smallest free‐living organisms without cell wall. Mycoplasma including Ureaplasma urealyticum (Uu) and Mycoplasma hominis (Mh) causes genital manifestations in both women and men.1 It was estimated that 40%‐80% of healthy adult female infected with Uu and 20%‐50% with Mh in cervix or vagina.2, 3 Meanwhile, Uu infects 20%‐29% healthy males in urogenital tract.4 Previous study reported that genital mycoplasmas were related to urogenital infections, including urethritis, vaginitis, cervicitis, and pelvic inflammatory disease.5 However, it is difficult to prove their pathogenic effect because of their existence in genital tract of healthy human. The prevalence of these organisms is significantly associated with age, socioeconomic status, physiological cycle, pregnancy, and multiple sex partners.6, 7
As Uu and Mh lack of peptidoglycan, β‐lactams are completely inactive against them. Generally, quinolones, tetracyclines, and macrolides are used for the treatment of mycoplasma infection.8 However, drug resistance of mycoplasma increased due to the improper use of the antibiotics. Since antibiotic resistance of many pathogens is continually changing, surveillance studies are required for assisting in the optimization of antimicrobial treatment.
The city of Meizhou, located in southern China, is known as the world's Hakka population capital, with unique population‐based culture and food, as well as different physiological characteristics.9 However, little was known regarding the prevalence and antimicrobial susceptibility of mycoplasma infection in Hakka population.
The purpose of this study was to determine the prevalence and antimicrobial resistance of Uu and Mh in patients of Hakka population and presented genital manifestations. Our study would provide useful information for local epidemiology of mycoplasma, and thus help make the prevention and treatment strategy.
2. MATERIALS AND METHODS
2.1. Patients
A total of 15 948 patients committing mycoplasma examination from April 15, 2014, to December 31, 2018, in the clinical laboratory of Meizhou People's Hospital were enrolled in this study. Eligible patients should meet four criterions: (a) native Hakka who lived in Meizhou for more than three generations; (b) age 16‐89; (c) with suspected symptoms of genital tract infection (including burning sensation or pain during urination, difficulty urinating, frequent urination, and pruritus of vagina); (d) negative for bacterial and fungal culture. This study was approved by the Ethics Committee of Meizhou People's Hospital (Reference No.: MPH‐HEC 2013‐A‐01). Written informed consent was obtained from all participants.
2.2. Specimen collection
Cervical and urethral swabs (Kangjian Medical, Jiangsu, China) were used to collected samples from urogenital tracts. In female patients, cervical samples were obtained by cervical swabs from the cervix area after cleaning the exocervical mucus. In male patients, urethral samples were slowly taken from urethra inside 2 cm after external meatus had been cleaned; semen and prostatic fluid were collected and placed in a sterile cup (Kangjian Medical, Jiangsu, China). All samples were sent at room temperature to the clinical laboratory for examination within 2 hour.
2.3. Culture and antimicrobial susceptibility test of Uu and Mh
The culture and susceptibility testing of Uu and Mh were performed using Mycoplasma ID/AST kit (DL medical), following the manufacture's protocol. Briefly, the specimen swab was inserted into medium flask and mixed intensively to make sample completely dissolve. Then transfer 100 μL of mixture medium to the mycoplasma ID/AST strip. The negative control was added with 100 μL medium. Then the wells were added two drops of the sterile mineral oil and inoculated at 37°C. The results were checked at 24 hours and 48 hours after incubation. The concentration of each organism and resistance or susceptibility to drug was determined on the color change. The susceptibility of mycoplasma toward twelve antibiotics were tested, including tetracycline (TET), levofloxacin (LEV), erythromycin (ERY), josamycin (JOS), doxycycline (DOX), ciprofloxacin (CIP), ofloxacin (OFX), minocycline (MIN), roxithromycin (ROX), azithromycin (AZM), clarithromycin (CLR), and sparfloxacin (SPA).
2.4. Statistical analysis
Data are presented as n (%) prevalence or mean ± SD and were statistically analyzed using SPSS 20.0 software (IBM Corp., Armonk, NY, USA). Quantitative data were analyzed using ANOVA test, and categorical data were analyzed using chi‐squared test. P < .05 was considered statistically significant.
3. RESULTS
3.1. Prevalence of Uu and Mh
Of the 16 118 individuals, 170 patients (112 non‐Hakka and 58 under 16 years of age) were excluded in this study as they did not meet the inclusion criteria. Finally, 15 948 participants were enrolled in this study for analysis, including 12 633 females, aged 34.0 ± 10.0 years, and 3315 males, aged 35.0 ± 11.0 years. Of these females, 5049 (40.0%) were positive for mycoplasma (included Uu, Mh and Uu & Mh infection). Among them, 4038 (32.0%) were positive for Uu, 135 (1.0%) for Mh and 876 (7.0%) for the both. Of clinical samples from males, 748 (22.6%) were positive for mycoplasma. Among them, 660 (20.0%) were positive for Uu, 17 (0.5%) for Mh, and 71 (2.1%) for the both. The positive rates of all three patterns of infection were significantly higher in females than in males, respectively (P < .05).
As shown in Figure 1, the total incidence as well as Uu infection was significantly increased in both women and men from 2014 to 2018 (P < .05). The positive rates of Mh or Uu &Mh infection were relatively stabilized in both women and men from 2014 to 2018 (P > .05).
Figure 1.
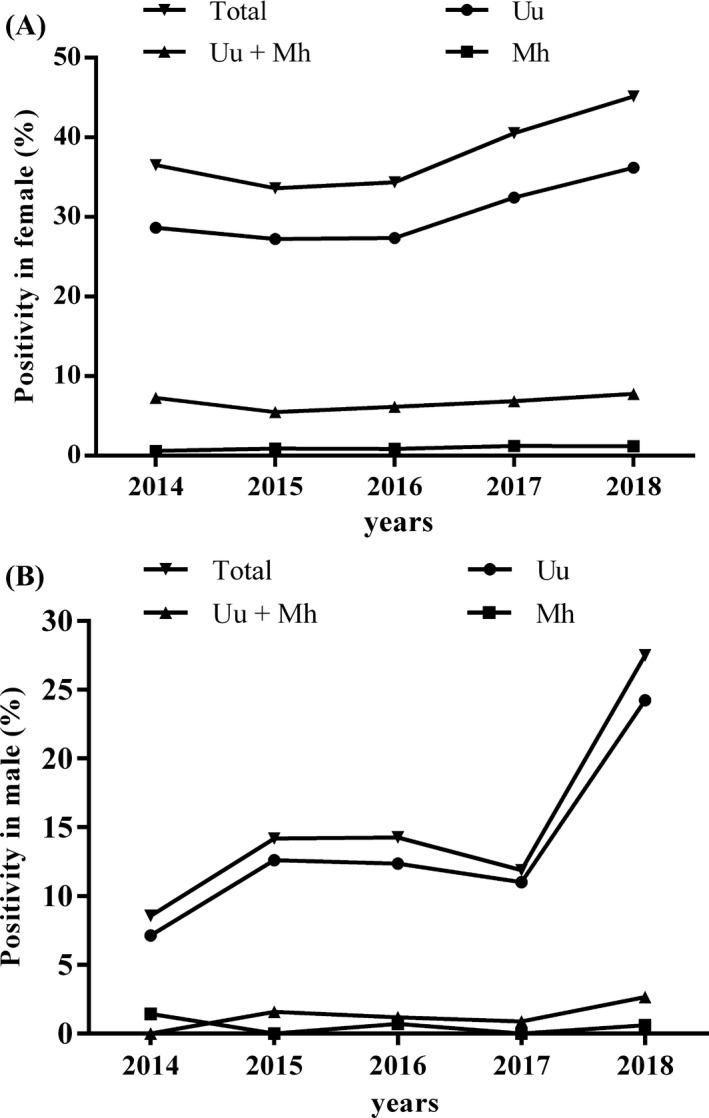
Trend of the prevalence of Uu and Mh in female and male patients
3.2. Age distribution of mycoplasma infection
The Figure 2 presents an age‐specific mycoplasma infection in Hakka population. As for female participants, the positive rates remained high in the age group of 16‐50 and began to go down gradually more than 50 year. To be noted, the infection rates were relatively high in the age groups of 16‐20 and 36‐50. Of the 3315 males enrolled, the positive rate was stable in the age group of 16‐60, and dramatically dropped after 60 years. Comparatively, the occurrence rate of mycoplasma infection was obviously higher in female than male in all of the age groups.
Figure 2.
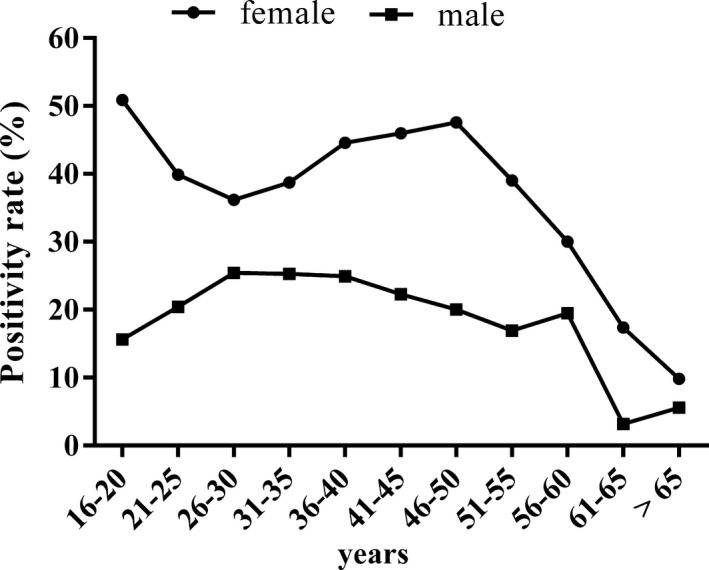
Age‐specific prevalence of Uu and Mh in female and male patients
3.3. Antimicrobial susceptibility patterns from 2014 to 2018
Our study found that Uu displayed low resistance rates to TET, LEV, ERY, JOS, DOX, OFX, MIN, ROX, AZM, CLR, and SPA, while relatively high resistance rates to CIP (>60%), as shown in Table 1. Mh displayed low resistance rates to TET, LEV, JOS, DOX, CIP, OFX, MIN, and SPA, while high resistance rates to ERY (>80%), ROX (>90%), AZM (>90%), and CLR (>85%; Table 2). Furthermore, Uu & Mh infection exhibited low resistance rates to TET, LEV, JOS, DOX, OFX, MIN, and SPA, but much higher to ERY (>95%), CIP (>85%), ROX (>90%), AZM (>90%), and CLR (>90%; Table 3). Increasing trend was not observed in resistance rates of three infection patterns to antibacterial agents from 2014 to 2018.
Table 1.
Antimicrobial resistance rate (%) of Uu infection in patients
| 2014 | 2015 | 2016 | 2017 | 2018 | |
|---|---|---|---|---|---|
| Case (%) | Case (%) | Case (%) | Case (%) | Case (%) | |
| TET | 12 (6.1) | 37 (6.9) | 39 (6.0) | 44 (11.9) | 176 (6.9) |
| LEV | 12 (6.1) | 31 (5.8) | 19 (2.9) | 34 (4.5) | 161 (6.3) |
| ERY | 25 (12.6) | 128 (23.9) | 87 (13.4) | 164 (21.5) | 267 (10.5) |
| JOS | 1 (0.5) | 2 (0.4) | 0 (0.0) | 0 (0.0) | 5 (0.2) |
| DOX | 9 (4.5) | 26 (4.9) | 22 (3.4) | 25 (3.3) | 117 (4.6) |
| CIP | 126 (63.6) | 396 (73.9) | 480 (73.8) | 589 (77.1) | 1881 (73.8) |
| OFX | 11 (5.6) | 27 (5.0) | 21 (3.2) | 44 (5.8) | 89 (3.5) |
| MIN | 9 (4.5) | 25 (4.7) | 21 (3.2) | 25 (3.3) | 104 (4.1) |
| ROX | 12 (6.1) | 53 (9.9) | 29 (4.5) | 86 (11.3) | 114 (4.5) |
| AZM | 10 (5.1) | 32 (6.0) | 10 (1.5) | 26 (3.4) | 58 (2.3) |
| CLR | 9 (4.5) | 26 (4.9) | 11 (1.7) | 25 (3.3) | 54 (2.1) |
| SPA | 15 (7.6) | 89 (16.6) | 76 (11.7) | 195 (25.5) | 261 (10.2) |
| n | 198 | 536 | 650 | 764 | 2550 |
Abbreviations: AZM, azithromycin; CIP, ciprofloxacin; CLR, clarithromycin; DOX, doxycycline; ERY, erythromycin; JOS, josamycin; LEV, levofloxacin; MIN, minocycline; OFX, ofloxacin; ROX, roxithromycin; SPA, sparfloxacin; TET, tetracycline; Uu, Ureaplasma urealyticum.
Table 2.
Antimicrobial resistance rate (%) of Mh infection in patients
| 2014 | 2015 | 2016 | 2017 | 2018 | |
|---|---|---|---|---|---|
| Case (%) | Case (%) | Case (%) | Case (%) | Case (%) | |
| TET | 0 (0.0) | 2 (12.5) | 3 (13.6) | 1 (3.4) | 14 (17.5) |
| LEV | 2 (40.0) | 5 (31.3) | 8 (36.4) | 9 (31.0) | 39 (48.8) |
| ERY | 5 (100.0) | 15 (93.8) | 20 (90.9) | 28 (96.6) | 65 (81.3) |
| JOS | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.3) |
| DOX | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| CIP | 3 (60.0) | 9 (56.3) | 11 (50.0) | 13 (44.8) | 48 (60.0) |
| OFX | 1 (20.0) | 6 (37.5) | 7 (31.8) | 9 (31.0) | 31 (38.8) |
| MIN | 0 (0.0) | 0 (0.0) | 1 (4.5) | 0 (0.0) | 2 (2.5) |
| ROX | 5 (100.0) | 16 (100.0) | 20 (90.9) | 28 (96.6) | 73 (91.3) |
| AZM | 5 (100.0) | 16 (100.0) | 20 (90.9) | 28 (96.6) | 73 (91.3) |
| CLR | 5 (100.0) | 15 (93.8) | 21 (95.5) | 28 (96.6) | 69 (86.3) |
| SPA | 2 (40.0) | 6 (37.5) | 7 (31.8) | 8 (27.6) | 25 (31.3) |
| n | 5 | 16 | 22 | 29 | 80 |
Abbreviations: AZM, azithromycin; CIP, ciprofloxacin; CLR, clarithromycin; DOX, doxycycline; ERY, erythromycin; JOS, josamycin; LEV, levofloxacin; Mh, Mycoplasma hominis; MIN, minocycline; OFX, ofloxacin; ROX, roxithromycin; SPA, sparfloxacin; TET, tetracycline.
Table 3.
Antimicrobial resistance rate (%) of Uu and Mh co‐infection in patients
| 2014 | 2015 | 2016 | 2017 | 2018 | |
|---|---|---|---|---|---|
| Case (%) | Case (%) | Case (%) | Case (%) | Case (%) | |
| TET | 11 (22.4) | 30 (28.6) | 32 (23.0) | 46 (28.6) | 119 (24.1) |
| LEV | 28 (57.1) | 56 (53.3) | 77 (55.4) | 102 (63.4) | 295 (59.8) |
| ERY | 49 (100) | 102 (97.1) | 136 (97.8) | 154 (95.7) | 490 (99.4) |
| JOS | 0 (0.0) | 8 (7.6) | 5 (3.6) | 2 (1.2) | 5 (1.0) |
| DOX | 3 (6.1) | 6 (5.7) | 9 (6.5) | 11 (6.8) | 34 (6.9) |
| CIP | 42 (85.7) | 95 (90.5) | 127 (91.4) | 152 (94.4) | 452 (91.7) |
| OFX | 25 (51.0) | 59 (56.2) | 73 (52.5) | 98 (60.9) | 260 (52.7) |
| MIN | 2 (4.1) | 3 (2.9) | 11 (7.9) | 12 (7.5) | 38 (7.7) |
| ROX | 48 (98.0) | 99 (94.3) | 131 (94.2) | 150 (93.2) | 462 (93.7) |
| AZM | 48 (98.0) | 97 (92.4) | 127 (91.4) | 148 (91.9) | 456 (92.5) |
| CLR | 48 (98.0) | 98 (93.3) | 129 (92.8) | 148 (91.9) | 455 (92.3) |
| SPA | 27 (55.1) | 67 (63.8) | 89 (64.0) | 110 (68.3) | 237 (48.1) |
| n | 49 | 105 | 139 | 161 | 493 |
Abbreviations: AZM, azithromycin; CIP, ciprofloxacin; CLR, clarithromycin; DOX, doxycycline; ERY, erythromycin; JOS, josamycin; LEV, levofloxacin; Mh, Mycoplasma hominis; MIN, minocycline; OFX, ofloxacin; ROX, roxithromycin; SPA, sparfloxacin; TET, tetracycline; Uu, Ureaplasma urealyticum.
As shown in Table 4, the drug resistance was also different between female and male patients. The mycoplasma, either Uu or Mh, that infected females presented higher resistance to TET, LEV, ERY, DOX, OFX, ROX, AZM, CLR, and SPA, as compared with those infected males.
Table 4.
Antimicrobial resistance rate (%) of Uu and Mh infection in patients
| Uu | Mh | Uu & Mh | ||||
|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | |
| TET | 271 (6.7) | 37 (5.6) | 19 (14.1) | 1 (5.9) | 223 (25.5) | 15 (21.1) |
| LEV | 233 (5.8) | 24 (3.6)* | 60 (44.4) | 3 (17.6)* | 527 (60.2) | 31 (43.7)* |
| ERY | 629 (15.6) | 42 (6.4)* | 123 (91.1) | 10 (58.8)* | 850 (97.0) | 61 (85.9)* |
| JOS | 6 (0.2) | 2 (0.3) | 0 (0.0) | 1 (5.9) | 17 (1.9) | 3 (4.2) |
| DOX | 174 (4.3) | 25 (3.8) | 0 (0.0) | 0 (0.0) | 56 (6.4) | 7 (9.9) |
| CIP | 3046 (75.4) | 426 (64.6)* | 78 (57.8) | 6 (35.3)* | 809 (92.4) | 59 (83.1)* |
| OFX | 178 (4.4) | 14 (2.1)* | 53 (39.3) | 1 (5.9)* | 492 (56.2) | 23 (32.4)* |
| MIN | 167 (4.1) | 17 (2.6) | 3 (2.2) | 0 (0.0) | 62 (7.1) | 4 (5.6) |
| ROX | 265 (6.6) | 29 (4.4)* | 130 (96.3) | 12 (70.6)* | 832 (95.0) | 58 (81.7)* |
| AZM | 125 (3.1) | 11 (1.7)* | 129 (95.6) | 13 (76.5)* | 816 (93.2) | 60 (84.5)* |
| CLR | 116 (2.9) | 9 (1.4)* | 125 (92.6) | 13 (76.5)* | 818 (93.4) | 60 (84.5)* |
| SPA | 616 (15.3) | 20 (3.0)* | 47 (34.8) | 1 (5.9)* | 517 (59.0) | 13 (18.3)* |
| n | 4038 | 660 | 135 | 17 | 876 | 71 |
Abbreviations: AZM, azithromycin; CIP, ciprofloxacin; CLR, clarithromycin; DOX, doxycycline; ERY, erythromycin; JOS, josamycin; LEV, levofloxacin; Mh, Mycoplasma hominis; MIN, minocycline; OFX, ofloxacin; ROX, roxithromycin; SPA, sparfloxacin; TET, tetracycline; Uu, Ureaplasma urealyticum.
P < .05.
4. DISCUSSION
To our knowledge, this is the first large‐scale study to investigate the prevalence of mycoplasmas infection and antimicrobial resistance in patients with genital symptoms among Hakka population in Meizhou.
The present study suggested that the incidence of mycoplasmas was 40.0% in females, which is lower than the previous findings in other areas of China,10, 11 but higher than those in Italy.12, 13 Some reasons may contribute to the variety. First, physical state (symptomatic, asymptomatic, and pregnant) of participants, as well as the socioeconomic conditions varied in different studies. Second, these studies used different sample types and sample size that may draw different conclusion.
Our study showed that the incidence of mycoplasmas infection in females increased annually during the past some years, which was similar to the results in Changzhou, China.14 Specifically, positive rate of Uu infection kept up‐going in the past five years, while that of Mh and Uu & Mh infection remained stable. The increasing positive rate in our study may be caused by several reasons. On one hand, we stopped testing urethral swab specimens since 2018, which was also a regular type of genital sample but proved much less positive rate for mycoplasmas infection. On the other hand, more and more people accepted genitourinary tests with the improved living condition and implement of national universal two‐child policy after 2016. However, as previous studies suggested, mycoplasmas infection may not cause diseases, but remained as a normal symbiotic colonization. In the present study, we investigated the prevalence of mycoplasmas in patients who showed urogenital symptoms and were excluded from other bacterial or fungal infections. The high positive rate in these samples highlighted the necessity of mycoplasmas examination, thus to facilitate the diagnosis and develop treatment strategy.
Previous research suggested that the infection level of Uu and Mh was associated with gender and age.15 In the current study, positive rates of three patterns of infection in females were much higher than in males. For one thing, the structure and environment of female genital system is more susceptible for the colonization of mycoplasmas. For another, the cervical specimen had the highest positive rate as reported in other studies.13
Our study found that in Hakka population, the mycoplasmas infection rates peaked in individuals at the age groups of 16‐20 and 36‐50, and dramatically dropped in patients older than 55. The age‐specific infection were similar to other studies.7, 16, 17 The reduced sexual activity of menopausal women may explained the decreased mycoplasmas infections in elderly people.
As for antimicrobial resistance, resistance rates of Uu infection to CIP and resistance rates of Mh infection to ERY, ROX, AZM, and CLR, as well as resistance rates of Uu & Mh infection to ERY, CIP, ROX, AZM, and CLR were higher, which were in consistence with results reported in other areas.18, 19 Interestingly, resistance rates of Uu, Mh and Uu & Mh infection to LEV, ERY, CIP, OFX, ROX, AZM, CLR, and SPA varied between females and males in our study. There are some reasons for this finding. First, female patients infected with mycoplasma were several times more than male patients; Second, female patients generally received more frequent antibiotic treatment in our study, and antibiotic selection pressures were different between females and males. Taking together, Uu and Mh species tested in our study subjects displayed relatively low resistance to TET, JOS, DOX, and MIN. Since TET caused severe side effects, JOS and MIN may be the main choice for Uu and Mh in Hakka population.
Although some novel findings were revealed in this study, there are some limitations needed to be clarified. First, the information of sexual behavior of the enrolled subjects was missing, which may partially influence the conclusion. Second, we did not collect lifestyle information thus could not confirm the role of lifestyle on mycoplasmas infection.
In conclusion, our study for the first time investigated the prevalence of mycoplasmas infection as well as drug resistance in Hakka population. Our data revealed the gender‐ and age‐specific infection distribution and the year‐by‐year positive rates. The findings added knowledge to local epidemiology of mycoplasmas and would be useful for prevention and treatment strategy.
AUTHORS' CONTRIBUTIONS
ZZ conceived and designed the experiments; XG contributed to the data collection and the manuscript draft. SL, XG, and RW helped to collect clinical data and conducted the clinical performances and researches; XG analyzed the data and wrote the paper.
Gu X, Liu S, Guo X, Weng R, Zhong Z. Epidemiological investigation and antimicrobial susceptibility analysis of mycoplasma in patients with genital manifestations. J Clin Lab Anal. 2020;34:e23118 10.1002/jcla.23118
Funding information
This study was supported by the grants from Science and Technology Program of Meizhou (Grant No. 2018B027), Key Scientific and Technological Project of Meizhou People's Hospital (Grant No. MPHKSTP‐20170101).
REFERENCES
- 1. Adelman JS, Kirkpatrick L, Grodio JL, Hawley DM. House finch populations differ in early inflammatory signaling and pathogen tolerance at the peak of Mycoplasma gallisepticum infection. Am Nat. 2013;181(5):674‐689. [DOI] [PubMed] [Google Scholar]
- 2. Waites KB, Brenda K, Schelonka RL. Mycoplasmas and ureaplasmas as neonatal pathogens. Clin Microbiol Rev. 2005;18(4):757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Waites KB, Schelonka RL, Li X, Grigsby PL, Novy MJ. Congenital and opportunistic infections: Ureaplasma species and Mycoplasma hominis . Semin Fetal Neonatal Med. 2009;14(4):190‐199. [DOI] [PubMed] [Google Scholar]
- 4. Paralanov V, Lu J, Duffy LB, et al. Comparative genome analysis of 19 Ureaplasma urealyticum and Ureaplasma parvum strains. BMC Microbiol. 2012;12(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meghan Arvind P, Paul N. Role of Mycoplasma and Ureaplasma species in female lower genital tract infections. Curr Infect Dis Rep. 2010;12(6):417‐422. [DOI] [PubMed] [Google Scholar]
- 6. Taylor‐Robinson D. Mollicutes in vaginal microbiology: Mycoplasma hominis, Ureaplasma urealyticum, Ureaplasma parvum and Mycoplasma genitalium . Res Microbiol. 2017;168(9‐10):875‐881. [DOI] [PubMed] [Google Scholar]
- 7. Bayraktar MR, Ozerol IH, Gucluer N, Celik O. Prevalence and antibiotic susceptibility of Mycoplasma hominis and Ureaplasma urealyticum in pregnant women. Int J Infect Dis. 2016;14(2):e90‐e95. [DOI] [PubMed] [Google Scholar]
- 8. Li X, Crabb DM, Duffy LB, Vanya P, Glass JI, Waites KB. Chromosomal mutations responsible for fluoroquinolone resistance in Ureaplasma species in the United States. Antimicrob Agents Chemother. 2012;56(5):2780‐2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zheng LB, Li YL, Lu SH, et al. Physical characteristics of Chinese Hakka. Sci Chin‐Life Sci. 2013;56(6):541‐551. [DOI] [PubMed] [Google Scholar]
- 10. He M, Xie Y, Zhang R, et al. Prevalence and antimicrobial resistance of Mycoplasmas and Chlamydiae in patients with genital tract infections in Shanghai, China. J Infect Chemother. 2016;22(8):548‐552. [DOI] [PubMed] [Google Scholar]
- 11. Zeng XY, Xin N, Tong XN, Wang JY, Liu ZW. Prevalence and antibiotic susceptibility of Ureaplasma urealyticum and Mycoplasma hominis in Xi'an, China. Eur J Clin Microbiol Infect Dis. 2016;35(12):1‐7. [DOI] [PubMed] [Google Scholar]
- 12. Foschi C, Salvo M, D'Antuono A, et al. Distribution of genital Mollicutes in the vaginal ecosystem of women with different clinical conditions. New Microbiol. 2018;41(3):225‐229. [PubMed] [Google Scholar]
- 13. Foschi C, Salvo M, Galli S, Moroni A, Marangoni A. Prevalence and antimicrobial resistance of genital Mollicutes in Italy over a two‐year period. New Microbiol. 2018;41(2):153‐158. [PubMed] [Google Scholar]
- 14. Zhu C, Liu J, Ling Y, et al. Prevalence and antimicrobial susceptibility of Ureaplasma urealyticum and Mycoplasma hominis in Chinese women with genital infectious diseases. Ind J Dermatol Venereol Leprol. 2012;78(3):406‐407. [DOI] [PubMed] [Google Scholar]
- 15. Tiejun S, Aiqing Y, Xinyou X, et al. Epidemiological investigation and antimicrobial susceptibility analysis of Ureaplasma species and Mycoplasma hominis in outpatients with genital manifestations. J Clin Pathol. 2014;67(9):817‐820. [DOI] [PubMed] [Google Scholar]
- 16. Verteramo R, Patella A, Calzolari E, et al. An epidemiological survey of Mycoplasma hominis and Ureaplasma urealyticum in gynaecological outpatients, Rome, Italy. Epidemiol Infect. 2013;141(12):2650‐2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ye G, Jiang Z, Wang M, Huang J, Jin G, Lu S. The resistance analysis of Ureaplasma urealyticum and Mycoplasma hominis in female reproductive tract specimens. Cell Biochem Biophys. 2014;68(1):207‐210. [DOI] [PubMed] [Google Scholar]
- 18. Francesco MAD, Caracciolo S, Bonfanti C, Manca N. Incidence and antibiotic susceptibility of Mycoplasma hominis and Ureaplasma urealyticum isolated in Brescia, Italy, over 7 years. J Infect Chemother. 2013;19(4):621‐627. [DOI] [PubMed] [Google Scholar]
- 19. Benu D, Neena M, Vishnubhatla S, et al. Ureaplasma serovars & their antimicrobial susceptibility in patients of infertility & genital tract infections. Indian J Med Res. 2012;136(6):991‐996. [PMC free article] [PubMed] [Google Scholar]


