Abstract
Short peptides are poorly immunogenic when delivered sublingually – under the tongue. Nanomaterial delivery of peptides could be utilized to improve immunogenicity towards designed sublingual vaccines, but nanomaterials have not been widely successful in sublingual vaccines owing to the challenges of transport through the sublingual mucosa. Here, we report that the sublingual immunogenicity of peptides is negligible, even in the presence of sublingual adjuvants or when PEGylated, but can be dramatically enhanced by assembly into supramolecular polymer-peptide nanofibers bearing low-molecular weight PEG, optimally between 2000–3000 Da. Neither PEGylation nor a sublingual adjuvant were capable of rendering peptides immunogenic without assembly into nanofibers. We found that PEG decreased nanofiber interactions with mucin and promoted longer residence time at the sublingual immunization site. Parallel investigations with shortened nanofibers indicated that the size of the assemblies had a surprisingly negligible influence over sublingual immunogenicity. In mice, optimized formulations were capable of raising strong and highly durable systemic antibody responses, antibodies in the upper respiratory and reproductive tracts, and systemic antigen-specific T-cell responses. These nanofiber-based sublingual vaccines were effective with both protein and nucleotide adjuvants and raised responses against both a model peptide epitope and a peptide epitope from M. tuberculosis. Further, PASylation (modification of nanofibers with peptide sequences rich in Pro, Ala, and Ser) could be substituted for PEGylation to also achieve sublingual immunogenicity. These findings indicated that surface properties supersede nanomaterial size in modulating sublingual nanomaterial immunogenicity, having important implications for the design of synthetic sublingual vaccines.
Keywords: sublingual, vaccine, mucosal, supramolecular, nanofiber, self-assembly
INTRODUCTION
Engineered nanomaterials are being investigated as potential vaccines and immunotherapies for infectious diseases, cancer, chronic inflammation, and autoimmunity, yet the sublingual route remains minimally explored for nanomaterial vaccination. Nanomaterial-based vaccines can be used to elicit immune responses with more tailored phenotypes than traditional vaccines based on inactivated or attenuated pathogens1–3 and have been constructed from platforms including self-assembling peptides,4–9 micelles,10–11 polymeric particles,12–17 and lipid particles.18–21 As this new generation of vaccines moves towards clinical translation, the choice of delivery route will be a critical consideration.
Mucosal vaccines, delivered across the mucosal barriers through which most pathogens enter the body, have key advantages over vaccines delivered via needles.22–25 Importantly, such vaccines can elicit protective antibodies in mucosal compartments to neutralize and clear pathogens from the body before they become established. Furthermore, depending on the chosen mucosal delivery route, they may have significant logistical and financial benefits. Sublingual vaccination, a subset of mucosal vaccination, has been shown to elicit antibody responses in an anatomically broad range of mucosal surfaces, such as the upper and lower respiratory tracts and the reproductive tract, in addition to raising systemic responses in the blood.26 It is also a favorable delivery route to encourage patient compliance, because in addition to avoiding the use of needles, it allows for pain-free and potentially self-administered vaccination using droplets, sprays, microneedle arrays,27 or dissolvable tablets and wafers.26 Because it does not in principle require needles or trained personnel, sublingual immunization could also be achieved at a lower overall cost compared to needle-based delivery, a critical constraint when designing vaccines for the developing world,28 and it eliminates the potential for needle re-use.29
Despite clear potential advantages of sublingually delivered nanomaterial-based vaccines, they have received surprisingly little attention, with only a few examples in the literature.18, 30–31 It is likely that this is due to the challenge of delivering nanomaterials through the salivary mucus layer in order that they be acquired by dendritic cells at the sublingual epithelium with enough efficiency and with adequate immunostimulation to induce a systemic response. The sublingual epithelium contains 6–8 layers in mice, within which 3–4% of cells have been reported to be dendritic cells.32–33 The protective mucus layer is composed largely of glycoprotein fibers that crosslink and entangle to form a network34 that hinders biomaterial transport via highly polyvalent, low-affinity adhesive interactions driven by charge and hydrophobicity.35 However, it has been shown that conjugation of hydrophilic polymers such as polyethylene glycol (PEG) can dramatically improve the mucosal delivery of nanomaterials by minimizing these adhesive interactions between the nanomaterial and the mucus network.35–39
We sought to develop a sublingually-active nanomaterial vaccine platform based on nanofiber-forming peptides shown previously to be immunogenic via other routes including the subcutaneous,40 intraperitoneal,4 and intranasal41 routes. We utilized the Q11 peptide system, in which short synthetic peptides self-assemble into fibers with lengths of hundreds of nanometers or more and widths of approximately 10–20 nm. We and others have shown that Q11 8, 40 and other fibrillar peptide nanomaterials 7, 42–43 can raise epitope-specific immune responses against chosen T- or B-cell epitopes co-assembled within them, even without supplemental adjuvants, and that the character of the immune response can be adjusted based on controllable parameters such as the ratio between T- and B-cell epitopes.5, 44
Here, we found that neither soluble peptide epitopes, PEGylated soluble peptide epitopes, nor self-assembled peptide nanofibers were immunogenic sublingually, even when delivered with mucosal adjuvants, and that nanofiber shortening was unable to improve immunogenicity. However, strikingly, modification of self-assembled nanofibers with PEG or with peptides rich in proline, alanine, and serine (PAS) rendered them strongly immunogenic when delivered sublingually with mucosal adjuvants, capable of raising class-switched antibodies in the blood and in multiple different mucosal tissues. This strategy was compatible with multiple different peptide epitopes and multiple different adjuvants and illustrated that even surprisingly large nanomaterials can be made immunogenic sublingually via polymer conjugation.
RESULTS
Despite the attractive properties of peptide nanofiber vaccines established in other routes of delivery, we expected that the extremely high aspect ratio and extended length of the nanofibers would make them non-immunogenic sublingually, and so we explored two distinct and potentially complementary approaches to render them immunogenic: PEGylation of the nanofibers and shearing them into much shorter fibers. We anticipated that PEG modification would be compatible with the Q11 peptide’s self-assembly into nanofibers, as we recently found that it is tolerant of a variety of changes in surface properties without a loss of immunogenicity, including N-terminal modification with a triethylene glycol block.45 Other fibrillizing peptides have also been shown to retain their self-assembly even with the inclusion of longer PEG chains,46–51 with small-angle neutron scattering (SANS) suggesting structures having a peptide nanofiber core surrounded by a PEG corona.48–49 We hypothesized that such a material, in which the entire supramolecular structure is densely coated in low molecular-weight PEG, would be ideal for promoting mucus penetration.
As an alternative to PEGylation, we also investigated whether shortening the nanofibers by shearing them would augment sublingual immunogenicity. While adhesive interactions between biomaterials and mucus are believed to be primarily responsible for inhibiting transport, the mucin network does have established pore sizes, suggesting that a material smaller than the pore size might be capable of penetrating more readily due to a reduction in steric obstruction.52–53
Design and Characterization of Epitope-Bearing Peptide-Polymer Conjugates
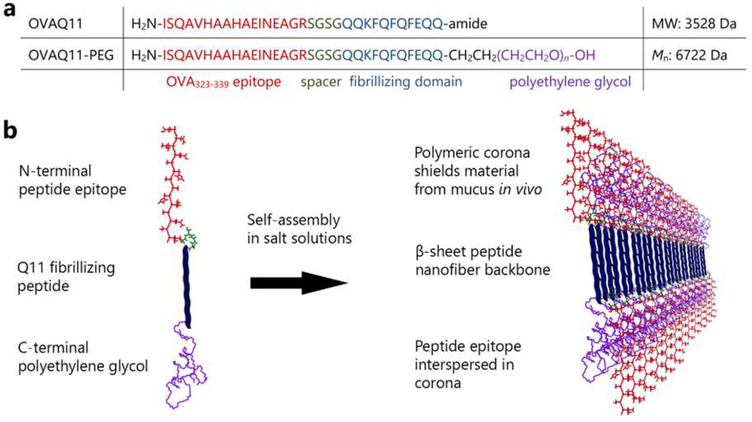
We initially designed a molecule consisting of the Q11 sequence and the model peptide epitope OVA323–339 connected to its N-terminus via a flexible (Ser-Gly)2 linker. A 3000 MW PEG was attached to the C-terminus. We postulated that this molecule would assemble into nanofibers with a Q11 peptide core and OVA epitopes interspersed among a PEG corona (Fig. 1). Owing to the use of a polydisperse PEG block, the resulting PEG-peptide conjugate (OVAQ11-PEG) was also polydisperse, exhibiting the expected molecular weight range (Fig. S1).
Figure 1. Design of supramolecular peptide-polymer nanofibers.
(a) Primary sequences of OVAQ11 and OVAQ11-PEG. (b) Peptide-polymers self-assemble to form supramolecular nanofibers. The hypothesized structure was produced using previously published knowledge regarding the anti-parallel β strand architecture in Q11 nanofibers54 and the propensity of similar self-assembling PEG-conjugated β-sheet peptides to form peptide cores with PEG coronas.48, 51
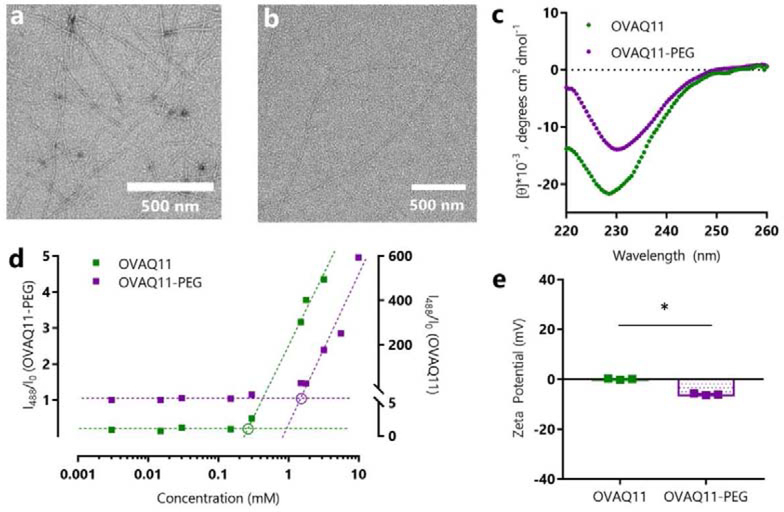
OVAQ11-PEG self-assembled in PBS to form high aspect ratio nanofibers morphologically similar to those observed for OVAQ11 (Fig. 2a, b). To characterize the secondary structure of these nanofibers, we used circular dichroism (CD) and fluorescent probes. The CD spectra of OVAQ11-PEG showed the same minima near 230 nm as previously reported for OVAQ11,40 suggesting a similar β-sheet secondary structure (Fig. 2c). To confirm this finding, we employed two fluorescent probes, Thioflavin T (ThT) and pyrene, which have been used previously in the characterization of PEG-peptide conjugates.55–57 Thioflavin T binds to β-sheet rich structures, although our previous work with OEG-Q11 has shown that the presence of just three ethylene oxide units can diminish the overall magnitude of fluorescence.45 We thus measured the fluorescence signal of OVAQ11-PEG and OVAQ11 assembled at a range of concentrations in solutions of ThT in PBS. We observed a linear increase of fluorescence at peptide concentrations above the critical aggregation concentrations (c.a.c.). To graphically estimate the c.a.c., we plotted the ThT fluorescence data against a log10 concentration axis, using the method reported by Hamley and colleagues 56 (Fig. 2d). We performed a similar procedure using pyrene, which is sensitive to changes in hydrophobicity. Upon assembly of OVAQ11-PEG, sequestration of hydrophobic amino acids, particularly phenylalanine, within the PEG-shielded nanofiber core would be expected to decrease the hydrophobicity of the solution, leading to an increase in fluorescence of pyrene’s first vibronic band. Both ThT and pyrene measurements estimated the c.a.c. of OVAQ11-PEG to be near 1 mM (Fig. 2d and Fig. S2). All subsequent experiments were thus performed with peptides prepared at concentrations of at least 2 mM. Additionally, due to our previous report that negative charge can diminish the immunogenicity of peptide nanofibers, we measured the zeta potential of OVAQ11-PEG, finding it to be slightly negative, about −6.6 mV (Fig. 2e). This is considerably less than the amount of negative charge previously found to partially diminish the immunogenicity of Q11-based nanofibers, about 17.3 mV,45 and so we did not expect any deleterious impact on immunogenicity. As expected, conjugation of PEG to the nanofibers did not result in any cytotoxicity in vitro (Fig. S3).
Figure 2.
OVAQ11-PEG self-assembled into β-sheet nanofibers, as indicated by negative stained TEM images of (a) OVAQ11 and (b) OVAQ11-PEG nanofibers assembled from 2 mM peptide and (c) Circular dichroism of peptides assembled at 3 mM in PBS and diluted to 0.1 mM in potassium fluoride immediately prior to analyzing. (d) β-sheet structure was further confirmed using Thioflavin T. Following the method of Hamley and coworkers,56 the graphical estimates of critical aggregation concentration correspond to the intersection of the pre- and post-assembly tangent lines (circled). (e) Zeta-potentials of OVAQ11-PEG and OVAQ11 indicated that surface charge was minimally altered by PEGylation. Peptides were prepared at 2 mM in 1X PBS and diluted to 0.2 mM in 1X PBS prior to measurement at 25 °C. * p < 0.05, unpaired, two-tailed T-test
Reduction of Nanofiber Length
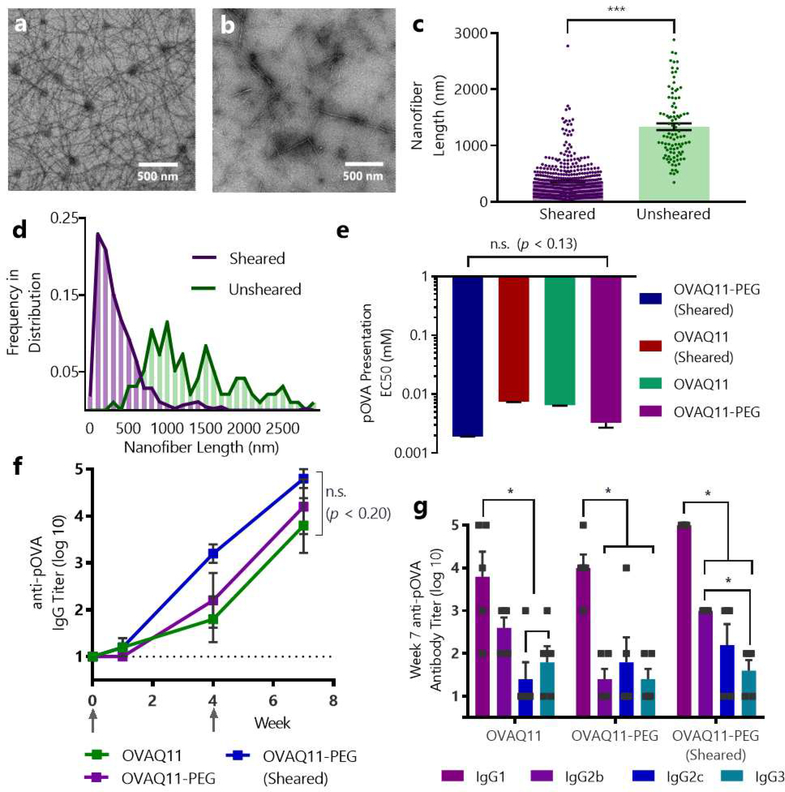
As an alternative to PEGylation, we also sought to test whether peptide nanofibers could be rendered immunogenic by the sublingual route by reducing their length. We previously found that β-sheet nanofiber length impacts immunogenicity when delivered intranasally,41 but the effects on subcutaneous or sublingual immunization is not known. While adhesive interactions are believed to dominate transport through mucus, the mucin network of human saliva has been reported to have defined pore sizes with a mode diameter of 700 nm,53 suggesting that a material with a size below this value might transport more readily.52 To this end, we physically sheared OVAQ11 nanofibers through a polycarbonate membrane containing 100 nm laser track-etched pores, a method previously reported to reduce the length of similar β-sheet peptides.46, 58 On TEM grids, unsheared OVAQ11 nanofibers formed an entangled mat of nanofibers with lengths of micrometers, as previously reported (Fig. 3a).40, 45 In contrast, sheared OVAQ11 nanofibers were significantly shorter (Fig. 3b–d, Fig. S4), with median fiber lengths of 276 nm compared to 1168 nm for unsheared nanofibers. Notably, close to 90% of the sheared fibers were below the reported 700 nm mucus pore size. To ensure that shearing was not leading to a decrease in overall peptide concentration, we sheared tryptophan-labelled Q11 (W-Q11) nanofibers and measured their absorbance at 210, 215, and 260 nm before and after shearing (Fig. S5), finding negligible loss of material from the shearing process.
Figure 3. Shearing of OVAQ11 nanofibers reduced nanofiber lengths, and neither PEGylation nor shearing diminished immunogenicity via traditional subcutaneous immunization.
Ten TEM images of OVAQ11 were obtained before and after shearing through a 100 nm track-etched polycarbonate membrane. ImageJ was used to determine the length of 450 individual sheared fibers and 96 non-sheared fibers. Representative images of unsheared (a) and sheared (b) OVAQ11 fibers, and (c) individual lengths of each nanofiber. ***p < 0.001, unpaired, two-tailed t-test. (d) Histogram showing the frequency distribution of fiber lengths before and after shearing. All TEM images and corresponding nanofiber traces are shown in Fig. S4. (e) Neither shearing nor PEGylation of nanofibers significantly affected presentation of pOVA in MHC class II molecules, as measured by DOBW reporter cells, which secrete IL-2 upon encountering DCs with pOVA-loaded MHC II. IL-2 concentration in the supernatant was measured by ELISA. EC50 corresponds to the concentration of material that gives the half-maximal antigen presentation; n.s. (p < 0.13), one-way ANOVA, n=3/group. (f) Neither shearing nor PEGylation disrupted the subcutaneous immunogenicity of OVAQ11 nanofibers. Mice were immunized subcutaneously on weeks 0 and 4 with two 50 μL injections of 2 mM peptide and serum was analyzed by ELISA; ns (p < 0.20); one-way ANOVA, n=5/group. Arrows indicate timepoints of immunizations. (g) Neither PEGylation nor shearing significantly altered the dominant subclasses of IgG raised by nanofibers. Shown is week 7 serum of mice from (f). *p < 0.05, two-way ANOVA with Tukey’s multiple comparisons test, n=5/group.
Neither PEGylation nor Shearing Diminished the Overall Immunogenicity of OVAQ11 via Traditional Routes
Prior to evaluating the PEGylated and sheared nanofibers sublingually, we tested whether either modification compromised the overall immunogenicity of the materials using standard subcutaneous delivery and in vitro methods. Because responses to Q11 have been shown to be T-cell dependent,59 it is critical that the material can be taken up and the conjugated peptide epitope can be processed and presented on major histocompatibility (MHC) molecules to T cells. To test in vitro whether PEGylation or shearing influenced this process for Q11 nanofibers, we incubated murine bone-marrow derived dendritic cells (BMDCs) with nanofibers and subsequently co-cultured the BMDCs with DOBW reporter T-cell hybridomas, whose T-cell receptors are specific for the pOVA epitope. These cells respond to MHC-restricted pOVA peptide by secreting IL-2. No significant difference in the EC50 values were observed between groups, suggesting that OVAQ11-PEG and sheared OVAQ11 could be efficiently internalized and processed, and the pOVA epitope presented to T cells (Fig. 3e).
To test whether shortening or PEGylation modified the overall immunogenicity of the nanofibers via traditional non-mucosal routes, we immunized mice subcutaneously with each material. Neither PEGylation nor shearing diminished the OVA-specific IgG titers raised (Fig. 3f). Additionally, as Q11 has previously been shown to elicit mildly Th2-biased phenotypes after subcutaneous immunization,5 we investigated the antibody subclasses raised by the modified nanofibers (Fig. 3g). All groups showed a predominantly IgG1 response, suggestive of a Th2-biased response. Taken together, these experiments indicated that neither shearing nor PEG conjugation diminished the overall immunogenicity of OVAQ11, and they provided further evidence of the robustness of the Q11 platform to physical changes without loss of immunogenicity.
PEG Conjugation to Nanofibers Enables Sublingual Peptide Immunization
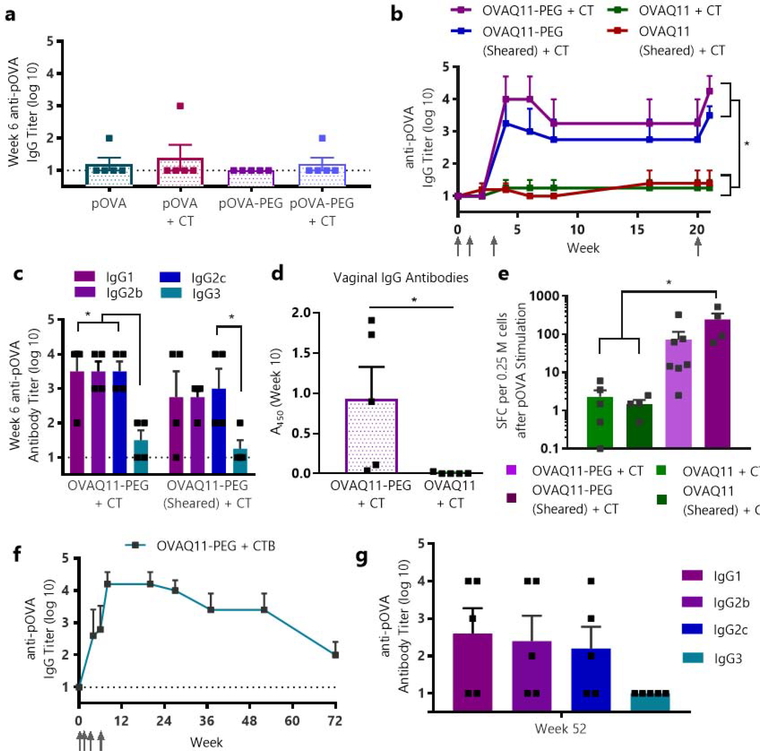
Having designed and characterized OVAQ11-PEG and observing no loss of immunogenicity in vitro or subcutaneously versus non-PEGylated OVAQ11, we next tested our hypothesis that these changes would enable sublingual immunization. To minimize the potential confounding effects of mice swallowing material, we immunized deeply anesthetized mice with a conservative volume of only 7 μL, well below the volumes expected to induce inadvertent swallowing.33 Sublingual immunization against short peptide epitopes using nanomaterials is challenging, and few reports exists in the literature.30 Consequently, sublingual immunization has previously been achieved primarily using whole proteins or whole pathogens along with a mucosal adjuvant such as cholera toxin (CT).33, 60 Consistent with this, we found that whole ovalbumin protein was capable of producing strong antibody responses when delivered sublingually with cholera toxin (Fig. S6), but cholera toxin was not capable of rendering the soluble peptide pOVA (OVA323–339) immunogenic, as the soluble pOVA peptide failed to raise responses regardless of whether it was formulated with CT (Fig. 4a). In addition, PEGylation of the pOVA peptide and delivery with CT likewise failed to raise responses sublingually (Fig. 4a). In short, neither PEGylation, adjuvanting with CT, nor the two strategies combined were sufficient to render pOVA immunogenic sublingually.
Figure 4. Supramolecular PEG-peptide nanofibers enabled sublingual immunization against peptide epitopes.
(a) Adjuvant plus soluble OVA peptide (pOVA) alone fails to elicit sublingual antibody responses, even when the peptide is PEGylated. Mice were primed and boosted at weeks 1 and 3 with 7 μL of 5.6 mM peptide with or without 2 μg of cholera toxin (CT) adjuvant, and serum pOVA-specific IgG was measured by ELISA at week 6, n=5/group. (b) PEGylated nanofibers were immunogenic sublingually with CT, regardless of whether they were sheared. Nanofibers lacking PEG were non-immunogenic sublingually. Mice were primed and boosted at weeks 1, 3, and 20 with 7 μL of 5.6 mM peptide with 2 μg of cholera toxin per mouse, and pOVA-specific IgG was measured by ELISA. *p < 0.05, one-way ANOVA, Tukey’s multiple comparisons test, n=4/group. Arrows indicate timepoints of immunizations. (c) Antibody subclasses at week 6 were balanced between IgG1, IgG2b, and IgG2c. *p < 0.05, two-way ANOVA, n=4/group. (d) Sublingually delivered OVAQ11-PEG nanofibers also raised IgG antibodies in the reproductive tract at week 10. *p<0.05, unpaired, two-tailed t-test, n=5/group. (e) PEGylated nanofibers elicited T cell responses, measured by splenocyte ELISPOT at week 21 (same mouse groups as shown in (b) and Fig. S7). SFC (spot-forming cells) after pOVA stimulation are shown. *p < 0.05, one-way ANOVA, Tukey’s multiple comparisons test, n = 4/group (OVAQ11 + CT, OVAQ11-PEG + CT), n = 7 (OVAQ11-PEG (Sheared) + CT), or n = 5 (OVAQ11 (Sheared) + CT). (f) OVA-Q11-PEG nanofibers remained strongly and durably immunogenic using the more clinically translatable adjuvant CTB. n=5 mice, immunized sublingually at weeks 0, 1, 3, and 6 with 7 μL of 5.6 mM OVAQ11-PEG with 10 μg of cholera toxin subunit B, pOVA-specific serum IgG measured by ELISA. (g) At week 52 for the mice immunized with CTB-adjuvanted nanofibers shown in (e), Ig responses were balanced between IgG1, IgG2b, and IgG2c.
In striking contrast to soluble peptide formulations, mice immunized sublingually with self-assembled nanofibers of OVAQ11-PEG raised high levels of circulating antibodies against the pOVA peptide, while mice immunized with non-PEGylated OVAQ11 nanofibers elicited no responses (Fig. 4b). The antibody responses were durable, with high titers still remaining four months after the initial immunization. Additionally, a recall response was evident after boosting mice at week 20, suggesting that immune memory may have developed. In contrast to PEG’s critical role in enabling sublingual nanofiber immunization, length reduction of OVAQ11 via shearing was not sufficient to produce efficacious formulations, nor did it improve the responses to sublingually delivered OVAQ11-PEG (Fig. 4b, S7). The inclusion of adjuvant was required in the formulation, yielding antibody subclasses that were balanced across IgG1, IgG2b, and IgG2c (Fig. S7, 4c). Again, it was clear that both Q11-driven assembly of the peptide epitope into nanofibers and PEGylation were necessary to render the pOVA peptide immunogenic sublingually, as both pOVA-PEG and OVAQ11 elicited no response even when adjuvanted with CT (Fig. 4a–b).
Sublingual OVAQ11-PEG Elicits Mucosal Antibody Responses in the Reproductive Tract
A major benefit of mucosal vaccination is the ability to raise antibodies not only in the systemic circulation, but also at mucosal surfaces through which pathogens enter the body, where they can neutralize pathogens prior to infection. The sublingual route has been shown to have a broader anatomical distribution of antibodies than other mucosal routes, with antibodies observed in both the upper and lower respiratory tracts and in the reproductive tract.26 Consistent with this, after sublingual immunization of mice with OVAQ11-PEG + CT we observed antigen-specific IgG in vaginal secretions, whereas no response was observed in mice immunized with OVAQ11 + CT (Fig. 4d). We also observed responses in the nasal wash, suggestive of antibodies in the respiratory tract (Fig. S8).
OVAQ11-PEG Elicits Antigen-Specific T-Cell Responses after Sublingual Immunization
The OVA323–339 epitope is unique in that it contains overlapping B- and T-cell epitopes, which allowed us to simultaneously examine the ability of OVAQ11-PEG to elicit antigen-specific T-cell responses. We have previously shown that raising simultaneous T- and B-cell responses is critical both for optimizing responses to Q11 and for raising therapeutic immune responses.5, 44 At week 21, one week after a final boost, mice were sacrificed and the spleens were harvested for analysis by ELISPOT (Fig. 4e, S9). The results were consistent with the adjuvanted humoral responses described above, as mice immunized with OVAQ11-PEG + CT raised OVA-specific IFNγ T-cell responses, yet mice immunized with OVAQ11 + CT raised minimal responses.
Sublingual Immunization with OVAQ11-PEG is Effective with a Clinically Relevant Protein Adjuvant
Our initial studies of sublingual immunization with OVAQ11-PEG were performed using the model mucosal adjuvant CT, which is commonly employed in mouse models. Subsequently, we investigated a more clinically translatable adjuvant, the B subunit of cholera toxin (CTB). CTB contains a strong T-cell epitope,61 interacts with the receptor GM1-ganglioside, and has received significant attention as a mucosal adjuvant.62 It has been previously approved for use in humans, as it is included in the oral cholera vaccine Dukoral63 and has been used as an intranasal adjuvant in human studies.64 Mice immunized with OVAQ11-PEG and the CTB protein raised antibody titers as strong as those with the full CT adjuvant after including an additional third boost at week 6 (Fig. 4f), indicating the compatibility of the PEGylated Q11 nanofiber system with this more clinically appropriate adjuvant. Strikingly, mice maintained class-switched antibody responses at 72 weeks after primary immunization and greater than a year after receiving their final boost (Fig. 4f, g).
Modulation of Conjugated PEG Size Tunes Sublingual Immune Responses
We next investigated how the length of the conjugated PEG affected sublingual immune responses by designing a set of self-assembling OVAQ11 nanofibers with 350, 1000, or 2000 MW mPEG (Fig. 5a–d). For synthetic reasons, we moved the OVA epitope to the C-terminus and the PEG chain to the N-terminus, and used a methoxy terminated mPEG rather than traditional hydroxy terminated PEG. Q11 vaccines have previously been shown to be effective with a C-terminal epitope8 and we verified that neither of these changes (mPEG, C-terminal epitope placement) altered uptake and presentation in vitro (Fig. S10).
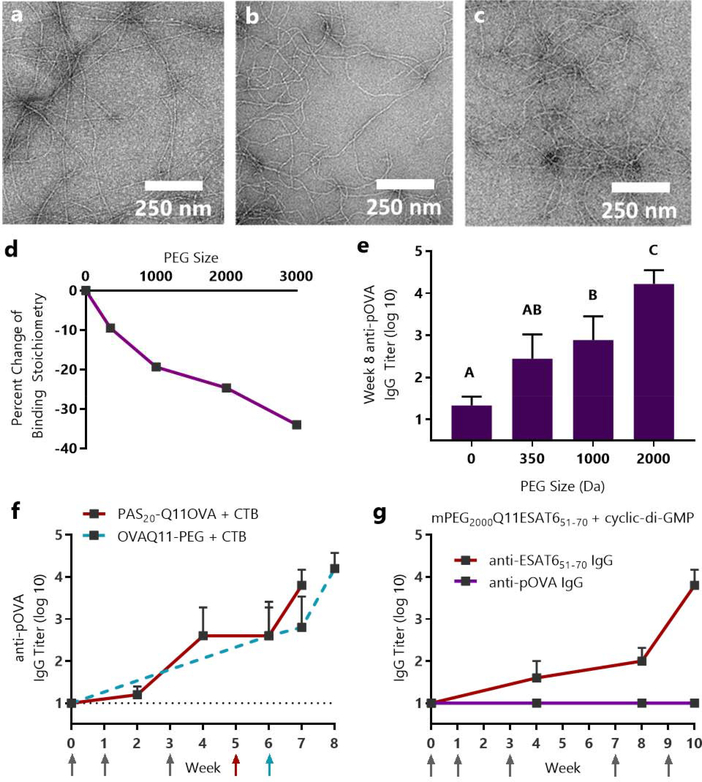
Figure 5. Sublingual immune responses and mucin binding to OVAQ11 were dependent on the length of conjugated PEG.
Q11OVA with N-terminal mPEG blocks of 350, 1000, and 2000 average molecular weight formed nanofibers, as evidenced by negative stained TEM: (a) mPEG350-Q11OVA (b) mPEG1000-Q11OVA (c) mPEG2000Q11OVA. (d) Isothermal titration calorimetry indicated that increasing PEG length diminished interactions with mucin. * p < 0.05, R2 = 0.94 by linear regression of PEG size vs. percent change in binding stoichiometry. (e) Increasing PEG length improved OVA-specific titers raised against OVAQ11-PEG nanofibers. Mice were immunized sublingually with 7 μL of 5 mM peptide with 2 μg of cholera toxin per mouse and boosted with the same dose at weeks 1, 3, and 6. IgG against the pOVA epitope was measured at week 8 by ELISA. A, B, C: Groups in (e) that do not share a letter are statistically different (p < 0.05) by 2-way ANOVA with Tukey’s multiple comparisons test, n=6 (0 PEG) or n=8/group from two independent experiments. Complete titer data is shown in Fig. S11. (f) Proline-Alanine-Serine modification (PASylation) had a similar effect as PEGylation, enabling sublingual immunization against PAS20-Q11OVA nanofibers in a fully peptidic formulation. Mice were immunized with 8 μL of 5 mM peptide and 10 μg CTB at weeks 0, 1, 3, and 5. Data from OVAQ11-PEG + CTB immunizations are re-presented from Figure 4f as a comparison. Arrows indicate timepoints of immunizations. (g) Sublingual immunization was achieved against the ESAT651–70 epitope from M. tuberculosis using cyclic-di-AMP adjuvant. CBA/J mice (n=5) were immunized at weeks 0, 1, 3, 7, and 9 with 8 μL of 5 mM mPEG2000-Q11ESAT651–70 with 10 μg of cyclic-di-AMP adjuvant per mouse; IgG against the ESAT651–70 and pOVA epitopes were measured by ELISA.
To quantify the impact of PEG size on the surface properties of Q11 nanofibers, we utilized isothermal titration calorimetry. This technique has been used previously to quantify mucin binding to biomaterials, and importantly, reduced in vitro mucin binding has been shown to correlate to mucus penetration in vivo.36 We found that mucin binding was reduced in a PEG-size dependent manner, supporting our hypothesis that PEG conjugation would shield nanofibers from interactions with mucus (Fig.5d). Furthermore, we found that sublingual immune responses displayed an inverse trend to mucin binding, with greater PEG sizes leading to stronger immune responses (Fig. 5e). This inverse relationship between nanofiber/mucin interactions and sublingual nanofiber immunogenicity strongly supports the hypothesis that PEG enables sublingual immunization by imparting mucus-inert surface properties to the nanofibers. mPEG chains as small as 1000 Da increased sublingual immune responses to Q11OVA, while 2000 Da mPEG was sufficient to induce high-titer antibody responses (Fig 5e). As all nanofibers displayed similar morphologies regardless of PEG size (Fig 5a–c), these results further supported the previous conclusion that alteration of nanofiber surface properties with PEG is critical for sublingual immune responses against peptide nanofibers, while length reduction is not.
To further support our finding that the surface properties of PEG are critical in rendering supramolecular assemblies immunogenic sublingually, we tested whether conjugating an alternate mucus-inert material to the nanofibers would have the same effect as PEG. We chose to replace PEG with a polypeptide sequence composed of proline, alanine, and serine (PAS). This PASylation approach was previously designed to mimic the biophysical properties of PEG to provide an alternative for improving circulation of biopharmaceutical proteins.65–66 We reasoned that by utilizing a lower molecular weight PAS sequence with a chain length similar to 2000 MW PEG we could impart favorable surface properties to Q11 nanofibers for sublingual delivery. This was indeed the case, as mice immunized with PAS20-Q11OVA and CTB adjuvant raised high titer antibody responses (Fig. 5f). While several polymeric alternatives to PEG have been explored for mucus penetration (reviewed recently67), this is to our knowledge the first use of a fully peptidic material to promote sublingual biomaterial delivery, in either immunization or drug delivery contexts. Concerns about the immunogenicity of PEG have increased in recent years due to loss of efficacy and adverse effects in some PEGylated therapeutics.68–69 In a 2003 study it was reported that over 25% of healthy blood donors had anti-PEG antibodies,70–71 representing an increase compared to the 0.2% figure reported in 198472 that could be due in part to continual PEG exposure in the form of foods and other commercial products. We believe that the use of short PAS sequences could hold potential beyond sublingual immunization as a PEG alternative for mucosal delivery that minimizes immunogenicity concerns.
We next sought to determine if our sublingual vaccine strategy could be used to raise antibodies against a clinically relevant peptide epitope. Based on our findings that conjugation of 2000 MW PEG was sufficient to raise sublingual immune responses, we synthesized an mPEG2000-Q11 variant containing the ESAT651–70 epitope from M. tuberculosis. This peptide epitope has been shown to be protective in a preclinical model of M. tuberculosis infection.73 We used the nucleotide adjuvant cyclic-di-AMP, a STING receptor agonist, in place of cholera toxin to test the amenability of our strategy to alternate formulations, as the optimal adjuvant can vary by vaccine target. Sublingual immunization with mPEG2000-Q11ESAT651–70 and cyclic-di-AMP produced antigen-specific antibodies against the ESAT651–70 epitope, showing that our strategy is not limited to model antigens or cholera toxin-based adjuvants (Fig. 5g). Additionally, CBA/J mice were used for these immunizations due to their haplotype compatibility with the ESAT651–70 epitope, demonstrating an ability to raise sublingual responses in multiple strains of mice.
PEG Conjugation Prolongs Nanofiber Residence at the Sublingual Immunization Site
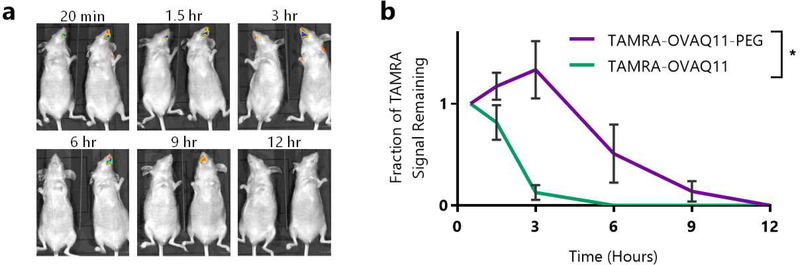
We hypothesized that by reducing interactions with mucus, PEG conjugation should also enhance nanofiber residence at the sublingual immunization site. Entrapment of nanofibers in mucus would lead to rapid mucus-mediated clearance, preventing the nanofibers from accessing APCs at the sublingual epithelium and thus limiting the initiation of a potential immune response. In contrast, PEGylated nanofibers are expected to more readily pass through the mucus and reach the epithelium. Directly above the epithelium is the unstirred mucus layer, where turnover is much less rapid than the mucus above, allowing materials that reach this layer to reside for longer periods of time at the adjacent epithelium.35, 74 To assess this hypothesis, we synthesized fluorescent, TAMRA-conjugated OVAQ11 and OVAQ11-PEG nanofibers, administered them sublingually without adjuvant, and monitored the radiant efficiency of the sublingual site over time using whole-mouse IVIS imaging (Fig. 6a). While TAMRA-OVAQ11 fibers were almost entirely cleared by 3 hours post-administration, TAMRA-OVAQ11-PEG fibers were detectable for at least 9 hours (Fig. 6b). The 9–12 hour residence time at the immunization site that we observed matches the timeline for a previously reported sublingual vaccine.30 We chose to use unadjuvanted nanofibers in this experiment to focus on material effects alone, as our platform works with a variety of adjuvants, each of which could alter the results. In combination with the observed effects of PEG size on mucin binding and antibody responses, this supports the hypothesis that PEG conjugation enables sublingual immunization by imparting favorable surface properties for mucus penetration.
Figure 6: PEG conjugation prolonged nanofiber residence at the sublingual immunization site.
(a) SKH-1 Elite mice (n=3/group) were administered 7 μL of 5 mM TAMRA-OVAQ11 or TAMRA-OVAQ11-PEG, and the radiant efficiency of the immunization site was monitored using an IVIS Lumina XR. Shown in (a) are representative images of one mouse given TAMRA-OVAQ11 (left in each image) and TAMRA-OVAQ11-PEG (right in each image). Images for all mice are shown in Fig. S12. (b) Quantification of IVIS imaging results. The radiant efficiency at each timepoint for each mouse was normalized to the value at the first timepoint (20 minutes) to determine the fraction of signal remaining. *p < 0.05, 2-way ANOVA.
DISCUSSION
In this study, we developed a strategy for sublingual immunization against peptide epitopes by combining the effects of supramolecular assembly with mucus-penetrating surface modifications, including PEGylation and PASylation. This approach was successful with a variety of adjuvants and epitope systems and in multiple strains of mice, illustrating its versatility and adaptability. Previous work with liposome-based sublingual vaccines showed moderate improvements with PEG-incorporating formulations,18 and virus-like particles did not require such surface changes,30 but we found that Q11 nanofiber vaccines, which have been previously developed to raise immune responses via subcutaneous40 or intranasal41 routes, failed to raise antibody responses sublingually even when formulated with the immunogenic model epitope OVA323–339 and the strong mucosal adjuvant cholera toxin. PEGylation or PASylation rendered these nanofibers highly immunogenic by the sublingual route, enabling strong, long-lived humoral responses and cellular responses and enabling the future development of supramolecular peptide materials as sublingually delivered vaccines. Importantly, PEGylation and adjuvants were not sufficient to render soluble (unassembled) peptides immunogenic, illustrating the strong influence of supramolecular assembly.
While simple, this approach affords control over two key parameters: PEG chain length and PEG surface density. PEG chain length is selected during synthesis, and although not investigated directly in this study, supramolecular peptide nanofibers allow co-assembly of multiple peptides in a range of ratios,75 so PEGylated peptides could in principle be co-assembled with other peptides to control the density of PEG chains on the surface of the nanofibers. For example, we found that PEG chain lengths of 2000 Da were sufficient to generate immune responses when incorporated on each peptide of the nanofiber assembly; it would be straightforward, in principle, to vary the stoichiometry of PEGylated peptides with other co-assembled peptides such as B- and T-cell epitopes to further tune and optimize the immune responses raised.
Our present study did not exhaustively investigate the mechanism by which PEG conjugation enables sublingual nanofiber vaccination, though the results are consistent with the hypothesis that its primary role is promoting mucus penetration. The molecular weight and high surface density of PEG coverage in the materials studied are consistent with the levels reported to be effective in promoting mucus penetration with other biomaterials,35 and the lack of effect in shortening the fibers corroborates findings that surface properties supersede biomaterial size in promoting such transport,76 at least within the range of sizes and physical properties relevant to supramolecular peptide materials.
While Q11 vaccines do not require addition of supplemental adjuvant in other vaccination routes, we found that adjuvant was required for consistent responses sublingually. This indicates that the adjuvant may be playing a delivery route-specific role rather than boosting the material’s immunogenicity in a more general way. The sublingual epithelium has been reported to contain a low (~3–4 %) number of antigen-presenting cells under normal conditions; however, sublingual application of cholera toxin leads to a transient increase in the level of these cells that peaks at 2 h after application and returns to basal levels by 6 h.33 The importance of nanofiber uptake by APCs for Q11-based vaccines is well-established; we reported previously that when Q11 fibers are modified such that APC uptake is disrupted, antibody responses are ablated.45 Dendritic cells that acquire antigen locally at the site of sublingual immunization are able to enter the systemic circulation, enabling responses in distant lymphoid organs.77 This is consistent with the strong serum antibody titers, splenic T cell responses, and mucosal antibodies found in this study. The simplest explanation thus appears to be that a primary role of cholera toxin (or its B subunit) in the present work is to increase the APC population available to the nanofibers.
We further hypothesize that PEGylation or PASylation promotes access of peptide nanofiber materials to sublingual APCs by prolonging the materials’ residence near the sublingual epithelium. While the upper layer of mucus is cleared rapidly, the unstirred layer below is more stable.74 We suspect that non-PEGylated nanofibers are quickly entrapped in the mucus and cleared without accessing the sublingual APCs, while PEGylated nanofibers pass through to the unstirred mucus layer and are maintained there long enough to be acquired by APCs. This is supported by the decreased interactions observed in vitro between PEGylated nanofibers and mucin. The precise nature of the nanofibers’ transport mechanism and the phenotype of immune cells which interact with them remains an important and interesting area of future study for the development of sublingual biomaterial vaccines.
Sublingual biomaterial vaccination is still a relatively unexplored area, and our findings hold important implications for future work. We demonstrate the feasibility of using supramolecular materials and PEG conjugation as strategies to overcome the challenge of promoting strong sublingual responses against short peptide epitopes. The modular nature of self-assembling peptide vaccines, such as those based on Q11, KFE8, or Coil29, makes them adaptable to a range of diseases through the choice of epitope,5–7, 78 as further demonstrated here by raising responses against the ESAT651–70 peptide epitope from M. tuberculosis.
Another important area for future investigation is a deeper characterization of the mucosal immune responses elicited by sublingually-delivered nanofibers. Sublingual vaccination has been shown to be capable of eliciting immune responses in a broad range of tissues,26, 33 including both IgG and IgA isotype antibodies. The focus of the present study was to engineer a biomaterial capable of overcoming the challenge of promoting sublingual vaccination both against peptide epitopes and with supramolecular materials, rather than the development of a mucosal vaccine for a specific disease. This led us to focus on serum IgG as a reliable readout of whether immune responses were being raised that was monitorable over time, though we did also measure IgG in mucosal secretions (Fig. 4d, S8). There is evidence that IgG, IgA, and IgM antibodies can all contribute to the protection of mucosal barriers,79 as well as that the neutralization capacity of antibodies is more critical to protection than isotype.80 The anatomical locations and isotypes of antibodies elicted by sublingually-delivered nanofibers, and how material modulation and adjuvant choice may impact them, is an important future direction enabled by the present work.
An important consideration in applying these findings to other self-assembling peptides is that PEG conjugation can impact assembly differently depending on the type of material. The self-assembling peptide YYKLVFF formed nanofibers with larger diameters and shorter lengths when conjugated to 3000 MW PEG than when conjugated to 1000 MW PEG.81 A longer self-assembling peptide, the Aβ(10–35) fragment of amyloid-β, maintained a similar morphology after conjugation to 3000 MW PEG.49 The observed dimensions may also be impacted by the method of imaging. The use of quick-freeze/deep-etch TEM, which preserves native morphology, showed Q11-PEG3000 to form longer nanofibers than unmodified Q11.47
Looking towards the applications of peptide-based vaccines, sublingual formulations hold promise for the developing world. The reuse of needles in developing nations is a significant concern; it was estimated in 2000 a full 40% of the 16 billion injections worldwide were performed with reused needles, leading to millions of new infections.29 Sublingual vaccines can be administered in a variety of needle-free forms, including drops and dissolvable tablets.26 Furthermore, this administration route does not necessitate highly trained personnel.82 This is an important factor considering the dramatically rising total cost of vaccine schedules in the developing world, which increased from under one dollar per child in 2001 to roughly $21 for boys and, with the inclusion of the HPV vaccine, $35 for girls in 2014.28 Our previous work has also shown Q11 to retain its immunogenicity even after six months of heating at 45°C, 83 which could address the costly requirement of cold-chain storage and transport. A heat-stable, sublingually delivered vaccine could hold significant promise towards vaccine development in the developing world.
Our finding that even micrometer-scale materials can be made immunogenic sublingually via PEG conjugation also holds broad significance to fields outside of vaccine delivery. PEG is used to improve the pharmacokinetics of drugs and nanoparticles, and it is also contained in orally delivered consumer products such as foods, dental care products, and laxatives. Despite the close anatomical proximity of the oral and sublingual mucosa, the immune responses that can be raised via oral and sublingual delivery vary greatly.26 Our findings suggest that a careful parsing of the relative contributions of oral and sublingual delivery is crucial in understanding the immunogenicity of PEG and other components of products delivered by mouth, and that broader classes of ingested materials containing PEG may be immunogenic than previously thought.
CONCLUSIONS
We developed an approach to immunize sublingually against peptide epitopes using the combined effects of supramolecular assembly and surface modification. We showed that PEGylation of self-assembled Q11 nanofibers reduced nanofiber interactions with mucin and enabled robust, long-lived antibody responses and T cell responses when combined with a mucosal adjuvant. Lacking supramolecular assembly, PEGylated peptides delivered with adjuvant were not immunogenic, and reducing the length of the nanofibers had no impact on sublingual antibody responses. These findings illustrate that supramolecular assembly into PEGylated or PASylated nanofibers is a simple and modular means of eliciting immune responses sublingually against short peptide epitopes.
METHODS
Peptide Synthesis and Purification
OVAQ11 (H2N-ISQAVHAAHAEINEAGRSGSGQQKFQFQFEQQ-NH2) and pOVA (H2N-ISQAVHAAHAEINEAGR-NH2) were synthesized using standard Fmoc solid-phase synthesis on Rink amide resin. For synthesis of OVAQ11-PEG and pOVA-PEG, peptides were synthesized using a PAP Tentagel resin (Peptides International, RTS-9002) which produces peptides C-terminally conjugated to a 3000 MW PEG block after cleavage. mPEG variants were synthesized by on-resin conjugation of 350, 1000, or 2000 MW mPEG-NHS (Creative PEGWorks PJK-208, PLS-215, or PLS-214) to the N-terminus of Q11OVA (H2N-SGSGQQKFQFQFEQQSGSGISQAVHAAHAEINEAGR-NH2) or Q11ESAT651–70 (H2N-SGSGQQKFQFQFEQQSGSGYQGVQQKWDATATELNNALQ-NH2). Fluorescent peptides were synthesized by on-resin conjugation of 5(6)-TAMRA (AnaSpec, AS-81120–01) to the N-terminus using DIC and Cl-HOBt as coupling reagents. Peptides were cleaved for 2 hours at room temperature in a 95/2.5/2.5 TFA/triisopropylsilane/water cocktail, followed by washing with cold diethyl ether. Peptides were purified by reverse-phase HPLC using either a C18 column (OVAQ11, pOVA, W-Q11, mPEG350-Q11OVA) or a C4 column (OVAQ11-PEG, pOVA-PEG, mPEG1000-Q11OVA, mPEG2000-Q11OVA, mPEG2000-Q11ESAT651–70) and lyophilized. Peptide identity was confirmed using matrix-assisted laser desorption/ionization mass spectrometry on a Bruker Autoflex Speed LRF MALDI-TOF spectrometer using α-cyano-4-hydroxycinnamic acid as the matrix.
To prepare immunization formulations, lyophilized peptides were first dissolved at 8 mM in sterile water and incubated at 4 °C overnight. The solutions were then brought to the final concentrations indicated in 1X PBS by addition of sterile water and sterile 10X PBS and incubated at room temperature for 3 h. To create sheared nanofibers, solutions were subsequently subjected to repeated passage (15 – 20 times) through a 100 nm polycarbonate track-etched (PCTE) membrane using an Avanti Polar Lipids Mini-Extruder (Avanti, 610000). ImageJ was used to determine the length of 450 individual sheared fibers and 96 non-sheared fibers.
Transmission Electron Microscopy (TEM)
Peptides were prepared at 2 mM and diluted with 1X PBS to 0.2 mM, then 5 μL of peptide solution was deposited onto Formvar/carbon-coated 400 mesh copper grids (Electron Microscopy Sciences, EMS400-Cu). The sample was allowed to incubate for 1 min, washed with ultrapure water, and negatively stained for 1 minute with 1 % w/v uranyl acetate in water prior to wicking away with filter paper. Samples were imaged on an FEI Tecnai G² Twin electron microscope. Nanofiber lengths were measured using ImageJ.
Secondary Structure Analysis Using Circular Dichroism and Fluorescent Probes
For CD, peptides were prepared at 3 mM in 1X PBS and diluted to 0.1 mM in potassium fluoride (KF) buffer just prior to analysis. CD spectra were collected on a Chiroscan Plus spectrometer from 220 nm to 260 nm in a 0.1 cm path length quartz cuvette.
For Thioflavin T (ThT) and pyrene assays, lyophilized peptides were dissolved in either a 4.0 × 10−2 g/L solution of ThT (Alfa Aesar, J61043) in 1X PBS or a 1.3 × 10−4 g/L solution of pyrene (Sigma, 82648) in 1X PBS and incubated at room temperature for 3 h. A 100 μL aliquot of each solution was added to a black 96-well plate and read using a Molecular Devices Spectramax M2 spectrophotometer. For pyrene, the excitation wavelength was 339 nm, and the emission wavelength was 373 nm. For Thioflavin T assays, following the method of Hamley and coworkers,56 OVAQ11-PEG and OVAQ11 were dissolved at several concentrations in PBS containing thioflavin T, and the fluorescence intensity was measured at 488 nm with an excitation wavelength of 440 nm. The intensity for each peptide concentration was divided by the intensity of a ThT solution containing no peptide (Io). The graphical estimates of critical aggregation concentration correspond to the intersection of the pre- and post-assembly tangent lines.
Zeta-potential Measurements
Peptides were prepared at 2 mM and diluted to 0.2 mM with 1X PBS just prior to analysis. Zeta-potential was measured at 25 °C using a Malvern Nan o ZetaSizer.
Mice and Immunizations
Except where indicated otherwise, female C57BL/6 mice were purchased from Envigo, and experiments were initiated for mice between 8–12 weeks of age (age matched within experiments). CBA/J mice were used for immunization with the ESAT651–70 epitope due to haplotype compatibility, while hairless SKH-1 Elite mice were used for IVIS imaging. Animal experiments were approved by the Institutional Care and Use Committees of Duke University and the University of Chicago. Some subcutaneous immunizations were performed at the University of Chicago; all other mouse experiments were performed at Duke University. For subcutaneous immunizations, mice were anesthetized using isoflurane and given two 50 μL injections of 2 mM peptide, one behind each shoulder, and booster immunizations of the same dose were given 4 weeks after the primary immunizations. For sublingual immunizations, mice were deeply anesthetized by a 100 μL intraperitoneal injection of a cocktail delivering 100 mg/kg ketamine and 10 mg/kg xylazine. A micropipette with a 20 μL tip was used to apply of the immunizing solution below the tongue, and the mice’s heads were placed in anteflexion for 20 min following administration to prevent swallowing of the material. Adjuvanted formulations included 2 μg of cholera toxin (List Biological Laboratories, 101B), 10 μg of CTB (List Biological Laboratories, 103B), or 10 μg of cyclic-di-AMP (Invivogen, vac-nacda) as described in figure captions.
In Vitro Presentation Assay
Bone marrow derived dendritic cells (BMDCs) were isolated from the femurs and tibias of naïve mice and added at 2 × 106/mL (5 mL) in complete RPMI medium to a 6-well plate. To each well, 1 μg of Flt-3L (Thermo Fisher, PHC9411) was added, and the BMDCs were incubated at 37 °C for 8 d. The BMDCs were added at 2 × 105/mL (50 μL) to a 96-well plate along with 50 μL of serially diluted peptides, and incubated at 37 °C for 2 h. T he plates were centrifuged at 500 × g for 5 min, the supernatant was aspirated, and 100 μL of DOBW cells (T cell hybridoma) resuspended at 5 × 10−5 cells/mL in complete DMEM media were added to each well and incubated overnight at 37 °C. DOBW cells produce IL-2 when they encounter pOVA presented in MHC class II (I-Ab). The plates were centrifuged for 5 min at 500 × g, the supernatant was collected, and the IL-2 concentration was measured using an ELISA kit (BD Bioscience, Cat# 55148). DOBW T cell hybridoma cells were provided by C. Harding.
In Vitro Cell Viability Assay
DC2.4 cells were seeded at 5 × 105/mL (100 μL) in serum-free medium onto a 96-well plate and incubated at 37 °C overnight. The supernatant was a spirated and either peptide solutions (prepared at 2 mM) or alum (as a positive assay control for cytotoxicity) were diluted in serum-free media, added to the wells (100 μL per well), and incubated with cells for 24 hours at 37 °C. Next, 10 μL of alamarBlue cell viability reagent (Thermo Fisher, DAL1025) was added to each well and plates were incubated for 5 hours at 37 °C. The flu orescence intensity was measured with an excitation wavelength of 570 nm and an emission wavelength of 610 nm.
Mucosal Antibody Responses
To measure antibody responses in the reproductive tract, mice were immunized at weeks 0, 1, 3, and 9 with 7 μL of 5.6 mM peptide with 2 μg cholera toxin adjuvant per mouse. Mice were sacrificed at week 10 (one week after the final immunization), and vaginal washes were collected. Samples were diluted 1:10 and pOVA-specific IgG was measured by ELISA. To collect vaginal washes, mice were anesthetized with isoflurane and the vagina was flushed with 40 μL of 1X PBS using a micropipette. Nasal wash was collected in post-mortem mice by flushing 200 μL of 1X PBS through the choanae.
Measurement of Antibody Responses Using ELISA
Serum was collected via the submandibular vein. For analysis of antigen-specific IgG by ELISA, plates were coated with a 20 μg/mL streptavidin solution and incubated overnight at 4 °C. Plates were washed with 0.5 g/L Tween-20 in PBS (1X PBST), blocked by Superblock blocking buffer solution (Thermo Fisher, Cat #37515), washed again, and coated with 20 μg/mL of biotin-pOVA or biotin-pESAT651–70 for 1 h at room temperature. Sera or mucosal secretions were diluted in 10 g/L BSA in 1X PBST and added to the plate, and antigen-specific IgG was detected by horseradish peroxidase (HRP) conjugated Fcγ fragment specific goat anti-mouse IgG (Jackson Immuno Research, Cat #115–035-071). Titers were calculated as previously described.40 For measuring antibody subclasses, HRP conjugated goat anti-mouse detection antibodies were purchased from Southern Biotech (IgG1: 1071–05, IgG2b: 1091–05, IgG2c: 1078–05, IgG3: 1101–05).
Measurement of T Cell Responses Using ELISPOT
To analyze T cell activation by ELISPOT, mice were sacrificed 7 d after the final booster immunization and spleens were harvested. Briefly, 0.25 million splenocytes in 200 μL were plated in each well of a 96-well ELISPOT plate (Millipore, MSIPS4510). The cells were stimulated with 5 μM pOVA peptide, left untreated as negative controls, or stimulated with Concanavalin A (ConA) (Sigma, C5275) as positive controls. To detect IFNγ secreting cell spots, a biotinylated anti-mouse IFNγ (Mabtech, 551881) detection antibody pair from BD Bioscience, streptavidin-alkaline phosphatase (Mabtech, 3310–0), and Sigmafast BCIP/NBT (Sigma, B5655) from Sigma Aldrich were used. Plates were imaged and counted by Zellnet Consulting using a Zeiss KS ELISPOT reader.
Isothermal Titration Calorimetry
OVAQ11, mPEG350-Q11OVA, mPEG1000-Q11OVA, mPEG2000Q11OVA, and OVAQ11-PEG3000 were prepared at 2 mM and diluted to 0.25 mM in 1X PBS just prior to analysis. A MicroCal Auto-iTC200 instrument was used to perform an automated isothermal titration of the peptide solutions into a 10 mg/mL solution of mucin from bovine submaxillary glands (Sigma, M3895) in 1X PBS. Malvern MicroCal software was used to fit the data to a one-site binding model and to derive the stoichiometry of binding between the peptide and mucin solutions, which was normalized to the value for OVAQ11 to yield a percentage change.
Sublingual Immunization Site Monitoring
Female SKH1-Elite mice (a hairless and immunocompetent strain) were purchased from Charles River. Mice were anesthetized as described above and 7 μL of 5 mM TAMRA-OVAQ11 or TAMRA-OVAQ11-PEG was applied below the tongue. The mouse’s heads were placed in anteflexion for 20 minutes to prevent swallowing of the material. The ventral view of each mouse was imaged using an IVIS Lumina XR imaging system with 535 nm excitation and a DsRed filter. The radiant efficiency of the administration site was quantified using Perkin Elmer Living Imaging software and the radiant efficiency at each timepoint for each mouse was normalized to the initial signal (20 minutes post administration) to give the fraction of signal remaining.
Statistical Analysis
Statistical analysis was performed as indicated in figure legends using GraphPad Prism software. Means ± standard error of the mean (s.e.m.) are presented. Statistically significant differences are indicated in each graph as *p < 0.05, **p < 0.01, and ***p < 0.001. Non-significant differences are indicated as n.s.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by the National Institutes of Health (NIBIB 5R01EB009701; NIAID 5R01AI118182). SHK is supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. DGE-1644868. The MALDI was performed on an instrument supported by NCBC grant 2017-IDG-1018. Circular dichroism and isothermal titration calorimetry were performed at the University of North Carolina at Chapel Hill using instruments supported by NIH grant P30CA016086. DC2.4 cells were kindly provided by Dr. Kenneth L. Rock (University of Massachusetts Medical Center, Worcester, MA, USA).
Footnotes
Competing Interests
JHC and SHK are listed as inventors on a patent application associated with the technology described.
DATA AND MATERIALS AVAILABILITY
The datasets generated and/or analyzed during the reported study are available from the corresponding author upon reasonable request.
SUPPORTING INFORMATION
Sequences of peptides used in this paper, MALDI spectrum of OVAQ11-PEG molecular weight distribution, pyrene fluorescence assay, complete set of TEM images and ImageJ traces used to quantify nanofiber length distributions, peptide concentration before and after shearing, additional sublingual immunizations, complete ELISPOT results, in vitro presentation and cytotoxicity assays, complete antibody results for immunization with mPEG-conjugated nanofibers, complete IVIS imaging image set
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bookstaver ML; Tsai SJ; Bromberg JS; Jewell CM, Improving Vaccine and Immunotherapy Design Using Biomaterials. Trends in Immunology 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly SH; Shores LS; Votaw NL; Collier JH, Biomaterial strategies for generating therapeutic immune responses. Advanced Drug Delivery Reviews 2017, 114, 3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotaling NA; Tang L; Irvine DJ; Babensee JE, Biomaterial strategies for immunomodulation. Annual Review of Biomedical Engineering 2015, 17, 317–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J; Pompano RR; Santiago FW; Maillat L; Sciammas R; Sun T; Han H; Topham DJ; Chong AS; Collier JH, The use of self-adjuvanting nanofiber vaccines to elicit high-affinity B cell responses to peptide antigens without inflammation. Biomaterials 2013, 34 (34), 8776–8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mora-Solano C; Wen Y; Han H; Chen J; Chong AS; Miller ML; Pompano RR; Collier JH, Active immunotherapy for TNF-mediated inflammation using self-assembled peptide nanofibers. Biomaterials 2017, 149, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rudra JS; Mishra S; Chong AS; Mitchell RA; Nardin EH; Nussenzweig V; Collier JH, Self-assembled peptide nanofibers raising durable antibody responses against a malaria epitope. Biomaterials 2012, 33 (27), 6476–6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Y; Norberg PK; Reap EA; Congdon KL; Fries CN; Kelly SH; Sampson JH; Conticello VP; Collier JH, A Supramolecular Vaccine Platform Based on α-Helical Peptide Nanofibers. ACS Biomaterials Science & Engineering 2017, 3 (12), 3128–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chesson CB; Huelsmann EJ; Lacek AT; Kohlhapp FJ; Webb MF; Nabatiyan A; Zloza A; Rudra JS, Antigenic peptide nanofibers elicit adjuvant-free CD8+ T cell responses. Vaccine 2014, 32 (10), 1174–1180. [DOI] [PubMed] [Google Scholar]
- 9.Rudra JS; Banasik BN; Milligan GN, A combined carrier-adjuvant system of peptide nanofibers and toll-like receptor agonists potentiates robust CD8+ T cell responses. Vaccine 2018, 36 (4), 438–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trent A; Ulery BD; Black MJ; Barrett JC; Liang S; Kostenko Y; David NA; Tirrell MV, Peptide amphiphile micelles self-adjuvant group A streptococcal vaccination. The AAPS Journal 2015, 17 (2), 380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuba E; Sakaguchi N; Kanda Y; Miyazaki M; Koiwai K, pH-Responsive Micelle-Based Cytoplasmic Delivery System for Induction of Cellular Immunity. Vaccines 2017, 5 (4), 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rincon-Restrepo M; Mayer A; Hauert S; Bonner DK; Phelps EA; Hubbell JA; Swartz MA; Hirosue S, Vaccine nanocarriers: Coupling intracellular pathways and cellular biodistribution to control CD4 vs CD8 T cell responses. Biomaterials 2017, 132, 48–58. [DOI] [PubMed] [Google Scholar]
- 13.Mueller SN; Tian S; DeSimone JM, Rapid and persistent delivery of antigen by lymph node targeting PRINT nanoparticle vaccine carrier to promote humoral immunity. Molecular Pharmaceutics 2015, 12 (5), 1356–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson JT; Keller S; Manganiello MJ; Cheng C; Lee C-C; Opara C; Convertine A; Stayton PS, pH-Responsive nanoparticle vaccines for dual-delivery of antigens and immunostimulatory oligonucleotides. ACS Nano 2013, 7 (5), 3912–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dowling DJ; Scott EA; Scheid A; Bergelson I; Joshi S; Pietrasanta C; Brightman S; Sanchez-Schmitz G; Van Haren SD; Ninković J, Toll-like receptor 8 agonist nanoparticles mimic immunomodulating effects of the live BCG vaccine and enhance neonatal innate and adaptive immune responses. Journal of Allergy and Clinical Immunology 2017, 140 (5), 1339–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng Q; Zhang P; Zeng X; Tostanoski LH; Jewell CM, Advanced manufacturing of microdisk vaccines for uniform control of material properties and immune cell function. Biomaterials Science 2018, 6 (1), 115–124. [DOI] [PubMed] [Google Scholar]
- 17.Wilson DS; Hirosue S; Raczy MM; Bonilla-Ramirez L; Jeanbart L; Wang R; Kwissa M; Franetich J-F; Broggi MA; Diaceri G, Antigens reversibly conjugated to a polymeric glyco-adjuvant induce protective humoral and cellular immunity. Nature Materials 2019, 1. [DOI] [PubMed] [Google Scholar]
- 18.Oberoi HS; Yorgensen YM; Morasse A; Evans JT; Burkhart DJ, PEG modified liposomes containing CRX-601 adjuvant in combination with methylglycol chitosan enhance the murine sublingual immune response to influenza vaccination. Journal of Controlled Release 2016, 223, 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spinner JL; Oberoi HS; Yorgensen YM; Poirier DS; Burkhart DJ; Plante M; Evans JT, Methylglycol chitosan and a synthetic TLR4 agonist enhance immune responses to influenza vaccine administered sublingually. Vaccine 2015, 33 (43), 5845–5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuai R; Ochyl LJ; Bahjat KS; Schwendeman A; Moon JJ, Designer vaccine nanodiscs for personalized cancer immunotherapy. Nature Materials 2017, 16 (4), 489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moon JJ; Suh H; Bershteyn A; Stephan MT; Liu H; Huang B; Sohail M; Luo S; Um SH; Khant H, Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nature Materials 2011, 10 (3), 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neutra MR; Kozlowski PA, Mucosal vaccines: the promise and the challenge. Nature Reviews Immunology 2006, 6 (2), 148. [DOI] [PubMed] [Google Scholar]
- 23.Lycke N, Recent progress in mucosal vaccine development: potential and limitations. Nature Reviews Immunology 2012, 12 (8), 592. [DOI] [PubMed] [Google Scholar]
- 24.Woodrow KA; Bennett KM; Lo DD, Mucosal vaccine design and delivery. Annual Review of Biomedical Engineering 2012, 14, 17–46. [DOI] [PubMed] [Google Scholar]
- 25.Shakya AK; Chowdhury MY; Tao W; Gill HS, Mucosal vaccine delivery: current state and a pediatric perspective. Journal of Controlled Release 2016, 240, 394–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kraan H; Vrieling H; Czerkinsky C; Jiskoot W; Kersten G; Amorij J-P, Buccal and sublingual vaccine delivery. Journal of Controlled Release 2014, 190, 580–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma Y; Tao W; Krebs SJ; Sutton WF; Haigwood NL; Gill HS, Vaccine delivery to the oral cavity using coated microneedles induces systemic and mucosal immunity. Pharmaceutica lResearch 2014, 31 (9), 2393–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen AK; Fields R; McQuestion M, The future of routine immunization in the developing world: challenges and opportunities. Global Health: Science and Practice 2014, 2 (4), 381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hauri AM; Armstrong GL; Hutin YJ, The global burden of disease attributable to contaminated injections given in health care settings. International Journal of STD & AIDS 2004, 15 (1), 7–16. [DOI] [PubMed] [Google Scholar]
- 30.Seth A; Kong IG; Lee S-H; Yang J-Y; Lee Y-S; Kim Y; Wibowo N; Middelberg AP; Lua LH; Kweon M-N, Modular virus-like particles for sublingual vaccination against group A streptococcus. Vaccine 2016, 34 (51), 6472–6480. [DOI] [PubMed] [Google Scholar]
- 31.Hwang HS; Puth S; Tan W; Verma V; Jeong K; Lee SE; Rhee JH, More robust gut immune responses induced by combining intranasal and sublingual routes for prime-boost immunization. Human Vaccines & Immunotherapeutics 2018, 14 (9), 2194–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thirion-Delalande C; Gervais F; Fisch C; Cuiné J; Baron-Bodo V; Moingeon P; Mascarell L, Comparative analysis of the oral mucosae from rodents and non-rodents: Application to the nonclinical evaluation of sublingual immunotherapy products. PloS One 2017, 12 (9), e0183398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Çuburu N; Kweon M-N; Song J-H; Hervouet C; Luci C; Sun J-B; Hofman P; Holmgren J; Anjuère F; Czerkinsky C, Sublingual immunization induces broad-based systemic and mucosal immune responses in mice. Vaccine 2007, 25 (51), 8598–8610. [DOI] [PubMed] [Google Scholar]
- 34.Lai SK; Wang Y-Y; Wirtz D; Hanes J, Micro-and macrorheology of mucus. Advanced Drug Delivery Reviews 2009, 61 (2), 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai SK; Wang Y-Y; Hanes J, Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Advanced Drug Delivery Reviews 2009, 61 (2), 158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu Q; Ensign LM; Boylan NJ; Schön A; Gong X; Yang J-C; Lamb NW; Cai S; Yu T; Freire E, Impact of surface polyethylene glycol (PEG) density on biodegradable nanoparticle transport in mucus ex vivo and distribution in vivo. ACS Nano 2015, 9 (9), 9217–9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang BC; Dawson M; Lai SK; Wang Y-Y; Suk JS; Yang M; Zeitlin P; Boyle MP; Fu J; Hanes J, Biodegradable polymer nanoparticles that rapidly penetrate the human mucus barrier. Proceedings of the National Academy of Sciences 2009, 106 (46), 19268–19273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stano A; van der Vlies AJ; Martino MM; Swartz MA; Hubbell JA; Simeoni E, PPS nanoparticles as versatile delivery system to induce systemic and broad mucosal immunity after intranasal administration. Vaccine 2011, 29 (4), 804–812. [DOI] [PubMed] [Google Scholar]
- 39.Cu Y; Booth CJ; Saltzman WM, In vivo distribution of surface-modified PLGA nanoparticles following intravaginal delivery. Journal of Controlled Release 2011, 156 (2), 258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rudra JS; Tian YF; Jung JP; Collier JH, A self-assembling peptide acting as an immune adjuvant. Proceedings of the National Academy of Sciences 2010, 107 (2), 622–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Si Y; Wen Y; Kelly SH; Chong AS; Collier JH, Intranasal delivery of adjuvant-free peptide nanofibers elicits resident CD8+ T cell responses. Journal of Controlled Release 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang Z-H; Shi L; Ma J-W; Sun Z-Y; Cai H; Chen Y-X; Zhao Y-F; Li Y-M, A totally synthetic, self-assembling, adjuvant-free MUC1 glycopeptide vaccine for cancer therapy. Journal of the American Chemical Society 2012, 134 (21), 8730–8733. [DOI] [PubMed] [Google Scholar]
- 43.Appavu R; Chesson CB; Koyfman AY; Snook JD; Kohlhapp FJ; Zloza A; Rudra JS, Enhancing the magnitude of antibody responses through biomaterial stereochemistry. ACS Biomaterials Science & Engineering 2015, 1 (7), 601–609. [DOI] [PubMed] [Google Scholar]
- 44.Pompano RR; Chen J; Verbus EA; Han H; Fridman A; McNeely T; Collier JH; Chong AS, Titrating T-Cell Epitopes within Self-Assembled Vaccines Optimizes CD4+ Helper T Cell and Antibody Outputs. Advanced Healthcare Materials 2014, 3 (11), 1898–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wen Y; Waltman A; Han H; Collier JH, Switching the immunogenicity of peptide assemblies using surface properties. ACS nano 2016, 10 (10), 9274–9286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Law B; Weissleder R; Tung C-H, Protease-sensitive fluorescent nanofibers. Bioconjugate Chemistry 2007, 18 (6), 1701–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collier JH; Messersmith PB, Self-Assembling Polymer–Peptide Conjugates: Nanostructural Tailoring. Advanced Materials 2004, 16 (11), 907–910. [Google Scholar]
- 48.Thiyagarajan P; Burkoth T; Urban V; Seifert S; Benzinger T; Morgan D; Gordon D; Meredith S; Lynn D, pH dependent self assembly of β-amyloid (10–35) and β-amyloid (10–35)-PEG3000. Journal of Applied Crystallography 2000, 33 (3), 535–539. [Google Scholar]
- 49.Burkoth TS; Benzinger TL; Urban V; Lynn DG; Meredith SC; Thiyagarajan P, Self-assembly of aβ (10–35)-peg block copolymer fibrils. Journal of the American Chemical Society 1999, 121 (32), 7429–7430. [Google Scholar]
- 50.Krysmann MJ; Castelletto V; Kelarakis A; Hamley IW; Hule RA; Pochan DJ, Self-assembly and hydrogelation of an amyloid peptide fragment. Biochemistry 2008, 47 (16), 4597–4605. [DOI] [PubMed] [Google Scholar]
- 51.Castelletto V; Newby G; Zhu Z; Hamley I; Noirez L, Self-assembly of PEGylated peptide conjugates containing a modified amyloid β-peptide fragment. Langmuir 2010, 26 (12), 9986–9996. [DOI] [PubMed] [Google Scholar]
- 52.Lai SK; Wang Y-Y; Hida K; Cone R; Hanes J, Nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses. Proceedings of the National Academy of Sciences 2010, 107 (2), 598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teubl BJ; Stojkovic B; Docter D; Pritz E; Leitinger G; Poberaj I; Prassl R; Stauber RH; Fröhlich E; Khinast JG, The effect of saliva on the fate of nanoparticles. Clinical Oral Investigations 2018, 22 (2), 929–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Collier JH; Messersmith PB, Enzymatic modification of self-assembled peptide structures with tissue transglutaminase. Bioconjugate Chemistry 2003, 14 (4), 748–755. [DOI] [PubMed] [Google Scholar]
- 55.Hamley IW; Dehsorkhi A; Castelletto V; Walter MN; Connon CJ; Reza M; Ruokolainen J, Self-Assembly and Collagen-Stimulating Activity of a Peptide Amphiphile Incorporating a Peptide Sequence from Lumican. Langmuir 2015, 31 (15), 4490–4495. [DOI] [PubMed] [Google Scholar]
- 56.Castelletto V; Gouveia RJ; Connon CJ; Hamley IW, Self-assembly and bioactivity of a polymer/peptide conjugate containing the RGD cell adhesion motif and PEG. European Polymer Journal 2013, 49 (10), 2961–2967. [Google Scholar]
- 57.Castelletto V; Hamley IW, Self assembly of a model amphiphilic phenylalanine peptide/polyethylene glycol block copolymer in aqueous solution. Biophysical Chemistry 2009, 141 (2–3), 169–174. [DOI] [PubMed] [Google Scholar]
- 58.Wagh A; Singh J; Qian S; Law B, A short circulating peptide nanofiber as a carrier for tumoral delivery. Nanomedicine: Nanotechnology, Biology and Medicine 2013, 9 (4), 449–457. [DOI] [PubMed] [Google Scholar]
- 59.Rudra JS; Sun T; Bird KC; Daniels MD; Gasiorowski JZ; Chong AS; Collier JH, Modulating adaptive immune responses to peptide self-assemblies. ACS Nano 2012, 6 (2), 1557–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song J-H; Nguyen HH; Cuburu N; Horimoto T; Ko S-Y; Park S-H; Czerkinsky C; Kweon M-N, Sublingual vaccination with influenza virus protects mice against lethal viral infection. Proceedings of the National Academy of Sciences 2008, 105 (5), 1644–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cong Y; Bowdon HR; Elson CO, Identification of an immunodominant T cell epitope on cholera toxin. European Journal of Immunology 1996, 26 (11), 2587–2594. [DOI] [PubMed] [Google Scholar]
- 62.Stratmann T, Cholera toxin subunit B as adjuvant––An accelerator in protective immunity and a break in autoimmunity. Vaccines 2015, 3 (3), 579–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matoba N, N-Glycosylation of Cholera Toxin B Subunit: Serendipity for Novel Plant-Made Vaccines? Frontiers in Plant Science 2015, 6, 1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bergquist C; Johansson E-L; Lagergård T; Holmgren J; Rudin A, Intranasal vaccination of humans with recombinant cholera toxin B subunit induces systemic and local antibody responses in the upper respiratory tract and the vagina. Infection and Immunity 1997, 65 (7), 2676–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schlapschy M; Binder U; Börger C; Theobald I; Wachinger K; Kisling S; Haller D; Skerra A, PASylation: a biological alternative to PEGylation for extending the plasma half-life of pharmaceutically active proteins. Protein Engineering, Design & Selection 2013, 26 (8), 489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Binder U; Skerra A, PASylation®: a versatile technology to extend drug delivery. Current Opinion in Colloid & Interface Science 2017, 31, 10–17. [Google Scholar]
- 67.Khutoryanskiy VV, Beyond PEGylation: alternative surface-modification of nanoparticles with mucus-inert biomaterials. Advanced Drug Delivery Reviews 2018, 124, 140–149. [DOI] [PubMed] [Google Scholar]
- 68.Zhang P; Sun F; Liu S; Jiang S, Anti-PEG antibodies in the clinic: Current issues and beyond PEGylation. Journal of Controlled Release 2016, 244, 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garay RP; El-Gewely R; Armstrong JK; Garratty G; Richette P, Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents. Taylor & Francis: 2012. [DOI] [PubMed] [Google Scholar]
- 70.Armstrong JK, The occurrence, induction, specificity and potential effect of antibodies against poly (ethylene glycol) In Pegylated protein drugs: Basic science and clinical applications, Springer: 2009; pp 147–168. [Google Scholar]
- 71.Armstrong J; Leger R; Wenby R; Meiselman H; Garratty G; Fisher T In Occurrence of an antibody to poly (ethylene glycol) in normal donors, Blood, 2003; pp 556A–556A. [Google Scholar]
- 72.Richter AW; Åkerblom E, Polyethylene glycol reactive antibodies in man: titer distribution in allergic patients treated with monomethoxy polyethylene glycol modified allergens or placebo, and in healthy blood donors. International Archives of Allergy and Immunology 1984, 74 (1), 36–39. [DOI] [PubMed] [Google Scholar]
- 73.Weinreich Olsen A; Hansen PR; Holm A; Andersen P, Efficient protection against Mycobacterium tuberculosis by vaccination with a single subdominant epitope from the ESAT-6 antigen. EuropeanJournal of Immunology 2000, 30 (6), 1724–1732. [DOI] [PubMed] [Google Scholar]
- 74.Cone RA, Barrier properties of mucus. Advanced Drug Delivery Reviews 2009, 61 (2), 75–85. [DOI] [PubMed] [Google Scholar]
- 75.Gasiorowski JZ; Collier JH, Directed intermixing in multicomponent self-assembling biomaterials. Biomacromolecules 2011, 12 (10), 3549–3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lai SK; O’Hanlon DE; Harrold S; Man ST; Wang Y-Y; Cone R; Hanes J, Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proceedings of the National Academy of Sciences 2007, 104 (5), 1482–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hervouet C; Luci C; Bekri S; Juhel T; Bihl F; Braud V; Czerkinsky C; Anjuere F, Antigen-bearing dendritic cells from the sublingual mucosa recirculate to distant systemic lymphoid organs to prime mucosal CD8 T cells. Mucosal Immunology 2014, 7 (2), 280. [DOI] [PubMed] [Google Scholar]
- 78.Rudra JS; Ding Y; Neelakantan H; Ding C; Appavu R; Stutz S; Snook JD; Chen H; Cunningham KA; Zhou J, Suppression of cocaine-evoked hyperactivity by self-adjuvanting and multivalent peptide nanofiber vaccines. ACS Chemical Neuroscience 2016, 7 (5), 546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ruprecht RM; Marasini B; Thippeshappa R, Mucosal Antibodies: Defending Epithelial Barriers against HIV-1 Invasion. Vaccines 2019, 7 (4), 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Astronomo RD; Santra S; Ballweber-Fleming L; Westerberg KG; Mach L; Hensley-McBain T; Sutherland L; Mildenberg B; Morton G; Yates NL, Neutralization takes precedence over IgG or IgA isotype-related functions in mucosal HIV-1 antibody-mediated protection. EBioMedicine 2016, 14, 97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Castelletto V; Newby G; Merino DH; Hamley I; Liu D; Noirez L, Self-assembly of an amyloid peptide fragment–PEG conjugate: lyotropic phase formation and influence of PEG crystallization. Polymer Chemistry 2010, 1 (4), 453–459. [Google Scholar]
- 82.Schulze K; Ebensen T; Riese P; Prochnow B; Lehr C-M; Guzmán CA, New horizons in the development of novel needle-free immunization strategies to increase vaccination efficacy In How to Overcome the Antibiotic Crisis, Springer: 2016; pp 207–234. [DOI] [PubMed] [Google Scholar]
- 83.Sun T; Han H; Hudalla GA; Wen Y; Pompano RR; Collier JH, Thermal stability of self-assembled peptide vaccine materials. Acta Biomaterialia 2016, 30, 62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.