Abstract
Background
Many pediatric patients with inflammatory bowel disease (IBD) lose response to infliximab (IFX) within the first year, and achieving a minimal target IFX trough concentration is associated with higher remission rates and longer durability. Population pharmacokinetic (PK) modeling can predict trough concentrations for individualized dosing. The object of this study was to refine a population PK model that accurately predicts individual IFX exposure during maintenance therapy using longitudinal real-practice data.
Methods
We exported data from the electronic health records of pediatric patients with IBD treated with originator IFX at a single center between January 2011 and March 2017. Subjects were divided into discovery and validation cohorts. A population PK model was built and then validated.
Results
We identified 228 pediatric patients with IBD who received IFX and had at least 1 drug concentration measured, including 135 and 93 patients in the discovery and validation cohorts, respectively. Weight, albumin, antibodies to IFX (ATI) detected by a drug-tolerant assay, and erythrocyte sedimentation rate (ESR) were identified as covariates significantly associated with IFX clearance and incorporated into the model. The model exhibited high accuracy for predicting target IFX trough concentrations with an area under the receiver operating characteristic curve (AUROC) of 0.86 (95% confidence interval [CI], 0.81–0.91) for population-based predictions without prior drug-level input. Accuracy increased further for individual-based predictions when prior drug levels were known, with an AUROC of 0.93 (95% CI, 0.90–0.97).
Conclusions
A population PK model utilizing weight, albumin, ordinal drug-tolerant ATI, and ESR accurately predicts IFX trough concentrations during maintenance therapy in real-practice pediatric patients with IBD. This model, which incorporates dynamic clinical information, could be used for individualized dosing decisions to increase response durability.
Keywords: Crohn’s disease, ulcerative colitis, pharmacokinetic model, individual dosing, biological drug
A population pharmacokinetic model utilizing weight, albumin, drug-tolerant antibodies to infliximab (IFX), and erythrocyte sedimentation rate accurately predicts IFX trough concentrations in real-practice pediatric patients with inflammatory bowel disease. This model, which incorporates dynamic clinical information, could be used for individualized dosing decisions to increase response durability.
INTRODUCTION
Pediatric patients with moderate to severe inflammatory bowel disease (IBD) are frequently treated with infliximab (IFX). Personalized dosing regimens using a dashboard system that applies a population pharmacokinetic model have improved IFX durability in adult patients1, 2 and are being developed in pediatric care. Infliximab is a chimeric monoclonal IgG1 antibody to tumor necrosis factor (TNF)–α, a pro-inflammatory cytokine important in the activation and proliferation of inflammation in IBD. Providers currently use standardized weight-based dosing; during clinical trials, this resulted in clinical remission in about one-half of patients.3–6 This uniform dosing results in a wide range of actual IFX exposure across the patient population. The predose trough concentration, which is reflective of IFX exposure, is associated with clinical outcomes, and achieving a minimal target IFX trough concentration is associated with higher rates of remission in adult and pediatric patients.7, 8
Emerging research supports that individual patient and disease factors strongly influence IFX pharmacokinetics (PK).9–11 As an intravenously administered drug, infliximab distributes in the circulation quickly and is usually eliminated over a period of weeks.12 Infliximab exhibits dose-proportional linear clearance that is affected by body weight; the clearance is also dependent on antigen and receptor expression.13 However, many other factors account for the variability observed in IFX clearance, including serum albumin concentration, the presence of antidrug antibodies, concomitant use of immunomodulatory medications, high levels of inflammation, and patient sex.14 A dosing regimen that incorporates individualized covariates would optimize drug exposure for each patient.2, 15
A population PK model leveraging predictive covariates and prior drug concentration results can predict trough concentrations to facilitate individualized dose adjustments.9, 16 This model-based approach with Bayesian estimation requires as few as 1 drug concentration measurement to estimate individual PK parameters that predict the future concentration profile over time with great accuracy.17, 18 In 2011, Fasanmade et al. reported a population PK analysis using data from 112 pediatric subjects in the REACH trial combined with data from 580 adult subjects from the ACCENT I trial.19 Covariates that influenced IFX clearance in this combined pediatric and adult analysis included weight, serum albumin concentration, the presence of antibodies to IFX (ATI) as detected by a drug-sensitive assay, and immunomodulator co-administration. Investigators could only apply weight and serum albumin concentration to the pediatric PK model as participants in the REACH trial all received concomitant immunomodulators, and very few patients developed ATI.19 In this study, we aimed to build a refined population PK model with real-practice pediatric IBD therapeutic drug monitoring data using patient- and disease-related factors, as they change to allow for more accurate prediction of individual IFX exposure during maintenance therapy as part of dose forecasting.
METHODS
Study Design
This study was a retrospective, single-center study of pediatric patients with IBD treated with originator IFX between January 2011 and March 2017. The electronic health record (EHR) system was queried for all patients receiving IFX infusions at Cincinnati Children’s Hospital Medical Center with diagnoses of Crohn’s disease (CD), ulcerative colitis (UC), or IBD-unclassified (IBD-U), as documented by their provider. As part of ongoing quality improvement efforts, diagnosis, Paris classification, Physician Global Assessment (PGA), and disease activity indexes (short PCDAI [sPCDAI] for Crohn’s disease and PUCAI for ulcerative colitis) were reviewed and entered into an electronic flowsheet when available. These clinic visit data were exported from the EHR. In addition, laboratory data at each IFX infusion visit, including IFX trough concentration and ATI levels, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), albumin, hematocrit, white blood cell count, platelet count, and fecal calprotectin, were collected. Earlier IFX trough concentrations were obtained using an enzyme-linked immunosorbent assay (ELISA; Prometheus, San Diego, CA, USA), and later trough concentrations were obtained with an electrochemiluminescence immunoassay (LabCorp, Austin, TX, USA). The lower limit of quantification (LLOQ) for IFX is 0.4 µg/mL. The level below the IFX LLOQ was treated as missing. To maintain uniformity regarding ATI, only ATI data from a drug-tolerant, highly sensitive electrochemiluminescence immunoassay (ECLIA) assay (LabCorp, Austin, TX, USA), which has a limit of detection of 22 ng/mL, were used for analysis. The lower limit of detection was developed against the serum of healthy subjects never exposed to IFX. Results of this ATI assay have been found to be 100% concordant with the drug-tolerant assay of the drug maker (Janssen), and the specificity each positive ATI result is confirmed through demonstration that the signal dissipates with the addition of excess soluble IFX.20 Internal biologic drug dosing guidelines with proactive therapeutic drug monitoring (TDM) of IFX concentrations were implemented at our center in October 2014. This included routine monitoring of IFX predose concentrations at the fourth infusion, with additional monitoring yearly if clinically in remission, and with clinical symptoms. In the guideline, dose or interval escalation is suggested if the IFX trough concentration is <5 μg/mL. The availability of baseline clinical disease activity indices (sPCDAI and PUCAI) before first recorded IFX infusion was used as the inclusion criterion to select patients for the discovery cohort used in the population PK modeling. Those subjects with a diagnosis of IBD-U were excluded from the discovery cohort because of the lack of a relevant clinical disease activity index for IBD-U. As clinical disease activity did not account for variation in estimated infliximab exposure, the remaining patients without available clinical disease activity index data were used as a validation cohort. The study was approved by the Cincinnati Children’s Hospital Medical Center Institutional Review Board.
Model Development
The PK model was constructed using maintenance IFX trough concentrations, focusing on estimating IFX clearance (CL) and determining influential covariates during maintenance therapy. To reliably estimate the volume of distribution and peripheral PK parameters from the IFX measurements, we would have required peak-level measurements (in addition to trough levels), which were not available. We therefore applied these fixed parameters from the previously published model, which were derived from the pediatric cohort of the REACH trial and were assumed to be the population median for this analysis.6, 19 These fixed parameters were linearly scaled by weight of the subject for individual estimates, which included the volume of distribution of the central compartment (V1) of 3.52 L/65 kg, the volume of distribution of the peripheral compartment (V2) of 1.9 L/65 kg, and an intercompartmental clearance (Q) of 0.0095 L/h/65 kg. Data were analyzed in NONMEM, a statistical software program that takes into account within-subject and between-subject variability to fit data to established or user-defined models. For each model, the population parameters and their distributions were estimated using the first-order conditional estimation method with interaction. Model selection was based on good modeling practices and goodness-of-fit indicators, including visual inspection of diagnostic scatter plots, comparisons of the minimum objective function value (OFV), and evaluation of the estimates of population parameters.21 The OFV is a goodness-of-fit statistic, expressed as minus twice the log of the likelihood, that indicates how well the model describes the data. The lower the value, the better the fit. Fixed effects have the same parameters for each patient, whereas random effects reflect the difference between an individual’s parameter and the population value. The population PK model was internally validated using a bootstrapping resampling technique.
Covariate Analysis
Demographic data including age and body weight (WT), all laboratory measurements at the time of each infusion as stated above in “Study Design,” and clinical evaluation within 28 days of infusion when available including disease activity index and PGA were each evaluated in the covariate analysis. FCP was measured in <5% of the records, and thus was not included as a covariate in the PK modeling analysis. A stepwise covariate modeling procedure was implemented using the base model focusing on IFX clearance. The full model including all covariate effects was created with a stepwise covariate model-building approach.22 The stepwise inclusion is based on a drop in NONMEM objective function value (OFV) >3.84 (P < 0.05). Model reduction using backward elimination was implemented at a drop in OFV of >10.83 (P < 0.001). The covariate analysis was described as:
where the typical value of a model parameter (TVP) was described as a function of m individual continuous covariates (covmi) and p individual categorical covariates (covpi), such that θ TVP is an estimated parameter describing the typical PK parameter value for an individual with covariates equal to the reference covariate values (covmi = refm, covpi = 0). θ (m+n) and θ (p+m+n) are estimated parameters describing the magnitude of the covariate–parameter relationships. A plot was implemented showing the effect of the statistically significant covariates on clearance.
Model Validation
The final model was evaluated by applying the model to predict IFX trough concentrations in the separate validation cohort. Population predicted (PRED) concentrations were based on significant covariates in the final PK model but did not incorporate prior IFX level measurements. The individual predictions (IPRED) included prior IFX measurement(s) and used Bayesian estimation to generate the predictions. Plots of observed IFX trough concentrations vs PRED and IPRED were used to assess the goodness of fit of the model. Predictive performance was examined by receiver operating characteristic (ROC) analysis coupled with the Youden index. A target 5-µg/mL IFX trough concentration was used to separate the observation into the binary outcome of achieving vs not achieving the target concentration. The ROC analysis determines how well the model predicts above or below the target of 5 µg/mL and whether the predicted cutoff that corresponds to the optimal balanced specificity and sensitivity is close to the target. The departure of the ROC curve from the diagonal line to the upper left corner indicates an improvement in predictive performance for the dichotomous outcomes. Area under the curve of the ROC curve (AUCOC) quantifies the performance of the prediction, ranging from 0.5 (random) to 1 (optimal).
Simulation
A total of 1000 subjects were randomly sampled with replacement from the study cohort. Simulations using the final model were performed to illustrate the impact of different dose intensification strategies (ie, dose increases or interval shortening) on predicted IFX trough concentrations. Four maintenance dose scenarios (5 mg/kg every 8 weeks, 5 mg/kg every 4 weeks, 10 mg/kg every 8 weeks, and 10 mg/kg every 6 weeks) were simulated to compare the target attainment (≥5 µg/mL) in this pediatric IBD cohort.
Software
Data management and ROC analysis were carried out using R (version 3.4.2). Population PK analysis and simulations were conducted by nonlinear mixed-effects modeling with NONMEM software, version 7.2.0 (ICON Development Solutions, Ellicott City, MD, USA). The simulations of different dose and interval combinations in a standard individual were performed using Berkeley-Madonna (version 8.3.18).
Statistics
Comparative statistics were run on demographic and clinical information between the discovery and validation cohorts. Statistical analysis included the Student t test for normally distributed data, for which the mean (SD) is presented. The Mann-Whitney test was used for non–normally distributed variables, for which the median (interquartile range) is presented. The mean score chi-square test was utilized for categorical variables. A P value of <0.05 was considered significant.
RESULTS
Demographics and Disease Activity
A total of 228 subjects with diagnoses of IBD (CD, UC, or IBD-U) received IFX between January of 2011 and March of 2017. One subject did not have any IFX concentrations measured and was excluded. There were 135 subjects in the discovery cohort and 93 in the validation cohort. The demographic and clinical characteristics of the discovery and validation cohorts at the time of the first predose trough concentration record are shown in Table 1, with the exception of disease indices, which were summarized at the time of the first IFX infusion for the discovery cohort. Median sPCDAI and PUCAI scores were in the mild range, and 46.2% of patients had quiescent disease activity by PGA at the time of IFX initiation. This level of disease activity is reflective of the fact that these patients were not treatment-naïve and their disease may have been partially controlled by corticosteroid or immunomodulator therapy or may have been corticosteroid dependent. To estimate how many levels were obtained for reactive vs proactive drug monitoring, we examined a subset of patients in the discovery cohort (n = 30) who had available PGA data within 28 days of the first IFX concentration measurement. Within this subset, 56.7% were quiescent, suggesting proactive monitoring, with active disease in the remaining 43.3%, suggesting reactive monitoring. These 2 cohorts had comparable demographic and clinical characteristics, except patients in the discovery cohort were slightly younger and patients with IBD-U were not included in the discovery cohort due to lack of available clinical disease activity index data. The validation cohort did not have clinical disease activity indices available, which we deemed acceptable because clinical disease activity indices did not account for variation in estimated IFX exposure and were not incorporated into the final model. At the first recorded predose trough concentration, 15 out of 135 patients in the discovery cohort and 18 out of 93 patients had missing or excluded ATI data.
TABLE 1.
Clinical and Demographic Characteristics at First Predose Infliximab Trough Concentration Measurement
| Variable | Discovery Cohort | Validation Cohort | P Value | ||
|---|---|---|---|---|---|
| n = 135 | n = 93 | ||||
| No. | Summary | No. | Summary | ||
| Female | 135 | 54 (40.0) | 93 | 37 (39.8) | 0.97 |
| Age, y | 135 | 14.5 ± 3.6 | 93 | 13.3 ± 3.8 | 0.01 |
| Body weight, kg | 135 | 56.26 ± 22.0 | 93 | 51.21 ± 19.9 | 0.08 |
| Crohn’s disease | 109 (80.7) | 63 (67.7) | <0.01 | ||
| Ulcerative colitis | 26 (19.3) | 17 (18.3) | |||
| IBD-unclassified | 0 (0) | 13 (14.0) | |||
| Disease activity indices at infliximab initiationa | |||||
| Short PCDAI | 109 | 20 (10.0–35.0) | 0 | ||
| PUCAI | 26 | 20 (6.3–33.8) | 0 | ||
| PGA (n = 117) | 117 | 0 | |||
| Quiescent | 54 (46.2) | ||||
| Mild | 32 (27.3) | ||||
| Moderate | 28 (23.9) | ||||
| Severe | 3 (2.6) | ||||
| PGA at 1st IFX level (within 28 d prior) | 117 | 0 | |||
| Quiescent | 17 (56.7) | ||||
| Mild | 10 (33.3) | ||||
| Moderate | 3 (10.0) | ||||
| Severe | 0 (0) | ||||
| Infliximab level if detectable, μg/mLa | 128 | 5.4 (2.48–12.0) | 90 | 5.85 (2.20–13.0) | |
| Infliximab below detection (0.4 μg/mL), No. | 7 | 7 (5.2) | 3 | 3 (3.2) | 0.67 |
| Detectable ATI level, No. | 135 | 84 (62.2) | 93 | 47 (50.5) | 0.55 |
| 22–<200 ng/mL | 69 (82.15) | 36 (76.6) | |||
| 200–<1000 ng/mL | 9 (10.71) | 11 (23.40) | |||
| ≥1000 ng/mL | 6 (7.14) | 0 (0) | |||
| No. infusionsa | 135 | 5.0 (4.0–10.0) | 93 | 4.0 (4.0–7.0) | 0.07 |
| Hematocrit, gm/dLa | 133 | 39.9 (36.5–43.1) | 92 | 40.5(35.4–42.9) | 0.89 |
| Platelets, k/mcL | 133 | 332.2 ± 101.3 | 92 | 320.2 ± 105.2 | 0.53 |
| WBC, k/mcL | 133 | 8.44 ± 3.59 | 92 | 7.76 ± 2.89 | 0.23 |
| ESR, mm/ha | 109 | 9.0 (6.75–12.25) | 109 | 9.0 (6.9–11.0) | 0.75 |
| CRP if abnormal, >0.29 mg/dLa | 111 | 0.29 (0.29–0.46) | 72 | 0.29 (0.29–0.63) | 0.90 |
| Albumin, gm/dL | 130 | 3.77 ± 0.51 | 91 | 3.77 ± 0.42 | 0.98 |
Values are No. (%), mean ± SD, or median (interquartile range).
aNon–normally distributed.
Model Development
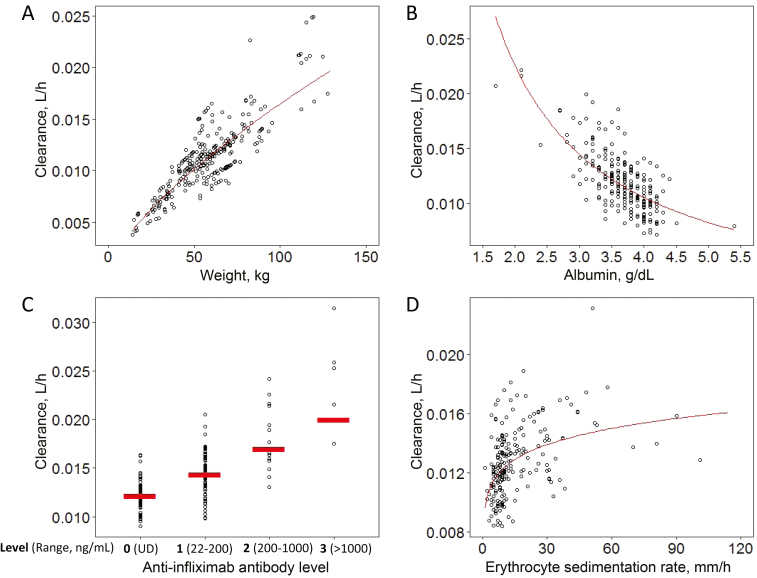
For the discovery cohort, a total of 289 IFX predose concentrations was available for analysis. The modeling process focused on estimating IFX clearance and evaluating covariate effects (eg, laboratory values, disease activity, anthropometric data) on clearance, between-subject variability and residual error. A base 2-compartmental model using patient weight and median PK parameter estimates as reported by Fasanmade et al.19 was assessed first. Then, we carried out stepwise covariate analysis as stated in the methods for all available variables with sufficient data (calprotectin was not included as a covariate (calprotectin was not included due to the high number of missing data). The following covariates, in addition to weight, were identified to be significant in the final model: albumin, ATI, and ESR. The following covariates, besides weight, were identified to be significant in the final model: albumin, ATI, and ESR. Analysis of the relationship between ATI and drug clearance revealed that clearance differed across 4 ordinal categories of ATI (<22 ng/mL, 22–<200 ng/mL, 200–1000 ng/mL, and >1000 ng/mL), and ATI therefore was included as an ordinal covariate. For all ATI measures >1000 ng/mL, we observed an exclusively undetectable level of IFX. A subject with 5 predose IFX concentration results was selected as a representative case to demonstrate how the model-predicted PK profile corresponds with the observed IFX levels (Fig. 1). For a given subject, the population-predicted trough concentrations incorporated dose, weight, and the model covariates albumin, ESR, and ordinal ATI, whereas the individual Bayesian predicted trough concentrations also incorporated the last IFX trough concentration result. In the example subject, the improved final population and individual prediction models predicted actual observed IFX concentrations better than the base model, indicating an improvement in the prediction of IFX concentrations when the model included albumin, ordinal ATI, and ESR information. The effect of weight, albumin, ATI, and ESR on IFX clearance was visualized by plotting these covariates against empirical Bayesian estimates of drug clearance (Fig. 2). Lower albumin, higher ESR, and higher ATI category were all associated with higher clearance. Notably, even ATI levels as low as 22–200 ng/mL were associated with increased IFX clearance. The median estimated clearance for patients with undetectable ATI (interquartile range) was 0.0122 (0.0115–0.0132) L/h, compared with 0.0143 (0.0129–0.0158) L/h, 0.0166 (0.0161–0.0209) L/h, and 0.0253 (0.0216–0.0259) L/h for those with ATIs of 22–200, 200–1000, and >1000 ng/mL, respectively (Fig. 2C).
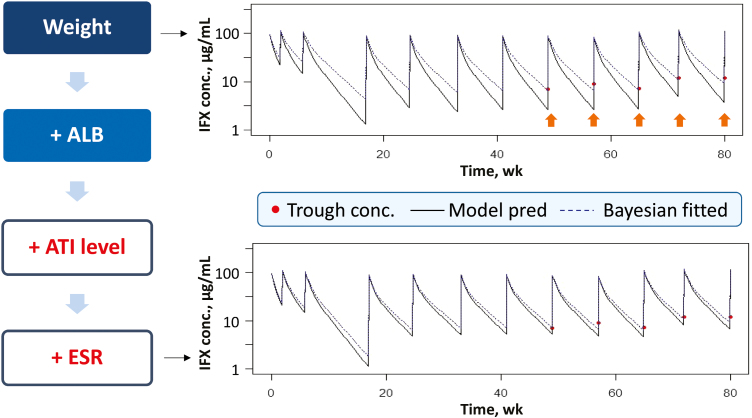
FIGURE 1.
Stepwise covariate analysis for a representative patient case. The example shows how well the model-predicted PK profile overlaid with the actual observations. The addition of serum albumin concentration, ATI category, and ESR improved the prediction of the observed IFX trough concentrations (B) in comparison with the base model, which included body weight as a covariate (A). The orange arrows highlight how the base model was not as accurate in predicting IFX trough concentrations, especially at >40 weeks.
FIGURE 2.
Covariate effects on individual IFX clearance estimates. The influence of weight (A), albumin (B), ATI (C), and ESR (D) was visualized by plotting against individual Bayesian estimates of clearance. The relationship of clearance with each covariate is normalized to exclude the effects of the other covariates. Lower albumin, higher ESR, and higher ATI category were associated with higher IFX clearance.
Goodness-of-fit criteria showed that the final model adequately predicted the observed IFX levels (GOF plots are shown for the validation cohort in Supplementary Figure 1) (data not shown). The mean IFX clearance estimate in this analysis (0.0122 L/h/65 kg) was in good agreement with the value reported by Fasanmade et al. (0.0121 L/h/65 kg).19 The PK parameters and the random effects were estimated with acceptable precision, as shown by a relatively small standard error of <30% (Supplementary Table 1). The final model reduced between-subject variability by 34% compared with the model using only weight as a covariate and 26% compared with the model using weight and albumin as covariates on clearance. All of the parameter estimates were within the 95% confidence intervals after bootstrapping, and none of the confidence intervals included 0 (data not shown). The effect of weight, albumin, and ESR on clearance was parametrized by power functions, whereas ATI level on clearance was included as an exponent as described below:
The effect of weight, albumin, and ESR on CL was parametrized by power functions, and ATI level on CL was included as an exponent. Based on our data, the ranges of effects of covariates on the CL population estimate are as follows: 0.36–1.6-fold by weight (15.1–127.6 kg), 2.2–0.62-fold by albumin (1.7–5.4 g/dL), 0.78–1.3-fold by ESR (1–101 mm/h), up to 1.6-fold by ATI (>1000 ng/mL).
Model Validation
In the validation cohort, the first IFX predose concentration result for each subject was used to predict subsequent trough concentrations. The other remaining IFX predose concentration results were used as part of the goodness-of-fit evaluation. As shown in Supplementary Figure 1, the observed concentrations vs population-predicted concentrations (not using prior levels) (A) and individual Bayesian-predicted concentrations (using prior levels) (B) were tightly grouped along the identity line. Conditional weighted residuals (CWRES) analysis indicated a good fit, as data were mostly symmetrically distributed around 0 with only 2 concentrations that fell outside of the goodness-of-fit criterion (|CWRES| ≥ 4) (Supplementary Figure 1C, D).
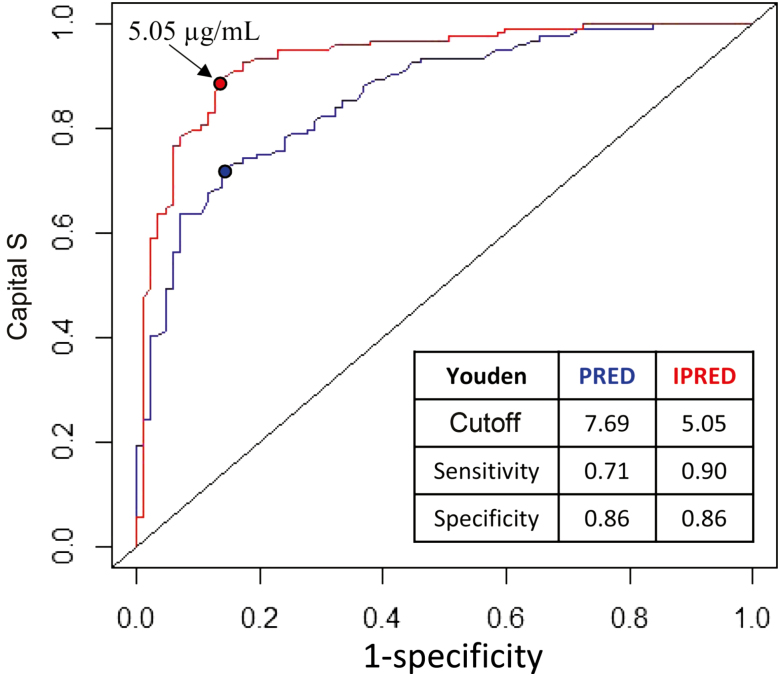
The predictive performance of the model for the population as a whole and predicted IFX trough concentrations at the individual patient level were then tested with a ROC curve (Fig. 3). ROC analysis describes the ability of the model to predict a binary outcome, namely whether the concentration will be above or below the target trough concentration of 5 µg/mL. The model had excellent predictive performance characteristics with an area under the curve of 0.86 (95% confidence interval [CI], 0.81–0.91) and 0.93 (95% CI, 0.90–0.97) for population and individual predictions, respectively. Based on the Youden index, the population prediction model has a sensitivity of 0.71 and specificity of 0.86 at a predicted cutoff of 7.69 µg/mL; the individual prediction model has a sensitivity of 0.90 and specificity of 0.86 at the predicted cutoff of 5.05 µg/mL.
FIGURE 3.
Receiver operating characteristics curves of the population and individual predicted infliximab trough concentrations. Infliximab concentration was transformed into a binary outcome using 5 µg/mL as the cutoff to indicate target concentration attainment. The embedded table presents the Youden index–predicted cutoffs with associated sensitivity and specificity.
Dosing Strategy Assessment Based on Model-Based Simulation Analysis
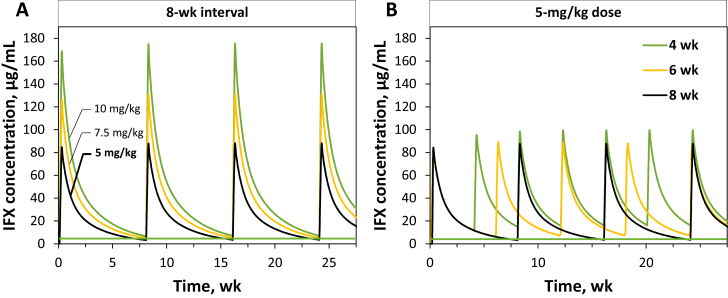
We performed a PK model–based simulation analysis to explore how early IFX target trough attainment could be improved by evaluating different starting doses and doing intervals. One thousand patients were randomly sampled with replacement from the combined discovery and validation cohorts, and their respective IFX PK profiles were simulated using the final model. Table 2 shows the percentage of simulated patients achieving a target IFX trough of 5 µg/mL with various combination of starting doses and dosing intervals. A standard starting dose of 5 mg/kg administered every 8 weeks resulted in below-target concentrations in the majority of patients, as only 24.2% of patients achieved a target trough of ≥5 µg/mL. From a pharmacokinetics perspective, the IFX trough concentration and overall drug exposure are influenced more by interval reduction than by dose increases. This was confirmed in the simulation analysis. For instance, when shortening the interval by 50% (5 mg/kg every 4 weeks), target attainment increased to 84.4%, whereas a doubling of the dose (10 mg/kg every 8 weeks) only increased the percentage of patients on target to 56.2%. A dose of 10 mg/kg every 6 weeks could achieve a target IFX concentration of ≥5 µg/mL in >80% of this population. In Figure 4, different doses and dosing intervals are simulated for a standard patient (WT 65 kg, ALB 3.5 g/dL, ATI <22 ng/mL, ESR 9 mm/h). The predicted IFX concentration time profiles show that interval shortening is predicted to have a more profound effect on trough concentrations than dose increases.
TABLE 2.
Percentage of 1000 Simulated Patients Who Achieve a Target IFX Trough Level of 5 μg/mL With Different Dosing Strategies
| Dose | Interval | |
|---|---|---|
| 5 mg/kg | 8 wk | 4 wk |
| 24.2% | 84.4% | |
| 10 mg/kg | 8 wk | 6 wk |
| 56.2% | 80.3% |
FIGURE 4.
Model-based prediction of infliximab concentrations with different dosing strategies in a simulated standard patient (weight 65 kg, ALB 3.5 g/dL, ATI <22 ng/mL, ESR 9 mm/h). The green line denotes an IFX target concentration of 5 µg/mL. A, A fixed interval of 8 weeks and IFX doses of 5 mg/kg, 7.5 mg/kg, and 10 mg/kg, respectively. B, A fixed IFX dose of 5 mg/kg and intervals of 4, 6, and 8 weeks, respectively.
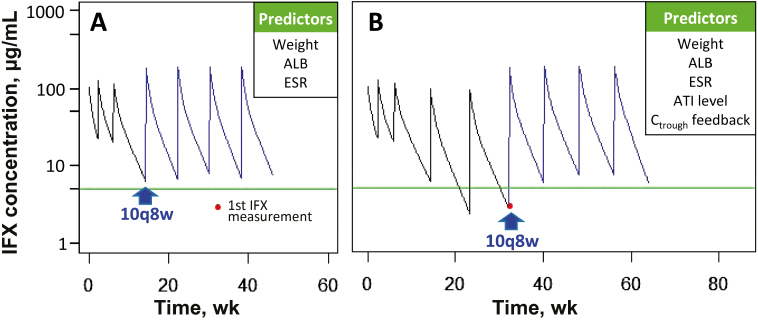
To illustrate how a model-based individualized dosing strategy could be used in clinical practice to adjust maintenance therapy, a representative case example of a 13-year-old subject who had not attained the target trough concentration of >5 µg/mL until 1.5 years after initial infusion was selected. Figure 5 shows 2 simulated dosing strategies based on patient data and available covariates. Using weight, albumin, and ESR measured at week 14 as predictors, the population model predicted that a dose increase to 10 mg/kg every 8 weeks would have resulted in an increase in trough concentrations to above the goal of 5 µg/mL (Fig. 5A). When subsequent IFX concentration measurements became available and were entered into the model, the updated model predicted the same dose and interval (10 mg/kg every 8 weeks) (Fig. 5B).
FIGURE 5.
Infliximab target concentration attainment using model simulation in an actual subject with a proactive vs reactive dosing strategy. At the first recorded infliximab trough concentration at 32 weeks, the patient was 13 years old, weighed 62.6 kg, and had an albumin of 3.5 g/dL, ESR of 10 mm/h, and ATI level of 22 ng/mL (level 1). Red dots indicate infliximab trough concentration measurements. The green line marks the infliximab target concentration of 5 µg/mL. A, How the utilization of the model with real-time covariates as indicated in the associated box could predict an individualized dosing strategy that would achieve a target concentration of 5 µg/mL. B, Updated clearance using concentration feedback as indicated by the associated box when it became available (at week 32) to predict an individualized dosing strategy for target attainment. In this case, a dose of 10 mg/kg every 8 weeks was predicted for target attainment in both scenarios.
DISCUSSION
We used real-world practice data to develop a pediatric population PK model that predicts IFX trough concentrations during maintenance therapy in patients with IBD. The final model included weight, albumin, ESR, and ordinal ATI as real-time covariates to predict individual IFX clearance at different occasions, and results were reproducible in a separate validation cohort. To our knowledge, this is the first population PK model to be built exclusively with pediatric data and using ordinal ATI data from a drug-tolerant assay.
This refined model improved on the previously published model because it was built with exclusively pediatric data. The Fasanmade model19 was built by combining adult data with the pediatric REACH trial data, a trial in which all of the pediatric participants had a diagnosis of CD and were receiving combination therapy with immunomodulators. Our cohort included patients with both CD and UC, and patients received immunomodulators or other medications as their provider saw fit. This is more representative of a generalizable pediatric IBD population in a clinical setting. Our analysis identified additional model covariates, including ordinal ATI and ESR, that further explained the interindividual variability in IFX clearance in pediatric patients. In summary, we developed a refined model that more accurately predicted IFX trough concentrations and was built with exclusively pediatric patients from an exclusively pediatric cohort with rich high-sensitivity ATI data.
In building this refined model, we were able to take advantage of ATI data measured with a drug-tolerant assay. This allowed for a more nuanced analysis of the influence of ATI on the clearance of IFX, as antidrug antibodies can still be detected in the presence of a detectable IFX concentration. In our cohort, a significant proportion of patients (66%) had detectable ATIs with this sensitive assay. In contrast, only 3 of 112 (2.7%) pediatric patients had a positive ATI level in the REACH trial, and this covariate could not be included in the Fasanmade pediatric model.19 A higher proportion of positive ATIs represents a more clinically realistic situation for practitioners. ATI data in our study were separated into ordinal categories that impacted interindividual variability in clearance. ATIs of >22 ng/mL resulted in consistent increases in clearance, with the highest clearance in those patients with an ATI >1000 ng/mL. We treated it as a categorical variable to homogenize the variability of CL in each of the ATI categories. For all ATI measures >1000 ng/mL, we observed exclusively undetectable levels of IFX. In simulations of adult infliximab PK models, dose adjustments based on previous IFX trough concentrations, in addition to patient-specific covariates, such as the ATI level, have been shown to be more effective in achieving a measurable IFX trough concentration than utilizing only the patient covariate levels. Dotan et al.14 showed in a simulation of an adult PK infliximab model that 38.3% of patients had troughs <1 mg/L when dosing was based on body weight, albumin level, and ATI status. When the pharmacokinetically guided dosing approach that included the trough levels was implemented, the vast majority of patients (96%) had a trough level >1 mg/L. This supports that ATI and other covariate data are most useful when the pharmacokinetic predictive approach incorporates both patient factors and previous IFX trough levels, as in our individual prediction simulations. A significant portion (57.5%) of our total cohort had detectable ATIs (>22 ng/mL) at the first recorded trough concentration measurement. The incidence of ATI detection in the literature varies widely and is dependent on the sensitivity of the assay utilized. In the post hoc analysis of the TAXIT study, 76 adult subjects with undetectable ATIs at screening had their sample re-analyzed using a drug-tolerant assay, and a similar incidence of positive ATI was found (63%).23 There are limited data available describing the clinical significance of lower levels of ATI, especially in the setting of therapeutic drug levels, as these may be transient but could be a herald of later development of clinically significant ATI. Using a similarly sensitive assay, 1 group found that, in IBD patients with disease relapse, IFX dose optimization was most likely to be successful in those with an IFX level <2 μg and ATI <200 ng/mL.24 Nonetheless, our data support that ATI levels as low as 22 ng/mL do have an effect on individual IFX clearance estimates, whereas ATIs >1000 ng/mL had a pronounced effect.
This population PK model incorporated a disease-related biomarker besides serum albumin as a significant predictor of IFX trough concentrations. CRP and ESR are widely used to monitor IBD activity with limitations in sensitivity and specificity.25–27 We incorporated ESR into our final model instead of CRP because it was a better predictor of individual clearance. This is likely in part attributed to many undetectable levels of CRP (36% of records), which made its effect not as informative as ESR. Measures of acute systemic inflammation like ESR and CRP have traditionally been used to assess disease severity and predict disease course, and our model supports the utility of ESR in predicting IFX trough concentrations.
Serum albumin concentration was also a predictor in our model. It has a well-established association with IFX trough concentrations.28 Albumin is the most abundant serum protein and has been a significant covariate in previously published PK models of IFX clearance in IBD.14, 19, 29 Both albumin and IgG antibodies like IFX undergo extensive catabolism with increasing inflammation.30 A lower albumin level may be reflective of leakage from inflamed intestines. In patients with more severe colitis as evidenced by disease scores and fecal calprotectin measurements, intestinal losses of infliximab have been shown to be increased.31 Consistent with these data, a lower serum albumin concentration predicted a lower IFX trough concentration in our model.
In the simulated patient population, shortening the interval to 50% was more effective to achieve a target concentration than doubling the dose. Similarly, in a Monte Carlo simulation analysis of 1000 pediatric patients utilizing the Fasanmade model, Frymoyer and others found that shortening the IFX interval had a larger impact on the resulting trough concentration than increasing the weight-based dose.32 This finding is supported by data from actual pediatric and adult populations. In a primarily adult UC cohort with low IFX trough concentrations, shortening the interval from every 8 to a median of 6 weeks resulted in clinical remission in 44% of patients compared with 25% of those who were instead escalated from a 5- to a 10-mg/kg dose.33 Pediatric data on interval shortening vs dose increase are limited. Two retrospective pediatric cohort studies reported that interval shortening resulted in more patients with an IFX trough concentration greater than the target of 3 μg/mL than increasing the weight-based dose.9, 34 In our study, we used 5 μg/mL as a goal IFX trough concentration based on the American Gastroenterological Associations 2017 guideline recommendations for proactive therapeutic drug monitoring and based on recent pediatric data.25, 35 When applied to real patients, dose individualization has been shown to better attain goal trough concentrations than standard dosing.36 Our simulation suggests that model-based dose individualization can be used to achieve target concentrations even without prior IFX trough concentration or ATI information.
Notable strengths of our study are the large number of patients and IFX trough concentrations, use of discovery and validation cohorts, the incorporation of a sensitive drug-tolerant ATI assay with a low limit of detection, and the application of Bayesian modeling. Our study also has some limitations owing to its retrospective design and electronic extraction of EHR data. As we studied patients administered IFX at our institution, data on each IFX dose were accurate and readily exported from the EHR. However, prescribed outpatient medication information in the EHR is frequently not updated in a timely fashion and does not often include accurate start and stop dates. Therefore, we could not evaluate concurrent use of immunomodulators as an independent predictor in this cohort. Previous literature has supported that combination therapy with IFX and an immunomodulator improved clinical outcomes and resulted in higher initial IFX trough concentrations in adult patients.37, 38 Although this variable could account for some unexplained variability in our model, concomitant immunomodulator use in our practice is low (~15% of patients treated with anti-TNF biologics). Finally, patients in the validation cohort differed from those in the discovery cohort in that they did not have disease activity indices entered into the EHR and included IBD-U patients. However, the cohorts were similar across other covariates (including laboratory values), disease activity indices were not a component of the final model, and we would expect any unknown differences between the 2 groups to reduce the likelihood of successful validation of the model (ie, bias the analysis toward a negative result).
Future directions for this work include incorporating the model into a dashboard system for clinician use in determining individualized dosing decisions.1, 39 Others have reported a dashboard system using another PK model of pediatric IBD that would suggest alternative dosing regimens in most patients.36 Constructing a dashboard with our refined model built-in, we envision a model-based dose individualization even when IFX concentration is not readily available. In the future, a personalized dosing calculator could be accessible via an Internet tool or via integration with an electronic health record.
In conclusion, we built and validated a refined population PK model in pediatric IBD that accurately predicts IFX trough concentrations during maintenance therapy using body weight, albumin, ordinal drug-tolerant ATI levels, and ESR as a novel covariate related to systemic inflammation. Simulations using this model indicate that changes in dosing interval have more effect on IFX trough concentrations than dose escalation. In the future, this model could be integrated into a dashboard system for efficient and tailored optimization of IFX to maximize the likelihood of a patient achieving a target trough concentration. Our findings support future prospective studies of PK model–based individualized IFX dosing.
Supplementary Material
Supported by: This study was funded in part by the National Institute of Child Health and Human Development, under award number 5T32HD69054, the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award numbers K23DK094832 and T32DK007727, and the Arnold W. Strauss Award at the Cincinnati Children’s Hospital Medical Center.
Conflicts of interest: Michael J. Rosen has a research collaboration with Prometheus Laboratories, Inc. The remaining authors have no potential conflicts of interest to declare.
Author contributions: The guarantors of the article are Dr. Michael Rosen and Dr. Alexander Vinks. Specific author contributions: L.E.B., Y.X., M.J.R., and A.V. designed the research study, L.E.B. and M.J.R. collected the data, L.E.B., Y.X., T.M., M.J.R., and A.V. analyzed the data, L.E.B. and Y.X. wrote the paper, and P.M., T.F., and M.D. contributed to the review of the manuscript.
REFERENCES
- 1. Mould DR, Upton RN, Wojciechowski J. Dashboard systems: implementing pharmacometrics from bench to bedside. Aaps J. 2014;16:925–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eser A, Primas C, Reinisch S, et al. . Prediction of individual serum infliximab concentrations in inflammatory bowel disease by a Bayesian dashboard system. J Clin Pharmacol. 2018;58:790–802. [DOI] [PubMed] [Google Scholar]
- 3. Hyams J, Walters TD, Crandall W, et al. . Safety and efficacy of maintenance infliximab therapy for moderate-to-severe Crohn’s disease in children: REACH open-label extension. Curr Med Res Opin. 2011;27:651–662. [DOI] [PubMed] [Google Scholar]
- 4. Crombé V, Salleron J, Savoye G, et al. . Long-term outcome of treatment with infliximab in pediatric-onset Crohn’s disease: a population-based study. Inflamm Bowel Dis. 2011;17:2144–2152. [DOI] [PubMed] [Google Scholar]
- 5. Wine E, Mack DR, Hyams J, et al. . Interleukin-6 is associated with steroid resistance and reflects disease activity in severe pediatric ulcerative colitis. J Crohns Colitis. 2013;7:916–922. [DOI] [PubMed] [Google Scholar]
- 6. Hyams J, Crandall W, Kugathasan S, et al. ; REACH Study Group Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn’s disease in children. Gastroenterology. 2007;132:863–873; quiz 1165. [DOI] [PubMed] [Google Scholar]
- 7. Merras-Salmio L, Kolho KL. Clinical use of infliximab trough levels and antibodies to infliximab in pediatric patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2017;64:272–278. [DOI] [PubMed] [Google Scholar]
- 8. Moore C, Corbett G, Moss AC. Systematic review and meta-analysis: serum infliximab levels during maintenance therapy and outcomes in inflammatory bowel disease. J Crohns Colitis. 2016;10:619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frymoyer A, Hoekman DR, Piester TL, et al. . Application of population pharmacokinetic modeling for individualized infliximab dosing strategies in Crohn disease. J Pediatr Gastroenterol Nutr. 2017;65:639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vande Casteele N, Khanna R, Levesque BG, et al. . The relationship between infliximab concentrations, antibodies to infliximab and disease activity in Crohn’s disease. Gut. 2015;64:1539–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cornillie F, Hanauer SB, Diamond RH, et al. . Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut. 2014;63:1721–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brandse JF, Mathôt RA, van der Kleij D, et al. . Pharmacokinetic features and presence of antidrug antibodies associate with response to infliximab induction therapy in patients with moderate to severe ulcerative colitis. Clin Gastroenterol Hepatol. 2016;14:251–8.e1. [DOI] [PubMed] [Google Scholar]
- 13. Ordás I, Mould DR, Feagan BG, et al. . Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther. 2012;91:635–646. [DOI] [PubMed] [Google Scholar]
- 14. Dotan I, Ron Y, Yanai H, et al. . Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: a population pharmacokinetic study. Inflamm Bowel Dis. 2014;20:2247–2259. [DOI] [PubMed] [Google Scholar]
- 15. Wojciechowski J, Upton RN, Mould DR, et al. . Infliximab maintenance dosing in inflammatory bowel disease: an example for in silico assessment of adaptive dosing strategies. Aaps J. 2017;19:1136–1147. [DOI] [PubMed] [Google Scholar]
- 16. Upton RN, Mould DR. Basic concepts in population modeling, simulation, and model-based drug development: part 3-introduction to pharmacodynamic modeling methods. CPT Pharmacometrics Syst Pharmacol. 2014;3:e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sheiner LB, Beal SL. Bayesian individualization of pharmacokinetics: simple implementation and comparison with non-Bayesian methods. J Pharm Sci. 1982;71:1344–1348. [DOI] [PubMed] [Google Scholar]
- 18. Jelliffe RW, Schumitzky A, Van Guilder M, et al. . Individualizing drug dosage regimens: roles of population pharmacokinetic and dynamic models, Bayesian fitting, and adaptive control. Ther Drug Monit. 1993;15:380–393. [PubMed] [Google Scholar]
- 19. Fasanmade AA, Adedokun OJ, Blank M, et al. . Pharmacokinetic properties of infliximab in children and adults with Crohn’s disease: a retrospective analysis of data from 2 phase III clinical trials. Clin Ther. 2011;33:946–964. [DOI] [PubMed] [Google Scholar]
- 20. Marini JC, Sendecki J, Cornillie F, et al. . Comparisons of serum infliximab and antibodies-to-infliximab tests used in inflammatory bowel disease clinical trials of remicade®. AAPS J. 2017;19:161–171. [DOI] [PubMed] [Google Scholar]
- 21. Byon W, Smith MK, Chan P, et al. . Establishing best practices and guidance in population modeling: an experience with an internal population pharmacokinetic analysis guidance. CPT Pharmacometrics Syst Pharmacol. 2013;2:e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mandema JW, Verotta D, Sheiner LB. Building population pharmacokinetic—pharmacodynamic models. I. Models for covariate effects. J Pharmacokinet Biopharm. 1992;20:511–528. [DOI] [PubMed] [Google Scholar]
- 23. Van Stappen T, Vande Casteele N, Van Assche G, et al. . Clinical relevance of detecting anti-infliximab antibodies with a drug-tolerant assay: post hoc analysis of the TAXIT trial. Gut. 2018;67:818–826. [DOI] [PubMed] [Google Scholar]
- 24. Paul S, Del Tedesco E, Marotte H, et al. . Therapeutic drug monitoring of infliximab and mucosal healing in inflammatory bowel disease: a prospective study. Inflamm Bowel Dis. 2013;19:2568–2576. [DOI] [PubMed] [Google Scholar]
- 25. Singh N, Rosenthal CJ, Melmed GY, et al. . Early infliximab trough levels are associated with persistent remission in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:1708–1713. [DOI] [PubMed] [Google Scholar]
- 26. Cornillie F, Hanauer SB, Diamond RH, et al. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut. 2014;63:1721–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner D, Levine A, Kolho KL et al. Combination of oral antibiotics may be effective in severe pediatric ulcerative colitis: a preliminary report. J Crohns Colitis. 2014;8:1464–1470. [DOI] [PubMed]
- 28. Fasanmade AA, Adedokun OJ, Olson A, et al. . Serum albumin concentration: a predictive factor of infliximab pharmacokinetics and clinical response in patients with ulcerative colitis. Int J Clin Pharmacol Ther. 2010;48:297–308. [DOI] [PubMed] [Google Scholar]
- 29. Brandse JF, Mould D, Smeekes O, et al. . A real-life population pharmacokinetic study reveals factors associated with clearance and immunogenicity of infliximab in inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:650–660. [DOI] [PubMed] [Google Scholar]
- 30. Hemperly A, Vande Casteele N. Clinical pharmacokinetics and pharmacodynamics of infliximab in the treatment of inflammatory bowel disease. Clin Pharmacokinet. 2018;57:2527–2542. [DOI] [PubMed] [Google Scholar]
- 31. Brandse JF, Van Den Brink GR, Wildenberg ME, et al. . Loss of infliximab into feces is associated with lack of response to therapy in patients with severe ulcerative colitis. Gastroenterology. 2015;149:350–355.e2. [DOI] [PubMed] [Google Scholar]
- 32. Frymoyer A, Piester TL, Park KT. Infliximab dosing strategies and predicted trough exposure in children with Crohn disease. J Pediatr Gastroenterol Nutr. 2016;62:723–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Seow CH, Newman A, Irwin SP, et al. . Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut. 2010;59:49–54. [DOI] [PubMed] [Google Scholar]
- 34. Hofmekler T, Bertha M, McCracken C, et al. . Infliximab optimization based on therapeutic drug monitoring in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2017;64:580–585. [DOI] [PubMed] [Google Scholar]
- 35. Feuerstein JD, Nguyen GC, Kupfer SS, et al. ; American Gastroenterological Association Institute Clinical Guidelines Committee American Gastroenterological Association Institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology. 2017;153:827–834. [DOI] [PubMed] [Google Scholar]
- 36. Dubinsky MC, Phan BL, Singh N, et al. . Pharmacokinetic dashboard-recommended dosing is different than standard of care dosing in infliximab-treated pediatric IBD patients. Aaps J. 2017;19:215–222. [DOI] [PubMed] [Google Scholar]
- 37. Drobne D, Bossuyt P, Breynaert C, et al. . Withdrawal of immunomodulators after co-treatment does not reduce trough level of infliximab in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2015;13:514–521.e4. [DOI] [PubMed] [Google Scholar]
- 38. Colombel JF, Sandborn WJ, Reinisch W, et al. ; SONIC Study Group Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395. [DOI] [PubMed] [Google Scholar]
- 39. Mould DR. Why therapeutic drug monitoring is needed for monoclonal antibodies and how do we implement this? Clin Pharmacol Ther. 2016;99:351–354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.