Abstract
Purpose
Osteoporosis and fragility fracture are major bone toxicities of aromatase inhibitors (AIs) for postmenopausal hormone receptor positive breast cancer. Except for a few small studies on bone turnover markers and reduced bone mineral density after AI treatment, data on the associations of bone markers and risk of osteoporosis or fracture from prospective studies are lacking.
Methods
In a prospective study of 1,709 women on AIs, two bone turnover markers, BALP and TRACP, and two bone regulatory markers, RANKL and OPG, were measured and examined in relation to risk of osteoporosis and fragility fractures during a median follow-up time of 6.1 years.
Results
Higher levels of BALP and TRACP were both associated with increased risk of osteoporosis and higher BALP/TRACP ratios were associated with lower risk of osteoporosis, but no associations were observed for fracture risk. Higher levels of OPG were associated with increased risk of fracture, whereas higher levels of RANKL were associated with lower risk. As a result, OPG/RANKL ratios were positively associated with fracture risk (hazard ratio [HR]=2.49, 95% confidence interval [CI] 1.34–4.61). After controlling for age and fracture history, the associations became non-significant but a suggestive trend remained (HR=1.80, 95% CI 0.96–3.37).
Conclusion
Our study provides suggestive evidence for the potential utility of OPG/RANKL ratios in predicting risk of fracture in women treated with AIs for breast cancer. Further validation may be warranted.
Keywords: breast cancer, osteoporosis, fracture, bone markers
Introduction
Adjuvant endocrine therapy is effective in lowering the risk of recurrence for women diagnosed with hormone receptor positive breast cancer. Its importance in the clinical management of breast cancer has become even more prominent in the context of the recent findings from the TAILORx clinical trial reporting that most patients with an intermediate recurrence score from the 21-gene test would benefit from endocrine therapy alone, forgoing the need of chemotherapy [1]. However, endocrine therapy is not free of side effects. Some long-term complications for postmenopausal patients, particularly from aromatase inhibitors (AIs), pose challenges to patients’ quality of life. A major side effect related to AI use is bone weakening. AIs almost completely block the peripheral conversion of androgens to estrogens in adipose tissue, which is a major source of estrogens in postmenopausal women. The resulting estrogen deficiency puts patients at high risk of osteoporosis and fractures. In a recent meta-analysis, it was shown that patients treated with AIs had a 35% higher fracture risk than those treated with tamoxifen [2]. Even the steroidal exemestane, the AI that was bone sparing in animal models due to its androgenic structure, caused a similar number of fragility fractures as the non-steroidal anastrozole in the MA-27 trial [3].
Clinical management of AI-associated bone loss in postmenopausal women with hormone receptor positive breast cancer usually involves recommendations for exercise and calcium/vitamin D supplementation, and therapeutic treatment such as bisphosphonates and denosumab. These intervention strategies are developed by considering bone mineral density (BMD) and conventional fracture risk factors, which are largely extrapolated from the literature on bone health in the general population without cancer. It is thus important to evaluate known and novel predictors of fracture risk in breast cancer patients treated with AIs.
In an ongoing observational study, we are investigating lifestyle, molecular markers and genetic factors as potential predictors for the risk of osteoporosis and fractures in a large population of patients who received AIs for their endocrine therapy for breast cancer in an integrated healthcare clinical setting [4]. This community-based clinical setting is different from most previous studies of bone health in the clinical trial setting. In this current study, we hypothesized that bone markers in circulation may provide important information about the baseline state of bone turnover and regulation, which cannot be directly assessed by dual energy x-ray absorptiometry (DXA) scans or surveying of other risk factors. Indeed, a significant proportion of fractures occur in postmenopausal women with apparently normal BMD, supporting the need of bone biomarkers in addition to DXA scans.
We measured four markers in 1,709 patients shortly after breast cancer diagnosis, including two bone turnover markers and two bone regulatory markers. In a previous study, we reported findings of these markers with bone health history before breast cancer diagnosis [5]. The present study focuses on these bone markers in relation to risk of osteopenia, osteoporosis and fractures identified prospectively after the initiation of AI therapy.
Patient Population and Methods
Patient population
This bone marker study was nested in the Pathways Study, a prospective cohort of breast cancer survivors at Kaiser Permanente Northern California (KPNC). Both the parent study and this ancillary study have been described in detail previously [4–6]. In brief, women newly diagnosed with invasive breast cancer at KPNC were enrolled, on average, two months post-diagnosis after written consent was obtained. Between January 2006 and April 2013, a total of 4,505 patients were enrolled by completing a baseline in-person interview after informed consent. Non-fasting blood samples were obtained from 90% of participants after the baseline interview, approximately 2 months after diagnosis, and shipped on dry ice overnight to the Data Bank and Biorepository (DBBR) laboratories at Roswell Park Comprehensive Cancer Center for processing. Serum samples were aliquoted into 0.5 straws and stored in liquid nitrogen until analysis. For this ancillary study, 1,709 patients who were treated with AIs and had blood samples collected for bone marker measurement were included. In this subcohort, blood samples were collected at a median 50 days before the start of AI treatment, whereas 515 patients had samples collected shortly after the start of AI treatment (median=32 days). The study was approved by Institutional Review Boards of Roswell Park Comprehensive Cancer Center and Kaiser Permanente Northern California for human subject protection.
Measurement of bone markers
Four bone markers were selected for measurement using serum samples and commercially available kits. These include two bone turnover markers, bone-specific alkaline phosphatase (BALP) for bone formation and tartrate-resistant acid phosphatase 5b (TRACP) for bone resorption (both from Quidel, San Diego, CA); and two bone regulatory markers, receptor activator of nuclear factor kappa-B ligand (RANKL) (Biovender, Ashville, NC) secreted by osteoclasts and osteoprotegerin (OPG) secreted by osteoblasts (R&D System, Minneapolis, MN). The assays were performed according to manufacturers’ protocols and each sample was tested in duplicates. When the coefficient of variation (CV) for a sample exceeded 15%, the assay was repeated. The average CV was 1.9% for BALP assay, 2.3% for TRACP assay, 5.8% for RANKL assay, and 5.5% for OPG assay. No significant differences were noted in the levels of any of the bone markers between those with blood samples collected before and after the start of AI therapy.
Bone mineral density, osteoporosis and fracture
To identify patients with osteopenia or osteoporosis after breast cancer diagnosis, bone mineral density (BMD) values for the femoral neck, total hip and lumbar spine were extracted from the radiology reports of DXA scans, which were performed at the discretion of physicians for usual clinical care, in the KPNC electronic medical record (EMR). Algorithms were developed for this purpose, with the performance validated by comparison to manual review of 239 patients with 532 BMD values as previously reported [4]. Among 1,709 patients included in this study, 1,509 (87.1%) had at least one DXA after breast cancer diagnosis, whereas 220 (12.9%) had no DXA scan after cancer diagnosis. T-scores were calculated based on BMD values, and osteopenia and osteoporosis were classified as previously described [5]. A patient was determined to be osteopenic or osteoporotic if the T-score was at any point after cancer diagnosis within the specified range. Similarly, any osteopenia/osteoporosis was defined as yes if at least one of the three sites measured (femur, hip and spine) fell within the specified range.
Fractures occurring after breast cancer diagnosis at the humerus, wrist, hip and spine were obtained from the EMR using ICD-9 codes. All encounter data were then manually reviewed by a medical record abstractor and subsequently validated by the study endocrinologist (J. Lo). Traumatic fractures, prevalent fractures and pathologic fractures including bone metastases were flagged and removed from the analysis. Major fragility fractures were defined as those at the humerus, wrist, hip or spine. Previous history of osteoporosis and fractures before breast cancer diagnosis were obtained from the EMR, and bisphosphonate use was also used as an indicator of history of osteoporosis as previously described [5].
Statistical analysis
Mean and standard deviation for continuous variables and count and percentage for categorical variables were used to summarize the patient population. The values of all four markers fit a normal distribution and thus were used without transformation. Two ratios, BALP/TRACP and OPG/RANKL were derived and log-transformed. For fracture outcomes, cumulative incidence curves were generated according to the quartile levels of the individual bone biomarkers and the two derived ratios for bone turnover and bone resorption. Cox proportional hazards models were developed with and without adjustment for age and prior fracture history, and hazards ratios (HRs) and 95% confidence intervals (CIs) were reported. The proportional hazards assumption was examined by the scaled Schoenfeld residuals and no violation was identified in any of the models tested. Because the incidence of frailty fractures was sparse in separate anatomic sites for patients treated with AIs, the analysis focused on major fractures as a combined outcome. In addition, osteopenia and osteoporosis were analyzed separately in comparison to patients without abnormal BMD results using multinomial logistic regression models. Odds ratios (ORs) and 95% CIs were reported for the quartiles of the levels of biomarkers and the two derived ratios. The analyses were conducted using R version 3.6.1.
Results
Table 1 describes the characteristics of the study population. As expected, most of the patients were Whites, diagnosed after menopause, with stage I/II, estrogen receptor positive and HER2 negative disease. A small percentage of the patients had a history of osteoporosis (7.8%) and any fracture (16.6%).
Table 1.
Descriptive characteristics of Pathways Study patient population who received aromatase inhibitor treatment
| Characteristic | N (%) |
|---|---|
| Age at diagnosis, years | |
| <50 | 54 (3.2) |
| 50–59 | 498 (29.1) |
| 60–69 | 717 (42.0) |
| ≥70 | 440 (25.7) |
| Menopausal status | |
| Premenopausal | 99 (5.8) |
| Postmenopausal | 1610 (94.2) |
| Race/ethnicity | |
| White | 1269 (74.3) |
| Black | 92 (5.4) |
| Asian | 159 (9.3) |
| Hispanic | 158 (9.2) |
| American Indian/Pacific Islander | 31 (1.8) |
| AJCC stage | |
| I | 1031 (58.1) |
| II | 579 (33.9) |
| III | 151 (8.8) |
| IV | 21 (1.2) |
| Estrogen receptor status | |
| Positive | 1695 (99.2) |
| Negative | 14 (0.8) |
| HER2 status | |
| Negative | 1484 (86.8) |
| Positive | 162 (9.5) |
| Not done | 63 (3.7) |
| Osteoporosis prior to diagnosis | |
| No | 1576 (92.2) |
| <5 years | 91 (5.3) |
| ≥5 years | 42 (2.5) |
| Any fracture prior to diagnosis | |
| No | 1426 (83.4) |
| <5 years | 153 (9.0) |
| ≥5 years | 130 (7.6) |
Abbreviations: AJCC, American Joint Cancer Committee; HER2, human epithelial growth factor 2
Almost half (48.9%) of the patients on AIs with were classified as osteopenic based on available DXA scans performed after breast cancer diagnosis, and another 15.8% were osteoporotic (Table 2). Spine was the most frequent anatomic site diagnosed with osteoporosis, followed by femur and hip. During a median follow-up time of 6.1 years (range 0.2–9.8 years), 117 (6.8%) incident, non-traumatic, non-pathologic fractures were ascertained, which are summarized in Table 2. Wrist was the most frequent anatomic site with fractures, followed by humerus, spine, and hip.
Table 2.
Bone health outcomes among breast cancer patients after treatment with aromatase inhibitors
| N (%) | |
|---|---|
| Any osteopenia/osteoporosis | |
| Osteopenia | 835 (48.9) |
| Osteoporosis | 270 (15.8) |
| None | 604 (35.3) |
| Hip osteopenia/osteoporosis | |
| Osteopenia | 564 (33.0) |
| Osteoporosis | 51 (3.0) |
| None | 1094 (64.0) |
| Femur osteopenia/osteoporosis | |
| Osteopenia | 715 (41.8) |
| Osteoporosis | 132 (7.7) |
| None | 862 (50.4) |
| Spine osteopenia/osteoporosis | |
| Osteopenia | 582 (34.1) |
| Osteoporosis | 174 (10.2) |
| None | 953 (55.8) |
| Any fracture | |
| Yes | 117 (6.8) |
| No | 1592 (93.2) |
| Hip fracture | |
| Yes | 17 (1.0) |
| No | 1692 (99.0) |
| Humerus fracture | |
| Yes | 31 (1.8) |
| No | 1678 (98.2) |
| Wrist fracture | |
| Yes | 47 (2.8) |
| No | 1662 (97.2) |
| Spine fracture | |
| Yes | 28 (1.6) |
| No | 1681 (98.4) |
Higher levels of BALP and TRACP were associated with higher risk of both osteopenia (Q4 BALP, age adjusted OR=1.33, 95% CI 0.97–1.81; Q4 TRACP, age adjusted OR=2.07; 95% CI 1.52–2.82) and osteoporosis (Q4 BALP, age adjusted OR=1.63, 95% CI 1.08–2.45; Q4 TRACP, age adjusted OR=2.07; 95% CI 1.34–3.19) with age adjustment; yet higher BALP/TRACP ratios were associated with lower risk of osteopenia (Q4, age adjusted OR=0.61; 95% CI 0.44–0.83) and osteoporosis (Q4, age adjusted OR=0.67; 95% CI 0.44–1.02) (Table 3). Higher OPG levels were also associated with lower risk of osteopenia (Q4, age adjusted OR=0.54, 95% CI 0.39–0.75) and osteoporosis (Q4, age adjusted OR=0.64, 95% CI 0.41–1.02), but no associations were found with RANKL levels and OPG/RANKL ratios. In analyses of osteopenia and osteoporosis at the femur, hip and spine separately, the associations with osteopenia at these sites remained largely the same. Similar associations were also found with osteoporosis at the spine but not at the femur or the hip (data not shown). Further adjustment for prior osteoporosis history did not noticeably change the results, and in some cases, resulted in stronger associations (data not shown).
Table 3.
Odds ratio of osteopenia and osteoporosis associated with bone biomarkers with at any site in patients treated with aromatase inhibitors
| Age adjusted |
Age and prior osteoporosis adjusted |
|||
|---|---|---|---|---|
| Osteopenia | Osteoporosis | Osteopenia | Osteoporosis | |
| BALP | ||||
| Q1 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 | 0.88 (0.64–1.19) | 0.79 (0.52–1.22) | 0.92 (0.67–1.25) | 1.01 (0.64–1.61) |
| Q3 | 1.36 (1–1.84) | 1.03 (0.67–1.58) | 1.44 (1.06–1.95) | 1.45 (0.92–2.3) |
| Q4 | 1.33 (0.97–1.81) | 1.63 (1.08–2.45) | 1.42 (1.04–1.94) | 2.44 (1.56–3.8) |
| TRACP | ||||
| Q1 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 | 1.53 (1.13–2.07) | 1.53 (0.99–2.36) | 1.59 (1.17–2.15) | 1.84 (1.15–2.94) |
| Q3 | 1.53 (1.13–2.07) | 1.7 (1.11–2.61) | 1.62 (1.19–2.21) | 2.33 (1.47–3.7) |
| Q4 | 2.07 (1.52–2.82) | 2.07 (1.34–3.19) | 2.23 (1.63–3.04) | 3.07 (1.93–4.89) |
| BALP/TRACP ratio | ||||
| Q1 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 | 0.73 (0.54–0.99) | 0.74 (0.49–1.1) | 0.74 (0.55–1) | 0.8 (0.53–1.22) |
| Q3 | 0.71 (0.53–0.96) | 0.66 (0.43–0.99) | 0.72 (0.53–0.97) | 0.68 (0.44–1.04) |
| Q4 | 0.61 (0.44–0.83) | 0.67 (0.44–1.02) | 0.6 (0.44–0.82) | 0.63 (0.41–0.98) |
| OPG | ||||
| Q1 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 | 0.95 (0.69–1.32) | 1.12 (0.71–1.75) | 0.93 (0.67–1.3) | 1.01 (0.63–1.61) |
| Q3 | 0.69 (0.5–0.95) | 0.87 (0.56–1.36) | 0.69 (0.5–0.94) | 0.86 (0.54–1.37) |
| Q4 | 0.54 (0.39–0.75) | 0.64 (0.41–1.02) | 0.54 (0.39–0.75) | 0.63 (0.39–1.01) |
| RANKL | ||||
| Q1 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 | 0.89 (0.66–1.19) | 0.85 (0.56–1.3) | 0.89 (0.66–1.2) | 0.86 (0.55–1.33) |
| Q3 | 0.9 (0.67–1.22) | 0.94 (0.61–1.44) | 0.89 (0.66–1.21) | 0.89 (0.57–1.4) |
| Q4 | 1.04 (0.76–1.41) | 1.34 (0.88–2.02) | 1.04 (0.76–1.41) | 1.31 (0.85–2.03) |
| OPG/RANKL ratio | ||||
| Q1 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 | 0.78 (0.56–1.08) | 0.96 (0.61–1.5) | 0.78 (0.56–1.08) | 0.98 (0.61–1.57) |
| Q3 | 0.83 (0.6–1.15) | 0.83 (0.52–1.3) | 0.85 (0.61–1.18) | 0.93 (0.58–1.5) |
| Q4 | 0.74 (0.54–1.02) | 0.72 (0.46–1.13) | 0.74 (0.54–1.02) | 0.74 (0.46–1.18) |
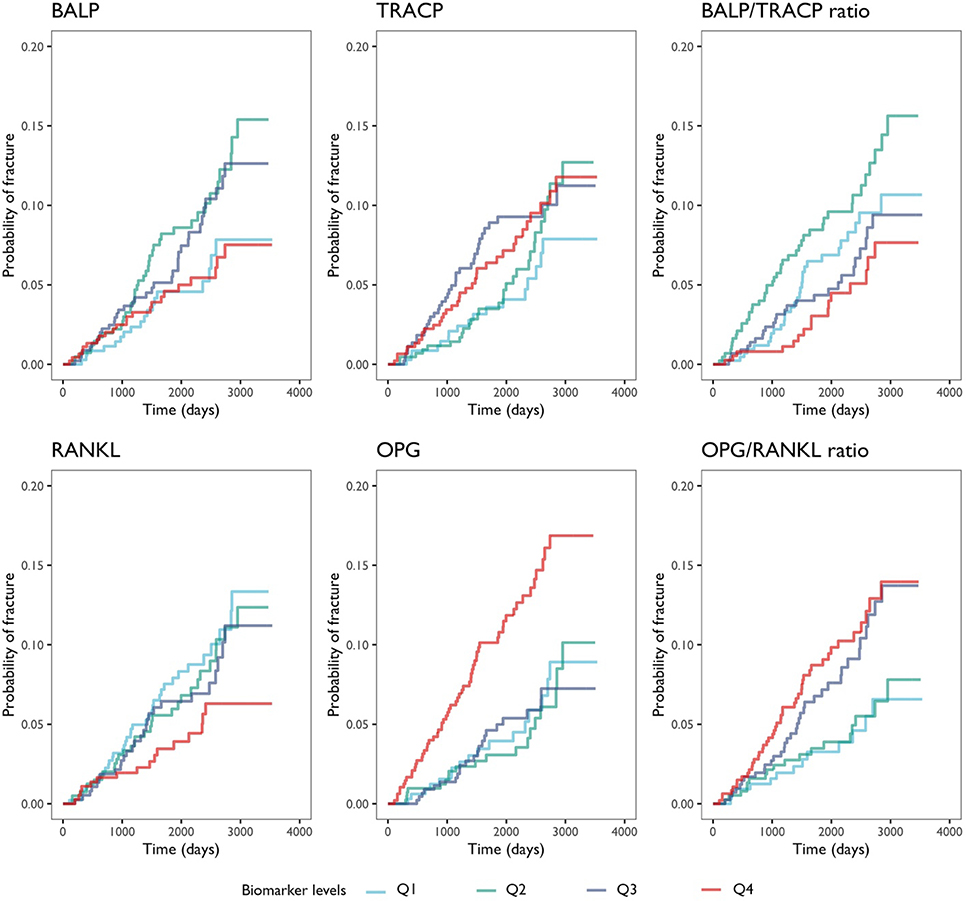
In unadjusted models, the levels of BALP, TRACP, or BALP/TRACP ratios were not associated with fracture risk (Table 4); whereas the fourth quartile of OPG and RANKL levels were associated with increased (HR=2.42, 95% CI 1.41–4.15) and decreased (HR=0.50, 95% CI 0.28–0.90) risks of fracture, respectively. As a result, the third and fourth quartiles of OPG/RANKL ratios were associated with increased risk of fracture (Q4 HR=2.49, 95% CI 1.34–4.61; Figure 1). After adjusting for age at diagnosis, the results became attenuated and non-significant, although the trends remained the same (Q4 OPG/RANKL ratios, adjusted HR=1.74, 95% CI 0.93–3.26). Further adjustment for prior fracture history did not noticeably change the risk estimates (HR=1.80, 95% CI 0.96–3.37).
Table 4.
Hazard ratio of fracture associated with bone biomarker levels in patients treated with aromatase inhibitors
| Outcome | Unadjusted |
Age adjusted |
Age and prior Fx adjusted |
|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| BALP | |||
| Q1 | 1.00 | 1.00 | 1.00 |
| Q2 | 1.87 (1.07–3.27) | 2.01 (1.15–3.52) | 2.05 (1.17–3.59) |
| Q3 | 1.62 (0.92–2.85) | 1.77 (1.00–3.11) | 1.80 (1.02–3.18) |
| Q4 | 1.02 (0.55–1.89) | 1.20 (0.65–2.23) | 1.27 (0.68–2.36) |
| TRACP | |||
| Q1 | 1.00 | 1.00 | 1.00 |
| Q2 | 1.34 (0.73–2.45) | 1.30 (0.71–2.37) | 1.34 (0.73–2.44) |
| Q3 | 1.82 (1.03–3.24) | 1.67 (0.94–2.97) | 1.75 (0.98–3.12) |
| Q4 | 1.65 (0.92–2.94) | 1.40 (0.78–2.51) | 1.47 (0.82–2.63) |
| BALP/TRACP ratio | |||
| Q1 | 1.00 | 1.00 | 1.00 |
| Q2 | 1.46 (0.92–2.32) | 1.57 (0.99–2.50) | 1.56 (0.98–2.48) |
| Q3 | 0.83 (0.49–1.41) | 1.03 (0.60–1.75) | 1.01 (0.59–1.72) |
| Q4 | 0.59 (0.32–1.09) | 0.83 (0.45–1.54) | 0.82 (0.44–1.53) |
| OPG | |||
| Q1 | 1.00 | 1.00 | 1.00 |
| Q2 | 0.90 (0.47–1.74) | 0.77 (0.40–1.49) | 0.80 (0.41–1.54) |
| Q3 | 0.94 (0.50–1.77) | 0.72 (0.38–1.38) | 0.75 (0.39–1.42) |
| Q4 | 2.42 (1.41–4.15) | 1.52 (0.86–2.70) | 1.61 (0.91–2.85) |
| RANKL | |||
| Q1 | 1.00 | 1.00 | 1.00 |
| Q2 | 0.88 (0.54–1.42) | 1.00 (0.62–1.61) | 1.02 (0.63–1.64) |
| Q3 | 0.82 (0.50–1.35) | 1.09 (0.66–1.81) | 1.09 (0.66–1.81) |
| Q4 | 0.50 (0.28–0.90) | 0.57 (0.32–1.02) | 0.57 (0.32–1.02) |
| OPG/RANKL ratio | |||
| Q1 | 1.00 | 1.00 | 1.00 |
| Q2 | 1.13 (0.55–2.31) | 1.05 (0.51–2.13) | 1.06 (0.52–2.16) |
| Q3 | 2.13 (1.13–4.02) | 1.78 (0.94–3.38) | 1.81 (0.96–3.44) |
| Q4 | 2.49 (1.34–4.61) | 1.74 (0.93–3.26) | 1.80 (0.96–3.37) |
Figure 1.
Cumulative incidences of major fragility fractures are plotted according to quartiles of each of the four biomarkers and the two contrived ratios. The curves are colored to indicate the first to the fourth quartile of each biomarker.
Discussion
In this large population of breast cancer patients in an integrated healthcare setting who received AI therapy for breast cancer, we found that bone turnover markers at diagnosis, including BALP and TRACP, were associated with risk of osteopenia and osteoporosis but not fragility fractures; whereas the bone regulatory markers, OPG and RANKL, were associated with risk of fracture. In comparison to single markers, the relative ratios between OPG and RANKL reflective of bone resorption and bone formation appeared to be more closely related to risk of osteoporosis or fracture.
Bone-damaging effects of AIs for breast cancer is well-established in many randomized clinical trials comparing AIs to tamoxifen or placebo where fracture events were recorded [7–15]. The role of bone biomarkers in either assessing fracture risk or monitoring response to antiresorptive therapy, however, is much less established. The limited existing evidence comes mostly from ancillary studies to clinical trials based on relatively small sample sizes [9,16,17]. Because those trials were not designed to evaluate bone outcomes as primary events, to the best of our knowledge, no studies have examined the associations of bone turnover markers with osteoporosis or fractures in AI users. In addition, despite their central role in coupling the processes of bone resorption and formation, we are unaware of any studies on OPG and RANKL as two bone regulatory markers in breast cancer patients. Thus, this is the first and largest prospective study evaluating the relationships of bone turnover and regulatory markers in relation to AI-related osteoporosis and fracture.
In postmenopausal women without breast cancer, some studies showed that elevated levels of bone turnover markers were associated with increased fracture risk independent of BMD or prior fracture [18–21]; however, a few other studies showed no such associations [22,23]. In our breast cancer patient population, higher levels of bone turnover markers were associated with higher risk of osteopenia and osteoporosis, i.e., low BMD, but not fracture. Thus, our findings suggest that patients with relatively high activity of bone turnover at cancer diagnosis may be at higher risk of BMD loss due to AIs, but do not support the utility of the two bone turnover markers for assessment of fracture risk in postmenopausal breast cancer patients undergoing AI therapy. Of note, biomarkers for bone formation and resorption were both associated with osteoporosis in the same direction. This suggests that both processes become more active in the event of accelerated bone turnover, as shown in previous studies evaluating changes in bone turnover markers after AI therapy [9,16,17]. It is the balance between the two contrasting processes as evaluated by BALP/TRACP ratio that may determine the net effect on BMD. These findings support future studies to evaluate the ratio instead of either marker separately to better assess the state and direction of bone turnover.
The RANKL/RANK/OPG pathway is the central signaling axis regulating the activities of osteoblasts and osteoclasts in bone turnover [24]. While RANKL binds to RANK receptor expressed on the precursor cells of osteoclasts to activate its differentiation and the receptor on mature osteoclasts to activate its functions in bone resorption, OPG works as a decoy receptor for RANKL and disrupts its activity before binding to RANK. Thus, the suppression of RANKL by OPG shifts the balance towards osteoblasts and bone formation. Our finding of higher OPG levels associated with lower risk of osteopenia and osteoporosis is consistent with its role in bone formation; yet the finding of higher OPG/RANKL ratio associated with higher risk of fracture contradicts this balance between osteoblasts and osteoclasts in favor of bone formation. A possible explanation is that the OPG/RANKL ratio reflects less the current state of bone turnover, but the direction to which the regulatory signaling axis attempts to adjust the current imbalance between the activities of osteoblasts and osteoclasts. Under this speculation, a higher OPG/RANKL ratio may be interpreted as a signal for stronger bone formation over resorption, in response to a current imbalance towards bone resorption. This hypothesis requires validation in future studies. The findings of the significant relationships of the two bone regulatory markers but not the bone turnover markers with AI-induced fracture also call for more research on OPG and RANKL in AI-induced bone loss among postmenopausal women.
Currently, there is no clear role of bone turnover markers in predicting risk of fractures or selecting candidates for BMD testing or anti-resorption therapy in either the general or postmenopausal breast cancer patients who undergo AI treatment, and guideline committees generally do not recommend its routine use [25,26]. This lack of clinical utility is primarily due to large within-individual variability in the rate of bone loss and fracture given a certain bone biomarker value, which makes the interpretation of the test results difficult in individual patients. The potential clinical utility of bone markers is further complicated by their biological variability and lack of standardization of the assays [27]. Some studies support the use of bone turnover markers to identify non-responders to antiresorptive therapies such as bisphosphonates in osteoporotic patients [28–30], which may be applicable to the breast cancer setting. In addition to prospective studies to establish an optimal strategy to incorporate bone biomarkers into clinical practice, continued research efforts to identify novel markers beyond the few commonly used bone turnover markers, such as the two bone regulatory markers, OPG and RANKL, are warranted.
Our study has several strengths, including a large contemporary breast cancer patient population, vigorous efforts to identify and confirm fragility fractures and to exclude traumatic and pathologic fractures, inclusion of the two bone regulatory markers OPG and RANKL, and the evaluation of the ratios of markers constructed based on biological rationale. Our study also has several limitations. The median follow-up time of 6.1 years is still relatively short, and thus the number of fragility fractures is limited given the relatively low rate of fracture, especially after stratification by fracture site. We were missing BMD data on 12.9% of the cohort due to lack of a clinically available DXA scan after breast cancer diagnosis. Further, DXA scans were conducted at the clinician’s discretion and not uniformly at predefined intervals, possibly resulting in under diagnosis of osteoporosis. In our analysis, a number of pre-analytic variables, including time of day (circadian rhythm), season, and fasting status, were not well controlled during the time of blood collection, which could lead to possible confounding effects. Moreover, we only included two bone turnover markers, whereas several other commonly tested markers, including N-terminal propeptide of type I procollagen (PINP) for bone formation and C-terminal telopeptide of type I collagen (CTX) for bone resorption, were not measured. We chose BALP and TRACP for their low intra-individual variability, low circadian variability, high thermostability, and robustness to non-fasting blood samples [31], whereas CTX is very sensitive to pre-analytic variations and might not be compatible to the conditions under which the blood samples were collected in this study. While BALP is considered one of the most useful clinical markers for bone formation, TRACP is relatively less studied than serum CTX or urinary N-terminal telopeptide crosslink (NTX) as markers for bone resorption. It remains to be determined which bone turnover markers are most predictive for osteoporosis and fracture in breast cancer patients. As a future direction, it will be important to confirm our findings of OPG/RANKL under more stringently controlled condition, with predefined fasting and time of day for blood collection and repeated sampling over time of follow up, with DXA scans regularly performed at a similar time frame.
In conclusion, our large prospective study in an integrated healthcare setting provides suggestive evidence for the potential utility of bone regulatory markers, OPG and RANKL, in predicting risk of fracture in women after AI treatment for breast cancer. Future studies are warranted to validate the findings and to potentially inform the development of a comprehensive risk assessment tool of clinical risk factors, BMD and bone biomarkers specifically for this patient population at high risk of fracture.
Acknowledgements
The Pathways Study was supported by the National Cancer Institute at the National Institutes of Health (R01 CA105274, PI: Kushi LH; U01 CA195565, PI: Kushi LH, Ambrosone CB; R01 CA166701, PIs: Kwan ML, Yao S). Electronic clinical data abstraction and integration was supported in part by Cancer Research Network (CRN) (U19 CA079689, U24 CA171524, PI: Kushi LH). Roswell Park DBBR is CCSG Shared Resource supported by P30 CA16056 (PI: Johnson C). The authors thank office and field staff for data collection, processing, and preparation, and DBBR staff for biospecimen processing. We thank all Pathways Study participants for their numerous contributions to this study. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the funding agencies.
Funding: This study was supported by National Cancer Institute grants R01 CA105274; R01 CA166701; U01 CA195565; and U24 CA171524; Roswell Park Data Bank and BioRepository (DBBR) is a Cancer Center Support Grant shared resource supported by National Cancer Institute grant P30 CA16056.
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
Compliance with Ethical Standards:
Ethical Approval: The study was approved by Institutional Review Boards of Roswell Park Comprehensive Cancer Center and Kaiser Permanente Northern California for human subject protection.
Informed Consent: Written consents were obtained from all study participants.
References
- 1.Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Goetz MP, Olson JA Jr, Lively T, Badve SS, Saphner TJ, Wagner LI, Whelan TJ, Ellis MJ, Paik S, Wood WC, Ravdin PM, Keane MM, Gomez Moreno HL, Reddy PS, Goggins TF, Mayer IA, Brufsky AM, Toppmeyer DL, Kaklamani VG, Berenberg JL, Abrams J, Sledge GW Jr. (2018) Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. The New England journal of medicine 379 (2):111–121. doi: 10.1056/NEJMoa1804710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tseng OL, Spinelli JJ, Gotay CC, Ho WY, McBride ML, Dawes MG (2018) Aromatase inhibitors are associated with a higher fracture risk than tamoxifen: a systematic review and meta-analysis. Ther Adv Musculoskelet Dis 10 (4):71–90. doi: 10.1177/1759720X18759291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coleman RE, Banks LM, Girgis SI, Kilburn LS, Vrdoljak E, Fox J, Cawthorn SJ, Patel A, Snowdon CF, Hall E, Bliss JM, Coombes RC, Intergroup Exemestane Study g (2007) Skeletal effects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES): a randomised controlled study. The Lancet Oncology 8 (2):119–127. doi: 10.1016/S1470-2045(07)70003-7 [DOI] [PubMed] [Google Scholar]
- 4.Kwan ML, Lo JC, Tang L, Laurent CA, Roh JM, Chandra M, Hahn TE, Hong CC, Sucheston-Campbell L, Hershman DL, Quesenberry CP Jr, Ambrosone CB, Kushi LH, Yao S (2014) Bone health history in breast cancer patients on aromatase inhibitors. PloS one 9 (10):e111477. doi: 10.1371/journal.pone.0111477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao S, Zhang Y, Tang L, Roh JM, Laurent CA, Hong CC, Hahn T, Lo JC, Ambrosone CB, Kushi LH, Kwan ML (2017) Bone remodeling and regulating biomarkers in women at the time of breast cancer diagnosis. Breast cancer research and treatment 161 (3):501–513. doi: 10.1007/s10549-016-4068-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwan ML, Ambrosone CB, Lee MM, Barlow J, Krathwohl SE, Ergas IJ, Ashley CH, Bittner JR, Darbinian J, Stronach K, Caan BJ, Davis W, Kutner SE, Quesenberry CP, Somkin CP, Sternfeld B, Wiencke JK, Zheng S, Kushi LH (2008) The Pathways Study: a prospective study of breast cancer survivorship within Kaiser Permanente Northern California. Cancer causes & control : CCC 19 (10):1065–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCloskey EV, Hannon RA, Lakner G, Fraser WD, Clack G, Miyamoto A, Finkelman RD, Eastell R (2007) Effects of third generation aromatase inhibitors on bone health and other safety parameters: results of an open, randomised, multi-centre study of letrozole, exemestane and anastrozole in healthy postmenopausal women. Eur J Cancer 43 (17):2523–2531. doi: 10.1016/j.ejca.2007.08.029 [DOI] [PubMed] [Google Scholar]
- 8.Goss PE, Ingle JN, Pritchard KI, Ellis MJ, Sledge GW, Budd GT, Rabaglio M, Ansari RH, Johnson DB, Tozer R, D’Souza DP, Chalchal H, Spadafora S, Stearns V, Perez EA, Liedke PE, Lang I, Elliott C, Gelmon KA, Chapman JA, Shepherd LE (2013) Exemestane versus anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27--a randomized controlled phase III trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 31 (11):1398–1404. doi: 10.1200/JCO.2012.44.7805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman RE, Banks LM, Girgis SI, Kilburn LS, Vrdoljak E, Fox J, Cawthorn SJ, Patel A, Snowdon CF, Hall E, Bliss JM, Coombes RC (2007) Skeletal effects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES): a randomised controlled study. The Lancet Oncology 8 (2):119–127 [DOI] [PubMed] [Google Scholar]
- 10.Rabaglio M, Sun Z, Price KN, Castiglione-Gertsch M, Hawle H, Thurlimann B, Mouridsen H, Campone M, Forbes JF, Paridaens RJ, Colleoni M, Pienkowski T, Nogaret JM, Lang I, Smith I, Gelber RD, Goldhirsch A, Coates AS, Collaborative BIG, International Breast Cancer Study G (2009) Bone fractures among postmenopausal patients with endocrine-responsive early breast cancer treated with 5 years of letrozole or tamoxifen in the BIG 1–98 trial. Ann Oncol 20 (9):1489–1498. doi: 10.1093/annonc/mdp033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coombes RC, Kilburn LS, Snowdon CF, Paridaens R, Coleman RE, Jones SE, Jassem J, Van de Velde CJ, Delozier T, Alvarez I, Del Mastro L, Ortmann O, Diedrich K, Coates AS, Bajetta E, Holmberg SB, Dodwell D, Mickiewicz E, Andersen J, Lonning PE, Cocconi G, Forbes J, Castiglione M, Stuart N, Stewart A, Fallowfield LJ, Bertelli G, Hall E, Bogle RG, Carpentieri M, Colajori E, Subar M, Ireland E, Bliss JM, Intergroup Exemestane S (2007) Survival and safety of exemestane versus tamoxifen after 2–3 years’ tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet 369 (9561):559–570. doi: 10.1016/S0140-6736(07)60200-1 [DOI] [PubMed] [Google Scholar]
- 12.Arimidex TAoiCTG, Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M (2008) Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. The Lancet Oncology 9 (1):45–53. doi: 10.1016/S1470-2045(07)70385-6 [DOI] [PubMed] [Google Scholar]
- 13.Jakesz R, Jonat W, Gnant M, Mittlboeck M, Greil R, Tausch C, Hilfrich J, Kwasny W, Menzel C, Samonigg H, Seifert M, Gademann G, Kaufmann M, Wolfgang J, Abcsg, the G (2005) Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet 366 (9484):455–462. doi: 10.1016/S0140-6736(05)67059-6 [DOI] [PubMed] [Google Scholar]
- 14.van de Velde CJ, Rea D, Seynaeve C, Putter H, Hasenburg A, Vannetzel JM, Paridaens R, Markopoulos C, Hozumi Y, Hille ET, Kieback DG, Asmar L, Smeets J, Nortier JW, Hadji P, Bartlett JM, Jones SE (2011) Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): a randomised phase 3 trial. Lancet 377 (9762):321–331. doi: 10.1016/S0140-6736(10)62312-4 [DOI] [PubMed] [Google Scholar]
- 15.Amir E, Seruga B, Niraula S, Carlsson L, Ocana A (2011) Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. Journal of the National Cancer Institute 103 (17):1299–1309. doi: 10.1093/jnci/djr242 [DOI] [PubMed] [Google Scholar]
- 16.Eastell R, Hannon RA, Cuzick J, Dowsett M, Clack G, Adams JE, group AT (2006) Effect of an aromatase inhibitor on bmd and bone turnover markers: 2-year results of the Anastrozole, Tamoxifen, Alone or in Combination (ATAC) trial (18233230). J Bone Miner Res 21 (8):1215–1223. doi: 10.1359/jbmr.060508 [DOI] [PubMed] [Google Scholar]
- 17.Perez EA, Josse RG, Pritchard KI, Ingle JN, Martino S, Findlay BP, Shenkier TN, Tozer RG, Palmer MJ, Shepherd LE, Liu S, Tu D, Goss PE (2006) Effect of letrozole versus placebo on bone mineral density in women with primary breast cancer completing 5 or more years of adjuvant tamoxifen: a companion study to NCIC CTG MA.17. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 24 (22):3629–3635 [DOI] [PubMed] [Google Scholar]
- 18.van Daele PL, Seibel MJ, Burger H, Hofman A, Grobbee DE, van Leeuwen JP, Birkenhager JC, Pols HA (1996) Case-control analysis of bone resorption markers, disability, and hip fracture risk: the Rotterdam study. BMJ 312 (7029):482–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garnero P, Hausherr E, Chapuy MC, Marcelli C, Grandjean H, Muller C, Cormier C, Breart G, Meunier PJ, Delmas PD (1996) Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res 11 (10):1531–1538. doi: 10.1002/jbmr.5650111021 [DOI] [PubMed] [Google Scholar]
- 20.Sornay-Rendu E, Munoz F, Garnero P, Duboeuf F, Delmas PD (2005) Identification of osteopenic women at high risk of fracture: the OFELY study. J Bone Miner Res 20 (10):1813–1819. doi: 10.1359/JBMR.050609 [DOI] [PubMed] [Google Scholar]
- 21.Ivaska KK, Gerdhem P, Vaananen HK, Akesson K, Obrant KJ (2010) Bone turnover markers and prediction of fracture: a prospective follow-up study of 1040 elderly women for a mean of 9 years. J Bone Miner Res 25 (2):393–403. doi: 10.1359/jbmr.091006 [DOI] [PubMed] [Google Scholar]
- 22.Johnell O, Kanis JA, Black DM, Balogh A, Poor G, Sarkar S, Zhou C, Pavo I (2004) Associations between baseline risk factors and vertebral fracture risk in the Multiple Outcomes of Raloxifene Evaluation (MORE) Study. J Bone Miner Res 19 (5):764–772. doi: 10.1359/JBMR.040211 [DOI] [PubMed] [Google Scholar]
- 23.Melton LJ 3rd, Crowson CS, O’Fallon WM, Wahner HW, Riggs BL (2003) Relative contributions of bone density, bone turnover, and clinical risk factors to long-term fracture prediction. J Bone Miner Res 18 (2):312–318. doi: 10.1359/jbmr.2003.18.2.312 [DOI] [PubMed] [Google Scholar]
- 24.Boyce BF, Xing L (2008) Functions of RANKL/RANK/OPG in bone modeling and remodeling. Archives of biochemistry and biophysics 473 (2):139–146. doi: 10.1016/j.abb.2008.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hlaing TT, Compston JE (2014) Biochemical markers of bone turnover - uses and limitations. Ann Clin Biochem 51 (Pt 2):189–202. doi: 10.1177/0004563213515190 [DOI] [PubMed] [Google Scholar]
- 26.Bell KJ, Hayen A, Irwig L, Hochberg MC, Ensrud KE, Cummings SR, Bauer DC (2012) The potential value of monitoring bone turnover markers among women on alendronate. J Bone Miner Res 27 (1):195–201. doi: 10.1002/jbmr.525 [DOI] [PubMed] [Google Scholar]
- 27.Vasikaran S, Eastell R, Bruyere O, Foldes AJ, Garnero P, Griesmacher A, McClung M, Morris HA, Silverman S, Trenti T, Wahl DA, Cooper C, Kanis JA, Group I-IBMSW (2011) Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int 22 (2):391–420. doi: 10.1007/s00198-010-1501-1 [DOI] [PubMed] [Google Scholar]
- 28.Bauer DC, Black DM, Garnero P, Hochberg M, Ott S, Orloff J, Thompson DE, Ewing SK, Delmas PD, Fracture Intervention Trial Study G (2004) Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: the fracture intervention trial. J Bone Miner Res 19 (8):1250–1258. doi: 10.1359/JBMR.040512 [DOI] [PubMed] [Google Scholar]
- 29.Bjarnason NH, Sarkar S, Duong T, Mitlak B, Delmas PD, Christiansen C (2001) Six and twelve month changes in bone turnover are related to reduction in vertebral fracture risk during 3 years of raloxifene treatment in postmenopausal osteoporosis. Osteoporos Int 12 (11):922–930. doi: 10.1007/s001980170020 [DOI] [PubMed] [Google Scholar]
- 30.Eastell R, Hannon RA, Garnero P, Campbell MJ, Delmas PD (2007) Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate: review of statistical analysis. J Bone Miner Res 22 (11):1656–1660. doi: 10.1359/jbmr.07090b [DOI] [PubMed] [Google Scholar]
- 31.Wheater G, Elshahaly M, Tuck SP, Datta HK, van Laar JM (2013) The clinical utility of bone marker measurements in osteoporosis. J Transl Med 11:201. doi: 10.1186/1479-5876-11-201 [DOI] [PMC free article] [PubMed] [Google Scholar]