Abstract
Leishmaniases are a group of infectious diseases caused by protozoan Leishmania parasites and are transmitted by the bites of infected phlebotomine sandflies. The heterogeneity of these diseases is influenced by both parasitic properties and host factors. Cutaneous leishmaniasis (CL) is a major public health problem in Morocco, where the geographical expansion of CL (particularly CL caused by Leishmania tropica), the heterogeneous appearance of lesions and the difficulty in diagnosing CL contribute to late diagnosis of CL and delayed treatment of patients. Therefore, the main objective of this study was to describe the epidemiological and clinical profiles of patients with CL diagnosed in Casablanca (Morocco), which is a non-endemic area for CL. A cross-sectional study was conducted between 2010 and 2016, during which epidemiological and clinical data were collected from patients that met the inclusion criteria through an information sheet. Then, samples were obtained from each patient for parasitological and molecular diagnosis, and only patients with positive polymerase chain reaction and genotyping results were included in the study. Overall, 106 cases of CL were genotyped, of which 61 (57.5%) were caused by L. tropica, 38 (35.9%) by L. major and 7 (6.6%) by L. infantum. While all age groups were affected, CL cases wherein L. tropica was the causative agent were most frequently diagnosed in children aged 0–9 years (p = 0.005), whereas those caused by L. major were more frequently diagnosed in elderly patients (p = 0.004). Multivariate logistic regression analysis showed that two clinical variables were significantly associated with CL caused by L. tropica: lesion size (p = 0.002) and occurrence of lesion on the face (p = 0.005). Furthermore, the results of our survey highlighted the association of Leishmania infection when travelling to endemic areas. The high number of endemic foci where patients with CL were infected with L. tropica illustrated the tendency of this form to spread and generate epidemics, exposing young people to a greater degree to the disease. The epidemic status of CL caused by L. tropica in Morocco and the increased movement of the population from rural to urban areas indicate a possible introduction of this species to urban areas.
Keywords: Cutaneous leishmaniasis, Leishmania tropica, Leishmania major, Clinical profile, Epidemiological profile, Morocco
1. Introduction
Leishmaniases are a group of infectious diseases caused by protozoan Leishmania parasites and are transmitted by the bites of infected female phlebotomine sandflies (Reithinger et al., 2007). These diseases are currently endemic in 98 countries worldwide (WHO, 2016), with up to 1 million new cases being estimated to occur annually (WHO, 2014). Leishmaniases are highly prevalent in North Africa (Aoun and Bouratbine, 2014), with cutaneous leishmaniasis (CL) being a major public health problem in Morocco, where it is caused by three Leishmania species: (1) L. major, which is the causative agent of zoonotic CL, the first form to be described in Morocco by Foley and Vialate in 1914 (Rhajaoui et al., 2004); (2) L. tropica, which is the causative agent of anthroponotic CL and (3) L. infantum, which is responsible for sporadic CL in northern areas where zoonotic visceral leishmaniasis has been endemic since 1920 (Hmamouch et al., 2014). The annual frequency of documented patients with CL fluctuates across years, with 8707 reported cases in 2010 (Ministry of Health, 2016).
Several issues are faced when attempting to prevent the spread of CL in Morocco, including the geographical expansion of the disease to new areas where it was previously unknown, particularly of CL caused by L. tropica; the heterogeneity of lesion appearance and the difficulty in diagnosing CL, all of which contribute to the late diagnosis of CL and delayed treatment of patients. Leishmania tropica is the Leishmania species with the widest geographical distribution in Morocco and the highest incidence in North Africa (Aoun and Bouratbine, 2014). Therefore, the objective of this study was to describe the epidemiological and clinical profiles of patients with CL diagnosed at Ibn Rochd University Hospital in Casablanca (Morocco), which is a non-endemic area for CL.
2. Patients and methods
2.1. Ethics statements
This study was conducted according to the principles specified in the Declaration of Helsinki and the local ethical guidelines of the Ethics Committee for Biomedical Research of the Faculty of Medicine and Pharmacy, University Hassan II of Casablanca, Morocco (International Review Board 00002504). Dermal tissue aspirates are routinely sampled by a dermatologist at the Department of Dermatology, University Hospital Ibn Rochd, Casablanca, for parasitological confirmation of CL prior to any treatment prescription. The confidentiality of personal and clinical data was guaranteed, and the entire dataset was processed anonymously.
2.2. Description of the study area
Casablanca is a large industrialised city situated on the Atlantic coast in the Casablanca-Settat region of northwest Morocco. This region is predominantly urban (urbanisation rate = 92.9% in 2008) and has high industrial activity (Haut Commissariat au Plan, 2010). According to the Moroccan Ministry of Health data, Casablanca is a non-endemic city for CL (Ministry of Health, 2016).
2.3. Epidemiological and clinical data
A cross-sectional study was conducted in Casablanca between 2010 and 2016, during which several variables were collected from patients who met the inclusion criteria, as outlined below.
2.3.1. Inclusion criteria
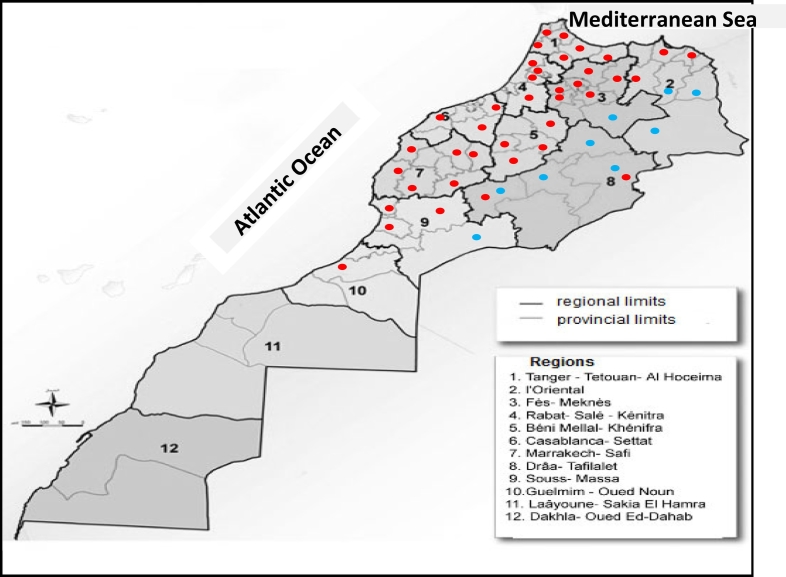
All patients who presented to the Department of Dermatology and Venereology of Casablanca Ibn Rochd University Hospital and met the following WHO criteria (WHO, 2014) were included: ≥1 lesions suggestive of CL presenting as papule or nodule, ulcerated or not, evolving for several weeks on the uncovered parts of their body, that had appeared since September and persisted after 3 weeks of usual therapeutics (i.e. antiseptics and local antibiotics) independently from any history of travel or residency in known endemic areas for CL in Morocco based on data from the Ministry of Health (Fig. 1).
Fig. 1.
Map showing the location of the endemic foci of cutaneous leishmaniasis due to Leishmania major (blue dots) and L. tropica (red dots) in Morocco according to Kahime et al. (2016).
No limitation in terms of age or sex of patients was considered. Finally, only patients with positive polymerase chain reaction (PCR) and genotyping results were analysed in this study, regardless of their epidemiological and clinical data.
2.3.2. Epidemiological and clinical variables
The following variables were recorded for each patient: age, sex, geographic origin of the infection, date of onset, disease duration at diagnosis, clinical presentation (papule, nodule, ulcer, vegetant/verrucous, lupoid, subcutaneous nodule and red halo), body location and size of dermal lesions. Any information related to travel to known endemic areas and the presence of similar cases in the family was also noted (Appendix A).
2.4. Diagnosis of CL
Samples were obtained from all patients who met the inclusion criteria for parasitological and molecular diagnosis. This was conducted at the Laboratory of Parasitology of the Faculty of Medicine and Pharmacy, University Hassan II of Casablanca.
Dermal fluid was collected from the border of active skin lesions in each patient using a syringe, and diagnosis was made through direct examination, parasite culture and restriction fragment length polymorphism analysis of PCR-amplified internal transcribed spacer 1 region (ITS1 PCR-RFLP), as described by Mouttaki et al. (2014). The disease was confirmed when the results of direct examination, culture and/or PCR were positive.
2.5. Statistical analysis
A simple descriptive analysis of data was first performed, in which quantitative variables are expressed as mean ± standard deviations and qualitative variables as percentages. Bivariate analysis was performed to examine possible associations among some variables using the chi-square and Fisher's exact tests for qualitative variables and Student's t-test for quantitative variables. Finally, multivariate logistic regression analysis was performed to estimate adjusted odds ratio and its 95% confidence interval for each selected factor (conservative threshold = 0.20). Outcome variable was genotyped Leishmania species, and regression model predictors were age and sex of patients, place of residence, similar cases in the family, clinical aspects, size and location of lesions, multiple or single lesions and incubation time. All statistical tests were bilateral and were performed using the SPSS 16.0 software using a threshold of Pearson's stability of 0.05.
3. Results
Over the 7-year study period, 106 cases of CL were diagnosed and genotyped, among which 61 (57.5%) were caused by L. tropica, 38 (35.9%) by L. major and 7 (6.6%) by L. infantum. Because only few cases were caused by L. infantum, only epidemiological data of patients with CL caused by L. tropica and L. major were included in subsequent analyses (n = 99; Table 1; Appendix B).
Table 1.
Epidemiological characteristics of patients diagnosed with cutaneous leishmaniasis caused by Leishmania tropica and L. major in Casablanca between 2010 and 2016.
| Patients n = 99 (%) |
p-value | Odds ratio | Multivariate logistic regression 95% CI | p-value | ||
|---|---|---|---|---|---|---|
|
L. tropica n = 61(61.6) |
L. major n = 38(38.4) |
|||||
| Age (mean ± SD) | 25.5 ± 23.5 | 41.2 ± 22.9 | 0.002 | 1.025 | 1.006–1.045 | 0.012 |
| Sex | ||||||
| Male | 25 (41.0) | 14 (36.8) | 0.423 | – | – | – |
| Female | 36 (59.0) | 24 (63.2) | ||||
| Place of residence | ||||||
| Urban and peri-urban | 16 (26.2) | 7 (18.4) | 0.984 | – | – | – |
| Rural | 42 (68.8) | 28 (73.7) | ||||
| Not determined | 3 (5.0) | 3 (7.9) | ||||
| Similar cases in the familya | 19 (31.1) | 11 (29.0) | 0.500 | – | – | – |
CI: confidence interval.
Only for patients living in endemic areas.
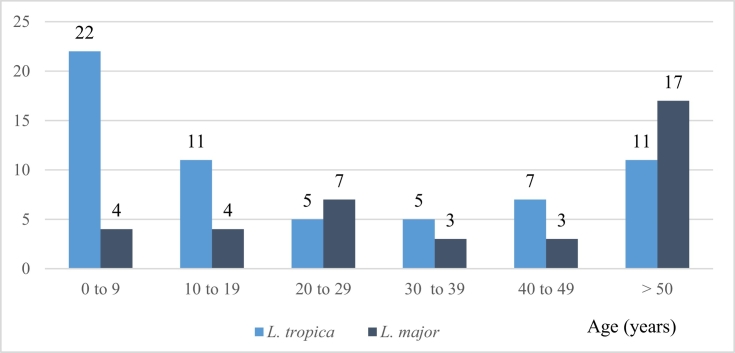
There was a slight female bias in the susceptibility of patients to CL caused by L. tropica and L. major, but this was not statistically significant. All age groups were affected by CL caused by both species, but CL caused by L. tropica was more frequently diagnosed in children aged 0–9 years (p = 0.005), whereas CL caused by L. major was more frequently diagnosed in elderly patients aged >50 years (p = 0.004) (Fig. 2). Consequently, the mean age of patients with CL caused by L. tropica was significantly lower than that of patients with CL caused by L. major (25 ± 23.5 vs. 41 ± 22.9, respectively; p = 0.002).
Fig. 2.
Distribution of patients in different age categories who were diagnosed with cutaneous leishmaniasis caused by Leishmania tropica and L. major in Casablanca between 2010 and 2016.
Among the patients who were included in the analysis, 75% (74/99) had a history of travelling to other known endemic areas in Morocco and 20% (20/99) resided in the endemic focus where they were infected, whereas the remaining 5% (5/99) had no history of residence in or travel to a known focus. Overall, the frequency of CL was not significantly associated with a history of residing in urban/peri-urban or rural endemic regions. Furthermore, 63.2% (24/38) of patients with CL caused by L. major were infected in the same southern endemic region (Daraa Tafilalet), whereas L. tropica infections occurred in 9 different endemic regions, with 26.2% (16/61) of these patients also being infected in Daraa Tafilalet (Fig. 1, Appendix C). There was no association between the residence of patients with CL in endemic foci and the presence of similar cases in their family.
Clinically, the appearance of lesions was heterogeneous (Table 2). Dermal lesions presented mainly as papules, nodules and ulcers, with no significant difference in terms of appearance of these lesions between CL caused by L. major and that by L. tropica. However, there was a significant difference in the location and size of lesions caused by the two species, with lesions resulting from L. major mainly occurring on the lower limbs (p = 0.001) and those resulting from L. tropica mainly occurring on the face (p = 0.001) and being significantly smaller (<2 cm; p = 0.001). Furthermore, 90.5% (86/95) of the patients presented with lesions that had been developing for >2 months at the time of diagnosis, whereas 33.7% (32/95) had lesions that had been developing for >6 months (Appendix D).
Table 2.
Clinical characteristics of patients diagnosed with cutaneous leishmaniasis caused by Leishmania tropica and L. major in Casablanca between 2010 and 2016.
| Characteristic | Patients n = 99 (%) |
p-value |
Odds ratio |
Multivariate logistic regression 95% CI |
p-value |
|
|---|---|---|---|---|---|---|
|
L. tropica n = 61(61.6) |
L. major n = 38 (38.4) |
|||||
| Clinical aspects (n = 215 lesions) | ||||||
| Cicatricial lesion/scar | 3 (2.3) | 3 (3.7) | 0.673 | – | – | – |
| Papule | 28 (21.0) | 12 (14.6) | 0.158 | 0.931 | 0.338–0.567 | 0.891 |
| Nodule | 49 (36.9) | 29 (35.4) | 0.635 | – | – | – |
| Ulcer | 19 (14.3) | 18 (22.0) | 0.105 | 1.012 | 0.357–2.864 | 0.983 |
| Vegetant/verrucous | 7 (5.3) | 6 (7.3) | 0.554 | – | – | – |
| Lupoid | 5 (3.7) | 3 (3.6) | 1.000 | – | – | – |
| Subcutaneous nodule | 1 (3.7) | 1 (1.2) | 1.000 | – | – | – |
| Purple red halo | 21 (15.8) | 10 (12.2) | 0.397 | – | – | – |
| Size | ||||||
| <2 cm | 38 (62.3) | 9 (23.7) | 0.001 | 4.378 | 1.694–11.313 | 0.002 |
| ≥2 cm | 23 (37.7) | 29 (76.3) | ||||
| Lesion body location (n = 123 lesions) | ||||||
| Face | 46 (62.2) | 15 (30.6) | 0.001 | 0.264 | 0.105–0.664 | 0.005 |
| Upper limb | 17 (23.0) | 15 (30.6) | 0.230 | – | – | – |
| Lower limb | 10 (13.5) | 17 (34.7) | 0.001 | 0.264 | 0.382–5.084 | 0.615 |
| Trunk | 1 (1.3) | 2 (4.1) | 0.557 | – | – | – |
| Single lesion | 30 (49.2) | 17 (44.7) | 0.667 | – | – | – |
| Multiple lesions | 31 (50.8) | 21 (55.3) | 0.667 | – | – | – |
| Incubation timea | ||||||
| <4 months | 23 (53.4) | 13 (44.8) | 0.471 | – | – | – |
| ≥4 months | 20 (46.6) | 16 (55.2) | ||||
CI: confidence interval.
Patients residing in endemic foci were excluded from this analysis.
Finally, multivariate linear regression analysis (ascending Wald) showed that three clinical variables were highly associated with CL caused by L. tropica: lesion size (p = 0.002), location on the face (p = 0.005) and young age of patients (Table 1; p = 0.012).
4. Discussion
Our analysis of patients with CL diagnosed at Ibn Rochd Hospital showed that three Leishmania species are responsible for CL in Casablanca, with L. tropica (57.5%) and L. major (35.9%) causing the highest number of cases, which reflects the national situation of Morocco. The low frequency of L. infantum genotype suggests that this zoonotic form occurs sporadically or is under-recognised and consequently under-diagnosed.
Moroccan foci of zoonotic CL are located in a large area stretching from the Atlantic coast south of the Anti-Atlas Mountains to the northeast of the country (Fig. 1). Zoonotic CL caused by L. major became an epidemic in southern arid regions at the end of the 1970s, with alternating endemic and epidemic cycles (Rhajaoui et al., 2007). However, over the last decade, there has been an unusual persistence of the epidemics, with annual and regional fluctuations (Riyad et al., 2013). The factors that influence the spatial and temporal dynamics of zoonotic CL are not well understood but may include the dynamics of rodent populations, dispersal of vectors, climate changes, vegetation and soil types as well as high density of human populations in areas where the sylvatic transmission of leishmaniasis is high (rodent–vector–rodent cycle) (Riyad et al., 2013; El Hamouchi et al., 2019).
In our passive survey of patients with CL in the non-endemic region of Casablanca between 2010 and 2016, 75% had a history of travel to endemic regions of Morocco, highlighting the potential risk of being infected with Leishmania when travelling to endemic areas. Furthermore, while 63.2% of patients with CL caused by L. major were infected in the same highly endemic region, patients with CL caused by L. tropica were infected in 9 different regions, illustrating the tendency of this species to expand geographically into new areas that were previously non-endemic (Ministry of Health, 2016). Over the last few decades, L. tropica foci have spread to several regions of Morocco including those that previously reported CL caused by L. major or L. infantum. Indeed, Ait Kbaich et al. (2017) recently reported the presence of all three Leishmania species responsible for CL in a region that was traditionally considered endemic for zoonotic CL caused by L. major, demonstrating the changing geographical patterns of these species, particularly L. tropica. Furthermore, El Alem et al. (2018) conducted an epidemiological study on cases of CL in 6 provinces in southwestern Morocco between 2000 and 2016, and ITS1 PCR-RFLP revealed the presence of L. tropica in all six of these provinces.
The North African form of CL caused by L. tropica remains clearly distinguishable from the anthroponotic urban and hyper-endemic CL observed in cities in the Middle East and Central Asia (Aoun and Bouratbine, 2014). During the 1980s, this North African form prevailed in scattered hypo-endemic rural foci and small towns located in the arid mountains where the disease was sporadic. However, since the late 1990s, several epidemics with hundreds of cases have been reported in several Moroccan cities (Rhajaoui, 2011), possibly as a result of migrations and population movements from endemic to non-endemic cities as well as human population growth in poor habitat. The introduction of leishmaniasis to a new area relies on a well-established sandfly population to act as a vector. The extended geographical distribution of Phlebotomus sergenti, which is the proven vector of L. tropica in Morocco, resulted in the spread of anthroponotic CL in both rural and urban/peri-urban areas (Kahime et al., 2016). Yahia et al. (2014) showed that nearly all mitochondrial DNA haplotypes and lineages of P. sergenti in Morocco have marked regionality, suggesting that they have undergone natural dispersal that was not human assisted. No autochthonous cases of anthroponotic CL have been detected in the Iberian Peninsula, despite P. sergenti commonly being found at sufficient densities to act as a vector. However, Merino-Espinosa et al. (2016) found that one lineage appears to have adaptive advantages (a wider tolerance to temperature and altitude) that would make it better suited to leading geographical expansion into the rest of Europe. Thus, this risk should be assessed by conducted entomological surveys in peri-urban areas of Morocco to search for vector species and determine Leishmania infection rates.
We detected a slightly but not significantly higher rate of CL caused by L. tropica and L. major in females than in males, consistent with the findings of previous studies (Masmoudi et al., 2007; Bennis et al., 2015). Furthermore, in a recent zoonotic CL outbreak in Morocco, significantly more females were infected than males (El Hamouchi et al., 2019). Several surveys have shown that women and children may consult medical professionals more frequently for aesthetic reasons and the potential social impact of the lesions (Bennis et al., 2017) because although these lesions heal spontaneously, they can cause facial scars, which can lead to a social stigma, particularly among women.
Patients who were included in our study were aged from 7 months to 85 years. The age at which people are affected by CL seems to depend on the parasite transmission intensity (force of infection) to which the population is exposed. In established endemic CL foci, the prevalence generally increases with age up to 15 years, after which it stabilises, probably reflecting the progressive acquisition of immunity (El Hamouchi et al., 2019). However, most patients with CL caused by L. tropica were aged <9 years, whereas most patients with CL caused by L. major were aged >50 years. The active geographical spread of L. tropica leading to new epidemic foci may, at least in part, explain why young patients are more exposed to infection because they will be immunologically naive and lack or have low specific anti-Leishmania immunity (Chraiet-Rezgani et al., 2016). To better understand why patients aged >50 years more often presented with CL caused by L. major, we compared the dates of diagnosis with the epidemiological situation in the province where they were infected the previous summer, according to the reports of the Ministry of Health. However, we found no correlation between these, suggesting that this finding is an artefact of our passive survey being conducted in a non-endemic area. Multivariate logistic regression analysis also showed that patients infected with L tropica were younger than those infected with L. major.
Clinically, there was clear heterogeneity in the appearance of lesions that was not significantly associated with Leishmania causing the infection (Table 2). However, the lesions caused by L. tropica were significantly smaller (<2 cm in diameter) than those caused by L. major, as previously reported (Chaara et al., 2014). Furthermore, the lesions caused by L. tropica mostly occurred on the face (p = 0.001) and were multiple in 50.8% (31/61) of patients. Multivariate logistic regression analysis showed that small facial lesions were significantly associated with L. tropica. Therefore, facial lesions could be a good criterion for predicting the responsible species. Aoun et al. (2012) suggested that lower night time temperatures in chronic CL foci in Tunisia can explain the predominance of facial lesions. Classically, L. major is characterised by multiple lesions that are often contiguous, which may be explained by the inability of P. papatasi to obtain a sufficient blood meal in a single bite (Aoun and Bouratbine, 2014). Thus, CL caused by L. tropica tended to be linked to multiple lesions, but L. major is also characterised by this; therefore, it cannot be used as a diagnostic feature. The clinical appearance of lesions also depends on the immune status of the host and T-lymphocyte response (Bailey and Lockwood, 2007) as well as parasite genotype and its burden (Aoun and Bouratbine, 2014), with experimental infections showing that sandfly salivary antigens affect local host immune responses (Reithinger et al., 2007).
Finally, no significant difference was found between the incubation periods of L. tropica and L. major (Table 2), although this may have been due to unreliable data being obtained through patients' anamneses. Furthermore, most patients presented with lesions that had been developing for >2 months (86.9%), with some having been developing for as much as 6 months (33.7%). These delayed medical consultations may be explained, at least in part, by misdiagnosis by physicians in Casablanca, which may have been due to the variety of differential diagnostic criteria for CL or a lack of knowledge about the disease.
5. Conclusion
Our survey of patients diagnosed with CL in Casablanca, which is a non-endemic city, highlighted the potential exposition to Leishmania infection when travelling to endemic CL areas. Thus, there is a real need for the implementation of prevention strategies as well as improved health education by informing people and physicians about CL in both endemic and non-endemic regions. The growing mobility of humans from rural to urban areas raises the possibility of emerging CL foci, particularly of L. tropica which is responsible for the anthroponotic form in Morocco. On the other hand, we found that the small size and facial location of dermal lesions were the only factors that were significantly associated with CL caused by L. tropica. However, an accurate diagnosis is essential because this will lead to the most appropriate treatment being used, which will improve and accelerate the healing process, helping to avoid drug resistance and leading to efficient preventive measures.
Acknowledgments
Acknowledgements
We thank Prof Samira Nani, Department of Epidemiology, Laboratory of Epidemiology, Faculty of Medicine and Pharmacy, University Hassan II of Casablanca, Morocco, for her review of the whole document and her help and approval of the statistical analysis.
We also thank Prof Fadila Guessous, Department of Microbiology, Immunology and Cancer Biology, Medical school, University of Virginia, Charlottesville, USA for reviewing the manuscript.
The authors would like to thank Enago (www.enago.com) for the English language review.
Funding
This study was funded by the University of Granada in Spain (Prg. CICODE 2010 and 2013) and the Moroccan Ministry of National Education, Professional Training, Higher Education and Scientific Research (CNRST, Prg. PPR/2015/27).
Appendix A.
Appendix B.
Results of parasitological diagnosis of patients with cutaneous leishmaniasis caused by Leishmania tropica and L. major in Casablanca between 2010 and 2016.
| Parasitological diagnosis | Patients n = 99 (%) |
|
|---|---|---|
|
L. tropica n = 61(61.6) |
L. major n = 38(38.4) |
|
| Direct examination | ||
| Positive | 31(50.8) | 15(39.5) |
| Negative | 14(23.0) | 19(50.0) |
| Not done | 16(26.2) | 4(10.5) |
| Culture | ||
| Positive | 37(60.6) | 16(42.1) |
| Negative | 24(39.4) | 22(57.9) |
Appendix C.
Regions visited by patients diagnosed with cutaneous leishmaniasis caused by Leishmania tropica and L. major in Casablanca between 2010 and 2016.
| Regions | Patients n = 99(%) |
|
|---|---|---|
|
L. tropica n = 61(61.6) |
L. major n = 38(38.4) |
|
| Beni Mellal Khenifra | 12(19.7) | 1(2.6) |
| Casablanca-Settat | – | 2(5.3) |
| Draa Tafilalet | 16(26.2) | 25(65.8) |
| Fes-Meknes | 9(14.7) | 2(5.3) |
| Marrakech-Safi | 10(16.4) | 3(7.9) |
| Oriental | 2(3.4) | – |
| Rabat Sale Kenitra | 2(3.3) | – |
| Sous Massa | 4(6.5) | 5(13.1) |
| Tanger Tetouan Al Hoceima | 6(9.8) | – |
Appendix D.
Evolution of lesions at diagnosis of patients diagnosed with cutaneous leishmaniasis caused by Leishmania tropica and L. major in Casablanca between 2010 and 2016.
| Evolution of lesions at diagnosis (months) | Patients n = 99(%) |
|
|---|---|---|
|
L. tropica n = 61(61.6) |
L. major n = 38(38.4) |
|
| <2 | 6(9.9) | 3(7.9) |
| 2–5 | 33(54.1) | 21(55.3) |
| ≥ 6 | 19(31.1) | 13(34.2) |
| ND | 3(4.9) | 1(2.6) |
ND: not determined.
References
- Ait Kbaich M., Mhaidi I., Ezzahidi A., Dersi N., El Hamouchi A., Riyad M., Akarid K., Lemrani M. New epidemiological pattern of cutaneous leishmaniasis in two pre-Saharan arid provinces, southern Morocco. Acta Trop. 2017;173:11–16. doi: 10.1016/j.actatropica.2017.05.016. [DOI] [PubMed] [Google Scholar]
- Aoun K., Bouratbine A. Cutaneous leishmaniasis in North Africa: a review. Parasite. 2014;21:14. doi: 10.1051/parasite/2014014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoun K., Ben Abda I., Bousslimi N., Bettaieb J., Siala E., Ben Abdallah R., Benmously R., Bouratbine A. Comparative characterization of skin lesions observed in the three endemic varieties of cutaneous leishmaniasis in Tunisia. Ann. Derm. Venereol. 2012;139(6–7):452–458. doi: 10.1016/j.annder.2012.04.154. [DOI] [PubMed] [Google Scholar]
- Bailey M.S., Lockwood D.N. Cutaneous leishmaniasis. Clin. Dermatol. 2007;25(2):203–211. doi: 10.1016/j.clindermatol.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Bennis I., De Brouwere V., Ameur B., El Idrissi Laamrani A., Chichaoui S., Hamid S., Boelaert M. Control of cutaneous leishmaniasis caused by Leishmania major in south-eastern Morocco. Tropical Med. Int. Health. 2015;20(10):1297–1305. doi: 10.1111/tmi.12543. [DOI] [PubMed] [Google Scholar]
- Bennis I., Thys S., Filali H., De Brouwere V., Sahibi H., Boelaert Psychosocial impact of scars due to cutaneous leishmaniasis on high school students in Errachidia province. Morocco. Infect. Dis. Poverty. 2017;6(1):46. doi: 10.1186/s40249-017-0267-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaara D., Haouas N., Dedet J.P., Babba H., Pratlong F. Leishmaniases in Maghreb: an endemic neglected disease. Acta Trop. 2014;132:80–93. doi: 10.1016/j.actatropica.2013.12.018. [DOI] [PubMed] [Google Scholar]
- Chraiet-Rezgani K., Bouafif-Ben Alaya N., Habboul Z., Hajjej Y., Aoun K. Epidemiological and clinical features of cutaneous leishmaniasis in Kairouan-Tunisia and characteristics in children. Bull. Soc. Pathol. Exot. 2016;109(2):80–83. doi: 10.1007/s13149-016-0475-4. [DOI] [PubMed] [Google Scholar]
- El Alem M.M.M., Hakkour M., Hmamouch A., Halhali M., Delouane B., Habbari K., Fellah H., Sadak A., Sebti F. Risk factors and prediction analysis of cutaneous leishmaniasis due to Leishmania tropica in southwestern Morocco. Infect. Genet. Evol. 2018;61:84–91. doi: 10.1016/j.meegid.2018.03.017. [DOI] [PubMed] [Google Scholar]
- El Hamouchi A., Daoui O., Ait Kbaich M., Mhaidi I., El Kacem S., Guizani I., Sarih M., Lemrani M. Epidemiological features of a recent zoonotic cutaneous leishmaniasis outbreak in Zagora province, southern Morocco. PLoS Negl. Trop. Dis. 2019;13(4) doi: 10.1371/journal.pntd.0007321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haut Commissariat au Plan, Royaume du Maroc, Direction Régionale du Grand Casablanca, 2010. Monographie de la région du grand Casablanca. 143 pg.
- Hmamouch A., Amarir F., Fellah H., Karzaz M., Bekhti K., Rhajaoui M., Sebti F. Coexistence of Leishmania tropica and Leishmania infantum in Sefrou province. Morocco. Acta Trop. 2014;130:94–99. doi: 10.1016/j.actatropica.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Kahime K., Boussaa S., Laamrani-El Idrissi A., Nhammi H., Boumezzough A. Epidemiological study on acute cutaneous leishmaniasis in Morocco. JAD. 2016;5(1):41–45. [Google Scholar]
- Masmoudi A., Ayadi N., Boudaya S., Meziou T.J., Mseddi M., Marrekchi S., Bouassida S., Turki H., Zahaf A. Clinical polymorphism of cutaneous leishmaniasis in Centre and south of Tunisia. Bull. Soc. Pathol. Exot. 2007;100(1):36–40. [PubMed] [Google Scholar]
- Merino-Espinosa G., Corpas-López V., Callejón-Fernández R., Porcel-Rodríguez L., Díaz-Sáez V., Gállego M., Ballart C., Molina R., Jiménez M., Morillas-Márquez F., Martín-Sánchez J. Differential ecological traits of two Phlebotomus sergenti mitochondrial lineages in southwestern Europe and their epidemiological implications. Tropical Med. Int. Health. 2016;21(5):630–641. doi: 10.1111/tmi.12686. [DOI] [PubMed] [Google Scholar]
- Ministry of Health, Morocco . 2016. Santé en Chiffres 2015. 168 pg. [Google Scholar]
- Mouttaki T., Morales-Yuste M., Merino-Espinosa G., Chiheb S., Fellah H., Martin-Sanchez J., Riyad M. Molecular diagnosis of cutaneous leishmaniasis and identification of the causative Leishmania species in Morocco by using three PCR-based assays. Parasit. Vectors. 2014;7:420. doi: 10.1186/1756-3305-7-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reithinger R., Dujardin J.C., Louzir H., Pirmez C., Alexander B., Brooker S. Cutaneous leishmaniasis. Lancet Infect. Dis. 2007;7(9):581–596. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- Rhajaoui M. Human leishmaniases in Morocco: a nosogeographical diversity. Pathol. Biol. 2011;59(4):226–229. doi: 10.1016/j.patbio.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Rhajaoui M., Fellah H., Pratlong F., Dedet J.P., Lyagoubi M. Leishmaniasis due to Leishmania tropica MON-102 in a new Moroccan focus. Trans. R. Soc. Trop. Med. Hyg. 2004;98(5):299–301. doi: 10.1016/S0035-9203(03)00071-3. [DOI] [PubMed] [Google Scholar]
- Rhajaoui M., Nasereddin A., Fellah H., Azmi K., Amarir F., Al-Jawabreh A., Ereqat S., Planer J., Abdeen Z. New clinico-epidemiologic profile of cutaneous leishmaniasis, Morocco. Emerg. Infect. Dis. 2007;13(9):1358–1360. doi: 10.3201/eid1309.070946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riyad M., Chiheb S., Soussi-Abdallaoui M. Cutaneous leishmaniasis caused by Leishmania major in Morocco: still a topical question. EMHJ. 2013;19(5):495–501. [PubMed] [Google Scholar]
- World Health Organization . 2014. Manual for case management of cutaneous leishmaniasis in the WHO Eastern Mediterranean Region. 50 pg. [Google Scholar]
- World Health Organization . 22 (91) 2016. Weekly Epidemiological Record; pp. 285–296. [Google Scholar]
- Yahia H., Ready P.D., Hamdani A., Testa J.M., Guessous-Idrissi N. Regional genetic differentiation of Phlebotomus sergenti in three Moroccan foci of cutaneous leishmaniasis caused by Leishmania tropica. Parasite. 2014;11(2):189–199. doi: 10.1051/parasite/2004112189. [DOI] [PubMed] [Google Scholar]