Abstract
Background
The type of pneumonia (coronavirus disease 2019, COVID-19) that is caused by the new coronavirus (severe acute respiratory syndrome coronavirus 2, SARS-CoV-2) is now spreading across the world in a pandemic. Many patients with COVID-19 were admitted to the University Hospital Aachen during an outbreak that first struck the Heinsberg district in February 2020.
Methods
A comparative presentation of the clinical features of the first 50 COVID-19 patients with and without acute respiratory distress syndrome (ARDS) who were hospitalized in the University Hospital Aachen.
Results
24 intubated patients were treated in the intensive care unit for ARDS of varying degrees of severity, while 26 patients who were breathing spontaneously without ARDS, but nevertheless needed supplemental oxygen, were treated in a separate isolation ward. The median age of the patients was 65 (IQR 58–76). The median latency from symptom onset to hospitalization was four days (IQR 1–8). Patients with ARDS had preexisting respiratory diseases more commonly than patients without ARDS (58% [95% confidence interval: 39; 76] versus 42% [26; 61]) and were more commonly overweight or obese (83% [64; 93] versus 42% [26; 61]). The two groups did not differ in viral burden but displayed significant differences in laboratory findings: ARDS patients had persistently elevated values for leukocytes, interleukin-6, lactate dehydrogenase, creatine kinase, and D-dimers over the period of observation. Patients without ARDS had persistently elevated inflammatory parameters and fever for at least one week, with an accompanying need for supplemental oxygen. Three of the patients with ARDS died of multiorgan failure, while four in the non-ARDS group died of respiratory insufficiency.
Conclusion
This initial description of a cohort of COVID-19 patients with and without ARDS in Germany reveals that those with ARDS more commonly have preexisting respiratory diseases and obesity, as well as persistently elevated inflammatory markers. COVID-19 patients without ARDS may likewise require prolonged hospitalization because of persistently elevated inflammatory values with a simultaneous need for supplemental oxygen.
In December 2019, an acute lung disease of unknown origin broke out in the Chinese province of Hubei. On 7 January 2020, Chinese scientists succeeded in isolating a new coronavirus (severe acute respiratory syndrome corona virus 2, SARS-CoV-2) and identifying it as the cause of the virus-induced pneumonia (1, 2). In line with the WHO‘s definition of the disease, it is now referred to as coronavirus disease 2019 (COVID-19) and, as a person-to-person droplet infection, is rapidly spreading worldwide (3). The clinical manifestation of the infection is highly variable, ranging from an asymptomatic or oligosymptomatic course to severe organ dysfunction and death. Initial symptoms include fever, non-productive cough, shortness of breath, myalgia, general fatigue, and occasionally also rhinorrhea, aching limbs, sore throat, and headache. Some patients develop severe lung failure (acute respiratory distress syndrome, ARDS), and in some cases also heart and kidney failure (4). According to the largest Chinese data set published to date, the mortality rate is between approximately 0.5% and 3% (taking into account laboratory-confirmed cases), with older individuals and those with comorbidities at higher risk of mortality (5, 6).
According to Wu et al., the development of ARDS in the setting of COVID-19 infection is a crucial factor for prognosis (7). Histopathologically, one sees diffuse alveolar damage caused by direct, viral cytotoxic effects on pneumocytes, as well as a concomitant inflammatory reaction. In addition, evidence is growing that some patients respond to the virus infection with a cytokine storm (8). In terms of clinical course, a distinction is currently made between two stages: During the repetitive stage, viral replication takes place over several days and, initially, the innate immune system mounts a response; this, however, is not sufficient to eliminate the virus. Mild symptoms may develop at this stage. This is then followed by the adaptive immune response, which is responsible for a decline in the virus titer. At the same time, however, one may see an increase in inflammatory mediators and cytokines, which in turn cause a clinical deterioration in the form of inflammatory tissue damage (9). A number of factors, such as age, hypertension, and diabetes as comorbidities, as well as the presence of coronary heart disease and lung disease, have been described and discussed as possible clinical predictors of severe disease (5, 6). In addition, certain laboratory parameters, such as persistently elevated white blood cell count or D-dimers, are associated with a poorer outcome. There is no causal treatment for COVID-19 to date; the search for factors associated with the course of the infection can help to better characterize the disease; ideally, predictors found in this way could also be helpful in clinical decision-making.
The German district of Heinsberg near Aachen is a region in Germany where, as early on as February 2020, a large number of individuals were infected and subsequently became ill. Therefore, the University Hospital of Aachen treated patients with COVID-19 of varying levels of severity very early on. This article compares the clinical characteristics of the first 50 COVID-19 patients, with and without ARDS, hospitalized at the Aachen University Hospital.
Methods
For the present study, we used the data on the first 50 patients hospitalized at the Aachen University Hospital during the period between February and March 2020 that tested positive for SARS-CoV-2 with initially obtained respiratory material. The 50 patients were divided into 24 patients with ARDS of varying severity (according to the Berlin definition and severity classification based on the degree of severity of hypoxemia [10]) and 26 patients without ARDS that were admitted to the normal (isolation) unit. Of the 50 patients, 16 were transferred to the Aachen University Hospital from external hospitals (14 patients with ARDS, two patients without ARDS).
In order to record comorbidities (including obesity, arterial hypertension, and existing respiratory disease), symptom onset, and start of hospitalization, medical reports from current and previous hospital stays, as well as self-reported history from non-intubated patients, were used. Overweight was documented at a body mass index of ≥ 25 and <30 kg/m2 and obesity at ≥ 30 kg/m2. The classification into diabetes or prediabetes was made on the basis of HbA1c values, i.e., >6.5% or 5.7–6.5%, respectively, or based on diagnoses previously recorded in the patient‘s medical records. Pre-existing medication was recorded on the basis of known regular medications and medication on admission. Febrile days were defined as the period from the onset of symptoms up to the final documented temperature over 38.5° C.
In order to represent vital parameters, the value medically deemed to be the worst for each parameter in the first 24 h was recorded 4 h following admission or intubation. The severity of ARDS was defined using the P/F ratio (Horowitz index). An index below 100 mmHg defined severe, below 200 mmHg moderate, and below 300 mmHg mild ARDS (10).
For pathogen diagnosis (viral, bacterial, fungal), bronchoscopy and bronchoalveolar lavage with a total volume of 160 ml was performed in all intubated patients. A portion of the lavage fluid was prepared for immunocytological analysis. In spontaneously breathing patients, the sputum obtained was used as sample material. Viral load was determined using real-time PCR (PCR, polymerase chain reaction) of respiratory material. The threshold cycle Ct represents the time at which the reaction enters the exponential phase of replication. It is inversely proportional to the viral concentration in patient material and reflects the relative differences in viral load in a logarithmic manner. Threshold cycles for the S-gene <20 were classified as high. Values >30 were considered low viral load; values between 20 and 30 were classified as moderate viral load. Serum, urine, and stool were also tested for SARS-CoV-2. The differential blood count obtained from the first blood sample following admission was analyzed for the presence of lymphocytopenia; this was defined as levels <22% (<25% microscopically) or <1.0/nL. In addition, other laboratory parameters regularly recorded over time, such as hemoglobin (Hb), white blood cell count, C-reactive protein (CRP), interleukin-6 (IL-6), lactate hydrogenase (LDH), D-dimers, creatinine kinase (CK), and creatinine, were also evaluated.
Initial instrument-based investigations—in this particular case chest X-rays and echocardiographic investigations—were also evaluated.
Results
The study population was made up of the first 50 COVID-19 patients hospitalized at the Aachen University Hospital. Of these patients, 60% came from the Heinsberg district. The median patient age was 65 years (interquartile range [IQR] 58–76); 34% were females. The main initial symptoms were fever, dyspnea, and cough. In all, 24 intubated patients were treated on an intensive care unit due to ARDS; 26 patients were treated outside an intensive care unit on an isolation unit. Four of the 26 patients without ARDS were initially admitted to the intensive care unit and later transferred from there to the isolation unit (table 1). For the total population, the median duration from onset of symptoms to hospitalization was 4 days (IQR 1–8). All affected patients had comorbidities (table 1). At the time of data collection, patients were hospitalized for a median of 8 days (IQR 5–11); eight patients could be discharged (all from the non-ARDS group; median duration of hospital stay: 7 days [IQR 6–11]); seven patients died (three in the ARDS group of multi-organ failure; four in the non-ARDS group of respiratory insufficiency [Table 1]—in these cases, the patients/relatives declined intensive care treatment).
Table 1. Patient characteristics.
| N (%) | |||
| All patients (N = 50) | ARDS patients (n = 24) | Non-ARDS patients (n = 26) | |
| Age—years: median (IQR) | 65 (58–76) | 62 (58–70) | 68 (59–81) |
| Female sex | 17 (34) | 9 (38) | 8 (31) |
| Comorbidities | |||
| Total | 50 (100) | 24 (100) | 26 (100) |
| Arterial hypertension | 35 (70) | 16 (67) | 19 (73) |
| Obesity (BMI ≥ 30 kg/m2) | 17 (34) | 11 (46) | 6 (23) |
| Overweight (BMI ≥ 25 kg/m2, <30 kg/m2) | 14 (28) | 9 (38) | 5 (19) |
| Diabetes mellitus | 29 (58) | 15 (63) | 14 (54) |
| Prediabetes | 13 (26) | 6 (25) | 7 (27) |
| Pre-existing respiratory disease | 25 (50) | 14 (58) | 11 (42) |
| – Chronic obstructive pulmonary disease | 11 (22) | 6 (25) | 5 (19) |
| – Obstructive sleep apnea syndrome | 7 (14) | 2 (8) | 5 (19) |
| – Bronchial asthma | 6 (12) | 4 (17) | 2 (8) |
| – Other pulmonary diseases | 16 (32) | 10 (42) | 6 (23) |
| Chronic kidney failure | 10 (20) | 4 (17) | 6 (23) |
| Nicotine abuse | 8 (16) | 2 (8) | 6 (23) |
| – Former nicotine abuse | 5 (10) | 2 (8) | 3 (12) |
| – Continued nicotine abuse | 3 (6) | 0 (0) | 3 (12) |
| Cerebral arterial occlusive disease | 7 (14) | 4 (17) | 3 (12) |
| Cancer | 7 (14) | 4 (17) | 3 (12) |
| Chronic hepatitis | 5 (10) | 2 (8) | 3 (12) |
| Chronic liver failure | 4 (8) | 0 (0) | 4 (15) |
| Peripheral arterial occlusive disease | 1 (2) | 0 (0) | 1 (4) |
| Pre-existing medication | |||
| Non-steroidal anti-inflammatory drugs | 5 (10) | 1 (4) | 4 (16) |
| ACE inhibitors | 12 (24) | 5 (20) | 7 (28) |
| Angiotensin-receptor blockers | 17 (34) | 9 (36) | 8 (32) |
| Antibiotic agent | 17 (34) | 7 (28) | 10 (40) |
| Antiviral drug | 4 (8) | 3 (12) | 1 (4) |
| Diuretics | 23 (46) | 8 (32) | 15 (60) |
| Systemic glucocorticoids | 10 (20) | 5 (20) | 5 (20) |
| Immunosuppressive drugs | 4 (8) | 1 (4) | 3 (12) |
| Inhalation drugs | 14 (28) | 6 (24) | 8 (32) |
| Lipid-lowering agents | 18 (36) | 9 (36) | 9 (36) |
| Initial symptoms | |||
| Fever | 41 (82) | 23 (92) | 18 (72) |
| Cough | 21 (42) | 11 (44) | 10 (40) |
| Rhinorrhea | 1 (2) | 1 (4) | 0 (0) |
| Sore throat | 2 (4) | 1 (4) | 1 (4) |
| Pharyngalgia | 1 (2) | 0 (0) | 1 (4) |
| Dyspnea | 24 (48) | 16 (64) | 8 (32) |
| Angina | 2 (4) | 1 (4) | 1 (4) |
| Myalgia | 6 (12) | 1 (4) | 5 (20) |
| Headache | 1 (2) | 0 (0) | 1 (4) |
| Fatigue | 6 (12) | 0 (0) | 6 (24) |
| Gastrointestinal symptoms | 9 (18) | 7 (28) | 2 (8) |
| – Diarrhea | 8 (16) | 6 (24) | 2 (8) |
| – Vomiting | 2 (4) | 1 (4) | 1 (4) |
| – Nausea | 1 (2) | 0 (0) | 1 (4) |
| Symptom onset to—in days: median (IQR) | |||
| Hospitalization | 4 (1– 8) | 7 (2– 10) | 3 (1– 7) |
| Intensive care treatment | 9 (4– 12) | 10 (4– 12) | 5 (0–7) |
| Course | |||
| Ventilation | – | 24 (100) | – |
| – Currently | – | 17 | – |
| Extracorporeal membrane oxygenation | – | 8 (33) | – |
| – Currently | – | 6 | – |
| Prone position | – | 17 (71) | – |
| Hemodialysis | – | 11 (46) | – |
| Antibiotic therapy | 31 (62) | 20 (83) | 11 (42) |
| Duration in days: median (IQR) | |||
| Febrile days | 10 (4–16) | 19 (13–24) | 7 (3– 10) |
| Hospitalization | 8 (5– 11) | 8 (5–15) | 7 (6– 11) |
| Treatment | |||
| – Intensive care treatment | 4 (2– 10) | 13 (6–19) | 2 (2– 3) |
| – Ventilation | – | 10 (7– 13) | – |
| – Extracorporeal membrane oxygenation | – | 7,5 (6– 9) | – |
| – Oxygen therapy | – | – | 8 (3– 11) |
| Outcome | |||
| Discharged | 8 (16) | 0 (0) | 8 (31) |
| Died | 7 (14) | 3 (13) | 4 (15) |
| Continued hospitalization | 35 (70) | 21 (88) | 14 (54) |
ARDS, acute respiratory distress syndrome; BMI, body mass index; IQR, interquartile range
Patients that developed ARDS more frequently had a pre-existing respiratory disease (58% versus 42%) and were more frequently obese (46% versus 23%) or overweight (38% versus 19%) compared to those without ARDS. There was no significant difference in relation to other comorbidities or to concomitant medication (table 1). In the ARDS group, 71% required complex ventilation in the prone position and 46% required hemodialysis for acute kidney failure. To date, eight of the ARDS patients required extracorporeal membrane oxygenation (ECMO); all non-ARDS patients required oxygen therapy. Antibiotic therapy was administered to 83% of the group with ARDS and 42% of the group without ARDS. Due to the current lack of evidence, no specific pharmacotherapy was administered.
Table 2 shows the vital parameters of patients 4 h following admission; the value medically deemed to be the worst in the first 24 h was recorded. The median body temperature in patients with ARDS was somewhat higher compared to those without ARDS (38.5 °C [37.8–38.9] versus 37.9 °C [36.8–38.5]). Interestingly, there was no significant difference in viral load between patient groups; bacterial superinfection of the lungs was seen in three patients in the ARDS group and in one patient in the non-ARDS group (table 2). Echocardiography revealed overall normal systolic pump function in 89% of patients with ARDS (table 2).
Table 2. Diagnostics.
| N (%) | |||
| All patients (N = 50) | ARDS patients (n = 24) | Non-ARDS patients (n = 26) | |
| Vital parameters—median (IQR) | |||
| Height (cm) | 175 (165–180) | 180 (164–180) | 173 (165–178) |
| Weight (kg) | 88 (75–98) | 90 (82–100) | 81 (71–89) |
| BMI (kg/m2) | 29 (25–31) | 29 (27–32) | 27 (24–30) |
| Respiratory rate (1/min) | 22 (18–26) | 24 (21–30) | 20 (16–23) |
| Oxygen saturation (%) | 95 (90–97) | 91 (88–97) | 95 (93–96) |
| Oxygen requirement (L/min) | 2 (0–6) | 0 (0–4) | 2 (0–2) |
| Temperature (°C) | 38.2 (37.3–38.7) | 38.5 (37.8–38.9) | 37.9 (36.8–38.5) |
| RRsys (mmHg) | 110 (94–128) | 95 (86–110) | 127 (110–140) |
| RRdia (mmHg) | 63 (53–76) | 55 (47–73) | 71 (60–80) |
| Heart rate (1/min) | 85 (75–94) | 90 (81–95) | 81 (74–88) |
| Echocardiography—(n = 19) | |||
| Left ventricular ejection fraction—overall normal | – | 17 (89) | – |
| Chest X-ray (n = 38) | |||
| Unilateral infiltrates | 3 (8) | 0 (0) | 3 (8) |
| Bilateral infiltrates | 35 (92) | 23 (61) | 12 (32) |
| Viral load (n = 48) | |||
| High | 11 (23) | 7 (14) | 4 (8) |
| Medium | 26 (54) | 12 (24) | 14 (27) |
| Low | 11 (23) | 5 (10) | 6 (12) |
| Virus detection | |||
| Respiratory material | 50 (100) | 24 (100) | 26 (100) |
| Serum (n = 22) | 11 (50) | 6 (27) | 5 (10) |
| Stool (n = 15) | 2 (13) | 1 (7) | 1 (2) |
| Urine (n = 24) | 7 (29) | 4 (17) | 3 (6) |
| Detection of other pathogens—respiratory material | |||
| Bacterial | 4 (8) | 3 (13) | 1 (4) |
| Fungal | 0 (0) | 0 (0) | 0 (0) |
| SOFA score | – | 8 (6– 9) | – |
| ARDS severity | |||
| Severe | – | 8 (35) | – |
| Moderate | – | 15 (65) | – |
| Mild | – | 0 (0) | – |
| Ventilation parameters, first 24 h—Median (IQR) | |||
| Max. fraction of inspired oxygen (FiO2) (%) | – | 65 (58–80) | – |
| Lowest P/F ratio (mmHg) | – | 116 (100–141) | – |
| Max. positive end-expiratory pressure (PEEP) (mbar) | – | 13 (12–15) | – |
| Lowest compliance (mL/cm H2O) | – | 39 (30–49) | – |
| Highest resistance | – | 13 (11–15) | – |
ARDS, acute respiratory distress syndrome; IQR, interquartile range; SOFA score, sepsis-related organ failure assessment score
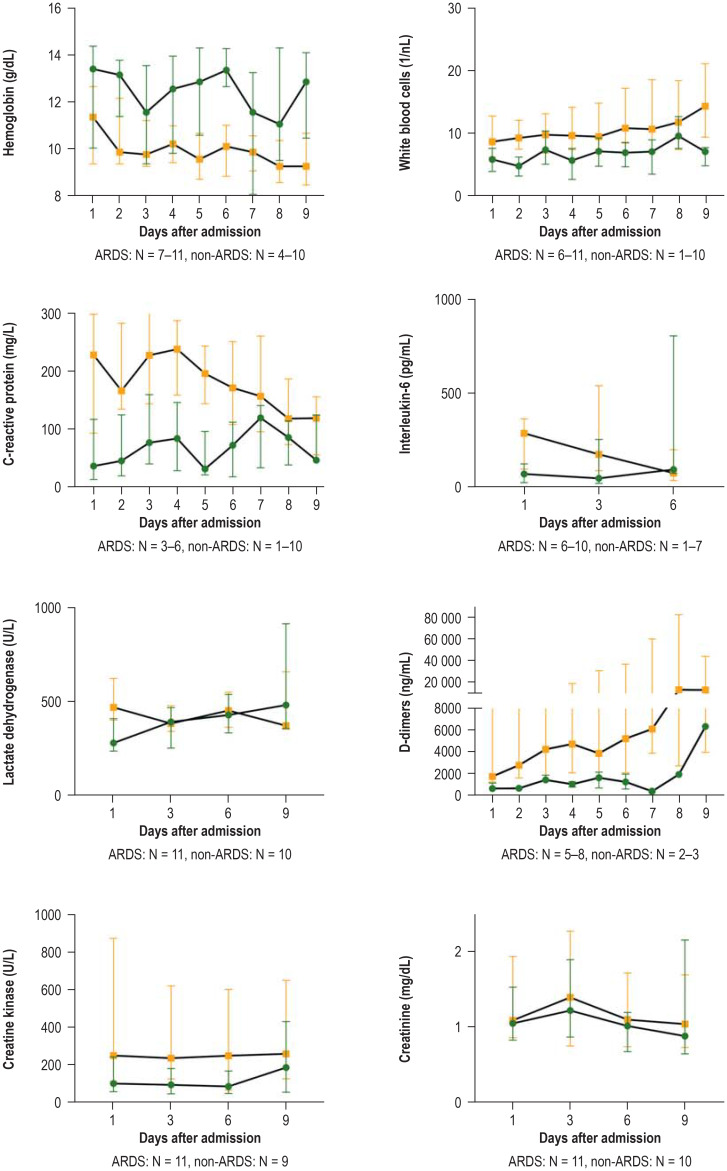
There were significant differences between the two groups in terms of the laboratory parameters analyzed on admission to the Aachen University Hospital. The ARDS group had higher white blood cell counts, higher CRP and IL-6 levels, higher serum levels of LDH and creatine kinase (CK), as well as higher D-dimer levels compared to patients without ARDS (table 3). The eFigure shows the dynamic trend in laboratory parameters over the course of hospital stay for both groups. The ARDS group exhibited persistently elevated levels of white blood cells, IL-6, LDH, CK, and D-dimers over the observation period of 6–7 days. Up until day 5 following admission, ARDS patients showed markedly elevated CRP values, which fell only in the further course (efigure).
Table 3. Laboratory parameters on admission.
| Median (IQR) | ||||
| Normal values | All patients (N = 50) | ARDS patients (n = 24) | Non-ARDS patients (n = 26) | |
| White blood cells (1/nL) | 4–10/nL | 6.7 (4.9–9.6) | 9.2 (6.3–11.6) | 5.8 (4.1–7) |
| Hemoglobin (g/dL) | M: 14–18 g/dL | 12.2 (10–13.8) | 11.4 (9.6–12.8) | 13.4 (10.1–14.2) |
| F: 12–16 g/dL | ||||
| D-dimers (ng/mL) | <500 ng/ml | 1682 (1 070–3 864) | 1986 (1 272–5 139) | 587 (475–837) |
| Creatine kinase (U/L) | M: <174 u/l | 134 (65–384) | 245 (120–754) | 96 (53–224) |
| F: <140 u/l | ||||
| Lactate dehydrogenase (U/L) | M: 135–225 U/L | 388 (263–491) | 444 (377–570) | 274 (234–369) |
| F: 135–214 U/L | ||||
| Creatinine (mg/dL) | 0.5–1.2 mg/dL | 1.1 (0.9–1.9) | 1.1 (0.8–1.9) | 1.2 (0.9–1.9) |
| C-reactive protein (mg/L) | <5 mg/l | 94 (28–173) | 110 (7–264) | 37 (15–113) |
| Procalcitonin (ng/mL) | <0.005 ng/ml | 0.2 (0.1–0.7) | 0.6 (0.2–4.5) | 0.1 (0.1–0.1) |
| Interleukin-6 (pg/mL) | ≤ 7.0 pg/mL | 122 (68–333) | 119 (47–338) | 10 (0–60) |
ARDS, acute respiratory distress syndrome; IQR, interquartile range
eFigure.
Representation of laboratory parameters over time.
For each parameter, sample number N refers to the smallest and largest sample size in each case; i.e., the number of available values differed on the individual days, but always refers to the same core of patients.
Patients with ARDS (acute respiratory distress syndrome) are marked in orange with boxes, non-ARDS patients in green with circles.
Due to the low case number and lack of normal distribution, data are presented as median with interquartile range (IQR).
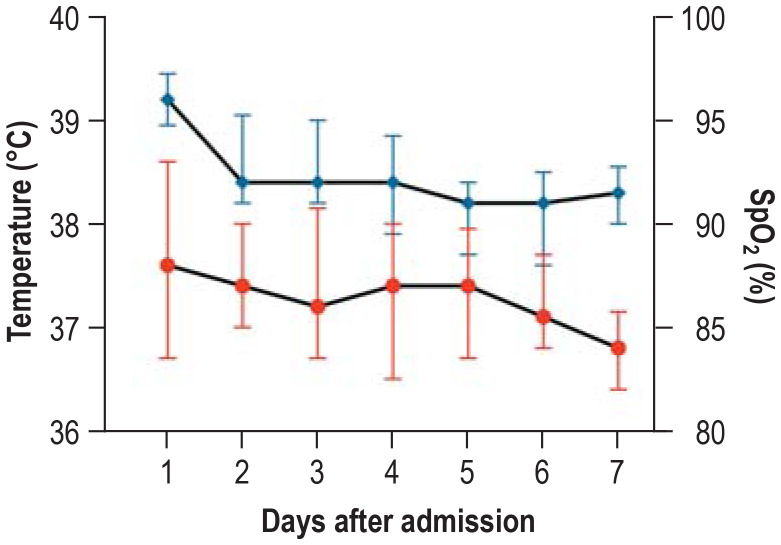
Patients without ARDS exhibited lower CRP levels compared to the ARDS group, but here, too, no normalization was seen during the observation period of 6–7 days. Body temperature fell from the third day following admission. However, all patients on the isolation unit required oxygen at all times throughout the entire observation period (figure).
Figure.
Vital parameters in non-ARDS patients over time (N = 16–26). Oxygen saturation (SpO2) under moderate oxygen therapy of 2 ± 2 L/min in blue with diamonds; body temperature in red marked with circles. Due to the low case number and lack of normal distribution, data are presented as median with interquartile range (IQR).
Discussion
The data presented here represent, to the best of our knowledge, the first case series in Germany of COVID-19 patients with ARDS compared to patients without ARDS. The main findings of our study suggest that:
Patients with pre-existing respiratory disease, as well as patients with obesity, are at higher risk for developing ARDS in the setting of COVID-19.
There is no difference in the extent of viral load between patients with and patients without ARDS.
Patients with ARDS are characterized in terms of laboratory parameters by persistently elevated white blood cell counts and elevated levels of CRP, IL-6, LDH, CK, and D-dimers.
Hospitalized COVID-19 patients without ARDS exhibit elevated inflammatory markers and body temperature, as well as a persistent oxygen requirement, over a period of 1 week.
The patient population described here shows some differences to the larger case series from China published to date (5, 6). Patients in the Chinese cohorts were on average younger at 56 years of age and only 45%–50% had comorbidities. At 65 years of age, our collective is older and all patients had pre-existing comorbidities. In particular, half of all patients, and 58% of patients with ARDS, had a pre-existing respiratory disease. The duration from the onset of symptoms to hospitalization was shorter in our collective at 4 days compared to the duration in the Chinese case series (7 and 11 days, respectively). The differences in patient characteristics could be attributable to: ethnic differences and varying living conditions or lifestyles; different health systems and patient treatment pathways; a differing initial test rate to SARS-CoV-2 and the corresponding consequences; or to the particular status of the Aachen University Hospital as a tertiary referral center to which severely ill patients are transferred.
In their multicenter collective of 191 hospitalized COVID-19 patients in China, Zhou et al. identified older age, higher SOFA score, and a D-dimer level >1 µg/L as potential risk factors for increased intrahospital mortality (5). Another study from China on 138 hospitalized patients with COVID-19 compared the characteristics of patients that required treatment on an intensive care unit with those that did not require intensive care treatment. Patients on the intensive care unit were on average older and exhibited elevated levels on admission for the laboratory parameters white blood cell count, D-dimers, LDH, creatinine, and highly sensitive troponin I (6). In an Italian collective of 1625 patients, the highest COVID-19-related mortality rate was seen in the group aged ≥ 80 years (11). Our study compares, for the first time, patients with and without ARDS and reveals that pre-existing respiratory disease and obesity were more frequently present in patients with ARDS. There was no difference in terms of age and the presence of arterial hypertension and diabetes between the two groups in our collective—in contrast to the groups in China, in which these comorbidities were seen more frequently in cases of severe disease. In a group of 21 COVID-19 patients requiring intensive care in the US, a total of 86% of patients had comorbidities, with chronic kidney failure, heart failure, and COPD being the most common concomitant diseases (12).
Interestingly, the presence of ARDS in our collective was not associated with a higher viral load. This contrasts with the data from Liu et al. in China, which suggest that severe cases are associated with an increased viral load (13); however, the analysis carried out there did not specifically investigate the association between viral load and the development of ARDS. There is not always a correlation between viral load and severity of the clinical picture; this is more likely due to, among other things, the disease stages, the material (for example, blood, sputum, or lavage, etc.), the collection of material, as well as the timing of determination in the course of the disease.
Patients with ARDS exhibited persistently and markedly elevated levels of white blood cells, CRP, IL-6, LDH, CK, and D-dimers. These findings are in accordance with the observations made in the above-mentioned Chinese studies, in which severe cases were associated with persistently elevated D-dimer, IL-6, and LDH levels, as well as elevated white blood cell counts. However, our findings expand current knowledge in the sense that persistently elevated CRP and CK levels in a patient collective with severe disease, like those with ARDS, had not been previously described. Added to this is the fact that bacterial superinfection was diagnosed in only a very small number of patients. In this respect, the severe lung damage and resulting markedly elevated inflammatory markers can be attributed to the viral disease alone. The majority of ARDS patients in our collective required a complex ventilation strategy including prone positioning and, frequently, dialysis for acute kidney failure; a proportion of patients required ECMO due to severe respiratory failure. Patients without ARDS also exhibited elevated CRP levels for several days, although less markedly compared to ARDS patients; furthermore, these patients exhibited elevated body temperature and oxygen dependency for at least 6 days. The latter findings could be relevant in the estimation of how long COVID-19 patients need to be hospitalized on non-intensive care units, since they suggest that these patients also require inpatient care for a prolonged period.
Our study has a number of limitations: Since the case number of COVID-19 infected patients was small, the data collected in this study can only be viewed as hypothesis-generating and need to be verified or falsified in larger cohorts. A further limitation lies in the fact that the comparison of the two groups involves patients at different stages of the disease, of which a large proportion of the ARDS patients in particular were transferred to the Aachen University Hospital from other hospitals. Furthermore, a number of patients were still hospitalized at the time of data analysis, meaning that the final clinical outcome cannot be evaluated for all patients. Nevertheless, the study looks at the largest collective of SARS-CoV-2-infected patients in Germany to date and provides the first description of clinical and laboratory characteristics of hospitalized patients with and without ARDS.
Key messages.
COVID-19 patients with ARDS have a higher prevalence of pre-existing respiratory disease and obesity; they are characterized by persistently elevated inflammatory markers.
In terms of intensive care treatment, many of these patients require a complex ventilation strategy including prone positioning and, in some cases, extracorporeal membrane oxygenation (ECMO).
COVID-19 patients without ARDS also require longer hospitalization due to persistently elevated inflammatory markers and fever combined with the requirement for oxygen therapy.
These results need to be confirmed in large collectives. They could be helpful in the risk stratification as well as in the assessment of the clinical course of COVID-19 patients.
Acknowledgments
Translated from the original German by Christine Rye.
Footnotes
Conflict of interests
The authors state that no conflict of interest exists
References
- 1.Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: Challenges for Global Health Governance. JAMA. 2020 doi: 10.1001/jama.2020.1097. doi: 10.1001/jama.2020.1097. [DOI] [PubMed] [Google Scholar]
- 2.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for disease control and prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.Kluge S, Janssens U, Welte T, Weber-Carstens S, Marx G, Karagiannidis C. [Recommendations for critically ill patients with COVID-19] Med Klin Intensivmed Notfmed. 2020 doi: 10.1007/s00063-020-00674-3. doi: 10.1007/s00063-020-00674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 doi: 101007/s00134-020-05991-x doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020 doi: 10.1001/jama.2020.3204. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. Jama. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 11.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4683. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 12.Arentz M, Yim E, Klaff L, et al. Characteristics and Outcomes of 21 critically Ill patients with COVID-19 in Washington State. JAMA. 2020 doi: 10.1001/jama.2020.4326. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Yan LM, Wan L, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30232-2. pii: S1473-3099(20)30232-2 doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]