Abstract
Experience of interpersonal trauma and violence alters self-other distinction and mentalising abilities (also known as theory of mind, or ToM), yet little is known about their neural correlates. This fMRI study assessed temporoparietal junction (TPJ) activation, an area strongly implicated in interpersonal processing, during spontaneous mentalising in 35 adult women with histories of childhood physical, sexual, and/or emotional abuse (childhood abuse; CA) and 31 women without such experiences (unaffected comparisons; UC). Participants watched movies during which an agent formed true or false beliefs about the location of a ball, while participants always knew the true location of the ball. As hypothesised, right TPJ activation was greater for UCs compared to CAs for false vs true belief conditions. In addition, CAs showed increased functional connectivity relative to UCs between the rTPJ and dorsomedial prefrontal cortex. Finally, the agent’s belief about the presence of the ball influenced participants’ responses (ToM index), but without group differences. These findings highlight that experiencing early interpersonal trauma can alter brain areas involved in the neural processing of ToM and perspective-taking during adulthood.
Keywords: childhood abuse, mentalising, temporoparietal junction (TPJ), theory of mind (ToM), trauma
Introduction
More than one in three women in a recent survey reported experiencing physical, sexual and/or psychological violence in childhood (E.U. Agency for Fundamental Rights, 2014). Notably, early traumatic experiences like childhood abuse (CA) disrupt the normal developmental trajectory and alter mentalising abilities, jeopardising social interaction later in life; CA impairs cognitive perspective-taking (cf. Benarous et al., 2015), reduces the ability to recognise and correctly interpret others’ emotions (Luke and Banerjee, 2013) and leads to less effective use of conflict-resolution strategies (Burack and Flanagan, 2006) in children. Nevertheless, while evidence mounts for the long-term consequences of CA on the neurobiology of affective and cognitive functioning (Mueller et al., 2010; Hart and Rubia, 2012; Philip et al., 2016; Teicher and Samson, 2016), the long-term influence on mentalising abilities is virtually unknown.
Despite the fundamental necessity of mentalising abilities for daily life, neurobiological research examining the effects of interpersonal trauma on social cognition is scarce and has mainly been conducted in psychiatric populations. While there is some behavioural evidence for reduced mentalising abilities following CA (Nazarov et al., 2014; Germine et al., 2015; Quidé et al., 2018), there is, to the best of our knowledge, only one imaging study that has investigated the neural correlates of cognitive ToM in CA survivors: Quidé et al. (2017) reported temporoparietal junction (TPJ) hypoactivation in previously maltreated adults with psychotic disorder. However, their study focused on the role of CA in impaired ToM in schizophrenia and did not include healthy controls. In addition, two studies focusing on affective ToM found a positive relationship between abuse severity and inferior frontal gyrus (IFG) activation in adolescents (van Schie et al., 2017) and between abuse severity and amygdala activation in chronically depressed adults (Hentze et al., 2016). Abu-Akel and Shamay-Tsoory (2011) proposed a model dissociating cognitive and affective ToM, concluding that there is evidence that these are separate processes with distinct yet connected neural networks. Meta-analytic findings support this model, suggesting that cognitive ToM tasks elicit more bilateral TPJ and precuneus activation, whereas affective tasks involve the bilateral IFG and posterior medial frontal cortex (Molenberghs et al., 2016).
Furthermore, all three above studies (Hentze et al., 2016; Quidé et al., 2017; van Schie et al., 2017) employed explicit—rather than spontaneous—mentalising tasks. In explicit mentalising tasks, participants are explicitly asked to reason about the beliefs of others. As such, these tasks can be used to measure people’s ability to mentalise. However, because adults rarely fail such tasks, recent work has started to use implicit (or spontaneous) mentalising tasks, in which participants are not explicitly asked to reason about the beliefs of others (Kovács et al., 2010). Instead, in these tasks, mentalising is inferred from the degree to which others’ beliefs influence participants’ performance on an unrelated task. In other words, implicit mentalising tasks measure not ability but propensity to mentalise (see Keysers and Gazzola, 2014, for a theoretical article regarding ability vs propensity). While previous research suggests that interpersonal trauma affects mentalising abilities (Hentze et al., 2016; Quidé et al., 2017; van Schie et al., 2017), evidence is lacking regarding its influence on mentalising propensity. As ability and propensity are not necessarily correlated (Keysers and Gazzola, 2014), an important question is therefore how CA influences brain activity during spontaneous mentalising in adulthood.
To address this question, the current fMRI study measured brain activity in a community sample of adult women with and without CA experiences while they performed a well-validated implicit cognitive ToM task (Kovács et al., 2010, 2014; Bardi et al., 2017; Nijhof et al., 2018). First, based on previous research identifying the right TPJ (rTPJ) as the central node in both explicit (Saxe et al., 2004; Decety and Lamm, 2007; Carter and Huettel, 2013; Krall et al., 2015) and implicit cognitive ToM networks (Bardi et al., 2017; Nijhof et al., 2018), and on evidence for CA-related hypoactivation in this region during explicit cognitive mentalising (Quidé et al., 2017), we tested the novel hypothesis that CA exposure is associated with rTPJ hypoactivation during implicit cognitive mentalising. Second, based on evidence for increased functional connectivity between the rTPJ and the dorsomedial prefrontal cortex (dMPFC), another region often activated in ToM tasks (Molenberghs et al., 2016), during spontaneous ToM (Burnett and Blakemore, 2009; Moessnang et al., 2017), we additionally tested the hypothesis that CA exposure leads to altered functional connectivity between these two regions.
Methods
Participants
Thirty-five adult women with CA experiences (CA group; Mage = 36.79 years, s.d. = 12.04) and 40 adult women without such experiences (unaffected comparisons, UC group; Mage = 35.64 years, s.d. = 11.50) participated in exchange for €30 (Table 1). Inclusion criteria for CAs were experience(s) of physical, sexual and/or emotional abuse occurring before age 17 (Supplementary Table S1). Exclusion criteria for UCs were history of other childhood trauma and/or experience(s) of interpersonal trauma in adulthood (e.g. emotional abuse, physical/sexual assault, etc.) due to similarities in the impact of CA and intimate partner violence (Bonomi et al., 2006). Because it emerged during questionnaires that nine women initially recruited as UCs experienced abuse in adulthood, yet not childhood, these participants were excluded, resulting in a final dataset of 31 adult women with no history of childhood or interpersonal trauma (Mage = 36.51 years, s.d. = 11.46). Inclusion criteria for both groups were MRI compatibility (i.e. no pregnancy or metal implants), fluency in Dutch, no history of severe head trauma or neurological condition, normal or corrected-to-normal vision and being 18–60 years old. Participants from both groups were recruited without regard to psychiatric history, as childhood maltreatment is a risk factor for a wide range of psychopathology (Green et al., 2010), and including only healthy survivors of CA could bias results (Teicher et al., 2014) (Table 1 and Supplementary Table S1).
Table 1.
Sample demographics, current psychopathology (MINI) and mean scores on measures of empathy (IRI), depression (BDI), trait and state anxiety (STAI), dissociation (DES) and resilience (RS)
| CA group (n = 35) | UC group (n = 31) | P value | Effect size (Cohen’s d) | |
|---|---|---|---|---|
| Demographic information | ||||
| Age | 36.79 (12.04) | 36.51 (11.46) | 0.92 | 0.02 |
| Empathy | 72.77 (12.45) | 64.48 (9.29) | 0.003* | 0.75 |
| Perspective-taking | 19.11 (4.91) | 18.03 (3.99) | 0.33 | 0.24 |
| Fantasy | 17.54 (5.69) | 16.13 (5.64) | 0.32 | 0.25 |
| Empathic concern | 21.29 (4.46) | 20.10 (4.22) | 0.27 | 0.27 |
| Personal distress | 14.83 (4.16) | 10.23 (4.46) | <0.001** | 1.07 |
| Depression | 15.85 (10.71) | 5.35 (5.30) | <0.001** | 1.23 |
| Trait anxiety | 48.51 (9.35) | 35.13 (9.22) | <0.001** | 1.44 |
| State anxiety | 39.71 (8.87) | 30.45 (7.22) | <0.001** | 1.14 |
| Dissociation | 20.58 (12.54) | 10.12 (7.73) | 0.001* | 0.98 |
| Resilience | 126.00 (17.58) | 138.61 (13.25) | 0.002* | 0.80 |
| Number of current psychological disorders | 2.37 (1.85) | 0.32 (0.65) | <0.001** | 1.44 |
| Current psychopathology | ||||
| Mood disorder | 15 (42.9%) | 2 (6.5%) | <0.001** | |
| Anxiety disorder | 22 (62.9%) | 3 (9.7%) | <0.001** | |
| PTSD | 8 (22.9%) | 0 (0.0%) | 0.005* | |
| Eating disorder | 2 (5.7%) | 1 (3.2%) | 0.63 | |
| Alcohol/substance disorder | 0 (0.0%) | 2 (6.5%) | 0.13 | |
| Psychotic disorder | 2 (5.7%) | 0 (0.0%) | 0.18 | |
| BPD | 12 (34.3%) | 0 (0.0%) | <0.001** | |
* P < 0.01
** P < 0.001
Participants were recruited via self-help groups (CAs only), flyers, social media and Ghent University’s student population and were matched for age, sex, handedness and level of education (χ2(2) = 1.96, P = 0.38). The study was approved by the IRB of Ghent University Hospital, and all participants provided written informed consent before commencing the study. At the end of the study women were able to discuss any feelings that may have arisen during the study and could also discuss with the experimenters the options of where they could turn to for help (should they so wish) such as local self-help groups, crisis helplines, clinical psychologists, etc.
Materials
Implicit ToM task
An adapted version (Deschrijver et al., 2016) of a previously validated task (Kovács et al., 2010, 2014) was used to measure implicit (spontaneous) ToM. The task comprises two phases—a belief phase, where the beliefs of the participant and an onscreen agent (Buzz Lightyear; Toy Story, 1995) are manipulated, and an outcome phase, where participants are required to react to the presence of a ball. The task consists of eight different movies and was presented using Presentation 18.2 (NeuroBehavioral Systems, Inc.).
At the beginning of every movie, Buzz Lightyear enters the scene and rolls a ball on a table with an occluder. Four different scenarios are then possible (Figure 1). In two scenarios, the ball stops behind the occluder. In the other two scenarios, the ball comes to rest off-screen. The agent then leaves the scene, believing the ball is either behind the occluder (A+) or not (A−). Subsequently, in half of the scenarios, the ball rolls again and changes location (i.e. if it were originally behind the occluder, it rolls and comes to a final stop off-screen and vice versa). In the other two scenarios, the ball remains stationary and does not change location. The agent then returns to the scene. Thus, while the participant always knows the ball’s true location (P+ or P−), in half of scenarios, the agent falsely believes the ball to be somewhere else to where it really is. At the end of each movie, the occluder falls. Each of the four scenarios was created with a ‘ball present’ and ‘ball absent’ ending, making the eventual presence of the ball at the end of the trial independent of both the participant’s and agent’s beliefs.
Fig. 1.
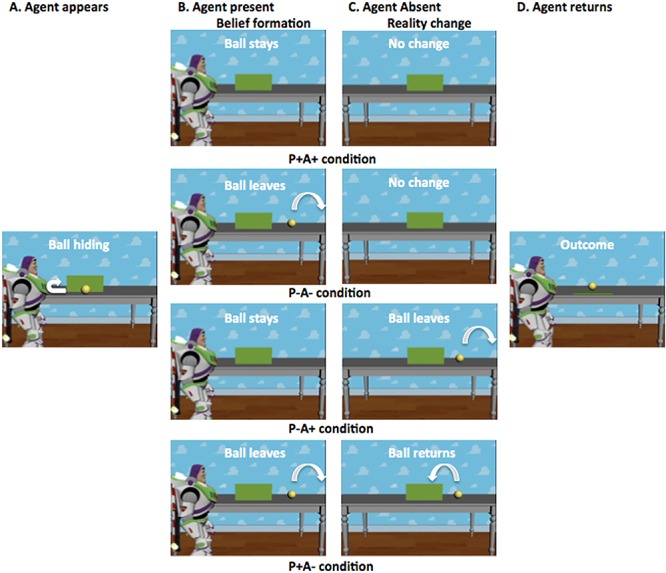
Schematic illustration of the trial procedure of the different conditions. Each trial begins with Buzz Lightyear entering the scene and rolling a ball on a table with an occluder. The ball either comes to rest behind the occluder or off-screen. Buzz (i.e. the agent) then leaves the scene, thinking the ball is either behind the occluder (A+) or not (A−). Subsequently, in half of the trials, the ball remains stationary. In the other half, it changes location. Finally, Buzz returns to the scene. Thus, while the participant always knows whether the ball is behind the occluder (P+) or not (P−), in half of the scenarios, Buzz falsely believes the ball to be somewhere else than where it really is. At the end of each movie, the occluder falls, revealing, independent of the participant’s or agent’s belief, that the ball is either present or absent. The current figure was taken from Nijhof et al. (2018), where it was published under a CC BY licence.
Participants were instructed to respond as quickly and as accurately as possible with their left index finger when the agent left the scene, to ensure that they paid attention to the whole movie, and with their right index finger if the ball was present at the end of the movie (after the occluder fell). Half of the movies ended with the ball present, regardless of the participant’s or agent’s belief. Crucially, participants were given no instructions to pay attention to the agent’s beliefs. Responses were registered using an MRI compatible response box (Cedrus).
Theory of mind localiser
To identify suitable mentalising-related brain regions and cohort-specific coordinates for these, including the rTPJ (primary ROI analysis), and to maintain independence of analysis, a Dutch translation of a well-validated ToM localiser was used (Dodell-Feder et al., 2011). Participants read 20 short stories pertaining to characters with false beliefs (‘false belief’ condition) or to inanimate objects such as maps or photographs which display false information (‘false photograph’ condition). Ten stories belonged to the ‘false belief’ condition, whereas the other 10 belonged to the ‘false photograph’ condition. Participants then read a statement on that story and responded using an MRI compatible response box (Cedrus) (index finger = true statement, middle finger = false statement).
Questionnaires
Stressful Life Events Screening Questionnaire
The 11-item Stressful Life Events Screening Questionnaire (SLESQ; Goodman et al., 1998) was used to assess traumatic exposure. We chose to use the SLESQ, instead of the more often used Childhood Trauma Questionnaire (CTQ; Bernstein and Fink, 1998), because it includes sub-items asking for additional details such as age at the time of the experience, frequency of the trauma, and duration of the trauma, and hence provides a more comprehensive report of traumatic experiences (cf. Supplementary Table S1). Participants were classified as CAs if they positively answered items 5 (rape), 6 (sexual assault), 7 (childhood physical abuse) and/or 9 (emotional abuse) and if their age at onset was <17 years. This cut-off was chosen to match other questionnaires assessing CA, such as the CTQ (Bernstein and Fink, 1998). Cronbach’s alpha (α) for the present sample was 0.76, indicating good internal consistency.
Interpersonal Reactivity Index
The 28-item Interpersonal Reactivity Index (IRI; Davis, 1984; Dutch translation by De Corte et al., 2007) measures empathic responsiveness (α = 0.79). It assesses four aspects of empathy: two cognitive (perspective-taking and fantasy) and two affective (empathic concern and personal distress).
Beck Depression Inventory-II
The 21-item Beck Depression Inventory-II (BDI-II; Beck et al., 1996; Dutch translation by van der Does, 2002) measures depressive symptoms covering cognitive, affective and somatic aspects of depression (α = 0.94).
STAI (trait and state)
The State-Trait Anxiety Inventory (STAI; Spielberger et al., 1983; Dutch translation by van der Ploeg et al., 2000) measures both state anxiety (20 items, α = 0.92) and trait anxiety (20 items, α = 0.94).
Dissociative Experiences Scale
The 28-item Dissociative Experiences Scale (DES; Bernstein and Putnam, 1986; Dutch translation by Ensink and van Otterloo, 1989) measures the extent to which respondents experience dissociative symptoms such as depersonalisation, derealisation and disturbances in memory and identity in daily life (α = 0.90).
Resilience Scale
The 25-item Resilience Scale (RS; Wagnild and Young, 1993) measures mental resilience and adaptability (α = 0.86).
Measure of psychopathology
Mini International Neuropsychiatric Interview
The Mini International Neuropsychiatric Interview (MINI; Sheehan et al., 1998; Dutch version by Overbeek et al., 1999) was administered by trained clinical psychology masters students. It assesses current and lifetime histories of Axis I disorders plus one Axis II disorder, antisocial personality disorder, according to DSM-IV criteria. Participants were categorised as ‘non-clinical’ if they endorsed only one of the two MINI screening questions, as ‘subclinical’ if they endorsed both screening questions but did not reach clinical threshold on the follow-up questions and as ‘clinical’ if they met both criteria. Due to much previous research on the link between CA and later borderline personality disorder (BPD; Herman et al., 1989; Stepp et al., 2016), an additional MINI-style subsection assessing BPD symptomatology was included as a rough indicator of BPD symptoms (Boccadoro et al., 2019a). Items were designed based on DSM-IV criteria, translated into Dutch, and back-translated. It was decided not to use a more thorough diagnostic interview (such as the Structured Clinical Interview for DSM-5 (SCID-5), First et al., 2015, or the Clinician Administered PTSD Scale (CAPS-5), Weathers et al., 2013) due to time constraints; furthermore, as our main focus was the influence of childhood trauma rather than psychopathology, the MINI was deemed sufficient.
Procedure
During fMRI, participants completed the implicit ToM task (~20 min duration with a short break in the middle), followed by the ToM localiser (~10 min duration) in fixed order. The implicit ToM task consisted of eight movies of each condition, resulting in 64 experimental trials, split into two blocks, and presented in randomised order. Prior to the task proper, feedback was given on four practice trials. Each trial lasted 13 800 ms. In each trial, the occluder fell at 13 250 ms. Between trials, there was a jitter of variable duration during which a black screen was displayed.
The ToM localiser consisted of 20 trials (10 per condition), presented in fixed order. Each trial comprised a short story displayed for 10 s, followed by a statement also displayed for 10 s. Beneath the statement, the words ‘true’ and ‘false’ (Dutch: ‘juist’ and ‘onjuist’) were displayed on either side of the screen to remind participants which finger to respond with. Between trials, there was a jitter of variable duration during which a white fixation cross was displayed on a black background.
Following scanning, participants completed the questionnaires outside the MRI scanner. Lastly, the MINI was conducted. Participants were then paid, debriefed, and thanked.
fMRI data acquisition
Images were acquired using a 3T Magnetom Siemens TrioTim MRI scanner at Ghent University Hospital. First, a T1-weighted high-resolution anatomical scan was performed [repetition time (TR) = 2250 ms, echo time (TE) = 4.18 ms, image matrix = 256 × 256, field of view (FOV) = 256 mm, flip angle = 9°, slice thickness = 1 mm, voxel size = 1.0 × 1.0 × 1.0 mm, number of slices = 176]. Functional images were acquired using a T2*-weighted Echo Planar Images (EPI) sequence (TR = 2000 ms, TE = 28 ms, image matrix = 384 × 384, FOV = 224 mm, flip angle = 80°, slice thickness = 3.0 mm, voxel size = 3.5 × 3.5 × 3.0 mm, number of slices = 34).
fMRI data processing
Data were preprocessed with SPM12 (Wellcome Department of Cognitive Neurology, London, UK) implemented in MATLAB R2012b (Mathworks, Inc.). The first five volumes of all EPI sequences were discarded due to scanner calibration. Data preprocessing began with coregistering the first EPI image with the corresponding participant’s structural image (and applying the resulting parameters to all remaining EPI images as well), in order to account for possible changes in head position during the short break between the acquisition of the structural and the functional images. Next, coregistered images were realigned and slice-time correction was applied. This was followed then by regular coregistration using the forward deformations from the segmentation step as per new SPM12 routine. Note that the extra coregistration at the beginning was added because visual inspection had revealed that the conventional approach of coregistering the structural image with the mean functional image following slice-time correction and/or realignment resulted in suboptimal coregistration that was subsequently resolved by first coregistering the structural image with the individual functional images. Structural images were segmented, and parameters generated during segmentation used to normalise the images to standard MNI space, following which voxel size was 2 × 2 × 2 mm. Finally, images were spatially smoothed with a Gaussian kernel of 8 mm (full-width-at-half-maximum). The high-pass filter was set at 128 Hz.
To quantify and additionally assess head motion, we calculated the framewise displacement (Power et al., 2014, 2015). The overall mean displacement was generally well below the recommended cut-off (<0.5 mm) for indicated motion artefacts with 0.09 mm for CA (s.d. = 0.05) and 0.08 mm for UC participants (s.d. = 0.03) suggesting no motion issues.
fMRI data analysis
All trials were modelled in SPM regardless of whether participants responded correctly. First-level statistical analyses were performed using the general linear model (GLM).
ToM localiser
For the ToM localiser, story and statement phases were conflated and analysed together. First-level models contained separate regressors for each condition (‘false belief’ and ‘false photograph’) as well as subject movement parameters. The second-level model contained three regressors of no interest: depression score (BDI-II), trait anxiety score (STAI-T) and age. Depression and anxiety scores were included as covariates because of group differences in the frequency of mood and anxiety disorder, while age was included as a covariate because of a relatively large age span of the study sample. Covariates were entered in the model as separate columns for the two groups and were centred across groups. For all covariates, the across-group mean was well within the range of scores of both groups. In order to identify primary (rTPJ) and secondary ToM regions, a one-sample t-test using the whole group was performed on the false belief > false photograph contrast at the whole-brain level (Supplementary Table S2 and Supplementary Figure S1). Coordinates of peak activation (FWE corrected at the peak level with a threshold of P < 0.05 and cluster size k > 10) indicating regions involved in cognitive ToM were then used in the ROI analyses for the implicit ToM task.
Implicit ToM task (belief phase)
For the implicit ToM task, only the belief phase was analysed (thus conflating ‘ball present’ and ‘ball absent’ conditions into one, resulting in four conditions). First-level models contained separate regressors for each condition as well as six movement parameters. In order to identify regions involved in ToM, a false belief (FB) > true belief (TB) contrast was generated. In this contrast, conditions where the agent falsely believed the ball to be somewhere other than its true location (i.e. where agent’s belief differed from participant’s) were classified as ‘false belief’ and conditions where both participant and agent believed the ball to be in the same location were classified as ‘true belief’.
To investigate differences in spontaneous ToM between CAs and UCs, first-level FB > TB contrasts were entered into a second-level model, and a UC > CA contrast was created. Regressors of no interest were depression score (BDI-II), anxiety score (STAI-trait) and age. An ROI analysis was carried out on the rTPJ (primary analysis) and other regions (secondary analysis) found to have been activated by the ToM localiser. To ensure independence of analysis, coordinates for the ROI analyses (6 mm spheres) were taken from coordinates of peak activation in the ToM localiser. Furthermore, FDR correction was applied for multiple comparisons to the secondary ROI analyses to account for the number of regions of interest (ROIs) being probed. As the temporal poles are well defined anatomically, here we instead used masks based on the AAL atlas created with WFU PickAtlas (Maldjian et al., 2003, 2004). Data were analysed by calculating UC > CA contrasts for the primary and secondary ROIs in the SPM toolbox MARSBAR (Brett et al., 2002). Cohen’s d was calculated from the t value as a measure of effect size after accounting for the covariates (Lakens, 2013). As a manipulation check (post hoc), we examined whether regions elicited during the implicit ToM task overlapped with our ROIs from the ToM localiser, which they did (Supplementary Table S3 and Supplementary Figures S4, S5), suggesting that the implicit ToM task was valid in eliciting cognitive ToM regions.
Next, to get a first indication of the degree to which comorbid psychopathology contributed to the results of the primary ROI analysis, we conducted subgroup analyses. Specifically, for each measured psychological disorder, we first fitted a one-way ANOVA in SPM including UCs, CAs without that disorder (CA−) and CAs with that disorder (CA+) as groups and then performed a ‘weak’ (UC > CA−) and a ‘strong’ test (CA− > CA+) of subgroup effects using the MARSBAR toolbox (Brett et al., 2002). The ‘weak test’ investigates if the UC > CA effect is still significant after excluding participants suffering from the tested disorder. If this is the case, we can conclude that the disorder in question cannot entirely explain the UC > CA effect. However, this does not necessarily mean that it does not contribute to the effect. This is then addressed with the ‘strong’ test, which examines if CAs with the tested disorder differ from CAs without that disorder. As such, combining both tests results in four possible outcomes: (i) significant weak test and nonsignificant strong test, indicating that there is no evidence that the tested disorder contributes to the rTPJ effect; (ii) nonsignificant weak test and significant strong test, indicating that the tested disorder contributes and potentially explains the effect; (iii) both tests significant, indicating that the disorder contributes but cannot explain the effect; and (iv) neither test significant, indicating that it can neither be confirmed nor disconfirmed that the disorder contributes.
Finally, to investigate functional connectivity between ToM regions, VOI information was extracted from the rTPJ ROI, and FB > TB contrasts were generated. It was decided to limit analyses to this region, as this region was the study’s main focus and, furthermore, the most consistently activated region in cognitive ToM tasks (Molenberghs et al., 2016). Psychophysiological interaction (PPI) analyses were then performed on this contrast, again using depression score, trait anxiety score and age as regressors of no interest. The dMPFC ROI was used as target region, based on previous research reporting rTPJ–dMPFC functional connectivity during spontaneous ToM (Burnett and Blakemore, 2009; Moessnang et al., 2017). Data were analysed with an UC ≠ CA contrast in the SPM gPPI and MARSBAR toolboxes (Brett et al., 2002; McLaren et al., 2012). The gPPI toolbox has been shown to improve model fit and have greater sensitivity and specificity than standard approaches (McLaren et al., 2012). A nondirectional contrast was used for the PPI analysis because, contrary to the ROI analysis (Quidé et al., 2017), there is no previous evidence available to inform directional hypotheses regarding the influence of CA on functional connectivity.
Behavioural data analysis (outcome phase)
As in previous work (Kovács et al., 2010; Deschrijver et al., 2016; Bardi et al., 2017; Nijhof et al., 2018), participants’ ToM index on the implicit ToM task was computed by calculating the difference in RT during the outcome phase between the condition where neither participant (P) nor agent (A) expect to see the ball (P−A−) and the condition where the participant does not expect to see the ball but the agent does (P−A+). In this way, it is possible to see how the agent’s perspective can spontaneously influence RT, with a larger ToM index indicating a stronger propensity to mentalise (Kovács et al., 2010; Deschrijver et al., 2016; Bardi et al., 2017; Nijhof et al., 2018). Independent t-tests were used to compare group performance, with Cohen’s d as a measure of effect size. Lastly, questionnaire data were correlated with the ToM index (also FDR-corrected) and ROI beta values were extracted and correlated with participants’ ToM index and questionnaire data. All analyses were performed in IBM SPSS Statistics 25.0 with P < 0.05 (two-tailed). Behavioural data for the ToM localiser were not analysed as the purpose of this task was to generate coordinates for fMRI ROI analyses.
Results
Sample characteristics
CAs had significantly higher levels of self-reported empathy (t(64) = 3.03, P = 0.003, d = 0.75), depression (t(49.21) = 5.07, P < 0.001, d = 1.23), dissociation (t(51) = 3.52, P = 0.001, d = 0.98), trait anxiety (t(63) = 5.80, P < 0.001, d = 1.44), state anxiety (t(64) = 4.61, P < 0.001, d = 1.14) and current psychopathology (t(64) = 5.85, P < 0.001, d = 1.44) (Table 1). UCs self-reported significantly higher levels of resilience (t(64) = 3.16, P = 0.002, d = 0.80) but were equally likely to be taking psychotropic medication (χ2(1) = 2.56, P = 0.110).
Regions of interest
First, ToM ROIs, including the rTPJ, were identified with the ToM localiser. The whole-brain false belief > false photograph contrast revealed significant clusters located in the bilateral TPJ, precuneus, dorsomedial prefrontal cortex (dMPFC), bilateral middle temporal gyri (MTG) and bilateral temporal poles (Supplementary Table S2 and Supplementary Figure S1), with no significant group differences in the resulting brain activity.
Neural activation during belief phase
Primary ROI analysis: rTPJ
As expected, analysis of the rTPJ ROI (peak MNI xyz: 46 −58 20) revealed that UCs (n = 31) had significantly more activation than CAs (n = 35) during the belief phase of the ToM task (t(58) = 1.70, P = 0.048) with a medium effect size (d = 0.42) (Figure 2). FB > TB activation in the rTPJ was not significantly correlated with any of the questionnaire or behavioral data.
Fig. 2.
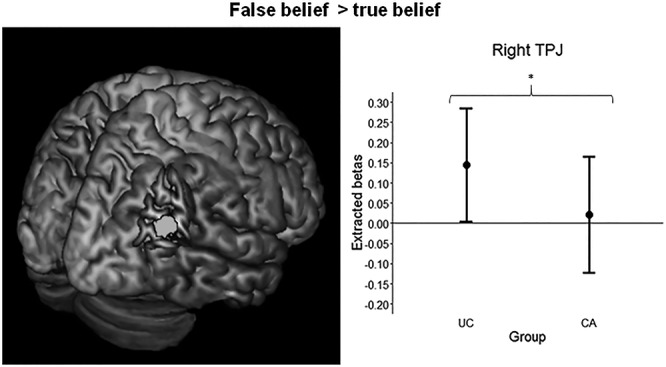
Right temporoparietal junction (TPJ) region of interest (6 mm sphere) on a standard brain (left panel). Mean extracted betas and 95% confidence intervals for the rTPJ in women with childhood abuse history (CA, n = 35) and unaffected comparison women (UC, n = 31). False belief (FB) > true belief (TB) (right panel). *P < 0.05.
Secondary ROI analysis
Exploratory ROI analyses of the other seven regions identified by the localiser revealed descriptively stronger activation for UCs than for CAs, with small to medium effect sizes, in all areas except the right temporal pole and right MTG (Table 2). However, significantly stronger activation for UCs was observed only in the dMPFC (t(58) = 2.03, P = 0.024, d = 0.50) and left MTG (t(58) = 1.92, P = 0.030, d = 0.47), and also these differences disappeared after FDR correction was applied (both ps = 0.105).
Table 2.
Mean extracted betas and s.d. for the secondary ROIs in women with childhood abuse history (CA, n = 35) and unaffected comparison women (UC, n = 31)†
| UC | CA | p uncorr | p corr | Effect size | |
|---|---|---|---|---|---|
| lTPJ | 0.09 (0.75) | −0.22 (0.57) | 0.135 | 0.189 | 0.28 |
| lMTG | −0.05 (0.43) | −0.16 (0.41) | 0.030 | 0.105 | 0.47 |
| rMTG | 0.00 (0.40) | 0.05 (0.41) | 0.590 | 0.590 | 0.06 |
| lTP | 0.06 (0.67) | −0.15 (0.58) | 0.056 | 0.131 | 0.40 |
| rTP | 0.03 (0.56) | −0.01 (0.54) | 0.252 | 0.294 | 0.17 |
| dMPFC | −0.10 (0.51) | −0.30 (0.48) | 0.024 | 0.105 | 0.50 |
| Precuneus | −0.10 (0.84) | −0.24 (0.66) | 0.095 | 0.166 | 0.33 |
† p uncorr refers to the uncorrected P value and pcorr to the corrected P value (FDR). Effect sizes are Cohen’s d. Notes: TPJ, temporoparietal junction; MTG, middle temporal gyrus; TP, temporal pole; dMPFC, dorsomedial prefrontal cortex
Comorbid psychopathology
Participants’ depressive and anxious symptoms were controlled for in all models. Furthermore, neither BDI (t(58) = 1.10, P = 0.139, r = 0.13) nor STAI scores (t(58) = −0.23, P = 0.590, r = −0.03) correlated positively with rTPJ brain activity. Further subgroup analyses were conducted on the rTPJ results for the two additional disorders that differed between both groups: PTSD and BPD (Table 1). Specifically, we conducted ‘weak’ (UC > CA−) and ‘strong’ (CA− > CA+) subgroup tests to explore, respectively, if the UC > CA contrast remained significant after excluding those participants that suffered from the tested disorder and if false belief activity was weaker for CAs with than without that disorder (see Methods). To increase statistical power, both clinical and subclinical cases were included in the CA+ group of the PTSD analysis (n = 13). However, the same results were obtained if the CA+ group included only clinical cases. For PTSD, neither the UC > CA− (t(54) = 0.89, P = 0.190, d = 0.25) nor the CA− > CA+ contrast (t(54) = 1.24, P = 0.110, d = 0.41) was significant (Supplementary Figure S2). For BPD, the UC > CA− contrast was significant (t(54) = 2.36, P = 0.011, d = 0.65), but the CA− > CA+ contrast was not (t(54) = −2.82, P = 0.997) (Supplementary Figure S3).
Functional connectivity during belief phase
The ToM contrast (FB > TB) PPI showed increased rTPJ–dMPFC functional connectivity for CAs compared with UCs (t(58) = 2.46, P = 0.017, d = 0.61). Follow-up tests revealed that the FB > TB PPI was significant for CAs (t(58) = 1.92, P = 0.030, d = 0.47) but not for UCs (t(58) = −1.53, P = 0.934).
Behavioural results during outcome phase
There was no significant difference in ToM index between UCs and CAs (t(62) = 0.98, P = 0.33, d = 0.25) (Table 3), and the correlational analyses between the ToM index and the questionnaire data did not reveal any significant associations.
Table 3.
Reaction times (RT) on implicit ToM task, ToM index (P−A− minus P−A+) and mean number of missed attention checks†
| CA (n = 33) | UC (n = 31) | P value | Effect size | |
|---|---|---|---|---|
| P−A− | 456.50 (48.85) | 454.12 (68.93) | 0.87 | 0.04 |
| P−A+ | 417.21 (43.74) | 425.45 (70.03) | 0.57 | 0.13 |
| P+A− | 404.65 (42.14) | 406.39 (60.39) | 0.89 | 0.03 |
| P+A+ | 433.68 (54.66) | 424.53 (63.95) | 0.54 | 0.15 |
| Overall RT | 436.90 (46.84) | 438.75 (72.98) | 0.91 | 0.03 |
| ToM index | 39.29 (39.43) | 28.68 (46.87) | 0.33 | 0.25 |
| N missed attention checks | 0.14 (0.55) | 0.16 (0.45) | 0.88 | 0.04 |
†No behavioural data was collected for two CAs. P refers to whether the participant knew the ball was behind the occluder (P+) or not (P−). A refers to whether the agent thought the ball was behind the occluder (A+) or not (A−)
Discussion
This study tested the simple hypothesis that early life maltreatment is associated with reduced activation of the rTPJ, a core structure in social cognition and ToM, in a community sample of adult women. Consistent with the main hypothesis, women with CA experiences showed less rTPJ activation during a spontaneous cognitive mentalising task compared to women without such experiences. Functional connectivity analyses further revealed increased functional connectivity between the rTPJ and dMPFC, another core region of the ToM network, in CAs compared with UCs, suggesting that also connectivity within the network may be altered as a result of childhood abuse. In keeping with previous studies on childhood maltreatment in psychiatric populations (Hentze et al., 2016; Quidé et al., 2017; van Schie et al., 2017) and populations known to have mentalising deficits (e.g. autism spectrum disorder: Nijhof et al., 2018; schizophrenia: Benedetti et al., 2009), the present data thus support the hypothesis that women who have been maltreated during childhood show aberrant functioning of the ToM network, specifically while computing other people’s beliefs when these differ from their own.
A similar pattern of reduced false belief related activity in CAs was also found, descriptively, in five other nodes of the ToM network. However, none of these group differences were significant after correcting for multiple comparisons. Therefore, this result should not be taken as evidence for widespread group differences across the ToM network, but rather as an indication for which regions to follow up on in future research. In this view, it will be important to directly test the degree to which CA-related deficits of the ToM network extend beyond the rTPJ. Based on the current study, the dMPFC, left MTG and left temporal pole seem especially promising, as they yielded, descriptively, the strongest group differences.
Importantly, as depression and anxiety symptoms were controlled for in all models, the reduced rTPJ activation for CAs cannot be explained by comorbid depression or anxiety symptoms. However, as UCs and CAs did not only differ with respect to mood and anxiety disorder but also with respect to PTSD and BPD, we additionally ran subgroup analyses exploring their contribution. The PTSD analysis revealed that, based on the current data, we can neither rule out nor confirm that rTPJ hypoactivation in CAs is related to the degree to which they are psychologically affected by their traumatic experience. As such, an important avenue for future research will be to more extensively explore the hypothesis that PTSD mediates the effects of CA on brain activity during mentalising. This seems especially relevant considering that a previous behavioural study by Nazarov et al. (2014) found that adult women with child abuse-related PTSD have reduced ToM compared with unaffected women. By investigating the influence of PTSD on ToM-related brain processes, future work might therefore provide insight into the potential mechanisms underlying such ToM deficits.
The BPD analysis suggested that BPD did not contribute to CA rTPJ hypoactivation. These results should be interpreted with care, however, as BPD was measured using a self-made MINI-style screening tool that has yet to be validated. Thus, while our results provide preliminary evidence that comorbid BPD cannot explain our results, this will have to be confirmed by future work using better-validated and perhaps continuous measures of BPD symptomatology.
Unexpectedly, whereas CAs displayed rTPJ hypoactivation during mentalising, there was no evidence for behavioural mentalising deficits. Specifically, the ToM index, which measures the degree to which participants’ responses were influenced by the agent’s belief (Kovács et al., 2010), did not differ between CAs and UCs. There are at least three possible reasons for this discrepancy. A first possibility is that limited statistical power prevented us from detecting a behavioural effect. This is supported by the results of another, purely behavioural, study in which we tested the same task on a different but larger sample and found a weaker ToM index in CAs (Hudson et al., 2019). A second possibility is that neural measures of mentalising are more sensitive to group differences than behavioural measures. Supporting this hypothesis, Nijhof et al. (2018), using the same spontaneous cognitive ToM task, similarly found reduced rTPJ activation but no behavioural deficits during mentalising in a group of participants with autism spectrum disorder. Finally, a third possibility is that compensatory mechanisms may hide underlying ToM deficits at the behavioural level (Livingston et al., 2019). For example, in a recent multi-study fMRI investigation (Boccadoro et al., 2019b), we found that the ToM task used in the current study also recruits brain networks involved in working memory and visuospatial attention. It is, therefore, conceivable that individuals with disturbed ToM capacities may rely more on these nonsocial networks when doing the task and that this hides ToM deficits caused by the disturbance of social brain networks. From this perspective, it seems desirable for future research to also consider other ToM tasks that rely less on nonsocial skills and may hence be more diagnostic of behavioural mentalising deficits (see, e.g. Nazarov et al., 2014; Germine et al., 2015; Quidé et al., 2018).
It should also be noted that evidence for rTPJ hypoactivation in the CA group, while statistically significant, was relatively weak. However importantly, this was accompanied by a clinically significant medium effect size. Therefore, although its findings will have to be confirmed in future research, this study may have important clinical implications. It appears that women with CA experiences are uniquely at risk of altered perspective-taking abilities well into adulthood. This may indicate that previous CA sufferers seeking psychotherapy require a specialised approach. This is of particular importance, as a recent study suggests that assaulted adolescent girls respond differently to trust violations, possibly due to alterations in their mentalising abilities (Lenow et al., 2014). Tailored therapy appears warranted to help improve such abilities, in order to reduce risk of revictimisation. Furthermore, reduced social competence has been linked to poorer social support networks in adolescents (Elliott et al., 2005) and adults (Stiller and Dunbar, 2007; Lewis et al., 2011), which in turn is associated with a higher risk for psychopathology in maltreated women (Schumm et al., 2006; Vranceanu et al., 2007; Sperry and Widom, 2013).
Despite these important implications, this study also has some notable limitations. Firstly, because we used a general psychopathology screening interview to assess comorbid psychopathology instead of more specialised interviews such as the CAPS for PTSD (Weathers et al., 2013), we have only limited information on psychological symptom severity, and the conclusions that can be drawn from this data are therefore limited. This is especially true for BPD, which was measured using a self-made, unvalidated screening tool. For these reasons, the reported comorbidity analyses, and particularly those relating to BPD, should not be taken as the end point but rather as an initial, preliminary exploration. Indeed, it is important to remember that the aim of this study was not to investigate the impact of different psychopathologies on mentalising and mentalising-related brain regions, but rather to investigate the impact of CA experience, which it did so successfully. Moreover, this study also gave valuable insight into a more representative sample of CA survivors—neither one extreme end of the spectrum (i.e. those with a clinical diagnosis) nor the other (i.e. those without any psychopathology). Secondly, our sample size consisted solely of female CA survivors, so generalisation to male survivors must be done carefully. Yet, this may also be considered as a strength because we thus excluded potential confounds related to biological sex, especially as studies are beginning to show neural evidence for sex differences in cognitive/affective ToM (Derntl et al., 2010; Adenzato et al., 2017).
Conclusions
In conclusion, here we demonstrate hypoactivation of a key mentalising brain region, the rTPJ, in women with a history of childhood abuse. Clinically, this study emphasises the importance of assessing individuals seeking psychotherapy for history of childhood abuse, as this may present a unique profile and set of risks independent of psychological diagnosis.
Supplementary Material
Acknowledgements
The authors would like to warmly thank all women who participated in our research, as well as all organisations and groups who helped with recruitment.
Funding
This work was supported by a 2–4-year grant (01J05415) from the Special Research Fund (BOF) at Ghent University to S.C.M. and M.B. E.C. was also supported by the Research Foundations Flanders (FWO18/PDO/049). The funding source had no involvement in study design; collection, analysis, and interpretation of data; writing of the report; or the decision to submit for publication.
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- Abu-Akel A., Shamay-Tsoory S. (2011). Neuroanatomical and neurochemical bases of theory of mind. Neuropsychologia, 49(11), 2971–84. doi: 10.1016/j.neuropsychologia.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Adenzato M., Brambilla M., Manenti R., et al. (2017). Gender differences in cognitive theory of mind revealed by transcranial direct current stimulation on medial prefrontal cortex. Scientific Reports, 7, 41219. doi: 10.1038/srep41219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardi L., Desmet C., Nijhof A., Wiersema J.R., Brass M. (2017). Brain activation for spontaneous and explicit false belief tasks overlaps: new fMRI evidence on belief processing and violation of expectation. Social Cognitive and Affective Neuroscience, 12(3), 391–400. doi: 10.1093/scan/nsw143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Brown G.K. (1996). Manual for the Beck Depression Inventory-II, San Antonio, TX: Psychological Corporation. [Google Scholar]
- Benarous X., Guilé J.M., Consoli A. (2015). Cohen D. a systematic review of the evidence for impaired cognitive theory of mind in maltreated children. Frontiers in Psychiatry, 6, 108. doi: 10.3389/fpsyt.2015.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F., Bernasconi A., Bosia M., Cavallaro R., Smeraldi E. (2009). Functional and structural brain correlates of theory of mind and empathy deficits in schizophrenia. Schizophrenia Research, 114(1–3), 154–60. doi: 10.1016/j.schres.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Bernstein D.P., Fink L.A. (1998). CTQ: Childhood Trauma Questionnaire: A retrospective self-report, San Antonio, TX: Psychological Corporation. [Google Scholar]
- Bernstein E.M., Putnam F.W. (1986). Development, reliability, and validity of a dissociation scale. The Journal of Nervous and Mental Disease, 174(12), 727–35. doi: 10.1097/00005053-198612000-00004. [DOI] [PubMed] [Google Scholar]
- Boccadoro S., Cracco E., Hudson A.R., et al. (2019b). Defining the neural correlates of spontaneous theory of mind (ToM): an fMRI multi-study investigation. NeuroImage, 203, 116193, doi: 10.1016/j.neuroimage.2019.116193. [DOI] [PubMed] [Google Scholar]
- Boccadoro S., Siugzdaite R., Hudson A.R., Maeyens L., Van Hamme C., Mueller S.C. (2019a). Women with early maltreatment experience show increased resting-state functional connectvitiy in the theory of mind (ToM) network. European Journal of Psychotraumatology, 10(1), 1647044. doi: 10.1080/20008198.2019.1647044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonomi A.E., Thompson R.S., Anderson M., et al. (2006). Intimate partner violence and women’s physical, mental, and social functioning. American Journal of Preventive Medicine, 30(6), 458–66. doi: 10.1016/j.amepre.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Brett M., Anton J.L., Valabregue R., Poline J.B. (2002). Region of interest analysis using the MarsBaR toolbox for SPM99. NeuroImage, 16(2), S497. [Google Scholar]
- Burack J.A., Flanagan T., Peled T., Sutton H.M., Zygmuntowicz C., Manly J.T. (2006). Social perspective-taking skills in maltreated children and adolescents. Developmental Psychology, 42(2), 207–17. doi: 10.1037/0012-1649.42.2.207. [DOI] [PubMed] [Google Scholar]
- Burnett S., Blakemore S.J. (2009). Functional connectivity during a social emotion task in adolescents and in adults. European Journal of Neuroscience, 29(6), 1294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D., Schilbach L., Vogeley K., et al. (2012). Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Structure & Function, 217(4), 783–96. doi: 10.1007/s00429-012-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R.M., Huettel S.A. (2013). A nexus model of the temporal-parietal junction. Trends in Cognitive Sciences, 17(7), 328–36. doi: 10.1016/j.tics.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M.H. (1984). A multidimensional approach to individual differences in empathy. JSAS Catalog of Selected Documents in Psychology, 10, 85. [Google Scholar]
- De Corte K., Buysse A., Verhofstadt L.L., Roeyers H., Ponnet K., Davis M.H. (2007). Measuring empathic tendencies: reliability and validity of the Dutch version of the interpersonal reactivity index. Psychologica Belgica, 47(4), 235–60. doi: 10.5334/pb-47-4-235. [DOI] [Google Scholar]
- Decety J., Lamm C. (2007). The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. The Neuroscientist, 13(6), 580–93. doi: 10.1177/1073858407304654. [DOI] [PubMed] [Google Scholar]
- Derntl B., Finkelmeyer A., Eickhoff S., et al. (2010). Multidimensional assessment of empathic abilities: neural correlates and gender differences. Psychoneuroendocrinology, 35(1), 67–82. [DOI] [PubMed] [Google Scholar]
- Deschrijver E., Bardi L., Wiersema J.R., Brass M. (2016). Behavioural measures of implicit theory of mind in adults with high functioning autism. Cognitive Neuroscience, 7(1–4), 192–202. doi: 10.1080/17588928.2015.1085375. [DOI] [PubMed] [Google Scholar]
- Dodell-Feder D., Koster-Hale J., Bedny M., Saxe R. (2011). fMRI item analysis in a theory of mind task. NeuroImage, 55(2), 705–12. doi: 10.1016/j.neuroimage.2010.12.040. [DOI] [PubMed] [Google Scholar]
- Does A.J.W. (2002). BDI-II-NL. Handleiding: De Nederlandse versie van de Beck Depression Inventory [Manual: The Dutch version of the Beck Depression Inventory], Lisse, The Netherlands: Harcourt Test Publishers. [Google Scholar]
- E.U. Agency for Fundamental Rights (2014). Violence Against Women: An EU-wide Survey. Main Results Report, Luxembourg: Publications Office of the European Union. [Google Scholar]
- Elliott G.C., Cunningham S.M., Linder M., Colangelo M., Gross M. (2005). Child physical abuse and self-perceived social isolation among adolescents. Journal of Interpersonal Violence, 20(12), 1663–84. doi: 10.1177/0886260505281439. [DOI] [PubMed] [Google Scholar]
- Ensink B.J., van Otterloo D. (1989). A validation study of the DES in the Netherlands. Dissociation: Progress in the Dissociative Disorders, 2(4), 221–3. [Google Scholar]
- First M.B., Williams J.B.W., Karg R.S., Spitzer R.L. (2015). Structured Clinical Interview for DSM-5—Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV), Arlington, VA: American Psychiatric Association. [Google Scholar]
- Germine L., Dunn E.C., McLaughlin K.A., Smoller J.W. (2015). Childhood adversity is associated with adult theory of mind and social affiliation, but not face processing. PLoS One, 10(6), e0129612. doi: 10.1371/journal.pone.0129612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman L.A., Corcoran C., Turner K., Yuan N., Green B.L. (1998). Assessing traumatic event exposure: general issues and preliminary findings for the stressful life events screening questionnaire. Journal of Traumatic Stress, 11(3), 521–42. doi: 10.1023/A:1024456713321. [DOI] [PubMed] [Google Scholar]
- Green J.G., McLaughlin K.A., Berglund P.A., et al. (2010). Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Archives of General Psychiatry, 67(2), 113–23. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H., Rubia K. (2012). Neuroimaging of child abuse: a critical review. Frontiers in Human Neuroscience, 6, 52. doi: 10.3389/fnhum.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze C., Walter H., Schramm E., et al. (2016). Functional correlates of childhood maltreatment and symptom severity during affective theory of mind tasks in chronic depression. Psychiatry Research: Neuroimaging, 250, 1–11. [DOI] [PubMed] [Google Scholar]
- Herman J.L., Perry J.C., Kolk B.A. (1989). Childhood trauma in borderline personality disorder. The American Journal of Psychiatry, 146(4), 490–5. [DOI] [PubMed] [Google Scholar]
- Hudson A., De Coster L., Spoormans H., et al. (2019). Childhood abuse and adult socio-cognitive skills: distinguishing between self and other following early trauma. Journal of Interpersonal Violence, 1–21. doi: 10.1177/0886260520906190. [DOI] [PubMed] [Google Scholar]
- Keysers C., Gazzola V. (2014). Dissociating the ability and propensity for empathy. Trends in Cognitive Sciences, 18(4), 163–6. doi: 10.1016/j.tics.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács A.M., Téglás E., Endress A.D. (2010). The social sense: susceptibility to others' beliefs in human infants and adults. Science, 330(6012), 1830–4. doi: 10.1126/science.1190792. [DOI] [PubMed] [Google Scholar]
- Kovács A.M., Kühn S., Gergely G., Csibra G., Brass M. (2014). Are all beliefs equal? Implicit belief attributions recruiting core brain regions of theory of mind. PLoS One, 9(9), e106558. doi: 10.1371/journal.pone.0106558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krall S.C., Rottschy C., Oberwelland E., et al. (2015). The role of the right temporoparietal junction in attention and social interaction as revealed by ALE meta-analysis. Brain Structure & Function, 220(2), 587–604. doi: 10.1007/s00429-014-0803-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakens D. (2013). Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Frontiers in Psychology, 4, 863. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenow J.K., Scott Steele J., Smitherman S., Kilts C.D., Cisler J.M. (2014). Attenuated behavioural and brain responses to trust violations among assaulted adolescent girls. Psychiatry Research, 223(1), 1–8. doi: 10.1016/j.pscychresns.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P.A., Rezaie R., Brown R., Roberts N., Dunbar R.I. (2011). Ventromedial prefrontal volume predicts understanding of others and social network size. NeuroImage, 57(4), 1624–9. doi: 10.1016/j.neuroimage.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston L.A., Colvert E., the Social Relationships Study Team, Bolton P., Happé F. (2019). Good social skills despite poor theory of mind: exploring compensation in autism spectrum disorder. Journal of Child Psychology and Psychiatry, 60, 102–10. doi: 10.1111/jcpp.12886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke N., Banerjee R. (2013). Differentiated associations between childhood maltreatment experiences and social understanding: a meta-analysis and systematic review. Developmental Review, 33(1), 1–28. doi: 10.1016/j.dr.2012.10.001. [DOI] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage, 19(3), 1233–9. doi: 10.1016/S1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Burdette J.H. (2004). Precentral gyrus discrepancy in electronic versions of the Talairach atlas. NeuroImage, 21(1), 450–5. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- McLaren D.G., Ries M.L., Xu G. (2012). Johnson SC. A generalised form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. NeuroImage, 61(4), 1277–86. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moessnang C., Otto K., Bilek E., et al. (2017). Differential responses of the dorsomedial prefrontal cortex and right posterior superior temporal sulcus to spontaneous mentalizing. Human Brain Mapping, 38, 3791–803. doi: 10.1002/hbm.23626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenberghs P., Johnson H., Henry J.D., Mattingley J.B. (2016). Understanding the minds of others: a neuroimaging meta-analysis. Neuroscience and Biobehavioral Reviews, 65, 276–91. doi: 10.1016/j.neubiorev.2016.03.020. [DOI] [PubMed] [Google Scholar]
- Mueller S.C., Maheu F.S., Dozier M., et al. (2010). Early-life stress is associated with impairment in cognitive control in adolescence: an fMRI study. Neuropsychologia, 48(10), 3037–44. doi: 10.1016/j.neuropsychologia.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarov A., Maheu F.S., Dozier M., et al. (2014). Theory of mind performance in women with posttraumatic stress disorder related to childhood abuse. Acta Psychiatrica Scandinavica, 129(3), 193–201. doi: 10.1111/acps.12142. [DOI] [PubMed] [Google Scholar]
- Nijhof A.D., Bardi L., Brass M., Wiersema J.R. (2018). Brain activity for spontaneous and explicit mentalizing in adults with autism spectrum disorder: an fMRI study. NeuroImage: Clinical, 18, 475–84. doi: 10.1016/j.nicl.2018.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeek T., Schruers K., Griez E. (1999). MINI: Mini International Neuropsychiatric Interview, Dutch Version 5.0.0. (DSM-IV), Maastricht, The Netherlands: Maastricht University. [Google Scholar]
- Philip N.S., Sweet L.H., Tyrka A.R., et al. (2016). Exposure to childhood trauma is associated with altered n-back activation and performance in healthy adults: implications for a commonly used working memory task. Brain Imaging and Behavior, 10(1), 124–35. doi: 10.1007/s11682-015-9373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploeg H.M., Defares P.B., Spielberger C.D. (2000). Handleiding bij de zelfbeoordelingsvragenlijst: Een Nederlandstalige bewerking van de Spielberger State-Trait Anxiety Inventory [Manual for the State-Trait Anxiety Inventory: A Dutch translation], Lisse, The Netherlands. [Google Scholar]
- Power J.D., Mitra A., Laumann T.O., Snyder A.Z., Schlaggar B.L., Petersen S.E. (2014). Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage, 84, 320–41. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Schlaggar B.L., Petersen S.E. (2015). Recent progress and outstanding issues in motion correction in resting state fMRI. NeuroImage, 105, 536–51. doi: 10.1016/j.neuroimage.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quidé Y., Ong X.H., Mohnke S., et al. (2017). Childhood trauma-related alterations in brain function during a theory-of-mind task in schizophrenia. Schizophrenia Research, 189, 162–8. doi: 10.1016/j.schres.2017.02.012. [DOI] [PubMed] [Google Scholar]
- Quidé Y., Cohen-Woods S., O'Reilly N., Carr V.J., Elzinga B.M., Green M.J. (2018). Schizotypal personality traits and social cognition are associated with childhood trauma exposure. The British Journal of Clinical Psychology, 57(4), 397–419. doi: 10.1111/bjc.12187. [DOI] [PubMed] [Google Scholar]
- Saxe R., Carey S., Kanwisher N. (2004). Understanding other minds: linking developmental psychology and functional neuroimaging. Annual Review of Psychology, 55, 87–124. doi: 10.1146/annurev.psych.55.090902.142044. [DOI] [PubMed] [Google Scholar]
- Schie C.C., Harmelen A.-L., Hauber K., Boon A., Crone E.A., Elzinga B.M. (2017). The neural correlates of childhood maltreatment and the ability to understand mental states of others. European Journal of Psychotraumatology, 8(1), 1272788. doi: 10.1080/20008198.2016.1272788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumm J.A., Briggs-Phillips M., Hobfoll S.E. (2006). Cumulative interpersonal traumas and social support as risk and resiliency factors in predicting PTSD and depression among inner-city women. Journal of Traumatic Stress, 19(6), 825–36. doi: 10.1002/jts.20159. [DOI] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., et al. (1998). The Mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry, 59(Suppl 20), 22–33; quiz 34–57. [PubMed] [Google Scholar]
- Sperry D.M., Widom C.S. (2013). Child abuse and neglect, social support, and psychopathology in adulthood: a prospective investigation. Child Abuse & Neglect, 37(6), 415–25. doi: 10.1016/j.chiabu.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R., Vagg P.R., Jacobs G.A. (1983). Manual for the State-Trait Anxiety Inventory (Self-evaluation questionnaire), Palo Alto, CA: Consulting Psychologists Press, Inc. [Google Scholar]
- Stepp S.D., Lazarus S.A., Byrd A.L. (2016). A systematic review of risk factors prospectively associated with borderline personality disorder: taking stock and moving forward. Personality Disorders, 7(4), 316–23. doi: 10.1037/per0000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiller J., Dunbar R.I. (2007). Perspective-taking and memory capacity predict social network size. Social Networks, 29(1), 93–104. doi: 10.1016/j.socnet.2006.04.001. [DOI] [Google Scholar]
- Teicher M.H., Samson J.A. (2016). Annual research review: enduring neurobiological effects of childhood abuse and neglect. Journal of Child Psychology and Psychiatry, 57(3), 241–66. doi: 10.1111/jcpp.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher M.H., Anderson C.M., Ohashi K., Polcari A. (2014). Childhood maltreatment: altered network centrality of cingulate, precuneus, temporal pole and insula. Biological Psychiatry, 76(4), 297–305. doi: 10.1016/j.biopsych.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranceanu A.M., Hobfoll S.E., Johnson R.J. (2007). Child multi-type maltreatment and associated depression and PTSD symptoms: the role of social support and stress. Child Abuse & Neglect, 31(1), 71–84. doi: 10.1016/j.chiabu.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagnild G.M., Young H.M. (1993). Development and psychometric evaluation of the resilience scale. Journal of Nursing Measurement, 1(2), 165–78. [PubMed] [Google Scholar]
- Weathers F.W., Blake D.D., Schnurr P.P., Kaloupek D.G., Marx B.P., Keane T.M. (2013). The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5), Interview available from the National Center for PTSD: www.ptsd.va.gov. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


