Diagnostic Approach
Effusions are commonly encountered in veterinary practice and often assist in determining a definitive diagnosis. The term effusion describes inappropriate accumulation of fluid within a body “potential” or “third” space outside of vascular or lymphatic conduits and visceral structures. These are often disclosed on physical examination; for example, overhydrated skin turgor and thickened lip folds, conjunctivae, tarsal webs, or scrotum may indicate localized edema or whole-body edema (i.e., anasarca); joint cavity distention indicates joint effusion often associated with arthritis; dyspnea and/or tachypnea, muted lung sounds, or cough may indicate pleural effusion; irregular femoral pulses, pulses alternans, and muted cardiac sounds concurrent with a jugular pulse may indicate pericardial effusion; and slippery visceral surfaces or a ballotable fluid wave may indicate abdominal effusion. Unfortunately, some effusions evade detection until imaging studies (radiography, ultrasonography) disclose their presence. Furthermore, centripetal adiposity can be confused with abdominal effusion but is easily differentiated on ultrasound examination. Collection, physicocytologic characterization, and chemical evaluation of effusions are crucial for accurate categorization. Classification schemes incorporating these features direct differential diagnoses considering the associated disease pathomechanisms.
Fluid Collection Techniques
Collection of Fluid
The site of fluid collection is prepared as for aseptic surgery. A 1-inch, 23- to 20-gauge needle, over-the-needle Teflon catheter, or butterfly needle–catheter is recommended. Fluid analysis usually requires a minimum of 3 to 5 ml of fluid. If ultrasound guidance is used, it is important to prevent sample contamination with ultrasound gel that induces artifacts (i.e., blue-appearing smudges on Diff-Quik or Wright-Giemsa-stained preparations). Aspiration methods should relieve negative pressure during needle withdrawal from the site of sample collection to avoid collection of contaminating and irrelevant tissues (cells).
Abdominocentesis
The abdomen is palpated immediately before abdominocentesis to avoid lacerating visceral structures, and any available images are consulted. The urinary bladder should be emptied before the procedure, particularly when 14-gauge catheters are used. In patients with tense abdominal distention, the abdomen is punctured laterally to avoid gravitational ventral midline seroma formation and adhesions associated with ovariohysterectomy. If septic peritonitis is suspected but unproven with routine abdominocentesis, a four-quadrant tap can be performed. Ultrasonographic guidance is helpful for sampling loculated fluid or fluid collecting in the abdominal gutters. Usually a 22- to 20-gauge, 1-inch needle or Teflon catheter is attached to extension tubing. Mobility afforded by the extension tubing helps avoid visceral laceration should the patient move during the centesis procedure. Alternatively, a butterfly catheter set is used. If an abdominal effusion is difficult to sample, a 14-gauge Teflon catheter is used to puncture the abdomen, and a closed-ended polypropylene Tomcat catheter is inserted through its lumen. The Teflon catheter provides a “sterile stent” through which the Tomcat catheter may be manipulated as the patient's position is altered. Local anesthesia (lidocaine block) may be needed in some patients to enable nonpainful abdominocentesis.
Abdominal Lavage
Sterile warmed physiologic saline (20 ml/kg) is administered intraperitoneally over 5 to 10 minutes through extension tubing and a Teflon catheter. The abdomen is massaged or the animal moved about for several minutes to mix infused fluid with that trapped within omental recesses and abdominal gutters. Lavage fluid is subsequently aspirated and analyzed. Dogs normally have less than 500 white blood cells (WBCs)/µl. Mild leukocytosis occurs after recent abdominal trauma or surgery. Diagnostic guidelines for interpretation of abdominal lavage fluid are listed in Box 10-1 . Iatrogenic injury and bacterial contamination are possible consequences of the lavage procedure, and dilutional influences on collected fluid can create diagnostic confusion. The convenient availability of ultrasound in clinical practice has reduced use of this technique by guiding targeted fluid centesis.
Box 10-1. Suggested Guidelines for Interpretation of Abdominal Lavage Effusion*.
| TURBIDITY | |
| Clear: | No disease or abdominal injury |
| Bloody: | Iatrogenic or hemorrhage |
| Chronic effusion: serosanguineous | |
| Blood darkens on repeat centesis: | Active hemorrhage: acquire packed cell volume (PCV) for relative change |
| Turbid: | Cannot clearly read newsprint through fluid: cytology indicated |
| PCV: | |
| <5% | Mild hemorrhage |
| >10% | Significant hemorrhage |
| WBC count: | |
| <500/µl | Normal dogs |
| >1000/µl | Mild to moderate inflammation |
| >2000/µl | Probable peritonitis: cytology indicated |
| PANCREATIC ENZYMES (lipase or amylase): | If >sera: pancreatic inflammation, injury, necrosis |
| TOTAL BILIRUBIN: | If >sera: bile spillage or enteric rupture |
| CREATININE: | If >sera: urinary tract rupture, urine spillage |
| VEGETABLE FIBERS: | Enteric rupture or sampled enteric lumen |
| MIXED BACTERIAL FLORA: | Enteric rupture, ruptured abscess, or sampled enteric lumen |
WBC, white blood cell.
Thoracocentesis
Thoracocentesis is usually performed in the seventh or eight intercostal space at the level of the costochondral junction; however, relevant imaging studies may better guide sample collection. The needle penetrates the middle of the intercostal space, avoiding the caudal rib margin where nerves and vessels are located. Harvesting fluid is optimal with the animal standing or in sternal recumbency. A 1-inch, 18- or 20-gauge butterfly catheter connected to a three-way stopcock and a 20- to 35-ml syringe are recommended. During initial needle placement, negative pressure is maintained on the syringe so that advancement of the needle immediately discloses effusion, thus avoiding inadvertent pulmonary puncture or laceration. Repeated centesis should be performed only after a local anesthetic block is applied to the puncture site.
Pericardiocentesis
Before pericardiocentesis, samples of blood are used to determine baseline packed cell volume (PCV), total solids (TS) concentration, platelet count, and activated coagulation time (ACT). This procedure is best performed with the patient in sternal recumbency and with local analgesia block. The site of thoracic penetration is surgically prepared and blocked with local anesthetic, and a small incision is made in the skin to facilitate movement of the catheter through the dermis. The right side is preferred to avoid large coronary arteries on the left side. However, echocardiography should assist in determining the optimal site for pericardiocentesis. Without access to echocardiography, the site selected corresponds to the palpable cardiac beat or just caudal to or below the elbow at the level of the costochondral junction (fifth to sixth intercostal space). The catheter is usually passed through the fifth or sixth right intercostal space (i.e., the cardiac notch between lung lobes) after local anesthetic is injected to the level of the pleura. A 12- to 16-gauge, 4- to 6-inch over-the-needle Teflon catheter is used with two or three extra holes aseptically snipped in the lateral aspect of the catheter approximately 1.5 to 3.0 cm from the tip. Extension tubing and a three-way stopcock are necessary in medium- and large-sized dogs. An electrocardiogram (ECG) is simultaneously recorded while the catheter is advanced; touching the myocardium elicits premature ventricular beats. Ultrasound guidance is routinely used for this procedure. Pleural effusion is usually present and first encountered, typically a modified transudate, amber to slightly red in color. Entrance into the pericardium may require an acute thrust associated with a “pop,” after which the catheter is slipped over the needle into the pericardial sac, and the needle removed and discarded. Collection of a hemorrhagic effusion is typical and necessitates immediate differentiation of centesis fluid from peripheral blood using comparisons between each fluid in PCV, platelet count, TS concentration, and supernatant color, and an ACT on the fluid to confirm its inability to clot. These assessments avoid inadvertent removal of large volumes of intracardiac blood. Pericardial hemangiosarcomas may initiate a hemorrhagic effusion upon pericardiocentesis that may be difficult to differentiate from iatrogenic cardiac puncture. Ultrasonographic imaging may assist in resolving this conundrum. Complications associated with pericardiocentesis include ventricular premature contractions, laceration of the coronary artery, and sudden death. After removal of pericardial fluid, the patient should be monitored for arrhythmias and acute recurrence of hemorrhagic tamponade (abrupt onset of tachycardia, poor pulse quality, pulsus paradoxus, tachypnea).
Collection of Edema Fluid
A 22- to 25-gauge needle is gently introduced into the affected tissue. Clear watery fluid often drains spontaneously by gravity but can be assisted by gentle massage or aspiration. If lymphatic cording is notable, direct puncture for lymph collection is possible.
Characterization of Fluid
Refer to Table 10-1 .
TABLE 10-1.
CHARACTERISTICS OF SELECTED TYPES OF EFFUSIONS
| Transudates |
Exudates |
||||||
|---|---|---|---|---|---|---|---|
| PURE TRANSUDATE | MODIFIED TRANSUDATE | HEMORRHAGIC EFFUSION | NONSEPTIC EXUDATE | SEPTIC EXUDATE | BILIOUS EFFUSION | CHYLOUS EFFUSION | |
| Color | Clear | Serous | Bloody | Serosanguineous | Purulent, creamy | Brown/green | Milky/white/pink |
| Watery | Serosanguineous | Serosanguineous | Dark yellow/green | Opalescent | |||
| Turbidity | Clear | Clear to cloudy | Opaque | Cloudy | Cloudy/flocculent | Opaque | Opaque |
| Total solids (g/dl) | <2.5 | 2.5–5.0 | >3.0 | >3.0 | >3.0 | >3.0 | >2.5 |
| Specific gravity | <1.017 | 1.017–1.025 | >1.025 | >1.025 | >1.025 | >1.025 | >1.018 |
| Nucleated cells/µl | <1000 | 500–10,000 | >1000 | >5000 | >5000 | >5000 | Variable |
| Differential | Mononuclear cells (mesothelial cells, lymphocytes, macrophages) | Mesothelial cells Macrophages Neutrophils (nondegenerate) RBCs (few) Lymphocytes |
Similar to blood Neutrophils (variable, nondegenerate) Lymphocytes (few) Macrophages (erythrophagocytosis) |
Neutrophils (nondegenerate) Macrophages (phagocytized debris) RBCs (variable) Mesothelial cells (increased in chronic) ± Neoplastic cells |
Neutrophils (degenerate, phagocytized bacteria) Mesothelial cells (variable) RBCs (variable) |
Neutrophils (predominate in acute) Macrophages (phagocytized and free bilirubin crystals: brown-granular material) Lymphocytes (few) |
Lymphocytes (predominate early) Neutrophils (increase in chronic) Mesothelial cells (variable) |
| Bacteria | No | No | No | No | May see bacteria | ± | Rare |
| Lipid | No | No | No | No | No | No | High triglycerides (fluid > sera) Cholesterol (fluid < sera) Stain positive with Sudan III or oil red O |
RBCs, Red blood cells.
Fluid Analysis
Collected fluids should be analyzed immediately to permit characterization and to direct further diagnostics (e.g., bacterial culture). Three- to 5-ml aliquots of fluid should be stored in an ethylenediaminetetraacetic acid (EDTA; purple top) tube and a sterile clot tube for cytologic and physicochemical assessments, respectively. A separate sample for culture is stored in a sterile clot tube, a Culturette containing transport medium, or broth culture medium. If only a few drops of fluid are collected, cytology has first priority. Cultures can be taken from the needle hub with a microtip culturette, or the needle and syringe can be washed with culture broth. PCV, total protein, and appearance of microcentrifuged supernatant of bloody effusions should be compared with peripheral blood. Physicochemical and cytologic assessment of effusions usually permits classification into one of several categories (see Table 10-1). The scheme presented divides effusions into transudates and exudates and then further subdivides each major category.
Physical Assessment of an Effusion
Color and turbidity of the fluid should be recorded (Figure 10-1 ). Turbid fluids contain cells or lipids. Chylous effusions are usually white, pink, or opalescent with a turbid supernatant. A red-tinged or maroon fluid reflects red blood cells (RBCs) or free hemoglobin. Blood-tinged fluids must be centrifuged to determine their PCV relative to systemic PCV and to permit supernatant evaluation. RBCs often accumulate in effusions secondary to inflammation or vascular congestion, where they cause a PCV less than or equal to 8%. If the PCV more closely resembles systemic blood and the supernatant is clear, acute hemorrhage or iatrogenic sample contamination is likely. Fluid PCV may be artifactually lowered by hemolysis caused by very high or low fluid tonicity, by freezing and thawing during sample storage, or consequent to high lipid concentrations or trauma (i.e., forced sample injection into a Vacutainer). Hemolysis should be suspected if a supernatant is maroon colored. Erythrophagocytosis (i.e., RBCs engulfed by macrophages) and macrophages containing hemosiderin (i.e., siderocytes) reflect blood contamination of at least 24 hours (Figure 10-2 ). However, erythrophagocytosis can also develop in fluids during storage (longer than a few hours). Chronically blood-contaminated samples lack platelets but have a xanthochromic (yellow-tinged) supernatant after centrifugation. Xanthochromia reflects presence of hemo-pigments (bilirubin pigments); jaundiced animals are expected to have yellow effusions. Bile peritonitis is usually associated with a brown-green or dark yellow-green effusion with large-volume bile spillage, containing both free and engulfed bilirubin crystals (Figure 10-3 ). Loculated bile peritonitis in the anterior abdomen (fluid entrapped in the omentum) may be associated with typical effusion near the site of bile leakage and a modified transudate or exudative effusion within the remainder of the abdomen. Septic effusions may emit a foul smell caused by anaerobic bacterial infection from bowel rupture.
FIGURE 10-1.

Examples of effusions with different physical characteristics. A, A pure low-protein transudate. It is colorless, clear, and transparent. B, A modified transudate that is red tinged and cloudy due to red blood cells (RBCs). It is nearly opaque. C, Chylous effusion that is white and opaque. D, Septic exudate that is cream colored and opaque. E, Hemoabdomen fluid that is dark red and opaque. Note that the supernatant in the microhematocrit tube is xanthochromic (yellow) due to RBC breakdown.
FIGURE 10-2.

Erythrophagocytosis, such as in this macrophage, or hemosiderin in phagocytes indicates the hemorrhage in a fluid was, at least in part, from a preexisting disease (i.e., pathologic hemorrhage) rather than an artifact of collecting the sample.
FIGURE 10-3.

A, Bilious effusion that is golden brown and semi-transparent. B, Cytology of bilious effusion showing inflammatory cells that have engulfed bile (brown material).
(Courtesy of Dr. Mark Johnson.)
Cell Counts and Cytology
Total and differential nucleated cell counts are performed using anticoagulated, noncentrifuged fluid. Total cell counts may be completed using a hemocytometer and manual count or a flow cytometry hematology analyzer. Very-small-volume samples may yield falsely low cell counts as a result of anticoagulant dilution. Poor sample mixing, sample contamination, prolonged storage, and medical therapy may each influence cell counts. Differential counts are best performed from concentrated cellular components. This can be simply done by sample centrifugation, smear preparation, and Diff-Quik staining. Smears of unconcentrated fluid allow estimation of cell numbers when cell counts exceed 1000/µl. At least six slides of collected fluid should immediately be made, rapidly air-dried to preserve cell morphology, and stained using a modified Wright stain such as Diff-Quik. If bacteria are visible, Gram stain is applied. If the fluid appears relatively acellular, a portion should be centrifuged and smears made of the sediment as soon as possible. Cytospin centrifugation provides the best cellular morphology. The clinician should remain alert for microorganism-contaminated stains, post-sampling degeneration of neutrophils associated with prolonged storage in EDTA or saline, or cell changes induced by exposure to urine or bile. Estimation of total nucleated cell count in a noncentrifuged sample can be done using the following formula and the stipulation that the objective used visualizes 1 to 10 nucleated cells per field of view: (average number of nucleated cells per microscopic field of view [count 10 areas]) × (objective power)2.1 For example, using a 40× objective, if 8 cells are counted per field of view: 8 cells × 402 = 12,800 cells/ml. The total nucleated cell count in fluid from body cavities of healthy dogs and cats is less than 3000 cells/ml (most are <1000 cells/ml).
Determining Fluid Protein Concentration
Protein concentration should be determined on supernatant of centrifuged fluid. Protein concentration can be estimated using a handheld refractometer or biochemically. While a refractometer may underestimate protein in fluid when protein is less than 2.0 g/dl, one study developed conversion values for estimating fluid protein concentration as low as 1.0 g/dl using a handheld refractometer (Table 10-2 ).25 In normal animals, protein content of body cavity fluid is less than 2.5 g/dl.
TABLE 10-2.
CONVERSION FOR ESTIMATING FLUID TOTAL PROTEIN USING A HANDHELD REFRACTOMETER ACCORDING TO GEORGE and O’NEILL (2001)
| REFRACTIVE INDEX | SPECIFIC GRAVITY | BODY FLUID PROTEIN (g/dl) | REFRACTIVE INDEX | SPECIFIC GRAVITY | BODY FLUID PROTEIN (g/dl) |
|---|---|---|---|---|---|
| <1.3376 | <1.013 | <1.0 | 1.3389 | 1.017 | 1.8 |
| 1.3376 | 1.013 | 1.0 | 1.3391 | 1.017 | 1.9 |
| 1.3378 | 1.014 | 1.1 | 1.3393 | 1.018 | 2.0 |
| 1.3380 | 1.014 | 1.3 | 1.3395 | 1.018 | 2.1 |
| 1.3382 | 1.015 | 1.4 | 1.3397 | 1.019 | 2.2 |
| 1.3384 | 1.015 | 1.5 | 1.3399 | 1.019 | 2.3 |
| 1.3385 | 1.016 | 1.6 | 1.3401 | 1.020 | 2.4 |
| 1.3387 | 1.016 | 1.7 | 1.3402 | 1.020 | 2.5 |
Distinguishing Different Types of Effusions
Refer to Table 10-1.
Transudates
These effusions have a low protein concentration and cell count, and are typically clear and colorless (FIGURE 10-4, FIGURE 10-5 ). Transudates reflect altered fluid dynamics associated with reduced interstitial fluid resorption (into capillaries), increased venous hydrostatic pressure with concurrent hypoalbuminemia, or severe hypoalbuminemia alone.
FIGURE 10-4.
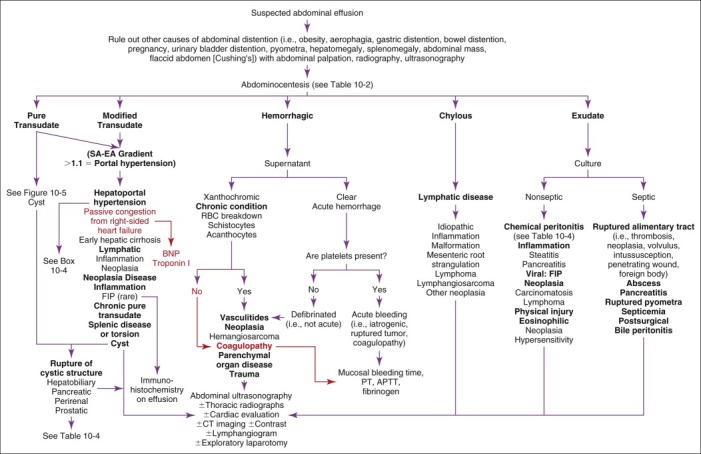
Diagnostic considerations in animals with suspected abdominal effusion. APTT, Activated partial thromboplastin time; BNP, B-type natriuretic peptide; CT, computed tomography; EA, effusion albumin; FIP, feline infectious peritonitis; PT, prothrombin time; RBC, red blood cell; SA, serum albumin.
FIGURE 10-5.

Diagnostic considerations in animals with abdominal transudates. AV, Arteriovenous; CT, computed tomography; CVP, central venous pressure; EA, effusion albumin; ECG, electrocardiogram; FIP, feline infectious peritonitis; GI, gastrointestinal; P/C, protein : creatinine ratio; SA, serum albumin.
Pure Transudates
These are poorly cellular (i.e., <1000 cells/µl), have TS concentrations less than 2.5 g/dl, and have a specific gravity (SG) less than 1.017. Classic examples include abdominal effusions associated with hypoalbuminemia plus portal hypertension resulting from hepatic insufficiency, and with severe hypoalbuminemia associated with sodium and water retention in protein-losing nephropathy (PLN) and protein-losing enteropathy (PLE), as well as with iatrogenic fluid overload or uroperitoneum from a ruptured urinary bladder or ureter.
Modified Transudates
These are associated with a higher TS concentration than pure transudates (generally 2.5 g/dl), a SG greater than 1.017, and moderate cellularity. Mesothelial cells are usually plentiful, and modified transudates reflect transudative vascular leakage from normal or noninflamed vasculature (increased capillary hydrostatic pressure or lymphatic obstruction). Modified transudates may be associated with neoplasia and many other disorders leading to transudative effusions, as well as uroperitoneum from a ruptured urinary bladder or ureter.
Hemorrhagic Effusions
These appear bloody, have a measurable hematocrit representing 10% to 25% of the systemic blood PCV, and have a TS concentration greater than 3.0 g/dl (Figure 10-6 ). If chronic, the supernatant evidences hemolysis or xanthochromia, and cytologic inspection reveals erythrophagocytosis, siderocytes, hematoidin (a yellow refractile crystalline or amorphous pigment, free of iron, formed from hematin), and lack of platelets. These effusions do not clot. Platelets appear only when bleeding has occurred 1 hour or less before sampling. Peracute or iatrogenic hemorrhage has no or only minor erythrophagocytosis, an absence of siderocytes, a clear supernatant, and platelets, and it may clot. If an acute hemorrhagic effusion is allowed to sit before slide preparation, erythrophagocytosis may occur in vitro, confusing the diagnosis.
FIGURE 10-6.

Diagnostic considerations in animals with hemoabdomen or hemothorax. ACT, Activated clotting time; APTT, activated partial thromboplastin time; CBC, complete blood count; CT, computed tomography; DDAVP, deamino d-arginine vasopressin; DIC, disseminated intravascular coagulation; PT, prothrombin time; Vit. K, vitamin K; vWF, von Willebrand factor.
Exudates
Exudates are characterized by high TS concentration (i.e., >3.0 g/dl), high SG (i.e., >1.025), and increased cellularity dominated by neutrophils and macrophages (i.e., >5000 cells/µl). These effusions are characterized as either septic or nonseptic and may be associated with inflammatory, necrotizing, infectious, or malignant disorders. Exudative effusions should be cultured aerobically and anaerobically for bacteria ± fungi. Immediate cytologic inspection of effusions is important to recognize septic exudates, which prioritizes bacterial or fungal culture submission. Evaluation of C-reactive protein (CRP) was recently shown to assist in discriminating exudative from transudative effusions; a cutoff value of 4 µg/ml had a sensitivity of 100% and a specificity of 94%.41
Bilious Effusions
Bilious effusions contain intracellular and extracellular bilirubin crystals (yellow, golden, or brownish debris that may appear refractile or crystalline; see Table 10-1). Large numbers of neutrophils are typical, and these may be highly segmented. Reactive mesothelial cells are common. If septic, bacteria may be visible within phagocytic cells or free in the effusion fluid. Comparing bilirubin concentration in the effusion to that in peripheral blood discloses a 5- to 10-fold higher concentration in the effusion. All effusions in jaundiced animals are yellow colored owing to the solubility and dispersal of bilirubin pigments.
Chylous Effusions
Chylous effusions usually have a TS concentration greater than 2.5 g/dl; a SG greater than 1.018; a predominant population of mononuclear cells (lymphocytes) or high numbers of neutrophils, or both; and a high triglyceride concentration relative to peripheral blood (see Figure 10-4 and Table 10-1). The fluid : serum triglyceride ratio is greater than 2 or 3:1 and commonly exceeds 10:1. Effusion cholesterol concentration is less than in peripheral blood. When centrifuged, chylous effusions have lactescent or opalescent supernatants with a buoyant triglyceride-rich chylomicron layer accumulating at the fluid surface when the sample is refrigerated. A qualitative test for high triglyceride content involves incubation of the suspect effusion pretreated with 1 to 2 drops of 1 N sodium hydroxide and an equal volume of ether. Ether-soluble triglycerides rise to the top of the tube and are discriminated as a white band. Alternatively, a wet mount of fluid may be stained with oil red O or Sudan black and subsequently evaluated for fat droplets (i.e., chylomicrons) (Figure 10-7 ). Chylous effusions reflect disruption of thoracic duct or smaller lymphatics, obstruction from neoplastic infiltrates within lymphatics or draining lymph nodes (e.g., lymphoma, thymoma), mediastinal inflammation or neoplasia, lymphatic occlusion by diaphragmatic or peritoneopericardial hernia, lung lobe torsion, congenital malformations (e.g., lymphangiectasia), cardiac disorders (including heartworm disease), or idiopathic disease (likely congenital malformations). Diagnosis in patients lacking a history of trauma may require a lymphangiogram (using radiography or computed tomography [CT]) and eventual surgical exploration of the appropriate body cavity after priming the lymphatics with a small high-fat meal (cream ingestion) that elucidates the location of lymphatics.
FIGURE 10-7.

A simple, cheap test for chylomicrons in thoracic fluid is to stain the smear with fat stain (e.g., Sudan stain or oil red O) to demonstrate neutral fat droplets in the phagocytes and free in the fluid, plus a drop of new methylene blue to stain the nuclei.
Pseudochylous Effusions
Pseudochylous effusions as described historically in veterinary literature are either extremely rare or nonexistent. Such effusions grossly resemble chylous effusions but have high cholesterol and low triglyceride concentrations relative to peripheral blood. Effusions associated with dense populations of neoplastic cells may appear “chylous” on gross inspection but fail to demonstrate other diagnostic features of chyle.
Malignant Effusions
Malignant effusions are usually characterized as modified transudates or exudates, are often blood tinged and xanthochromic, and are definitively diagnosed by finding neoplastic cells. However, it is important to recognized that effusions secondary to tumors may or may not contain malignant cells. Caution: Reactive mesothelial cells may be misinterpreted as malignant (e.g., binucleated cells, signet ring–shaped cells similar to carcinoma cells, high nuclear : cytoplasmic ratio, large and variably sized nucleus and nucleoli) (Figure 10-8 ). Immunocytochemical staining assists in differentiation of cell origin in cases in which cytologic characterization of a malignant cell population remains uncertain. Differentiating mesothelioma cells from reactive mesothelial cells is problematic, requiring tissue biopsy for definitive diagnosis. Hemangiosarcomas commonly cause malignant effusion in dogs with associated effusions usually containing large numbers of foamy macrophages, reactive and quiescent mesothelial cells, erythrophagocytes, xanthochromic supernatant, and an absence of platelets unless associated with active or iatrogenic hemorrhage. Being vascular tumors, hemangiosarcomas are usually accompanied by circulating acanthocytes and schistocytes (see Chapters 2 and 3Chapter 2Chapter 3). Hemangiosarcomas often produce hemorrhagic effusions lacking cytologic evidence of neoplasia, as these cells do not exfoliate easily. When observed, hemangiosarcoma cells are large spindle-shaped to polyhedral cells, with a round to oval nucleus having one or more prominent nucleoli, a dark-blue cytoplasm (modified Wright-Giemsa staining), and many small, discrete nonstaining vacuoles (Figure 10-9 ). Carcinomatosis (i.e., miliary tumors implanted on peritoneal or pleural surfaces) frequently cause body cavity effusion. Radiography discloses ill-defined serosal margins with fluid confirmed by ultrasonography that can assist in fluid sample collection. These effusions have a high SG and a large amount of protein and may be hemorrhagic. Cytology may disclose neoplastic cells, but in some cases reactive mesothelial cells and carcinoma cells may be indistinguishable, requiring immunohistochemical differentiation.
FIGURE 10-8.

Cytology of abdominal effusion showing reactive mesothelial cells. These cells have peripheral cytoplasmic blebs resembling a “brush border” and very prominent nucleoli and are found in rafts, closely mimicking carcinoma cells.
FIGURE 10-9.

Cytology of a hemangiosarcoma. Many of the cells are spindloid and contain numerous small punctate cytoplasmic vacuoles.
(Courtesy of Dr. Mark Johnson.)
Eosinophilic Effusions
Eosinophilic effusions contain greater than 10% eosinophils, with approximately 50% classified as modified transudates and the remainder as nonseptic exudates. Eosinophilic effusions cannot be predicted from circulating eosinophil counts. Canine eosinophilic pleural effusions may be associated with heartworm disease, disseminated eosinophilic granulomatosis, systemic mastocytosis, interstitial pneumonia, lymphoma, hemangiosarcoma, and carcinoma. In cats, eosinophilic effusions may be associated with lymphosarcoma, systemic mastocytosis, and hypereosinophilic syndrome. Pulmonary infiltrates are commonly detected by radiography. In some animals with pulmonary disease, pneumothorax precedes development of the eosinophilic pleural effusion. Animals with multifocal eosinophilic hepatic granulomas my develop a transudative or eosinophilic abdominal effusion.
Specific Body Cavity Effusions: Diagnostic Considerations
Abdominal Effusions
Diagnostic considerations in animals with suspected abdominal effusion encompass a wide spectrum of disorders (see Figure 10-4). Pure transudates are typically associated with severe hypoalbuminemia (see Chapter 12; see also Figure 10-5) and are usually caused by PLN, PLE, hepatic failure, protein loss from exudative cutaneous lesions, repeated body cavity lavage, or repeated large-volume or therapeutic abdominocentesis of a large-volume effusion. Anorexia and emaciation alone do not produce hypoalbuminemia severe enough to elicit edema or effusion. Important rule outs include causes of pathologic proteinuria (see Chapter 7) and hepatic insufficiency (see the discussion of bile acids in Chapter 9). Because PLE may occur without signs of enteric disease, enteric biopsy may be needed for diagnosis. Adjunctive scrutiny of the serum cholesterol concentration (see Chapter 8) is helpful in differentiating the cause of a pure transudate associated with hypoalbuminemia: PLE and hepatic failure usually cause hypocholesterolemia, whereas PLN usually causes hypercholesterolemia. The contribution of portal hypertension as an important pathomechanism of abdominal effusion can be discerned by calculating the serum albumin–effusion albumin (SA-EA) gradient, defined by the serum albumin concentration minus the ascitic fluid albumin concentration. Finding an SA-EA value greater than 1.1 indicates that portal hypertension has played an etiopathologic role in that patient's effusion. The gradient correlates directly with only a single physiologic variable (portal pressure) as compared to the ascites fluid total protein concentration which is influenced by serum protein concentration as well as portal pressure. This ratio is not affected by diuresis, paracentesis, or type of hepatic disease causing portal hypertension, and has a higher utility than traditional effusion classification schemes for differential diagnosis of the cause of effusion in human patients.42 The utility of the SA-EA gradient has also been examined in dogs.38 Noteworthy is that detection of portal hypertension with the SA-EA gradient is not synonymous with a diagnosis of hepatic failure, as there are numerous disorders (Figure 10-10 ; see also Figure 10-4) that can lead to prehepatic, hepatic (presinusoidal, sinusoidal, post-sinusoidal), or post-hepatic portal hypertension. Considerable overlap of SA-EA gradients has been shown in humans and animals for different disorders. In the absence of liver-related causes, malignancy is the second most important cause of an increased SA-EA gradient and portal hypertension. Differential diagnosis of causes of abdominal effusion is best done considering collective physical findings and diagnostic features (Table 10-3 ).
FIGURE 10-10.

Diagram of blood flow through the liver identifying the sites of pre-hepatic, post-hepatic, and hepatic causes of ascites, as well as pre-sinusoidal, sinusoidal, and post-sinusoidal causes.
TABLE 10-3.
CHARACTERISTICS OF DIFFERENT TYPES OF PORTAL HYPERTENSION
| DIAGNOSTIC FEATURE | Post-hepatic Portal Hypertension |
Hepatic Portal Hypertension |
Prehepatic Portal Hypertension |
||
|---|---|---|---|---|---|
| CARDIAC/PERICARDIAL (FILLING/PUMP FAILURE) | CVC OCCLUSION | NUMEROUS CAUSES | INTRAHEPATIC AV FISTULA | NUMEROUS CAUSES | |
| Serum Albumin : Effusion Albumin | >1.1 | >1.1 | >1.1 | >1.1 | >1.1 |
| CVP | ↑ | Normal | Normal | Normal | Normal |
| ECG | Abnormal | Normal | Normal | Normal | Normal |
| Radiography: | |||||
| Cardiac silhouette | ↑ /normal | Normal | Normal | Normal | Normal |
| Caudal vena caval size | ↑ | ↓, normal, mass lesion | Normal | Normal | Normal |
| Liver Size | ↑ | ↑ | Normal, variable, ↓ | ↑ individual lobe | Normal |
| Ultrasonography | |||||
| Liver pattern | Hypoechoic/normal | Hypoechoic/normal | Variable | Anechoic foci | Normal |
| Size: Doppler confirms flow | |||||
| Hepatic vein | Distended | Distended | Normal | Normal | Normal |
| Portal vein | Prominent | Prominent | Variable | Segmentally larger | Normal |
| CBC: | |||||
| PCV | ↑ /normal | ↑ /normal | Variable | ↓ /normal | Variable |
| MCV | Normal | Normal | ↓ | ↓ | ↓ with shunting/normal |
| Poikilocytes | Rare | Rare | Common | Common | Variable |
| Schistocytes, acanthocytes | ↑ if vascular lesions | ↑ if vascular lesions | Rare | Rare | Rare |
| Chemistry Profile: | |||||
| Albumin | Normal | Normal | ↓ /normal | ↓ /normal | ↓ /normal |
| Liver enzymes | ↑ ALP, ↑ ALT, ↑ AST | ↑ ALP, ↑ ALT, ↑ AST | Variable | Variable | Variable |
| Glucose | Normal | Normal | Normal/ ↓ | Normal/ ↓ | Normal |
| Serum Bile Acids: | Normal | Normal | ↑ postprandial | ↑ postprandial | ↑ if shunting |
| Ascites: | Common | Common | Chronic disease | Common | Variable |
| Pure transudate | Uncommon | Uncommon | Common | Possible | Possible |
| Modified transudate | Common | Common | Rare | Possible | Possible |
ALP, Alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; AV, arteriovenous; CBC, complete blood count; CVC, caudal vena cava; CVP, central venous pressure; ECG, electrocardiogram; MCV, mean corpuscular volume; PCV, packed cell volume.
Abdominal modified transudates are often associated with increased venous (capillary) hydrostatic pressure (see Figure 10-5) and an SA-EA greater than 1.1. Concurrent hepatomegaly suggests impaired blood flow at the level of the hepatic venules, vena cava craniad to the diaphragm, pericardium, right atrium, or pulmonary arterial bed (Box 10-2 ; see Table 10-3, and Figure 10-10). The clinician should look for jugular pulse, pulsus paradoxus, hepatojugular reflex, poor femoral pulse quality, muffled cardiac sounds, exercise intolerance, and physiologically inappropriate tachycardia, which might indicate pericardial tamponade or pericardial restriction. The hepatojugular reflex is elicited by applying gentle abdominal compression to the liver or cranial abdomen for 10 to 15 seconds (increases venous return to the heart) and observing jugular vein distention or pulsation (indicating reduced right heart function or filling). Hepatomegaly caused by venous congestion may be difficult to palpate because of abdominal distention due to ascites or secondary to patient conformation (deep-chested dog).
Box 10-2. Differential Diagnostic Considerations for Abdominal Effusions Associated with Portal Hypertension.
| POST-SINUSOIDAL/ POST-HEPATIC PORTAL HYPERTENSION |
HEPATIC/SINUSOIDAL IMPAIRED SINUSOIDAL/PORTAL FLOW PORTAL HYPERTENSION |
PRESINUSOIDAL/ PREHEPATIC PORTAL HYPERTENSION |
|---|---|---|
| Right-Sided Cardiac Disturbance: | Cirrhosis: | Prehepatic Portal Vein Occlusion: |
| Cardiomyopathy | Regenerative nodules | Portal vein thrombosis |
| Tricuspid insufficiency | Collagenization of sinusoids | Portal vein stenosis: |
| Dirofilariasis | Parenchymal collapse | Trauma |
| Pulmonary thromboembolism | Chronic diffuse hepatitis | Congenital |
| Intracardiac neoplasia | Chronic cholangiohepatitis | Congenital portal atresia |
| Atrial, valvular, mural, infiltrative | Postnecrotic fibrosis | Extraluminal portal vein occlusion: |
| Cor triatriatum dextor | Breed-specific hepatopathies | Neoplasia |
| Pericardial Disease: | Drug-related hepatopathies | Lymph nodes |
| Pericardial tamponade: | Biliary Cirrhosis: | Abscess |
| Atrial hemangiosarcoma | Severe peribiliary fibrosis | Granuloma |
| Coagulopathy | Bridging fibrosis—acquired, congenital: | Peritonitis |
| Trauma | Chronic cholangitis/cholangiohepatitis | |
| Benign pericardial effusion | Chronic major bile duct obstruction | |
| Infectious | Malformations: associated with increased extracellular matrix deposition | |
| Restrictive pericarditis | Feline polycystic liver disease = ductal plate malformation | |
| Constrictive pericarditis | Juvenile hepatic fibrosis = ductal plate malformation | |
| Obstructed Caudal Vena Cava/Hepatic Vein: | Miscellaneous Causes: | |
| Congenital “kinked” vena cava | Noncirrhotic portal hypertension | |
| Post-traumatic vena caval stenosis | Congenital portal atresia | |
| Vena caval syndrome (dirofilariasis) | Portal/sinusoidal disseminated neoplasia | |
| Vena caval/hepatic vein thrombosis | Portal/sinusoidal thromboembolism | |
| Diaphragmatic hernia: vascular entrapment | Diaphragmatic hernia: liver entrapment | |
| Hepatic amyloidosis | ||
| Portal Blood Flow (arterialization of portal vasculature): | ||
| Hepatic artery/portal vein fistula: | ||
| Congenital | ||
| Traumatic | ||
| Neoplastic | ||
| Splanchnic | ||
| Microanastomosis (cirrhosis): intrahepatic sinusoidal shunting |
Thoracic radiographs evaluate shape and size of cardiac and pericardial silhouettes; the tortuosity and filling of the pulmonary arterial bed; and the shape, distention, and position of the vena cava, an important capacitance vessel. If cardiomegaly is present, echocardiographic evaluations differentiate cardiac from pericardial disease. A vascular interrogation using abdominal ultrasonography (color flow Doppler) may reveal distended hepatic veins and an exaggerated flow pattern because of cardiac outflow obstruction, abdominal masses (i.e., neoplasia, granuloma), or obstructed portal flow (e.g., thrombi causing a luminal filling defect). Central venous pressure (CVP) values greater than 8 cm H2O are suggestive, and values greater than or equal to 14 cm H2O are diagnostic of right-sided cardiac dysfunction, filling, or impaired flow of blood into the lungs (e.g., pulmonary hypertension, thromboembolism). CVP may be normal in dogs with cor triatriatum dexter (abnormal congenital occlusive webbing within the right atrium) which causes abdominal effusion secondary to passive congestion. Assessment of CVP is not commonly done because it is subject to many mechanical variables that invalidate its interpretation (e.g., catheter end position variability; kinking or folding of the catheter and catheter occlusion) and because advanced imaging modalities allow better assessment of potential disease mechanisms. Furthermore, it may lead to iatrogenic hemorrhage in patients with coagulopathies (e.g., hepatic insufficiency, rodenticide toxicity, vasculitis, thrombocytopenia).
Modified transudates associated with chronic hepatic disease can develop before severe hypoalbuminemia concordant with onset of presinusoidal, sinusoidal, or post-sinusoidal portal hypertension (see Box 10-2). Pooling of albumin in the abdominal effusion is one factor contributing to onset of systemic hypoalbuminemia in these patients. Abdominal ultrasonography usually discloses one or more of the following features: microhepatia, irregular lobe margins, altered parenchymal echogenicity, distended extrahepatic portal vasculature and/or retrograde portal blood flow, splenic congestion, or tortuous portosystemic shunts (caudal to the kidneys, adjacent to the splenic vasculature) (see Table 10-3). Modified transudates may also reflect fibrosis in the porta hepatis, neoplasia occluding (strangulating) portal vasculature, or portal venous thromboembolism (detected by color flow Doppler examination). An SA-EA gradient greater than 1.1. develops in all of these disorders (see Figure 10-5).
Splenic infarction, thromboembolism, or torsion may produce an abdominal effusion characterized as a modified transudate or exudate. Part or all of the spleen may appear large on ultrasonography, and color flow Doppler interrogation may disclose impaired perfusion (e.g., vascular thrombi, impaired venous or arterial flow). Splenic and other abdominal masses (i.e., neoplasia, granulomas) may also produce modified transudates, these are usually detected by ultrasonography.
Effusions can be suspected on the basis of radiographic images demonstrating lack of distinct visceral margins. However, ultrasonography is more accurate for fluid detection. Radiographic images made after abdominal fluid evacuation (drainage) allow appraisal of hepatic size, detection of a mass effect, or altered visceral positions. Visceral margins will remain ill defined because of retention of small fluid volumes. After large-volume paracentesis, most effusions reaccumulate within hours to days, so radiographic studies must be done immediately.
Finding an exudative effusion mandates a search for infection, necrosis, or malignancy. Identification of phagocytized organisms (neutrophils or macrophages) is definitive for sepsis but may require careful, tedious inspection of several cytology slides. Finding plant fibers, enteric debris, or a mixed “fecal flora” in an abdominal effusion suggest loss of enteric integrity or gut rupture. Degenerate WBCs (Figure 10-11 ; see Chapter 16) suggest infection, although some organisms do not alter neutrophil morphology (e.g., Actinomyces). Degenerate changes in WBCs also may result from specimen handling (i.e., storage too long before cytology smear preparation). Recent abdominal trauma (e.g., exploratory laparotomy) causes mild, transient, fluid accumulation characterized by a neutrophilia with degenerative changes.
FIGURE 10-11.
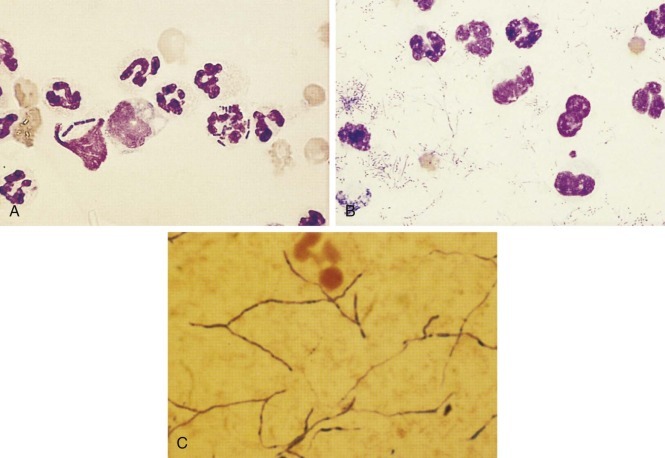
A, Two neutrophils in this canine abdominal fluid contained rod-shaped bacteria. Note that the neutrophils do not look degenerate even though the exudate was septic. One should not consider the lysed neutrophil with bacteria to be degenerate. B, The degenerate neutrophils in this thoracic fluid have karyolytic (swollen) nuclei as evidence of degeneration caused by the bacterial sepsis about them. The branching filamentous bacterium was Actinomyces.C, The gram-positive, branching, beaded organisms were Actinomyces. Gram-negative organisms are difficult to find on Gram-stained smears of exudate. Many gram-negative rods are illustrated here, but typically only one bacterium is found per several microscope fields in exudates and, if pale, a rare red bacteria would be easily hidden among red-staining leukocytes or debris.
Finding and identifying infecting organisms can be difficult, especially when bacterial numbers are low or bacteria are within granulomas or loculated within abscesses. Certain organisms are notoriously difficult to find (e.g., Nocardia and Actinomyces). Therefore all exudative effusions should be cultured aerobically and anaerobically for bacteria (see Chapter 15). Samples should be immediately submitted in sterile clot tubes or transferred to appropriate transport medium. If samples in transport medium cannot be immediately submitted, they should be refrigerated to slow bacterial growth to avoid medium substrate use and microbe death. Finding irrefutable evidence of an infectious organism may indicate a need for emergency exploratory surgery. However, animals with suppurative cholangitis or hepatitis may be placed at great risk by surgical exploration that will have no direct survival benefit (aside from tissue biopsy and culture). Animals with obstructive biliary disorders associated with sepsis should be given intravenous antimicrobials before surgery and intraoperatively; a combination of metronidazole, enrofloxacin, and ticarcillin is recommended. Positive culture results should be reconciled with the antimicrobials in use and the regimen tailored appropriately. Animals with abdominal contamination secondary to surgery or iatrogenic infections (e.g., contamination during paracentesis) should have antibiotic therapy tailored to results of culture and sensitivity; these cases have higher risk for resistant nosocomial pathogens. Infections with fungal agents may also underlie exudative effusions. Fungal cultures should be considered in animals with body cavity effusions characterized as granulomatous and in animals with unexplained effusions within geographic regions where endemic fungi are recognized (e.g., Southwest United States for Coccidioides; regions where blastomycosis or histoplasmosis are common).
Exudates without cytologic evidence of sepsis necessitate review of history for trauma and possible urinary, biliary, or cyst rupture (Table 10-4 ; see also Figure 10-4). Neutrophilic abdominal effusion can persist for weeks after blunt abdominal trauma or surgery. Radiography of bony structures sometimes reveals evidence of injury. If trauma is considered unlikely, physical assessments should be made looking for other evidence of inflammation. In cats, feline infectious peritonitis (FIP) must be considered. Although clinical presentations are quite variable, most cats with FIP are chronically ill with systemic signs, hyperglobulinemia (i.e., ≥5 g/dl), and a high-protein abdominal effusion (i.e., >3.5 g/dl). Evaluation of the effusion by protein electrophoresis may assist in achieving a diagnosis; one study demonstrated that finding greater than 32% gamma globulins in effusions with a total protein greater than 3.5 g/dl had a 100% positive predictive value for FIP in a suspect population of cats.47 Estimation of acute phase proteins also may be helpful. Concentrations of α1-acid glycoprotein (AGP) greater than 1.5 g/L in plasma, sera, or effusion from cats with spontaneous or “field” FIP were shown in one study to help distinguish FIP-affected cats from cats with similar clinical signs.15 Unfortunately, not all cats with FIP have raised AGP values. The total nucleated cell count in body cavity effusions from cats with FIP is highly variable, ranging from 1000 to 30,000 cells/µL. Cytology reveals nondegenerative neutrophils as the predominant cell type and numerous macrophages and variable numbers of lymphocytes and plasma cells. Inconsistencies in signs and clinical pathologic findings in FIP-affected cats (see Chapter 15) makes conclusive diagnosis impossible in cats with atypical features (e.g., long-term survival, modified transudative effusions) without immunocytochemistry or immunohistochemistry using anti-Coronavirus antibody. Serologic polymerase chain reaction (PCR) detection of Coronavirus antigen or its antibody does not provide a definitive diagnosis. Other considerations regarding abdominal effusions are summarized in Figure 10-4.
TABLE 10-4.
CHARACTERISTICS, CAUSES, AND DIAGNOSIS OF CHEMICAL PERITONITIS
| BILE PERITONITIS | UROABDOMEN | PANCREATITIS | RUPTURED “CYSTS” | |
|---|---|---|---|---|
| Appearance | Golden brown-green, serosanguineous, turbid | Light to dark yellow ± serosanguineous, clear (some acute), turbid (chronic) | White, yellow, serosanguineous, turbid | Clear to turbid, pale to yellow, colorless |
| Causes | Blunt abdominal trauma Necrotizing cholecystitis Cholelithiasis |
Trauma: avulsed ureter or bladder, ruptured bladder Urolithiasis |
Pancreatitis | Perirenal cysts Polycystic renal/hepatic disease Pancreatic cysts Paraprostatic/prostatic cysts |
| Clinical features | Vague abdominal pain Lethargy Pale or acholic feces Increased hepatic enzymes Jaundice (chronicity) Septic peritonitis Gallbladder: may be difficult to visualize on ultrasonography |
Dehydration Azotemia Anuria/oliguria Abdominal distention Hyponatremia Hyperkalemia Hyperphosphatemia Metabolic acidosis |
Anorexia Vomiting Abdominal pain Lethargy Fever Jaundice Increased: hepatic enzymes/TLI/PLI cholesterol/bilirubin Cardiac arrhythmias Pleural effusion Acute renal failure |
Vary with underlying tissue involved and severity of lesion |
| Definitive diagnosis | Free and phagocytized bilirubin crystals Fluid bilirubin > serum bilirubin. May require ultrasound-directed fluid aspiration, CT imaging, or (rarely) hepatobiliary scintigraphy |
Intravenous urogram Retrograde ureterocystography, ultrasound or CT imaging Fluid creatinine > serum creatinine |
Macrophages contain refractile lipid inclusions Fluid lipase/amylase/TLI/PLI greater than serum lipase/amylase/TLI/PLI |
Ultrasonography Tissue biopsy Cyst aspiration + fluid analysis/cytology |
CT, computed tomography; PLI, pancreatic lipase (species specific); TLI, trypsin-like immunoreactivity.
Neoplasia becomes an important differential for modified transudates or exudates after eliminating other causes. Neoplastic cells are sometimes identified cytologically; however, there often are too few or no exfoliated neoplastic cells in small-volume samples. Use of cytospin preparations or examination of centrifuged fluid sediment increases the likelihood of finding malignant cells. Sometimes neoplastic cells are only found using sediment derived from large volumes (e.g., >50-ml samples) of effusion. It is important to remember that “reactive” mesothelial cells resemble carcinoma cells and may be erroneously classified without immunocytochemical or immunohistochemical investigations. Ultrasonography can aid in aspiration of mass lesions for cytologic evaluation that may yield a more diagnostic population of cells for definitive identification.
Chylous abdominal effusions usually suggest intestinal lymphangiectasia; lymphoproliferative disease of the gut, mesenteric lymphatics, or lymph nodes; intra-abdominal neoplasia “strangulating” the mesenteric root; or idiopathic disease (these may be malformations). Rarely, vitamin E–responsive steatitis or biliary cirrhosis has accompanied feline chyloabdomen (see Figure 10-4). While small lymphocytes are the initial cell type associated with chylous effusions, neutrophilic inflammation becomes established with chronicity. Repeated large-volume removal of chylous effusions depletes systemic protein concentrations (i.e., chyle contains 1 to 6 g protein/dl), further disrupting Starling's forces that may augment further fluid accumulation. Secondary infections are rare in animals with chylous effusions because chyle imparts a bacteriostatic influence. Animals with chyloabdomen should be evaluated for pleural effusion, lymphadenopathy, and metastatic neoplasia by thoracic radiography. Ultrasonography may reveal mesenteric root masses or mesenteric lymphadenopathy.
Hemoabdomen may be associated with trauma (e.g., ruptured spleen or hepatic parenchyma; avulsed renal pedicle or mesenteric vessels), vascular neoplasia (e.g., hemangiosarcoma, other vascular tumors, or tumors with necrotic centers such as hepatomas and hepatocellular carcinomas), increased hepatic fragility as occurs in feline hepatic amyloidosis, or coagulopathies (see Chapter 5; see also Figure 10-6). Physical examination usually reveals abrasions or pain in traumatized animals, with follow-up radiographs sometimes disclosing broken bones. Inspection for signs of bleeding or coagulopathy should include detection of petechiae (e.g., fundic examination, mucous membranes), rectal and fecal examination looking for melena or hematochezia, palpation for hemarthrosis (i.e., swollen, painful joints), and urinalysis looking for hematuria. Without history or physical findings suggesting trauma, a complete blood count (CBC), including a platelet count and a coagulation profile, becomes essential. PCV reveals whether the erythron mass is reduced. However, in acute severe hemorrhage, change in PCV is contingent on fluid redistribution and whether a regenerative RBC response (increased reticulocytes, broad RBC distribution width [RDW]) has been realized (3 to 5 days after blood loss). Schistocytes and acanthocytes (see Chapters 2 and 3Chapter 2Chapter 3) suggest microangiopathic damage reflecting vascular neoplasia (e.g., hemangiosarcoma) or disseminated intravascular coagulation (DIC). Scanning a blood smear should detect thrombocytopenia severe enough to cause hemorrhage (i.e., fewer than 3 platelets/400× field of view, see Chapter 5). Severe acute hemorrhage not caused by thrombocytopenia initially increases the platelet count. An ACT and buccal mucosal bleeding time (BMBT) (see Chapter 5) detect many hemostatic defects; BMBT is only pursued in animals with an adequate platelet count (>100,000/µl). Samples for effusion characterization should be obtained before initiating fluid or blood replacement therapy. Thoracic radiographs may reveal pleural fluid, lymphadenopathy (e.g., sternal lymph node), or frank metastasis. Ultrasonography may discover vascular tumors, usually associated with hepatic or splenic hematomas, and may disclose the site of active bleeding. Animals with persistent abdominal hemorrhage may require blood component therapy (whole blood, fresh frozen plasma, or cryoglobin if severe von Willebrand factor [vWF] deficiency is suspected) in addition to empirical vitamin K1 treatment (0.5 to 1.5 mg/kg subcutaneously [SC] or intramuscularly [IM], 3 doses q8hr) and synthetic vasopressin (1 to 5 µg/kg SC or intravenously [IV] diluted), followed by exploratory laparotomy if hemostatic risks can be attenuated.
Bilious effusions caused by gallbladder or common bile duct rupture may derive from blunt abdominal trauma, necrotizing cholecystitis or choledochitis, cholelithiasis, or gallbladder mucocele (progresses to gallbladder ischemic necrosis). Biliary tree leakage may be immediate or delayed after blunt or surgical injury (see Table 10-4). Affected animals may be asymptomatic or symptomatic. Symptomatic patients demonstrate variable low-grade abdominal pain, lethargy, fever, and jaundice associated with a mild to modest abdominal effusion and may have concurrent bacterial contamination. Patients with aseptic bile peritonitis may be asymptomatic with the exception of jaundice and equivocal cranial abdominal discomfort. Clinical signs may correlate with the extent/severity of bile leakage (e.g., experimentally, the lethal dose of sterile bile injected intraperitoneally into dogs ranged from 20 to 30 ml/kg body weight). Pale or acholic feces indicate deviation of bile from the intestines (extrahepatic bile duct obstruction or severe small bile duct “ductopenia” as develops in cats with immune-mediated cholangiohepatitis). Serum alkaline phosphatase (SAP), gamma-glutamyl transpeptidase (GGT), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) activities and total bilirubin concentrations are invariably increased. Appearance and severity of jaundice depend on underlying cause and severity and chronicity of bile leakage. Bile induces a chemical peritonitis associated with cytokine release and alterations in fluid transport across peritoneal membranes. Cell membranes exposed to high concentrations of bile acids (functionally acting as detergents that disrupt cell membranes), bilirubin, and lysolecithin develop leaky gap junctions permitting translocation of enteric bacteria and endotoxin, leading to endotoxemia and/or septic peritonitis. Focal bile peritonitis may be restricted by omental adhesions to biliary structures. These present as pericholecystic effusions or effusions within the porta hepatis on ultrasonography. Cautious targeted aspiration using a spinal needle and ultrasonographic guidance may successfully collect diagnostic fluid in these cases. A ruptured gallbladder may be indicated by its sudden absence on ultrasound evaluation. In some cases, ultrasound imaging can identify the location of bile leakage (i.e., focal fluid accumulation, hyperechoic foci, adhesions, loss of normal gallbladder wall layering). Focal ileus of small intestine or colon may be identified adjacent to a site of biliary rupture, reflecting chemical peritonitis. Bile peritonitis is cytologically characterized by high numbers of neutrophils and macrophages and the presence of free and phagocytized bile (see Figure 10-3). The fluid specimen is often turbid with a golden-brown or golden-green color. A higher bilirubin concentration relative to peripheral blood is found. Fluid samples for accurate diagnosis of bile peritonitis should be collected from the immediate area of leakage for best assessment owing to the propensity for omental encapsulation of the most diagnostic fluid (i.e., bile particulates, bacteria).
Uroabdomen occurs when urine leaks and pools within the peritoneal cavity. Affected animals may appear to void normally if the rent reflects a single ruptured ureter or when bladder avulsion surrounded by a fibrous tract provides a voiding conduit. Avulsion of a ureter at the renal pedicle may cause retroperitoneal effusion. Trauma is the major cause of urinary system leakage, but cystocentesis, traumatic diagnostic cystoscopy, or neoplasia may also be causal factors. The degree of azotemia varies depending on severity and chronicity of urinary leakage into the abdomen. If virtually all urine accumulates in the abdomen, rapid-onset azotemia and hyperkalemia are expected. Most patients develop a vaguely painful abdomen, lethargy, fever, and dehydration. Some animals develop pathologic arrhythmias associated with electrolyte aberrations (i.e., severe hyperkalemia and acidosis). Markedly increased blood urea nitrogen (BUN) and creatinine concentrations, hyperphosphatemia, hyponatremia, hyperkalemia, and metabolic acidosis are expected. The effusion is slightly turbid, blood tinged, and yellow. Fluid creatinine concentration is markedly higher relative to peripheral blood, whereas there may be no substantial difference between fluid and serum urea nitrogen concentrations. The small size of the urea molecule allows rapid systemic dispersal in body water, negating its diagnostic utility in uroabdomen. Ultrasonography or an IV urogram (followed by a retrograde urethral cystogram as necessary) usually locates the damaged area. Diagnosis may be more descriptive if contrast studies use CT. Urinary drainage and abdominal lavage rapidly correct electrolyte and acid-base derangements if surgical intervention is delayed.
Pancreatitis sometimes causes diffuse peritonitis and copious effusion. Clinical pathologic changes are discussed in Chapter 9; ultrasonography provides important diagnostic information, such as altered pancreatic echogenicity, marginal irregularity, altered duct morphology (distention), associated distal bile duct obstruction, focal pain on imaging probe pressure application, focal ileus (duodenal corrugation, amotility), and peripancreatic fat hyperechogenicity (saponification). Effusions are grossly turbid and sometimes have a lipid surface interface after refrigeration and centrifugation. Inflammation is characterized by large numbers of neutrophils and macrophages; the latter often contain many small to large, clear or refractile vacuoles (engulfed lipid). Pancreatic enzyme activity in effusion may be markedly higher than in the peripheral blood (i.e., lipase or amylase). This suggests enzyme leakage from pancreatic ducts.
Ruptured “cystic” lesions in the liver, kidneys, pancreas, or prostate occasionally cause transudative abdominal effusions (see Figure 10-4). Fluid within large cysts is sometimes misidentified as free abdominal effusion before leakage. Cystic fluid is evaluated for underlying malignancy or infection but often is characterized as a transudate. Polycystic hepatic or renal disease with well-developed cysts is more common in cats (i.e., Persians, Himalayans) than dogs. Cystadenomatous malformations in cats usually cannot be drained owing to their multicompartmented structure. These may cause effusions when located adjacent to or within the porta hepatitis due to pressure imposed on vasculature. Perirenal pseudocysts are more common in cats, especially older males. Although rare, pancreatic cysts may be benign, may represent postpancreatitis abscessation or formation of a pseudocyst, or may be the result of malignancy (i.e., pancreatic adenocarcinoma). In intact male dogs, paraprostatic or prostatic cysts may be large and may become infected. If a perirectal cyst is identified, position of the urinary bladder should be confirmed (decompressed by voluntary voiding or catheterization). Thereafter cystic fluid should be aspirated and analyzed for creatinine. Ultrasound-guided cyst aspiration and sampling of suspected abdominal effusion are the least invasive and most cost-effective methods of determining whether resection or drainage of cysts is needed. Examination of cystic fluid relative to any abdominal effusion is important to identify infection or neoplasia or other biochemical components that might incite chemical peritonitis.
Pleural Effusions
The general approach to differential diagnosis of pleural effusion is similar to that described for abdominal effusions (Figure 10-12 ). However, the pleura is not as readily accessible by exploratory surgery and is less easily visualized by ultrasonography. Animals with pleural effusion often have rapid, shallow breathing with accentuated abdominal effort. Radiographs should include right and left lateral and ventrodorsal views. Radiographically, pleural fluid is characterized as either free (will move upon patient repositioning) or encapsulated (nonmovable with positional change).
FIGURE 10-12.

Diagnostic considerations in animals with suspected thoracic effusion. ADH, Antidiuretic hormone; APTT, activated partial thromboplastin time; FIP, feline infectious peritonitis; PT, prothrombin time; RBC, red blood cell; Sp grav, specific gravity.
Pure transudates are less common in the pleural space than in the abdomen (Figure 10-13 ). These effusions signal severe hypoalbuminemia and thoracic vascular hypertension. Pure transudates also occur in animals overhydrated with crystalloid fluids; this is most common in overweight animals where calculations are erroneously based on gross body weight. Overhydration causing pleural and pulmonary fluid retention is most symptomatic in patients with incipient cardiac disease (e.g., asymptomatic cardiomyopathy).
FIGURE 10-13.

Diagnostic considerations in animals with pleural transudates. BNP, B-type natriuretic peptide; CT, computed tomography; ECG, electrocardiogram; FIP, feline infectious peritonitis.
Modified transudates are the most common type of pleural effusion (see Figure 10-13). Obstructive effusions can be serous to serosanguineous, have a SG ranging from 1.015 to 1.040, and have a TS concentration greater than or equal to 2.5 g/dl. Cellularity is usually mixed with RBCs, lymphocytes, and fewer neutrophils, eosinophils, macrophages, and mesothelial cells; with chronicity these progressively appear more inflammatory. Physical examination may disclose features suggesting a primary underlying disease (e.g., gallop rhythm, cardiac murmur, loss of normal compression of the anterior chest in cats with an enlarged heart or mediastinal mass). A history of trauma plus a vague “emptiness” of the abdomen and auscultation of borborygmi within the thorax suggest diaphragmatic hernia. Dogs with right-sided cardiac failure usually develop ascites, whereas cats with cardiac failure often develop focal pulmonary infiltrates and/or pleural effusion. Benign or neoplastic pericardial effusion, constrictive or restrictive pericarditis, and right atrial hemangiosarcoma cause pleural effusion associated with pericardial tamponade in dogs (Box 10-3 ). Occasionally, animals with a cranial mediastinal mass and associated pleural effusion demonstrate features of cranial vena cava syndrome (i.e., submandibular edema; jugular engorgement and pulses; injection of the conjunctival blood vessels indicating impaired cranial venous return, lymphatic return, or both). Thymomas, thymic cysts, lymphoma, invasive thyroid adenocarcinoma, and bilateral jugular thrombosis are the most common causes of this syndrome.
Box 10-3. Differential Diagnosis of Pericardial Effusions.
Idiopathic Pericarditis (~20% of cases)
Neoplasia (~70%–80% of cases)
Mass Lesion Location and Differential Diagnosis:
Right atrium (most common):
Hemangiosarcoma (~88%; ~25%–30% have splenic neoplasm also)
Neuroendocrine
Thyroid adenocarcinoma
Mesothelioma
Lymphoma (primary & multicentric)
Sarcoma
Heart base (second most common):
Neuroendocrine (~40%)
Thyroid adenocarcinoma
Mesothelioma
Hemangiosarcoma
Pericardial mass
Right ventricular mass
Cranial mediastinal mass
Left atrial mass
Infiltrative Disease
Lymphosarcoma
Pyogranulomatous inflammation:
Coccidioides, Histoplasma
Congenital
Peritoneopericardial diaphragmatic hernia
Pericardial cyst
Miscellaneous
Right-sided heart failure
Left atrial rupture: idiopathic (mitral valve insufficiency)
Right atrial rupture: traumatic
Anticoagulant rodenticide toxicosis
Uremic pericarditis
Bacterial or fungal infection
Constrictive pericarditis
Thoracic radiography should be performed before thoracocentesis to minimize the possibility of iatrogenic lung laceration, to identify cardiomegaly (suggesting cardiomyopathy or pericardial effusion), and to detect gas entrapped within visceral structures (diaphragmatic hernia) or an abscess. Removing effusion followed by radiography may permit identification of masses and consolidated lung lobes or diaphragmatic incontinuity. Thoracic ultrasonography is enhanced by thoracic effusions, and ultrasound-guided aspiration of masses may provide a definitive diagnosis. Most animals require sedation before needle-targeted thoracic sampling of lesions deeper than the peripheral pleural space.
Thoracic exudates (Figure 10-14 ) necessitate careful examination for infectious agents. A variety of bacterial organisms may be identified in septic pleural effusions. Actinomyces and Nocardia are often found in dogs, particularly in geographic locales where foxtail grass awns occur. Multiple bacterial species are commonly found in feline pyothorax. Septic exudates typically are turbid, cream colored, seropurulent or brown tinged and contain degenerate WBCs. Exudates associated with pure Actinomyces infection may have minimal or no WBC degeneration; dual infections with Nocardia are common, and mixed populations of both organisms may be found in thick or thin red-brown exudates containing degenerate WBCs and “sulfur granules.” Inclusion of colored or whitish flecks or “granules” from an exudate on cytologic smears improves identification of Actinomyces and Nocardia organisms. These are presumptively identified when beaded, branching filaments (see Figure 10-11) are found. Most Nocardia spp. stain acid fast with a modified acid-fast stain, whereas Actinomyces spp. do not. While both organisms produce exudates containing sulfur granules, only Actinomyces produces these within tissues. In animals with pyothorax, thoracic radiography may disclose pulmonary parenchymal involvement. When effusions are managed with chest tube insertion, pleural fluid persists as long as the tube is retained. This necessitates sequential cytologic evaluation of pleural fluid to estimate treatment response and to guide propriety of tube removal.
FIGURE 10-14.

Diagnostic considerations in animals with thoracic exudates. CT, Computed tomography; FIP, feline infectious peritonitis; WBCs, white blood cells.
Nonseptic exudates with a hemorrhagic component are associated with lung lobe torsion (suggested by radiographic evidence of a malpositioned main stem bronchus, persistent air within a twisted bronchus, and usually prolific fluid production). Bronchoscopy may grossly visualize a twisted bronchus. Ultrasonography may confirm lung lobe torsion by interrogation of lung lobe perfusion. Nonseptic exudates also are associated with idiopathic pleuritis, infectious pneumonia (e.g., Mycoplasma pneumonia), and various tumors. FIP can cause a pyogranulomatous pleural effusion that is light yellow and viscous, with a high protein concentration. A background of proteinaceous material (homogeneous pink background on Wright-Giemsa stains) is common on cytologic evaluation.
Thoracic neoplasia often induces pleural effusion associated with exuberant mesothelial cell exfoliation. The most common neoplasm causing pleural effusion in dogs and cats is mediastinal lymphoma. In dogs, lymphocytes are the predominate cell population and these may lack obvious malignant characteristics. Aspiration of mediastinal masses or lymph nodes or other more accessible enlarged nodes may be diagnostic. In cats, exfoliated lymphoblasts are common. It is important to differentiate thymomas from lymphoma, as the former may have a better prognosis. Differentiation of thymoma from lymphoma may require tissue sampling. Malignant or benign thymic cysts also may cause pleural effusion. Mesotheliomas pose a great diagnostic challenge and commonly require tissue biopsy for definitive diagnosis.
Hemorrhagic pleural effusion (see Figure 10-6) is usually caused by trauma or neoplasia. With trauma, radiographs may reveal rib fractures, pulmonary consolidation, or pneumothorax. Nontraumatic hemorrhagic pleural effusions usually result from bleeding neoplasia; however, coagulopathy also must be considered (e.g., minor trauma can cause substantial bleeding in dogs with severe von Willebrand's disease or vitamin K depletion from warfarin-like rodenticides; see Chapter 5). Other nontraumatic causes of hemothorax include lung lobe torsion, pulmonary abscessation, pulmonary infarction, dirofilariasis, and (rarely in the United States) Spirocerca lupi–associated aortic aneurysm. In dogs, hemorrhagic pleural effusion derived from disseminated pulmonary hemangiosarcoma is difficult to diagnose antemortem. Pulmonary aspirates or open-chest biopsy impose high risk for tension pneumothorax and worsening hemorrhage.
Chylous effusions are more common in the thorax than in the abdomen (see Figure 10-12). These may be idiopathic or associated with underlying disease as described previously. Some breeds (e.g., Afghan hounds) may have a congenital propensity for chylous pleural effusions. Thoracic ultrasonography or postdrainage radiography can assist in identifying underlying conditions. Contrast lymphangiography performed by cannulating mesenteric lymphatics or the thoracic duct may elucidate the site of chyle leakage. An alternative strategy is to feed a high-fat (i.e., cream) small meal shortly before surgical exploration to fill lymphatics with grossly identifiable chyle.
Bicavity Effusions
The list of differentials includes several diseases (Box 10-4 ). Tumors and cardiac disease are particularly common causes.
Box 10-4. Conditions Associated with Bicavity Effusions.
Cardiovascular Conditions
Idiopathic hemorrhagic pericardial effusion
Constrictive pericardial disease
Biventricular cardiac failure:
Congestive cardiomyopathy
Hypertrophic cardiomyopathy
Idiopathic pulmonary hypertension
Right ventricular thromboembolism
Caudal vena cava thromboembolism
Congenital obstruction: caudal vena cava
Pancreatitis
Bile Peritonitis
End-Stage Hepatic Disease
Feline Infectious Peritonitis
Neoplastic Conditions
Right atrial fibroma
Metastatic adenocarcinoma
Lymphoma
Hemangiosarcoma
Mesothelioma
Cholangiocellular carcinoma
Chemodectoma
Prostatic adenocarcinoma
Diffuse carcinomatosis
Pericardial Effusions
The pericardial cavity is a potential space between the parietal and visceral layers of the serous pericardium normally containing from 1 to 15 ml of plasma ultrafiltrate. Normal intrapericardial pressure equates with intrapleural pressure, vacillating by ±4 mm Hg with ventilation.45 Accumulation of fluid within the pericardial sac exceeding its flexible capacitance impairs right atrial/ventricular filling pressures (normally 4 to 8 mm Hg) causing cardiac tamponade. This compromises venous return, ventricular filling, stroke volume, and cardiac output. Initially, a compensatory increase in heart rate and peripheral vascular resistance moderates systemic effects of cardiac tamponade, maintaining normal blood pressure and cardiac output. However, as fluid accumulates and intrapericardial pressure increases, impaired left atrial and ventricular filling lead to left-sided cardiac dysfunction and cardiogenic shock secondary to a marked fall in cardiac output and peripheral blood pressure. The volume of pericardial fluid provoking symptomatic tamponade varies with speed of accumulation, underlying cause, and total accumulated volume. Rapid accumulation of volumes as small as 25 to 100 ml can abruptly cause tamponade, whereas slower accumulations can generate volumes as large as 2 L in large-breed dogs (which are most commonly affected) before onset of clinical signs. Dogs with chronic pericardial effusion eventually demonstrate clinical signs consistent with right-sided heart failure: lethargy, exercise intolerance, tachypnea, weight loss, and abdominal distention. Signs are progressive in development concordant with fluid accumulation that exceeds compliance of the pericardial sac. Dogs with acute symptomatic pericardial effusion present with acute collapse, syncope, or weakness precipitated by physical exertion and require emergency pericardiocentesis. The clinician should look for jugular pulse, pulsus paradoxus, hepatojugular reflex, poor femoral pulse quality, muffled cardiac sounds, exercise intolerance, and physiologically inappropriate tachycardia consistent with pericardial tamponade or restrictive pericardial disease. Hepatojugular reflex is elicited by applying gentle abdominal compression to liver or cranial abdomen for 10 to 15 seconds (increases venous return to the heart) and observing jugular vein distention or pulsation (indicating reduced right heart function or filling). Hepatomegaly caused by venous congestion may be difficult to palpate because of abdominal distention due to ascites formation or secondary to patient conformation (deep-chested dog). The unique finding of pulsus paradoxus is best detected with the patient laterally recumbent. This represents an exaggerated change in arterial pressure during respiration: fall in pressure during inspiration and stronger pulse during expiration that coordinate with exaggerated right atrial and ventricular filling during inspiration and reduced stroke volume from reduced left ventricular volume.
Pericardial effusion is usually associated with pericardial irritation and inflammation, neoplasia causing hemorrhage, or central venous congestion. Animals with congestive cardiomyopathy may have small to moderate amounts of pericardial fluid that can lead to diagnostic confusion in delineating the cause of clinical signs. Pericardial effusion is associated with a severe globoid or round enlargement of the cardiac silhouette with the size increasing with the volume of accumulated effusion (large size with chronicity, perhaps near-normal shape and size with rapid onset). The caudal vena cava, an important capacitance vessel, is usually large on the lateral thoracic radiograph reflecting hepatic congestion and hepatomegaly due to passive congestion. Pleural effusion and evidence of metastatic disease may be apparent. Edges of the cardiac silhouette may appear unusually “sharp” due to decreased motion artifact associated with the diminished cardiac contraction.46 Abdominal radiographs may disclose hepatomegaly or reduced abdominal detail due to ascites derived from severe passive congestion (right-sided congestive heart failure) caused by pericardial tamponade. Sinus tachycardia is a common electrophysiologic finding along with low-voltage QRS complexes (50% of dogs with pericardial effusion). Electrical alternans (cyclic change in R-wave amplitude) reflects motion of the heart suspended in the pericardial sac. Finding a normal ECG does not dismiss pericardial effusion from considered differential diagnoses. The gold standard for diagnosing pericardial effusion is echocardiography; effusion in the pericardial sac is obvious and can be identified by entry-level ultrasound operators. Fluid volumes as small as 10 to 15 ml can be detected using ultrasonography. Diastolic collapse of the right atrium or ventricle is a classic feature.46 Mass lesions may be identified but often require an operator with specialized training. A cavitated mass associated with the right atrium is highly consistent with hemangiosarcoma, the most common neoplasm in large-breed dogs associated with pericardial tamponade linked with a hemorrhagic/xanthochromic effusion. Small hemangiosarcomas located beyond the echocardiographic “window” may remain undetected.
Pericardial effusions characterized as pure transudates may reflect severe hypoalbuminemia. Modified transudates are found with idiopathic pericarditis, right-sided heart failure, pericardial cysts (congenital malformations), uremic pericarditis, and syndromes associated with vascular leakage. Exudates are found with FIP and bacterial or fungal pericarditis. Septic pericarditis is rare but has been reported secondary to migrating grass awns. Apparent chylopericardium also is rare but may develop secondary to mediastinal venous hypertension derived from cardiomyopathy. Trauma, neoplasia, coagulopathy, or rare spontaneous left atrial rupture can cause hemopericardium. Pericardial tamponade is most common in dogs and is most often associated with a hemorrhagic effusion. However, it also may be associated with a modified transudate or exudate. A benign pericardial effusion is detected in approximately 50% of dogs. In the remainder, the most common diagnosis is neoplasia with right atrial hemangiosarcoma most common (approximately 60% to 75% of neoplastic causes) followed by chemodectoma (approximately 10% of neoplastic causes), mesothelioma (approximately 5% of neoplastic causes), and rarely metastatic adenocarcinoma. Idiopathic pericarditis is associated with extensive fibrosis and a mixed inflammatory response having greatest intensity at the cardiac surface. Perivascular lymphoplasmacytic aggregates are found at the pleural surface and within fibrosed pericardium.13 It is notable that some cases of idiopathic pericarditis resolve after a single pericardiocentesis.
Cytologic differentiation of benign from malignant pericardial effusion remains problematic. Irrespective of the definitive diagnosis, hemorrhagic effusions are most common. One study of 50 dogs with pericardial effusion confirmed that cytologic characterizations could not reliably distinguish between neoplasia and other underlying causes.48 Use of fluid pH to differentiate benign from neoplastic pericardial effusion in dogs remains controversial. Determining pH requires availability of an accurate portable pH meter and prompt analysis of sample supernatant as a bedside test. In humans, inflammatory pericardial effusions have a significantly lower mean pH than noninflammatory disorders. In dogs, idiopathic effusion tends to have a lower pH compared to neoplastic effusions, but there is broad overlap that thwarts clinical utility of pH as a diagnostic parameter. Measurement of serum cardiac troponin I (cTnI) may assist in differentiating idiopathic and neoplastic causes of pericardial effusion. Dogs with effusion secondary to neoplasia had a median value of 2.77 (range 0.09 to 47.18) ng/dl, contrasting with dogs with idiopathic pericardial effusion that had a median cTnI value of 0.05 (range 0.03 to 0.09) ng/dl. Notably, there was overlap in values between groups that compromises use of cTnI as a stand-alone diagnostic parameter.
Radiographs, ultrasonography, and CT imaging coupled with thoracoscopy or exploratory surgery are usually needed to differentiate benign from malignant disease. Pericardial biopsy performed during laparoscopic pericardectomy is the least invasive yet reliable method of tissue retrieval for definitive diagnosis. While this procedure may provoke lethal hemorrhage upon sampling of a highly vascular tumor, it remediates pericardial effusion by creating a pericardial window (fluid drains into the pleural space). Most cats with pericardial disease have cardiac disease (i.e., cardiomyopathy, valve abnormalities), neoplasia (i.e., lymphoma, metastatic carcinomas), chronic renal disease (uremic pericarditis), coagulopathies, or more rarely, bacterial infection or restrictive pericarditis. Intrapericardial cysts are a rare cause of pericardial effusion and tamponade. These are associated with a serosanguineous modified transudate or exudate in dogs; diagnosis is achieved by echocardiography. These lesions reflect entrapped omentum or falciform ligament in the pericardial structure during embryonic development and usually are asymptomatic unless associated with effusion.
Joint Effusions
The joint capsule is constructed of three layers: an outermost fibrous layer providing joint stability and flexibility, a subsynovium that serves as the source of joint fluid, and an inner synovial lining comprising two cell types: type A synoviocytes (macrophage-like cells) and type B synoviocytes (fibroblast-like cells that produce hyaluronic acid). Synoviocytes modify synovial fluid, provide viscous hyaluronic acid for joint lubrication, remove large molecules (i.e., plasma proteins), and participate in maintenance of articular cartilage. Synovial fluid, a plasma ultrafiltrate enriched with viscous hyaluronic acid important for joint mobility, nourishes articular cartilage and functions as a boundary lubricant for periarticular tissues.32 Synovial effusions reflect cartilage injury secondary to degenerative joint disease, trauma, immune-mediated mechanisms, and infection. On injury, chondrocytes and synoviocytes release cytokines that induce vasodilation of subsynovial capillaries leading to enhanced vascular permeability and extravasation of protein and inflammatory cells into the joint space. Inflammation is fostered by accumulated leukocytes and release of degradative enzymes and mediators from multiple cell types. Number and cellular composition of leukocytes infiltrating the synovium and migrating into synovial fluid determine characteristics of a joint effusion and clinical features of associated arthritis. Generally, arthropathies are considered inflammatory or degenerative, exudative or transudative, septic or nonseptic, hemorrhagic or nonhemorrhagic, and erosive or nonerosive, and are cytologically classified similar to other body cavity effusions with additional assessment of joint fluid viscosity.
Joint fluid should be analyzed in patients with swollen, fluctuant, or painful joints not historically associated with degenerative joint disease. Because joint pain may be subtle, joints should be carefully palpated for mobility, swelling, and discomfort. Atraumatic arthrocentesis requires familiarity with the anatomic landmarks of the involved joint. Synovial fluid analysis distinguishes noninflammatory from inflammatory conditions, but interpretation of findings must be integrated with realization that many immune-mediated and systemic inflammatory conditions also involve joints. Thus, joint inflammation does not indicate a primary polyarthropathy. Examination and bacterial culture of joint fluid may be helpful in animals with fever of unknown origin associated with sepsis or immune-mediated disorders. Assessment of joint fluid and interpretation of changes in appearance and cytology follow recommendations made for other body fluids with additional assessment of fluid viscosity (Figure 10-15 ).
FIGURE 10-15.
Diagnostic considerations in animals with joint effusion. ANA, Antinuclear antibodies; FeLV, feline leukemia virus; FIV, feline immunodeficiency virus; WBC, white blood cells.
Volume
Very small amounts of fluid are available from most normal joints; therefore collecting relatively large volumes of synovial fluid suggests effusion. Arthrocentesis in healthy animals commonly yields less than 0.1 to 0.25 ml. Joint disease can be accompanied by an increase or decrease in synovial fluid volume. Increased volume of synovial fluid is usually detected during physical examination as joint distention in acute and inflammatory joint conditions, whereas chronic and noninflammatory disorders produce joint enlargement due to soft tissue swelling or thickness despite normal to reduced joint fluid volume. When arthrocentesis is performed to evaluate fever or unexplained lameness in an animal without swollen joints, at least two or three joints should be sampled (carpal and tarsal usually preferred) because of expected low fluid yield.
Gross Appearance
Normal synovial fluid is clear, colorless, viscous, and free of flocculent debris. It does not clot, although it does demonstrate sol-gel reversibility on agitation (thixotropism).17, 32 Volume is noted subjectively. If greater than 0.25 ml is collected from a single joint, an aliquot should be placed in a pediatric EDTA tube (to avoid clotting) and in a sterile plain tube (for culture and mucin clot test). Fluid samples for cell counts and cytologic smears can be stored for 24 hours in a refrigerator, but cytologic smears should be promptly made. Homogeneously red or red-tinged fluid implicates joint hemorrhage associated with trauma, inflammation, or bleeding. Fluid containing “streaks” of blood or bleeding only at the end of centesis indicates hemorrhage subsequent to arthrocentesis, platelets should be seen cytologically. Xanthochromia (yellow-orange fluid discoloration) indicates prior hemorrhage and hemoglobin breakdown. White or light yellow fluid or sediment indicates increased nucleated cell counts associated with inflammation, sepsis, or neoplasia (rare). Increased fluid turbidity reflects suspended particulates, including RBCs, WBCs, infectious agents, fibrin, neoplastic cells (rare), and crystals (rare).
Viscosity
Viscosity of synovial fluid should be grossly assessed at the time of collection because this reflects the amount and polymerization of hyaluronic acid and joint lubrication. Decreased viscosity reflects reduced hyaluronic acid production associated with synovial membrane damage, dilution (plasma or fluid), degradation (by WBCs or bacteria), intra-articular injection of drugs, or joint lavage. Crude estimation of viscosity is done by visual observation: a drop of fluid suspended between thumb and opposing finger or suspended from a needle should form a strand at least 1 inch long before breakage. A more qualitative assessment is done using the mucin clot test conducted with synovial fluid collected in a plain or heparinized Vacutainer. This test is invalid in EDTA-preserved samples. A mixture of synovial fluid and 2.5% glacial acetic acid (1 part fluid:4 parts acid) is combined in a test tube, mixed well, and the precipitate semiquantitatively graded: good = tight ropy clot with clear solution, fair = soft clot with turbid solution, poor = friable clot with cloudy solution, and very poor = flocculent material in a cloudy solution.7 When only a few drops of fluid are collected, hyaluronic acid content (hence viscosity) can also be estimated crudely based on the density of the background staining observed microscopically.32 Normal viscosity is associated with a homogeneous pink, fine to coarsely granular background staining and numerous apparent crescents or folds of this material on cytology smears (Figure 10-16 ). Fluid viscosity is reduced in most types of joint effusion.
FIGURE 10-16.

Cytology of joint fluid from a dog. Note the pink-staining granular background, which represents the mucin in the joint fluid. This is a normal finding.
(Courtesy of Dr. Mark Johnson.)
pH
pH of synovial fluid can be determined using narrow-range pH paper immediately after collection (plain tube sample); normal pH ranges from 7.2 to 7.4. Experimental work shows a drop in pH shortly after onset of joint sepsis (to 6.9), but this has not been evaluated for diagnostic accuracy in clinical patients.
Total Protein
Total protein of synovial fluid is best measured using a quantitative biochemical method; however, refractometry is also used. A normal reference range of 1.5 to 3.0 g/dl is reported.32 An increased total protein concentration reflects inflammation and/or exudation of plasma proteins. However, excessive EDTA relative to joint fluid can falsely increase synovial fluid protein concentrations estimated by refractometry.
Fluid Analysis
Culture, nucleated cell counts, differential cell counts, and estimation of fluid viscosity are recommended (Table 10-5 ; see also Figure 10-15). Viscosity is reduced in most types of joint effusion. If only small amounts of fluid are obtained (e.g., drops), smears to determine relative cellularity and the predominant cell type have first priority. Determination of the total nucleated cell count/µl of fluid is a useful characteristic that helps differentiate inflammatory from noninflammatory arthropathies. Sequential arthrocentesis has been proposed for assessment of response to therapy, although repetitious sampling of a joint can lead to an increase in mononuclear cell count.
TABLE 10-5.
ANALYSIS OF JOINT EFFUSIONS
| Noninflammatory Joint Disease |
Inflammatory Joint Disease |
|||||
|---|---|---|---|---|---|---|
| DEGENERATIVE JOINT DISEASE | NEOPLASTIC JOINT INVOLVEMENT | HEMARTHROSIS | INFECTIOUS INFLAMMATION | NONINFECTIOUS INFLAMMATION | NORMAL | |
| Color | Light yellow | Light yellow–blood tinged | Bloody, xanthochromic | Variable: yellow, blood tinged, bloody | Variable: yellow, blood tinged | Straw colored |
| Turbidity | Clear–slightly turbid | Mild to moderate turbidity | Turbid | Turbid to purulent | Variable: slight to moderate turbidity | Clear |
| Viscosity | Normal | Normal to reduced | Reduced | Reduced | Reduced | Viscous |
| Mucin Clot Test | Normal firm | Normal firm | Normal to slightly friable | Friable | Friable | Firm |
| CYTOLOGY: | ||||||
| RBCs | Few | Few to many | Many Erythrophagocytosis | Moderate | Few to moderate | Rare |
| WBCs/µl | <3000 | Variable | Variable | 40,000–250,000 | Many but variable | 0–2900 |
| Neutrophils | Few (<20%) | Moderate | Moderate | Many (usually >90%) | Many but variable | 0–10% |
| Degenerative changes | Absent | Absent | Absent | May be present | None to mild | None |
| Lymphocytes | Few to moderate | Few to moderate | Rare | Few | Few to moderate | Few |
| Synoviocytes | Common | Few | Rare | Few to moderate | Few to moderate | Few |
| Macrophages | Few to moderate | Few to moderate | Moderate if chronic | Few to moderate | Few to moderate | Rare |
| Microorganisms | None | None | None | May be present | None | None |
| Neoplastic cells | None | Variable | None | None | None | None |
| Others | Blood contamination deduced by presence of platelets | May not visualize infecting bacteria | May see LE cells, tart cells, ragocytes | Blood contamination noted by blood streaks or blood near end of aspiration; platelets | ||
LE, Lupus erythematosus; RBCs, red blood cells; WBCs, white blood cells.
Total Nucleated Cell Count
Normal canine stifle joint fluid contains less than 3000 nucleated cells/µl, with less than 10% to 12% neutrophils (frequently <5%) in the absence of hemorrhage.32 Mononuclear cells account for the remainder of nucleated cells, comprising 60% to 97% of the total nucleated cell count. RBC quantification provides little useful information. Total nucleated cell counts can be determined by manual methods using a hemocytometer or by electronic cell counters with fluid treated before counting with hyaluronidase to eliminate problems associated with fluid viscosity (pipetting, dilution, acid precipitation of hyaluronic acid) and interactions with normal diluting fluids. One to two drops of a 150-U/ml hyaluronidase suspension added to a small aliquot of synovial fluid diminishes viscosity within a few minutes but increases the rate of cell sedimentation, requiring careful mixing before sample evaluations. Hyaluronidase treatment also invalidates the mucin clot test. Finding greater than 3000 WBCs/µl or that greater than 15% of cells are neutrophils indicates inflammation.
Cytologic Features
Cytologic interpretation of joint fluid is hampered by fluid viscosity, which causes cell aggregation and preparation of thick smears in which many nucleated cells appear pyknotic (small, darkly stained, difficult to recognize morphology). As a result, neutrophils may not be distinguishable from lymphocytes. The edge of the smear may be the only location where cell morphology may be distinguishable (area that dried quickly, thin specimen). Because RBCs are rare in normal joint fluid, their presence reflects joint trauma or hemorrhage associated with inflammation, or they are secondary to arthrocentesis. Iatrogenic hemorrhage will display platelets. The chronic presence of RBCs in synovial fluid results in erythrophagocytosis and accumulation of hemoglobin degradation pigments (blue/black granules in macrophages). Neutrophils, lymphocytes, monocytes, and macrophages may be identified in joint fluid from both normal and diseased joints. Normal joint fluid has one to three nucleated cells per high-power field (400×) where each nucleated cell represents approximately 1000 cells/µl; less than 10% of cells are neutrophils and these have a normal cytologic appearance. The remaining cells (90%) consist of small lymphocytes, monocytes, macrophages, and a few synoviocytes. Phagocytosed cytoplasmic debris is common in joint fluid from animals with degenerative joint disease and resolving or chronic inflammation. Infectious agents, including bacteria, fungi, and protozoa, are sometimes found within macrophages in septic arthritis. Infrequent but informative cells in different disorders include multinucleated giant cells, osteoclasts, ragocytes, and lupus erythematosus (LE) cells. Multinucleated giant cells are rarely observed but represent fusion macrophages; these have multiple round to oval nuclei in a gray granular cytoplasm and may demonstrate phagocytosed debris or organisms. Osteoclasts are 5 to 10 times the size of a neutrophil and have an irregular cell margin, abundant fine granular light blue-gray cytoplasm, as well as several round nuclei that contain singular nucleoli; these indicate cartilage damage with exposure of bone.32 Ragocytes are neutrophils containing small, round, purple, variably sized cytoplasmic granules; these are common in joint fluid from animals with inflammatory and immune-mediated arthritis. Cytoplasmic granules are thought to represent phagocytosed droplets of immune complexes.12 While these may be confused with phagocytosed coccoid bacteria, they are differentiated on the basis of granule size variability. LE cells found in patients with immune-mediated joint disease (e.g., systemic lupus erythematosus [SLE], rheumatoid arthritis) are phagocytes containing engulfed degenerate nucleoprotein with a homogeneous pink appearance that fills the cytoplasmic compartment. Tart cells may be confused with LE cells; these are neutrophils that display phagocytized nuclear material that retains the color and texture of normal chromatin. Tart cells can be seen in joint fluid associated with any inflammatory arthropathy.
Inflammatory Arthropathies
Inflammatory arthropathies share common characteristics of suppurative synovial inflammation (see Figure 10-15). Both infections and immune-mediated disorders generate similar pathologic responses that involve complement activation, vascular fluid leakage, inflammatory mediator release, and increased synovial fluid causing joint capsule distention and pain. Increased total protein, fibrin, and coagulation proteins may cause joint fluid to clot on collection if it is not promptly placed in an EDTA tube. While highest cell counts are encountered with septic effusions, there is broad overlap in neutrophil counts among dogs with septic and immune-mediated arthritis. Neutrophilic inflammation may also be seen in joints affected by degenerative arthritis with as many as 12,000 cells/µl and up to a 56% distribution of neutrophils. Neutrophil morphology may be well preserved in septic and nonseptic inflammatory arthritis, and identification of infectious agents may be difficult. Inflammatory arthritides are associated with turbid synovial fluids (high cell counts) ranging in color from yellow to orange. Septic joint effusion may appear yellowish-green reflecting large numbers of degenerating neutrophils. Such fluid has reduced viscosity secondary to enzymatic degradation of hyaluronic acid and glycoproteins, a mucin clot test ranging from poor to very poor, and diminished background staining on cytology preparations.
Immune-Mediated Arthropathies
Immune-mediated arthropathies are more common than infectious arthropathies in dogs and cats. Presence or absence of radiographic erosive lesions assists in subclassifying these disorders (see Figure 10-15). Erosive lesions should be followed up with submission of a rheumatoid factor (RF) titer in dogs. Rheumatoid factor is an antibody against the Fc portion of immunoglobulin G (IgG) and requires species-specific antisera. Synovial fluids from animals with immune-mediated arthritis have an increased total nucleated cell count with a neutrophilic or mixed inflammatory cell response. Ragocytes are common, whereas LE cells are rarely observed. Foamy macrophages are common and contain cell debris, RBCs, and disintegrating nuclei. Disintegrating cellular components can also be identified in the background. Finding LE cells and ragocytes supports a diagnosis of SLE-associated immune-mediated arthropathy. Owing to cyclic activity of immune-mediated arthritis, associated joint effusions are widely variable lending confusion to initial diagnosis and during sequential assessments by arthrocentesis. Repeated arthrocentesis for disease monitoring may increase mononuclear cell counts. Similar confusion exists in assessment of canine rheumatoid arthritis, an erosive immune-mediated disorder, because affected animals may present with either a predominantly neutrophilic or mononuclear joint inflammation. Lymphoplasmacytic synovitis, an immune-mediated arthropathy found in some dogs with anterior cruciate rupture and degenerative joint disease, is histologically characterized by synovial hyperplasia and nodular aggregates of lymphocytes. Joint fluid from affected individuals is characterized by a moderately increased cell count (5000 to 20,000/µl) with predominance of neutrophils or small lymphocytes.
Infectious Arthropathies
While most canine polyarthritis is nonspecifically immune mediated, it may reflect response to infection. Sepsis is suggested by degenerative neutrophils and is confirmed by observing or culturing an infectious agent. Neutrophils are not reliably degenerative in septic joint effusion. Perusal of a cytologic preparation should focus initially on clumps of cells and cells on the smear margin. While phagocytosed bacteria confirm septic arthritis, bacteria are only observed in approximately 50% of culture-positive fluid samples. Because joint sepsis and immune-mediated arthritis can produce similar total nucleated cell counts and distribution, bacterial cultures are important for identifying sepsis. If minimal amounts of fluid are aspirated, the syringe and needle can be rinsed with broth-enrichment medium and this rinse material cultured. Synovial fluid preincubated in blood culture medium for 24 hours may be more sensitive for detection of infectious agents (i.e., liquid blood culture medium is thought to prevent sample coagulation, dilutes bacterial growth inhibitors, inactivates or dilutes antibiotics, and curtails in vitro leukocyte phagocytosis of bacteria). If anaerobic bacteria are suspected, the sample must be protected from room air by prompt collection into anaerobic transport medium. If mycoplasma are suspected, specialized culture medium is necessary. In chronic low-grade infections, culturing synovial tissue biopsies may be more productive than joint fluid cultures. In cases in which cultures are negative but infection is still considered likely, blood and urine cultures may yield an infectious agent. Sudden detection of a cardiac murmur may herald the onset of infectious endocarditis and should be explored with echocardiography. Many dogs with septic joints have minimal or no degenerative neutrophils or negative cultures due to concurrent antibiotic therapy. Some infectious agents may not cause degenerative neutrophil changes and are not cytologically detectable (e.g., mycoplasma, rickettsia, L-form bacteria, viruses [calicivirus in cats, postvaccinal arthritides]). A pyogranulomatous response is usually associated with fungal and bacterial L-form infections (L-forms are bacteria lacking cell walls). Ehrlichia morulae are only found in very small numbers of neutrophils (~1%) in synovial fluid during the acute stages of infection. Protozoa (e.g., Leishmania), fungal hyphae (i.e., Aspergillus), or yeast forms (e.g., Blastomyces) of fungi causing systemic infections also may be observed in synovial fluid macrophages. Feline polyarthritis (i.e., chronic progressive polyarthritis) is statistically linked with feline syncytium-forming virus (FeSFV) and feline leukemia virus (FeLV) infections. Viral-induced arthritis may present either as a mild or marked mononuclear effusion, as observed in feline calicivirus or as a suppurative response. Omphalophlebitis may be the source of infection in neonatal animals. Serologic testing for borreliosis (i.e., Lyme disease) may help diagnose inflammatory arthritis of unknown cause (see Chapter 15). Response to therapy may be the most compelling basis for diagnosing Lyme disease or rickettsial polyarthropathies. However, administration of doxycycline for these agents also has an anti-inflammatory influence that may modulate joint pain and effusion in the absence of infection.
Exudative Joint Disease
Exudative joint disease without evidence of sepsis is categorized as erosive or nonerosive based on radiographs (see Figure 10-15). Rheumatoid arthritis is classically considered erosive. Nonerosive arthritides are more difficult to definitively diagnose and categorize. SLE and arthritis associated with a variety of underlying diseases are most common. Ragocytes, LE cells, or both indicate immune-mediated disease (Figure 10-17 ). Submission of sera for antinuclear antibody (ANA) and RF titers (see Chapter 12), blood for an LE cell prep, and synovial biopsies for histologic evaluation may implicate immuno-destructive processes. Chronic, progressive feline polyarthritis usually occurs in males and has two forms. A periosteal-proliferative form is most prevalent, primarily affecting young adult cats causing tenosynovitis followed by nondeforming periarticular periosteal proliferation and subchondral bone erosions. A deforming or rheumatoid-like arthritis form has an insidious onset in older cats where it is associated with severe subchondral bone destruction, joint instability, and deformity. Feet, carpi, and tarsi are severely and symmetrically affected.
FIGURE 10-17.

This canine synovial fluid smear has one large lupus erythematosus (LE) cell at the far left, which is a neutrophil containing a large round violet LE body that is composed of nuclear proteins from a dead lysed cell bound to antinuclear antibodies. Many other neutrophils have multiple, smaller inclusions that are probably antigen-antibody complexes, and these white blood cells are called ragocytes. Both are indicative of immune-mediated joint disease.
Noninflammatory/Degenerative Arthropathies
The most common cause of noninflammatory joint disease is degenerative arthritis secondary to trauma or joint instability. Other causes include hemarthrosis, neoplasia, genetic or developmental disorders, dietary or nutritional deficiencies or excesses, and miscellaneous causes (e.g., hypertrophic osteopathy). Noninflammatory arthritides (i.e., degenerative joint disease, traumatic joint injury, hemarthrosis, neoplastic joint conditions) are often characterized by low-grade synovial mononuclear inflammation. Fluid analysis may be normal or show only minor changes: mild vasodilation may slightly increase fluid volume and total protein content, causing mild dilution or no change in hyaluronic acid and fluid viscosity, and a normal or fair mucin clot test. Color of joint fluid in these conditions varies from clear and light yellow to bloody or xanthochromic, reflecting intra-articular hemorrhage. Total nucleated cell counts may be normal to mildly increased but are rarely greater than 5000 cells/µl. Cytologic features include a predominance of monocular cells and a normal to slightly increased number of neutrophils; total and differential cell counts must be reconciled with blood contamination. Mononuclear cells may appear enlarged and have an abundant foamy vacuolated cytoplasm consistent with phagocytic activity. Traumatic or coagulopathic hemarthrosis often produces erythrophagocytosis and hemosiderin-laden macrophages. Cartilage fragments, chondrocytes, and osteoclasts may indicate severe cartilage damage. Diagnosis of degenerative osteoarthritis is made by reconciling physical findings, radiographic images, and synovial fluid analyses. Bony changes evident radiographically indicate an advanced lesion associated with irreversible cartilage damage.
Edema and Anasarca
Edema is defined as a clinically evident increase in interstitial fluid volume. Anasarca refers to gross, generalized edema. Ascites refers to effusion in the abdominal cavity and hydrothorax to fluid accumulated in the pleural cavity. Depending on cause and etiopathogenesis, edema may be localized or generalized. Forces maintaining homeostatic balance of fluid distribution between the intravascular plasma volume and the interstitial fluid space are referred to as Starling's forces. Finding edema or anasarca signals imbalance of Starling's forces favoring fluid distribution into the interstitial compartment: (1) reduced colloid oncotic pressure (i.e., decreased plasma proteins), (2) altered hydrostatic pressure (i.e., opposing pressures within the interstitium and vasculature) favoring interstitial fluid accumulation, (3) reduced vascular integrity (i.e., leakiness of veins, arteries, capillaries, lymphatics), and/or (4) increased pathophysiologic signals conserving systemic water and sodium (e.g., enhanced angiotensin-converting enzyme activity, aldosterone and antidiuretic hormone [ADH] elaboration, systemic or splanchnic hypotension). To determine the cause of regionalized or local edema, consideration must be given to local anatomy of vasculature and soft tissues and the presence of local inflammatory, structural, or neoplastic conditions. A large number of disease processes can participate in edema formation and development of anasarca (FIGURE 10-18, FIGURE 10-19 ).
FIGURE 10-18.

Diagnostic considerations in animals with regional or localized edema. AV, Arteriovenous; CT, computed tomography.
FIGURE 10-19.

Diagnostic considerations in animals with anasarca. ADH, Antidiuretic hormone; CVP, central venous pressure; ECG, electrocardiogram.
Severe hypoalbuminemia leads to edema when TS concentration is less than 1.0 g/dl, whereas venous congestion, lymphedema, or inflammation generates edema fluid while TS concentration is greater than 2.5 g/dl. In the latter circumstance, total and differential cell counts may distinguish inflammatory (e.g., vasculitis) from noninflammatory causes.
Regional edema is usually caused by inflammation or vascular or lymphatic obstructions (see Figure 10-18). Lymphadenopathy is an indication for lymph node aspirates for cytology and culture. Congenital or acquired arteriovenous (AV) fistulae are rare but may cause localized edema. These may be detected on the basis of a palpable or auscultable fremitus or bruit, ultrasonography, or CT angiography. Acquired lymphatic insufficiency after trauma, surgery, or regional infections commonly causes regional edema. Lymphangiograms are usually not indicated in acute disorders with a plausible short-term duration. A fine-needle aspiration of involved tissues or regional lymph nodes and aspiration of edema fluid may help distinguish the underlying cause. Lymphatic cording is occasionally palpated in animals with lymphatic obstruction or inflammation; lymph fluid can be easily aspirated from such prominent lymphatics. Congenital malformation or degenerative lymphatic disorders may cause lymphedema in young animals; lymph nodes are sometimes atrophied or absent. Limb edema and tail edema are most commonly recognized. Affected animals develop overtly swollen appendages (single or multiple) but lack significant physical disability.
Generalized edema or anasarca is usually dependent, affecting distal extremities or the brisket (see Figure 10-19). Overhydration, right-sided congestive heart failure, and marked hypoalbuminemia are the most common causes (Box 10-5 ). Iatrogenic overhydration does not typically induce marked generalized edema unless another underlying factor (e.g., hypoalbuminemia, anuria/oliguria) coexists, an inappropriately large volume of fluids has been administered, or a concurrent disease condition or drug therapy has stimulated ADH release. Iatrogenic fluid overload imparts generalized edema associated with an obvious change in skin turgor (e.g., jelly-like consistency); morbidly obese patients have the highest risk when calculation of maintenance fluid requirements is based on gross body weight. Overhydration typically resolves within 48 hours of discontinuing fluid administration, although animals demonstrating cough or tachypnea may benefit from short-term furosemide administration. Animals with a normal serum albumin concentration that are highly sensitive to iatrogenic fluid overload may have incipient cardiac disease (e.g., high-output cardiac failure associated with severe anemia, chronic valvular insufficiency), anuric/oliguric renal failure, or disease processes or drug therapies stimulating excessive ADH release (syndrome of inappropriate antidiuretic hormone secretion [SIADH]).
Box 10-5. Conditions Associated with Anasarca.
Iatrogenic Overhydration
Excessive fluid volume calculated or administered
Fluid administration based on whole body weight in morbid obesity leads to overhydration (esp. cats)
Congestive Heart Failure
Failure of sodium restriction
If coupled with crystalloid fluid administration at normal maintenance rate
Increased body sodium and water retention driven by:
Increased ADH elaboration
Increased renin-angiotensin-aldosterone system activity
Acute Renal Failure
Anuria coupled with fluid administration
Hepatic Failure
Hypoalbuminemia
Portal hypertension
Increased body sodium and water retention driven by:
Increased ADH elaboration
Increased renin-angiotensin-aldosterone system activity
Nephrotic Syndrome
Hypoalbuminemia
Increased body sodium and water retention driven by:
Increased ADH elaboration
Increased renin-angiotensin-aldosterone system activity
Vasculitis
Increased vascular permeability: multiple causes
Immune-mediated (e.g., systemic lupus erythematosus)
Infectious diseases (e.g., rickettsial infections)
Hypersensitivity reactions
ADH, Antidiuretic hormone.
Serum albumin must be less than or equal to 1.5 g/dl to cause anasarca. This also may generate a pure transudative body cavity effusion. Patients with generalized edema associated with vasculitis usually demonstrate perivascular hemorrhage in some organ system (i.e., petechial hemorrhages, microhematuria, retinal hemorrhages) or microangiopathic effects (i.e., schistocytes, acanthocytes). Total body water retention secondary to SIADH (see Chapter 6) usually coexists with another disorder that overshadows its presence. SIADH is also induced by several drug therapies. With hypoalbuminemia, fluid retention is aggravated by sodium and water retention driven by the renin-angiotensin-aldosterone system, as occurs in severe renal, hepatic, or cardiac disease. Systemic hypertension often reflects abnormal activity of this system. History, physical examination, serum albumin determination, thoracic radiographs, cardiac evaluations (i.e., ECG, echocardiography), systemic blood pressure, CVP determination (not routinely used), urinalysis, abdominal ultrasonography, and CT imaging assist in identifying the cause of anasarca (see Figure 10-19). Finding a jugular pulse or abnormal hepatojugular reflex suggests intrathoracic disease within the cranial mediastinum or involving the heart or pericardium. If an underlying cause is not found, physical examination and laboratory data are reviewed looking for evidence of vasculitis (i.e., petechiation, microvascular lesions on fundic examination, microscopic hematuria, schistocytes on blood smear). Skin biopsies may be performed in areas of bruising or cutaneous lesions to investigate vasculitis; both affected and unaffected tissue and marginal interfaces should be sampled. Collecting edema fluid and analyzing its protein content may help implicate inflammatory causes.
Scrotal Effusions
Scrotal effusion usually develops when abdominal fluid enters the scrotum via the inguinal rings. Severe orchitis or testicular torsion may also be responsible and can be identified by ultrasonography and aspiration cytology. Because scrotal edema may be caused by vasculitis (e.g., Rocky Mountain spotted fever), evaluation for systemic infection is important. If effusion is localized to the scrotum, scrotal ablation and castration may be diagnostic and therapeutic.
Footnotes
After instillation of 20 ml/kg and mixing throughout peritoneal cavity.
References and Suggested Readings
- 1.Alleman AR. Abdominal, thoracic and pericardial effusions. Vet Clin North Am Small Anim Pract. 2003;33:89. doi: 10.1016/s0195-5616(02)00057-8. [DOI] [PubMed] [Google Scholar]
- 2.Aronson MG. Clinical and pathologic features of thymoma in 15 dogs. J Am Vet Med Assoc. 1984;184:1355. [PubMed] [Google Scholar]
- 3.Atilola M, Lumsden J, Rooke F. A comparison of manual and electronic counting for total nucleated cell counts on synovial fluid from canine stifle joints. Can J Vet Res. 1986;50:282. [PMC free article] [PubMed] [Google Scholar]
- 4.Berg R, Wingfield W. Pericardial effusion in the dog: a review of 42 cases. J Am Anim Hosp Assoc. 1984;20:721. [Google Scholar]
- 5.Berg RI, Sykes JE, Kass PH. Effect of repeated arthrocentesis on cytologic analysis of synovial fluid in dogs. J Vet Intern Med. 2009;23:814. doi: 10.1111/j.1939-1676.2009.0340.x. [DOI] [PubMed] [Google Scholar]
- 6.Bolton GR, Ettinger SJ. Peripheral edema. In: Ettinger SJ, editor. Textbook of veterinary internal medicine. ed 3. WB Saunders; Philadelphia: 1989. [Google Scholar]
- 7.Boon D. Synovial fluid analysis: a guide for small-animal practitioners. Vet Med. 1997;92:443. [Google Scholar]
- 8.Bowers R. Discussion on Miles RM & Jeck HS: experimental bile peritonitis. Surgery. 1953;34:445. [PubMed] [Google Scholar]
- 9.Braun JP, Guelfi JF, Pages JP. Comparison of four methods for determination of total protein concentrations in pleural and peritoneal fluid from dogs. Am J Vet Res. 2001;62:294. doi: 10.2460/ajvr.2001.62.294. [DOI] [PubMed] [Google Scholar]
- 10.Center SA. Pathophysiology of liver disease: normal and abnormal function. In: Guilford WG, editor. Strombeck's small animal gastroenterology. WB Saunders; Philadelphia: 1996. [Google Scholar]
- 11.Center SA. Proteins invoked by vitamin K absence and clotting times in clinically ill cats. J Vet Intern Med. 2000;14:292. doi: 10.1892/0891-6640(2000)014<0292:pibvka>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Davis MJ, Denton J, Freemont AJ. Comparison of serial synovial fluid cytology in rheumatoid arthritis: delineation of subgroups with prognostic implications. Ann Rheum Dis. 1988;47:559. doi: 10.1136/ard.47.7.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Day MJ, Martin MWS. Immunohistochemical characterization of the lesions of canine idiopathic pericarditis. J Small Anim Pract. 2002;43:382. doi: 10.1111/j.1748-5827.2002.tb00088.x. [DOI] [PubMed] [Google Scholar]
- 14.de Laforcade A, Rozanski E, Freeman L. Biochemical analysis of pericardial fluid and whole blood in dogs. J Vet Intern Med. 2005;19:833. doi: 10.1892/0891-6640(2005)19[833:baopfa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Duthie W, Eckersall PD, Addie DD. Value of α1-acid glycoprotein in the diagnosis of feline infectious peritonitis. Vet Rec. 1997;141:299. doi: 10.1136/vr.141.12.299. [DOI] [PubMed] [Google Scholar]
- 16.Edwards NJ. The diagnostic value of pericardial fluid pH determination. J Am Anim Hosp Assoc. 1996;32:63. doi: 10.5326/15473317-32-1-63. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez F, Grindem C, Lipowitz A. Synovial fluid analysis: preparation of smears for cytologic examination of canine synovial fluid. J Am Anim Hosp Assoc. 1983;19:727. [Google Scholar]
- 18.Fine DM, Tobias AH, Jacob KA. Use of pericardial fluid pH to distinguish between idiopathic and neoplastic effusions. J Vet Intern Med. 2003;17:525. doi: 10.1111/j.1939-1676.2003.tb02473.x. [DOI] [PubMed] [Google Scholar]
- 19.Forrester SD, Fossum TW. Pleural effusions: pathophysiology and diagnostic considerations. Compend Contin Educ. 1988;10:121. [Google Scholar]
- 20.Fossum TW. Chylothorax in cats: 37 cases (1969–1989) J Am Vet Med Assoc. 1991;198:672. [PubMed] [Google Scholar]
- 21.Fossum TW. Lymphedema: clinical signs, diagnosis, and treatment. J Am Vet Med Assoc. 1992;6:312. doi: 10.1111/j.1939-1676.1992.tb00361.x. [DOI] [PubMed] [Google Scholar]
- 22.Fossum TW, Birchard SJ, Jacobs RM. Chylothorax in 34 dogs. J Am Vet Med Assoc. 1986;188:1315. [PubMed] [Google Scholar]
- 23.Fossum TW, Jacobs RM, Birchard SJ. Evaluation of cholesterol and triglyceride concentrations in differentiating chylous and nonchylous pleural effusions in dogs and cats. J Am Vet Med Assoc. 1986;188:49. [PubMed] [Google Scholar]
- 24.Freeman K, Todhunter R, Lust G. Cytology of polychrome-stained equine synovial fluid. Acta Cytol. 1991;35:512. [PubMed] [Google Scholar]
- 25.George JA, O’Neill SL. Comparison of refractometer and biuret method of total protein measurement in body cavity fluids. Vet Clin Pathol. 2001;30:16. doi: 10.1111/j.1939-165x.2001.tb00250.x. [DOI] [PubMed] [Google Scholar]
- 26.Gibson N, Carmichael S, Li A. Value of direct smears of synovial fluid in the diagnosis of canine joint disease. Vet Rec. 1999;144:463. doi: 10.1136/vr.144.17.463. [DOI] [PubMed] [Google Scholar]
- 27.Gores BR. Chylous ascites in cats: nine cases (1978–1993) J Am Vet Med Assoc. 1994;205:1161. [PubMed] [Google Scholar]
- 28.Griffin D, Vasseur P. Synovial fluid analysis in dogs with cranial cruciate ligament rupture. J Am Anim Hosp Assoc. 1992;28:277. [Google Scholar]
- 29.Jackson J, Richter KP, Launer DP. Thoracoscopic partial pericardiectomy in 13 dogs. J Vet Intern Med. 1999;13:529. doi: 10.1892/0891-6640(1999)013<0529:tppid>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 30.Kolata RJ. Diagnostic abdominal paracentesis and lavage: experimental and clinical evaluations in the dog. J Am Vet Med Assoc. 1976;168:697. [PubMed] [Google Scholar]
- 31.Lozano MD. Immunocytochemistry in the differential diagnosis of serous effusions. Cancer Cytopathol. 2001;93:68. [PubMed] [Google Scholar]
- 32.MacWilliams PS, Friedrichs KR. Laboratory evaluation and interpretation of synovial fluid. Vet Clin North Am Small Anim Pract. 2003;33:158. doi: 10.1016/S0195-5616(02)00083-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marchevsky A, Read R. Bacterial septic arthritis in 19 dogs. Aust Vet J. 1999;77:233. doi: 10.1111/j.1751-0813.1999.tb11708.x. [DOI] [PubMed] [Google Scholar]
- 34.Martin MWS, Green MJ, Stafford-Johnson MJ. Idiopathic pericarditis in dogs: no evidence for an immune mediated etiology. J Small Anim Pract. 2006;47:387. doi: 10.1111/j.1748-5827.2006.00027.x. [DOI] [PubMed] [Google Scholar]
- 35.Miles RM, Jeck HS. Experimental bile peritonitis. Surgery. 1953;34:445. [PubMed] [Google Scholar]
- 36.Montgomery R, Long I, Milton J. Comparison of aerobic culturette, synovial membrane biopsy, and blood culture medium in detection of canine bacterial arthritis. Vet Surg. 1989;18:300. doi: 10.1111/j.1532-950x.1989.tb01089.x. [DOI] [PubMed] [Google Scholar]
- 37.O’Brien PJ, Lumsden JH. The cytologic examination of body cavity fluids. Semin Vet Med Surg. 1988;3:140. [PubMed] [Google Scholar]
- 38.Parra MD, Papasouliotis K, Ceron JJ. Concentrations of C-reactive protein in effusion in dogs. Vet Rec. 2006;158:753. doi: 10.1136/vr.158.22.753. [DOI] [PubMed] [Google Scholar]
- 39.Parry BW. Synovial fluid. In: Cowell RL, Tyler RD, Meinkoth JH, editors. Diagnostic cytology and hematology of the dog and cat. ed 2. Mosby; St. Louis: 1999. p. 104. [Google Scholar]
- 40.Pedersen NC, Pool RR, O’Brien T. Feline chronic progressive polyarthritis. Am J Vet Res. 1980;41:522. [PubMed] [Google Scholar]
- 41.Pembleton-Corbett JR, Center SA, Schermerhorn T. Serum-effusion albumin gradient in dogs with transudative abdominal effusion. J Vet Intern Med. 2000;14:613. doi: 10.1892/0891-6640(2000)014<0613:sagidw>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 42.Runyon BA, Montano AA. The serum-ascites albumin gradient is superior to the exudates-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992;117:215. doi: 10.7326/0003-4819-117-3-215. [DOI] [PubMed] [Google Scholar]
- 43.Rush JE, Keene BW, Fox PR. Pericardial disease in the cat: a retrospective evaluation of 66 cases. J Am Anim Hosp Assoc. 1990;26:39. [Google Scholar]
- 44.Shaw SP, Rush JE. Canine pericardial effusion: diagnosis, treatment, and prognosis. Compend Contin Educ Small Anim Pract. 2007;29:405. [PubMed] [Google Scholar]
- 45.Shaw SP, Rush JE. Canine pericardial effusion: pathophysiology and cause. Compend Contin Educ Vet. 2007;29:400. [PubMed] [Google Scholar]
- 46.Shaw P, Rozanski EA, Rush JE. Cardiac troponins I and T in dogs with pericardial effusion. J Vet Intern Med. 2004;18:322. doi: 10.1892/0891-6640(2004)18<322:ctiati>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 47.Shelly SM, Scarlett-Kranz J, Blue JT. Protein electrophoresis on effusions from cats as a diagnostic test for feline infectious peritonitis. J Am Anim Hosp Assoc. 1988;24:495. [Google Scholar]
- 48.Sisson D, Thomas WP, Ruehl WW. Diagnostic value of pericardial fluid analysis in the dog. J Am Vet Med Assoc. 1984;184:51. [PubMed] [Google Scholar]
- 49.Steyn PF, Wittum TE. Radiographic, epidemiologic, and clinical aspects of simultaneous pleural and peritoneal effusions in dogs and cats: 48 cases (1982–1991) J Am Vet Med Assoc. 1993;202:307. [PubMed] [Google Scholar]
- 50.Suess RP. Constrictive pleuritis in cats with chylothorax: 10 cases (1983–1991) J Am Anim Hosp Assoc. 1994;30:70. [Google Scholar]


