Abstract
A substantial amount of research focuses on the error-related negativity (ERN)—a negative deflection in the event-related potential waveform that occurs when individuals commit errors on lab-based tasks. The ERN has been link to concurrent and prospective risk for psychopathology and is thought to index sensitivity or reactivity to errors. The ERN can be potentiated in the lab with punishment and has been shown to be increased among offspring of harsh or controlling parents. A separate line of work has demonstrated that the ERN is increased among individuals high in perfectionism. In the current study, we integrate these separate lines of work by examining parenting styles, perfectionism and the ERN in a sample of young adults. Results suggest that the ERN is increased among offspring of controlling parents (both maternal and paternal). Additionally, the ERN is increased among individuals who report being high in perfectionism—specifically, the concerns over mistake and the personal standard perfectionism subscales of the Frost Multidimensional Perfectionism Scale. Moreover, results supported a mediation model wherein the indirect pathway from controlling parenting style to perfectionism (personal standard subscale) was mediated by the ERN—for paternal parenting.
Keywords: error-related negativity, ERN, parenting, perfectionism, neural marker
Introduction
Monitoring one’s behavior and awareness of errors is necessary for optimal adaption to a changing environment (Falkenstein et al., 2000; Botvinick et al., 2001; Holroyd and Coles, 2002; van Veen and Carter, 2002). A substantial amount of research has focused on an event-related potential (ERP) related to error monitoring: the error-related negativity (ERN; Falkenstein et al., 1991; Gehring et al., 1993). The ERN is elicited when individuals commit errors on lab-based speeded and reaction time tasks and appears as a negative deflection in the waveform occurring ∼50 ms after an error at fronto-central electrode sites.
The ERN has been proposed to reflect the activation of a general error monitoring system (Falkenstein et al., 1991; Gehring et al., 1993). Errors may be conceptualized as motivationally salient, internal events that may threaten an individual’s safety—requiring immediate attention and corrective action. Errors prompt a variety of physiological changes consistent with this view, i.e. skin conductance, heart rate changes, pupil dilation, potentiated startle reflex and corrugator activity, suggesting a defensive motivational response (Weinberg et al., 2012b, 2016). Source localization and functional magnetic resonance imaging (fMRI) studies that suggest the ERN is generated in the anterior cingulate cortex (Dehaene et al., 1994; Kiehl et al., 2000; Menon et al., 2001; Mathalon et al., 2003; Beckmann et al., 2009; Agam et al., 2011)—an area of the brain associated with the integration of pain, threat and punishment to alter future behavior (Shackman et al., 2011).
The magnitude of the ERN has been associated with a variety of individual differences. Most notably, the ERN has been shown to be increased in anxious individuals in over 50 studies to date (Ladouceur et al., 2006, 2018; Weinberg et al., 2010, 2012a, 2015; Moser et al., 2013; Riesel et al., 2014, 2015, 2019; Meyer, 2016). Additionally, the ERN has been shown to predict increases in anxiety across time (Meyer et al., 2015, 2017a, 2018b)—suggesting that it may be a neural risk marker for the development of anxiety disorders.
Additionally, some work has suggested that the ERN is sensitive to the motivational salience of errors (Amodio et al., 2008). For example, the ERN is larger when errors are more significant or costly (Hajcak et al., 2005; Chiu and Deldin, 2007; Ganushchak and Schiller, 2008; Endrass et al., 2010), when performance is being evaluated (Hajcak et al., 2005; Kim et al., 2005) and when accuracy is emphasized over speed (Gehring et al., 1993; Falkenstein et al., 2000).
Indeed, the ERN has been linked to traits related to increased sensitivity to errors—e.g. perfectionism (Schrijvers et al., 2010; Stahl et al., 2015; Drizinsky et al., 2016; Barke et al., 2017; Perrone-McGovern et al., 2017). Perfectionism, in part, is conceptualized as a hypervigilance and overreactivity to mistakes. More broadly, perfectionism has been defined as the tendency toward ‘high standards of performance which are accompanied by tendencies for overly critical evaluation of one’s own behavior’ (Frost et al., 1990). Results from one study suggest that maladaptive perfectionism is related to the ERN (Perrone-McGovern et al., 2017), suggesting that the ERN may index the degree to which an individual is distressed by their performance or behavior not meeting their own standards. Additionally, Stahl et al. (2015) found that personal standard perfectionism and evaluative concern perfectionism are both error-related neural activities. Another fMRI study found increased error-related neural activation in participants high in personal standard perfectionism (Barke et al., 2017).
Perfectionism is commonly conceptualized as being multidimensional in nature (Frost et al., 1990; Stöber, 1998). In line with this theoretical formulation, the Frost Multidimensional Perfectionism Scale (FMPS) contains six subscales: concern over mistakes, personal standards, parental expectations, parental criticism, doubts about actions and organization (Frost et al., 1990). The previous work has linked increased personal standard perfectionism to increased error-related neural activity (Stahl et al., 2015; Drizinsky et al., 2016; Barke et al., 2017). Personal standard perfectionism refers to the tendency to set high criteria or standards regarding one’s own performance. Individuals who score high on personal standard perfectionism are highly motivated to reach their own internally set goals. For example, one item on the FMPS personal standard perfectionism subscale is the following: ‘If I do not set the highest standards for myself, I am likely to end up a second-rate person.’ Personal standard perfectionism has been linked to academic performance (Blankstein et al., 2008) and has been suggested to reflect self-imposed expectations that may only be maladaptive in combination with self-criticism (Dunkley et al., 2006).
The second subscale that may be linked to error-related neural activity is the concern over mistake subscale of the FMPS. This scale measures negative reactions to errors and is thought to be central to the construct of perfectionism (Frost et al., 1990). Individuals high in concern over mistake perceive their mistakes as more serious, ruminate about their mistakes more often, believe others will think more poorly of them for making mistakes and have lower self-esteem than others (Frost et al., 1997). Additionally, the concern over mistake FMPS subscale has been linked to depression, obsessive–compulsive symptoms and procrastination (Frost et al., 1993). Items from this scale include the following: ‘I hate being less than best at things,’ and ‘If I fail at work/school, I am a failure as a person.’ The concern over mistake subscale has been suggested to reflect maladaptive responses to perceived mistakes or failures.
In line with the view that the ERN reflects sensitivity to errors, work in the lab suggests that the ERN is increased when errors are punished via shock or loud noise and that this effect persists after punishment ends (Riesel et al., 2012; Meyer and Gawlowska, 2017). Thus, learning-related experiences that increase the cost associated with errors appear to shape the ERN. Taken together, individual differences related to sensitivity to errors (i.e. perfectionism) are related to the ERN and within-subject manipulations (i.e. punishment) that increase the threat value of errors that increase the ERN.
Building on work in the lab suggesting that the ERN can be modulated by punishment, we (and others) have begun to examine how parenting may impact the ERN in offspring. Parents characterized by a controlling or punitive parenting style tend to punish children’s mistakes more intensely and more frequently (Robinson et al., 2001), which can result in children’s excessive concern related to making mistakes (Kawamura et al., 2002). We have proposed that one mechanism that may shape an increased ERN is exposure to a controlling parenting style. In a large study (N = 295), parenting style was assessed when children were 3 years old via structure observations in the lab. Additionally, parents completed a self-reported measure of parenting style. When the children were 6 years old, the ERN was measured, and results suggested that both observational and self-report measures of authoritarian parenting (high control and low warmth) are related to an increased ERN in children (Meyer et al., 2015). The link between parenting and the ERN has also been found among even younger children (4 years old; Brooker and Buss, 2014) and emerging adult females (Banica et al., 2019). Moreover, we have also found that the ERN is larger when parents characterized by high control are in the room with their children while the ERN is measured (Meyer et al., 2019)—highlighting the impact of parenting in shaping the ERN.
Parenting has also been studied in the context of the development of perfectionism (Flett et al., 2002; Kawamura et al., 2002; Snell Jr et al., 2005; Soenens et al., 2006; Lee et al., 2012; Hibbard and Walton, 2014). For example, high parental control and harshness have been linked to the development of perfectionism in children (Kawamura et al., 2002; Soenens et al., 2006; Hibbard and Walton, 2014). Researchers have suggested that parents who criticize children for being less than perfect impose unnecessarily high expectations and standards. Children may internalize these expectations and learn to be overly critical of their own performance and overly sensitive to their own mistakes.
While separate lines of work have linked controlling parenting styles to both perfectionism and ERN, no study to our knowledge has integrated these separate areas of research. Given the link between the ERN and perfectionism, as well as controlling parenting and the ERN, in the current study, we wished to extend the previous work to integrate all three of these constructs into one unified model. We propose a model wherein parenting impacts perfectionism via the ERN. Thus, we propose that an increased ERN may be an underlying neural mechanism that partly explains the association between parenting and perfectionism. For example, is it possible that parenting styles shape the ERN early in development and that the ERN functions as a risk marker or a diathesis toward developing anxiety and/or perfectionism later in life. As a first step, in the present study, we examine associations between parenting styles (maternal and paternal) and the ERN in a sample of young adults. Based on previous work, we hypothesized that controlling parenting styles would be associated with an increased ERN in offspring. We hypothesized that no other parenting styles would be associated with the ERN. Next, we examine whether the ERN is associated with self-reported perfectionism. Based on the previous work, we hypothesized that perfectionism (personal standard perfectionism as well as concern over mistakes) would be associated with an increased ERN. Next, we examine mediation models wherein we hypothesized that the indirect path from controlling parenting styles to perfectionism (personal standards and concern over mistakes) via the ERN would be significant. We hypothesized that relationships between response monitoring neural activity and both controlling parenting and perfectionism subscales would be specific to error-related activity (i.e. we hypothesized that no significant relationships between the CRN and parenting or perfectionism would emerge).
Method
Participants
Participants were undergraduate students who received course credit for participation in the study. Of the 80 participants, 13 participants made too few errors (i.e. less than six) and were therefore excluded from all analyses. Additionally, one participant’s self-report data was lost due to experimenter error. Thus, the final sample consisted of 66 participants, 22 males and 44 females, between the ages of 18–51 years old, M = 19.72, s.d. = 4.55. Overall, 38% of participants were Hispanic or Latino. Additionally, 5% of participants were Asian, 5% were Black, 6% were identified as Other, and 83% were Caucasian. Estimated annual family income was $10 000–25 000 for 9% of participants, $25 000–40 000 for 14%, $40 000–$75 000 for 27% and more than $75 000 for 50%. All participants were given verbal and written information about the procedures of the study, and written consent was obtained. Study procedures were approved by the institutional review board.
Self-report: parental behavior inventory (Children’s Report of Parental Behavior Inventory)
To assess parenting styles of participants’ parents, we utilized the Children’s Report of Parental Behavior Inventory (CRPBI; Schludermann and Schludermann, 1970). The CRPBI contains 30 items (Schludermann and Schludermann, 1988) and assesses parenting along 3 dimensions: (i) acceptance (e.g. my parent enjoys doing things with me); (ii) control (e.g. my parent often tells me how to behave); and (iii) firmness (e.g. my parent is strict with me). This measure has demonstrated good psychometric properties, alphas between 0.77 and 0.90 (Schludermann and Schludermann, 1970, 1988; McClure et al., 2001; Meyer et al., 2019).
Self-report: FMPS
To assess perfectionism, we utilized the FMPS (Stöber, 1998). The FMPS contains 35 items and provides six subscales for perfectionism: (i) concern over mistakes, (ii) personal standards, (iii) parental expectations, (iv) parental criticism, (v) doubts about actions and (vi) organization. This measure has demonstrated good psychometric properties, alphas between 0.70 and 0.91 (Stahl et al., 2015). In the current study, we focus on the personal standard perfectionism subscale, which reflects a tendency to set high standards for one’s own performance and includes items as follows: ‘I set higher goals than most people,’ and ‘if I do not set the highest standards for myself, I am likely to end up a second-rate person.’ Additionally, we also utilize the concern over mistake subscale, which reflects a tendency to be self-critical and to react negatively to errors. This subscale includes items as follows: ‘I hate being less than best at things,’ and ‘If I fail at work/school, I am a failure as a person.’
EEG task
EEG was recorded while participants completed an arrowhead version of the Flanker task (Eriksen and Eriksen, 1974). On each trial, participants were shown five arrowheads and are instructed to press the left or right mouse button (depending on which direction the center arrow is pointing) as quickly as possible. There were two compatible conditions, ‘< < < < <’ and ‘> > > > >,’ and two incompatible conditions, ‘< < > < <’ and ‘> > < > >.’. The stimuli were presented randomly such that 50% of trials were incompatible. Stimuli were presented for 200 ms, and the interval between the offset of one stimulus and the onset of the subsequent stimulus varied randomly between 2300 and 2800 ms. Participants completed a practice block of 10 trials and were instructed to be both as accurate and as fast as possible. The task consisted of 11 blocks of 30 trials (330 total trials). Each block was initiated by the participant with a mouse click. To make the task as quick as possible, it terminated after participants committed 30 errors or 330 correct responses. To encourage both fast and accurate responding, participants received feedback based on their performance at the end of each block. If performance was 75% correct or lower, the message ‘Please try to be more accurate’ was displayed. If performance was above 90% correct, the message ‘Please try to respond faster’ was displayed. If performance was between 75 and 90% correct, the message ‘You’re doing a great job’ was displayed.
Psychophysiological recording and data analysis
EEG was recorded continuously using an elastic cap and the ActiveTwo BioSemi system (BioSemi, Amsterdam, the Netherlands). Thirty-four electrode sites were used, as well as two electrodes for the left and right mastoids. Electrooculogram (EOG) generated from eye movements and blinks was recorded using four electrodes: horizontal eye movements were measured via two electrodes placed 1 cm outside the outer edge of the left and right eyes. Vertical eye movements and blinks were recorded via two electrodes placed 1 cm above and below the right eye. The EEG signal was preamplified with a gain of one by a BioSemi ActiveTwo system. The data was digitized at a 24 bit resolution with a sampling rate of 1024 Hz using a low-pass fifth-order sinc filter with a half-power cutoff of 204.8 Hz. Each active electrode was measured online with respect to a common mode sense (CMS) active electrode producing a monopolar (non-differential) channel. Offline data was referenced to the average of the left and right mastoids and band-pass filtered between 0.1 and 40 Hz. Eyeblink and ocular corrections were conducted per Gratton et al. (1983). An automatic procedure was employed to detect and reject artifacts. The criteria applied was a voltage step of more than 50.0 μV between sample points, a voltage difference of 300.0 μV within a trial and a maximum voltage difference of less than 0.50 μV within 100 ms intervals. These intervals were rejected from individual channels on each trial.
The EEG data were segmented for each trial beginning 500 ms before the response and continuing for 800 ms after the response. The response-locked ERPs were averaged separately for each trial type (e.g. correct and incorrect responses) to derive the correct response negativity (CRN) and the ERN. Baseline correction was performed using the interval from −500 to −300 ms before response onset. Average activity between 0 and 100 ms at Cz was exported for each subject, where error-related brain activity was maximal. Additionally, we calculated a residualized-based difference score (i.e. we conducted a regression wherein the CRN was entered predicting the ERN and unstandardized residual scores were saved; ERNresid) for each participant (Meyer et al., 2017b). Behavioral measures included the number of error and correct trials for each subject at each assessment. Additionally, average reaction times (RTs) on error and correct trials were calculated separately, as well as RTs on correct trials following error trials to calculate post-error RT slowing.
Statistical analyses were conducted using SPSS (Version 17.0) General Linear Model software, with Greenhouse-Geisser correction applied to P values associated with multiple-df, repeated-measure comparisons when necessitated by the violation of the assumption of sphericity. Repeated-measure ANOVAs were utilized to examine behavioral data (accuracy, RTs and post-error slowing) as well as error-related brain activity. Additionally, Pearson’s r correlations were used to examine the relationships between error-related brain activity and parenting, as well as perfectionism. We also conducted follow-up mediation analyses using a nonparametric bootstrapping method (MacKinnon et al., 2004). We used an SPSS macro (PROCESS; Preacher and Hayes, 2004), which provides a bootstrap estimate of the indirect effect between the independent and dependent variable, an estimate of standard error and 95% confidence intervals for the population value of the indirect effect. When confidence intervals for the indirect effect do not include zero, this indicates a significant indirect effect at the P < 0.05 level. Direct and indirect effects were tested using 5000 bootstrap samples.
Results
Behavioral data
Overall, participants were faster on error trials, M = 433 ms, s.d. = 118, compared to correct trials, M = 491, s.d. = 73, F(1, 63) = 39.46, P < 0.001. Additionally, participants were slower on trials following errors, M = 484, s.d. = 62, compared to trials following correct responses, M = 476, s.d. = 59, F(1, 63) = 3.50, P = 0.07, at a trend level. Overall, participants committed an average of 18 errors, s.d. = 6.37, range = 6–33.
Error-related brain activity and parenting
Neural activity was more negative during error trials, M = 2.48, s.d. = 9.11, compared to correct trials, M = 8.06, s.d. = 6.41, F(1, 63) = 42.23, P < 0.001. Means and s.d. of error-related brain activity (i.e. the ERN, CRN and ERNresid), maternal and paternal CRPBI scales, and correlations are presented in Table 1. The CRPBI scales were moderately correlated with each other, such that acceptance was negatively correlated to both control and firmness, for both the maternal and paternal scales. Additionally, control and firmness were positively correlated, for both maternal and paternal scales. Overall, maternal and paternal scales were moderately correlated with each other. Paired-samples t-tests suggested that participants rated maternal acceptance higher than paternal acceptance, t(65) = 2.44, P < 0.05. Additionally, maternal control was rated higher than paternal control, t(65) = 3.15, P < 0.01. However, maternal and paternal firmness did not differ, t(65) = 0.73, P = 0.47.
Table 1.
Means, standard deviations (SDs), and bivariate correlations between main study variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Mean (s.d.) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. ERN | - | 2.48 (9.11) | |||||||||
| 2. CRN | 0.66** | - | 8.06 (6.41) | ||||||||
| 3. ERNresid | 0.76** | 0.00 | - | 0.00 (6.95) | |||||||
| 4. Maternal acceptance | 0.10 | 0.10 | 0.05 | - | 25.61 (4.23) | ||||||
| 5. Paternal acceptance | −0.11 | −0.17 | 0.01 | 0.42* | - | 23.90 (5.88) | |||||
| 6. Maternal control | −0.21* | −0.16 | −0.13 | −0.56** | −0.12 | - | 16.83 (4.93) | ||||
| 7. Paternal control | −0.32** | −0.23* | −0.24* | −0.35** | −0.21* | 0.58** | - | 15.09 (4.87) | |||
| 8. Maternal firmness | −0.15 | −0.03 | −0.16 | −0.42** | −0.08 | 0.66** | 0.26* | - | 19.20 (5.03) | ||
| 9. Paternal firmness | −0.05 | 0.01 | −0.08 | −0.31** | −0.39** | 0.25* | 0.39** | 0.48** | - | 18.74 (4.87) | |
| 10. Concern over mistakes | −0.25* | −0.16 | −0.19t | −0.07 | −0.06 | 0.12 | 0.21 | 0.05 | 0.13 | - | 21.07 (6.02) |
| 11. Personal standards | −0.27* | −0.12 | −0.34** | 0.16 | 0.21 | 0.03 | 0.09 | 0.13 | 0.06 | 0.56** | 24.77 (4.80) |
t P < .07.
* P < .05.
** P < .01.
ERN, error-related negativity; CRN, correct related negativity; ERNresid, residualized difference score for error-related brain activity. Maternal/paternal acceptance, control, and firmness are scales from the Children’s Report of Parental Behavior Inventory (CRPBI). Concern over mistakes and Personal standards are scales from the Frost Multidimensional Perfectionism Scale (FMPS).
Error-related brain activity was associated with both maternal and paternal control, such that more parental control related to an increased ERN in offspring.1 Maternal control related to a larger ERN, but was not significantly related to either the CRN or the ERNresid. However, paternal control related to a larger ERN, CRN and ERNresid. Figure 1 depicts the error, correct and difference (error minus correct) waveforms for high (top quartile) and low (bottom quartile) maternal control (top) and paternal control (bottom) participants. Additionally, topographical headmaps (right) depict error minus correct from 0 to 100 ms for high and low control groups. Neither acceptance nor firmness related to error-related brain activity, for either the maternal or paternal scales.
Fig. 1.
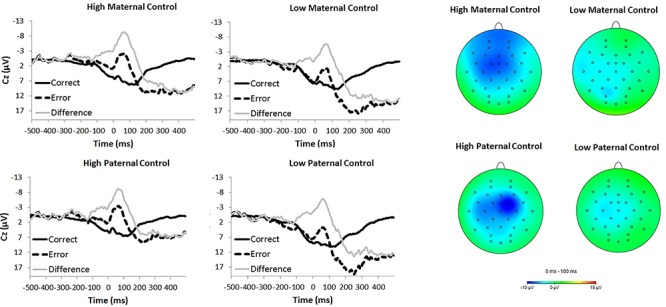
On the left, waveforms are presented for error trials (dotted black line), correct trials (solid black line) and the difference (error minus correct; gray line), for individuals who reported their parent (maternal on top; paternal on bottom) was high or low on the control scale of the CRPBI (based on a median split). On the right, topographical headmaps are presented for activity from 0 to 100 ms after responses (error minus correct). Headmaps are presented based on a median split on the control scale of the CRPBI for maternal (top) and paternal (bottom) parenting style.
Perfectionism
Means and s.d. for the concern over mistake and personal standard subscales on the FMPS, as well as correlations with other study variables, are presented in Table 1. As would be expected, subscales on the FMPS were moderately correlated. Moreover, the pattern of associations between FMPS subscales were in the expected direction—i.e. higher scores on the concern over mistake subscale were associated with higher scores on the personal standard subscale.
Error-related brain activity was related to both subscales of the FMPS. Both the ERN and ERNresid was larger among participants who reported being higher on the personal standard subscale of the FMPS. Additionally, the ERN (and the ERNresid at a trend level) was larger among participants who reported being higher on the concern over mistake subscale of the FMPS. Neither perfectionism subscale was significantly related to the CRN. Taken together, these results suggest that the ERN is increased among individuals who report having more controlling parents, as well as individuals who report being higher in perfectionism (i.e. higher on the concern over mistake and personal standard subscales).2
Mediation models
We examined mediation models wherein the relationship between parental control (maternal and paternal) and perfectionism (concern over mistake and personal standard subscales) was mediated by the ERNresid. In the first mediation model, we examined a model wherein the relationship between maternal control and the concern over mistake scales of the FMPS was mediated by the ERNresid, and results did not support a significant mediation model, effect = 0.03, 95% CI [−0.02–0.16]. In the second mediation model, we examined a model wherein the relationship between maternal control and the personal standard perfectionism scales of the FMPS was mediated by the ERNresid, and results did not support a significant mediation model, effect = 0.04, 95% CI [0.04–0.14].
Next, we examined two mediation models wherein paternal control was entered as the predictor. In the first mediation model, we examined a model wherein the relationship between paternal control and the concern over mistake scales of the FMPS was mediated by the ERNresid. Results did not support this mediation model, effect = 0.04, 95% CI [−0.011–0.204]. In the second mediation model (Figure 2), we examined a model wherein the relationship between paternal control and the personal standard perfectionism scales of the FMPS was mediated by the ERNresid. Results supported the mediation model, i.e. the indirect path from paternal control to personal standard perfectionism (FMPS) via the ERN reached significance, effect = 0.08, 95% CI [0.003–0.206]. To test the specificity of this model, the mediator and the outcome were reversed (i.e. the ERNresid was entered as the outcome variable, and personal standard perfectionism (FMPS) was entered as the mediator). Results suggested that the indirect path in this alternative model (i.e. mediation) did not reach significance, effect = −0.05, 95% CI [−0.199–0.064].
Fig. 2.
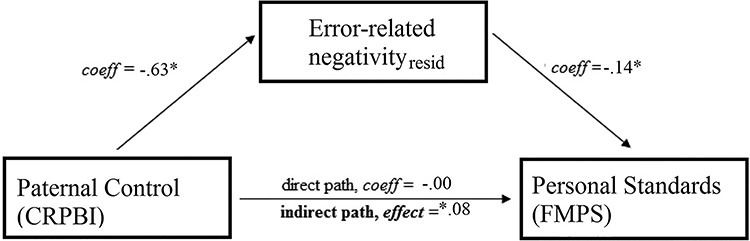
A depiction of a mediation model wherein the relationship between paternal control (as reported on the CRPBI) and personal standards (as reported on the Frost Multidimensional Perfectionism Scale; FMPS) is mediated by the ERN residualized difference score.
Discussion
Consistent with our hypothesis, results from the current study suggest that controlling parenting styles (both maternal and paternal) were associated with increased error-related brain activity in offspring. Additionally, individuals who reported being higher in perfectionism (concern over mistakes and personal standards) also displayed an increased ERN. Moreover, results supported a mediation model wherein the association between controlling parenting styles (paternal) and personal standard perfectionism was mediated by the ERN, suggesting that an increased ERN may be an underlying neural mechanism partly explaining the relationship between parenting and personal standard perfectionism.
In line with the previous work suggesting a link between parenting styles and the ERN in offspring (Brooker and Buss, 2014; Meyer et al., 2015, 2019; Banica et al., 2019), results from the current study supported a link between maternal and paternal controlling parenting styles and an increased ERN in offspring. This is the second study to find this association in young adults (Banica et al., 2019). And, while parental control was associated with error-related brain activity, neither parental acceptance nor firmness related to the ERN, suggesting specificity between controlling parenting styles and the ERN. It should be noted that the CRPBI factor of control indexes is: ‘covert, psychological methods of controlling the child’s activities and behaviors that would not permit the child to develop as an individual apart from the parent’ (Schaefer, 1965). Thus, young adults who report their parents are high on this particular dimension are also characterized by an increased ERN.
Consistent with the previous work (Schrijvers et al., 2010; Stahl et al., 2015; Drizinsky et al., 2016; Barke et al., 2017; Perrone-McGovern et al., 2017), in the current study, we observed increased error-related brain activity among individuals who reported being more perfectionistic. Specifically, the subscales measuring concern over mistakes and personal standards on the FMPS were associated with an increased ERN. The concerns over mistake subscale indexes sensitivity and overreactivity to errors. This scale contains items as follows: ‘I should be upset if I make a mistake’, ‘if I fail partly, it is as bad as being a complete failure,’ and ‘the fewer mistakes I make, the more people will like me.’ The personal standard subscale on the FMPS indexes having higher than average goals for behavior that are inflexible. This scale contains items as follows: ‘I expect higher performance in my daily tasks than most people,’ ‘if I do not set the highest standards for myself, I am likely to end up a second-rate person,’ and ‘it is important to me that I am thoroughly competent in everything I do.’ Thus, the ERN appears to index increased distress in response to mistakes and high expectations for performance.
Results also supported a mediation model wherein the indirect pathway from controlling parenting style to perfectionism (i.e. the personal standard subscale on the FMPS) via the ERN was significant, for paternal parenting. Although preliminary, these findings support the notion that one mechanism whereby parenting may impact personal standard perfectionism in offspring may be by potentiating the neural response to errors. This finding is consistent with other work suggesting the ERN mediates the association between parenting and anxiety disorders in children (Meyer et al., 2015, 2019). However, the current study was entirely cross-sectional, and, therefore, conclusions regarding causality and the direction of effects are limited. Future longitudinal work, utilizing multiple time points across development, should examine whether parenting impacts the ERN and thereby perfectionism in offspring.
Additionally, it should be noted that personal standard perfectionism has sometimes been conceptualized as an adaptive facet of perfectionism. For example, personal standard perfectionism been linked to academic performance (Blankstein et al., 2008), and some have suggested it reflects self-imposed expectations that may only be maladaptive in combination with self-criticism (Dunkley et al., 2006). In line with this, the ERN has previously been linked to academic performance (Hirsh and Inzlicht, 2010). Thus, in the current study, increased personal standard perfectionism is linked to an increased ERN—and both may reflect an adaptive level of concern for expectations and performance. This may be especially true in light of the fact that the sample in the current study was not a clinical population.
One surprising finding was a significant correlation between correct-related neural activity (i.e. the CRN) and paternal controlling parenting style. This association was such that more paternal control was related to a larger (i.e. more negative) CRN. While it is unclear what individual differences in the CRN reflect, some have suggested that the magnitude of the CRN may reflect error processing on correct trials (i.e. individuals may perceive themselves to have made an erroneous response on a correct trial; Wessel, 2012). It is possible that the relationship observed between paternal control and the CRN was due to the error processing that occurred during correct trials.
Another finding that was contrary to our hypotheses was the lack of significance of the mediation model wherein the ERN mediated the relationship between parental control and the concern over mistake subscale. This model failed to reach significance when using either maternal or paternal control. While results from the current study suggest that the ERN (and the ERNresid) is increased in individuals who report being high on the concerns over mistake subscale, the lack of significance in these mediation models suggests that the hypothesized mechanism whereby parenting impacts the ERN and thus relates to an increased concern over mistakes is not supported by this data. It is possible that factors other than parenting may be more important in shaping the ERN and thereby concern over mistakes in young adults. For example, it is possible that the school context (i.e. grades, difficulty of courses, teacher criticism, etc.) may play an important role in this model. Or, it is possible that romantic partner criticism or peer group criticality may impact the ERN and thereby concern over mistakes in young adults. Future work should investigate alternative stressors or contexts in young adult populations in terms of their impact on error-related brain activity and thereby concern over mistakes.
Previous work has found associations between the ERN and a broad range of psychopathology (Pailing and Segalowitz, 2004; Olvet and Hajcak, 2008; Moser et al., 2013; Pasion and Barbosa, 2019), temperamental styles (Torpey et al., 2013; Meyer et al., 2018a) and personality traits (Pasion et al., 2016; Taylor et al., 2018). In the current study, we focus on perfectionism (i.e. personal standard perfectionism and concern over mistakes). It is possible that the pattern of results observed is better explained by some higher-order psychological construct (e.g. neuroticism or negative affect). Future studies should examine whether the association between perfectionism and the ERN persists after controlling for broad personality and temperamental styles, as well as psychopathology.
There are a number of limitations to the current study to consider. Specifically, both perfectionism and parenting were measured via self-reported measures. Future work should replicate findings using alternative types of measurement—e.g. observational measurement, interview-based measures, lab-based behavioral measures, etc. Additionally, as noted above, this study utilized cross-sectional data. Future studies should use a longitudinal design to investigate causal mediation. Moreover, the current study was conducted among young adults. Future work should investigate whether parenting impacts perfectionism via the ERN in children across development.
The findings from the current study are novel insofar as they integrate separate lines of work on the ERN and parenting and the ERN and perfectionism. Results suggest that there may be significant links between controlling parenting styles, error-related brain activity and perfectionism in young adults. Considering that perfectionism has been linked to risk for psychopathology and suggested to be transdiagnostic risk marker (Limburg et al., 2017), it is important to understand neural substrates and mechanisms associated with this trait.
Conflict of interest
None declared.
Footnotes
The relationship between both maternal and paternal control and the ERN remained significant after controlling for accuracy and reaction time, all Ps < 0.05.
The relationships between the concerns over mistake and personal standard subscales on the FMPS with the ERN remained significant after controlling for accuracy and reaction time, all Ps < 0.05.
References
- Agam Y., Hämäläinen M.S., Lee A.K., et al. (2011). Multimodal neuroimaging dissociates hemodynamic and electrophysiological correlates of error processing. Proceedings of the National Academy of Sciences, 108(42), 17556–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio D.M., Master S.L., Yee C.M., Taylor S.E. (2008). Neurocognitive components of the behavioral inhibition and activation systems: implications for theories of self-regulation. Psychophysiology, 45(1), 11–9. doi: 10.1111/j.1469-8986.2007.00609.x. [DOI] [PubMed] [Google Scholar]
- Banica I., Sandre A., Weinberg A. (2019). Overprotective/authoritarian maternal parenting is associated with an enhanced error-related negativity (ERN) in emerging adult females. International Journal of Psychophysiology, 137, 12–20. [DOI] [PubMed] [Google Scholar]
- Barke A., Bode S., Dechent P., Schmidt-Samoa C., Van Heer C., Stahl J. (2017). To err is (perfectly) human: behavioural and neural correlates of error processing and perfectionism. Social Cognitive and Affective Neuroscience, 12(10), 1647–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann M., Johansen-Berg H., Rushworth M.F. (2009). Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. The Journal of Neuroscience, 29(4), 1175–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankstein K.R., Dunkley D.M., Wilson J. (2008). Evaluative concerns and personal standards perfectionism: self-esteem as a mediator and moderator of relations with personal and academic needs and estimated GPA. Current Psychology, 27(1), 29–61. [Google Scholar]
- Botvinick M.M., Braver T.S., Barch D.M., Carter C.S., Cohen J.D. (2001). Conflict monitoring and cognitive control. Psychological Review, 108(3), 624–52. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Brooker R.J., Buss K.A. (2014). Harsh parenting and fearfulness in toddlerhood interact to predict amplitudes of preschool error-related negativity. Developmental Cognitive Neuroscience, 9, 148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu P., Deldin P. (2007). Neural evidence for enhanced error detection in major depressive disorder. American Journal of Psychiatry, 164(4), 608–16. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Posner M.I., Don M.T. (1994). Localization of a neural system for error detection and compensation. Psychological Science, 5(5), 303–5. [Google Scholar]
- Drizinsky J., Zülch J., Gibbons H., Stahl J. (2016). How personal standards perfectionism and evaluative concerns perfectionism affect the error positivity and post-error behavior with varying stimulus visibility. Cognitive, Affective, & Behavioral Neuroscience, 16(5), 876–87. [DOI] [PubMed] [Google Scholar]
- Dunkley D.M., Blankstein K.R., Masheb R.M., Grilo C.M. (2006). Personal standards and evaluative concerns dimensions of “clinical” perfectionism: a reply to Shafran et al. (2002, 2003) and Hewitt et al. (2003). Behaviour Research and Therapy, 44(1), 63–84. [DOI] [PubMed] [Google Scholar]
- Endrass T., Schuermann B., Kaufmann C., Spielberg R., Kniesche R., Kathmann N. (2010). Performance monitoring and error significance in patients with obsessive-compulsive disorder. Biological Psychology, 84(2), 257–63. [DOI] [PubMed] [Google Scholar]
- Eriksen B., Eriksen C. (1974). Effects of noise letters upon the identification of a target letter in a nonsearch task. Attention, Perception, & Psychophysics, 16(1), 143–9. doi: 10.3758/bf03203267. [DOI] [Google Scholar]
- Falkenstein M., Hohnsbein J., Hoormann J., Blanke L. (1991). Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology, 78(6), 447–55. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M., Hoormann J., Christ S., Hohnsbein J. (2000). ERP components on reaction errors and their functional significance: a tutorial. Biological Psychology, 51(2–3), 87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Flett G.L., Hewitt P.L., Oliver J.M., Macdonald S. (2002). Perfectionism in children and their parents: A developmental analysis. In: Flett G.L., Hewitt P.L., editors. Perfectionism: Theory, research, and treatment (p. 89–132), American Psychological Association 10.1037/10458-004 [DOI]
- Frost R.O., Marten P., Lahart C., Rosenblate R. (1990). The dimensions of perfectionism. Cognitive Therapy and Research, 14(5), 449–68. [Google Scholar]
- Frost R.O., Heimberg R.G., Holt C.S., Mattia J.I., Neubauer A.L. (1993). A comparison of two measures of perfectionism. Personality and Individual Differences, 14(1), 119–26. [Google Scholar]
- Frost R.O., Trepanier K.L., Brown E.J., et al. (1997). Self-monitoring of mistakes among subjects high and low in perfectionistic concern over mistakes. Cognitive Therapy and Research, 21(2), 209–22. [Google Scholar]
- Ganushchak L.Y., Schiller N.O. (2008). Motivation and semantic context affect brain error-monitoring activity: an event-related brain potentials study. NeuroImage, 39(1), 395–405. [DOI] [PubMed] [Google Scholar]
- Gehring W.J., Goss B., Coles M.G.H., Meyer D.E., Donchin E. (1993). A neural system for error detection and compensation. Psychological Science, 4(6), 385–90. [Google Scholar]
- Gratton G., Coles M.G.H., Donchin E. (1983). A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology, 55(4), 468–84. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hajcak G., Moser J.S., Yeung N., Simons R.F. (2005). On the ERN and the significance of errors. Psychophysiology, 42(2), 151–60. [DOI] [PubMed] [Google Scholar]
- Hibbard D.R., Walton G.E. (2014). Exploring the development of perfectionism: the influence of parenting style and gender. Social Behavior and Personality: An International Journal, 42(2), 269–78. [Google Scholar]
- Hirsh J.B., Inzlicht M. (2010). Error-related negativity predicts academic performance. Psychophysiology, 47(1), 192–6. [DOI] [PubMed] [Google Scholar]
- Holroyd C.B., Coles M.G.H. (2002). The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychological Review, 109(4), 679–709. doi: 10.1037/0033-295x.109.4.679. [DOI] [PubMed] [Google Scholar]
- Kawamura K.Y., Frost R.O., Harmatz M.G. (2002). The relationship of perceived parenting styles to perfectionism. Personality and Individual Differences, 32(2), 317–27. [Google Scholar]
- Kiehl K.A., Liddle P.F., Hopfinger J.B. (2000). Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiology, 37(2), 216–23. [PubMed] [Google Scholar]
- Kim E.Y., Iwaki N., Uno H., Fujita T. (2005). Error-related negativity in children: effect of an observer. Developmental Neuropsychology, 28(3), 871–83. [DOI] [PubMed] [Google Scholar]
- Ladouceur C.D., Dahl R.E., Birmaher B., Axelson D.A., Ryan N.D. (2006). Increased error-related negativity (ERN) in childhood anxiety disorders: ERP and source localization. Journal of Child Psychology and Psychiatry, 47(10), 1073–82. [DOI] [PubMed] [Google Scholar]
- Ladouceur C.D., Tan P.Z., Sharma V., et al. (2018). Error-related brain activity in pediatric anxiety disorders remains elevated following individual therapy: a randomized clinical trial. Journal of Child Psychology and Psychiatry, 59(11), 1152–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.A., Schoppe-Sullivan S.J., Dush C.M.K. (2012). Parenting perfectionism and parental adjustment. Personality and Individual Differences, 52(3), 454–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limburg K., Watson H.J., Hagger M.S., Egan S.J. (2017). The relationship between perfectionism and psychopathology: a meta-analysis. Journal of Clinical Psychology, 73(10), 1301–26. [DOI] [PubMed] [Google Scholar]
- MacKinnon D.P., Lockwood C.M., Williams J. (2004). Confidence limits for the indirect effect: distribution of the product and resampling methods. Multivariate Behavioral Research, 39(1), 99–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalon D.H., Whitfield S.L., Ford J.M. (2003). Anatomy of an error: ERP and fMRI. Biological Psychology, 64(1–2), 119–41. doi: 10.1016/s0301-0511(03)00105-4. [DOI] [PubMed] [Google Scholar]
- McClure E.B., Brennan P.A., Hammen C., Le Brocque R.M. (2001). Parental anxiety disorders, child anxiety disorders, and the perceived parent–child relationship in an Australian high-risk sample. Journal of Abnormal Child Psychology, 29(1), 1–10. [DOI] [PubMed] [Google Scholar]
- Menon V., Adleman N.E., White C.D., Glover G.H., Reiss A.L. (2001). Error-related brain activation during a Go/NoGo response inhibition task. Human Brain Mapping, 12(3), 131–43. doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A. (2016). Developing psychiatric biomarkers: A review focusing on the error-related negativity as a biomarker for anxiety. Current Treatment Options in Psychiatry, 3(4), 356–64. [Google Scholar]
- Meyer A., Gawlowska M. (2017). Evidence for specificity of the impact of punishment on error-related brain activity in high versus low trait anxious individuals. International Journal of Psychophysiology, 120, 157–63. [DOI] [PubMed] [Google Scholar]
- Meyer A., Proudfit G.H., Bufferd S.J., et al. (2015). Self-reported and observed punitive parenting prospectively predicts increased error-related negativity in six-year-old children. Journal of Abnormal Child Psychology, 43(5), 821–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A., Hajcak G., Torpey-Newman D.C., Kujawa A., Klein D.N. (2015). Enhanced error-related brain activity in children predicts the onset of anxiety disorders between the ages of 6 and 9. Journal of Abnormal Psychology, 124(2), 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A., Danielson C.K., Danzig A.P., et al. (2017a). Neural biomarker and early temperament predict increased internalizing symptoms after a natural disaster. Journal of the American Academy of Child & Adolescent Psychiatry, 56(5), 410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A., Lerner M.D., De Los Reyes A., Laird R.D., Hajcak G. (2017b). Considering ERP difference scores as individual difference measures: issues with subtraction and alternative approaches. Psychophysiology, 54(1), 114–22. [DOI] [PubMed] [Google Scholar]
- Meyer A., Hajcak G., Torpey-Newman D., et al. (2018a). Early temperamental fearfulness and the developmental trajectory of error-related brain activity. Developmental Psychobiology, 60(2), 224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A., Nelson B., Perlman G., Klein D.N., Kotov R. (2018b). A neural biomarker, the error-related negativity, predicts the first onset of generalized anxiety disorder in a large sample of adolescent females. Journal of Child Psychology and Psychiatry, 59(11), 1162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A., Carlton C., Chong L.J., Wissemann K. (2019). The presence of a controlling parent is related to an increase in the error-related negativity in 5–7 year-old children. Journal of Abnormal Child Psychology, 47(6), 935–45. [DOI] [PubMed] [Google Scholar]
- Moser J.S., Moran T.P., Schroder H.S., Donnellan M.B., Yeung N. (2013). On the relationship between anxiety and error monitoring: a meta-analysis and conceptual framework. Frontiers in Human Neuroscience, 7, 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet D.M., Hajcak G. (2008). The error-related negativity (ERN) and psychopathology: toward an endophenotype. Clinical Psychology Review, 28(8), 1343–54. doi: 10.1016/j.cpr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pailing P.E., Segalowitz S.J. (2004). The error-related negativity as a state and trait measure: motivation, personality, and ERPs in response to errors. Psychophysiology, 41(1), 84–95. [DOI] [PubMed] [Google Scholar]
- Pasion R., Barbosa F. (2019). ERN as a transdiagnostic marker of the internalizing-externalizing spectrum: a dissociable meta-analytic effect. Neuroscience & Biobehavioral Reviews, 103, 133–49. [DOI] [PubMed] [Google Scholar]
- Pasion R., Cruz A.R., Barbosa F. (2016). Dissociation of boldness and disinhibition psychopathic traits in ERN modulation. Personality and Individual Differences, 95, 6–10. [Google Scholar]
- Perrone-McGovern K., Simon-Dack S., Esche A., et al. (2017). The influence of emotional intelligence and perfectionism on error-related negativity: an event related potential study. Personality and Individual Differences, 111, 65–70. [Google Scholar]
- Preacher K.J., Hayes A.F. (2004). SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments, & Computers, 36(4), 717–31. [DOI] [PubMed] [Google Scholar]
- Riesel A., Weinberg A., Endrass T., Kathmann N., Hajcak G. (2012). Punishment has a lasting impact on error-related brain activity. Psychophysiology, 49(2), 239–47. [DOI] [PubMed] [Google Scholar]
- Riesel A., Kathmann N., Endrass T. (2014). Overactive performance monitoring in obsessive–compulsive disorder is independent of symptom expression. European Archives of Psychiatry and Clinical Neuroscience, 264(8), 707–17. [DOI] [PubMed] [Google Scholar]
- Riesel A., Endrass T., Auerbach L.A., Kathmann N. (2015). Overactive performance monitoring as an Endophenotype for obsessive-compulsive disorder: evidence from a treatment study. American Journal of Psychiatry, 0(0). doi: 10.1176/appi.ajp.2014.14070886. [DOI] [PubMed] [Google Scholar]
- Riesel A., Klawohn J., Grützmann R., et al. (2019). Error-related brain activity as a transdiagnostic endophenotype for obsessive-compulsive disorder, anxiety and substance use disorder. Psychological Medicine, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson C., Mandleco B., Olsen S.F., Hart C. (2001). The parenting styles and dimensions questionnaire (PSDQ). Handbook of Family Measurement Techniques, 3, 319–21. [Google Scholar]
- Schaefer E.S. (1965). A configurational analysis of children's reports of parent behavior. Journal of Consulting Psychology, 29(6), 552. [DOI] [PubMed] [Google Scholar]
- Schludermann E., Schludermann S. (1970). Replicability of factors in children's report of parent behavior (CRPBI). The Journal of Psychology, 76(2), 239–49. [Google Scholar]
- Schludermann S., Schludermann E. (1988). Questionnaire for Children and Youth (CRPBI-30), Winnipeg: University of Manitoba, (unpublished manuscript). [Google Scholar]
- Schrijvers D.L., De Bruijn E.R., Destoop M., Hulstijn W., Sabbe B.G. (2010). The impact of perfectionism and anxiety traits on action monitoring in major depressive disorder. Journal of Neural Transmission, 117(7), 869–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman A.J., Salomons T.V., Slagter H.A., Fox A.S., Winter J.J., Davidson R.J. (2011). The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience, 12(3), 154–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell W.E. Jr., Overbey G.A., Brewer A.L. (2005). Parenting perfectionism and the parenting role. Personality and Individual Differences, 39(3), 613–24. [Google Scholar]
- Soenens B., Vansteenkiste M., Duriez B., Goossens L. (2006). In search of the sources of psychologically controlling parenting: the role of parental separation anxiety and parental maladaptive perfectionism. Journal of Research on Adolescence, 16(4), 539–59. [Google Scholar]
- Stahl J., Acharki M., Kresimon M., Völler F., Gibbons H. (2015). Perfect error processing: perfectionism-related variations in action monitoring and error processing mechanisms. International Journal of Psychophysiology, 97(2), 153–62. [DOI] [PubMed] [Google Scholar]
- Stöber J. (1998). The Frost multidimensional perfectionism scale revisited: more perfect with four (instead of six) dimensions. Personality and Individual Differences, 24(4), 481–91. [Google Scholar]
- Taylor J.B., Visser T.A., Fueggle S.N., Bellgrove M.A., Fox A.M. (2018). The error-related negativity (ERN) is an electrophysiological marker of motor impulsiveness on the Barratt Impulsiveness Scale (BIS-11) during adolescence. Developmental Cognitive Neuroscience, 30, 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torpey D.C., Hajcak G., Kim J., et al. (2013). Error-related brain activity in young children: associations with parental anxiety and child temperamental negative emotionality. Journal of Child Psychology and Psychiatry, 54(8), 854–62. doi: 10.1111/jcpp.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veen V., Carter C.S. (2002). The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiology & Behavior, 77(4–5), 477–82. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Weinberg A., Olvet D.M., Hajcak G. (2010). Increased error-related brain activity in generalized anxiety disorder. Biological Psychology, 85(3), 472–80. doi: 10.1016/j.biopsycho.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Weinberg A., Klein D.N., Hajcak G. (2012a). Increased error-related brain activity distinguishes generalized anxiety disorder with and without comorbid major depressive disorder. Journal of Abnormal Psychology., 121(4), 885. doi: 10.1037/a0028270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A., Riesel A., Hajcak G. (2012b). Integrating multiple perspectives on error-related brain activity: the ERN as a neural indicator of trait defensive reactivity. Motivation and Emotion, 36(1), 84–100. [Google Scholar]
- Weinberg A., Kotov R., Proudfit G.H. (2015). Neural indicators of error processing in generalized anxiety disorder, obsessive-compulsive disorder, and major depressive disorder. Journal of Abnormal Psychology, 124(1), 172. [DOI] [PubMed] [Google Scholar]
- Weinberg A., Meyer A., Hale-Rude E., et al. (2016). Error-related negativity (ERN) and sustained threat: conceptual framework and empirical evaluation in an adolescent sample. Psychophysiology, 53(3), 372–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel J.R. (2012). Error awareness and the error-related negativity: evaluating the first decade of evidence. Frontiers in Human Neuroscience, 6, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]


