Abstract
The coronavirus disease (COVID-19) is highly pathogenic viral infection caused by SARS-CoV-2. Currently, COVID-19 has caused global health concern. It is assumed that COVID-19 has zoonotic origin based on the large number of infected people who were exposed to the wet market in Wuhan City, China. The phylogenetic analysis has revealed that SARS-CoV-2 has significant sequence similarity with severe acute respiratory syndrome-like (SARS-like) bat viruses, thus bats could be primary possible reservoir. The intermediate host and there subsequent transfer is not known yet, although human to human transfer is widely confirmed. The transmission of COVID-19 infection from one person to another resulted in the isolation of patients who were subsequently given a variety of treatments. To monitor the current outbreak, robust steps have been taken around the globe to reduce the transmission of COVID-19 infection particularly banning international and domestic flights, inducting lockdowns in vulnerable areas, social distancing etc. No clinically approved antiviral drug or vaccine against COVID-19 is reported yet. However, in clinical trials, few broad-spectrum antiviral drugs were evaluated against COVID-19 infection which resulted in clinical recovery. In this article emergence and pathogenicity of COVID-19 infection along with potential therapeutic strategies are analyzed to combat the COVID-19 pandemic.
Keywords: COVID-19, Pathogenesis, Phylogenetic analysis, Therapeutic strategies, Transmission
Introduction
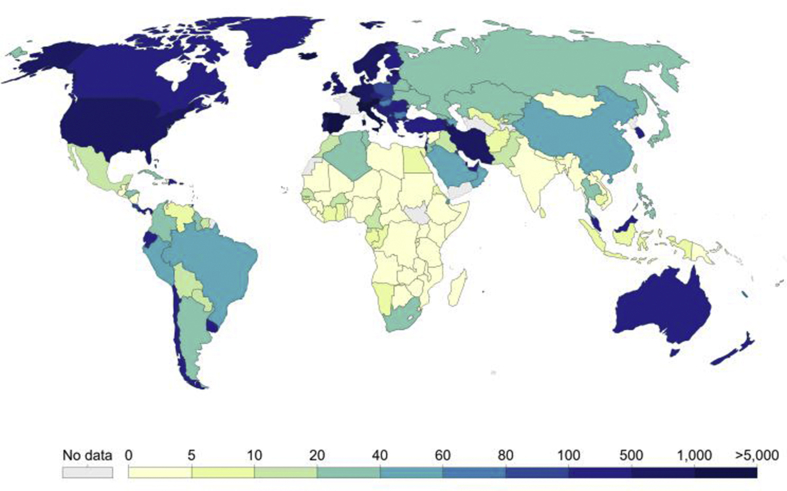
Coronavirus is one of the major pathogens which targets primarily the human respiratory system. Earlier coronaviral outbreaks (CoVs) include Middle East respiratory syndrome (MERS)-CoV and severe acute respiratory syndrome (SARS)-CoV which have significantly caused great threat to human beings. In December 2019, a number of patients were admitted to hospitals with an initial diagnosis of pneumonia. These patients were found to have epidemiological links with wet animal and seafood wholesale market in Wuhan, Hubei Province, China [1,2]. Early studies predicted the onset of a possible coronavirus outbreak. The WHO on 11th February 2020 estimated the reproduction number for COVID-19 significantly largely ranging between 2.24 to 3.58 [3]. In December 2019 the first cases for COVID-19 infections were reported [4]. From 18th December, 2029 to 29th December 2019, 5 patients having acute respiratory distress syndrome were hospitalized among which one patient died [5]. Upto 2 January 2020, 41 patients were identified to possess laboratory confirmed COVID-19 infection. Almost half of these patients had underlying diseases particularly cardiovascular diseases, hypertension and diabetes [6]. It was believed that these patients were infected in hospital possibly due to nosocomial infection. Thus it was concluded COVID-19 is not a super-hot spreading virus (spread to many others from one patient) but it most likely spread as many patients get infected by unknown mechanisms at different locations in the hospital. Furthermore only those patients were tested that were clinically sick and probably there were several more patients who were infected. As of January 22, 2020 a total of 571 COVID-19 infection cases were reported in 25 provinces of China [7]. Upto 22 January 2020 China National Health Commission reported the details of first 17 deaths. On 25th January 2020 a total of 1975 COVID-19 infection cases were confirmed along with 56 deaths in mainland China [8]. Another report of 24th January 2020, estimated 5502 COVID-19 infections in China [9,10]. The first COVID-19 infection case was reported on 10th January 2020 which was led by identification, description, clinical diagnosis and management of the case. It included initial mild symptoms of the patient which leaded to pneumonia on 9th day of illness [11]. Moreover on 30 January 2020 first case of COVID-19 human-to-human transmission was reported in US (https://www.cdc.gov/media/releases/2020/p0130). Until 5th April 2020, total number of COVID-19 infected cases in USA mounted to 311,635 surpassing the infected cases of Italy (124,632) and China (81,669). Out of these 288,356 cases are active and 8454 are death cases while as rest i.e. 14825 cases have recovered (https://www.worldometers.info/coronavirus/). A study published in Nature reported that Chinese health authorities concluded that 31,161 people had contracted the infection in China as of 7 February 2019, and that more than 630 people had died of the infection (http://www.natur.com/articles/d41586-020-00154). On 5th April 2020, total number of infected cases in Italy has reached to 124,632 out of which 20,996 have recovered and 88,274 cases are active. Out of these 15,362 are death cases (https://www.worldometers.info/coronavirus/). Currently total number of corona virus cases in India reported so far 3374. So far there are 77 COVID-19 deaths cases reported in India. At the time of preparation of this manuscript i.e. 5th April 2020 the WHO has reported 1056159 COVID-19 confirmed cases throughout the world including 57,206 confirmed death cases. So far COVID-19 has spread to 208 countries around the globe (https://www.who.int/emergencies/diseases/novel-coronavirus-2019). The number of confirmed COVID-19 cases per million people around the globe as on 4th April, 2020 is represented in Fig. 1.
Fig. 1.
Total confirmed cases of COVID-19 per million people around the globe as on 4th April, 2020 (European CDC).
Symptoms
The COVID-19 infection symptoms starts to appear after an incubation period of 5-6 days [12]. Wang et al. [8] reported that time period between onset of COVID-19 symptoms to death ranges between 6-41 days with a 14-day median. This time period depends on patient's immune system and age of the patient. It is found shorter among patients of more than 70 years of age relative to those below 70 years patients [8]. The common symptoms at the onset of infection are fatigue, cough and fever while as other symptoms include lymphopenia, haemoptysis, headache, sputum production and diarrhea [13]. The chest CT scan reveals pneumonia nevertheless there are some other clinical features like acute cardiac injury, acute respiratory distress syndrome, RNAaemia and incidence of grand-glass opacities that leads to death [6]. In certain cases, several peripheral ground-glass opacities have been identified in subpleural regions of both lungs which likely induce both localized and systemic immune response leading to increased inflammation [14]. Unfortunately interferon inhalation therapy showed no therapeutic benefit but instead aggravated the disorder by worsening pulmonary opacity [14].
It is pertinent to mention here that symptoms between earlier betacoronavirus infections and COVID-19 such as bilateral ground-glass opacity on CT scans, dry cough, dyspnea and fever are similar [6]. More importantly, COVID-19 showed unique clinical features like targeting the lower airway as is evident by symptoms like sore throat, rhinorrhoea and sneezing [15,16]. Additionally, on the basis of chest radiographs results some cases were found to possess infiltrate in the upper lobe of the lung associated with increasing dyspnea with hypoxemia [17]. Although COVID-19-infected patients reported gastrointestinal (GI) symptoms like diarrhoea, a small percentage of SARS-CoV or MERS-CoV patients experienced similar GI distress. Hence, analyzing faecal and urine samples is necessary to eliminate a possible alternative route of transmission, specifically by health care staff, patients, etc. [15,16]. Therefore, development of methods for identifying the different modes of transmission such as feacal and urine samples is urgently needed to establish strategies for inhibiting and/or reducing transmission and developing therapeutics to manage the disease.
Epidemiology
The first four cases of an acute respiratory syndrome of unknown etiology were identified among people linked to a local seafood market (“wet market”) in Wuhan City China on 29 December 2019 [12]. The research is under progress to learn more about COVID-19-related transmissibility, frequency and other features [18]. It has been observed that early detected cases had some kind of history of interaction with the seafood market [19]. Eventually human-to-human transmission by close contact was found as secondary cause of COVID-19 infection. There has been rise in the frequency of infected individuals with no history of wildlife exposure or visiting Wuhan and several cases of infection have been identified among medical professionals as well [20]. It has become apparent that COVID-19 infection occurs upon exposure to virus and both normal and immunosuppressed population seem susceptible. Most of adult patients were between 35 -55 years of age with fewer cases found among children and infants [19,21]. A research on the dynamics of early transmission of the virus reported that the median age of patients was 59 years ranging from 15 - 89 years with the majority being males (59%) [12]. People with low immune function particularly old aged people and those with renal and hepatic dysfunction were considered to be high risk group [12].
COVID-19 was found to have higher rates of transmissibility and pandemic risk than SARS-CoV, as COVID-19's effective reproductive number (R) is 2.9 which is much higher than recorded effective reproductive number (R) of SARS (1.77). Different COVID-19 studies have estimated the basic reproduction (R0) ranges between 2.6 to 4.71. The COVID-19 average incubation duration was estimated to be 4.8 ± 2.6 ranging from 2-11 days [22]. The Chinese health authorities have recently reported average incubation of 7 days ranging from 2- 14 days. The findings of epidemiological studies based on important indicators are summarized on the Table 1.
Table 1.
Epidemiological indicators of COVID-19 infection
| Indicators | Description |
|---|---|
| Patient age | Cases ranged between 25 - 89 years, with most patients aged between 35 - 55 years and fewer cases among children and infants [19] |
| Median age of patients is 59 years, ranging from 51- 89 [12] | |
| Average age of patients was 55.5 years; age distribution: ≤ 39: 10%; 40–49: 22%, 50–59: 30%; 60–69: 22%, ≥70: 15% [1] | |
| Cases range from 2 - 72 years | |
| Patients Sex | More cases were males [12,19] |
| 68% males [12] | |
| 59% males [1] | |
| Age of the deaths | Median age of death was 75 (with a range between 48 and 89 years [8] |
| History of Exposure | Huanan Seafood Market in Wuhan [1] |
| Wuhan residents or people who visited Wuhan [8] | |
| Incubation time | 4.8 ± 2.6 days (2–11 days) [22] |
| 5.2 days (4.1–7 days) [12] | |
| Average of 7 days (2–14 days) [13] | |
| Average of 10 days [17] | |
| Basic Reproduction (R0) | 2.6 (uncertainty range: 1.5–3.5) [9] |
| 3.8 (95% CI: 3.6–4.0) [10] | |
| 2.2 (1.4–3.8) [12] | |
| 2.68 (95% CI: 2.47–2.86) [22] | |
| Susceptible populations | Elderly people [8] |
| People with poor immune function | |
| People with chronic co-morbidities [8] | |
| Surgery history before admission [8] |
Pathogenesis and fatality rate
Coronaviruses are single-stranded, zoonotic RNA viruses that cause symptoms ranging from common cold to more extreme respiratory, enteric, hepatic, and neurological symptoms [23,24]. Besides, SARS-CoV-2 there are six other coronaviruses reported in humans. These are HCoV-OC43, HCoV-229E, SARS-CoV, HCoV-HKU1, MERS-CoV and HCoVNL63 [25,26]. Over the last two decades Coronavirus has caused two major pandemics: SARS [27] and MERS [28]. In order to find out the source of the COVID-19 outbreak CDC researchers of China collected 585 environmental samples from Huanan Seafood Market in Wuhan, Hubei Province. They found 33 samples were SARS-CoV-2 suggested that it came from wild animals sold on the market [29]. Researchers also used the 'lung fluid, blood, and throat swab samples from 15 patients for laboratory research. Such laboratory studies revealed that virus-specific nucleic acid sequences in the sample vary from those of known human coronavirus species. The laboratory findings also showed SARS CoV-2 is identical to some of the beta (β) coronaviruses genera found in bats which is located in the SARS/SARS-like CoV groups [30]. For the conduction of next-generation sequencing from cultured isolates and bronchoalveolar lavage fluid nine inpatients were enrolled by researchers in Wuhan with viral pneumonia and without any common respiratory pathogens. The results indicated SARS-CoV-2 was more distinct from MERS-CoV (with approximately 50% sequence identity) and SARS-CoV (with approximately 79% sequence identity) than from two bat-derived SARS-like coronaviruses bat-SL-CoVZXC21 (with 87.2% sequence identity) and bat-SL-CoVZC45 (with 87.9% sequence identity) [31]. The studies also showed that COVID-19 S-protein strongly interacted with human ACE2 molecules in spite of dissimilarity of its sequence with that of SARS-CoV [32,33].
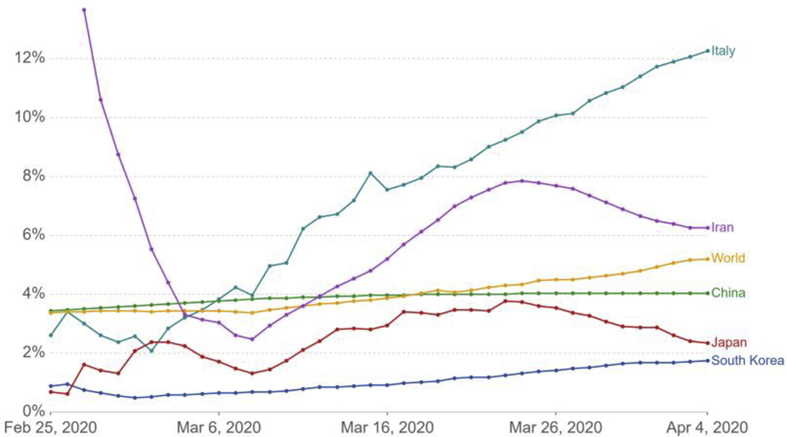
The case fatality rate (CFR) of any disease is not fixed it varies with location and with time. It is reported that CFR of COVID-19 varies widely between countries ranging from 0.2 % in Germany to 7.7 % in Italy. It may not be accurate comparison of the true likelihood particularly like that of COVID-19. Since, we are not aware weather the same criterion of testing is applied between the countries or many cases are symptomatic vs asymptomatic. So for better decision making and to understand differences in CFR we need better data. However, if we are careful to acknowledge its limitations CFR can help us to understand pandemic better and what should be done to combat it.The countries having small number of confirmed cases till now are left as CFR at small sample size is not significant. As we look towards the trajectory in case of Iran on 24th of February 2020 it had 2 confirmed cases and 2 deaths an implausible CFR of 100%. As the number of cases increased into hundreds over time, its CFR dropped to the level seen in other countries. The CFR of ongoing COVID-19 pandemic is represented in Fig. 2.
Fig. 2.
The growth kinetics COVID-19 infection in different countries around the globe as on 4th April, 2020 (European CDC).
Transmission
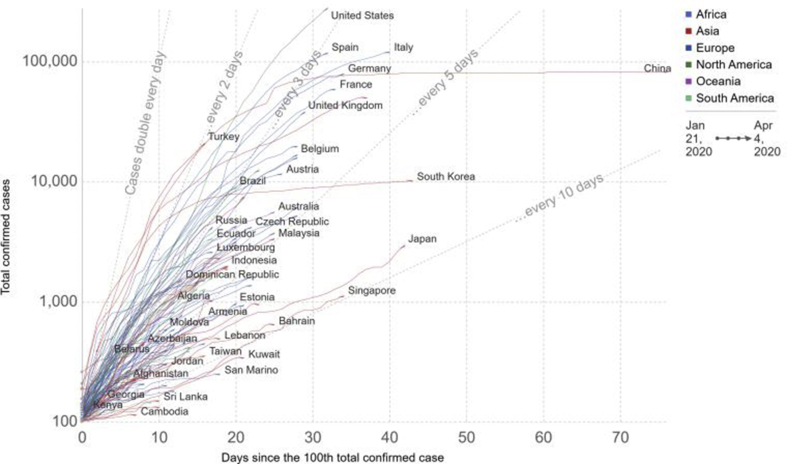
It is anticipated that there is probable zoonotic origin of COVID19, based on the large number of infected people who were exposed to the wet animal market in Wuhan City where live animals are routinely sold. Efforts were made to look for a host reservoir or intermediate carriers from which the infection could spread to humans [10]. The two snake species were identified which were thought the possible COVID-19 reservoirs. However, there is no clear cut evidence for the reservoirs of coronavirus other than mammals and birds till date. The COVID-19 genomic sequence analysis showed its 88 % identity with two bat-derived severe acute respiratory syndrome (SARS)-like coronaviruses suggesting that bats are the most likely link between COVID-19 and humans [34]. Several studies have indicated that transmission from person to person is a possible way for COVID-19 infection to spread. As it was observed COVID-19 infection occurred in many people who did not even visited Wuhan's wet animal market [13]. Transmission of disease from person to person occurs mainly through direct contact or by droplets released by an infected person through coughing or sneezing. The Fig. 3 how quickly the disease has spread in each country since the 100th confirmed case. A study was conducted on COVID-19 infected women having third trimester and no evidence was analyze which can confirm that transmission can occur from mother to child. Nevertheless pregnant mothers underwent cesarean sections, so it remains uncertain if transmission can occur during vaginal delivery. This study is important as pregnant women's are comparatively more vulnerable to respiratory infections and extreme pneumonia [34]. The first step of COVID-19 viral infection is the binding of the receptor expressed by host cells followed by fusion with the cell membrane. The lung epithelial cells are the primary target of the virus. It has been stated that human-to-human transmissions of SARS-CoV occurs through binding between cellular receptor known as the angiotensin-converting enzyme 2 (ACE2) receptor and receptor-binding domain of virus spikes [34]. It is important to mention here that receptor binding domain sequence of COVID-19 is similar to that of SARS-CoV. These results indicate that the most likely entry into the host cells is through the ACE2 receptor [35].
Fig. 3.
The case fatality rate of ongoing COVID-19 pandemic.
Phylogenetic analysis
The COVID-19 has been classified as β CoV of group 2B by the World Health Organization [36]. The COVID-19 genome sequencing from nine patients showed 99.98% sequence identity [37]. Another study in five patients showed 99.8–99.9% nucleotide identity and sequencing studies revealed the presence of a new beta-CoV strain [5]. The COVID19 genetic sequence showed more than 50 % identity to MERS-CoV and 80% to SARS-CoV [37] and both MERS-CoV and SARS-CoV originate in bats [38]. COVID-19 is the seventh member of the coronavirus family that infects humans and has been categorized under the subfamily orthocoronavirinae. It was a clade within the subgenus sarbecovirus [23]. On the basis phylogenetic reports and genetic sequence studies COVID-19 varies significantly from SARS-CoV and can thus be regarded as a new betacoronavirus that infects humans. The COVID-19 most likely developed from bat origin coronaviruses. The another evidence which supports that COVID-19 is of bat origin is the high of homology ACE2 receptor from a diversity of animal species there by implicating these animal species are possible intermediate hosts or animal models for COVID-19 infections [35]. Additionally these viruses have single intact open reading frame on gene 8 which is another sign of bat-origin CoVs. Nevertheless, the amino acid sequence of receptor-binding domain resembles with that of SARS-CoV indicating that these viruses might use the same receptor [5].
Clinical manifestation and diagnosis
The complete clinical manifestation is not yet clear, as the symptoms recorded so far vary from mild to extreme, with some cases even leading to death [18]. The most frequently reported symptoms include cough-producing phlegm, runny nose, myalgia, fever, pneumonia, and complicated dyspnea, while the less widely documented symptoms include headache, vomiting and hemoptysis, [6,18]. The patients with mild symptoms usually recover within a week while as severe cases have been reported to suffer from progressive respiratory failure due to alveolar damage which can lead to death as well [18]. Usually elderly patients and middle aged patients with some pre-existing diseases (coronary heart disease, Parkinson's disease, diabetes, hypertension, tumor surgery and cirrhosis) were seen to more prone to death [38]. The case definition guidelines mentioned the following symptoms: decrease in lymphocytes and white blood cells, fever and new pulmonary infiltrates on chest radiography with no improvement in symptoms after 3 days of antibiotic treatment [38]. In patients with suspected infection, the following techniques for diagnosis have been suggested: conducting real-time fluorescence (RT-PCR) to detect SARS-CoV-2 positive nucleic acid in sputum, throat swabs, and lower respiratory tract sample secretions [39].
Therapeutics options
There is no vaccine currently available for COVID-19 and neither there is specific antiviral treatment recommended so far. The treatment is symptomatic and the patients with severe infection are given oxygen therapy. In cases of respiratory failure mechanical ventilation is required whereas hemodynamic support is important for septic shock management. The WHO on 28th January, 2020 released a document summarizing WHO guidelines and clinical data obtained from the treatment of previous HCoVs epidemics. The document address steps for identification and sorting of patients with serious acute respiratory disease, guidelines for laboratory diagnosis, early supportive therapy and monitoring, management of septic shock and respiratory failure and methods of treatment for pregnant patients.
Amongst these recommendations, we focus on approaches to tackle respiratory failure, including safe mechanical ventilation and non-invasive ventilation (NIV) or high-flow nasal oxygen (HFNO).
Protective mechanical ventilation and intubation
During intubation special precautions are required. An professional operator who uses personal protective equipments (PPE) such as protective goggles, disposable double socks, disposable gloves, FFP3 or N95 mask and disposable gown long sleeve raincoat can conduct the operation. The rapid sequence intubation (RSI) should be performed if possible. The preoxygenation (100% O2) should be performed for 5 minutes using the method of continuous positive airway pressure (CPAP). Heat and moisture exchanger (HME) must be located between the circuit of fan and mask or between the ventilation balloon and the mask.
In case of mechanical ventilation lower inspiratory pressures, reaching a plateau pressure (Pplat) < 28 to 30 cm H2O and lower tidal volumes (4 to 6 ml/kg predicted body weight, PBW) should be kept. The positive end expiratory pressure (PEEP) should be kept as high as possible so as to maintain driving pressure (Pplat-PEEP) as low as possible (<14 cm H2O). Furthermore to prevent atelectasis and loss of PEEP disconnections from the ventilator must be avoided. The paralytics is not recommended until PaO2/FiO2 < 150 mm Hg. The use of conservative fluid management strategy and prone ventilation for >12 hours per day for patients suffering from acute respiratory distress syndrome is strongly recommended.
Non-invasive ventilation
With regard to high flow nasal oxygen (HFNO) or non-invasive ventilation (NIV) the panel of experts points out that these approaches performed by systems with good interface fitting do not cause widespread dispersion of exhaled air and their use can be considered at low risk of airborne transmission [40]. In practical sense non-invasive ventilation can be used only in non severe forms of respiratory failure. Nevertheless, if the patient does not improve or even worsen within a limited period of time (1–2 hours) the mechanical ventilation must be preferred.
Prospective therapeutic strategies
At first broad-spectrum antibiotics, interferons-α nebulization and anti-viral drugs were used to reduce the viral load [41] but only remdesivir has shown promising viral impact [42]. Remdesivir alone and in combination with chloroquine or β-interferon significantly blocked the SARS-CoV-2 replication and patients were found to recover clinically [43]. Several other anti-virals drugs are currently being evaluated against infection. Moderate results were exhibited by Favipiravir, Nitazoxanide, Ribavirin, Baricitinib, Penciclovir, Ritonavir and Arbidol when tested against infection in vitro clinical isolates and in patients [44]. Many other formulations have also been tested against COVID-19 infections in humans and mice such as combinations of antibiotic or antivral drugs with the traditional Chinese medicines [45]. The convalescent plasma therapy was also tested by doctors in Shanghai China and in USA as well in which blood plasma was isolated from clinically treated COVID-19 patients and injected it in the infected patients who showed positive results with rapid recovery [46].
Vaccination
Currently no vaccine is available against COVID-19 infection, however vaccines or strategies used to develop a vaccine against SARS-CoV can be effective. The recombinant DNA from the Urbani strain of SARS-CoV (AY278741) was administered to hamsters and mice which leaded to production of neutralizing antibodies and protection against SARS-CoV [47,48]. The DNA was found to inactivate whole virus or live-vectored strain of SARS-CoV (AY278741) and there by significantly reduced the viral infection in animal models [[49], [50], [51]]. Numerous other SARS-CoV strains are also used to develop inactivated or live-vectored vaccines that have effectively reduced the viral load in animal models. These strains include BJ01 (AY278488) [52,53], Tor2 (AY274119) [54,55], FRA (AY310120) [56], NS1 (AY508724) [57], ZJ01 (AY297028) [57], GZ50 (AY304495) [58], Utah (AY714217) [59] and GD01 (AY278489) [53]. Moreover there are few vaccines against SARS-CoV-2 in pipeline. The Chinese Centre for Disease Control and Prevention (CDC) is focusing on creating an inactivated vaccine for viruses [60]. A sample mRNA based vaccine (prepared by Stermirna Therapeutics) will be available soon [61]. GeoVax and Bravovax [62] is working for the formation of Modified Vaccina Ankara (MVA) based vaccine while as Clover Biopharmaceuticals are developing a recombinant 2019-nCoV S protein subunit-trimer based vaccine [63].
Although research teams around the world are working to explore key characteristics, pathogenesis, and combating the disease, appropriate attention should be drawn on therapeutic options and cross-resistance of other vaccines. For instance vaccines for other diseases like for measles and rubella can create cross-resistance for SARS-CoV-2. This concept of cross resistance is based on the findings that children are found less vulnerable to infection as compared to elder population and children predominantly vaccinated for measles.
Prevention and control
Strategies for prevention and control are reported at three levels: national level, case-related population level, and general population level. In case of national level the China's National Health Commission issued the “No.1 announcement” which officially included COVID-19 in the management of legally infectious diseases in Class B and allowed for class A infectious disease preventive and control measures to be implemented [64]. Under this policy medical institutes can adopt observation protocols and isolation treatment to prevent and control the spread of the COVID-19. In China the National Health Commission issued national guidelines on the prevention and control of COVID-19 infection on 22 January 2020 for medical institutes to prevent nosocomial infection [65]. On 28 January 2020, during the Chinese Spring Festival the National Health Commission released guidelines for rapid prevention and control measures to effectively contain the spread of the disease through a "major isolation and great disinfection” program [66]. National-level policies with targeted measures for elderly population (published on 31 January 2020) and for rural areas (released on 28 January 2020) were also released [67]. Several public health interventions have been implemented that can avoid or delay the COVID-19 transmission; these include case isolation, identification and surveillance of contacts, environmental disinfection, and the use of personal protective equipments [68]. As of now no specific antiviral therapy has been confirmed COVID-19 infection. With regard to patients diagnosed with COVID-19, it was recommended that appropriate symptomatic treatment and supportive care be applied [6]. The studies have also reported the prevention of psychological health issues and nosocomial infection associated with COVID. A series of measures have been suggested to minimize nosocomial infection, including awareness training for prevention and control, isolation, disinfection, protection at different levels in infection areas and protection of reported cases [68]. With respect to psychological health, some suggested psychological interventions are set up for medical staff, suspected cases and confirmed cases [69].
Since there is no vaccine preventing COVID-19 at this moment the best prevention for general population is to avoid exposure to the virus [70]. The airborne measures and other protections were discussed and suggested for protection. The infection prevention and control measures that can minimize the risk of exposure include use of face masks; covering coughs and sneezes with tissues that are then safely disposed of, regular hand wash with soap or disinfection with hand sanitizer having 60% alcohol in it, maintaining an appropriate distance as much as possible, avoidance of contact with infected people and refraining from touching nose, mouth and eyes with unwashed hands [18].
The WHO also released detailed recommendations on the use of face masks within the population, during home care, and in COVID-19 health care settings [71]. Health care staff are advised to use particulate respirators such as those licensed N95 or FFP2 while conducting aerosol-generating procedures and to use surgical masks when providing any treatment for suspected or confirmed cases. As per these guidelines an individual without respiratory symptoms when in public is not required to wear medical mask. To avoid any increase in risk of transmission proper use and disposal of masks is important [71].
In addition to papers published in scientific journals, the China CDC has provided guidelines to raise awareness among the general public about the prevention and control of COVID-19. The guidelines main messages include causes, how to choose and wear face masks, proper hand washing practices, preventive measures at different locations (e.g., at home, on public transportation, and in public space), methods of disinfection and home medical monitoring [72]. In addition to scientific knowledge on how to treat the outbreak of COVID-19, the guidelines also recommends ways of reducing fear among the general population [72].
Obstacle in research
Animal models play a crucial role in uncovering the mechanisms of viral pathogenicity from the entrance to transmission and in the scheming of therapeutic strategies. Previously, various animal models were used for examining replication of SARS-CoV which showed the symptoms of severe infection [73]. In case MERS-CoV no pathogenesis was observed in small animals as against seen in case of SARS-CoV. Mice was found not susceptible to MERS-coronavirus infection because of noncompatibility of the DPP4 receptor [74]. As the entire genome of COVID-19 is more than 80% similar previous human SARS-like bat CoV, hence animal models previously used for SARS-CoV may be useful to study the pathogenicity of COVID-19. Novel coronaviruses and SARS both recognize human ACE2 cell receptor. Conclusively TALEN or CRISPR-mediated genetically modified hamsters or other small animals may be used to test the pathogenicity of novel coronaviruses. It was observed that SARS-CoV having a mutation for spike glycoprotein, replicate and cause severe disease in Rats (F344) [75]. Thus spike glycoprotein targeting therapeutics is a suitable option against coronaviruses. In recent studies to formulate some therapeutic strategy against COVID-19 clinical isolates and mice models were used [45]. Artificial intelligence prediction were also used in similar studies to investigate the inhibitory role of the drug against SARS-CoV-2 [44]. Randomized clinical trials were also used in COVID-19 patients [11]. It is now essential for scientists worldwide to collaborate on the design of an effective model and to investigate the in vivo mechanisms associated with COVID-19 pathogenesis.
Future perspectives
Combating the current outbreak requires rigorous steps to reduce the transmission of COVID-19 infection from person to person. Special attention and efforts should be applied for protecting or reducing transmission in vulnerable populations, including infants, health care providers and the elderly. For medical personnel, health care professionals, public health individuals and researchers involved in the 2019-nCoV guidelines to follow are published [76]. The early death cases in COVID-19 outbreak occurred mainly in the elderly possibly due to a poor immune system that allows for more rapid progression of viral infection [12]. The decontaminating regents should be provided by public services and facilities regularly. The physical contact with contaminated things particularly urine and facecal samples should be considered in dealing with the virus as these can potentially serve as an alternative route of transmission [15]. Most of the countries around the world particularly US, China, India, UAE etc have implemented major control and prevention measures like implementing state wise or country wise lockdown and travel screenings so as to control virus spread. The epidemiological changes occurring in COVID-19 infection should be monitored carefully taking into account potential transmission routes and subclinical infections, as well as the adaptation, evolution, possible intermediate animals and reservoir and spread of virus among humans. A large number of issues still remain to be addressed. Some of which are who and how many were tested, what percentage of these came out positive, weather this rate remains constant or it varies. What proposition of people tested positive for COVID-19 but showed clinical symptoms. So far only few paediatric cases were reported, it is because real lack of susceptibility or due to inadequate testing.
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- 1.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu H., Stratton C.W., Tang Y.W. Outbreak of pneumonia of unknown etiology in wuhan China: the mystery and the miracle. J Med Virol. 2020;92(4):401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao S., Lin Q., Ran J., Musa S.S., Yang G., Wang W. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int J Infect Dis IJID: Off Publ Int Soc Infect Dis. 2020;92:214–217. doi: 10.1016/j.ijid.2020.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du A., Toit Outbreak of a novel coronavirus. Nat Rev Microbiol. 2020;18:123. doi: 10.1038/s41579-020-0332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ren L.L., Wang Y.M., Wu Z.Q. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J. 2020 doi: 10.1097/CM9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci Trends. 2020 doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 8.Wang W., Tang J., Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J Med Virol. 2020;92(4):441–447. doi: 10.1002/jmv.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishiura H., Jung S.M., Linton N.M., Kinoshita R., Yang Y., Hayashi K. The extent of transmission of novel coronavirus in wuhan, China, 2020. J Clin Med. 2020:9. doi: 10.3390/jcm9020330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bassetti M., Vena A D. Roberto giacobbe, the novel Chinese coronavirus (2019- nCoV) infections: challenges for fighting the storm. Eur J Clin Invest. 2020 doi: 10.1111/eci.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holshue M.L., DeBolt C.S., Lindquist K.H., Lofy J. First case of 2019 novel coronavirus in the United States, N. Engl J Med. 2020 doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Q., Guan X., Wu P., Wang X., Zhou L. Early transmission dynamics in wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlos W.G., Dela C., Cao B., Pasnick S. (2019-nCoV) coronavirus. Am J Respir Crit Care Med. 2020;201(4):7–8. doi: 10.1164/rccm.2014P7. [DOI] [PubMed] [Google Scholar]
- 14.Lei J., Li J., Li X., Qi X. CT imaging of the 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020:200236. doi: 10.1148/radiol.2020200236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A., Al-Rabiah F.A., Al-Hajjar S., AlBarrak A. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G.M. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 17.Phan L.T., Nguyen T.V., Luong Q.C., Nguyen T.V. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N Engl J Med. 2020 doi: 10.1056/NEJMc2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CDC 2019 Novel coronavirus, Wuhan, China. 2020. https://www.cdc.gov/coronavirus/2019-nCoV/summary.html
- 19.Medical expert group of Tongji hospital . 3rd ed. Herald Med; 2020. Quick guide to the diagnosis and treatment of pneumonia for novel coronavirus infections.http://kns.cnki.net/kcms/detail/42.1293.r.20200130.1803.002.html [Google Scholar]
- 20.Gralinski L.E., Menachery V.D. Return of the coronavirus: 2019-nCoV. Viruses. 2020;12:135. doi: 10.3390/v12020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang C., Wang X. Prevalence, nosocomial infection and psychological prevention of novel coronavirus infection. Chin Gen Pract Nurs. 2020;18:2–3. [Google Scholar]
- 22.Liu T., Hu J., Kang M., Lin L., Zhong H., Xiao J. 2020. Transmission dynamics of 2019 novel coronavirus (2019-nCoV) [DOI] [Google Scholar]
- 23.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO Coronavirus. 2020. https://www.who.int/health-topics/coronavirus
- 25.Su S., Wong G., Shi W., Liu J., Lai A.C.K., Zhou J. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trend Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y., Liu Q., Guo D. Coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020 doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peiris J.S., Guan Y., Yuen K. Severe acute respiratory syndrome. Nat Med. 2004;10:S88–S97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zaki A.M., Boheemen S.V., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 29.Chinese Center for Disease Control and Prevention . 2020. 585 environmental samples from the south China seafood market in wuhan, Hubei province, China.http://www.chinacdc.cn/yw_9324/202001/t20200127_211469.html [Google Scholar]
- 30.Lu H., Tang C.W., Tang Y. Outbreak of pneumonia of unknown etiology in Wuhan China: the mystery and the miracle. J Medl Virol. 2020 doi: 10.1002/jmv/25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roujian L., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020 doi: 10.1016/S0140–6736(20)30251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu X., Chen P., Wang J., Feng J., Zhou H., Li X. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci Chin Life Sci. 2020 doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Health Commission of People’s Republic of China Prevent guideline of 2019-nCoV. 2020. http://www.nhc.gov.cn/xcs/yqfkdt/202001/bc661e49b5bc487dba182f5c49ac445b.shtml
- 34.Jaimes J.A., Millet J.K., Stout A.E., Andre N.M., Whittaker G.R. A tale of two viruses: the distinct spike glycoproteins of feline coronaviruses. Viruses. 2020;12 doi: 10.3390/v12010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS. J Virol. 2020 doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hui D.S., IA E., Madani T.A., Ntoumi F., Kock R., Dar O. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li T., Wei C., Li W., Hongwei F., Shi J. Beijing Union Medical College Hospital on "pneumonia of novel coronavirus infection" diagnosis and treatment proposal (V2.0) Med J Peking Union Med Coll Hosp. 2020 http://kns.cnki.net/kcms/detail/11.5882.r.20200130.1430.002.html [Google Scholar]
- 39.National Health Commission of People’s Republic of China Pneumonia diagnosis and treatment of 2019-nCoV infection from Chinese NHC and CDC 2020. 2020. http://www.nhc.gov.cn/xcs/zhengcwj/202001/4294563ed35
- 40.Hui D.S., Chow B.K., Lo T., Tsang O.T.Y., Ko F.W., Ng S.S., Gin T., Chan M.T.V. Exhaled air dispersion during high-flow nasal cannula therapy versus CPAP via different masks. Eur Respir J. 2019;53(4) doi: 10.1183/13993003.02339-2018. [DOI] [PubMed] [Google Scholar]
- 41.Ng C.S., Kasumba D.M., Fujita T., Luo H. Spatio-temporal characterization of the antiviral activity of the XRN1-DCP1/2 aggregation against cytoplasmic RNA viruses to prevent cell death. Cell Death Differ. 2020:1–20. doi: 10.1038/s41418-020-0509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio. 2018;9(2) doi: 10.1128/mBio.00221-18. e00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020:1–3. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richardson P., Griffin I., Tucker C., Smith D., Oechsle O., Phelan A. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. The Lancet. 2020 doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11(1):1–14. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Derebail V.K., Falk R.J. Mass Medical Soc; 2020. ANCA-associated vasculitis—refining therapy with plasma exchange and glucocorticoids. [DOI] [PubMed] [Google Scholar]
- 47.Bisht H., Roberts A., Vogel L., Subbarao K., Moss B. Neutralizing antibody and protective immunity to SARS coronavirus infection of mice induced by a soluble recombinant polypeptide containing an N-terminal segment of the spike glycoprotein. Virology. 2005;334(2):160–165. doi: 10.1016/j.virol.2005.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kam Y.W., Kien F., Roberts A., Cheung Y.C., Lamirande E.W., Vogel L. Antibodies against trimeric S glycoprotein protect hamsters against SARS-CoV challenge despite their capacity to mediate FcγRII-dependent entry into B cells in vitro. Vaccine. 2007;25(4):729–740. doi: 10.1016/j.vaccine.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kapadia S.U., Rose J.K., Lamirande E., Vogel L., Subbarao K., Roberts A. Long-term protection from SARS coronavirus infection conferred by a single immunization with an attenuated VSV-based vaccine. Virology. 2005;340(2):174–182. doi: 10.1016/j.virol.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bisht H., Roberts A., Vogel L., Bukreyev A., Collins P.L., Murphy B.R. Severe acute respiratory syndrome coronavirus spike protein expressed by attenuated vaccinia virus protectively immunizes mice. Proc Natl Acad Sci. 2004;101(17):6641–6646. doi: 10.1073/pnas.0401939101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buchholz U.J., Bukreyev A., Yang L., Lamirande E.W., Murphy B.R., Subbarao K. Contributions of the structural proteins of severe acute respiratory syndrome coronavirus to protective immunity. Proc Natl Acad Sci. 2004;101(26):9804–9809. doi: 10.1073/pnas.0403492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang L., Zhu Q., Qin E., Yu M., Ding Z., Shi H. Inactivated SARS-CoV vaccine prepared from whole virus induces a high level of neutralizing antibodies in BALB/c mice. DNA Cell Biol. 2004;23(6):391–394. doi: 10.1089/104454904323145272. [DOI] [PubMed] [Google Scholar]
- 53.Qin E., Shi H., Tang L., Wang C., Chang G., Ding Z. Immunogenicity and protective efficacy in monkeys of purified inactivated Vero-cell SARS vaccine. Vaccine. 2006;24(7):1028–1034. doi: 10.1016/j.vaccine.2005.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weingartl H., Czub M., Czub S., Neufeld J., Marszal P., Gren J. Immunization with modified vaccinia virus Ankara-based recombinant vaccine against severe acute respiratory syndrome is associated with enhanced hepatitis in ferrets. J Virol. 2004;78(22):12672–12676. doi: 10.1128/JVI.78.22.12672-12676.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.See R.H., Zakhartchouk A.N., Petric M., Lawrence D.J., Mok C.P., Hogan R.J. Comparative evaluation of two severe acute respiratory syndrome (SARS) vaccine candidates in mice challenged with SARS coronavirus. J Gen Virol. 2006;87(3):641–650. doi: 10.1099/vir.0.81579-0. [DOI] [PubMed] [Google Scholar]
- 56.Stadler K., Roberts A., Becker S., Vogel L., Eickmann M., Kolesnikova L. SARS vaccine protective in mice. Emerging Infect Dis. 2005;11(8):1312. doi: 10.3201/eid1108.041003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou J., Wang W., Zhong Q., Hou W., Yang Z., Xiao S.-Y. Immunogenicity, safety, and protective efficacy of an inactivated SARS-associated coronavirus vaccine in rhesus monkeys. Vaccine. 2005;23(24):3202–3209. doi: 10.1016/j.vaccine.2004.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qu D., Zheng B., Yao X., Guan Y., Yuan Z.-H., Zhong N.-S. Intranasal immunization with inactivated SARS-CoV (SARS-associated coronavirus) induced local and serum antibodies in mice. Vaccine. 2005;23(7):924–931. doi: 10.1016/j.vaccine.2004.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spruth M., Kistner O., Savidis-Dacho H., Hitter E., Crowe B., Gerencer M. A doubleinactivated whole virus candidate SARS coronavirus vaccine stimulates neutralising and protective antibody responses. Vaccine. 2006;24(5):652–661. doi: 10.1016/j.vaccine.2005.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheung E. China coronavirus: Hong Kong researchers have already developed vaccine but need time to test it, expert reveals: south China morning post. 2020. https://www.scmp.com/news/hongkong/health-environment/article/3047956/chinacoronavirus-hong-kong-researchers-have [cited 2020 29 January]; Available from:
- 61.Xinhua. China fast-tracks novel coronavirus vaccine development xinhua. 2020. http://www.xinhuanet.com/english/2020-01/28/c_138739378.htm [cited 202 29 January]; Available from:
- 62.Geo-Vax Geovax and bravovax (wuhan, China) to collaborate on development of coronavirus vaccine. 2020. https://www.geovax.com/news/geovax-and-bravovax-wuhan-china-to-collaborate [cited 2020 3 March]; Available from:
- 63.Clover B. Clover initiates development of recombinant subunit- trimer vaccine for wuhan coronavirus 2019. http://www.cloverbiopharma.com/index.php?m=content&c=index&a=show&catid=11&id=40 -ncov). [cited 2020 6 March]; Available from:
- 64.National Health Commission of People’s Republic of China Pneumonia infected with novel coronavirus is included in the management of legal infectious diseases. 2020. http://www.nhc.gov.cn/jkj/s7915/202001/e4e2d5e6 f01147e0a8df3f6701d49f33.shtml
- 65.National Health Commission of People’s Republic of China . Notice on printing and distributing the technical guide for prevention and control of novel coronavirus infection in medical institutions. 1st ed. 2020. http://www.nhc.gov.cn/yzygj/s7659/202001/b91fdab7c304431eb082d6784 7d27e14.shtml [Google Scholar]
- 66.National Health Commission of People’s Republic of China Notice on printing and distributing the work plan for prevention and control of pneumonia caused by novel coronavirus infection in the near future. 2020. http://www.nhc.gov.cn/tigs/s7848/202001/808bbf75e5ce415aa19f74c78ddc653f.shtml Accessed.
- 67.National Health Commission of People’s Republic of China Notice on further prevention and control of pneumonia caused by novel coronavirus infection in rural areas. 2020. http://www.nhc.gov.cn/jkj/s3578/202001/f8d45f6af1d24ef18151c1d91cf8a028.shtml Accessed.
- 68.Wei Q., Ren Z. Disinfection measures for pneumonia foci infected by novel coronavirus in 2019. Chin J Disinfect. 2020;37:59–62. [Google Scholar]
- 69.Xu M., Zhang Y. Investigation on the psychological status of the first batch of clinical first-line support nurses to fight against pneumonia caused by novel coronavirus. Chin Nurs Res. 2020;34:1–3. [Google Scholar]
- 70.Ou F., Wu H., Yang Y., Tan W., Zhang J., Gu J. Countermeasures for rapid spread of new coronavirus pneumonia in Wuhan. Chin Gen Pract Nurs. 2020 http://kns.cnki.net/kcms/detail/14.1349.R.20200131.1319.002.html [Google Scholar]
- 71.WHO . 2020. Advice on the use of masks in the community, during home care and in health care settings in the context of the novel coronavirus 2019- nCoV outbreak (Interim guidance) WHO/nCov/IPC_Masks/2020. [Google Scholar]
- 72.National Health Commission of People’s Republic of China Guidelines for public protection against novel coronavirus infection. 2020. http://www.nhc.gov.cn/jkj/s7915/202001/bc661e49b5bc487dba182f5c49ac445b.shtml
- 73.Gretebeck L.M., Subbarao K. Animal models for SARS and MERS coronaviruses. Curr Opin Virol. 2015;13:123–129. doi: 10.1016/j.coviro.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cockrell A.S., Peck K.M., Yount B.L., Agnihothram S.S., Scobey T., Curnes N.R. Mouse dipeptidyl peptidase 4 is not a functional receptor for Middle East respiratory syndrome coronavirus infection. J Virol. 2014;88(9):5195–5199. doi: 10.1128/JVI.03764-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nagata N., Iwata N., Hasegawa H., Fukushi S., Yokoyama M., Harashima A. Participation of both host and virus factors in induction of severe acute respiratory syndrome (SARS) in F344 rats infected with SARS coronavirus. J Virol. 2007;81(4):1848–1857. doi: 10.1128/JVI.01967-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jin Y.H., Cai L., Cheng Z.S., Cheng H., Deng T., Fan Y.P. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil Med Res. 2020;7:4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]