Abstract
The genetically engineered Chimeric Antigen Receptor bearing T-cell (CAR T cell) therapy has been emerged as the new paradigm of cancer immunotherapy. However, recent studies have reported an increase in the number of relapsed haematological malignancies. This review provides newer insights into how the efficacy of CAR T cells might be increased by the application of new genome editing technologies, monitoring the complexity of tumor types and T cells sub-types. Next, tumor mutation burden along with tumormicroenvironment and epigenetic mechanisms of CAR T cell as well as tumor cell may play a vital role to tackle the cancer resistance mechanisms. These studies highlight the need to consider traditional cancer therapy in conjunction with CAR T cell therapy for relapsed or cases unresponsive to treatment. Of note, this therapy is highly expensive and requires multi-skill for successful implementation, which results in reduction of its accessibility/affordability to the patients. Here, we also propose a model for cost minimization of CAR T cell therapy by a collaboration of academia, hospitals and industry.
Keywords: Immunotherapy, CAR T cells, Tumor mutation burden, Epigenetics, Combination therapy, Affordable, Cell biology, Immunology, Genetics, Proteins, Biochemistry, Molecular biology, Cancer research, Health sciences, Oncology, Clinical research
Immunotherapy, CAR T cells, Tumor mutation burden, Epigenetics, Combination therapy, Affordable; Cell biology; Immunology; Genetics; Proteins; Biochemistry; Molecular biology; Cancer research; Health sciences; Oncology; Clinical research
1. Introduction
Understanding of the intricate relationship between the immune system and the tumor cells has provided the accelerated development of cancer treatment such as rejuvenation of host immunity, training the immune cells against the cancer cells, removing the exhaustions of immune cells against tumor antigens etc.
Turning on immune cell against the cancer cell either through antibodies mediated therapeutics or re-infusion of trained immune cells into the cancer patient are excellent examples of cancer immunotherapy [1, 2, 3]. As tumor cells utilize various escape mechanisms for their survival from immune surveillance, the vital question is how the immune cells can be genetically reprogrammed to recognise and kill tumor cells. Extensive research employing immune cells in cancer treatment has led to the development of antibodies based immunotherapies [2], allogeneic stem cell transplantation [4] and Chimeric Antigen Receptors (CARs) T cell therapy [5].
Among all the cell-based therapies, the genetically engineered CAR bearing T-cells (CAR T cell) for targeting cancer cells has emerged as the successful therapy in hematological malignancies. Its unprecedented role in cancer treatment [6, 7, 8, 9] has been due to better understanding of transcriptomics, proteomics, genomics, and Cell Based Assays. In 2017, two anti-CD19 CAR T-cell therapies have been approved by the US Food and Drug Administration (FDA) Tisagenlecleucel (Kymriah, Novartis) and Axicabtageneciloleucel (Yescarta, Kite Pharma). However, the long term follow ups have shown [10, 11] the development of resistance against CAR T cell therapy, which needs further evaluation [12]. Plenty of opportunities to improve the efficacy and safety of this therapy may establish a paradigm of CAR T cell therapy not only in haematological malignancies but also in solid tumors which frequently exhibit resistance to it. Hence, we have discussed the main keys of CAR T cells focussing on ‘how to improvise it to minimize failures and relapses’ for a better outcome. This includes the discussion on the effect of tumor mutation burden (TMB), tumor microenvironment (TME), role of epigenetic mechanisms and possible combination therapies.
Until the CAR T cell therapy is made affordable, its potential to reduce the disease burden will remain untapped. Hence, manufacturing cost reduction should be prioritized by assessing the need (therefore the demand), availability of skilled staff and infrastructure, which may contribute towards cost minimization and thus a wider use. Recent observations regarding the cancer cases, which are increasing remarkably every year, are quite alarming (Figure 1). Thus, the need of establishing better therapies at an affordable cost is our priority. We propose a triangular collaboration among hospital, academia and industry which may further improve the quality and affordability of this cellular therapy, especially for economically developing nations.
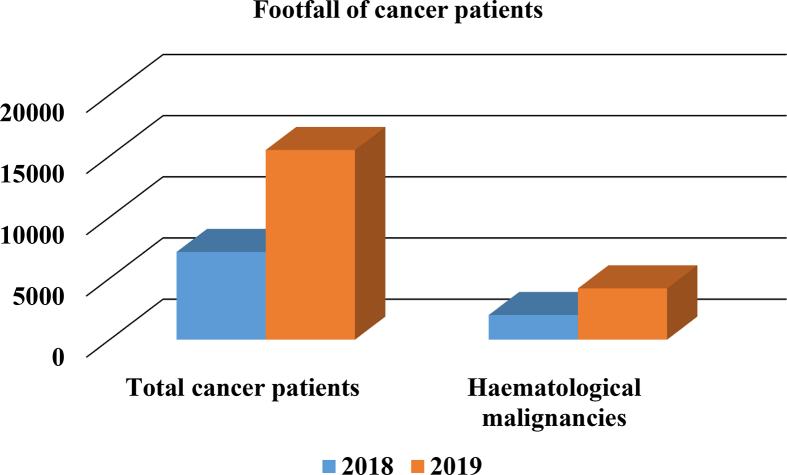
Figure 1.
Increasing haematological malignancies at All India Institute of Medical Sciences Patna: Approximately two-fold increase in haematological malignancies cases suggest a priority to establish CAR T cell therapy at affordable cost in a small city of Patna.
2. Strategies in CAR T cell therapy
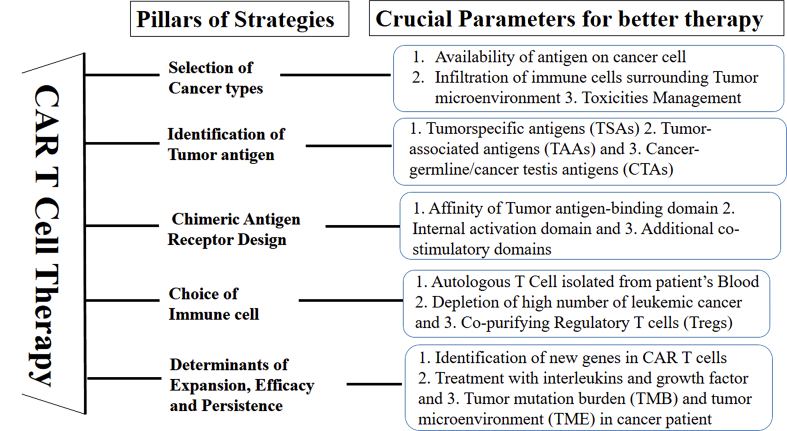
Successful CAR T cell therapy has been developed by careful considerations of crucial factors such as Selection of cancers where it can act optimally; Identification of appropriate tumor antigen(s); Selection of gene constructs to design chimeric receptors; Selection of immune cells for engineering the receptors against tumor antigen; Selection of other determinants of efficacy against cancer cells such as affinity of CAR for tumor antigen(s); selection of appropriate growth factors in the development of CAR T cells; and potential toxicities associated with the therapy including cytokine release syndrome and neurotoxicity. With the advent of new technology such as real time cellular assay and next generation sequencing, these crucial factors may be exploited to further improve CAR T cells’ performance (Figure 2).
Figure 2.
Strategies for better CAR T Cell therapy: Crucial factors are essential to consider for better treatment.
The CAR T cell therapy is based on the nature of cancer cells (suppressive or infiltrative) carrying specific tumor antigen(s) [13]. Hematological malignancies have been treated successfully by CAR T cell therapy as compared to solid tumors [11]. For instance, CD19-antigen selection in hematologic malignancies was near to perfection due to the readily available antigen for its recognition by anti-CD19 CAR T cells. However, in the case of solid tumors, CAR T cells generally failed to interact with the tumor antigens due to physical barriers and an immune suppressive tumor microenvironment (TME) [12]. Still successful approaches for solid tumors have been proposed and reported [14].
So far, the most successful antigen has been CD19, a tumor associated antigen (TAA) and not the tumor specific antigens or Cancer-germline/cancer testis antigens [15]. Though, other TAAs (CD20, CD22 or CD19 and CD22 together, CD 30) have also been used in various cancer immunotherapies [16, 17, 18, 19]. Various other tumor antigens may also give directions and opportunities to design receptors appropriately [1].
The effective cytotoxic T cell in CAR T cell carries a genetically engineered receptor protein on its surface, equivalent to T Cell Receptor (TCR) but recognises tumor antigen independent of Major Histocompatibility Complex which is an added feature. Many types of CARs have been designed and successfully used [20]. To reduce the cost of CAR T cell therapy, the concept of Universal CAR (UniCAR) and universal T cell has emerged [21]. The UniCAR contains a bridging molecule which serves as a connector through a domain directed against a tumor antigen and an epitope being recognized by the signalling domain of a conventional CAR. Thus, T cells with UniCARs may be prepared from allogenic healthy donors avoiding the patient's blood which may have low T cell count. The graft-versus-host disease, which is adverse immunologic response due to transplant, may be abolished by genetic elimination or disruption of the TCR gene and/or HLA class I loci of the allogenic T cells [21]. However, this needs further validation before being practised. This approach may be very useful in cost reduction of the CAR T cells as it will abolish the initial steps involving patient.
Earlier, a similar technique where immune cells isolated from the tumor region, grown outside of the body and re-infuse back to the patient to kill cancer cells, was described as Adoptive cell transfer (ACT) [1]. The choice of immune cells for ACT is a critical step as there are several of them, namely, Natural Killer cells, Tumor-infiltrating lymphocytes, T cells and dendritic cells [22]. Hence, any of these cells can be genetically modified to reprogram them against tumor cells. However, T cells have successfully been used in most cases for developing cell based immunotherapies [6] owing to their intrinsic properties of proliferation, cytotoxicity and memory. The efficacy and persistence of CAR T cell in-vivo decides the successful outcome of the therapy. Therefore, the factors contributing towards their effector functions are taken into consideration in the existing approaches. The cellular components (other T cell subtypes) the use of growth factors and interleukins for CAR T cells' activation and proliferation have been found to affect the performance of CAR T cells in-vivo [23, 24, 25]. Therefore, leukemic cells must be depleted before isolating T cells for CAR T cell preparation [9, 24]. Equally important is the ratio of CD4+ to CD8+ or total T-cell isolated from the patients [17, 26]. Some studies have reported that it could be difficult to isolate sufficient number of T cells from patients with relapsed/refractory cases or those that had multiple rounds of chemotherapy. Also, due to heterogeneity among the patient's blood samples, the proliferation and efficacy of CAR T cells prepared, have shown different functional ability, although sufficient quantity of CD3+ lymphocytes were isolated to manufacture CAR T cells [27].
In summary, it is essential to better understand the different strategies of CAR T cell therapy (summarised in Figure 2) for the development of newer approaches for cancer treatment.
3. Failure/relapses
Failures and relapses in most cancer treatments have been reported and CAR T cell therapy is no exception as individual immunity and co-morbid conditions vary among cohorts [28]. Understanding these events is the next milestone for better results of this therapy.
Long term survival studies in CAR T cell therapy have indicated cases of disease relapse within one year of treatment [10, 11]. In a rare case, one patient who initially did not respond to therapy showed complete remission after clonal evolution of one of the CAR T cell clones with hypomorphic mutation in one of its tumor suppressor genes [29]. On the contrary, a relapsed case was reported in a B cell acute lymphoblastic leukemia with aberrant myeloperoxidase expression after CAR T cell therapy [30]. These findings suggest the importance of mechanistic studies on CAR T cell therapy with more cases to understand the altered gene expression exhibiting two opposite phenomenon- one remission and the other, relapse after the therapy.
To get a complete picture of the events occurring in failure and relapses, the strategies employed by the cancer cells to escape CAR T cell need special attention [31, 32]. In general, tumor cells escape by - Lineage switching [33, 34]; loss of tumor antigen, for example CD 19, or epitope hiding from recognition [35]; Immunomodulation of the host immune cells to escape from surveillances [36]; T cell exhaustion and epigenomic landscape modulation [37]. Examples, such as lineage markers including myeloid conversion in patients following CD19 CAR therapy is seen in murine adult acute lymphoblastic leukemia (ALL) models after the long-term effects of CD19 CAR-T cells [33]. Also, a CD19-negative myeloid phenotype is responsible for the immune escape of mixed-lineage leukemia (MLL) from CD19 CAR-T-cell therapy [35].
4. New essentials of CAR T cell therapy
The CAR T cell therapy has shown a great success in paediatric, young and adult patients with relapsed or refractory B-cell ALL, however, some cancers have shown resistance against it [11]. To make the treatment better, the question is what are the possible contributors that may be modulated in CAR T cell therapy? In this section, the most recent approaches will be discussed, and these may hold future promise to improve CAR T cell therapy (summarized in Figure 2).
4.1. Understanding complexity of tumor types and T cells
Since immunotherapy depends on how quickly and effectively tumor cells are being recognized and killed without any toxicities to normal cells, it must be a priority to understand the molecular beacons of tumor cells and T cells. Therefore, molecular characterization of tumor types and T cells by next generation sequencing (to know any abnormal gene expression) could be considered as a routine procedure to avoid failures. Immuno-phenotyping, T cell receptor sequencing, determination of tumor tissue (grade, age, pathology examination by imaging and gene expression signature analysis) may indicate the feasibility of this therapy. Hematological cases are easy to characterize in terms of above mentioned parameter; however, complexed tumor types may need better tools for the same [38].
Identification of genes essential for CAR T cells’ stability, efficacy and in-vivo persistence may further contribute in enhancing their anti-tumor activities. It is plausible to apply genome editing tools such as zinc-finger nucleases, transcription activator-like effector nucleases and clustered regulatory interspaced short palindromic repeat and CRISPR-associated protein 9 (CRISPR/Cas9) techniques to manipulate T cells for better efficacy [39, 40, 41]. These techniques might also be useful in tracing the lineage of CAR T cells in-vivo using appropriate reporter system [3, 42]. Other important aspect of CAR T cell is to maintain controlled activity in-vivo for clinical safety. This needs regulated expression of CAR constructs, which can be achieved by the tetracycline regulatory system known as inducible CD19CAR T cells [43]. Apart from this, anti-tumor efficacy in CAR T cell may be augmented by disruption of programmed cell death protein 1 [PD-1]) (discussed below) using CRISPR/Cas9 [39]. The CAR T cell with all the desired parameters needs to be used in an appropriate ratio to target tumor cells (target to effector cell ratio) for better efficacy [29, 44].
4.2. Dynamic tumor Antigen(s) and tumor microenvironment
Tumor cells evolve under immune surveillance by constantly changing their surface proteins or intracellular signalling molecules to avoid their recognition from cell mediated immunity [45]. In such a situation, how a CAR T cell will target a tumor cell having high or low mutational burden, should be the first line to understand. Hence, tumor mutation burden (TMB) along with tumor microenvironment (TME) during cancer progression need to be systematically studied for selection of target antigen(s) [46]. Prior to CAR T cell preparation, depletion of tumor cells or reducing the tumor load with chemotherapeutics are the supporting evidence that TMB is critical for CAR T cell therapy [25]. It also plays a vital role in maintaining the plasticity of T cell [47]. Moreover, continuous exposure of T cells to tumor antigen(s) causes their exhaustion, a phenomenon observed with T cells when exposed to lymphocytic choriomeningitis virus [48]. Furthermore, the immunosuppressive strategies adopted by tumor cells such as expressing PDL-1 (programmed cell death protein ligand-1); secreting immunosuppressive molecules such as TGF-β, IL-10 etc.; and change in nutrients in tumor microenvironment and hypoxia, also promote T cell exhaustion. However, a recent study has reported reduced exhaustion of T cells by reduction in mTORC1 activity mediated by IL15 [49]. Results of this study have shown a reduced expression of exhaustion markers, thus conferring better antitumor activity to CAR T cells [49]. Number of recently discovered T cell subtypes (Regulatory T cells (Tregs), Th17 etc) have also been reported to affect the continued persistence and cytotoxic activities of effector T cells [50, 51]. However, it is not clear as to how these regulatory T cells interplay with CAR T cells to control their tumor toxic activities. It is proposed that it may be through a non-classical immune synapse formation through releasing a battery of enzymes like perforin and granzyme [52].
Next generation sequencing technologies provide newer opportunities to identify highly tumor-specific markers and neoantigens as a result of somatic mutations [53, 54, 55]. Targeting a single antigen by CAR T cell therapy may not be as effective as combination with other tumor specific antigen(s). It is plausible that combining newly discovered neoantigen with highly tumor-specific CAR T cell may further improve treatment outcomes [32, 56, 57]. In summary, it is essential to examine tumor cells for their dynamic antigenic landscaping along with the other immune associated factors during the course of therapy to make it a useful approach (Figure 3).
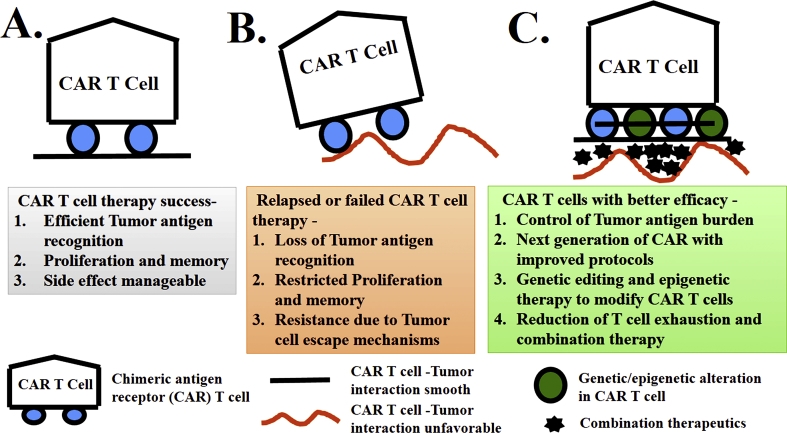
Figure 3.
Present and future of next generation “CAR” T cell therapy: A) Unique features behind the success of CAR T cell therapy; B) Recent clinical trials show relapsed and failed cases of CAR T cell therapy and C) Probable future approach to make CAR T cells with better efficacy.
4.3. Epigenetic modulation: can it shape a better CART cell therapy?
While genetics has led to several breakthroughs in our understanding of tumor progression, epigenetics is also now known to play a central role in tumorogenesis [58, 59, 60]. On an evolutionary scale, it is easy to acquire rapid, reversible and stable epigenetic changes over genetic change in a life cycle of an organism [61]. In the same way a normal cell may acquire stable inheritable phenotypes (a tumor cell) independent of a DNA sequence (genetic code) [62]. Various epigenetic mechanisms involved in this transformation are depicted in Figure 4A. In this review, it is discussed how the epigenetic mechanisms may affect efficacy of CAR T cells or cause resistance against tumor cells. The chromatin may be the very first signalling platform influencing gene expression in CAR T cells. A battery of chromatin modifiers (Writer, Eraser and Readers) may interplay to maintain proper chromatin structure temporally and spatially (Figure 4B). Their role in posttranslational modifications (PTMs) of histones or modification of bases of DNA has been correlated with gene expression or repression (also historically known as histone code) [58, 63, 64]. Henceforth, chromatin may act as the signalling platform to integrate signal to control the repressive, poised or active gene expression in the cell [65]. Such chromatin states (characterized by histone PTMs) and DNA modifications also control the gene expression pattern in naïve, effector and memory T cells also [29, 66, 67, 68]. For instance, mice responsible three gene loci -IFNg(Ifng), granzyme B (Gzmb), and perforin 1(Prf1) for effector function of T cells, have common nucleosomal and H3K27 methylation signatures in memory CD8+ T cells [69]. However, during the primary infection, there is a distinct change in chromatin state (reduced nucleosomal density near the transcription start sites and reduced H3K27 methylation) at the genes loci of Ifng and Gzmb and this change remains in the memory CD8+ T cells [69]. Reduced nucleosomes and reduced H3K27 methylation correlate with open chromatin structure at these gene loci. Moreover, increased histone acetylation associated with open chromatin was found significantly higher in the genes responsible for human memory CD8+ T cells than in naïve CD8+ T cells [70]. Thus, an open chromatin structure at gene loci may play a vital role for memory function of T cells [70] (Figure 4C, D).
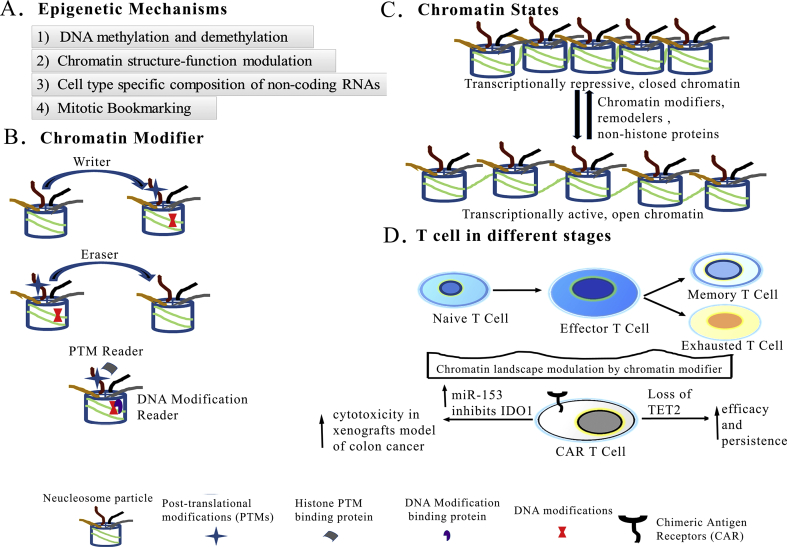
Figure 4.
Epigenetic mechanisms control gene expression. A) General epigenetic mechanisms. B) Epigenetic players: Nucleosome particle consists of DNA wrapped around octamer of Histone H3, H4, H2A, H2B and “Tails” protruding out through the core particle. Enzymes which “add”, “remove” posttranslational modification on histones or base modification on DNA are called as “Writers”, “Erasers” respectively and proteins which “bind” posttranslational modification or DNA modification are called “Readers”. C) Chromatin structure may act as signalling platform to co-ordinate epigenetic pathways and control the transcriptional activity. The reversible transition between open and closed chromatin structure is determined by chromatin modifiers, remodeller and various non-histone proteins. D) Gene expressions of naïve, effector, memory and exhausted T cells may be controlled by distinct chromatin structure. Loss of TET2 and inhibition of Indoleamine 2,3-dioxygenase 1 (IDO1) expression by miR-153 increase activities of CAR T cells.
For a reader proteins, the best example is BET (bromodomain and extra-terminal motif) proteins, which usually bind to acetylated histone marks through its bromodomain [71]. Interestingly, JQ1, a specific inhibitor of BET proteins has shown a prolonged persistence and enhance antitumor activity of T cells [68].
Another study on human ovarian cancers has shown that “writers” (enhancer of zeste homologue 2 (EZH2) which write histone H3 lysine 27 trimethylation (H3K27me3) and DNA methyltransferase1 (DNMT1) which writes DNA methylation), repress the production of chemokines (T helper 1 (TH1)-type chemokines CXCL9 and CXCL10) by tumor cells [72]. These chemokines are involved in trafficking of effector T cells to the tumor microenvironment [72], thus their low levels prevent the T cell function. Also, promoter hypermethylation of genes that contain neo-antigenic mutations has been identified as an epigenetic mechanism of immune-editing in lung cancer model [73]. Another chromatin modifier, TET2 (Eraser) encodes methyl cytosinedioxygenase, an enzyme that catalyses DNA demethylation activating gene expression [74]. Whereas, the transcriptional silencing of tumour-suppressor genes in cancer cells can be mediated through DNA methylation [62]. Interestingly, disruption of TET2 promotes a progeny of a CAR T cell (targeting the CD19 protein) to proliferate and extensively cure chronic lymphocytic leukaemia in a patient [29]. This is due to inhibiting differentiation of CAR T cells, which arose from the TET2 depleted single CAR T cell clone (Figure 4D). However, more studies are required to understand different chromatin states and the role of reader proteins in changing gene expression in T cells. Therefore, to establish a possible chromatin state of CAR T cells may be important to contribute to their efficacy [37]. In view of this, we hypothesize that understanding the functions of chromatin modifiers of both CAR T cells as well as tumor cells may improve cancer immunotherapy.
Apart from these facts, non-coding miRNA profiling in naïve, effector and memory T cells may also be crucial for better understanding of gene expression in these cells. In colon cancer cells, miR-153 inhibits Indolamine 2,3-dioxygenase 1 (IDO1) expression which catalyzes the conversion of tryptophan to kynurenine [75, 76]. Combining miR-153 over expression with CAR T cells targeting the epidermal growth factor receptor variant III has shown effective tumor killing effects in the xenografts model of colon cancer [76] (Figure 4D).
Thus, epigenetic mechanisms such as chromatin states, DNA methylation states and non-coding RNA profile in CAR T cells are some of the key points to be considered for further investigation in relation with tumor for better treatment outcomes.
4.4. Combination of CAR T cell therapy with other cancer therapeutics
Cancer development is a gradually evolving process accompanied by multiple mutations in various biochemical processes [77, 78, 79]. It suggests that combination therapies (Table 1) may have to be developed to tackle the cancer progression [80]. The CAR T cell therapy is a cellular immunity based therapy and may be combined either with inhibitors of immune supressing pathways (e.g. T cell exhaustion) or inhibitors of signalling pathways so that specific toxicity to cancer cells can be achieved [81].
Table 1.
Combination therapy: CAR T cell therapy combined with classical cancer therapeutics.
| Broad Classification | Therapeutics combined with CAR T cell therapy | Biochemical pathway | Reference |
|---|---|---|---|
| Immunomodulators | Ipilimumab (a fully human monoclonal antibody against CTLA-4) | CTLA-4 inhibits earlier activation stage of T-cell | [86] |
| nivolumab (PD-1) | PD-1 for T-cell exhaustion | [90, 91] | |
| local expression of cytokine | immunosuppressive environment inhibition or T cell activation | [92] | |
| Indoleamine 2,3-dioxygenase (IDO) Inhibitors | immunosuppressive metabolites (kynurenine and 3-hydroxyanthranilic acid | [94] | |
| Chemical Inhibitor | BRAF and MEK inhibitors | Kinase signalling pathway | [97] |
| carboplatin | chemotherapeutic agent | [98] | |
| Radiation | local subtherapeutic irradiation | DNA Damage or Genome Instability | [99] |
| Vaccination | amphiphile CAR-T ligands (amph-ligands) | priming CAR-Ts in the native lymph node microenvironment | [100] |
| mircoRNA therapeutics | miR-153 | miR-153 inhibits Indoleamine 2,3-dioxygenase 1 expression | [76] |
4.4.1. Nanoparticle and photothermal
Nanoparticle-based therapy which also has it use in diagnostics, is being developed for various cancer types [82]. As CD19 has been the most common choice of antigen, anti-CD19 antibodies grafted in the nanoparticles may be another approach for therapies and cancer diagnostic imaging [83]. Since solid tumors are compact and inaccessible to infiltration, mild heat treatment may increase permeability to the tumor microenvironment. So combining photothermal therapy with CAR T cells may increase the chances to infiltration of CAR T cells inside solid tumors [38] and thus, making them effective.
4.4.2. Immunomodulators
Combining CAR T cell therapy with immunomodulators, chemical inhibitors or radiation are the other options to improve disease outcome (Table 1). The immune-checkpoint inhibitors have shown their role in maintaining the antitumor activity of T cells, thus the success [84]. The two checkpoint receptors, the CTLA-4 (cytotoxic T-lymphocyte associated protein 4) and PD-1 (programmed death1), are expressed by activated T cells to negatively control their cytotoxic activities with non-overlapping functions [2, 85]. The CTLA-4 inhibits initial activation of T-cell, whereas over expressed PD-1 exhausts T-cell in a later stage. The Regulatory T cells also express CTLA-4 to inhibit anti-tumor activity of activated T cells [85]. Interestingly, Ipilimumab (a fully human monoclonal antibody against CTLA-4) has shown to promote antitumor activity in patients with metastatic melanoma [86]. The mechanism involved in effector T cell exhaustion by PD 1 is mediated by IFNγ produced by them. The IFNγ increases the expression of the PD-L1 protein on T cells as well as other cell types, including tumor cells, which can engage the PD-1 receptor on T cells to suppress their antitumor immunity [87, 88]. This is the mechanism used by tumor cells to protect themselves from CD8+ T cell–mediated lysis [89]. Use of PD1 or PDL1 blockade can get back antitumor activity of effector T cells (67). In fact, combined PD-1 (nivolumab) and CTLA-4 (ipilimumab) antibodies blockade has achieved objective response rate in advanced melanoma patients [90]. Hence, it is obvious to think that these antibodies blockade approach may be combined with CAR T cell therapy to get effective response in cancer treatment. In fact, deletion of PD1 enhanceing antitumor efficacy of CAR T cells, has also been reported [39]. Also, the anti-HER2 CAR T cells alone were not that effective on tumor cells bearing HER2 antigen and expressing PDL1 as with anti-PD-1 antibody in the mouse model [91].
In a different approach, the immunosuppressive environment may be inhibited or T cell may be activated by local expression of cytokines [92]. Moreover, the IFNγ secreted by activated T cell may promote the expression of of immunosuppressive molecule, IDO [93]. This enzyme is overexpressed in several human cancers [94] and catalyzes the conversion of tryptophan into immunosuppressive metabolites (kynurenine and 3-hydroxyanthranilic acid) which blocks antigen-specific T cell proliferation [95]. So, metabolites take key part in inhibiting T cell proliferation, and this is thought to be a key mechanism with which cancers evade immunity. In fact, IDO activity of tumor cells inhibit CD19-CAR T cells. This can be circumvented by administration of fludarabine and cyclophosphamide before CD19-CAR T cell infusion to patient [94]. Therefore, IDO inhibitors may also be combined with CAR T cell therapy to further improve its cytotoxic effect. In a recent study on animal model, CAR T cell therapy has been tested with an antidote, Dasatinib, a drug that controls the cytokine storm. This combined approach has shown to reduce the toxicities associated with the therapy as the drug has on/off control in CAR T cell immunotherapy [96].
These findings suggest the need of more pre-clinical trials to test the combinatorial approach in CAR T cell therapy.
4.4.3. Chemotherapy, radiation and vaccine
The RAS/RAF/MEK/ERK MAPK pathway is a key signalling pathway in immune cells as well as in tumor cells and its inhibitors are well known in cancer treatment [101, 102]. It has been shown that BRAF and MEK inhibitors influence the function of CAR T cells [97]. In another study, combining carboplatin, a chemotherapeutic agent, with CAR T cell (using ErbB-Retargeted T Cells) therapy showed enhanced anti-tumor effect in a mouse model of ovarian cancer [98]. The next obvious question is whether CAR T cell therapy can be combined with irradiation the rapy. It has been shown that there was increased accumulation of CAR T cells surrounding the tumor site upon local subtherapeutic irradiation in a mouse model of glioblastoma [99]. Recently, Ma et al. has shown how fruitful the vaccination approach is in combination with CAR T cell therapy in the immunocompetent mouse tumor model [100]. Injecting the amphiphile CAR-T ligands (amph-ligands), as the vaccine primed CAR-T cells in the native lymph node microenvironment leads to massive CAR-T expansion and enhanced antitumor efficacy [100].
These findings suggest that CAR T cell therapy if supported by the discussed approaches, may further improve its remission rate.
5. Affordability for CAR T cell therapy: a clinical programme bridging hospital and academia
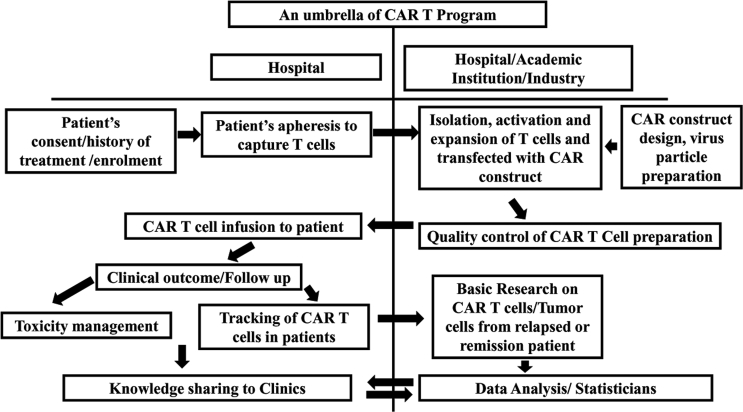
In the era of personalized medicine, autologous CAR T cell therapy has shown promising results. However, the cost (approximately $470, 000 per patient) of this therapy as quoted by Novartis is exorbitantly high, which raises concerns of affordability by common man in under-developed and developing nations [103]. Not all can afford this successful therapy and be benefitted. Therefore, the complexity of the process of making of this live drug needs to be addressed for cost minimization. In the existing system, the manufacturing unit for CAR T cell, which are limited in number, are not always in the proximity of the healthcare centres where the patient is being treated, thus involves sample transport and other pre-analytical issues and affect the cost. Usually, blood samples are transported from hospital to the CAR T cell manufacturing unit and shipped back to hospital to re-infuse, which takes longer time, and also affects the quality and cost of the CAR T cell preparation [104, 105, 106]. Cancer centres in developed nations are using the CAR T cells for cancer treatment being manufactured by pharma companies. However, an affordable CAR T cell has been successfully made by academic institutions as well [107]. Therefore, working together with hospitals and research centre in academia could be a solution for making this therapy cost effective. This may not only help in addressing tremendous challenges associated with complexities of CAR T cell therapy in cancer management but also reduce time of treatment. Thus, this unifying model (Figure 5) will bring brighter perspective for cancer treatment to individuals of all socio-economic status at lower cost.
Figure 5.
An umbrella of CAR T program showing how collaboration between hospital, industry and academic institution may increase the chances of this therapy to economically poor region of the world. Arrows are the flow of steps or knowledge.
5.1. Need assessment for manufacturing of CAR T cell
Need assessment for CAR T cell therapy, is a key point in understanding the demand of the same. This will help in giving the necessary volume to the manufacturing unit to minimize the cost. Therefore, the tertiary healthcare centres should be invited to develop this therapy. In manufacturing part of the CAR T cell, the concept of universal CAR seems to be beneficial and needs to be explored, it can tremendously reduce the cost and the complexity of the procedure [21].
5.2. Contribution of academicians in protocol optimization for CAR T cell therapy
Collaboration of clinicians and scientific community can greatly contribute in protocol optimization for the development of autologous CAR T cell. The researchers play a critical role not only in preparing the desired cells but also in maintaining the quality of the preparation. Keeping the physiology of T cell as close as in-vivo situations while manipulating it, is very critical and should be taken care of. Chemical and physical stress may change the phenotype of T cells, therefore, appropriate methods must be adopted to transfect CAR construct to autologous T cells (as using patients’ own cells reduces many risks associated with graft vs host disease) [27]. The cryopreservation of CAR-T cells should be avoided as it affects their potency [108]. Monocytes and granulocytes may inhibit the growth of lymphocytes isolated from peripheral blood, so counter-flow elutriation closed system developed for the separation of monocytes and granulocytes should be used for expansion of CAR T cells [109]. Also, T lymphocytes extraction from a patient who has a low leukocyte counts is quite challenging especially with multiple cycles of chemotherapy or marrow transplantation. A mononuclear cell apheresis technique is preferably used in such cases [27]. The role of basic research becomes even more important when the CAR T cells are being used for the treatment on the patient, as certain changes may be desired during the process. The analytical evaluation of the clinical outcomes is an effort of the team as these may vary according to the history of chemotherapeutics, tumor mutational load, number of purified or T cell isolate, etc. [12]. Thus, a collaborative effort between clinician and basic researchers can tackle and counter all the possible pitfalls associated with the development of autologous CAR T cell, its use and management thereafter [110].
5.3. Partnering the hospital, academia and industry under the CAR T cell clinical programme
CAR T cell therapy, unlike other conventional therapies, needs constellations of oncologist, hematologist, basic researchers, toxicologist, epidemiologist and statisticians. Hence, there is a need to develop a unique program with regulatory requirements and distinct operational processes [111] along with the infrastructure and trained personnel to follow up with the patients [112, 113]. CAR T cell therapy has acute toxicities and may be life threatening [114, 115]. Thus it needs multicentric facilities and management system for optimum care to the patients [116, 117]. An academic institution associated with healthcare can play a key role in creating most effective CAR T cell and explore better options for combining CAR T cell therapy with other therapies. Also, in underdeveloped and developing nations, neither hospitals nor academic institutions alone can handle the cost of CAR T cell therapy. Thus, sharing infrastructure between them can be advantageous (Figure 5). Apart from this, regulatory tools for approval of CAR-T cell therapy are essential. Various medicinal/gene products have great potential for the treatment for various diseases as advanced therapeutics. These have also been licensed and therefore their market may grow exponentially in future. Adoptive T-cell transfer (ACT) is also a subclass of such advanced gene therapy product. The first chimeric antigen receptor (CAR)-T cells received regulatory approval with remarkable clinical results (https://www.ema.europa.eu/news/first-two-car-t-cell-medicines-recommended-approval European-Union) received EU approval also in 2018. More than 400 CAR-T clinical trials are still going on [118]. With this great achievement in cancer immunotherapy, regulatory needs have to be approved by competent authorities for smooth implementation of CAR T cell clinical programme.
6. Conclusion
In a nutshell, treatment of cancer by CAR T cell raises great hopes to the patient care systems. Hence, improving it further for the gaps observed, it is pertinent to understand the molecular complexities of CAR T cells along with tumor cells. The major concerns about the therapy are the relapses/failure and the affordability. This review has taken newer routes to tackle the constantly changing landscapes of antigens on immune cells and tumor cells. It also, emphases the epigenetic mechanisms which may improve efficacy and performance of CAR T cell. Our recommendation in this review emphasizes a path forward i.e. an approach for a collaborative effort between academia, hospitals and industry, to increase its accessibility and affordability for cancer patients. Also, the helping hands from government bodies in initiating the health care policies will play a crucial role in supporting the cancer treatment of low- and middle-income groups of patients.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
The work was supported by Ramalingaswami Re-entry Fellowship to Dr. Rajesh Kumar Yadav awarded by Department of Biotechnology, Government of India (Award Letter: D.O. No.BT/HRD/35/02/2006).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank Naina Gour, Postdoctoral fellow, John Hopkins University and Mansi Krishan, Food safety scientist Danone, North America for critically reading this manuscript. We sincerely apologize to researchers whose works have not been discussed due to space limitations.
References
- 1.Klebanoff C.A., Rosenberg S.A., Restifo N.P. Prospects for gene-engineered T cell immunotherapy for solid cancers. Nat. Med. 2016;22:26–36. doi: 10.1038/nm.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiner L.M., Murray J.C., Shuptrine C.W. Antibody-based immunotherapy of cancer. Cell. 2012;148:1081–1084. doi: 10.1016/j.cell.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li D., Li X., Zhou W.L., Huang Y., Liang X., Jiang L., Yang X., Sun J., Li Z., Han W.D., Wang W. Genetically engineered T cells for cancer immunotherapy. Signal Transduct. Tar. 2019;4:35. doi: 10.1038/s41392-019-0070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maris M.B., Sandmaier B.M., Storer B.E., Chauncey T., Stuart M.J., Maziarz R.T., Agura E., Langston A.A., Pulsipher M., Storb R., Maloney D.G. Allogeneic hematopoietic cell transplantation after fludarabine and 2 Gy total body irradiation for relapsed and refractory mantle cell lymphoma. Blood. 2004;104:3535–3542. doi: 10.1182/blood-2004-06-2275. [DOI] [PubMed] [Google Scholar]
- 5.Dunbar C.E., High K.A., Joung J.K., Kohn D.B., Ozawa K., Sadelain M. Gene therapy comes of age. Science. 2018;359 doi: 10.1126/science.aan4672. [DOI] [PubMed] [Google Scholar]
- 6.Kalos M., Levine B.L., Porter D.L., Katz S., Grupp S.A., Bagg A., June C.H. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 2011;3 doi: 10.1126/scitranslmed.3002842. 95ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grupp S.A., Kalos M., Barrett D., Aplenc R., Porter D.L., Rheingold S.R., Teachey D.T., Chew A., Hauck B., Wright J.F., Milone M.C., Levine B.L., June C.H. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brentjens R.J., Davila M.L., Riviere I., Park J., Wang X., Cowell L.G., Bartido S., Stefanski J., Taylor C., Olszewska M., Borquez-Ojeda O., Qu J., Wasielewska T., He Q., Bernal Y., Rijo I.V., Hedvat C., Kobos R., Curran K., Steinherz P., Jurcic J., Rosenblat T., Maslak P., Frattini M., Sadelain M. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kochenderfer J.N., Dudley M.E., Feldman S.A., Wilson W.H., Spaner D.E., Maric I., Stetler-Stevenson M., Phan G.Q., Hughes M.S., Sherry R.M., Yang J.C., Kammula U.S., Devillier L., Carpenter R., Nathan D.A., Morgan R.A., Laurencot C., Rosenberg S.A. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park J.H., Riviere I., Gonen M., Wang X., Senechal B., Curran K.J., Sauter C., Wang Y., Santomasso B., Mead E., Roshal M., Maslak P., Davila M., Brentjens R.J., Sadelain M. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N. Engl. J. Med. 2018;378:449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maude S.L., Laetsch T.W., Buechner J., Rives S., Boyer M., Bittencourt H., Bader P., Verneris M.R., Stefanski H.E., Myers G.D., Qayed M., De Moerloose B., Hiramatsu H., Schlis K., Davis K.L., Martin P.L., Nemecek E.R., Yanik G.A., Peters C., Baruchel A., Boissel N., Mechinaud F., Balduzzi A., Krueger J., June C.H., Levine B.L., Wood P., Taran T., Leung M., Mueller K.T., Zhang Y., Sen K., Lebwohl D., Pulsipher M.A., Grupp S.A. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah N.N., Fry T.J. Mechanisms of resistance to CAR T cell therapy. Nat. Rev. Clin. Oncol. 2019 doi: 10.1038/s41571-019-0184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filley A.C., Henriquez M., Dey M. CART immunotherapy: development, success, and translation to malignant gliomas and other solid tumors. Front. Oncol. 2018;8:453. doi: 10.3389/fonc.2018.00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J., Li W., Huang K., Zhang Y., Kupfer G., Zhao Q. Chimeric antigen receptor T cell (CAR-T) immunotherapy for solid tumors: lessons learned and strategies for moving forward. J. Hematol. Oncol. 2018;11:22. doi: 10.1186/s13045-018-0568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gubin M.M., Artyomov M.N., Mardis E.R., Schreiber R.D. Tumor neoantigens: building a framework for personalized cancer immunotherapy. J. Clin. Invest. 2015;125:3413–3421. doi: 10.1172/JCI80008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fry T.J., Shah N.N., Orentas R.J., Stetler-Stevenson M., Yuan C.M., Ramakrishna S., Wolters P., Martin S., Delbrook C., Yates B., Shalabi H., Fountaine T.J., Shern J.F., Majzner R.G., Stroncek D.F., Sabatino M., Feng Y., Dimitrov D.S., Zhang L., Nguyen S., Qin H., Dropulic B., Lee D.W., Mackall C.L. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 2018;24:20–28. doi: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nirali N Shah S.L.H., Shalabi Haneen, Yates Bonnie, Kane Eli, Fellowes Vicki, Delbrook Cynthia, Ren Jiaqiang, Jin Jianjian, David Stroncek, Terry J. Fry. CD4/CD8 T-cell selection enhances CD22 CAR-T cell transduction and in-vivo CAR-T expansion: updated results on phase I anti-CD22 CAR dose expansion cohort. Blood. 2017;130:809. [Google Scholar]
- 18.Zhang W.Y., Wang Y., Guo Y.L., Dai H.R., Yang Q.M., Zhang Y.J., Zhang Y., Chen M.X., Wang C.M., Feng K.C., Li S.X., Liu Y., Shi F.X., Luo C., Han W.D. Treatment of CD20-directed Chimeric Antigen Receptor-modified T cells in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: an early phase IIa trial report. Signal Transduct. Tar. 2016;1:16002. doi: 10.1038/sigtrans.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang C.M., Wu Z.Q., Wang Y., Guo Y.L., Dai H.R., Wang X.H., Li X., Zhang Y.J., Zhang W.Y., Chen M.X., Zhang Y., Feng K.C., Liu Y., Li S.X., Yang Q.M., Han W.D. Autologous T cells expressing CD30 chimeric antigen receptors for relapsed or refractory hodgkin lymphoma: an open-label phase I trial. Clin. Canc. Res. 2017;23:1156–1166. doi: 10.1158/1078-0432.CCR-16-1365. [DOI] [PubMed] [Google Scholar]
- 20.Zhang C., Liu J., Zhong J.F., Zhang X. Engineering CAR-T cells. Biomark. Res. 2017;5:22. doi: 10.1186/s40364-017-0102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao J., Lin Q., Song Y., Liu D. Universal CARs, universal T cells, and universal CAR T cells. J. Hematol. Oncol. 2018;11:132. doi: 10.1186/s13045-018-0677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel S., Burga R.A., Powell A.B., Chorvinsky E.A., Hoq N., McCormack S.E., Van Pelt S.N., Hanley P.J., Cruz C.R.Y. Beyond CAR T cells: other cell-based immunotherapeutic strategies against cancer. Front. Oncol. 2019;9:196. doi: 10.3389/fonc.2019.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee D.W., Kochenderfer J.N., Stetler-Stevenson M., Cui Y.K., Delbrook C., Feldman S.A., Fry T.J., Orentas R., Sabatino M., Shah N.N., Steinberg S.M., Stroncek D., Tschernia N., Yuan C., Zhang H., Zhang L., Rosenberg S.A., Wayne A.S., Mackall C.L. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kochenderfer J.N., Feldman S.A., Zhao Y., Xu H., Black M.A., Morgan R.A., Wilson W.H., Rosenberg S.A. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J. Immunother. 2009;32:689–702. doi: 10.1097/CJI.0b013e3181ac6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vormittag P., Gunn R., Ghorashian S., Veraitch F.S. A guide to manufacturing CAR T cell therapies. Curr. Opin. Biotechnol. 2018;53:164–181. doi: 10.1016/j.copbio.2018.01.025. [DOI] [PubMed] [Google Scholar]
- 26.Turtle C.J., Hanafi L.A., Berger C., Gooley T.A., Cherian S., Hudecek M., Sommermeyer D., Melville K., Pender B., Budiarto T.M., Robinson E., Steevens N.N., Chaney C., Soma L., Chen X., Yeung C., Wood B., Li D., Cao J., Heimfeld S., Jensen M.C., Riddell S.R., Maloney D.G. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J. Clin. Invest. 2016;126:2123–2138. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ceppi F., Rivers J., Annesley C., Pinto N., Park J.R., Lindgren C., Mgebroff S., Linn N., Delaney M., Gardner R.A. Lymphocyte apheresis for chimeric antigen receptor T-cell manufacturing in children and young adults with leukemia and neuroblastoma. Transfusion. 2018;58:1414–1420. doi: 10.1111/trf.14569. [DOI] [PubMed] [Google Scholar]
- 28.Hinrichs C.S. Self-defeating CAR-Ts protect leukemic cells. Sci. Transl. Med. 2018;10 [Google Scholar]
- 29.Fraietta J.A., Nobles C.L., Sammons M.A., Lundh S., Carty S.A., Reich T.J., Cogdill A.P., Morrissette J.J.D., DeNizio J.E., Reddy S., Hwang Y., Gohil M., Kulikovskaya I., Nazimuddin F., Gupta M., Chen F., Everett J.K., Alexander K.A., Lin-Shiao E., Gee M.H., Liu X., Young R.M., Ambrose D., Wang Y., Xu J., Jordan M.S., Marcucci K.T., Levine B.L., Garcia K.C., Zhao Y., Kalos M., Porter D.L., Kohli R.M., Lacey S.F., Berger S.L., Bushman F.D., June C.H., Melenhorst J.J. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature. 2018;558:307–312. doi: 10.1038/s41586-018-0178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li N., Wang S.A., Lin P., Jabbour E., Thompson P., Chen Z., Li S., Xu J., You M.J., Bueso-Ramos C.E., Medeiros L.J., Yin C.C. Relapsed B-acute lymphoblastic leukemia with aberrant myeloperoxidase expression following CAR T-cell therapy: a diagnostic challenge. Am. J. Hematol. 2019 doi: 10.1002/ajh.25478. [DOI] [PubMed] [Google Scholar]
- 31.Mueller K.T., Maude S.L., Porter D.L., Frey N., Wood P., Han X., Waldron E., Chakraborty A., Awasthi R., Levine B.L., Melenhorst J.J., Grupp S.A., June C.H., Lacey S.F. Cellular kinetics of CTL019 in relapsed/refractory B-cell acute lymphoblastic leukemia and chronic lymphocytic leukemia. Blood. 2017;130:2317–2325. doi: 10.1182/blood-2017-06-786129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah N.N., Maatman T., Hari P., Johnson B. Multi targeted CAR-T cell therapies for B-cell malignancies. Front. Oncol. 2019;9:146. doi: 10.3389/fonc.2019.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacoby E., Nguyen S.M., Fountaine T.J., Welp K., Gryder B., Qin H., Yang Y., Chien C.D., Seif A.E., Lei H., Song Y.K., Khan J., Lee D.W., Mackall C.L., Gardner R.A., Jensen M.C., Shern J.F., Fry T.J. CD19 CAR immune pressure induces B-precursor acute lymphoblastic leukaemia lineage switch exposing inherent leukaemic plasticity. Nat. Commun. 2016;7:12320. doi: 10.1038/ncomms12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah N.N., Fry T.J. Anti-CD19 resistance can "stem" from progenitors. Blood. 2017;130:1961–1963. doi: 10.1182/blood-2017-09-804070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gardner R., Wu D., Cherian S., Fang M., Hanafi L.A., Finney O., Smithers H., Jensen M.C., Riddell S.R., Maloney D.G., Turtle C.J. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood. 2016;127:2406–2410. doi: 10.1182/blood-2015-08-665547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tokarew N., Ogonek J., Endres S., von Bergwelt-Baildon M., Kobold S. Teaching an old dog new tricks: next-generation CAR T cells. Br. J. Canc. 2019;120:26–37. doi: 10.1038/s41416-018-0325-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghoneim H.E., Zamora A.E., Thomas P.G., Youngblood B.A. Cell-intrinsic barriers of T cell-based immunotherapy. Trends Mol. Med. 2016;22:1000–1011. doi: 10.1016/j.molmed.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Q., Hu Q., Dukhovlinova E., Chen G., Ahn S., Wang C., Ogunnaike E.A., Ligler F.S., Dotti G., Gu Z. Photothermal therapy promotes tumor infiltration and antitumor activity of CAR T cells. Adv. Mater. 2019 doi: 10.1002/adma.201900192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rupp L.J., Schumann K., Roybal K.T., Gate R.E., Ye C.J., Lim W.A., Marson A. CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci. Rep. 2017;7:737. doi: 10.1038/s41598-017-00462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong M.B., Wang G., Chow R.D., Ye L., Zhu L., Dai X., Park J.J., Kim H.R., Errami Y., Guzman C.D., Zhou X., Chen K.Y., Renauer P.A., Du Y., Shen J., Lam S.Z., Zhou J.J., Lannin D.R., Herbst R.S., Chen S. Systematic immunotherapy target discovery using genome-scale in vivo CRISPR screens in CD8 T cells. Cell. 2019;178:1189–1204 e23. doi: 10.1016/j.cell.2019.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mirzaei H.R., Pourghadamyari H., Rahmati M., Mohammadi A., Nahand J.S., Rezaei A., Mirzaei H., Hadjati J. Gene-knocked out chimeric antigen receptor (CAR) T cells: tuning up for the next generation cancer immunotherapy. Canc. Lett. 2018;423:95–104. doi: 10.1016/j.canlet.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 42.Roszik J., Rabinovich B., Cooper L.J. Imaging of T cells expressing chimeric antigen receptors. Immunotherapy. 2011;3:1411–1414. doi: 10.2217/imt.11.138. [DOI] [PubMed] [Google Scholar]
- 43.Gu X., He D., Li C., Wang H., Yang G. Development of inducible CD19-CAR T cells with a tet-on system for controlled activity and enhanced clinical safety. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19113455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan L., Liu B. Critical factors in chimeric antigen receptor-modified T-cell (CAR-T) therapy for solid tumors. OncoTargets Ther. 2019;12:193–204. doi: 10.2147/OTT.S190336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vitale I., Sistigu A., Manic G., Rudqvist N.P., Trajanoski Z., Galluzzi L. Mutational and antigenic landscape in tumor progression and cancer immunotherapy. Trends Cell Biol. 2019;29:396–416. doi: 10.1016/j.tcb.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 46.Vitale I., Sistigu A., Manic G., Rudqvist N.P., Trajanoski Z., Galluzzi L. Mutational and antigenic landscape in tumor progression and cancer immunotherapy. Trends Cell Biol. 2019 doi: 10.1016/j.tcb.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Wellenstein M.D., de Visser K.E. Cancer-cell-intrinsic mechanisms shaping the tumor immune landscape. Immunity. 2018;48:399–416. doi: 10.1016/j.immuni.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 48.Moskophidis D., Lechner F., Pircher H., Zinkernagel R.M. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 49.Alizadeh D., Wong R.A., Yang X., Wang D., Pecoraro J.R., Kuo C.F., Aguilar B., Qi Y., Ann D.K., Starr R., Urak R., Wang X., Forman S.J., Brown C.E. IL-15-mediated reduction of mTORC1 activity preserves the stem cell memory phenotype of CAR-T cells and confers superior antitumor activity. Canc. Immunol. Res. 2019 doi: 10.1158/2326-6066.CIR-18-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sehrawat S., Rouse B.T. Interplay of regulatory T cell and Th17 cells during infectious diseases in humans and animals. Front. Immunol. 2017;8:341. doi: 10.3389/fimmu.2017.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luckheeram R.V., Zhou R., Verma A.D., Xia B. CD4(+)T cells: differentiation and functions. Clin. Dev. Immunol. 2012;2012:925135. doi: 10.1155/2012/925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benmebarek M.R., Karches C.H., Cadilha B.L., Lesch S., Endres S., Kobold S. Killing mechanisms of chimeric antigen receptor (CAR) T cells. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kiyotani K., Chan H.T., Nakamura Y. Immunopharmacogenomics towards personalized cancer immunotherapy targeting neoantigens. Canc. Sci. 2018;109:542–549. doi: 10.1111/cas.13498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu Y.C., Zheng Z., Robbins P.F., Tran E., Prickett T.D., Gartner J.J., Li Y.F., Ray S., Franco Z., Bliskovsky V., Fitzgerald P.C., Rosenberg S.A. An efficient single-cell RNA-seq approach to identify neoantigen-specific T cell receptors. Mol. Ther. 2018;26:379–389. doi: 10.1016/j.ymthe.2017.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kahles A., Lehmann K.V., Toussaint N.C., Huser M., Stark S.G., Sachsenberg T., Stegle O., Kohlbacher O., Sander C., Cancer Genome Atlas Research N., Ratsch G. Comprehensive analysis of alternative splicing across tumors from 8,705 patients. Canc. Cell. 2018;34:211–224 e6. doi: 10.1016/j.ccell.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sasada T. Cancer immunotherapy targeting neoantigens derived from tumor-specific gene mutations. Nihon Rinsho. 2017;75:189–195. [PubMed] [Google Scholar]
- 57.Perna F., Berman S.H., Soni R.K., Mansilla-Soto J., Eyquem J., Hamieh M., Hendrickson R.C., Brennan C.W., Sadelain M. Integrating proteomics and transcriptomics for systematic combinatorial chimeric antigen receptor therapy of AML. Canc. Cell. 2017;32:506–519 e5. doi: 10.1016/j.ccell.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Linggi B.E., Brandt S.J., Sun Z.W., Hiebert S.W. Translating the histone code into leukemia. J. Cell. Biochem. 2005;96:938–950. doi: 10.1002/jcb.20604. [DOI] [PubMed] [Google Scholar]
- 59.Valencia A.M., Kadoch C. Chromatin regulatory mechanisms and therapeutic opportunities in cancer. Nat. Cell Biol. 2019;21:152–161. doi: 10.1038/s41556-018-0258-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yadav T., Quivy J.P., Almouzni G. Chromatin plasticity: a versatile landscape that underlies cell fate and identity. Science. 2018;361:1332–1336. doi: 10.1126/science.aat8950. [DOI] [PubMed] [Google Scholar]
- 61.Timp W., Feinberg A.P. Cancer as a dysregulated epigenome allowing cellular growth advantage at the expense of the host. Nat. Rev. Canc. 2013;13:497–510. doi: 10.1038/nrc3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Esteller M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nat. Rev. Genet. 2007;8:286–298. doi: 10.1038/nrg2005. [DOI] [PubMed] [Google Scholar]
- 63.Cosgrove M.S., Wolberger C. How does the histone code work? Biochem. Cell. Biol. 2005;83:468–476. doi: 10.1139/o05-137. [DOI] [PubMed] [Google Scholar]
- 64.Jenuwein T., Allis C.D. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 65.Badeaux A.I., Shi Y. Emerging roles for chromatin as a signal integration and storage platform. Nat. Rev. Mol. Cell Biol. 2013;14:211–224. [PubMed] [Google Scholar]
- 66.Kersh E.N., Fitzpatrick D.R., Murali-Krishna K., Shires J., Speck S.H., Boss J.M., Ahmed R. Rapid demethylation of the IFN-gamma gene occurs in memory but not naive CD8 T cells. J. Immunol. 2006;176:4083–4093. doi: 10.4049/jimmunol.176.7.4083. [DOI] [PubMed] [Google Scholar]
- 67.Scharer C.D., Barwick B.G., Youngblood B.A., Ahmed R., Boss J.M. Global DNA methylation remodeling accompanies CD8 T cell effector function. J. Immunol. 2013;191:3419–3429. doi: 10.4049/jimmunol.1301395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kagoya Y., Nakatsugawa M., Yamashita Y., Ochi T., Guo T., Anczurowski M., Saso K., Butler M.O., Arrowsmith C.H., Hirano N. BET bromodomain inhibition enhances T cell persistence and function in adoptive immunotherapy models. J. Clin. Invest. 2016;126:3479–3494. doi: 10.1172/JCI86437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zediak V.P., Johnnidis J.B., Wherry E.J., Berger S.L. Cutting edge: persistently open chromatin at effector gene loci in resting memory CD8+ T cells independent of transcriptional status. J. Immunol. 2011;186:2705–2709. doi: 10.4049/jimmunol.1003741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Araki Y., Fann M., Wersto R., Weng N.P. Histone acetylation facilitates rapid and robust memory CD8 T cell response through differential expression of effector molecules (eomesodermin and its targets: perforin and granzyme B) J. Immunol. 2008;180:8102–8108. doi: 10.4049/jimmunol.180.12.8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jain A.K., Barton M.C. Bromodomain histone readers and cancer. J. Mol. Biol. 2017;429:2003–2010. doi: 10.1016/j.jmb.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 72.Peng D., Kryczek I., Nagarsheth N., Zhao L., Wei S., Wang W., Sun Y., Zhao E., Vatan L., Szeliga W., Kotarski J., Tarkowski R., Dou Y., Cho K., Hensley-Alford S., Munkarah A., Liu R., Zou W. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature. 2015;527:249–253. doi: 10.1038/nature15520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosenthal R., Cadieux E.L., Salgado R., Bakir M.A., Moore D.A., Hiley C.T., Lund T., Tanic M., Reading J.L., Joshi K., Henry J.Y., Ghorani E., Wilson G.A., Birkbak N.J., Jamal-Hanjani M., Veeriah S., Szallasi Z., Loi S., Hellmann M.D., Feber A., Chain B., Herrero J., Quezada S.A., Demeulemeester J., Van Loo P., Beck S., McGranahan N., Swanton C., consortium T.R. Neoantigen-directed immune escape in lung cancer evolution. Nature. 2019 doi: 10.1038/s41586-019-1032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Melamed P., Yosefzon Y., David C., Tsukerman A., Pnueli L. Tet enzymes, variants, and differential effects on function. Front. Cell Dev. Biol. 2018;6:22. doi: 10.3389/fcell.2018.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hornyak L., Dobos N., Koncz G., Karanyi Z., Pall D., Szabo Z., Halmos G., Szekvolgyi L. The role of indoleamine-2,3-dioxygenase in cancer development, diagnostics, and therapy. Front. Immunol. 2018;9:151. doi: 10.3389/fimmu.2018.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang Q., Xia J., Wang L., Wang X., Ma X., Deng Q., Lu Y., Kumar M., Zhou Z., Li L., Zeng Z., Young K.H., Yi Q., Zhang M., Li Y. miR-153 suppresses Ido1 expression and enhances CAR T cell immunotherapy. J. Hematol. Oncol. 2018;11:58. doi: 10.1186/s13045-018-0600-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garraway L.A., Lander E.S. Lessons from the cancer genome. Cell. 2013;153:17–37. doi: 10.1016/j.cell.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 78.Morgan M.A., Shilatifard A. Chromatin signatures of cancer. Genes Dev. 2015;29:238–249. doi: 10.1101/gad.255182.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Burrell R.A., McGranahan N., Bartek J., Swanton C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature. 2013;501:338–345. doi: 10.1038/nature12625. [DOI] [PubMed] [Google Scholar]
- 80.Joshi S., Durden D.L. Combinatorial approach to improve cancer immunotherapy: rational drug design strategy to simultaneously hit multiple targets to kill tumor cells and to activate the immune system. J. Oncol. 2019;2019:5245034. doi: 10.1155/2019/5245034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sharma P., Allison J.P. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Amreddy N., Babu A., Muralidharan R., Panneerselvam J., Srivastava A., Ahmed R., Mehta M., Munshi A., Ramesh R. Recent advances in nanoparticle-based cancer drug and gene delivery. Adv. Canc. Res. 2018;137:115–170. doi: 10.1016/bs.acr.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jakobczyk H., Sciortino F., Chevance S., Gauffre F., Troadec M.B. Promises and limitations of nanoparticles in the era of cell therapy: example with CD19-targeting chimeric antigen receptor (CAR)-modified T cells. Int. J. Pharm. 2017;532:813–824. doi: 10.1016/j.ijpharm.2017.07.075. [DOI] [PubMed] [Google Scholar]
- 84.Grywalska E., Pasiarski M., Gozdz S., Rolinski J. Immune-checkpoint inhibitors for combating T-cell dysfunction in cancer. OncoTargets Ther. 2018;11:6505–6524. doi: 10.2147/OTT.S150817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sharma P., Hu-Lieskovan S., Wargo J.A., Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hodi F.S., O'Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Haanen J.B., Gonzalez R., Robert C., Schadendorf D., Hassel J.C., Akerley W., van den Eertwegh A.J., Lutzky J., Lorigan P., Vaubel J.M., Linette G.P., Hogg D., Ottensmeier C.H., Lebbe C., Peschel C., Quirt I., Clark J.I., Wolchok J.D., Weber J.S., Tian J., Yellin M.J., Nichol G.M., Hoos A., Urba W.J. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat. Rev. Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 88.Dong H., Strome S.E., Salomao D.R., Tamura H., Hirano F., Flies D.B., Roche P.C., Lu J., Zhu G., Tamada K., Lennon V.A., Celis E., Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 89.Zou W., Wolchok J.D., Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 2016;8:328rv4. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wolchok J.D., Kluger H., Callahan M.K., Postow M.A., Rizvi N.A., Lesokhin A.M., Segal N.H., Ariyan C.E., Gordon R.A., Reed K., Burke M.M., Caldwell A., Kronenberg S.A., Agunwamba B.U., Zhang X., Lowy I., Inzunza H.D., Feely W., Horak C.E., Hong Q., Korman A.J., Wigginton J.M., Gupta A., Sznol M. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.John L.B., Devaud C., Duong C.P., Yong C.S., Beavis P.A., Haynes N.M., Chow M.T., Smyth M.J., Kershaw M.H., Darcy P.K. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin. Canc. Res. 2013;19:5636–5646. doi: 10.1158/1078-0432.CCR-13-0458. [DOI] [PubMed] [Google Scholar]
- 92.Rosewell Shaw A., Porter C.E., Watanabe N., Tanoue K., Sikora A., Gottschalk S., Brenner M.K., Suzuki M. Adenovirotherapy delivering cytokine and checkpoint inhibitor augments CAR T cells against metastatic head and neck cancer. Mol. Ther. 2017;25:2440–2451. doi: 10.1016/j.ymthe.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gajewski T.F., Schreiber H., Fu Y.X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ninomiya S., Narala N., Huye L., Yagyu S., Savoldo B., Dotti G., Heslop H.E., Brenner M.K., Rooney C.M., Ramos C.A. Tumor indoleamine 2,3-dioxygenase (Ido) inhibits CD19-CAR T cells and is downregulated by lymphodepleting drugs. Blood. 2015;125:3905–3916. doi: 10.1182/blood-2015-01-621474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Frumento G., Rotondo R., Tonetti M., Damonte G., Benatti U., Ferrara G.B. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J. Exp. Med. 2002;196:459–468. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mestermann K., Giavridis T., Weber J., Rydzek J., Frenz S., Nerreter T., Mades A., Sadelain M., Einsele H., Hudecek M. The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells. Sci. Transl. Med. 2019;11 doi: 10.1126/scitranslmed.aau5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dorrie J., Babalija L., Hoyer S., Gerer K.F., Schuler G., Heinzerling L., Schaft N. BRAF and MEK inhibitors influence the function of reprogrammed T cells: consequences for adoptive T-cell therapy. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19010289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Parente-Pereira A.C., Whilding L.M., Brewig N., van der Stegen S.J., Davies D.M., Wilkie S., van Schalkwyk M.C., Ghaem-Maghami S., Maher J. Synergistic chemoimmunotherapy of epithelial ovarian cancer using ErbB-retargeted T cells combined with carboplatin. J. Immunol. 2013;191:2437–2445. doi: 10.4049/jimmunol.1301119. [DOI] [PubMed] [Google Scholar]
- 99.Weiss T., Weller M., Guckenberger M., Sentman C.L., Roth P. NKG2D-Based CAR T cells and radiotherapy exert synergistic efficacy in glioblastoma. Canc. Res. 2018;78:1031–1043. doi: 10.1158/0008-5472.CAN-17-1788. [DOI] [PubMed] [Google Scholar]
- 100.Ma L., Dichwalkar T., Chang J.Y.H., Cossette B., Garafola D., Zhang A.Q., Fichter M., Wang C., Liang S., Silva M., Kumari S., Mehta N.K., Abraham W., Thai N., Li N., Wittrup K.D., Irvine D.J. Enhanced CAR-T cell activity against solid tumors by vaccine boosting through the chimeric receptor. Science. 2019;365:162–168. doi: 10.1126/science.aav8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Braicu C., Buse M., Busuioc C., Drula R., Gulei D., Raduly L., Rusu A., Irimie A., Atanasov A.G., Slaby O., Ionescu C., Berindan-Neagoe I. A comprehensive review on MAPK: a promising therapeutic target in cancer. Cancers (Basel) 2019;11 doi: 10.3390/cancers11101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu Y., Shepherd E.G., Nelin L.D. MAPK phosphatases--regulating the immune response. Nat. Rev. Immunol. 2007;7:202–212. doi: 10.1038/nri2035. [DOI] [PubMed] [Google Scholar]
- 103.Hay A.E., Cheung M.C. CAR T-cells: costs, comparisons, and commentary. J. Med. Econ. 2019;22:613–615. doi: 10.1080/13696998.2019.1582059. [DOI] [PubMed] [Google Scholar]
- 104.Levine B.L., Miskin J., Wonnacott K., Keir C. Global manufacturing of CAR T cell therapy. Mol. Ther. Meth. Clin. Dev. 2017;4:92–101. doi: 10.1016/j.omtm.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang X., Riviere I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol. Ther. Oncolytics. 2016;3:16015. doi: 10.1038/mto.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Harrison R.P., Zylberberg E., Ellison S., Levine B.L. Chimeric antigen receptor-T cell therapy manufacturing: modelling the effect of offshore production on aggregate cost of goods. Cytotherapy. 2019;21:224–233. doi: 10.1016/j.jcyt.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 107.Castella M., Boronat A., Martin-Ibanez R., Rodriguez V., Sune G., Caballero M., Marzal B., Perez-Amill L., Martin-Antonio B., Castano J., Bueno C., Balague O., Gonzalez-Navarro E.A., Serra-Pages C., Engel P., Vilella R., Benitez-Ribas D., Ortiz-Maldonado V., Cid J., Tabera J., Canals J.M., Lozano M., Baumann T., Vilarrodona A., Trias E., Campo E., Menendez P., Urbano-Ispizua A., Yague J., Perez-Galan P., Rives S., Delgado J., Juan M. Development of a novel anti-CD19 chimeric antigen receptor: a paradigm for an affordable CAR T cell production at academic institutions. Mol. Ther. Meth. Clin. Dev. 2019;12:134–144. doi: 10.1016/j.omtm.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang L., Gong W., Wang S., Neuber B., Sellner L., Schubert M.L., Huckelhoven-Krauss A., Kunz A., Gern U., Michels B., Hinkelbein M., Mechler S., Richter P., Muller-Tidow C., Schmitt M., Schmitt A. Improvement of in vitro potency assays by a resting step for clinical-grade chimeric antigen receptor engineered T cells. Cytotherapy. 2019 doi: 10.1016/j.jcyt.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 109.Stroncek D.F., Lee D.W., Ren J., Sabatino M., Highfill S., Khuu H., Shah N.N., Kaplan R.N., Fry T.J., Mackall C.L. Elutriated lymphocytes for manufacturing chimeric antigen receptor T cells. J. Transl. Med. 2017;15:59. doi: 10.1186/s12967-017-1160-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Janetzki S., Britten C.M., Kalos M., Levitsky H.I., Maecker H.T., Melief C.J., Old L.J., Romero P., Hoos A., Davis M.M. MIATA"-minimal information about T cell assays. Immunity. 2009;31:527–528. doi: 10.1016/j.immuni.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Taylor L., Rodriguez E.S., Reese A., Anderson K. Building a program: implications for infrastructure, nursing education, and training for CAR T-cell therapy. Clin. J. Oncol. Nurs. 2019;23:20–26. doi: 10.1188/19.CJON.S1.20-26. [DOI] [PubMed] [Google Scholar]
- 112.Callahan C., Barry A., Fooks-Parker S., Smith L., Baniewicz D., Hobbie W. Pediatric survivorship: considerations following CAR T-cell therapy. Clin. J. Oncol. Nurs. 2019;23:35–41. doi: 10.1188/19.CJON.S1.35-41. [DOI] [PubMed] [Google Scholar]
- 113.Buitrago J., Adkins S., Hawkins M., Iyamu K., Oort T. Adult survivorship: considerations following CAR T-cell therapy. Clin. J. Oncol. Nurs. 2019;23:42–48. doi: 10.1188/19.CJON.S1.42-48. [DOI] [PubMed] [Google Scholar]
- 114.Riegler L.L., Jones G.P., Lee D.W. Current approaches in the grading and management of cytokine release syndrome after chimeric antigen receptor T-cell therapy. Therapeut. Clin. Risk Manag. 2019;15:323–335. doi: 10.2147/TCRM.S150524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brudno J.N., Kochenderfer J.N. Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev. 2019;34:45–55. doi: 10.1016/j.blre.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Beaupierre A., Lundberg R., Marrero L., Jain M., Wang T., Alencar M.C. Management across settings: an ambulatory and community perspective for patients undergoing CAR T-cell therapy in multiple care settings. Clin. J. Oncol. Nurs. 2019;23:27–34. doi: 10.1188/19.CJON.S1.27-34. [DOI] [PubMed] [Google Scholar]
- 117.Anderson K., Latchford T. Associated toxicities: assessment and management related to CAR T-cell therapy. Clin. J. Oncol. Nurs. 2019;23:13–19. doi: 10.1188/19.CJON.S1.13-19. [DOI] [PubMed] [Google Scholar]
- 118.Sadelain M. CD19 CAR T cells. Cell. 2017;171:1471. doi: 10.1016/j.cell.2017.12.002. [DOI] [PubMed] [Google Scholar]