This cluster randomized clinical trial tests the effects of receipt of a paper-based mammography screening decision aid for women 75 years and older on their screening decisions.
Key Points
Question
How does use of a workbook mammography screening decision aid (DA) for women 75 years and older affect their screening decisions?
Findings
In this cluster randomized clinical trial of 546 women aged 75 to 89 years, receipt of the decision aid before a visit with their clinician led to women 75 years and older being more knowledgeable about mammography screening, having more discussions with their primary care physician about screening, and fewer women being screened.
Meaning
Use of a mammography screening decision aid may help women 75 years and older make more informed decisions about mammography screening and, as a result, may reduce overscreening.
Abstract
Importance
Guidelines recommend that women 75 years and older be informed of the benefits and harms of mammography before screening.
Objective
To test the effects of receipt of a paper-based mammography screening decision aid (DA) for women 75 years and older on their screening decisions.
Design, Setting, and Participants
A cluster randomized clinical trial with clinician as the unit of randomization. All analyses were completed on an intent-to-treat basis. The setting was 11 primary care practices in Massachusetts or North Carolina. Of 1247 eligible women reached, 546 aged 75 to 89 years without breast cancer or dementia who had a mammogram within 24 months but not within 6 months and saw 1 of 137 clinicians (herein referred to as PCPs) from November 3, 2014, to January 26, 2017, participated. A research assistant (RA) administered a previsit questionnaire on each participant’s health, breast cancer risk factors, sociodemographic characteristics, and screening intentions. After the visit, the RA administered a postvisit questionnaire on screening intentions and knowledge.
Interventions
Receipt of the DA (DA arm) or a home safety (HS) pamphlet (control arm) before a PCP visit.
Main Outcomes and Measures
Participants were followed up for 18 months for receipt of mammography screening (primary outcome). To examine the effects of the DA, marginal logistic regression models were fit using generalized estimating equations to allow for clustering by PCP. Adjusted probabilities and risk differences were estimated to account for clustering by PCP.
Results
Of 546 women in the study, 283 (51.8%) received the DA. Patients in each arm were well matched; their mean (SD) age was 79.8 (3.7) years, 428 (78.4%) were non-Hispanic white, 321 (of 543 [59.1%]) had completed college, and 192 (35.2%) had less than a 10-year life expectancy. After 18 months, 9.1% (95% CI, 1.2%-16.9%) fewer women in the DA arm than in the control arm had undergone mammography screening (51.3% vs 60.4%; adjusted risk ratio, 0.84; 95% CI, 0.75-0.95; P = .006). Women in the DA arm were more likely than those in the control arm to rate their screening intentions lower from previsit to postvisit (69 of 283 [adjusted %, 24.5%] vs 47 of 263 [adjusted %, 15.3%]), to be more knowledgeable about the benefits and harms of screening (86 [adjusted %, 25.5%] vs 32 [adjusted %, 11.7%]), and to have a documented discussion about mammography with their PCP (146 [adjusted %, 47.4%] vs 111 [adjusted %, 38.9%]). Almost all women in the DA arm (94.9%) would recommend the DA.
Conclusions and Relevance
Providing women 75 years and older with a mammography screening DA before a PCP visit helps them make more informed screening decisions and leads to fewer women choosing to be screened, suggesting that the DA may help reduce overscreening.
Trial Registration
ClinicalTrials.gov Identifier: NCT02198690
Introduction
Twelve million women in the United States are 75 years and older, a number that is rising, and breast cancer risk increases with age.1,2 Although mammography screening is associated with a 20% reduction in breast cancer mortality among women aged 40 to 74 years,3,4 its effectiveness in women 75 years and older is unknown because none of the screening randomized clinical trials to date have included these women.5 In women aged 50 to 74 years, it takes on average 10.7 years for mammography screening to prevent 1 in 1000 women from dying of breast cancer.6 Meanwhile, the harms of screening are immediate, including pain, anxiety, false-positive results, and overdiagnosis (detection of nonlethal tumors) resulting in overtreatment.7
Because of the uncertainty of a mortality benefit for women 75 years and older, guidelines recommend that these women be informed of the benefits and harms of mammography before being screened and that women with less than a 10-year life expectancy not be screened.8,9,10,11 Despite these recommendations, 56% of community-dwelling women 75 years and older report recent mammography screening, including many women with short life expectancy.12
To help older women weigh the benefits and harms of screening, we previously developed a paper-based mammography screening decision aid (DA) for women 75 years and older (eMethods in Supplement 1) based on the Ottawa Decision Support Framework.13,14,15,16 Development of the DA has been described previously.17 The DA is tailored based on age (75-84 years vs ≥85 years) and includes information on breast cancer risk factors, life expectancy by age, competing mortality risks, screening outcomes, and a values clarification exercise. The DA also asks users 10 questions about their health from a validated mortality index by Schonberg et al18 to calculate a health score; higher scores are associated with shorter life expectancy. The DA does not inform users of their estimated life expectancy because some older women find this information objectionable.19 However, it informs users with higher scores that having a mammogram is unlikely to help them live longer. The DA does not distinguish the effects of mammography screening on overall vs breast cancer–specific mortality because older women found this distinction confusing. The DA informs users that it is uncertain if mammography screening reduces breast cancer mortality in older women. Written at a sixth-grade reading level, the DA uses large fonts and lots of white space. In a pilot pretest-posttest study,14 DA use was associated with women having increased knowledge of the benefits and harms of mammography and with lower screening intentions. This study aimed to test the effects of the DA in a large cluster randomized clinical trial. The DA describes the benefits and harms of mammography, whereas educational materials have traditionally focused on the benefits of mammography; therefore, we hypothesized that DA use would lead to fewer women 75 years and older being screened.
Methods
Study Design
A cluster randomized clinical trial was conducted of the mammography screening DA with clinician (herein referred to as PCP) as the unit of randomization. In eTable 1 in Supplement 1, we justify the few revisions made to the trial protocol since publication17 (the trial protocol is available in Supplement 2). The following 11 primary care practices in Massachusetts or North Carolina participated in the study: an academic internal medicine practice and an academic geriatrics practice in Boston, Massachusetts; 7 different community practices in the Boston area; and an academic internal medicine practice and an academic family medicine practice in North Carolina. eTable 2 in Supplement 1 lists details of these sites. Institutional review boards at Boston’s Beth Israel Deaconess Medical Center and at The University of North Carolina at Chapel Hill approved this study before data collection. Oral informed consent was obtained from participants. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.
Study Sample
From November 3, 2014, to January 26, 2017, English-speaking women aged 75 to 89 years who were scheduled for a routine visit or physical examination with their PCP (physician or nurse practitioner) in the next 4 to 12 weeks were invited to participate in the study. To identify women likely to be contemplating screening, participants had to have had a mammogram in the past 24 months but not within 6 months. Women with dementia were excluded as determined by the following: a problem list, PCP communication, or score of at least 19 (indicative of dementia) on the Short Blessed Test20; invasive or noninvasive breast cancer or atypia; lacking capacity; less than seventh-grade education; documentation of having stopped screening; first visit with the PCP; and not seeing their PCP during the study.
Recruitment
Patients were identified through PCP appointment logs. After obtaining PCP approval, a research assistant (RA) (A.R.J. or G.M.A.) sent eligible patients an informational letter with a number to call to opt out of being contacted. The RAs called patients who did not opt out to assess their willingness to participate and to reconfirm eligibility. Women who declined participation were asked to report their age, race/ethnicity, educational level, and perceived health. Patients were informed that they would be asked to complete a previsit questionnaire, to come early to a visit to read the study’s educational materials, and to complete a postvisit questionnaire as well as that their medical records would be reviewed for receipt of preventive services within 18 months and that they would receive $40 for participating.
Interventions and Randomization
For the first patient participating for each PCP, an RA randomized the patient’s PCP stratified by site (Boston academic or 2 different Boston community groups vs North Carolina) and panel size (<25, ≥25, or ≥75 women in the panel) to either the DA (DA arm) or to the American Geriatrics Society Health in Aging Foundation’s 2-page home safety (HS) pamphlet (control arm) (eMethods in Supplement 1), which served as an attention control.21 Randomization assignments were determined using a permuted block randomization scheme with randomly varying block sizes and were placed in sequentially numbered, sealed envelopes by the statistician (R.B.D.), stratified by site and panel size. Subsequent participants for each PCP received the same intervention. We stratified by site to account for institutional differences in the approach to screening and by panel size to help ensure balance in the number of patients recruited per arm. The PCPs were emailed before each visit to inform them that their patient would be coming early to receive the study’s educational materials. For PCPs randomized to the DA, the email included a hyperlink to an optional 3-minute training video (4 PCPs watched the video). Recruitment was capped at 20 patients per PCP. When providing study materials, RAs asked participants to read every line. The RAs referred patients with questions to their PCP.
Data Collection
Study questionnaires are included in the eMethods in Supplement 1. The previsit questionnaire, administered a median of 34 days (interquartile range, 34 days) before the PCP visit by an RA, assessed participants’ sociodemographics, life expectancy,18 numeracy,22 literacy,23 screening intentions,24 perceived risk, subjective norms around mammography,25 and risk factors to calculate 5-year breast cancer risk using the Gail Breast Cancer Risk Assessment Tool.26,27
Outcomes
Primary Outcome
The primary outcome was receipt of mammography screening within 18 months.8 To assess this outcome, RAs reviewed electronic medical record (EMR) notes, radiology records, and screening sheets (on which mammograms that were performed outside the medical system were entered manually) (eTable 3 in Supplement 1). Initially, all medical records were dually abstracted; however, after reviewing 280 medical records, agreement was consistently 100% between abstractors. Therefore, the remainder were singly abstracted, with 20% randomly dually abstracted to ensure quality. If it was unclear from the EMR whether a patient had been screened (eg, the patient had moved or there were no notes in the last 6 months of follow-up and no documentation of death), then participants (or, if necessary, a proxy) were contacted to assess screening.
Secondary Outcomes
The postvisit questionnaire, usually administered immediately after the visit, assessed the following: knowledge of the benefits and harms of mammography,14 decisional conflict around screening (including 5 subscales),28 preferred decision-making role,29 whether participants discussed mammography or HS with their PCP, and changes in screening intentions.24 The PCP notes were reviewed 6 months after participation to identify if PCPs documented discussing mammography or HS.
Acceptability and Safety
The postvisit questionnaire assessed women’s anxiety30 and asked about the acceptability of the educational materials received.31 Women in the DA arm were asked whether the DA presented balanced information on mammography and whether the DA helped prepare them for decision-making with their PCP.32 From medical records, we abstracted whether women experienced breast pain, underwent diagnostic mammography, were diagnosed as having breast cancer, or died during follow-up.
Statistical Analysis
All analyses were performed using SAS, version 9.4 (SAS Institute Inc), statistical software. Sample size was based on an assumed intraclass correlation coefficient of 0.1 and that 5 patients on average would be recruited from 100 PCPs. With an α level of .05, an estimated 516 women needed to be recruited for the study to have 0.90 power to detect a 15% difference in receipt of screening between arms. Anticipating some loss to follow-up, we aimed to recruit 550 women.
To examine the effects of the DA on receipt of screening (primary outcome), marginal logistic regression models were fit using generalized estimating equations with sandwich estimates of standard error to allow for clustering by PCP. The model was fit with 3 independent variables, including intervention group, PCP site, and panel size, to estimate relative risks and 95% CIs. Adjusted probabilities and risk differences were estimated to account for clustering by PCP using methods described by Spiegelman and Hertzmark.33 Similar methods were used for the other categorical outcomes. For continuous outcomes, random-effects linear regression models were used to allow for clustering by PCP and to fit each of these models with the 3 independent variables. The mean differences between arms were further estimated with 95% CIs and adjusted probabilities.33 In secondary analyses, effect modification was examined by PCP site and patient age, educational level, life expectancy, and breast cancer risk,
Because we did not specify a priori how to define statistical significance for secondary outcomes, 95% CIs are given for these outcomes but not P values. We used a 2-sided P value and a threshold of .05 to determine statistical significance. Data on screening were missing for only one woman who withdrew after the follow-up questionnaire, so a complete case analysis was conducted. All analyses were completed on an intent-to-treat basis.
Results
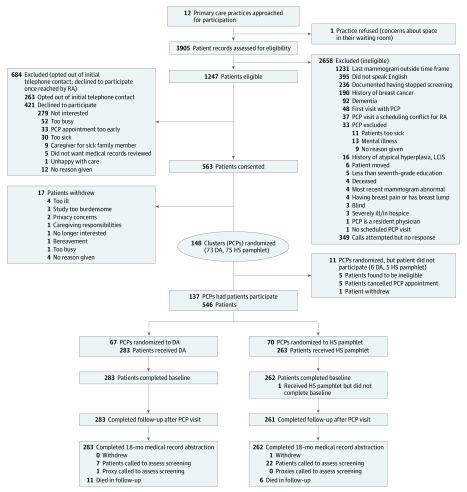
Of 3905 patient records reviewed, 1596 patients were deemed eligible and were sent a study informational letter. Of these, 349 were not reached, 263 opted out of initial telephone contact, 421 declined to participate, and 563 agreed to participate; 17 subsequently withdrew, leaving 546 participants (mean [SD] age, 79.8 [3.7] years) without breast cancer or dementia who had a mammogram within 24 months but not within 6 months and saw 1 of 137 PCPs (Figure). A mean (SD) of 4.0 (3.8) women participated per PCP. Women who opted out or declined to participate were similar in age to participants but were less educated and in worse health; women who declined were also less likely to be of non-Hispanic white race/ethnicity and to have had a recent mammogram (eTable 4 and eTable 5 in Supplement 1). Among 546 participants, 283 (51.8%) received the DA. Patients in each arm were well matched by baseline characteristics (Table 1).18,20,22,23,24,26,34 Of 546 participants, 428 (78.4%) were non-Hispanic white, 321 (of 543 [59.1%]) had completed college, 192 (35.2%) had less than a 10-year life expectancy, and 110 (20.2%) were from North Carolina. Among 137 PCPs who had patients participate in the study, 79 (57.7%) were female, 41 (29.9%) were from North Carolina, and 67 (48.9%) were randomized to the DA (eTable 6 in Supplement 1).
Figure. Consolidated Standards of Reporting Trials (CONSORT) Diagram of Screening, Enrollment, and Follow-up of Trial Participants.
DA indicates decision aid; HS, home safety; LCIS, lobular cancer in situ; PCP, primary care provider; and RA, research assistant.
Table 1. Participant Characteristics.
| Variable | No. (%) | |
|---|---|---|
| DA (n = 283) | HS pamphlet (n = 263) | |
| Age, mean (SD), y | 79.7 (3.7) | 79.8 (3.7) |
| Recruitment site | ||
| 2 Academic practices at BIDMC | 75 (26.5) | 69 (26.2) |
| 4 Community practices affiliated with BIDMC | 49 (17.3) | 52 (19.8) |
| 3 Atrius Health community practices in the Boston area | 118 (41.7) | 73 (27.8) |
| 2 Academic practices at UNC | 41 (14.5) | 69 (26.2) |
| Race/ethnicity | ||
| Non-Hispanic white | 224 (79.2) | 204 (77.6) |
| Non-Hispanic black | 52 (18.4) | 48 (18.3) |
| Hispanic | 1 (0.4) | 7 (2.7) |
| Other | 6 (2.1) | 4 (1.5) |
| Educational level | ||
| Less than high school | 13 (4.6) | 14 (5.3) |
| High school | 52 (18.4) | 36 (13.7) |
| Some college | 58 (20.5) | 49 (18.6) |
| College degree or beyond | 160 (56.5) | 161 (61.2) |
| Missing | 0 | 3 (1.1) |
| MacArthur Scale of Subjective Social Status socioeconomic ladder | ||
| No. | 277 | 255 |
| Mean (SD)a | 6.7 (2.1) | 6.7 (2.0) |
| Income | ||
| ≤$35 000 | 83 (29.3) | 87 (33.1) |
| >$35 000 to $65 000 | 64 (22.6) | 53 (20.2) |
| >$65 000 | 95 (33.6) | 77 (29.3) |
| Declined to answer | 41 (14.5) | 46 (17.5) |
| Marital status | ||
| Currently married | 113 (39.9) | 100 (38.0) |
| Single/divorced/separated/widowed | 169 (59.7) | 160 (60.8) |
| Missing | 1 (0.4) | 3 (1.1) |
| Living arrangement | ||
| Lives alone | 153 (54.1) | 139 (52.9) |
| Lives with others | 129 (45.6) | 121 (46.0) |
| Missing | 1 (0.4) | 3 (1.1) |
| Short Blessed Testb | ||
| 0-8, No impairment | 279 (98.6) | 256 (97.3) |
| 9-18, Mild to moderate impairment | 3 (1.1) | 3 (1.1) |
| Missing | 1 (0.4) | 4 (1.5) |
| Life expectancyc | ||
| ≥10 y | 179 (63.3) | 174 (66.2) |
| <10 y | 104 (36.7) | 88 (33.5) |
| Missing | 0 | 1 (0.4) |
| Falls in the past year | ||
| ≥1 | 83 (29.3) | 68 (25.9) |
| None | 199 (70.3) | 192 (73.0) |
| Missing | 1 (0.4) | 3 (1.1) |
| Medical literacy assessed using REALM-7d | ||
| 7 Medical terms correctly pronounced | 246 (86.9) | 235 (89.4) |
| <7 Medical terms correctly pronounced | 26 (9.2) | 23 (8.7) |
| Missing | 11 (3.9) | 5 (1.9) |
| Subjective numeracy, 1 (not good with numbers) to 7 (extremely good with numbers)e | ||
| No. | 283 | 262 |
| Mean (SD) | 3.5 (1.0) | 3.4 (1.0) |
| 5-y Probability of breast cancer based on the Gail Breast Cancer Risk Assessment Tool, %f | ||
| No. | 283 | 263 |
| Mean (SD) | 2.2 (1.1) | 2.1 (1.1) |
| First-degree female history of breast cancer | ||
| ≥1 | 56 (19.8) | 50 (19.0) |
| None | 226 (79.9) | 212 (80.6) |
| Missing | 1 (0.4) | 1 (0.4) |
| Intentions to be screened at baseline, 1 (intends to be screened) to 15 (does not intend to be screened) (n = 541)g | ||
| Mean (SD) | 3.0 (4.3) | 2.9 (4.2) |
| Use of postmenopausal hormone therapy | ||
| Currently using | 12 (4.2) | 9 (3.4) |
| Previously used for <5 y | 51 (18.0) | 48 (18.3) |
| Previously used for ≥5 y | 80 (28.3) | 84 (31.9) |
| Never used | 133 (47.0) | 115 (43.7) |
| Missing | 7 (2.5) | 7 (2.7) |
| My family thinks I should have a mammogram | ||
| Strongly agree/agree | 109 (38.5) | 105 (39.9) |
| Strongly disagree/disagree/neutral | 98 (34.6) | 110 (41.8) |
| Missingh | 76 (26.9) | 48 (18.3) |
| My friends think I should have a mammogram | ||
| Strongly agree/agree | 96 (33.9) | 86 (32.7) |
| Strongly disagree/disagree/neutral | 93 (32.9) | 116 (44.1) |
| Missingh | 94 (33.2) | 61 (23.2) |
| My PCP thinks I should have a mammogram | ||
| Strongly agree/agree | 195 (68.9) | 159 (60.5) |
| Strongly disagree/disagree/neutral | 50 (17.7) | 68 (25.9) |
| Missingh | 38 (13.4) | 36 (13.7) |
| How was the baseline survey administered? | ||
| RA administered over the telephone | 277 (97.9) | 254 (96.6) |
| RA administered in person | 4 (1.4) | 6 (2.3) |
| Missing | 2 (0.7) | 3 (1.1) |
| How was the follow-up survey administered? | ||
| RA administered over the telephone | 64 (22.6) | 43 (16.3) |
| RA administered in person | 217 (76.7) | 218 (82.9) |
| Missing | 2 (0.7) | 2 (0.8) |
Abbreviations: BIDMC, Beth Israel Deaconess Medical Center, Boston, Massachusetts; DA, decision aid; HS, home safety; PCP, primary care provider; RA, research assistant; REALM-7, Rapid Estimate of Adult Literacy in Medicine 7; UNC, The University of North Carolina at Chapel Hill.
On the MacArthur Scale of Subjective Social Status, individuals are asked to place an X on 1 of 10 rungs of a ladder (1 is the least well-off, and 10 is the most well-off) to represent how well-off (based on money/educational level/job) they feel compared with others in the United States.34
On the Short Blessed Test, scores range from 0 to 28. Scores 9 to 18 suggest some cognitive impairment, and scores greater than 18 suggest severe cognitive impairment.20
On the validated mortality index by Schonberg et al18 to calculate a health score, scores for women range from 0 to 24. Scores of 10 or higher are associated with greater than 50% chance of 10-year mortality. Therefore, women who score 10 or higher are estimated to have less than a 10-year life expectancy.
REALM-7 scores range from 0 to 7, with higher scores indicating greater literacy.23
Subjective Numeracy Scale scores range from 1 (not at all good with numbers) to 7 (extremely good with numbers).22
The Gail Breast Cancer Risk Assessment Tool considers a woman’s age, race/ethnicity, first-degree family history of breast cancer, age at menarche, age at first birth, and history of breast biopsies (specifically, a finding of atypical hyperplasia) in assessing women’s probability of breast cancer in the next 5 years. The average 75-year-old white woman has a 2.2% 5-year risk.26
Intentions to be screened on a scale of 1 point (will have a mammogram) to 15 points (will not have a mammogram).24
Questions on norms were added after the study began; data are missing for patients enrolled before questions were added.
Primary Outcome
At 18 months’ follow-up, statistically significantly fewer women in the DA arm had undergone mammography screening than in the control arm (51.3% vs 60.4%) (adjusted risk difference, −9.1%; 95% CI, −1.2% to −16.9%) (adjusted risk ratio, 0.84; 95% CI, 0.75-0.95; P = .006; PCP intraclass correlation coefficient, 0.18) (Table 2).24,28,29,33 Examining effect modification (Table 3)33 in Massachusetts, where the crude number (adjusted %) undergoing screening in the control arm was 143 (71.9%), 12.6% (95% CI, 3.1%-22.0%) fewer women in the DA arm were screened (150 [59.3%]); in North Carolina, where screening in the control arm was only 16 (21.6%), 2.2% (95% CI, −14.7% to 19.0%) more women in the DA arm were screened (11 [23.8%]). However, the P value for interaction was not statistically significant. No apparent effect modification on receipt of screening was found by patient age, educational level, life expectancy, or breast cancer risk.
Table 2. Primary Outcome and Secondary Outcomes of the Effects of the Decision Aid (DA).
| Variable | Crude No. (adjusted %) | Risk ratio (95% CI)a | Adjusted risk difference, mean (95% CI) | |
|---|---|---|---|---|
| DA (n = 283) | HS pamphlet (n = 263) | |||
| Primary outcome | ||||
| Receipt of mammography screening within 18-mo follow-upb | 161 (51.3) | 159 (60.4) | 0.84 (0.75 to 0.95) (P = .006) | NA |
| Missing, crude No. | 0 | 1 | NA | NA |
| Secondary outcomes (categorical)c | ||||
| Change in intentions to be screened with mammographyd | NA | NA | 1.41 (1.01 to 1.98) | NA |
| Moved away from screening | 69 (24.5) | 47 (15.3) | NA | NA |
| No change/moved toward screening | 209 (75.5) | 209 (84.7) | NA | NA |
| Missing, crude No. | 5 | 7 | NA | NA |
| Documented discussion of risks/benefits of mammography within 6 mo | 86 (25.5) | 32 (11.7) | 2.17 (1.39 to 3.40) | NA |
| Missing, crude No. | 0 | 1 | NA | NA |
| Preferred decision-making rolee | NA | NA | 0.65 (0.45 to 0.95) | NA |
| Prefers PCP to make the final decision | 33 (11.9) | 48 (15.8) | NA | NA |
| Prefers to share decision with PCP or to make the decision on her own | 247 (88.1) | 208 (84.2) | NA | NA |
| Missing, crude No. | 3 | 7 | NA | NA |
| Talked with PCP about mammography at visit | 146 (47.4) | 111 (38.9) | 1.16 (0.95 to 1.42) | NA |
| Missing, crude No. | 4 | 3 | NA | NA |
| Talked with PCP about home safety at visit | 60 (23.4) | 67 (25.9) | 0.87 (0.62 to 1.24) | NA |
| Missing, crude No. | 3 | 5 | NA | NA |
| Secondary outcomes (continuous) | ||||
| Knowledge, least squares mean (SE)f | 7.9 (0.1) | 6.3 (0.1) | NA | 1.6 (1.3 to 1.9) |
| Missing, crude No. | 1 | 1 | NA | NA |
| Decisional Conflict Scale total, least squares mean (SE)g | 19.5 (0.8) | 20.0 (0.8) | −0.5 (−2.6 to 1.7) | |
| Missing, crude No. | 1 | 7 | NA | NA |
| Informed subscale, least squares mean (SE)h | 19.4 (1.0) | 22.4 (1.0) | NA | −3.0 (−5.5 to −0.5) |
| Missing, crude No. | 1 | 2 | NA | NA |
| Values clarity subscale, least squares mean (SE)i | 21.8 (1.0) | 23.1 (1.0) | NA | −1.3 (−3.9 to 1.3) |
| Missing, crude No. | 1 | 4 | NA | NA |
| Support subscale, least squares mean (SE)j | 18.1 (0.9) | 17.7 (0.9) | NA | 0.3 (−2.1 to 2.7) |
| Missing, crude No. | 1 | 3 | NA | NA |
| Uncertainty subscale, least squares mean (SE)k | 20.6 (1.2) | 19.0 (1.2) | 1.6 (−1.6 to 4.8) | |
| Missing, crude No. | 1 | 5 | NA | NA |
| Effective decision subscale, least squares mean (SE)l | 18.0 (0.9) | 18.6 (1.0) | NA | −0.7 (−3.1 to 1.8) |
| Missing, crude No. | 1 | 5 | NA | NA |
Abbreviations: HS, home safety; NA, not applicable; PCP, primary care provider.
Models were adjusted for PCP site (4 sites) and PCP panel size (<25, ≥25, or ≥75 women in the panel).
The predicted probabilities are reported; these were estimated using methods by Spiegelman and Hertzmark.33 Crude numbers are given and do not correspond with the predicted probabilities.
All outcomes were assessed via the follow-up questionnaire completed immediately after the visit.
Intentions to be screened on a scale of 1 point (will have a mammogram) to 15 points (will not have a mammogram).24 We calculated the change in intentions to be screened for each participant from baseline to follow-up. We then examined the proportion of women in each arm who moved away from screening vs no change/moved toward screening.
The Controlled Preferences Scale assessed the preferred role in decision-making around mammography screening (examined passive decision-making vs active/shared decision-making).29
Knowledge was assessed using 11 questions (9 true or false and 2 multiple choice) estimating the mean difference correct and 95% CI to determine the presence of statistically significant differences between arms.
The Decisional Conflict Scale measures uncertainty in a decision, feeling informed in a decision, clear about personal values, supported, and whether one feels that decision-making is effective and likely to be implemented (scores range from 0 [no decisional conflict] to 100 [extremely high decisional conflict]), estimating the mean difference correct and 95% CI to determine the presence of statistically significant differences between arms. Five subscales are then detailed.28
Informed subscale scores range from 0 (feels extremely certain about best choice) to 100 (feels extremely uncertain about best choice).
Values clarity subscale scores range from 0 (feels extremely clear about personal values) to 100 (feels extremely unclear about personal values).
Support subscale scores range from 0 (feels extremely supported in decision-making) to 100 (feels extremely unsupported in decision-making).
Uncertainty subscale scores range from 0 (feels extremely certain about best choice) to 100 (feels extremely uncertain about best choice).
Effective decision subscale scores range from 0 (good decision) to 100 (bad decision).
Table 3. Assessment of Effect Modification on Receipt of Mammography Screening Within 18 Months.
| Variable | Crude No. (adjusted %) | Adjusted risk ratio (95% CI)a | P value for interaction | |
|---|---|---|---|---|
| DA (n = 283) | HS pamphlet (n = 263) | |||
| Receipt of mammography screening by site | ||||
| Boston, Massachusetts, area (n = 436) | 150 (59.3) | 143 (71.9) | 0.83 (0.72-0.95) | .40 |
| North Carolina (n = 110) | 11 (23.8) | 16 (21.6) | 1.13 (0.55-2.33) | |
| Receipt of mammography screening by life expectancyb | ||||
| ≥10 y (n = 353) | 114 (59.7) | 124 (68.6) | 0.83 (0.73-0.95) | .50 |
| <10 y (n = 192) | 47 (40.9) | 35 (41.2) | 0.94 (0.69-1.28) | |
| Receipt of mammography screening by 5-y breast cancer risk | ||||
| ≥3% (n = 87) | 25 (51.5) | 34 (74.0) | 0.68 (0.51-0.91) | .13 |
| <3% (n = 459) | 136 (52.6) | 125 (54.6) | 0.89 (0.76-1.05) | |
| Receipt of mammography screening by age | ||||
| <85 y (n = 463) | 144 (55.3) | 146 (63.0) | 0.83 (0.73-0.94) | .44 |
| ≥85 y (n = 83) | 17 (35.4) | 13 (32.1) | 1.01 (0.62-1.63) | |
| Receipt of mammography screening by educational levelc | ||||
| College degree or beyond (n = 321) | 94 (53.5) | 102 (59.1) | 0.85 (0.73-1.00) | .90 |
| Less than college degree (n = 222) | 67 (51.5) | 56 (57.3) | 0.84 (0.70-1.00) | |
Abbreviations: DA, decision aid; HS, home safety.
Models were adjusted for primary care provider site (4 sites) and primary care provider panel size (<25, ≥25, or ≥75 women in the panel). The predicted probabilities are reported and were estimated using methods by Spiegelman and Hertzmark.33 Crude numbers are given and do not correspond with the predicted probabilities.
Follow-up on receipt of mammography screening by life expectancy is missing for 1 patient in the HS pamphlet arm.
Three patients had missing responses to educational level.
Secondary Outcomes
Findings from the secondary outcomes paralleled those from the primary outcome (Table 2). Women in the DA arm were more likely than those in the control arm to rate their screening intentions lower from previsit to postvisit (69 of 283 [adjusted %, 24.5] vs 47 of 263 [adjusted %, 15.3%]). These women had rated their screening intentions even lower immediately after reading the DA, but their intentions went up slightly after seeing their PCP (eTable 7 in Supplement 1). Women in the DA arm were more knowledgeable about the benefits and harms of mammography than those in the control arm (86 [adjusted %, 25.5%] vs 32 [adjusted %, 11.7%]) (eTable 8 in Supplement 1 lists women’s responses to knowledge questions), preferred to be more involved in decision-making, and were more likely than those in the control arm to have a documented discussion about mammography with their PCP (146 [adjusted %, 47.4%] vs 111 [adjusted %, 38.9%]). Overall decisional conflict scores were similar by arm; however, DA arm participants were more likely to perceive themselves as informed.
Acceptability and Safety
After accounting for missing responses to the follow-up questionnaire, participants in the DA arm found the DA to be helpful and clear, and almost all of these women (94.9%) would recommend the DA (Table 4).30,35 Few participants (3.6%) needed help reading the DA, and 85.2% did not find it anxiety provoking (similar to what women described when reading the HS pamphlet). On average, women thought that the DA somewhat prepared them for decision-making with their PCP. Most participants (75.2%) thought that the length of the DA was just right, but 19.9% thought that it was too long. Overall, among participants who completed the questionnaire, 54.0% thought that the information on mammography was balanced; however, 21.5% thought that it was slanted toward mammography, and 24.5% thought that it was slanted away from mammography (women with lower educational level were more likely to think that the DA was slanted toward mammography [eTable 9 in Supplement 1]). The DA arm participants tended to be less likely than participants in the control arm to undergo diagnostic mammography within 18 months (14 [4.9%] vs 23 [8.7%]), and breast cancer diagnoses were rare (5 total, 3 in the DA arm); no one died of breast cancer during follow-up. Eleven (3.9%) DA arm participants died of other causes within 18 months compared with 6 (2.3%) control arm participants.
Table 4. Acceptability and Safety of the Decision Aid (DA) and the Home Safety (HS) Pamphlet.
| Variable | No. (%) | |
|---|---|---|
| DA (n = 283) | HS pamphlet (n = 263) | |
| Categorical outcomes from the follow-up questionnairea | ||
| Length of the materialsb | ||
| Too short | 8 (2.8) | 10 (3.8) |
| Just right | 213 (75.3) | 221 (84.0) |
| Too long | 55 (19.4) | 27 (10.3) |
| Missing | 7 (2.5) | 5 (1.9) |
| Clarity of informationc | ||
| All/most of the information was clear | 262 (92.6) | 257 (97.7) |
| Some information was unclear | 12 (4.2) | 0 |
| Most of the information was unclear | 6 (2.1) | 1 (0.4) |
| Missing | 3 (1.1) | 5 (1.9) |
| Understanding the informationd | ||
| Understood all/most | 276 (97.5) | 256 (97.3) |
| Understood some | 5 (1.8) | 1 (0.4) |
| Missing | 2 (0.7) | 6 (2.3) |
| Balance | ||
| Slanted toward mammography | 59 (20.8) | NA |
| Completely balanced | 148 (52.3) | NA |
| Slanted away from mammography | 67 (23.7) | NA |
| Missing | 9 (3.2) | NA |
| Anxiety provokinge | ||
| Made me very/extremely anxious | 8 (2.8) | 9 (3.4) |
| A little anxious | 33 (11.7) | 26 (9.9) |
| Not at all | 236 (83.4) | 224 (85.2) |
| Missing | 6 (2.1) | 4 (1.5) |
| Recommend the educational materials | ||
| Would recommend | 260 (91.9) | 244 (92.8) |
| Would not recommend | 14 (4.9) | 9 (3.4) |
| Missing | 9 (3.2) | 10 (3.8) |
| Helpfulness of the educational materials | ||
| Helpful | 238 (84.1) | 240 (91.3) |
| Not helpful | 35 (12.4) | 19 (7.2) |
| Missing | 10 (3.5) | 4 (1.5) |
| Needed someone else to read them the materials | ||
| Had another person read them the materials | 10 (3.5) | 11 (4.2) |
| Did not need help | 272 (96.1) | 250 (95.1) |
| Missing | 1 (0.4) | 2 (0.8) |
| What is your preferred format for health educational materials? | ||
| A paper pamphlet like the one you read for this study | 193 (68.2) | 181 (68.8) |
| To read information on the computer/internet | 29 (10.2) | 25 (9.5) |
| A web-based or mobile application | 6 (2.1) | 5 (1.9) |
| Both on paper and computer/internet | 4 (1.4) | 2 (0.8) |
| Prefer information verbally | 4 (1.4) | 2 (0.8) |
| No preference | 41 (14.5) | 43 (16.3) |
| Missing | 6 (2.1) | 5 (1.9) |
| Continuous outcomes from the follow-up questionnaire | ||
| No. | 283 | 263 |
| Spielberger State-Trait Anxiety Inventoryf | ||
| No. | 274 | 256 |
| Mean (SD) | 25.8 (8.6) | 27.1 (9.8) |
| Helped prepare to make decision with PCP (n = 282)g | ||
| Mean (SD) | 2.9 (1.0) | NA |
| Breast cancer outcomes from 18-mo medical record abstraction | ||
| No. | 283 | 263h |
| Breast pain within 18 mo | 7 (2.5) | 4 (1.5) |
| Diagnostic mammography within 18 mo | 14 (4.9) | 23 (8.7) |
| Breast cancer within 18 mo | 3 (1.1) | 2 (0.8) |
| Breast cancer death within 18 mo, No. | 0 | 0 |
| Non–breast cancer death within 18 mo | 11 (3.9) | 6 (2.3) |
Abbreviations: NA, not applicable; PCP, primary care provider.
Crude probabilities are given to show more than 2 levels within a category.
Compared Just right vs other.
Compared All/most of the information was clear vs other.
Compared Understood all/most vs other.
Compared Not at all vs other.
On the 6-item Spielberger State-Trait Anxiety Inventory, each item is scored from 1 (not at all) to 4 (very much), items are summed, and the total score is multiplied by 20 and then divided by 6 (score range is 20-80, with higher scores indicating worse anxiety). The adjusted mean difference was −1.2 (95% CI, −3.0 to 0.2).30
Ten-item index on the Preparation for Decision Making Scale. Each item is scored from 1 point (not at all) to 5 points (a great deal).35
Data are missing for 1 patient in the HS pamphlet arm.
Discussion
In this multicenter, cluster randomized clinical trial, receipt of a mammography screening DA led to women 75 years and older being more knowledgeable about screening, having more discussions with their PCP about screening, fewer intending to be screened, and 9.1% fewer being screened over 18 months. No statistically significant differential effect of the DA was observed based on patient 10-year life expectancy. However, guidelines recommend not screening women 75 years and older at low or average risk regardless of their life expectancy,8,9 and overscreening older women for breast cancer is increasingly recognized as a major health issue.36 Although deprescribing low-value medical interventions in older adults has proven to be challenging,37 we found that providing older women with a DA before a PCP visit may help these women make more informed decisions, leading to fewer women choosing screening. Most women found the DA to be helpful, clear, and not anxiety provoking.
Randomized clinical trials of educational materials promoting screening have generally found a 5% increase in screening,38,39 suggesting that the 9% reduction in mammography screening found in this trial was considerable. In Boston-area practices, where screening among women 75 years and older was common (71.9% in the control arm were screened), DA use reduced screening by 12.6%. In North Carolina, where screening was already low (21.6% in the control arm were screened), DA use increased screening by 2.2%. We had anticipated there would be institutional differences in mammography screening of older women and thus had randomized PCPs by site; however, the differences found between Massachusetts and North Carolina were considerable. Patients in North Carolina differed from those in Massachusetts by many factors known to be associated with lower screening rates12,40 (eg, lower educational level or health literacy [eTable 10 in Supplement 1]); a major difference was that North Carolina participants were much less likely to report that others (eg, PCPs) expected them to be screened. Therefore, differences in cultural norms may explain much of the regional variations seen in screening rates and, as a result, the differential effect of the DA. In the Boston area, payment incentives have led to many health system programs (eg, same-day mammograms and automatic scheduling) to improve screening rates among women aged 50 to 74 years.34,41 These programs designed to increase screening in age-appropriate populations may lead to high screening rates in women 75 years and older and may need to be fine-tuned. For example, letters reminding women to have mammograms may need to be revised for women 75 years and older to encourage discussion with their PCP or use of the DA described herein.
Although hundreds of DAs exist, few are used, mainly because they are inaccessible, are not current, are too long, require high literacy, have not been rigorously tested, or are not disseminated.42,43,44,45 Because the DA used herein is effective, implementation should be pursued as a next step. In preparation, our group previously interviewed primary care administrators and staff on DA implementation.46 Staff thought that it would be feasible to deliver the DA with only minimal training; however, they wanted to know that the DA was supported by professional organizations and their practice’s PCPs. They recommended using Medicare wellness visits, creating EMR alerts for DA use, and making the DA available online. In response, we added a link to the DA on ePrognosis47 for easy access. We also plan to update the DA as needed.
Although the DA informed women of their likelihood of benefiting from screening based on their health, it presented screening as a decision regardless of life expectancy. Because almost all breast cancer guidelines recommend not screening women with less than a 10-year life expectancy,8,9,10,11 we have added a posttrial statement to the DA indicating that experts generally do not recommend mammograms for women with scores exceeding 6 on the health scale (this score is equivalent to scores >9 on the validated Schonberg mortality index and suggestive of less than a 10-year life expectancy) (revised DA in the eDiscussion in Supplement 1). Although the DA used informed women of major breast cancer risk factors (eg, family history, history of breast biopsies), the DA was not tailored based on risk.48 There is little consensus on what risk threshold places women 75 years and older at high risk. For postmenopausal women in general, a threshold of at least a 3% 5-year risk has been recommended to define high risk49; however, increasingly higher thresholds are used, such as at least a 6% 5-year risk.50 Only 3 women in our study had a 5-year risk of at least 6%, suggesting that it was reasonable for almost all women in the study to consider stopping screening. We plan for the next generation of this DA to be interactive, web based, and tailored to breast cancer risk and life expectancy.
Limitations
This study has limitations. Patient populations and the number of PCPs differed at each site; however, these differences were accounted for in the study design and analyses. Participants spoke English and did not have dementia. However, we have translated the DA to Spanish47 and are testing a modified version among caregivers of older women with dementia. Although our study sample was not nationally representative, it included 2 diverse geographical locations. Participants were more likely than nonparticipants to be in good health and highly educated; however, the effects of the DA on screening did not differ by these factors. After the first patient participating for each PCP, RAs were not blinded to patient randomization assignment; however, RAs attempted to recruit all eligible patients. The DA was designed for patients to read before a visit. Many women discussed mammography with their PCP during the visit, but we did not capture the effects of the DA on visit length. We are surveying PCPs to obtain their feedback on the DA.
Conclusions
The DA used in this cluster randomized clinical trial was found to be helpful, improved knowledge and discussions about mammography, and led to fewer women aged 75 and older being screened. Older women should have the opportunity to make informed screening decisions based on their values and a realistic understanding of the benefits and harms of mammography.
eTable 1. Differences Between Published Protocol and Final Study Protocol and Justification
eTable 2. Description of Recruitment Sites
eTable 3. Data Collection
eTable 4. Sample Characteristics of Women Who Opted-Out of Initial Telephone Contact vs Participants
eTable 5. Sample Characteristics of Women Who Declined to Participate Over the Telephone vs Participants
eTable 6. Sample Characteristics of PCPs
eTable 7. Changes in Intentions to Be Screened Within the Decision Aid Arm
eTable 8. Proportion of Correct Responses to the 11 Knowledge Questions
eTable 9. Characteristics of Women Who Perceived the DA to Be Slanted
eTable 10. Participant Characteristics by Health Systems in the Study
eMethods. Supplemental Methods
eDiscussion. Supplemental Discussion
Trial Protocol
Data Sharing Statement
References
- 1.Cancer Query System SEER incidence statistics. Accessed September 8, 2019. https://seer.cancer.gov/canques/incidence.html
- 2.Vincent GK, Velkoff VA The next four decades: the older population in the United States: 2010 to 2050. US Dept of Commerce, Economics and Statistics Administration, US Census Bureau; 2010. Appendix Table A-3. Projections of the older population by selected age group and sex, and sex ratios for the United States: 2010 to 2050. Published May 2010. Accessed March 9, 2020. https://www.census.gov/prod/2010pubs/p25-1138.pdf
- 3.Pace LE, Keating NL. A systematic assessment of benefits and risks to guide breast cancer screening decisions. JAMA. 2014;311(13):1327-1335. doi: 10.1001/jama.2014.1398 [DOI] [PubMed] [Google Scholar]
- 4.Nyström L, Andersson I, Bjurstam N, Frisell J, Nordenskjöld B, Rutqvist LE. Long-term effects of mammography screening: updated overview of the Swedish randomised trials. Lancet. 2002;359(9310):909-919. doi: 10.1016/S0140-6736(02)08020-0 [DOI] [PubMed] [Google Scholar]
- 5.Nelson HD, Fu R, Cantor A, Pappas M, Daeges M, Humphrey L. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force recommendation. Ann Intern Med. 2016;164(4):244-255. doi: 10.7326/M15-0969 [DOI] [PubMed] [Google Scholar]
- 6.Lee SJ, Boscardin WJ, Stijacic-Cenzer I, Conell-Price J, O’Brien S, Walter LC. Time lag to benefit after screening for breast and colorectal cancer: meta-analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ. 2013;346(1):e8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walter LC, Schonberg MA. Screening mammography in older women: a review. JAMA. 2014;311(13):1336-1347. doi: 10.1001/jama.2014.2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siu AL; U.S. Preventive Services Task Force . Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement [published correction appears in Ann Intern Med. 2016;164(6):448]. Ann Intern Med. 2016;164(4):279-296. doi: 10.7326/M15-2886 [DOI] [PubMed] [Google Scholar]
- 9.Qaseem A, Lin JS, Mustafa RA, Horwitch CA, Wilt TJ; Clinical Guidelines Committee of the American College of Physicians . Screening for breast cancer in average-risk women: a guidance statement from the American College of Physicians. Ann Intern Med. 2019;170(8):547-560. doi: 10.7326/M18-2147 [DOI] [PubMed] [Google Scholar]
- 10.Oeffinger KC, Fontham ET, Etzioni R, et al. ; American Cancer Society . Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society [published correction appears in JAMA. 2016;315(13):1406]. JAMA. 2015;314(15):1599-1614. doi: 10.1001/jama.2015.12783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Practice Bulletin No. 179 summary: breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130(1):241-243. doi: 10.1097/AOG.0000000000002151 [DOI] [PubMed] [Google Scholar]
- 12.Schonberg MA, Breslau ES, McCarthy EP. Targeting of mammography screening according to life expectancy in women aged 75 and older. J Am Geriatr Soc. 2013;61(3):388-395. doi: 10.1111/jgs.12123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Connor AM, Tugwell P, Wells GA, et al. A decision aid for women considering hormone therapy after menopause: decision support framework and evaluation. Patient Educ Couns. 1998;33(3):267-279. doi: 10.1016/S0738-3991(98)00026-3 [DOI] [PubMed] [Google Scholar]
- 14.Schonberg MA, Hamel MB, Davis RB, et al. Development and evaluation of a decision aid on mammography screening for women 75 years and older. JAMA Intern Med. 2014;174(3):417-424. doi: 10.1001/jamainternmed.2013.13639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Should I continue having mammograms? for women age 75 to 84 years. Accessed March 2, 2020. https://eprognosis.ucsf.edu/decision_aids/Mammography_75-84.pdf
- 16.Should I continue having mammograms for women age 85 or older? Accessed March 6, 2020. https://www.bidmc.org/-/media/files/beth-israel-org/research/research-by-department/medicine/general-medicine-research/research-faculty/decision-aid-8512020.pdf
- 17.Schonberg MA, Kistler CE, Nekhlyudov L, et al. Evaluation of a mammography screening decision aid for women aged 75 and older: protocol for a cluster-randomized controlled trial. J Clin Trials. 2014;4(1):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schonberg MA, Li V, Marcantonio ER, Davis RB, McCarthy EP. Predicting mortality up to 14 years among community-dwelling adults aged 65 and older. J Am Geriatr Soc. 2017;65(6):1310-1315. doi: 10.1111/jgs.14805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schoenborn NL, Janssen EM, Boyd C, et al. Older adults’ preferences for discussing long-term life expectancy: results from a national survey. Ann Fam Med. 2018;16(6):530-537. doi: 10.1370/afm.2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry. 1983;140(6):734-739. doi: 10.1176/ajp.140.6.734 [DOI] [PubMed] [Google Scholar]
- 21.Popp L, Schneider S. Attention placebo control in randomized controlled trials of psychosocial interventions: theory and practice. Trials. 2015;16(1):150. doi: 10.1186/s13063-015-0679-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fagerlin A, Zikmund-Fisher BJ, Ubel PA, Jankovic A, Derry HA, Smith DM. Measuring numeracy without a math test: development of the Subjective Numeracy Scale. Med Decis Making. 2007;27(5):672-680. doi: 10.1177/0272989X07304449 [DOI] [PubMed] [Google Scholar]
- 23.Davis TC, Long SW, Jackson RH, et al. Rapid Estimate of Adult Literacy in Medicine: a shortened screening instrument. Fam Med. 1993;25(6):391-395. [PubMed] [Google Scholar]
- 24.O’Connor AM. User Manual: Measures of Decision/Choice Predisposition Ottawa Hospital Research Institute; 1996. Modified 2003. Accessed September 5, 2019. https://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_ChoicePredisposition_Decision.pdf
- 25.Tiro JA, Diamond PM, Perz CA, et al. Validation of scales measuring attitudes and norms related to mammography screening in women veterans. Health Psychol. 2005;24(6):555-566. doi: 10.1037/0278-6133.24.6.555 [DOI] [PubMed] [Google Scholar]
- 26.National Cancer Institute. Breast Cancer Risk Assessment Tool Accessed August 23, 2019. https://bcrisktool.cancer.gov/calculator.html
- 27.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879-1886. doi: 10.1093/jnci/81.24.1879 [DOI] [PubMed] [Google Scholar]
- 28.O’Connor AM. User Manual: Decisional Conflict Scale (16 Item Statement Format) Ottawa Hospital Research Institute; 1993. Updated 2010. Accessed September 5, 2019. https://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decisional_Conflict.pdf
- 29.Degner LF, Kristjanson LJ, Bowman D, et al. Information needs and decisional preferences in women with breast cancer. JAMA. 1997;277(18):1485-1492. doi: 10.1001/jama.1997.03540420081039 [DOI] [PubMed] [Google Scholar]
- 30.Marteau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI). Published correction appears in . Br J Clin Psychol. 1992;31(3):301-306. https://onlinelibrary.wiley.com/doi/10.1111/bjc.12243. doi: 10.1111/j.2044-8260.1992.tb00997.x [DOI] [PubMed] [Google Scholar]
- 31.O’Connor AM, Cranney A. User Manual: Acceptability Ottawa Hospital Research Institute; 1996. Modified 2002. Accessed August 14, 2019. https://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Acceptability.pdf
- 32.Graham ID, O’Connor AM. User Manual: Preparation for Decision Making Scale Ottawa Hospital Research Institute; 1995. Modified 2010. Accessed September 5, 2019. https://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_PrepDM.pdf
- 33.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199-200. doi: 10.1093/aje/kwi188 [DOI] [PubMed] [Google Scholar]
- 34.Williams BA, Lindquist K, Sudore RL, Covinsky KE, Walter LC. Screening mammography in older women: effect of wealth and prognosis. Arch Intern Med. 2008;168(5):514-520. doi: 10.1001/archinternmed.2007.103 [DOI] [PubMed] [Google Scholar]
- 35.Bennett C, Graham ID, Kristjansson E, Kearing SA, Clay KF, O’Connor AM. Validation of a Preparation for Decision Making scale. Patient Educ Couns. 2010;78(1):130-133. doi: 10.1016/j.pec.2009.05.012 [DOI] [PubMed] [Google Scholar]
- 36.Department of Health and Human Services. Reducing overscreening for breast, cervical, and colorectal cancers among older adults (R01 clinical trial optional). Accessed June 25, 2019. https://grants.nih.gov/grants/guide/pa-files/PA-18-005.html
- 37.Steinman MA, Landefeld CS. Overcoming inertia to improve medication use and deprescribing. JAMA. 2018;320(18):1867-1869. doi: 10.1001/jama.2018.16473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeGroff A, Sharma K, Satsangi A, et al. Increasing colorectal cancer screening in health care systems using evidence-based interventions. Prev Chronic Dis. 2018;15(1):E100. doi: 10.5888/pcd15.180029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brouwers MC, De Vito C, Bahirathan L, et al. What implementation interventions increase cancer screening rates? a systematic review. Implement Sci. 2011;6(1):111. doi: 10.1186/1748-5908-6-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cromwell J, Trisolini MG, Pope GC, Mitchell JB, Greenwald LM, eds. Pay for Performance in Health Care: Methods and Approaches RTI Press; 2011. RTI Press publication BK-0002-1103. Accessed June 28, 2019. https://www.rti.org/sites/default/files/resources/bk-0002-1103-mitchell.pdf
- 41.RTI International. Accountable Care Organization 2015 Program Analysis Quality Performance Standards Narrative Measure Specifications RTI Press; 2015. RTI project 0213195.001.004. Accessed March 6, 2020. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/sharedsavingsprogram/Downloads/ACO-NarrativeMeasures-Specs.pdf
- 42.Elwyn G, Légaré F, van der Weijden T, Edwards A, May C. Arduous implementation: does the Normalisation Process Model explain why it’s so difficult to embed decision support technologies for patients in routine clinical practice? Implement Sci. 2008;3(1):57. doi: 10.1186/1748-5908-3-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4(1):CD001431. doi: 10.1002/14651858.CD001431.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joseph-Williams N, Elwyn G, Edwards A. Knowledge is not power for patients: a systematic review and thematic synthesis of patient-reported barriers and facilitators to shared decision making. Patient Educ Couns. 2014;94(3):291-309. doi: 10.1016/j.pec.2013.10.031 [DOI] [PubMed] [Google Scholar]
- 45.Stacey D, Suwalska V, Boland L, Lewis KB, Presseau J, Thomson R. Are patient decision aids used in clinical practice after rigorous evaluation? a survey of trial authors. Med Decis Making. 2019;39(7):805-815. doi: 10.1177/0272989X19868193 [DOI] [PubMed] [Google Scholar]
- 46.Schonberg MA, Jacobson AR, Aliberti GM, et al. Primary care–based staff ideas for implementing a mammography decision aid for women 75+: a qualitative study. J Gen Intern Med. 2019;34(11):2414-2420. doi: 10.1007/s11606-019-05239-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.University of California San Francisco. ePrognosis. Accessed August 28, 2019. https://eprognosis.ucsf.edu/
- 48.Schonberg MA, Li VW, Eliassen AH, et al. Accounting for individualized competing mortality risks in estimating postmenopausal breast cancer risk. Breast Cancer Res Treat. 2016;160(3):547-562. doi: 10.1007/s10549-016-4020-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moyer VA; U.S. Preventive Services Task Force . Medications to decrease the risk for breast cancer in women: recommendations from the U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(10):698-708. [DOI] [PubMed] [Google Scholar]
- 50.Eklund M, Broglio K, Yau C, Connor JT, Stover Fiscalini A, Esserman LJ. The WISDOM personalized breast cancer screening trial: simulation study to assess potential bias and analytic approaches. J Natl Cancer Inst Cancer Spectr. 2019;2(4):pky067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Differences Between Published Protocol and Final Study Protocol and Justification
eTable 2. Description of Recruitment Sites
eTable 3. Data Collection
eTable 4. Sample Characteristics of Women Who Opted-Out of Initial Telephone Contact vs Participants
eTable 5. Sample Characteristics of Women Who Declined to Participate Over the Telephone vs Participants
eTable 6. Sample Characteristics of PCPs
eTable 7. Changes in Intentions to Be Screened Within the Decision Aid Arm
eTable 8. Proportion of Correct Responses to the 11 Knowledge Questions
eTable 9. Characteristics of Women Who Perceived the DA to Be Slanted
eTable 10. Participant Characteristics by Health Systems in the Study
eMethods. Supplemental Methods
eDiscussion. Supplemental Discussion
Trial Protocol
Data Sharing Statement