Abstract
Covid-19 disease is caused by SARS-CoV-2, a virus belonging to the coronavirus family. Covid-19 is so new that there is currently no specific vaccine or treatment. Clinical trials are currently underway. In vitro tests are also being conducted to assess the efficacy of chloroquine and hydroxychloroquine for the treatment of this epidemic, which is considered a pandemic by the WHO. We note that the content of this review is dated. The information it contains is subject to change and modification as the epidemic progresses.
Keywords: Chloroquine, coronavirus, Covid-19, epidemic, hydroxychloroquine, treatment
Introduction
In mid-December 2019, in the city of Wuhan, in central China, an epidemic of coronavirus was declared. It is caused by a new type of coronavirus first named 2019-nCoV, then renamed SARS-CoV-2, never observed before its appearance in this metropolis of 11 million inhabitants.
Chinese health officials officially announced the discovery on 7th January, 2020. On 30th January, the World Health Organization (WHO) declared a public health emergency of international concern. On February 11, WHO gave a name to the disease caused by this new coronavirus: Covid-19. On 11th March, WHO upgraded the epidemic to a pandemic, the first triggered by a coronavirus [1,2].
On this subject which worries the whole world, several questions can arise on the coronavirus itself, the world epidemiological situation and the effectiveness of using chloroquine and hydroxychloroquine in the treatment. Answers to these questions will be found throughout this review.
What is a coronavirus? What is COVID-19?
Covid-19 disease, which appeared in China in late 2019, is caused by SARS-CoV-2, a virus that belongs to the great coronavirus. Very frequent, they can cause a simple cold as well as a serious respiratory infection such as pneumonia, causing fatal epidemics as it was the case with Sras or Mers and now with Covid-19 (for Coronavirus Disease) [1]. COVID-19 is the infectious disease caused by the last coronavirus that was discovered. This new virus and this disease were unknown before the outbreak appeared in Wuhan (China) in December 2019 [1]. Coronaviruses, which owe their name to the crown shape of the proteins that coat them, are part of a large family of viruses, some of which infect different animals, others humans. They are likely to cause a wide range of diseases. For humans, these diseases begin with a simple cold to a severe lung infection, responsible for acute respiratory distress [1].
What is the epidemiological situation of the coronavirus?
According to WHO, after an epidemic outbreak in China in January-February, the epidemic situation has changed globally since 23rd February, 2020. With the intensification of outbreaks in South Korea, Japan, and Singapore, and the appearance of new outbreaks in Iran and Italy. In these countries, we are witnessing community dissemination, with no identified link with cases imported from China. Two months after its appearance in China, the epidemic seems to have peaked there. In March 2020, the Chinese authorities announced that the number of new cases was declining sharply in the country. However, all the countries of the European Union are now affected by COVID-19 [1], especially Italy, Spain and France. On 16th March 2020, the WHO counted almost as many cases in China as outside of China: 165 515 confirmed cases worldwide, including 81 077 in China and 86 438 outside China (in 143 different countries). And 3218 deaths in China and 3388 outside China [3].
WHO has therefore called these countries to act for contain the coronavirus, which has killed more than 45 525 people and more than 896 450 cases of infection worldwide, according to data reported by the authorities on 2 April, 2020 [3].
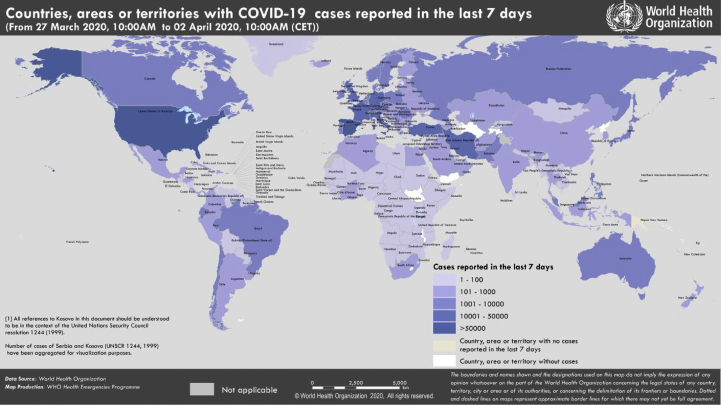
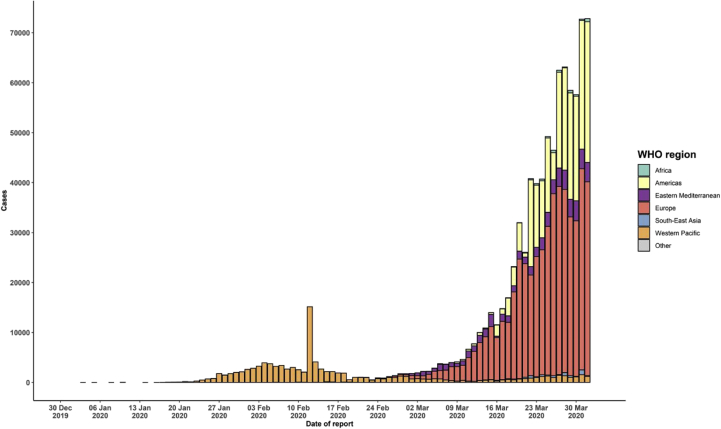
The WHO Regional Director for Europe said the number of cases is expected to increase further. He urged countries to continue implementing a containment strategy while accelerating their efforts to fight the disease. It is essential to act quickly, and every day can make a difference [4]. Indeed, on 31st March 2020 Italy became at the top of the world ranking of deaths due to Covid-19, followed by Spain, but is just behind the United States in terms of cases tested positive for the virus. According to the newspaper, the world, in Africa, the sub-Saharan countries remain, until 28th March, still little affected by the Covid-19 in comparison with Europe, even if the number of cases increases rapidly. Indeed, the African countries multiply the measures much more precociously than Europe [5]. In the Maghreb, the States take early measures going from the simple prudence to the closing of the borders and confinement [5]. Indeed, the Situation on the scale reported by WHO (02 April, 2020) is shown in Table 1 and Fig. 1, Fig. 2 [3].
Table 1.
The global epidemiological situation (02 April, 2020)
| Confirmed cases | Deaths | Countries, areas or territories with cases |
|---|---|---|
| 896 450 | 45 525 | 195 |
Fig. 1.
Countries, territories or areas with reported confirmed cases of COVID-19, 02 April 2020 (WHO).
Fig. 2.
Epidemic curve of confirmed COVID-19, by date of report and WHO region through 02 April 2020 (WHO).
Are there any drugs that can prevent or cure COVID-19?
The Center of Disease control (CDC), in a public document on its website published on 21st March 2020, informed that there are no US Food and Drug Administration (FDA)-approved drugs specifically for the treatment of patients with COVID-19. At present clinical management includes infection prevention and control measures and supportive care, including supplementary oxygen and mechanical ventilator support when indicated. An array of drugs approved for other indications as well as several investigational drugs are being studied in several hundred clinical trials that are underway across the globe [6]. Very recent studies have tested and shown the effectiveness and use of two drugs (chloroquine and hydroxychloroquine) for the treatment of COVID-19 disease. According to the US Centers for Disease Control and Prevention, hydroxychloroquine and chloroquine are oral prescription drugs that have been used for treatment of malaria and certain inflammatory conditions. Chloroquine has been used for malaria treatment and chemoprophylaxis, and hydroxychloroquine is used for treatment of rheumatoid arthritis, systemic lupus erythematosus and porphyria cutanea tarda. WHO includes it on its list of essential medicines, meaning it should be kept affordable and accessible at all times. Both drugs have in-vitro activity against SARS-CoV, SARS-CoV-2, and other coronaviruses, with hydroxychloroquine having relatively higher potency against SARS-CoV-2 [[7], [8], [9]]. Studies revealed that it has potential broad-spectrum antiviral activities by increasing endosomal pH required for virus/cell fusion, as well as interfering with the glycosylation of cellular receptors of SARS-CoV [6,10]. According to Colson et al., 2020 the activity of hydroxychloroquine on viruses is probably the same as that of chloroquine since the mechanism of action of these two molecules is identical, and hydroxychloroquine was prescribed for long periods, which would be therefore the first choice in the treatment of SARS-CoV-2. For optimal treatment, it may be necessary to administer a loading dose followed by a maintenance dose [8].
In systematic review appeared on line at March 2020 [11]. It was reported that a study (published online 04th February 2020) was carried out by a Chinese group on the effect of chloroquine in vitro, using Vero E6 cells infected with SARS-CoV-2 has a multiplicity of infection (MOI) of 0.05. The study demonstrated that chloroquine was very effective in reducing viral replication, with an effective concentration (EC) 90 of 6.90 μM which can be easily achievable with standard dosage, thanks to its favorable penetration in tissues, including in the lungs [7]. The authors described that chloroquine is known to block viral infection by increasing endosomal pH and by interfering with the glycosylation of the SARSCoV cellular receptor. The authors also speculated on the possibility that the known immunomodulating effect of the drug could improve the antiviral effect in vivo [7].
The article published online on 19th February, draws its results from a clinical trial conducted in more than ten Chinese hospitals (in Wuhan - epicenter of the epidemic -, Beijing and Shanghai in particular) to measure the effectiveness of chloroquine on treatment of pneumonia associated with Covid-19 [13]. In this study, it was confirmed that the anti-viral and anti-inflammatory activities of chloroquine may account for its potent efficacy in treating patients with COVID-19 pneumonia. According to Chinese researchers, a treatment of 500 mg of chloroquine per day for ten days would be sufficient. According to Chinese researchers, a treatment of 500 mg of chloroquine per day for ten days would be sufficient.
Chinese researchers point out that the results obtained so far on more than 100 patients have shown that chloroquine phosphate was more effective than the treatment received by the comparative group to contain the development of pneumonia, to improve the condition of the lungs, to make the patient negative for the virus again and to shorten the duration of the illness [13]. However, the brief study does not quantify this difference in effectiveness. Based on the results of this Chinese clinical study, the Director of the Mediterranean Infection Institute in 3eille, 3eille center of expertise on infectious diseases, assured, Tuesday, 25th February, 2020, to France-Press Agency (FPA) that an ordinary treatment with chloroquine, a drug commonly used against malaria, has shown signs of efficacy against the coronavirus. He considers that the use of chloroquine is a preferred route according to him rather than the search for a vaccine which could not be available anyway for long months. While opinions diverge and critics on the effectiveness of its treatment with chloroquine to treat patients with coronavirus, a second study was published on-line on 27th March, 2020 by the team of researchers from 3eille on 80 patients, aged 18 and 88 and suffering from coronavirus [14]. The objective of this study is to defend chloroquine and to prove the effectiveness of the protocol adopted and the relevance of the combination of hydroxychloroquine and azithromycin. According to this study [14], only one person (86 years old) who received this treatment has died and another is still in serious condition. The remaining 78 patients experienced clinical improvement in their medical condition and left intensive care after five days. The majority (65/80, 81.3%) of the patients had favorable results and were discharged. Only 15% required oxygen therapy [14]. According to the newspaper “Capital”, critics of the study were however formulated by other scientists. Their main critic is that the study does not include a control group in order to make a comparison [15]. The Mediterranean Infection Institute in 3eille published on the same day on 27th March, 2020 a new article demonstrated that the combination of hydroxychloroquine and azithromycin has a synergistic effect in vitro on SARS-CoV-2 at concentrations compatible with that obtained in human lung [16]. Tested in China, and partially in France and the United States, chloroquine, an antimalarial substance, is carefully administered in Switzerland as a treatment against the Covid-19 pandemic. The clinical effect is encouraging since the severity of the disease has decreased in the sample of patients subjected to the pilot experiment. This treatment can be administered to patients who suffer from severe forms of Covid-19, but should not be used for less severe forms, warns Public Health France. France, which has long procrastinated on the use of chloroquine, has just authorized its use on Tuesday, 245th March, 2020 with very restricted conditions. The United States has also approved the use of chloroquine, which has shown very encouraging preliminary results [17]. In Morocco, the technical and scientific committee of the Ministry of Health decided to prescribe chloroquine for all patients, and not only severe cases, with treatment, dosage, monitoring and control procedures to be carried out from 23rd March, 2020 [18].
Conclusion
Despite efforts in clinical trials to assess the efficacy of drugs, previously used in other diseases, for the treatment of COVID-19. However, given the current serious global epidemiological situation and given the urgent therapeutic need. It is interesting to do more research to find a specific vaccine or drug for this epidemic in order to manage this health crisis effectively, to limit the rapid spread and to save humanity.
Declaration of Competing Interest
S. Saqrane and M. A. El Mhammedi declare to have no conflict of interest.
Acknowledgment
The authors would like to thank University Sultan Moulay Slimane of Beni Mellal, Morocco for supporting this work.
References
- 1.https://www.pasteur.fr/fr/centre-medical/fiches-maladies/coronavirus-wuhan
- 2.https://www.who.int/fr/emergencies/diseases/novel-coronavirus2019/advice-for-public/q-a-coronaviruses
- 3.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200402-sitrep-73-covid-19.pdf?sfvrsn=5ae25bc7_4
- 4.http://www.euro.who.int/fr/health-topics/health-emergencies/coronavirus-covid 19/news/news/2020/3/who-announces-covid-19-outbreak-a-pandemic
- 5.https://www.lemonde.fr/afrique/article/2020/03/16/covid-19-le-tour-d-afrique-des mesures_6033295_3212.html
- 6.Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect Dis. 2003;3:722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020 Mar;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colson P., Rolain J.M., Lagier J.C., Brouqui P., Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020 Mar 4:105932. doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., Liu X., Zhao L., Dong E., Song C., Zhan S., Lu R., Li H., Tan W., Liu D. In Vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020 Mar 9 doi: 10.1093/cid/ciaa237. pii: ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan Y., Zou Z., Sun Y., Li X., Xu K.F., Wei Y., Jin N., Jiang C. Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell Res. 2013;23:300–302. doi: 10.1038/cr.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortegiani A., Ingoglia G., Ippolito M. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020 March doi: 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020 doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 14.https://www.mediterranee-infection.com/wp-content/uploads/2020/03/COVID-IHU-2-1.pdf
- 15.https://www.capital.fr/economie-politique/didier-raoult-publie-une-nouvelle-etude-pour-defendre-la-chloroquine-1366067
- 16.https://www.mediterranee-infection.com/wp-content/uploads/2020/03/La-Scola-et-al-V1.pdf
- 17.https://fr.le360.ma/societe/coronavirus-plusieurs-jours-apres-le-maroc-la-france-autorise-la-chloroquine-211583
- 18.https://lobservateur.info/news/un-medicament-antipaludique-serait-efficace-contre-le-coronavirus/